Abstract
Object:
Epilepsy surgery is effective for lesional epilepsy but can be associated with significant morbidity when seizures originate from eloquent cortex that is resected. Here, the objective is to describe chronic subthreshold cortical stimulation and evaluate its early surgical safety profile in adult patients with epilepsy originating from seizure foci not amenable to surgical resection.
Methods:
Adult patients with focal drug resistant epilepsy underwent intracranial electroencephalography monitoring for evaluation of surgical resection. Those with seizure foci in eloquent cortex were not resection candidates and were offered a short therapeutic trial of continuous subthreshold cortical stimulation via intracranial monitoring electrodes. After a successful trial, electrodes were explanted and permanent stimulation hardware was implanted.
Results:
Ten adult patients (6 males) from 2014 to 2016 were included. Intracranial pathologies included: cortical dysplasia (n = 3), encephalomalacia (n = 3), cortical tubers (n = 1), Rasmussen encephalitis (n = 1) and linear migrational anomaly (n = 1) based on radiographic imaging. The duration of intracranial monitoring ranged from 3–20 days. All patients experienced an uneventful postoperative course and were discharged home with median length of stay 10 days. No postoperative surgical complications developed (median length of follow-up: 7.7 months). Seizure severity and seizure frequency improved in all patients.
Conclusion:
Our institutional experience shows that chronic subthreshold cortical stimulation can be safely and effectively performed in appropriately selected patients without postoperative complications in this small group. Future investigation will provide further insight to recently published results regarding mechanism and efficacy of this novel and promising intervention.
Keywords: intractable epilepsy, medically refractory epilepsy, cortical stimulation, cortical electrodes
Introduction
Approximately one-third of the three million people in the United States with epilepsy continue to have seizures despite taking anti-seizure medications (ASMs)6, termed drug resistant epilepsy.10 Surgical resection has been established as a safe and effective treatment for these patients20, however, epileptic foci located within eloquent cortex represent a specific challenge as postoperative neurological morbidity may outweigh potential benefits.
Several recent studies have shown brain stimulation to be a viable strategy for select patients with intractable epilepsy.7,9,18 Many different cortical and subcortical brain regions, including the thalamus, the cerebellum and the hippocampus, have been selected as neurostimulation targets utilizing either duty-cycle13 or responsive stimulation paradigms.3 Despite their demonstrated efficacy in randomized controlled trials, currently approved stimulation approaches uncommonly yield seizure-free outcomes.3,13 Chronic subthreshold cortical stimulation (CSCS) may represent a more effective option. Currently available responsive neurostimulation device detection algorithms3 use a low spectral bandwidth (0.1 – 100 Hz)21 and do not probe small spatial scales (<1 mm3) and abnormal microdomain oscillations17, limiting early detection. Similarly, duty-cycle stimulation paradigms (e.g. 1 minute on and 5 minute off) leave ample time for pathological rhythms to develop and evolve to large scale seizures. We have previously published our preliminary experience with CSCS focusing on seizure impact and outcome.11 Herein, we describethe safety, feasibility and technique of CSCS in ten adult patients.
MATERIALS AND METHODS
After Institutional Review Board approval, data were obtained from the medical records and operative notes. All patients were diagnosed with seizures refractory to multiple ASMs and had epileptic foci residing within eloquent cortex based on intracranial electroencephalography (iEEG) monitoring and cortical stimulation mapping. A trial of focal CSCS was performed via the monitoring electrodes in each patient prior to permanent implantation, as previously described.11 Stimulated electrodes included subdural contacts and depth electrode contacts determined from prolonged iEEG to be in the seizure onset zone.
After intracranial EEG electrodes were explanted, permanent stimulation was performed with hardware typically used for spinal cord stimulation or deep brain stimulation, which were repurposed for implantation and stimulation targeting the detected seizure focus with CSCS. The 16-electrode Medtronic 37702 PrimeAdvanced and Medtronic 97712 RestoreUltra NeuroStimulators were used as the implantable pulse generators. Employed Medtronic hardware included: 2×8 surgical leads Model 39286 (length 65 cm) with sixteen 4×1.5 mm rectangular contacts; DBS lead Model 3387 (length 40 cm) with four 1.5 mm electrodes at the tip separated by 1.5 mm; DBS lead Model 3389 (length 40 cm) with four 1.5 cm electrodes at the tip separated by 0.5 mm; DBS lead Model 3391 (length 40 cm) with four 3 mm electrodes at the tip separated by 4 mm; Resume II lead Model 3587 (length 25 cm) with conducting electrode tip. Dually tunneled 2 to 1 40 cm lead extensions were also used to take advantage of all 16 channels of the battery. All patients were fully informed of the procedure’s benefits and risks and consented to the operation and the study.
RESULTS
Ten adult patients (6 male) received CSCS between 2014 and 2016; six patients were included in a prior brief report [11]. Ages ranged from 19–56 years with seizure history duration of 2–50 years. Intracranial pathologies comprised: cortical dysplasia (n = 3), encephalomalacia (n = 3), cortical tubers (n = 1), Rasmussen encephalitis (n = 1) and linear migrational anomaly (n = 1). The duration of intracranial monitoring ranged from 3–20 days with a 10 day median length of stay. An expanded description of CSCS is included in the initial case presentation. No postoperative surgical complications developed (median length of follow-up: 7.7 months). Specifically, no infections were seen or focal deficits from permanent implants. Seizure severity and seizure frequency improved for all patients. Two were class 1 (free of disabling seizures), five were Engel class 2 (rare disabling seizures), and three were class 3 (worthwhile improvement of seizure burden) [Table 1].
Table 1.
