Abstract
Background:
In healthy adults, successful between-session recall of extinction learning depends on the hippocampus and ventromedial prefrontal cortex (vmPFC), especially when tested in the extinction context. Poor extinction recall and dysfunction within hippocampal-vmPFC circuitry are associated with fear-based disorders (e.g., anxiety, posttraumatic stress disorder). Despite the early age of onset of virtually all fear-based disorders and the protracted development of the hippocampus and vmPFC across the first two decades of life, little is known about extinction recall and the underlying neural correlates in children.
Methods:
Here, we tested extinction recall in 43 pre-adolescent children (ages 6–11 yrs) by coupling functional magnetic resonance imaging and virtual reality with a novel interpersonal threat-related two-day (ABBA) fear-extinction paradigm. Conditioned fear responding was assessed at behavioral, subjective, physiological, and neural levels.
Results:
Although children demonstrated intact within-session extinction, there was poor between-session recall of extinction learning (retention index: 13.56%), evidenced by elevations in skin conductance, avoidant behavioral responses, and subjective ratings. Elevations in conditioning fear responding were accompanied by activation in the hippocampus and insula, and increased connectivity of the hippocampus with the insula and dorsal anterior cingulate cortex - regions implicated in the return of fear in adult studies. Children who kept more distance from the extinguished cue during extinction subsequently demonstrated heightened hippocampal-cingulate coupling during recall, suggesting that avoidant behavior interferes with extinction retention.
Conclusions:
Poor extinction recall in children may have implications for developmental vulnerability to fear-based disorders, and for the application of therapeutic strategies that rely on principles of extinction (e.g., exposure therapy) to pediatric samples.
Keywords: fMRI, hippocampus, fear, ventromedial prefrontal cortex, anterior cingulate cortex, insula, context, skin conductance, avoidance, Fear learning, Pavlovian fear conditioning, extinction retention, insula, anterior cingulate cortex
1. Introduction
In healthy adults, successful between-session recall of extinction learning has been shown to depend on hippocampal-ventromedial prefrontal cortex (vmPFC) circuitry, especially when tested in the extinction context (Åhs, Kragel, Zielinski, Brady, & LaBar, 2015; Kalisch et al., 2006; Milad et al., 2005; Milad, Wright, et al., 2007). For example, neuroimaging studies have linked better extinction recall with a higher magnitude of blood-oxygen level-dependent (BOLD) response in the hippocampus and vmPFC, higher hippocampal-vmPFC functional connectivity, and increased cortical thickness of the vmPFC (Kalisch et al., 2006; Milad et al., 2005; Milad, Wright, et al., 2007). In contrast, conditioned fear responding after extinction learning in adults is associated with re-exposure to the environmental context in which fear learning (but not extinction) occurred, or to a novel context (Mineka, Mystkowski, Hladek, & Rodriguez, 1999; Vansteenwegen et al., 2005). Conditioned fear responding is associated with activation in the hippocampus, amygdala, insula, and dorsal anterior cingulate cortex (dACC), as well as positive hippocampal-dACC functional connectivity (Åhs et al., 2015; A. Hermann, Stark, Milad, & Merz, 2016; Hermann, Stark, Blecker, Milad, & Merz, 2017). Taken together, these studies underscore the pivotal role of the hippocampus in gating the expression of extinction or fear associations.
Converging evidence in animal models and adult patients with fear-based disorders (e.g., anxiety, posttraumatic stress disorder [PTSD]) points to deficits in between-session recall of extinction learning, causing inappropriate and persistent fear expression (Åhs et al., 2015; Milad et al., 2009). Neuroimaging studies suggest that poor extinction recall in adults with fear-based disorders is linked to dysfunction of hippocampal-vmPFC-amygdala circuitry (Garfinkel et al., 2014; Milad et al., 2009), particularly lower activation and connectivity between the hippocampus and vmPFC when re-exposed to a previously extinguished cue.
Despite the substantial uptick in fear-based disorders during childhood and adolescence (Kessler et al., 2005; McLaughlin et al., 2012), and the protracted development of the hippocampus and the vmPFC across the first two decades of life (Keding & Herringa, 2015; Uematsu et al., 2012), research on the maintenance and retrieval of extinction learning in children is limited. Developmental studies in animal models indicate that fear learning emerges in early life, but the capacity to express and extinguish fear can change dramatically across development. For example, work by Sullivan and colleagues (Landers & Sullivan, 2012) indicates that extinction learning is ‘relapse resistant’ in pre-weanling ‘infant’ rat pups (prior to P24), suggesting that extinction at this young age may cause an erasure of the original fear memory, rather than a separate competing memory trace, as observed in adults. In contrast, post-weanling ‘adolescent’ mice (P29-P39; Pattwell et al., 2011) and rats (P35-P45; Baker and Richardson, 2015; Kim et al., 2011; McCallum et al., 2010; Zbukvic et al., 2017) demonstrate poorer extinction learning and recall relative to younger (i.e., pre-adolescent) and older (i.e., young adult) animals. Thus, there appears to be a developmental suppression of extinction during adolescence, that may relate to relatively immature dopaminergic signaling in the vmPFC (Ganella et al., 2017; Zbukvic et al., 2017). Interestingly, despite the presumed immaturity of the hippocampus and vmPFC during childhood, rodent models suggest that extinction recall may be intact during pre-adolescence (e.g., P25-P30 rats; Santini et al., 2008). However, one study found elevated fear responding when pre-adolescent children (5–10 year-olds; Michalska et al., 2016) were re-exposed to a previously-extinguished cue (i.e., colored cartoon bell) during a discrimination task, which may be similar to a test of extinction recall. These data suggest that extinction recall in pre-adolescent children may differ from patterns observed in mice.
The present study sought to examine the between-session recall of extinction learning in pre-adolescent children (ages 6–11 yrs). Within the same pediatric sample, we have previously demonstrated that children are able to acquire and subsequently extinguish fear within-session (Marusak et al., 2017). These results are consistent with previous pediatric studies (Gao, Raine, Venables, Dawson, & Mednick, 2010; Schiele et al., 2016). The present study extends this previous work by evaluating the between-session recall of extinction learning, tested 24 hours following fear and extinction learning. We have previously adapted a well-validated contextual cued Pavlovian fear-extinction paradigm (Milad et al., 2009) for use in children (Marusak et al., 2017). Our novel paradigm couples virtual reality (VR) with environmental contexts that allow for robust context manipulation, and includes active navigation in the VR environment to augment hippocampal engagement (Burgess, Maguire, & O’Keefe, 2002). Active navigation in the VR environment also allows for unique access to behavioral response patterns (e.g., avoidant behavior) during fear-extinction, which provides additional information on dimensions of fear responding beyond physiology (skin conductance responses, SCRs) or subjective ratings (fear and unconditioned stimulus [US]-expectancy ratings). Another adaptation of the paradigm is that it was designed to model the cues and environmental contexts that youth frequently experience during real-life threat exposures. Given that interpersonal threat exposures (e.g., violence, abuse) are extremely common among children and adolescents and are strongly linked to the development of fear-based disorders (Kessler et al., 2010; McLaughlin et al., 2012), virtual human beings (‘avatars’) were chosen as conditioned stimuli (CS) to model the interpersonal nature of these common threat exposures (Marusak et al., 2017).
In addition to behavioral, subjective, and physiological measures of conditioned fear, we evaluated neural activation and functional connectivity within fear-extinction circuitry during extinction recall, as it is presently unclear if the brain patterns observed during extinction recall in adults (i.e., activation and positive coupling within hippocampal-vmPFC circuitry) are also evident in children. Based on rodent studies, we predicted that pre-adolescent children would show intact extinction recall, evidenced by low levels of conditioned fear responding (i.e., low SCR and fear- and US-expectancy ratings) to a previously extinguished cue in the extinction context (CXT-), but show renewal of conditioned fear responses when subsequently tested in the fear conditioning context (CXT+). In addition, given evidence that the vmPFC is relatively immature during childhood, and connections between the vmPFC and limbic areas continue to develop across childhood and adolescence (Gee et al., 2013), we predicted that extinction recall would be mediated more directly by subcortical circuitry (i.e., hippocampal-amygdala circuitry) rather than indirectly, via vmPFC, as observed in adults.
