Abstract
Objectives
Physical activity shows promise for reduced risk of Alzheimer’s disease (AD) and protection against cognitive decline among individuals with and without AD. Older adults face many barriers to adoption of physically active lifestyles and people with AD face even further challenges. Physical activity is a promising non-pharmacological approach to improve depressive symptoms, but little is known about the impact of depressive symptoms as a potential barrier to engagement in physical activity. The present study aimed to investigate depressive symptoms as a potential barrier for participation in physical activity across a range of dementia severity.
Method
We used longitudinal structural equation modelling to investigate the bi-directional relationship between depressive symptoms and physical activity in 594 older adults with and without AD over a 2 year longitudinal follow up. Participants ranged from no cognitive impairment to moderately severe AD.
Results
We found that depressive symptoms predicted reduced engagement in subsequent physical activity, but physical activity did not predict subsequent reductions in depressive symptoms.
Conclusion
We conclude that depressive symptoms may be an important barrier to engagement in physical activity that may be addressed in clinical practice and intervention research.
Introduction
Older adults spend 65–80% of waking time in sedentary activities [1,2], and individuals with Alzheimer’s disease (AD) are even less active [3,4]. Physical activity shows promise for reduced risk of AD and protection against cognitive decline among individuals with and without AD [5,6]. However, older adults face many barriers to adoption of physically active lifestyles. In one study, 87% of older adults reported one or more barriers to exercise [7]. In addition to the barriers to physical activity faced by most people, (e.g., perceived effort, discomfort of sweating, muscle soreness), older adults face unique barriers such as chronic health problems, fear of falling, inadequate environmental support, and lack of self-efficacy about exercise [7,8].
People with AD likely face even further challenges to engaging in physical activity. Aside from a few small qualitative studies [9–11], there is little research addressing the barriers to physical activity specific to individuals with dementia [7]. The barriers reported in qualitative studies fall into several categories including physical, social, emotional, environmental, and cognitive. The physical barriers are likely similar to those reported in older adults without dementia such as decreased energy and impaired body function [11]. Malthouse and Fox [9] reported that people with AD generally had positive attitudes about the benefits of physical activity, however few of them were willing to push themselves hard enough to sweat and did not like the feeling of being forced to exercise.
However, barriers due to cognitive disability and socio-emotional factors are more unique to persons with AD. For example, a hallmark cognitive symptom of AD is disorientation to place. This makes it difficult and unsafe for individuals with AD to walk for exercise without a companion or in an unfamiliar environment. A key social-emotional barrier to physical activity is the need to have a caregiver to arrange for transportation, accompaniment, and assurance of safety. AD patients reported feeling a loss of freedom and safety in their inability to exercise unsupervised and loss of identity in their inability to continue with activities they previously enjoyed [11]. The development of “dementia-friendly” communities would be beneficial for allowing people to regain some sense of independence.
Individuals with AD have been found to have a reduced ability to accurately perceive bodily states that impact effort during physically demanding activity [12]. This may result in over- or under- exertion during exercise leading to unsafe conditions or insufficient effort to achieve benefits of exercise. There is also evidence that cognitive and motor declines in dementia may occur in parallel due to underlying brain changes [13]. Some possible mechanistic explanations of this process include changes in brain and spinal cord areas that regulate motor movement, and vulnerability of muscle to systemic dysregulation of catabolic, inflammatory, immune, endocrine, and metabolic processes. Loss of lean mass, muscle strength, and motor performance are common in older adults and a prominent features of frailty, sarcopenia, and metabolic syndrome, all of which are associated with risk of AD [14].
Limitations of attention and memory in people with AD may require additional prompts for carrying out multistep daily tasks [15]. With increasing disease severity, individuals with AD become less likely to initiate activities of daily living, social engagement, or other daily activities, and become increasingly dependent on support of caregivers to carry out these tasks [16]. Reduced initiation in activity engagement may be related to depressive symptoms, cognitive impairment, or other disease-related neurological changes [17,18]. Rates of depression are higher among individuals with AD compared to those with normal cognitive status, though the direction of the causal relationship between dementia and depression remains unclear [19,20].