Summary of cases
| Case # | Gender | Age at surgery (years) | Duration of seizure history (years) | Pathology | Duration of monitoring (days) | TLOS (days) | Postoperative Complications | Follow-up length (months) | Engel Class |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 28 | 16 | Cortical dysplasia and bilateral MTS | 3 | 8 | None | 8.1 | 2 |
| 2 | Male | 25 | 15 | Presumptive linear migrational anomaly | 10 | 12 | None | 6.7 | 2 |
| 3 | Male | 56 | 50 | Multiple right-sided encephalomalacias | 3 | 9 | None | 10 | 3 |
| 4 | Male | 19 | 5 | Encephalomalacia with gliosis | 20 | 19 | None | 4 | 1 |
| 5 | Female | 19 | 2 | Right precentral deep cortical dysplasia | 6 | 9 | None | 8.9 | 2 |
| 6 | Male | 26 | 11 | Encephalomalacia | 4 | 6 | None | 5.9 | 2 |
| 7 | Female | 22 | 19 | Cortical tubers, SEN, SEGA | 9 | 11 | None | 5.6 | 3 |
| 8 | Female | 27 | 21 | Cortical dysplasia | 8 | 11 | None | 9 | 1 |
| 9 | Female | 39 | 21 | Rasmussen encephalitis – Left parietal atrophy | 3 | 6 | None | 7.3 | 2 |
| 10 | Male | 23 | 12 | None | 7 | 11 | None | 20.5 | 3 |
MTS: mesial temporal sclerosis; SEGA: subependymal giant cell astrocytoma; SEN: subependymal nodules; TLOS: total length of stay
Case Presentations
Case #1
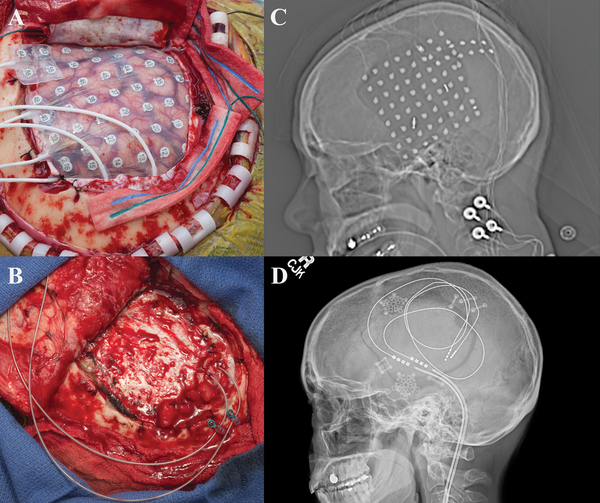
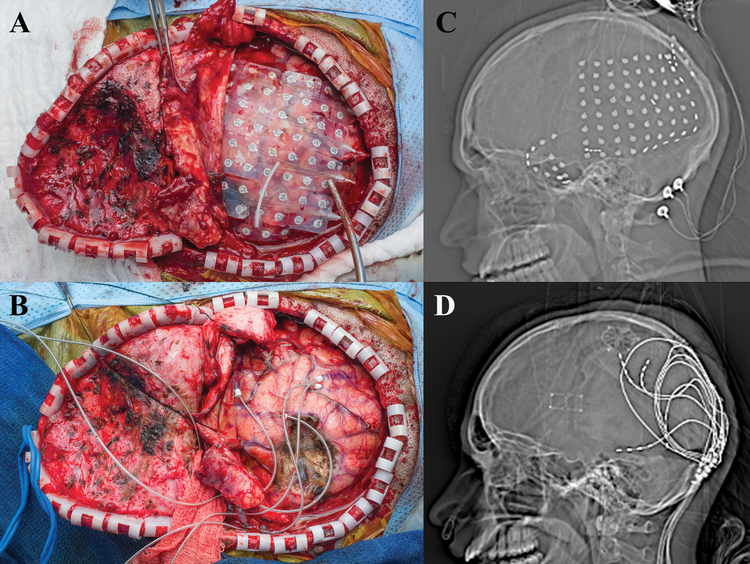
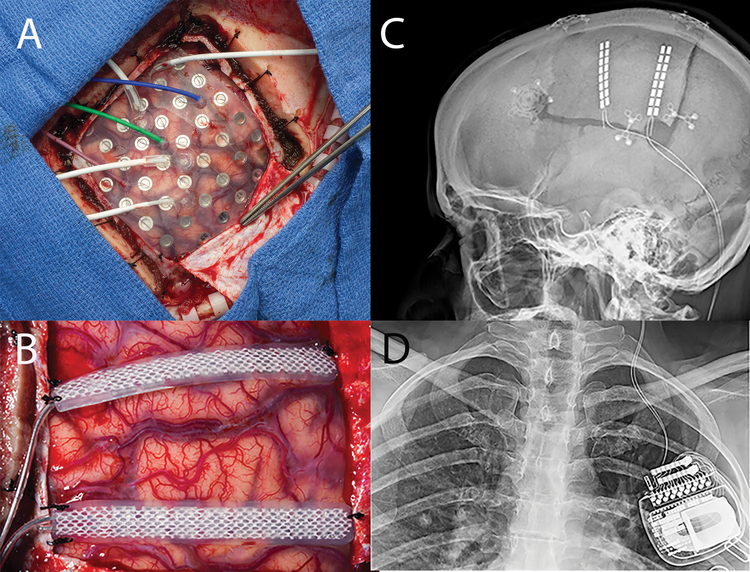
A 27-year old, left-handed female presented with a medically refractory focal dyscognitive seizures associated with behavioral and speech arrest, and occasionally associated with jerking movements of the left arm. MRI revealed a likely area of focal cortical dysplasia in the right superior temporal gyrus. Prior to intracranial monitoring there was concern that this area was very close to cortex critical for language processing, as evaluated by fMRI. For intracranial EEG (iEEG) monitoring, the patient underwent a right frontotemporoparietal craniotomy with wide subdural electrode coverage as well as penetrating depth electrodes into the cortical dysplasia [Figure 1]. A subdural 8×8 contact grid with was placed over the right temporal region with 4-contact anterior and posterior temporal depth electrodes targeting the anterior hippocampus/amygdala and cortical dysplasia, respectively. Two 1×4 contact subdural strips were placed posteriorly extending radially from the anterior and inferior posterior margin of the subdural grid [Figure 1]. Grids, strips and depth electrodes contained contacts separated by 1 cm.