Although to our knowledge recall of extinction learning has yet to be tested in pre-adolescent children, the results of the discrimination task performed by Michalska et al. (2016) hint at poor between-session recall in pre-adolescent children. Thus, it is possible that children will alternatively show poor recall of extinction learning, evidenced by an increase in indicators of conditioned fear (e.g., SCR, subjective ratings, avoidant behavior) during recall on Day 2. In this case, we would expect to observe activation in areas implicated in the expression of conditioned fear (i.e., hippocampus, amygdala, insula, dACC), and positive hippocampal-dACC coupling (Hermann et al., 2017).
2. Materials and Methods
2.1. Participants
This study reports on a total of 43 children (24 female, ages 6–11 yrs). A subset of the total sample (n = 9, 5 female, ages 6–9 yrs) completed extinction recall and fear renewal sessions in the laboratory using VR (to match Day 1 testing), to examine potential effects of the scan environment on extinction recall. The behavior-only subset did not differ from the full sample on age, pubertal maturation, gender, or race (p’s > 0.05). All study procedures were approved by the Wayne State University Institutional Review Board (IRB), and study methods were performed in accordance with IRB guidelines and regulations. Participants and their parents provided written informed assent/consent prior to study participation. Please see the Supplemental Material for more information on the study sample. Given our interest in examining overall extinction recall in children, age was not controlled for in analyses. However, exploratory analyses tested for effects of age as a continuous factor, and by splitting children into two age groups (younger, under 10 years of age; older, ages 10+) following prior work (Glenn et al., 2012; Jovanovic et al., 2014).
2.2. Testing Procedures
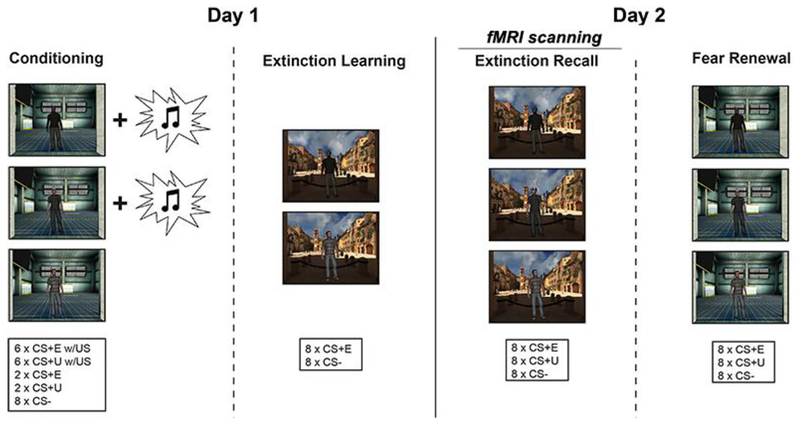
Children underwent a novel two-day fear-extinction paradigm (see Figure 1). Day 1 consisted of fear conditioning and extinction learning in VR, separated by a 10-min delay; Day 2 consisted of tests of extinction recall and fear renewal. Given our focus on extinction recall, the recall session (Day 2) was conducted during fMRI scanning using an adapted 2D version of the task. Day 1 sessions (fear conditioning, extinction learning) were conducted in the lab using an advanced VR platform (see Supplemental Material). Following the recall session, participants completed a fear renewal test on a laptop outside of the scanning environment. The behavior-only subset (n = 9) underwent identical experimental procedures, except that Day 2 testing occurred in the lab in VR (i.e., same setup as Day 1) rather than at the scan center. Testing sessions were conducted within the same 1–3 hr time window in subsequent visits (e.g., between 12:30 PM and 2:30 PM) to control for time-of-day effects.
Figure 1. Schematic of experimental protocol.
Day one consisted of fear conditioning and extinction learning in separate virtual reality (VR) environmental contexts, separated by a 10-minute delay. 24 hours later, participants completed a test of extinction recall in the extinction context, while undergoing functional magnetic resonance imaging (fMRI) scanning. Renewal of fear was subsequently tested in the conditioning context, on a laptop with headphones. A subset of participants (n = 9) completed all four sessions in VR, to examine the potential effect of the fMRI scan environment on extinction recall.
Prior to the testing sessions, participants viewed a brief video to prepare them for the study visits (available at www.tnp2lab.org/think-study-video). Prior to the fear conditioning session on Day 1, participants completed a brief practice tutorial (60 s) in VR, during which they received recorded instructions about how to navigate and explore the virtual environment. Then, they were given the opportunity to move through space within a third virtual environment that was not used in the experimental paradigm (i.e., indoor art gallery) using a joystick in their dominant hand, as well as practice looking around the visual field by turning their head left and right (limited to 90° with visual bounds while sitting in a stationary chair). Participants also practiced submitting their subjective responses using the joystick, which they would need to make during the experimental paradigm (described below).
The scan session (Day 2) began with training in a child-friendly mock scanner, and participants practiced submitting ratings and moving in the virtual environment using MRI scanning. Participants were scanned with a research-dedicated Siemens 3T MAGNETOM Verio system (MRI Research Facility, Wayne State University) using a 32-channel head coil. Foam padding was used to reduce motion during the scan, and a trained member of the research team remained in the room with the child during the scan, to ensure comfort and adherence. The extinction recall session was at the end of the <60 min scan protocol, to reduce the potential influence of the novel scan environment on fear responding.
2.3. Fear-Extinction Paradigm
The fear-extinction paradigm was a novel version of an established cued Pavlovian fear-extinction paradigm with context manipulations for adults (Milad, Wright, et al., 2007), adapted and validated for children in our lab (Marusak et al., 2017). This two-day design manipulates context using an ABBA design such that the context in which fear is first acquired (CXT+) is different from the context in which fear is subsequently extinguished (CXT-). Given that extinction recall has been shown to be context dependent in adults (Hermann et al., 2017; Ji & Maren, 2007; Kalisch et al., 2006), the between-session test of extinction recall was conducted in the CXT-. Following the recall session, all children underwent a test of fear renewal. Fear renewal was conducted to determine whether good extinction recall was actual recall of the extinction memory trace or rather, an erasure of fear, as observed in some rodent studies in juvenile animals. In the case of the latter, then we would not expect subsequent fear renewal when placed back in the original fear conditioning context. Thus, fear renewal occurred after recall in the CXT+. During conditioning, two conditioned stimuli (CS) were paired with the unconditioned stimulus (US) – a white noise burst – and only one was subsequently extinguished (CS+E). Thus, one CS remained unextinguished (CS+U). A third CS was presented but never paired with the US (i.e., CS-). An overview of the experimental paradigm and number of trials is given in Figure 1.
The environmental contexts were two separate 3-D immersive VR environments, matched on size and layout but comprised of different colors, textures, sounds (ambient noise), and background scenes (see Figure 1). The CSs were three virtual adult males—modeling the interpersonal nature of early threat exposures that children commonly experience (i.e., violence, abuse). The CSs varied in hair color/style, clothing, and race (African American, Asian, Caucasian) to match the demographics of the study sample. The US was an aversive white noise burst (500 ms, 95 dB) — a stimulus that is appropriate for use in children and well-tolerated even in anxious youth (Shechner et al., 2015).