Physical activity and exercise are a promising non-pharmacological approach to improve depressive symptoms in older adults with or without dementia, without the side effects or drug interactions commonly found with pharmacological therapy in this population [21]. A great deal of research has demonstrated benefits of physical activity and exercise for improving depressive symptoms in non-cognitively-impaired older adults and in individuals with AD and other forms of dementia [22,23].
Despite the extensive research into the protective nature of exercise for dementia, and the promise of physical activity for reducing depressive symptoms, to date, little is known about the impact of depressive symptoms on engagement in physical activity. It is plausible, that given the prevalence of depressive symptoms among individuals with dementia, these symptoms may present additional challenges to engaging in physical activity. For example, the presence of symptoms such as dysphoric mood, apathy, and psychomotor retardation may further prevent older adults with or without AD from engaging in physical activity. This is of particular clinical relevance as the presence of such depressive symptoms may limit the efficacy of physical activity as a non-pharmacological intervention.
This study had two aims. Aim 1 was to evaluate the effect of engagement in physical activity on subsequent depressive symptoms, thereby confirming previous findings that physical activity is associated with reduced depressive symptoms. Aim 2 was to examine the reverse hypothesis that depressive symptoms act as a potential barrier to subsequent engagement in physical activity.
Methods
Participants
All study procedures approved by the University of Kansas Medical Center Human Subjects Committee #11132. Written consent was obtained for all participants and/or their legally authorized representative. All participants were enrolled in a Midwestern Alzheimer’s Disease Center Clinical Cohort and received standard clinical and cognitive evaluations annually. Detailed information about evaluation procedures and data collection have been published previously [24]. Briefly, experienced study clinicians trained in dementia assessment conducted a standard clinical evaluation that includes a Clinical Dementia Rating (CDR) [25] and a trained psychometrician administered a comprehensive cognitive testing battery. The clinical and psychometric test results were reviewed and discussed at a weekly consensus conference that included clinicians, a neuropsychologist, and raters to determine a final consensus diagnosis. AD diagnosis was made using National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria (NINCDS-ADRDA) [26]. Dementia severity was determined using the CDR. Because they represented a very small proportion of the sample, we excluded patients with dementia due to other causes including vascular dementia, Lewy Body dementia, and frontotemporal dementia. To assign an etiology for the disease, we followed the National Institute on Aging-Alzheimer’s Association (NIA-AA) workgroup diagnostic guidelines for Alzheimer’s disease which include the category “mild cognitive impairment due to Alzheimer’s disease” to identify individuals with symptoms consistent with AD pathophysiology, but not yet meeting criteria for dementia [27]. Participants with active ischemic heart disease or uncontrolled insulin dependent diabetes mellitus were excluded.
The present study includes 594 individuals categorized as having either no impairment (CDR = 0; n = 345), mild cognitive impairment due to AD (CDR = 0.5; n = 154), and AD (CDR = 1 or greater; n = 95). Data are reported from three waves of annual assessment. Baseline participant characteristics are shown in Table 1. All characteristics described in Table 1 differed statistically by CDR status and p < .001.
Table 1. Baseline participant characteristics.
| Total Sample N = 594 M (SD) |
CDR = 0 N = 345 M (SD) |
CDR = 0.5 N = 154 M (SD) |
CDR = 1+ N = 95 M (SD) |
|
|---|---|---|---|---|
| Age | 72.70 (7.39) | 72.35 (6.49) | 71.89 (8.11) | 75.29 (8.64) |
| Education (years) | 16.13 (2.96) | 16.53 (2.84) | 15.92 (3.07) | 15.01 (2.90) |
| Physical Activity (RAPA score) | 3.86 (1.90) | 3.98 (1.84) | 4.06 (1.95) | 3.08 (1.90) |
| Geriatric Depression Scale Score | 1.39 (2.05) | 0.81 (1.29) | 2.59 (2.29) | 1.57 (1.69) |
| N (%) | N (%) | N (%) | N (%) | |
| Female | 353 (59.4) | 234 (67.8) | 67 (43.5) | 52 (54.7) |
| Neuropsychiatric Medication Use | 203 (34.2) | 85 (24.6) | 70 (45.5) | 48 (50.5) |
Notes: RAPA scores range from 0 (rarely or never do physical activities) to 7 (I do 20 minutes or more a day of vigorous physical activities, 3 or more days a week). A score of 4 corresponds to the statement, “I do moderate physical activity every week, but less than 30 minutes a day or 5 days a week”. Responses to the Geriatric Depression Scale in our sample ranged from 0 to 13. Classes of medications included benzodiazepines, tricyclics, selective serotonin reuptake inhibitors (SSRIs), atypical antipsychotics, serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion.