Figure 1:
a) Intraoperative view status post right frontotemporoparietal craniotomy with placement of grid and strip electrodes for electrocorticogrqphy. b) Intraoperative view status post right frontotemporoparietal craniotomy with placement final depth electrodes into the superior temporal lobe and inferior parietal lobule used for chronic stimulation c) Scout view revealing the placement of grid and multiple strip electrodes. d) Scout view demonstrating final placement of depth electrodes used for chronic stimulation.
During iEEG monitoring the most frequent interictal discharges and typical clinical seizures emanated from the mid-posterior portion of the grid. During direct cortical stimulation for language mapping, language function was interrupted during stimulation testing of several electrodes localized to the seizure onset zone (SOZ), precluding resection. A trial of cortical stimulation was initiated using the four contacts from the posterior depth electrode as well as six neighboring grid electrodes. Empirically, stimulation was deemed more likely effective for seizure reduction if the rate of the most frequent epileptiform interictal discharges decreased significantly during trial CSCS. The choice of which electrodes to stimulate was influenced by cortical geometry as well as the location of the most frequent epileptiform discharges and the SOZ. After the successful CSCS trial via the iEEG monitoring electrodes, two depth electrodes (Medtronic 3387s-40 and 3389s-40) were placed into the superior temporal lobe and inferior parietal lobule, respectively, and connected to a Medtronic 37702 internal generator. No complications developed postoperatively and patient was discharged home on POD 3. In the month prior to implantation and stimulation, the patient recorded 68 seizures, eight of which were disabling. Following the initiation of chronic stimulation, the patient was seizure-free for two months and then started to have mild seizures. As of her 9 month follow-up she continued to have intermittent mild seizures typified by unusual feelings but did not have any disabling seizures.
Case #2
A 28-year old, right-handed man presented with a 16-year old history of intractable focal reflex seizures involving the left lower extremity. Common triggers included standing or walking, and the patient was wheelchair bound as a result. The patient had undergone partial resection of cortical dysplasia in the parietal lobe in 2010 at an outside institution and insertion of a vagus nerve stimulator in 2014. He continued to have focal reflex seizures with weight-bearing on his left leg following these interventions, leaving him wheelchair bound. MRI was remarkable for encephalomalacia in the prior resection cavity, and visible extension of his cortical dysplasia into the leg motor area. The patient underwent right frontal craniotomy with subdural grid insertion and interhemispheric subdural strip electrodes. iEEG demonstrated the SOZ to be concordant with eloquent motor cortex [Figure 2]. Rather than resecting the residual cortical dysplasia and leaving him unable to walk a trial CSCS was performed with the implanted electrodes, and he was able to bear weight on the left leg without seizures. He was then taken back to the operative theater and 4 leads (Medtronic Resume II 3587) were placed, two inter-hemispherically and two over the right motor cortex [Figure 2]. The electrodes were connected to a Medtronic 37702 internal generator with dual tunneled 2 to 1 lead extensions. The patient had an uneventful postoperative course and was discharged home on POD 3. Within the week, he was able to walk in the local shopping mall in public which was an emotional achievement for the patient. Since commencing CSCS, he has had rare leg movements that were likely seizure-related, but has otherwise been seizure free.
Figure 2:
a) Scout view demonstrating intracranial monitoring electrodes. b) Intraoperative view status post right frontal craniotomy with a 4×6 grid and 2 strip electrodes placed interhemispherically. c) Intraoperative view where the 4×6 monitoring grid has been replaced with two Resume II electrodes sutured together over the R motor cortex, as well as two interhemispheric Resume II leads. These are further secured to the dura with silk sutures to ensure they do not move. d) Scout view demonstrating stimulation electrodes in final position, two over the motor cortex and two interhemispheric. e) T1 weighted sagittal MRI demonstrating placement of interhemispheric strip electrodes over the right paracentral lobule and area of prior resection.
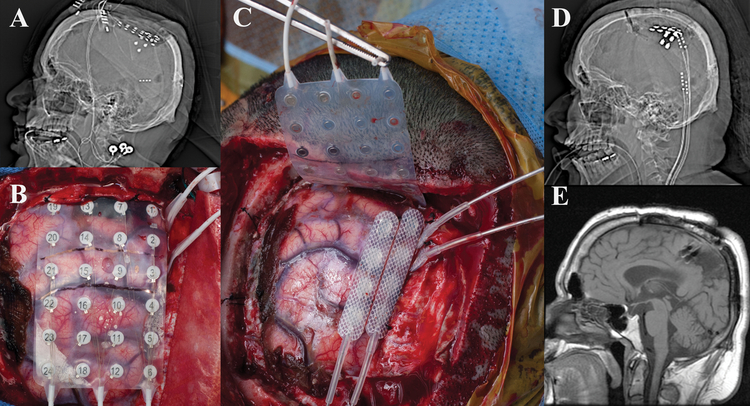
Case #3
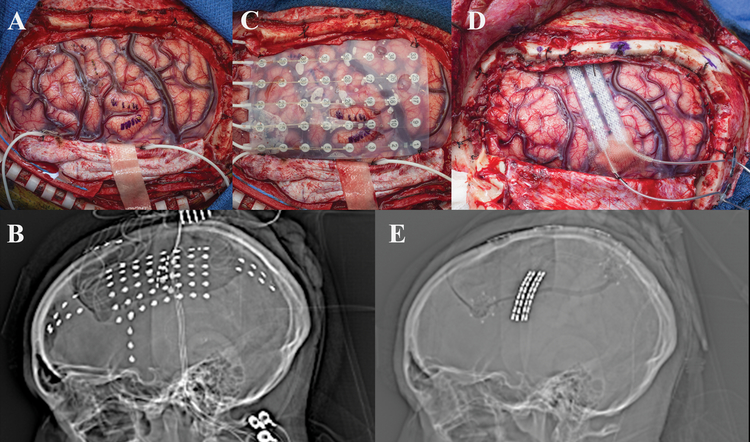
A 25-year old, right-handed male presented with a 15-year old history of intractable focal dyscognitive seizures manifesting as tinnitus and twitching in the right upper extremity, which often secondarily generalized. Pediatric history was notable for La Crosse Encephalitis. MRI was notable for a presumptive linear migrational anomaly deep to the left precentral gyrus. No previous surgical intervention for his epilepsy had been performed. The patient underwent a wide left frontotemporoparietal craniotomy with extensive frontal, temporal, and parietal subdural electrode coverage [Figure 3]. iEEG demonstrated two separate seizure foci in eloquent regions of the brain involving motor and speech. These foci were targeted with CSCS resulting in suppression of interictal epileptiform discharges. He was taken back to the operative theater and two Medtronic 39286 leads were placed over the left motor strip and attached to a Medtronic 37702 internal generator [Figure 3]. Patient was discharged home on POD 2 without any complications. His seizure frequency decreased by approximately half, and his disabling seizures are now rare.