During the fear conditioning session (~7.3 min), participants were presented with two CSs (CS+; e.g., African American and Caucasian males) that co-terminated with the aversive white noise burst US through a pair of headphones at a partial reinforcement rate of 75% (i.e., partial reinforcement extinction effects; Milad et al., 2009, 2007). A second CS (e.g., Asian male) was presented during fear conditioning but was never paired with the US (CS-). Fear conditioning consisted of 6 presentations of each CS+ that co-terminated with the US, intermixed with an additional 2 non-reinforced presentations of each the CS+, and 8 presentations of the CS-, all presented within the conditioning context (CXT+; Figure 1). During the subsequent extinction session (~6.8 min), one of the two CS+’s was extinguished (CS+E) by presenting it in the absence of the US. There were 8 CS+ and 8 CS- trials presented in the extinction context (CXT-; Figure 1a). The second CS+ was not shown during extinction (i.e., CS+U). During extinction recall and fear renewal sessions on Day 2 (7.2 min each), the CS+E, CS+U, and CS- were each presented 8 times in the CXT- and CXT+, respectively.
At the beginning of each session, participants received recorded instructions to approach the person when they appeared, and that they may hear a loud sound: “During the game, you will meet virtual human beings. When they appear, you will be told to move directly towards them…During the game you might also hear a loud sound.” Each experimental session began by participants rating the CS-CXT for fear and US-expectancy (described below). Following the rating, each trial began in a virtual hallway (CXT+ or CXT-) and participants were free to move through the virtual environment using the joystick. Of note, active navigation is thought to augment the spatial representation of contexts and more robustly engage hippocampal-dependent learning (Burgess et al., 2002). After 3–8 s, the participant was set back to the start position (17 virtual meters from the CS) to control for differences in participant movement in the context. Concurrently, the CS rounded the corner, turned to face the participant, and a written prompt appeared at the lower part of the visual field that read “move toward the person”. After 4 s, the trial terminated to a black screen (ITI; 4–9 s). The designation of the virtual avatars (CS+E, CS+U, or CS-) and the context (CXT+ or CXT-) was counterbalanced across the participants. In addition, the order of trials was pseudo-randomized, such that no more than two presentations of the same virtual person CS occurred in a row. Electrodes and headphones remained in place during all sessions (removed only during breaks).
2.4. Measures of Conditioned Fear
Measures of conditioned fear included subjective reports of (1) fear and (2) US expectancy, physiological measures of (3) SCRs, and (4) behavioral movement in the VR environment.
2.4.1. Fear and US-expectancy Ratings:
Participants were asked to rate each CXT-CS combination on a 5-point Likert scale for fear (“How scary is this?”; 1 = not scary, 5 = very scary) and US expectancy (“Do you think that you will hear a loud sound with this?”; 1 = definitely not, 5 = definitely yes). Ratings were submitted at three timepoints during each session: beginning (‘start’), after the first half (‘early’) and after the second half (‘late). As our focus was on cued fear-learning within a contextual environment, the questions were designed to be vague, to not lead the participant to focus on the CS in the absence of the CXT, or vice versa. Of note, ratings at the ‘start’ timepoint are available only for a subset of youth (n = 14).
2.4.2. Skin Conductance Responses:
SCRs for each CS presentation were measured during each session and analyzed following our prior work (Marusak et al., 2017; Rabinak et al., 2014). Briefly, SCRs were measured using two electrodes (EL509, BIOPAC Systems, Inc., Goleta, CA) attached between the first and second phalanges of the second and third digits of the non-dominant hand (left in all but 2 participants). A third, ground electrode, was additionally placed on the palm during fMRI scanning (extinction recall session). AcqKnowledge software v.4.4 (BIOPAC Systems) was used to acquire the SCR trace (1000 samples per second) and to calculate SCRs using event-related electrodermal response analysis. The recorded waveforms were low pass filtered using a Blackman window (cutoff frequency = 25 Hz, coefficients = 160) and mean value smoothed over 100 adjacent data points prior to scoring. SCRs were calculated on the maximum of the SCR trace within a 0.5–4.5 s latency window following CS onset, and accounting for a baseline (2 s window prior to CS onset). Raw SCRs were square root transformed to normalize the distributions across participants. Analyses focused on SCRs to the last trial of extinction learning, and the first trial of extinction recall and fear renewal, tested 24 hours later. In addition, an extinction retention index was calculated following prior work (Milad et al., 2009; Rabinak et al., 2014), to normalize each participant’s SCR during recall to that exhibited during the conditioning. The index was calculated as follows: 100- ([the average SCR during the first two trials of the extinction recall session divided by the largest SCR during the conditioning session] × 100).
2.4.3. Behavioral Action Tendencies:
Participant movement following CS onset (3 s window, to avoid contamination from the US at 3.5 s) was recorded during the first half (‘early’) and second half (‘late’) of each session in two directions: 1) forward-to-backward distance from the CS, and 2) left-to-right distance travelled. Distance is provided in virtual meters. Of note, participant movement in the virtual environment for Day 2 sessions was limited to forward-to-backward movement, due to restrictions of the scan environment (i.e., stationary head, MR-compatible button-box). Further, because of the change in response device, behavior was not directly contrasted between sessions. Analysis of behavioral data focused on the first half of the recall and renewal sessions.
2.5. BOLD fMRI
2.5.1. BOLD fMRI data acquisition:
During extinction recall, whole-brain blood oxygen level-dependent (BOLD) functional images were acquired with a multi-echo/multi-band (ME/MB) echo-planar imaging sequence, customized for the scanner and for pediatric neuroimaging (51 slices, 186 mm field of view (FOV), 64 × 65 matrix size yielding 2.9 mm isotropic resolution, in-plane GRAPPA acceleration factor 2, flip angle (FA) = 83 degrees, repetition time (TR) = 1.5 s, and echo time triplet (TEs) = 15, 31, 46 ms). A whole-brain anatomical image was collected during the scan session for co-registration, using a magnetization prepared rapid acquisition GRE (MP-RAGE) sequence (128 slices, 256 mm FOV, 384 × 384 matrix size yielding 0.7 × 0.7 × 1.3 mm resolution, in-plane GRAPPA acceleration factor 2, FA = 9 degrees, TR = 1.68 s, TE = 3.51 ms).
2.5.2. fMRI preprocessing and denoising:
ME/MB fMRI datasets for each participant were submitted to ME-ICA software (v3, beta 1; https://bitbucket.org/prantikk/me-ica) for preprocessing and denoising. ME-ICA uses independent components analysis (ICA) to decompose ME fMRI datasets into independent components, and categorizes these components as BOLD or non-BOLD (noise) based on their TE-dependence (Kundu, Inati, Evans, Luh, & Bandettini, 2012). The BOLD components are then optimally combined into a denoised fMRI timeseries that reflects the T2* weighted averaging of timeseries, for each echo. Previous studies demonstrate that the combination of ME fMRI acquisition and ME-ICA denoising is superior than conventional (i.e., single-echo) fMRI and denoising strategies (e.g., motion parameter regression) in terms of removal of non-BOLD artifact, enhanced specificity, and improved fMRI effect sizes and thus statistical power (Kundu et al., 2015, Lombardo et al., 2016). These benefits are particularly relevant for pediatric imaging where artifact due to excess head motion poses significant challenges (Kotsoni et al., 2006). By collecting earlier TE’s, ME fMRI also allows for coverage of regions of interest (ROIs) in areas prone to signal dropout (e.g., vmPFC, medial temporal areas; Olman et al., 2009). The preprocessing pipeline in MEICA includes, in brief: (1) skull-stripping and warping of the anatomical image to the Montreal Neurological Institute (MNI) template, (2) co-registration of the first echo timeseries for motion correction and for anatomical-functional co-registration, (3) de-obliquing of the functional data, and (4) 12-parameter affine anatomical-functional co-registration. The first 4.5 s of data (prior to first stimulus onset) was removed to allow for signal equilibration. No temporal filtering or smoothing was applied to the data. See Kundu et al. (2012) for further information. Head motion was relatively low across the sample, with mean framewise displacement (FD) 0.47 mm (SD = 0.38) even prior to ME-ICA denoising.