All study procedures were conducted in accordance with the Helsinki Declaration and approved by the institutional Human Subjects Committee. Participants and their legally authorized representatives completed an informed consent process before participating in the study. The potential participant’s understanding of the research was established by obtaining verbal responses that paraphrase the material in a way that indicates understanding. These procedures have been approved by our institutional review board.
Measures
The Geriatric Depression Scale (GDS) was administered by a trained coordinator to an informant knowledgeable about the participant’s behavior. The GDS is a widely used measure of depressive symptoms in older adults. We used the 15 item version to evaluate depressive symptoms [28]
To assess physical activity engagement, we used the Rapid Assessment of Physical Activity (RAPA), which was designed to assess physical activity in older adults [29]. It defines light, moderate, and vigorous activities for participants according to the degree of perceived heart and respiratory rates achieved during activity for pleasure, work, or transportation. It classifies respondents according to their highest level of activity over a typical week according to the intensity level, duration, and frequency of activity. There is a lack of research validating physical activity assessment tools for use in people with dementia [30]. Thus, we relied on a common practice which is to have questionnaires completed by both patients and their caregivers with guidance from the study personnel.
Covariates included self-reported age, sex, and years of education, clinically assessed body mass index (BMI), and use of antidepressant and antianxiety medications. Classes of medications included benzodiazepines, tricyclics, selective serotonin reuptake inhibitors (SSRIs), atypical antipsychotics, serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion.
Statistical analysis
We report findings from three waves of assessment, each one year apart. We examined longitudinal relationships between depressive symptoms and physical activity to help establish causal direction. We used confirmatory factor analysis (CFA) to summarize the score for the GDS using a single factor. CFA is advantageous for providing improved measurement accuracy by aggregating common variance across multiple items and attenuating error idiosyncratic to individual items. Using a total score assumes that each item is equally valuable in contributing to the construct of depression, whereas CFA does not make that assumption. To evaluate model fit we used Root Mean Squared Error of Approximation (RMSEA), a measure of the discrepancy between predicted and observed model values. Values closer to 0 indicate better fit (preferred values <0.09) We also report comparative fit index (CFI) which estimates the relative fit of a model compared to an alternative model (CFI >0.90 indicates good fit). We used longitudinal structural equation modeling to evaluate the association between depressive symptoms (GDS) and physical activity (RAPA) adjusting for age, sex, education, BMI, CDR, and use of antidepressant and antianxiety medications. To help establish causal direction of influence, we tested the effect of physical activity on subsequent depressive symptoms and tested the reverse, the effect of depressive symptoms on subsequent physical activity. Because we are using structural equation modeling to estimate all the pathways simultaneously, we do not have the same inflation of type 1 error associated with multiple testing. We used Full Information Maximum Likelihood to statistically represent missing data.
Results
Baseline demographic characteristics are given in Table 1. A one factor model provided good fit to the items on the GDS (χ2 [df] = 112.737 [90], RMSEA = 0.021, CFI = 0.984). All items loaded on the single factor with estimates of .56 or higher. At baseline, we had data for 594 participants. At the second and third waves of data collection, we had 423 and 311 observations, respectively.