Figure 3:
a) Intraoperative view status post left frontotemporoparietal craniotomy showing recording electrode placement. b) Scout view demonstrating intracranial monitoring grid and strip electrodes. c) Three dimensional reconstruction showing placement of grid and three strip electrodes utilized for electrocorticography. d) Intraoperative view showing replacement of grid and strip electrodes with two strip electrodes over the motor cortex and parietal seizure foci. Note that prior to permanent placement of the electrodes the tunneling channel is present so that this manipulation is not performed with permanent electrodes in place (metal tube in picture at 10 o’clock) e) Scout view demonstrating two strip electrodes over the motor cortex and parietal seizure foci.
Case #4
A 56-year old, right-handed male, presented with a 50-year old history of medically intractable focal reflex seizures described as an electric shock-like sensation in the right foot and leg followed by tonic-clonic movements that occasionally spread into the right arm. Seizures were triggered by ambulation or by placing a sock or shoe on the right foot. Notably, he had not worn a shoe on the right side during his adult years because of the reflex sensory-motor seizures. Pediatric history was notable for head injury secondary to child abuse. Neuroimaging revealed multifocal regions of encephalomalacia in the left frontal and temporal head regions. Patient underwent a left frontal craniotomy centered over motor cortex with interhemispheric subdural strip electrodes [Figure 4]. After implantation, he was found to have focal seizures emanating from his leg motor area, and therefore was not a resective surgery candidate. A trial of CSCS was initiated in the hospital with the implanted electrodes. With CSCS, his usual triggers of placing a shoe on and standing on the right foot, failed to activate seizure activity. He was subsequently taken back to the operative theater and two Medtronic 3391 leads were placed subpially down the long axis of the leg motor area in the interhemispheric area; the latter was done due to an overlying vein which interfered with strip placement [Figure 4]. In addition, two strip leads Medtronic 39286 were placed over the proximal leg region of the convexity [Figure 4], which were connected to a Medtronic 37702 internal generator. Patient had an uncomplicated postoperative course and was discharged home on POD 6. He did not have any complications, and after years of not walking was able to walk out of the hospital. After about eight months, more prominent reflex seizures returned and at 10 month follow-up he continues to walk.
Figure 4:
a) Intraoperative view status post left frontal craniotomy with grid placement. b) Intraoperative view showing replacement of recording electrodes with depth electrodes into the motor cortex along with a strip electrode over the region. These electrodes are stabilized at the bone edge with a dogbone plate securing them c) Scout view showing final placement of depth and strip electrodes over the motor cortex. d) Intraoperative view status post plating of the craniotomy bone demonstrating the lead extensions over the craniotomy site and tunneling to the left.
Case #5
A 19-year old, right handed male presented with a 5-year old history of intractable focal dyscognitive seizures, during which the patient would experience intense fear followed by clonic movements of the head to the right as well as clonic movements of the right arm and leg that sometimes progressed to generalized tonic-clonic seizures. MRI showed encephalomalacia in the left posterolateral occipital cortex. The patient underwent a left temporoparietooccipital craniotomy with extensive cortical coverage with subdural electrodes [Figure 5]. A large SOZ was identified which included critical speech and motor cortex. After a CSCS trial, a topectomy of the area of cortical abnormality was performed. Four Medtronic 3391 leads were then tunneled subpially into the left posterior parietal and posterior temporal region and these were connected to a Medtronic 37702 internal generator utilizing dual lead extensions for two channel output [Figure 5]. Patient did not experience any postoperative complications and was discharged home on POD 4. He continues to be seizure-free four months following initiation of stimulation.
Figure 5:
a) Intraoperative view status post left temporoparietooccipital craniotomy with grid placement. b) Scout view, demonstrating placement of the monitoring grids. c) Intraoperative view status post topectomy of the area of encephalomalacia along with subpial placement of the four 3391 depth electrodes. Note we place a plastic tether to limit further insertion of the electrodes, these are further attached to the bone edge with a dogbone plate to prevent electrode migration. d) Scout view showing final placement of depth electrodes.
Case #6
A 19-year old, right-handed female presented with a 2-year old history of intractable focal motor seizures that involved eye twitching, usually in both eyes, which would occasionally spread to the left face. Past medical history was unremarkable. MRI revealed a right precentral, deep cortical dysplasia. The patient underwent a right frontoparietal craniotomy with extensive cortical coverage in addition to depth electrodes placed in the facial motor region targeting the deep cortical dysplasia [Figure 6]. iEEG confirmed a seizure focus in the face motor region. A trial of CSCS was shown to suppress interictal and ictal epileptiform activity. Therefore, two 1×8 subdural electrode strips were placed over the right motor cortex and connected to a Medtronic 37702 generator [Figure 6]. The patient experienced an uneventful postoperative course and was discharged home after 3 days without complications. In the six weeks following initiation of CSCS, the patient experienced rare focal seizures, and over the subsequent 7 months has been seizure-free after further programming.