2.5.3. fMRI first and second level activation analyses:
Statistical parametric maps were estimated for each participant using a general linear model (GLM) framework in SPM8. Experimental conditions (CS+E, CS+U, CS-, Context) were modeled during the extinction recall session using an event-related design and by convolving the BOLD signal for each event with a canonical hemodynamic response function (HRF). All trials (8 for each CS-type) were modeled for each experimental condition. Our main contrasts of interest were CS+E>CS+U, CS+E>CS-, and CS+E (>implicit), but effects of CS-type (CS+E, CS+U, CS-) were also evaluated at the group level using a one-way random-effects ANOVA in SPM8. We also performed separate group-level random-effects one-sample t-tests, to assess neural activity for CS+E, CS+U, and CS- (each > implicit) across the sample, using SPM8.
2.5.4. Task-based functional connectivity analysis:
Functional connectivity during CS+E, CS+U, and CS- trials was evaluated using a generalized psychophysiological interaction analysis (gPPI). The deconvolved time series from the hippocampus was extracted for each participant to create the physiological variable. We extracted from 6 mm radii spheres around the group peak of activation in the hippocampus for the one-sample t-test for CS+E (>implicit). Condition onset times for CS+E, CS+U, CS-, and context, were separately convolved with the canonical HRF for each condition, creating psychological regressors. Interaction terms (PPIs) were computed by multiplying extracted time series from the psychological regressors with the physiological variable. Activity within the hippocampal seed was regressed on a voxel-wise basis against the interaction, with the physiological and psychological variables serving as regressors of noninterest. Individual contrast images for CS+E, CS+U, and CS- (each >implicit) were then entered into a group-level one-sample t-test, to assess hippocampal-dependent functional connectivity for each trial type during recall.
2.5.5. Regions of interest analysis:
Activation and connectivity analyses focused on anatomical ROIs shown to be involved in extinction learning and/or recall and fear renewal in adults, and in developmental studies in animal models (Åhs et al., 2015; Hermann et al., 2016; Milad, Wright, et al., 2007; Shechner, Hong, Britton, Pine, & Fox, 2014): amygdala, hippocampus, vmPFC, dACC, and insula. ROIs were defined bilaterally using anatomical landmarks provided in MARINA software (Walter et al., 2003) based on masks from the atlas of (Tzourio-Mazoyer et al., 2002). Effects were considered significant within ROIs using a small-volume family-wise error correction, pFWE < 0.05.
2.5.6. Exploratory whole-brain threshold:
For completeness, significant whole-brain results are presented in the Supplemental Material, at a combined voxel-level (p < 0.005) and cluster-level (71 voxel) whole-brain correction. This threshold was derived by computing the spatial autocorrelation of the data via AFNI’s 3dFWHMx and subsequently performing Monte Carlo simulations (10,000 iterations) using 3dClustSim (compile date July 22, 2016; National Institute of Mental Health, Bethesda, MD; https://afni.nimh.nih.gov).
3. Results
3.1. Children show intact within-session extinction learning
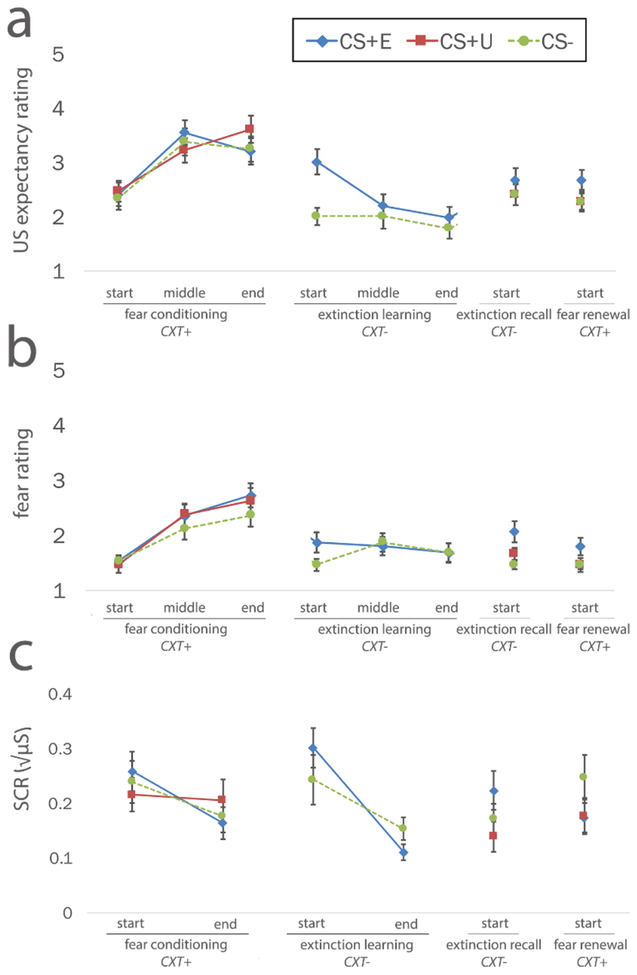
As previously reported (Marusak et al., 2017), children show intact fear-conditioning and within-session extinction (Figure 2), evidenced by an increase in US expectancy ratings to the CS+E and CS+U (but not the CS-) during fear conditioning, higher US expectancy for the CS+E relative to the CS- at the start of extinction, and declines in subjective ratings and SCRs to the CS+E and CS- over the course of extinction. These patterns are similar to previous reports in children (Gao et al., 2010; Schiele et al., 2016).
Figure 2. Children show intact within-session extinction learning, but poor between-session extinction recall.
Children show within-session extinction learning, evidenced by significant reductions in SCRs and fear ratings to the CS+E from fear conditioning to the end of extinction learning. Children show poor between-session recall, evidenced by elevations in SCRs and fear ratings to the CS+E from the end of extinction learning to the beginning of extinction recall. Extinction recall was tested in the extinction context (CXT-) 24 hours after fear conditioning and extinction learning sessions. Of note, fear ratings are on a scale from 1–5 where 1 = low fear and 5 = high fear. Similar results were obtained for US-expectancy ratings (see text). Abbreviations: SCRs, skin conductance responses; CXT-, extinction context; CXT+, fear conditioning context; CS+E, extinguished conditioned stimulus; CS+U, unextinguished conditioned stimulus; CS-, safety conditioned stimulus (unpaired with the US); US, unconditioned stimulus. Error bars represent standard error of the mean.
3.2. Children show poor between-session recall of extinction learning
3.2.1. US expectancy ratings:
24 hours after fear conditioning and extinction learning, children were re-exposed to the CS+E within the extinction context (CXT-) to test recall of extinction learning. CS-type (CS+E, CS-) × time (end of extinction, beginning of recall) ANOVA revealed a significant main effect of time on US expectancy ratings (F[1,14] = 16, p = 0.001, ηp2 = 0.58), such that expectancy increased from the end of extinction learning to the start of extinction recall. The main effect of CS-type and CS-type × time interaction were not significant (p’s > 0.17). Within the recall session, there was also no main effect of CS-type (CS+E, CS+U, CS-; p = 0.77). Exploratory age analyses showed an age-related decrease in US expectancy at the start of extinction recall (r(15) = -0.54, p = 0.037), suggesting that extinction recall ability improves with age.
3.2.2. Fear ratings:
For fear ratings, in contrast, the main effect of CS-type was significant (F[1,14] = 5, p = 0.042, ηp2 = 0.55), such that fear was higher for the CS+E relative to the CS-. The main effect of time and time × CS-type interaction were not significant for fear ratings (p’s > 0.069). Within the recall session, there was no main effect of CS-type (CS+E, CS+U, CS-; p = 0.1). There were no main effects or interactions with age on fear ratings.