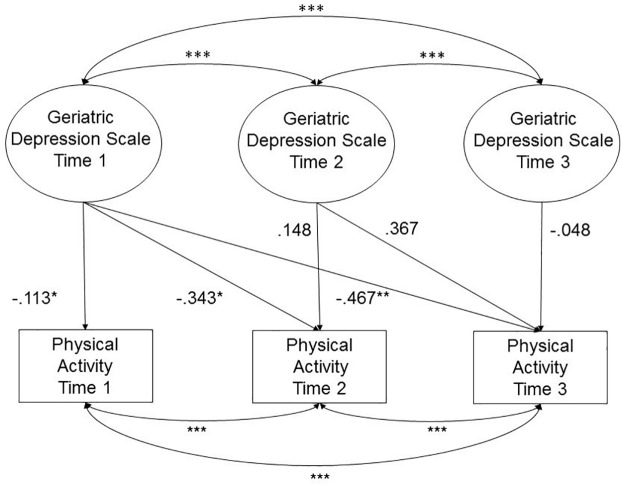
To evaluate the direction of influence between physical activity and depressive symptoms, we tested the relationships between each at three waves of assessment adjusting for age, sex, education, CDR, BMI, and use of neuropsychiatric medications. A summary of the neuropsychiatric medications used in the sample can be found in S1 Table. Results of both models are reported in Table 2. The first three columns represent the effects of physical activity and covariates on depressive symptoms across three waves (Model 1). Physical activity did not predict depressive symptoms at any of the three time points. The second three columns represent the effects of depressive symptoms on physical activity across the three waves (Model 2). Higher levels of baseline depressive symptoms on the GDS predicted lower levels of physical activity at all three waves (Wave 1 β = -.188, p < .05, Wave 2 β = -.165, p < .05, β = -.213, p < .01). See Fig 1 for an illustration of Model 2 in which the GDS at each time point predicts concurrent and subsequent physical activity scores. Higher dementia severity (CDR) was consistently associated with a higher number of depressive symptoms. Higher BMI was consistently associated with lower rates of physical activity.
Table 2. Results of structural equation models of physical activity as a predictor of depressive symptoms on the GDS and depressive symptoms on the GDS as a predictor of physical activity across three waves (standardized estimates).
| Depressive symptoms Time 1 | Depressive symptoms Time 2 | Depressive symptoms Time 3 | Physical activity Time 1 | Physical activity Time 2 |
Physical activity Time 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Physical activity time 1 | -.128 | -.018 | -.015 | — | — | — | ||||||
| Physical activity time 2 | — | -.043 | -.107 | — | — | — | ||||||
| Physical activity time 3 | — | — | .023 | — | — | — | ||||||
| Depressive symptoms time 1 | — | — | — | -.188 | * | -.165 | * | -.213 | ** | |||
| Depressive symptoms time 2 | — | — | — | — | -.059 | .055 | ||||||
| Depressive symptoms time 3 | — | — | — | — | — | .010 | ||||||
| Age | -.053 | -.037 | -.031 | -.127 | -.165 | ** | -.193 | *** | ||||
| Sex | .019 | .001 | -.072 | -.069 | -.115 | -.078 | ||||||
| Education (years) | -.107 | -.049 | -.005 | .106 | .017 | .137 | * | |||||
| CDR | .218 | ** | .287 | *** | .192 | * | -.080 | -.046 | -.143 | * | ||
| BMI | -.050 | -.038 | .080 | -.187 | ** | -.160 | ** | -.203 | *** | |||
| Neuropsychiatric Medication | .124 | .139 | .166 | * | -.071 | -.100 | -.150 | ** |
* p < .05
** p < .01
*** p < .001; Note: Classes of medications included benzodiazepines, tricyclics, selective serotonin reuptake inhibitors (SSRIs), atypical antipsychotics, serotonin-norepinephrine reuptake inhibitors (SNRIs), and bupropion.
Fig 1. Geriatric depression scale predicts physical activity adjusting for age, sex, education, BMI, CDR, neuropsychiatric medications.
Presented in standardized estimates. * p < .05, ** p < .01, *** p < .001.
Discussion
To the best of our knowledge, this is the first study to investigate the directional relationship between depressive symptoms and participation in physical activity across a range of dementia severity using a longitudinal approach. In contrast to commonly reported benefits of physical activity for improvement of depressive symptoms, we found that in our sample, depressive symptoms predicted reduced engagement in later physical activity participation over a two year follow up. Thus, we conclude that depressive symptoms may be an important barrier to engagement in physical activity that may be addressed in clinical practice and intervention research. Identifying and treating depressive symptoms may serve as a way to reduce barriers to physical activity which has been shown to have numerous benefits for health and cognitive function in older adults with and without cognitive impairment.