Figure 6:
a) Intraoperative view status post right frontoparietal craniotomy with placement of strip electrodes. b) Scout view revealing the placement of grid and multiple strip electrodes. c) Intraoperative view status post right frontoparietal craniotomy with placement of strip electrodes and large grid for coverage of the right hemisphere. d) Intraoperative view showing replacement of recording electrodes with two strip electrodes over the motor cortex utilized for trial stimulation e) Scout view demonstrating two strip electrodes over the motor cortex.
Case #7
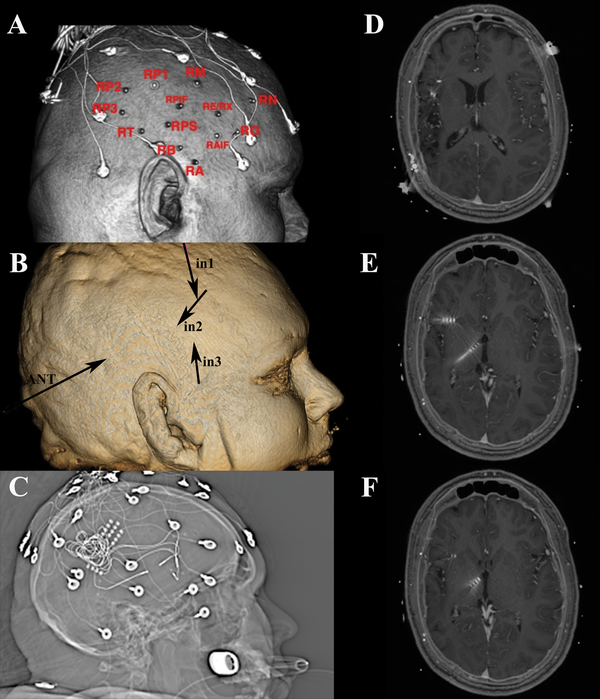
A 26- year old, right-handed male presented with an 11-year history of focal dyscognitive seizures described as a feeling of cold sensation in the entire body, tunnel vision, adventitious movements in his upper and lower extremities, and loss of awareness. Neonatal history was significant for stroke in the right middle cerebral artery (MCA) territory as well as apneic seizures with cyanosis soon after delivery. MRI revealed a wide area of encephalomalacia related to the perinatal stroke. He underwent stereo EEG implantation of four 4-contact electrodes targeting the encephalomalacia and insula [Figure 7]. Although the initial implantation suggested an anterior insular focus, the patient was unwilling to consider an insular resection. CSCS was offered to the patient. Four Medtronic 3387s leads were implanted targeting the anterior insular SOZ as determined by iEEG; three into the right frontal operculum terminating in the anterior insula and one into the anterior thalamic nucleus. These electrodes were then externalized percutaneously [Figure 7]. Subsequently, he underwent extraoperative scalp EEG monitoring during CSCS, which showed significant suppression of interictal epileptiform discharges and seizure frequency when seizure medications were withdrawn compared to baseline. He was subsequently taken back to the operative theater where the four leads were internalized and connected to a Medtronic 37702 internal generator. The patient did not experience any postoperative complications and was discharged home on POD 2. In the subsequent six months he has had two typical seizures, compared with clusters of up to 60 seizures about every 4 weeks prior to CSCS.
Figure 7:
a) Scalp reconstruction revealing placement of monitoring electrodes in region of right encephalomalacia. b) Three dimensional skull model revealing insertion and vector for depth permanent depth electrodes. c) Scout view revealing monitoring electrodes d,e,f) Axial T-1 weighted MRI sequences revealing locations of the four permanent depth electrodes used for chronic stimulation.
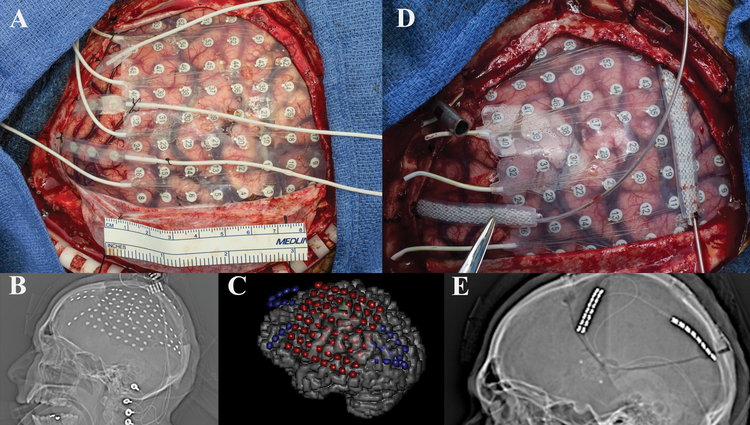
Case #8
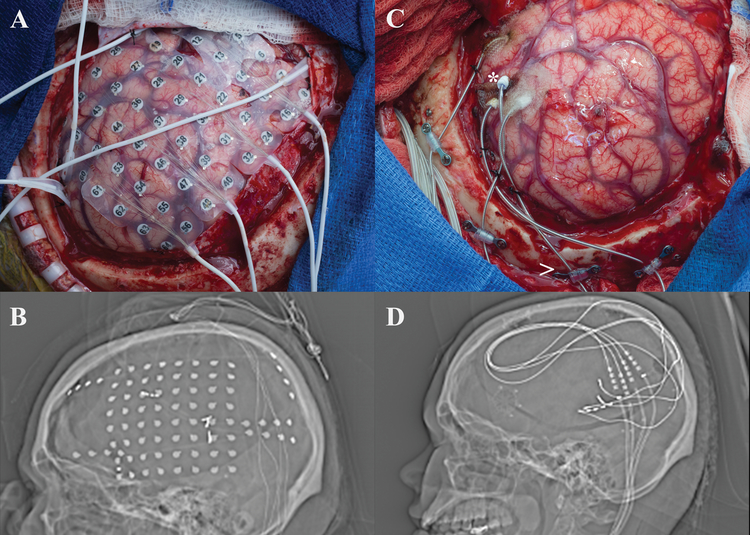
A 22 year-old, right-handed female with tuberous sclerosis presented with a 19 year history of daily focal dyscognitive seizures characterized by tinnitus, hand wringing automatisms, and behavioral arrest followed by aphasia and confusion. She also complained of nocturnal events consisting of arousals followed by confusion and semi-purposeful motor activities. Past surgical history was notable for VNS placement in 2013 with limited seizure control. MRI showed three tubers, the most prominent of which enhanced after contrast administration and was located in the left posterior temporal region. The patient underwent a left frontotemporoparietal craniotomy and four depth electrodes were placed in Heschl’s gyrus around the cortical tuber as well as wide subdural cortical coverage [Figure 8]. Extraoperative iEEG demonstrated seizures originating from the perilesional tissue around the tuber, however, overlying cortex was found to be critical for speech during cortical stimulation mapping. CSCS was trialed and successful when targeting the perilesional cortex. Three Medtronic 3387 leads and one 3391 lead were placed around the tuber within Heschl’s gyrus [Figure 8]. These leads were connected to a Medtronic 37702 internal generator. The patient had an uneventful postoperative course and was discharged home after 3 days. Seizures have continued, although reduced in frequency. Family estimates an 80% improvement in seizure severity.