3.2.3. SCRs:
Consistent with the expectancy ratings, there was a main effect of time on SCRs (F[1,35] = 6.45, p = 0.016, ηp2 = 0.69) such that SCRs were higher at the start of recall relative to the end of extinction. The main effect of CS-type and the CS-type × time interaction were not significant (p’s > 0.11). Within the recall session, there was also no main effect of CS-type (CS+E, CS+U, CS-; p = 0.09). In further support of poor overall extinction recall, the extinction retention index varied across children but was low on average (13.56 ± 80.7%). Overall, the extinction retention index observed in our pediatric population was significantly lower (t(38) = 4.7, p < 0.001) than previously reported in healthy adult populations using similar experimental paradigms (e.g., 74–85%; Holt et al., 2009; Milad et al., 2009; Milad, Wright, et al., 2007; Rabinak et al., 2014). There were no main effects or interactions with age on SCRs.
3.2.4. Movement:
For behavioral data (i.e., movement in the virtual environment), the main effect of CS-type was tested within the extinction recall session and not between sessions, given that response devices differed between sessions (joystick vs. MRI response-box). During the recall session, there was a main effect of CS-type (CS+E, CS-; F[1,41] = 8.6, p = 0.005, ηp2 = 0.17) such that children kept more distance from the CS+E (M = 9.7 virtual m, SD = 3.9) relative to the CS- (M = 9.26 virtual m, SD = 4.1). Although the main effect of age was not significant, there was a significant age × CS-type interaction (F[1,32] = 3.22, p = 0.013, ηp2 = 0.72) such that younger (but not older) children kept more distance from the CS+E relative to the CS- (t(22) = 3.4, p = 0.002). There was no difference in distance from the CS+E vs. CS+U (M = 9.4 virtual m, SD = 3.9) during recall (p = 0.066). There was also no main effect of time (early, late) or time × CS-type interaction movement during extinction recall.
3.2.5. Effects of scan environment:
Importantly, SCRs and fear and US-expectancy ratings did not differ in the behavior-only subset compared to the fMRI sample (p’s > 0.18), suggesting that the scan environment wasn’t the reason for poor extinction recall.
Taken together, fear indicators point to poor between-session recall of extinction learning. Given the poor extinction recall, it is not surprising that children also demonstrated conditioned fear responding to the cues when re-exposed to the conditioning context (CXT+; Supplemental Material).
3.3. Activation and connectivity within hippocampal-dACC-insula circuitry during extinction recall
3.3.1. Neural activation:
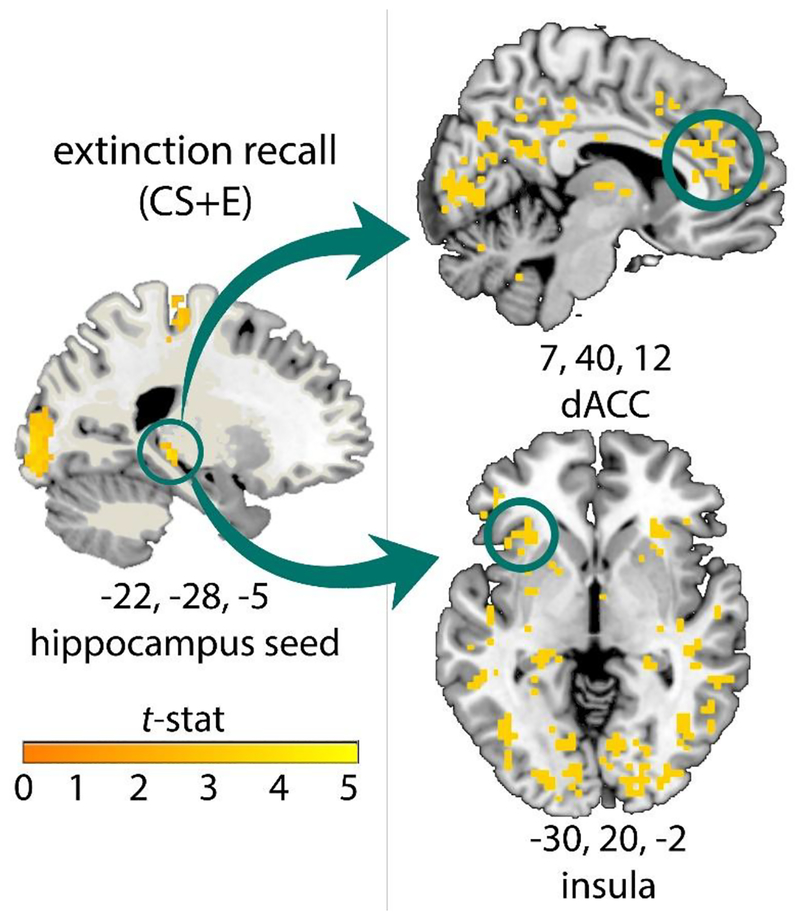
Given the lack of main effects of CS-type on conditioned fear responding (e.g., SCRs, subjective ratings), it is not surprising that there were no significant effects of CS+E>CS+U or CS+E>CS-, or main effects of CS-type on neural activity in any a priori ROI (pFWE > 0.1). Therefore, we examined neural response to each CS type alone. Children demonstrated significant activation to the CS+E during extinction recall in the left hippocampus (pFWE = 0.003, Z = 4.41, x = -22, y = -28, z = -5, 3 voxels), right hippocampus (pFWE = 0.013, Z = 4.21, x = 16, y = -31, z = -2, 7 voxels), left insula (pFWE = 0.022, Z = 4.24, x = -42, y = -3, z = 12, 21 voxels), and right insula (pFWE = 0.040, Z = 4.11, x = 39, y = 15, z = 3, 47 voxels, Figure 3). Significant activation was not observed in amygdala or vmPFC ROIs. Of note, a similar pattern of neural activation was observed for CS+U trials, such that significant activation was observed in the hippocampus (pFWE = 0.012, Z = 4.51, x = -22, y = -28, z = -5, 2 voxels), and the right (pFWE = 0.004, Z = 4.5, x = 33, y = 12, z = 9, 44 voxels) and left insula (pFWE = 0.011, Z = 4.29, x = -42, y = 3, z = 3, 34 voxels; see Figure S1). There was also significant activation in the hippocampus alone for CS- trials (pFWE = 0.006, Z = 4.27, 2 voxels, x = -22, y = -28, z = -5; see Figure S2). There was no main effect of age on neural response in these regions.
Figure 3. Functional neural activation in the hippocampus and insula during a test of extinction recall in children, tested ~24 hours after extinction learning.
Results significant using small-volume family-wise error correction (pFWE < 0.05), within anatomically defined regions of interest (Tzourio-Mazoyer et al., 2002). No masking was applied to images. Displayed at p < 0.005, >5 voxels. Abbreviation: CS+E, previously extinguished cue; FWE, familywise error corrected.