Although many studies have investigated barriers to physical activity among older adults, few studies have directly addressed depressive symptoms as a barrier and despite the high rate of comorbidity with dementia, we found no studies that considered this potential barrier in individuals with AD. The EPESE study [31] provided evidence that depressive symptoms predicted subsequent declines in physical performance including poorer balance, slower walking speed, and slower speed when rising from a chair. A possible explanation offered was a lower rate of walking, gardening, and vigorous exercise activities among individuals with a greater number of depressive symptoms. A prospective, population-based study of Norwegian adults reported that mood and exercise were correlated over a three year period, but did not find evidence for a consistent directional relationship between them [32]. One study, using a focus group methodology, reported negative affect as a barrier to engagement in exercise among older adults [33]. Our study adds to the sparse existing literature by considering the longitudinal and potentially bi-directional relationship between depressive symptoms and physical activity across individuals with a range of cognitive status.
There are several possible mechanisms that could reasonably explain how depressive symptoms may act as a barrier to physical activity, including physiological and psychological causes. Compared to younger adults, depression in older adults is characterized by fewer affective symptoms and more somatic symptoms.[34] Somatic symptoms of depression such as fatigue, change in body weight, slowed motor response, or poor sleep may contribute to reduced physical activity. Depression is associated with increased perception of pain [35], another known barrier to engagement in physical activity. Pharmacological treatments for depression may also result indirectly in reductions in physical activity. Common side effects of anti-depressant medications include weight gain, orthostatic hypotension, and other cardiovascular side effects that may alter the body’s response to physical activity [36,37].
Psychological mechanisms by which depressive symptoms prevent physical activity include apathy, anhedonia, and reduced self-efficacy. Apathy, characterized by reduced initiation and persistence, has been associated with reductions in self-care, functional impairment in activities of daily living, [18] and poorer cognitive function, all of which are likely barriers to activity. Anhedonia, a reduced ability to experience pleasure, may also reduce engagement in activities that were previously found to be pleasurable. Individuals with anhedonia tend to expect that they will not enjoy activities, thus becoming less likely to engage in them. Self-efficacy, an individual’s belief in their ability to successfully perform a specific behavior, has been identified as an important barrier to exercise in older adults.
Our findings have important implications for clinical practice and intervention research. To capitalize on the benefits of physical activity for health, depressive symptoms, and cognitive function, we must first remove barriers to engagement in physical activity that are inherent in our population, including the barriers created by the very symptoms we are hoping to treat. Before embarking on a program of physical activity, existing depressive symptoms need to be identified and treated. There are a number of approaches for the treatment of depressive symptoms in people with dementia, including both pharmacological and non-pharmacological approaches [38]. These include multi-sensory stimulation, Behavioral Therapy-Pleasant Events [38], and the combination of Shiatsu and physical activity [39].
To encourage adoption of physical activity routines among individuals with depressive symptoms, we need to educate people with depressive symptoms about the possible benefits for improvement of mood, physical, and cognitive symptoms. Because dysphoric mood is accompanied by a bias toward negative information and expectation that activities will not be pleasurable, physical activity routines should capitalize on experiences pleasurable to individuals. This might include activities that are socially engaging, interactive with nature or pets, or accompanied by music [40,41]. Physical activities must be appropriate to the physical and cognitive ability level of individuals, and ensure safety. Walking is the most common physical activity among older adults [3,42] and may be safe for individuals with AD in the right physical setting (e.g., safe, navigable) [43] or with a companion. Caregivers and other social supports play a critical role in the initiation and maintenance of any lifestyle routine.
Limitations and future directions
A limitation of the study is the reliance on a self- and caregiver- reported measure of physical activity. Objective measures of physical activity such as accelerometry would improve accuracy of estimates. Our sample is largely Caucasian and well educated, reducing our ability to generalize our findings to broader samples of older adults. As is typical in clinical samples, we have a larger number of individuals without impairment compared to the number with impairment. This imbalance of sample size could potentially impact the results of our statistical analysis, though the analytic approach we used is generally robust to unbalanced sample sizes. Finally, our sample includes participants on the mild end of the dementia spectrum, thus we cannot draw conclusions about more severe stages of dementia.
Our understanding of how to increase physical activity engagement in older adults with and without AD would benefit from future research on ways to reduce depressive symptoms as a barrier to physical activity. To effectively increase the levels of physical activity among older people with and without AD, further research is needed to determine how to motivate persons within this population to exercise regularly despite the complexity of chronic health and mental health conditions [44]. Future research should consider individual symptoms specifically related to reduced physical activity and how to best treat them with medications or psychosocial interventions [38].
Supporting information
(DOCX)
Data Availability
The data are available on KU Scholarworks at the following handle http://hdl.handle.net/1808/27118.