Figure 8:
a) Intraoperative view status post left frontotemporoparietal craniotomy with placement of grid and multiple strip recording electrodes. b) Scout view revealing the placement of grid and multiple strip electrodes. c) Intraoperative view status post left frontotemporoparietal craniotomy with placement of depth electrodes utilized for stimulation therapy (the * denotes the placement of the electrode into Heschl’s gyrus) d) Scout view demonstrating final placement of depth electrodes used for chronic stimulation.
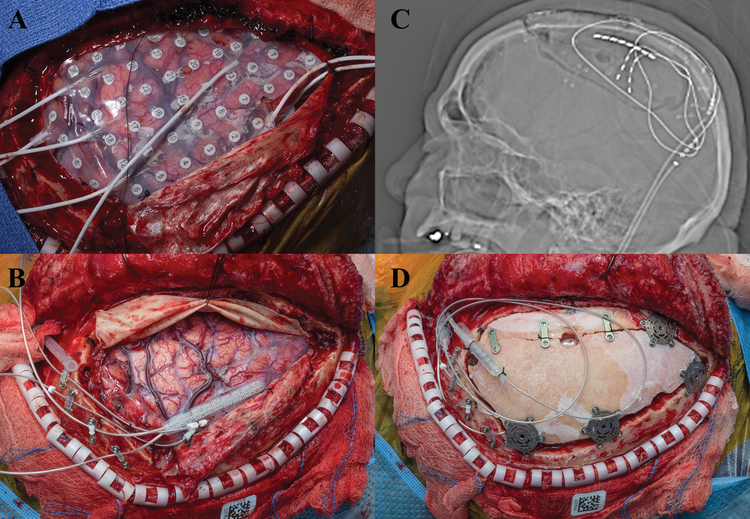
Case #9
A 39-year old, right-handed female presented with a 21-year old history of intractable focal motor seizures of her right face and hand exacerbated by talking, and secondary generalized tonic-clonic seizures. Pediatric history was remarkable for Rasmussen encephalitis involving the left parietal cortex. MRI was notable for left parietal atrophy and mild parietal white matter signal abnormality without any focal abnormalities. No prior surgery had been performed. The patient underwent left-sided craniotomy with a 6×8 frontoparietal subdural electrode grid placement for monitoring [Figure 9]. Extraoperative iEEG demonstrated epileptiform activity over the left motor cortex and clinical findings consistent with epilepsia partialis continua. The patient was taken back to the operating room three days after the first procedure, where two Medtronic 39286 leads were placed over the left primary and supplementary motor area and attached to a Medtronic 97712 internal generator [Figure 9]. Patient was discharge home on POD 6 without postoperative complications. As of 7 months post-intervention, she continues to have seizures, although CSCS largely controls her facial seizures that were most bothersome to her.
Figure 9:
a) Grid implant over motor cortex with PMT electrodes. b) 2 Spinal Cord stimulator strips sutured to dura over the prior test stimulation sites, note the hypervascularity of the cortex consistent with the diagnosis of Rasmussen. c) Scout x-ray, lateral view of head demonstrating implant, note the newer spinal implants have one of the two electrodes leads cut as they exit the dura and nicely do not require a lead extension. d) A/P Chest x-ray, Spinal Cord stimulator battery implanted in the chest ipsilateral to the side of the implant.
Care #10
A 23-year old, right-handed male presented with a 12-year old history of intractable focal sensory and motor seizures manifesting as electric shock-like sensation and followed by jerking of the left upper extremity. MRI did not identify any brain abnormalities or epileptogenic foci. The patient underwent right-sided frontoparietal craniotomy with extensive frontal and parietal subdural electrode monitoring [Figure 10]. Extraoperative iEEG and cortical mapping demonstrated overlap of the SOZ and right primary motor cortex. He was taken back to the operating room after seven days, where four Medtronic 3587 leads were placed over the right motor strip and attached to a Medtronic 37702 internal generator [Figure 10]. Patient was discharge home on POD 11 without postoperative complications. At follow-up of 20 months, the patient reported an overall decrease of 80–90% in epilepsy severity.