3.3.2. Functional connectivity:
Given the known pivotal role of the hippocampus in context-dependent retrieval of fear and extinction learning in adults (Kalisch et al., 2006; Maren, Phan, & Liberzon, 2013), we performed a gPPI analysis to evaluate connectivity of the hippocampus during extinction recall. A left hippocampal seed was defined based on the group activation peak shown in Figure 3. During CS+E trials, we observed positive left hippocampal coupling with the right dACC (pFWE = 0.001, Z = 4.67, x = 7, y = 40, z = 12, 47 voxels), left dACC (pFWE = 0.004, Z = 4.41, x = -1, y = 38, z = 23, 37 voxels), and left insula (pFWE = 0.025, Z = 4.17, x = -30, y = 20, z = -2, 50 voxels; Figure 4). Similar results were observed using a right hippocampal seed, defined based on group activation peak reported above (right dACC: two peaks: pFWE < 0.001, Z = 4.91, x = 10, y = 23, z = 26, 61 voxels and pFWE = 0.022, Z = 4.02, x = 10, y = 48, z = 15, 5 voxels; left insula: two peaks: pFWE = 0.020, Z = 4.22, x = -27, y = 20, z = -8, 47 voxels, and pFWE = 0.026, Z = 4.16, x = 33, y = 23, z = -2, 68 voxels). There were no ROIs showing reduced coupling with the hippocampus during CS+E trials. Similar patterns were observed for CS+U and CS- trials. During CS+U trials, we observed positive left hippocampal coupling with the right dACC (pFWE = 0.001, Z = 4.17, x = 1, y = 20, z = 26, 10 voxels), left dACC (pFWE = 0.001, Z = 4.5, x = -4, y = 46, z = 15, 30 voxels), left insula (pFWE = 0.001, Z = 4.71, x = -39, y = 9, z = 6, 8 voxels), and right insula (pFWE = 0.001, Z = 4.39, x = 30, y = 17, z = -8, 5 voxels; Figure S3). During CS- trials, there was positive left hippocampal coupling with the left dACC (pFWE = 0.001, Z = 4.38, x = -1, y = 12, z = 29; see Figure S4). There was no main effect of age on neural response in these regions.
Figure 4. Positive hippocampal coupling with the dACC and insula during a test of extinction recall in children, tested ~24 hours following extinction learning.
Task-based functional connectivity was estimated using a gPPI analysis, by creating a 6-mm radius sphere around the group peak of activation for CS+E (see Figure 3). Similar results were obtained using a right hippocampal seed (see text). Results significant using small-volume family-wise error correction (pFWE < 0.05), within anatomically defined regions of interest (Tzourio-Mazoyer et al., 2002). No masking was applied to images. Abbreviations: dACC, dorsal anterior cingulate cortex; CS+E, previously extinguished cue; gPPI, generalized psychophysiological interaction; FWE, familywise error corrected.
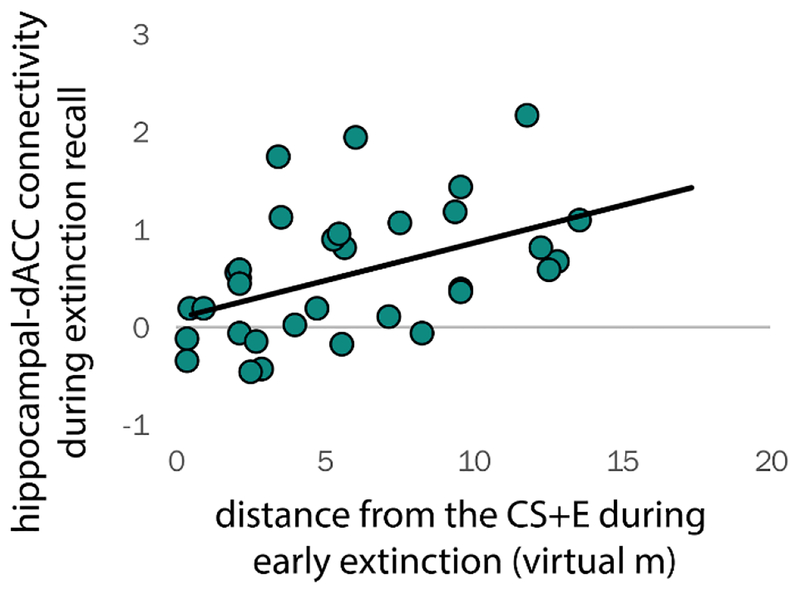
3.4. Association between measures
Children who kept more distance from the CS+E during early extinction subsequently demonstrated higher left hippocampal coupling with the right dACC (r[33] = 0.46, p = 0.007) and the left insula (r[33] = 0.36, p = 0.041) during CS+E extinction recall trials (Figure 5). Children who reported higher US expectancy ratings to the CS+E at the start of recall subsequently kept more distance from the CS+E (r[15] = 0.58, p = 0.023), which in turn, was associated with higher fear ratings to the CS+E at the start of the renewal session (r[15] = 0.546, p = 0.035). In addition, higher fear ratings to the CS+E at the end of extinction were associated with higher fear and US expectancy ratings to the CS+E at the start of recall the next day (p’s < 0.002), suggesting that poorer within-session extinction is associated with poor between-session extinction recall in children.
Figure 5. Children who kept more distance from the CS+E during extinction showed higher hippocampal-dACC coupling during a test of extinction recall, performed 24 hours later.
Task-based functional connectivity was estimated using a gPPI analysis, by creating a 6-mm radius sphere around the group peak of activation in the hippocampus for CS+E (see Figure 3). Significant hippocampal-dACC connectivity was detected for the CS+E during extinction recall across the sample (see Figure 4). Bivariate correlations conducted by comparing virtual distance with individual-participant hippocampal-dACC connectivity values, from the group peak shown in Figure 4. Abbreviations: dACC, dorsal anterior cingulate cortex; gPPI, generalized psychophysiological interaction.
4. Conclusions
This is the first study to our knowledge to test brain and behavioral correlates of extinction recall in pre-adolescent children. We utilized a novel paradigm that includes robust VR context manipulations and was designed to model interpersonal threat-related experiences as well as assess conditioned fear responding at multiple levels (Marusak et al., 2017). While children demonstrated within-session extinction, there was poor between-session recall of extinction learning, evidenced by elevations in SCRs and subjective ratings when re-exposed to the CS+E in the CXT- 24 hours later, and an overall low extinction retention index (13.56%). Behaviorally, children kept more distance from the CS+E than the CS- during recall. Elevations in conditioned fear responding were accompanied by activation and increased functional coupling within hippocampal-insula-dACC circuitry – a pattern associated with the return of fear in adult studies (e.g., Hermann et al., 2016). Finally, children who kept more distance from the CS+E during extinction learning subsequently showed greater hippocampal-dACC coupling, suggesting that an avoidant behavioral pattern may interfere with extinction. These results provide new evidence that pre-adolescent children may be impaired in their ability to recall extinction learning.
Poor recall and activation of hippocampal-dACC-insula circuitry may suggest relatively immature hippocampal-vmPFC circuitry during pre-adolescence. In support of this prediction, research shows age-related decreases in gray matter volume of the vmPFC over the course of childhood and adolescence (e.g., Keding and Herringa, 2015), and a peak in volume of the hippocampus around ages 9–11 (Uematsu et al., 2012). In addition, there are age-related increases in hippocampal connectivity with regions of the default mode network (DMN; includes mPFC), across childhood (Blankenship et al., 2017), and connectivity within the DMN continues to increase through adolescence (Fair et al., 2007; Sherman et al., 2014). Given recent evidence supporting a novel role for the DMN in the contextualization of safety memories (Marstaller, Burianová, & Reutens, 2017), immaturity within the DMN during childhood may contribute to an impaired ability to use contextual information to modify fear responding, or to retrieve the extinction memory within a safe context. Our exploratory age analyses showed an age-related decrease in US expectancy ratings to the CS+E, suggesting that extinction recall ability improves with age. This change in subjective ratings was accompanied by a potential increase in exploratory behavior towards the CS+E, as younger (but not older) children kept more distance from the CS+E than the CS- during extinction recall. Overall, our exploratory analyses suggest a more complex pattern of age-related change in different measures of conditioned fear during childhood.