Funding Statement
ASW and JMB are supported by University of Kansas Alzheimer’s Disease Center, supported by the National Institute on Aging (P30AG035982; R01AG033673). MEM is supported by the Australian National Health and Medical Research Council (NHMRC) and Australian Research Council (ARC) Dementia Research Development Fellowship #1102028. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. 10.1093/aje/kwm390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harvey JA, Chastin SF, Skelton DA. Prevalence of sedentary behavior in older adults: a systematic review. Int J Environ Res Public Health. 2013;10(12):6645–6661. 10.3390/ijerph10126645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watts AS, Vidoni ED, Loskutova N, Johnson DK, Burns JM. Measuring physical activity in older adults with and without early stage Alzheimer’s disease. Clin Gerontol. 2013;36(4):356–374. 10.1080/07317115.2013.788116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varma VR, Watts A. Daily Physical Activity Patterns During the Early Stage of Alzheimer’s Disease. J Alzheimers Dis. 2017;55(2):659–667. 10.3233/JAD-160582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil. 2004;85(10):1694–1704. [DOI] [PubMed] [Google Scholar]
- 6.Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269(1):107–117. 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- 7.Schutzer KA, Graves BS. Barriers and motivations to exercise in older adults. Prev Med. 2004;39(5):1056–1061. 10.1016/j.ypmed.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 8.Mathews AE, Laditka SB, Laditka JN, Wilcox S, Corwin SJ, Liu R, et al. Older adults’ perceived physical activity enablers and barriers: a multicultural perspective. J Aging Phys Act. 2010;18(2):119–140. [DOI] [PubMed] [Google Scholar]
- 9.Malthouse R, Fox F. Exploring experiences of physical activity among people with Alzheimer’s disease and their spouse carers: a qualitative study. Physiotherapy. 2014;100(2):169–175. 10.1016/j.physio.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 10.Yu F, Swartwood RM. Feasibility and perception of the impact from aerobic exercise in older adults with Alzheimer’s disease. Am J Alzheimers Dis Dementias®. 2012;27(6):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cedervall Y, Torres S, \AAberg AC. Maintaining well-being and selfhood through physical activity: experiences of people with mild Alzheimer’s disease. Aging Ment Health. 2015;19(8):679–688. 10.1080/13607863.2014.962004 [DOI] [PubMed] [Google Scholar]
- 12.García-Cordero I, Sedeño L, Fuente L, Slachevsky A, Forno G, Klein F, et al. Feeling, learning from and being aware of inner states: interoceptive dimensions in neurodegeneration and stroke. Phil Trans R Soc B. 2016. November 19;371(1708):20160006 10.1098/rstb.2016.0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer’s disease. Expert Rev Neurother. 2011;11(5):665–676. 10.1586/ern.11.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428–433. 10.1001/archneurol.2010.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wherton JP, Monk AF. Problems people with dementia have with kitchen tasks: The challenge for pervasive computing. Interact Comput. 2010. Jul 1;22(4):253–66. [Google Scholar]
- 16.Vikström S, Josephsson S, Stigsdotter-Neely A, Nyg\a ard L. Engagement in activities: Experiences of persons with dementia and their caregiving spouses. Dementia. 2008;7(2):251–270. [Google Scholar]
- 17.Yeager CA, Hyer L. Apathy in Dementia: Relations with Depression, Functional Competence, and Quality of Life. Psychol Rep. 2008. June 1;102(3):718–22. 10.2466/pr0.102.3.718-722 [DOI] [PubMed] [Google Scholar]
- 18.Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 19.Snowden MB, Atkins DC, Steinman LE, Bell JF, Bryant LL, Copeland C, et al. Longitudinal Association of Dementia and Depression. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2015. September;23(9):897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett S, Thomas AJ. Depression and dementia: Cause, consequence or coincidence? Maturitas. 2014. October 1;79(2):184–90. 10.1016/j.maturitas.2014.05.009 [DOI] [PubMed] [Google Scholar]
- 21.Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci. 2003;58(3):M249–M265. [DOI] [PubMed] [Google Scholar]