Figure 10:
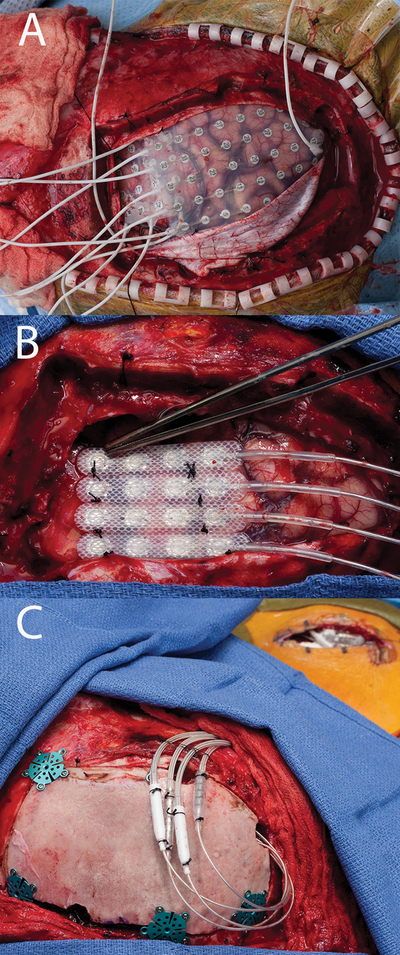
a) Grid implant. b) final electrode configuration with resume 2 leads sutured together in a row of 4 placed over the test stimulation site. c) Implant with battery in place, notice the lead extension connectors in this circumstance are placed over the craniotomy, these are typically sutured down higher on the head in order for the patient not to have discomfort when laying on them. Further, the incision to the right of the field is the battery pocket. The lead extensions are typically tunneled behind the ear with the patient in pinions which requires thought in patient positioning.
DISCUSSION
Based on our institutional experience, CSCS for patients with drug resistant epilepsy originating from eloquent cortex can be performed safely, with no apparent additional morbidity related to trial stimulation or permanent implantation phase. At present, no complications have been reported in these adult patients. This is a critical finding, as iEEG monitoring is associated with an increased risk of infection, even without permanent hardware implantation. To minimize risk of infection in our patients, patients are treated prophylactically with cefepime and vancomycin while hospitalized and 4 weeks of cefadroxil therapy following permanent implantation. In addition, despite placement of electrodes around and into eloquent cortex, we have not experienced any temporary or permanent neurologic complications. On clinical examination on most recent follow-up, none of the patients exhibited any signs of focal neurologic deficit (e.g. weakness or numbness) associated with the stimulated area. Further, we have not experience electrode migration over time. Admittedly with a small sample size of ten patients, we have seen an overall acceptable risk profile with this approach in which we are modifying spine and deep brain stimulation hardware for the purpose of focal cortical and subcortical CSCS. . Surgical intervention for intractable epilepsy requires a careful assessment of risk and benefit. For example, resection of motor and/or sensory cortex in children with benign rolandic epilepsy is associated with Engel Class I or II outcomes in ~ 40–77% and up to 28% postoperative complication risk.2 CSCS appears to be associated with an acceptable risk, and is generally reversible if unsuccessful, however no patient has required explantation of the hardware at this time.
Chronic subthreshold cortical stimulation has been primarily studied in animal models.5 The first report in human subjects was published by Matsumoto and colleagues in 2002 in a 31 year-old man with medically intractable mesial temporal lobe epilepsy who underwent low-frequency electrical cortical stimulation by means of subdural electrodes. The authors found that interictal epileptiform discharges at the seizure focus occurred less frequently after 250 seconds of stimulation.23 Similar results were presented by the same team in 2006, where a total of 4 patients (one with occipital cortical atrophy, one with frontal lobe tumor, one with calcified mass in the right Sylvian fissure, and one with a history of perinatal asphyxia) underwent the same procedure in the absence of postoperative adverse events.22 Notably, none of the aforementioned patients received permanent implantation of stimulation hardware. Later, Velasco and colleagues reported on an adolescent and a teenager each with non-lesional refractory motor epilepsy that were treated with duty-cycle (1 min on & 4 minute off) therapeutic neurostimulation of the right motor area.19 In their series, neither of the patients experienced side effects and seizure frequency decreased by more than 90%, while preserving motor function. Lastly, our own previously reported experience with pediatric and adult patients has demonstrated the safety, feasibility, and efficacy of CSCS.4,11
Class 1 evidence for neurostimulation for epilepsy comes from the two landmark trials, the SANTE (Stimulation of the Anterior Nucleus of Thalamus for treatment of refractory Epilepsy) Trial and the Responsive Neurostimulation (RNS) System Pivotal Trial. These trials demonstrated the efficacy of neurostimulation, and that the majority of serious adverse events (SAEs) occur within the first postoperative month.7,9,12. The SANTE trial showed a 40% reduction in a seizure frequency compared to baseline in the active stimulation group versus 15% in the non-stimulated group.7 Similarly, the multicenter RNS Trial showed a 38% reduction in seizure frequency compared to the sham group.9,12. During the subsequent open-label phase of the RNS trial there was an overall 66% reduction in seizure frequency, 56% responder rate, and improved quality of life.3 Similarly, in the open-label phase of the SANTE trial there was a 69% reduction in seizures, 68% responder rate, and improved quality of life.13 However, patients infrequently experience a seizure free outcome. In the RNS trial, 13% of patients had at least a 12 month period of seizure freedom and in the SANTE trial 16% of the patients had at least a 6 month period of seizure freedom.
According to a recent systematic review by Gooneratne and colleagues8, the most common SAE associated with responsive cortical stimulation is implant site infection (9.4%) followed by intracranial hemorrhage of any type (4.7%). Notably, death has been observed in 11 patients so far, seven of which have been attributed to possible, probable, or definite sudden unexpected death in epilepsy (SUDEP),8 which were not greater than expected compared to the medically intractable focal epilepsy population.15 Similarly, in the SANTE trial device-related adverse events occurred in 35.5% of patients undergoing deep brain stimulation of the anterior thalamic nucleus. Infection and intracranial hemorrhage were observed in 10% and 4.6% of treated subjects, respectively. Although a total of seven deaths have been reported, none of them were device related and three were attributed to SUDEP.8 Other less frequent device-related complications that have been described as well, include lead damage, repeat surgery for revision of lead location and exacerbation of seizure frequency and/or duration. Notably, alternative options for seizures originating in eloquent brain regions such as multiple subpial transections appear to be less effective and greater risk of neurologic injury.16
CSCS presents an alternative to thalamic stimulation and RNS. In contrast to thalamic stimulation, CSCS directly targets the SOZ with the hypothesis that seizures can prevented prior to spread. In contrast to the current FDA-approved RNS device, CSCS as implemented above avoids the complications of a skull-based device and does not necessarily preclude MR imaging. In contrast to RNS, CSCS is not responsive stimulation. It remains unclear to what extent the responsive aspect of current RNS systems is efficacious. Although the mechanism by which CSCS is efficacious is unknown, one possibility is that stimulation is continually decreasing cortical excitability on a relatively short time scale and is thus required chronically. It may also provide improved efficacy by virtue of continuous neuromodulation that does not depend on seizure detection, which is limited by current device sensing paradigms that use narrow spatial and spectral bandwidths, and duty-cycle stimulation paradigms that leave the epileptic network free to generate seizures during the off portion of the cycle. Optimizing the multitude of parameters involved in CSCS remains a challenge, and highlights the need for improved biomarkers of cortical excitability.