Rodent models suggest that retrieval of the fear memory is associated with inputs to the amygdala from the hippocampal and prelimbic cortex – the homolog of the dACC (Knapska et al., 2012; Milad, Quirk, et al., 2007; Orsini, Kim, Knapska, & Maren, 2011; Quirk & Mueller, 2008). Therefore, the observed activation and connectivity within hippocampal-dACC-insula circuitry in children, overall, may reflect retrieval of the fear memory. Circuitry for fear retrieval may be stronger than circuitry for extinction recall in children. Consistent with this interpretation, the insula and dACC are some of the earliest structures to develop during childhood (Lyall et al., 2015). Further, the insula, dACC, and amygdala are highly interconnected, forming key nodes of the so-called ‘salience network’, involved in detecting the emotional significance of environmental stimuli and autonomic interoception (Craig, 2009; Seeley et al., 2007). Function of the salience network is established during early life, but increases in within- and between-network connectivity are observed through adolescence (see Menon, 2013). Salience network activation during extinction recall may suggest that this network is over-responsive or poorly-regulated during childhood. For example, because the CS+E had emotional salience at some point in time, salience network responding to the previous salience or the uncertainty of the CS+E may be stronger than the recall of extinction learning in children. Given also that cortical thickness of the dACC and insula are positively correlated with conditioned fear responding in adults (Hartley et al., 2011; Milad et al., 2007a), pruning during adolescence may associated with reduced reactivity of these regions, and may result in a shift in balance that favors extinction recall.
Conditioned fear responding after extinction is consistent with the idea that extinction learning does not result in an erasure of the original fear memory in children, as observed in infant rat pups (Landers & Sullivan, 2012). However, poor between-session recall in pre-adolescence also does not align well with studies in juvenile animals showing intact extinction recall. This may suggest an altered maturational trajectory of fear-extinction neural circuitry in humans versus rodents. For example, in humans, development of the DMN may be necessary for the contextualization of safety memories, contributing to the inhibition of fear responses when re-exposed to a safety context. Alternatively, the protracted development of hippocampus and vmPFC in humans may not support recall of extinction during childhood, whereas in rodents this functionality is intact at a young age. In general, the development of fear-extinction neural circuitry is thought to be nonlinear in nature (Hartley & Lee, 2014). Future studies building on this work should examine extinction recall across a wider age range, or in a longitudinal framework, to better understand developmental patterns.
Consistent with calls for inclusion of different indicators of conditioned fear in fear-extinction studies (Beckers, Krypotos, Boddez, Effting, & Kindt, 2013; Lonsdorf et al., 2017), our novel paradigm was designed to assess conditioned fear responding at neural, behavioral, subjective, and physiological levels. We have previously reported on the divergence among these fear indicators during fear conditioning and extinction learning sessions (Marusak et al., 2017), and observed some areas of divergence during recall, here. In particular, SCRs and US expectancy ratings to both the CS+E and CS- increased from the end of extinction learning on Day 1 to the beginning of recall on Day 2, whereas fear ratings and virtual distance remained higher for the CS+E relative to the CS-. The divergence in findings highlights the importance of collecting multiple measures of conditioned fear. An increase in conditioned fear responding to the safety cue (CS-) on Day 2 was unexpected, and may reflect poor differentiation or generalization of fear across cues – a pattern previously observed in pediatric participants during other sessions (e.g., conditioning; Lau et al., 2011; Schiele et al., 2016).
Interestingly, there were some associations between measures of conditioned fear during recall. In particular, children who reported higher US expectancy to the CS+E at the start of recall subsequently kept more distance from the CS+E, suggesting that subjective awareness may predict behavioral avoidance. Although neural activation or connectivity was not related with measures of conditioned fear within the recall session, children who kept more distance from the CS+E during extinction subsequently showed increased hippocampal-dACC coupling during recall. This suggests that behavioral patterns during extinction are associated with neural patterns during recall. In particular, a novel hypothesis from these data is that a passive avoidant behavioral pattern (i.e., failure to approach the CS+E when it no longer signals danger) may negatively impact the recall or consolidation of extinction learning, leading to greater coupling within fear-renewal circuitry during subsequent exposure. A strikingly similar pattern was observed in a previous Pavlovian fear-extinction study in adults using VR (Cornwell, Overstreet, Krimsky, & Grillon, 2013). Although recall was not tested in that study, adults who displayed a passive avoidant response showed poorer extinction ability, as measured by physiological startle response (Cornwell et al., 2013). Taken together, these findings suggest that individual differences in behavioral action tendencies may not only represent another expression of conditioned fear, but may also serve to maintain or exacerbate fear. Thus, behavioral action tendencies may be relevant in understanding the pathogenesis of fear-related disorders, and may help to explain variation in treatment response.
Limitations of this study should be considered. We did not perform fMRI during fear conditioning and extinction learning sessions, so it is unclear if neural patterns during these sessions would relate to patterns observed during recall. In addition, when designing the paradigm for children, we aimed to limit the number of trials needed so that children could acquire and extinguish fear responses. Thus, although children did show intact within-session extinction, future studies should test whether additional trials may help to enhance between-session recall. Similarly, certain aspects of the paradigm (e.g., three CSs, two CXTs) may have made for a too complicated overall learning experience for children. However, we observed intact fear conditioning and within-session learning, and observed patterns are similar to previous reports in pediatric samples, using less complicated designs (Gao et al., 2010; Schiele et al., 2016). Similarly, given that the CSs were virtual humans, we cannot rule out the possibility that the observed neural response may reflect more general processing of social stimuli rather than related social behaviors including interpersonal threat. Nonetheless, consistent with prior studies using stimuli relevant to the population of interest (e.g., face stimuli in studies of social anxiety disorder; Molapour, Golkar, Navarrete, Haaker, & Olsson, 2015), the stimuli were chosen to model interpersonal nature of real-life threat exposures that children commonly experience, and are linked to the development of psychopathology. In addition, adult males, particularly in high violence exposure environments, may be a pre-potent threat cue that may help to explain some of the observed fear generalization and/or poor differentiation between the CS’s. However, previous pediatric studies using non-social stimuli show similar patterns of poor differentiation between the CS’s, and that children and adolescents are more likely than adults to generalize across cues (Lau et al., 2011; Schiele et al., 2016). Another limitation of the study is the lack of an adult comparison group, which precludes our ability to comment on the relative maturity of this system. However, we used an adapted version of a widely used experimental paradigm to examine between-session recall of extinction learning, and were therefore able to compare patterns observed in previous reports in healthy adults and adults with fear-based conditions (e.g., PTSD; Milad et al., 2009; Milad, Wright, et al., 2007; Rabinak et al., 2014).
Poor between-session extinction recall in children may have implications for the development of fear-based disorders, and for therapy. For example, poor between-session extinction contributes to relapse after successful exposure-based therapy, and such therapies may be less effective in pediatric samples than they are in adults (Drysdale et al., 2014).
Supplementary Material
HIGHLIGHTS.
In healthy adults, extinction recall depends on hippocampal-vmPFC circuitry
Poor recall and hippocampal-vmPFC dysfunction are linked to anxiety disorders
Anxiety frequently begins in childhood; yet few studies examine recall in children
Skin conductance, behavioral, and subjective data indicate poor recall overall
Poor recall was accompanied by activation of the hippocampus and insula
Acknowledgements
The authors would like to thank Laura Crespo, Kelsey Sala-Hamrick, Shelley Paulisin, Limi Sharif, Klaramari Gellci, Sajah Fakhoury, Allesandra Iadipaolo, Xhenis Brahimi, Farah Sheikh, Brian Silverstein, and Suzanne Brown of Wayne State University (WSU) for assistance in participant recruitment and data collection. The authors would like to thank Dr. Moriah Thomason for sharing some of the included behavioral data. Thanks also to the children and families who generously shared their time to participate in this study.