- 22.Rhyner KT, Watts A. Exercise and depressive symptoms in older adults: A systematic meta-analytic review. J Aging Phys Act. 2016;24(2):234–246. 10.1123/japa.2015-0146 [DOI] [PubMed] [Google Scholar]
- 23.de Souto Barreto P, Demougeot L, Pillard F, Lapeyre-Mestre M, Rolland Y. Exercise training for managing behavioral and psychological symptoms in people with dementia: A systematic review and meta-analysis. Ageing Res Rev. 2015. Nov;24:274–85. 10.1016/j.arr.2015.09.001 [DOI] [PubMed] [Google Scholar]
- 24.Graves RS, Mahnken JD, Swerdlow RH, Burns JM, Price C, Amstein B, et al. Open-source, rapid reporting of dementia evaluations. J Regist Manag. 2015;42(3):111. [PMC free article] [PubMed] [Google Scholar]
- 25.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993; [DOI] [PubMed] [Google Scholar]
- 26.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–939. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc. 2011;7(3):270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol J Aging Ment Health. 1986; [Google Scholar]
- 29.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MMB. Peer reviewed: the Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4). [PMC free article] [PubMed] [Google Scholar]
- 30.Farina N, Hughes LJ, Watts A, Lowry RG. Use of physical activity questionnaires in people with dementia: A scoping review. J Aging Phys Act. 2018;1–24. [DOI] [PubMed] [Google Scholar]
- 31.Penninx BW, Guralnik JM, Ferrucci L, Simonsick EM, Deeg DJ, Wallace RB. Depressive symptoms and physical decline in community-dwelling older persons. Jama. 1998;279(21):1720–1726. [DOI] [PubMed] [Google Scholar]
- 32.Sexton H, Søgaard AJ, Olstad R. How are mood and exercise related? Results from the Finnmark study. Soc Psychiatry Psychiatr Epidemiol. 2001;36(7):348–353. [DOI] [PubMed] [Google Scholar]
- 33.Lees FD, Clark PG, Nigg CR, Newman P. Barriers to exercise behavior among older adults: a focus-group study. J Aging Phys Act. 2005;13(1):23–33. [DOI] [PubMed] [Google Scholar]
- 34.Fiske A, Wetherell JL, Gatz M. Depression in Older Adults. Annu Rev Clin Psychol. 2009;5(1):363–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barsky AJ, Goodson JD, Lane RS, Cleary PD. The amplification of somatic symptoms. Psychosom Med. 1988;50(5):510–519. [DOI] [PubMed] [Google Scholar]
- 36.Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10(20):2463–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmermann U, Kraus T, Himmerich H, Schuld A, Pollmächer T. Epidemiology, implications and mechanisms underlying drug-induced weight gain in psychiatric patients. J Psychiatr Res. 2003;37(3):193–220. [DOI] [PubMed] [Google Scholar]
- 38.Verkaik R, van Weert J, Francke AL. The effects of psychosocial methods on depressed, aggressive and apathetic behaviors of people with dementia: a systematic review. Int J Geriatr Psychiatry. 2005;20(4):301–314. 10.1002/gps.1279 [DOI] [PubMed] [Google Scholar]
- 39.Lanza G, Centonze SS, Destro G, Vella V, Bellomo M, Pennisi M, et al. Shiatsu as an adjuvant therapy for depression in patients with Alzheimer’s disease: A pilot study. Complement Ther Med. 2018;38:74–78. 10.1016/j.ctim.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 40.Clair AA, Memmott J. Therapeutic uses of music with older adults. ERIC; 2008. [Google Scholar]
- 41.Souter MA, Miller MD. Do animal-assisted activities effectively treat depression? A meta-analysis. Anthrozoös. 2007;20(2):167–180. [Google Scholar]
- 42.McPhillips JB, Pellettera KM, Barrett-Connor E, Wingard DL, Criqui MH. Exercise patterns in a population of older adults. Am J Prev Med. 1989; [PubMed] [Google Scholar]
- 43.Watts A, Ferdous F, Diaz Moore K, Burns JM. Neighborhood integration and connectivity predict cognitive performance and decline. Gerontol Geriatr Med. 2015;1:2333721415599141 10.1177/2333721415599141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nyman SR, Adamczewska N, Howlett N. Systematic review of behaviour change techniques to promote participation in physical activity among people with dementia. Br J Health Psychol. 2017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
The data are available on KU Scholarworks at the following handle http://hdl.handle.net/1808/27118.