CONCLUSIONS
In the present case series, we presented our own institutional experience with ten adult patients treated with CSCS for focal drug resistant epilepsy involving eloquent cortex. There is morbidity risk associated with subdural grid placement (overall 2.1–13.6%), including hemorrhage, extra-axial fluid and blood collections, and infection requiring additional surgical intervention in up to 3% of complications.1,14 Evidence here suggests that this procedure can be safely performed in appropriately selected patients. While the current study provides only class IV evidence and is limited to ten patients with short follow-up, preliminary results demonstrate that CSCS shows promise as a safe and effective alternative to resective or ablative therapies.
Acknowledgments
Disclosures: Authors Drs. Stead, Brinkmann, Worrell, and Van Gompel report support from NIH funded public-private-partnership grant (UH2-NS095495: Neurophysiologically Based Brain State Tracking & Modulation in Focal Epilepsy) between Mayo Clinic and Medtronic and a Medtronic supported Investigational device exemption study (Chronically-recorded deep brain nuclei/ hippocampal high frequency oscillations as biomarkers of neurologic disease). The patients in this report received treatment based on compassionate, off label use with a commercially available, FDA approved devices.
References
- 1.Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA: Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systematic review and meta-analysis. Epilepsia 54:828–839, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Benifla M, Sala F Jr, Jane J, Otsubo H, Ochi A, Drake J, et al. : Neurosurgical management of intractable rolandic epilepsy in children: role of resection in eloquent cortex. Clinical article. J Neurosurg Pediatr 4:199–216, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King-Stephens D, Nair D, et al. : Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology 84:810–817, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Child ND, Stead M, Wirrell EC, Nickels KC, Wetjen NM, Lee KH, et al. : Chronic subthreshold subdural cortical stimulation for the treatment of focal epilepsy originating from eloquent cortex. Epilepsia 55:e18–21, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Della Paschoa OE, Kruk MR, Hamstra R, Voskuyl RA, Danhof M: Seizure patterns in kindling and cortical stimulation models of experimental epilepsy. Brain Res 770:221–227, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS, Sander JW, Sisodiya SM, Walker MC: Adult epilepsy. Lancet 367:1087–1100, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. : Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51:899–908, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Gooneratne IK, Green AL, Dugan P, Sen A, Franzini A, Aziz T, et al. : Comparing neurostimulation technologies in refractory focal-onset epilepsy. J Neurol Neurosurg Psychiatry 87:1174–1182, 2016 [DOI] [PubMed] [Google Scholar]
- 9.Heck CN, King-Stephens D, Massey AD, Nair DR, Jobst BC, Barkley GL, et al. : Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia 55:432–441, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwan P, Schachter SC, Brodie MJ: Drug-resistant epilepsy. N Engl J Med 365:919–926, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Lundstrom BN, Van Gompel J, Britton J, Nickels K, Wetjen N, Worrell G, et al. : Chronic Subthreshold Cortical Stimulation to Treat Focal Epilepsy. JAMA Neurol:2016. Available: http://archneur.jamanetwork.com/article.aspx?articleid=2553322. Accessed 22 September 2016 [DOI] [PubMed] [Google Scholar]
- 12.Morrell MJ RNS System in Epilepsy Study Group: Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77:1295–1304, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. : Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84:1017–1025, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt RF, Wu C, Lang MJ, Soni P, Williams KA Jr, Boorman DW, et al. : Complications of subdural and depth electrodes in 269 patients undergoing 317 procedures for invasive monitoring in epilepsy. Epilepsia 57:1697–1708, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Shorvon S, Tomson T: Sudden unexpected death in epilepsy. Lancet 378:2028–2038, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Spencer SS, Schramm J, Wyler A, O’Connor M, Orbach D, Krauss G, et al. : Multiple Subpial Transection for Intractable Partial Epilepsy: An International Meta-analysis. Epilepsia 43:141–145, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Stead M, Bower M, Brinkmann BH, Lee K, Marsh WR, Meyer FB, et al. : Microseizures and the spatiotemporal scales of human partial epilepsy. Brain 133:2789–2797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tellez-Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S: Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology 66:1490–1494, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Velasco AL, Velasco F, Velasco M, María Núñez J, Trejo D, García I: NEUROMODULATION OF EPILEPTIC FOCI IN PATIENTS WITH NON-LESIONAL REFRACTORY MOTOR EPILEPSY. Int J Neural Syst 19:139–147, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wiebe S, Blume WT, Girvin JP, Eliasziw M: A Randomized, Controlled Trial of Surgery for Temporal-Lobe Epilepsy. N Engl J Med 345:311–318, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Worrell GA, Jerbi K, Kobayashi K, Lina JM, Zelmann R, Le Van Quyen M: Recording and analysis techniques for high-frequency oscillations. Prog Neurobiol 98:265–278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto J, Ikeda A, Kinoshita M, Matsumoto R, Satow T, Takeshita K, et al. : Low-frequency electric cortical stimulation decreases interictal and ictal activity in human epilepsy. Seizure 15:520–527, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto J, Ikeda A, Satow T, Takeshita K, Takayama M, Matsuhashi M, et al. : Low-frequency electric cortical stimulation has an inhibitory effect on epileptic focus in mesial temporal lobe epilepsy. Epilepsia 43:491–495, 2002 [DOI] [PubMed] [Google Scholar]