Research reported in this publication was supported by the Wayne State University Department of Pharmacy Practice and a Karmanos Cancer Institute and American Cancer Society IRG #14-238-04-IRG (CAR). Dr. Marusak is supported by American Cancer Society award 129368-PF-16-057-01-PCSM. Dr. Rabinak is supported by National Institute of Mental Health grants K01 MH101123 and R61 MH111935.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Craig AD (2009). How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. https://doi.org/10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Åhs F, Kragel PA, Zielinski DJ, Brady R, & LaBar KS (2015). Medial prefrontal pathways for the contextual regulation of extinguished fear in humans. NeuroImage, 122, 262–271. https://doi.org/10.1016/j.neuroimage.2015.07.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KD, & Richardson R (2015). Forming competing fear learning and extinction memories in adolescence makes fear difficult to inhibit. Learning & Memory, 22, 537–543. https://doi.org/10.1101/lm.039487.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers T, Krypotos A-M, Boddez Y, Effting M, & Kindt M (2013). What’s wrong with fear conditioning? Biological Psychology, 92(1), 90–96. https://doi.org/10.1016/j.biopsycho.2011.12.015 [DOI] [PubMed] [Google Scholar]
- Blankenship SL, Redcay E, Dougherty LR, & Riggins T (2017). Development of hippocampal functional connectivity during childhood. Human Brain Mapping, 38(1), 182–201. https://doi.org/10.1002/hbm.23353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, & O’Keefe J (2002). The human hippocampus and spatial and episodic memory. Neuron. https://doi.org/10.1016/S0896-6273(02)00830-9 [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Overstreet C, Krimsky M, & Grillon C (2013). Passive avoidance is linked to impaired fear extinction in humans. Learning & Memory, 20(3), 164–9. https://doi.org/10.1101/lm.028902.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Hartley CA, Pattwell SS, Ruberry EJ, Somerville LH, Compton SN, … Walkup JT (2014). Fear and anxiety from principle to practice: Implications for when to treat youth with anxiety disorders. Biological Psychiatry. https://doi.org/10.1016/j.biopsych.2013.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Dosenbach NUF, Church JA, Cohen AL, Brahmbhatt S, Miezin FM, … Schlaggar BL (2007). Development of distinct control networks through segregation and integration. Proceedings of the National Academy of Sciences, 104(33), 13507–13512. https://doi.org/10.1073/pnas.0705843104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganella DE, Lee-Kardashyan L, Luikinga SJ, Nguyen DLD, Madsen HB, Zbukvic IC, … Kim JH (2017). Aripiprazole Facilitates Extinction of Conditioned Fear in Adolescent Rats. Front Behav. Neurosci, 11(1662–5153 (Linking)), 76 https://doi.org/10.3389/fnbeh.2017.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Raine A, Venables PH, Dawson ME, & Mednick SA (2010). The development of skin conductance fear conditioning in children from ages 3 to 8 years. Developmental Science, 13(1), 201–212. https://doi.org/10.1111/j.1467-7687.2009.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, & Liberzon I (2014). Impaired Contextual Modulation of Memories in PTSD: An fMRI and Psychophysiological Study of Extinction Retention and Fear Renewal. Journal of Neuroscience, 34(40), 13435–13443. https://doi.org/10.1523/JNEUROSCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A Developmental Shift from Positive to Negative Connectivity in Human Amygdala-Prefrontal Circuitry. Journal of Neuroscience, 33(10), 4584–4593. https://doi.org/10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, & Hajcak G (2012). The Development of Fear Learning and Generalization in 8 to 13 year-olds. Developmental Psychobiology, 54(7), 675–684. https://doi.org/10.1002/dev.20616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, & Phelps EA (2011). Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral Cortex, 21(9), 1954–1962. https://doi.org/10.1093/cercor/bhq253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. a, & Lee FS (2014). Sensitive Periods in Affective Development: Nonlinear Maturation of Fear Learning. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 40(1), 1–11. https://doi.org/10.1038/npp.2014.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann A, Stark R, Blecker CR, Milad MR, & Merz CJ (2017). Brain structural connectivity and context-dependent extinction memory. Hippocampus, (April), 1–22. https://doi.org/10.1002/hipo.22738 [DOI] [PubMed] [Google Scholar]
- Hermann A, Stark R, Milad MR, & Merz CJ (2016). Renewal of conditioned fear in a novel context is associated with hippocampal activation and connectivity. Social Cognitive and Affective Neuroscience, 11(9), 1411–1421. https://doi.org/10.1093/scan/nsw047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, … Goff DC (2009). Extinction Memory Is Impaired in Schizophrenia. Biological Psychiatry, 65(6), 455–463. https://doi.org/10.1016/j.biopsych.2008.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, & Maren S (2007). Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. https://doi.org/10.1002/hipo.20331 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, & Bradley B (2014). Development of fear acquisition and extinction in children: Effects of age and anxiety. Neurobiology of Learning and Memory, 113, 135–142. https://doi.org/10.1016/j.nlm.2013.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, & Dolan RJ (2006). Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of Neuroscience, 26(37), 9503–9511. https://doi.org/10.1523/JNEUROSCI.2021-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding TJ, & Herringa RJ (2015). Abnormal Structure of Fear Circuitry in Pediatric Post-traumatic Stress Disorder. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 40(September), 537–45. https://doi.org/10.1038/npp.2014.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime Prevalence and Age-of-Onset Distributions of DSM-IV Disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593 https://doi.org/10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- Kessler RC, McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, … Williams DR (2010). Childhood adversities and adult psychopathology in the WHO world mental health surveys. British Journal of Psychiatry, 197(5), 378–385. https://doi.org/10.1192/bjp.bp.110.080499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Li S, & Richardson R (2011). Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cerebral Cortex, 21(3), 530–538. https://doi.org/10.1093/cercor/bhq116 [DOI] [PubMed] [Google Scholar]
- Knapska E, Macias M, Mikosz M, Nowak A, Owczarek D, Wawrzyniak M, … Kaczmarek L (2012). Functional anatomy of neural circuits regulating fear and extinction. Proceedings of the National Academy of Sciences, 109(42), 17093–17098. https://doi.org/10.1073/pnas.1202087109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Inati SJ, Evans JW, Luh W-M, & Bandettini PA (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage, 60(3), 1759–1770. https://doi.org/10.1016/j.neuroimage.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers MS, & Sullivan RM (2012). The development and neurobiology of infant attachment and fear. Developmental Neuroscience, 34(2–3), 101–114. https://doi.org/10.1159/000336732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, … Pine DS (2011). Distinct neural signatures of threat learning in adolescents and adults. Proc Natl Acad Sci U S A, 2011/03/04(11), 4500–4505. https://doi.org/10.1073/pnas.1005494108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, … Merz CJ (2017). Don’t fear ‘fear conditioning’: Methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neuroscience and Biobehavioral Reviews. https://doi.org/10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Lyall AE, Shi F, Geng X, Woolson S, Li G, Wang L, … Gilmore JH (2015). Dynamic Development of Regional Cortical Thickness and Surface Area in Early Childhood. Cerebral Cortex, 25(8), 2204–2212. https://doi.org/10.1093/cercor/bhu027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, & Liberzon I (2013). The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience, 14(June), 417–28. https://doi.org/10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marstaller L, Burianová H, & Reutens DC (2017). Adaptive contextualization: A new role for the default mode network in affective learning. Human Brain Mapping, 38(2), 1082–1091. https://doi.org/10.1002/hbm.23442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Peters CA, Hehr A, Elrahal F, & Rabinak CA (2017). A novel paradigm to study interpersonal threat-related learning and extinction in children using virtual reality. Scientific Reports. https://doi.org/10.1038/s41598-017-17131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum J, Kim JH, & Richardson R (2010). Impaired extinction retention in adolescent rats: effects of D-cycloserine. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 35(10), 2134–2142. https://doi.org/10.1038/npp.2010.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K. a, Greif Green J, Gruber MJ, Sampson N. a, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69(11), 1151–1160. https://doi.org/10.1001/archgenpsychiatry.2011.2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2013). Developmental pathways to functional brain networks: Emerging principles. Trends in Cognitive Sciences. https://doi.org/10.1016/j.tics.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Michalska KJ, Shechner T, Hong M, Britton JC, Leibenluft E, Pine DS, & Fox NA (2016). A developmental analysis of threat/safety learning and extinction recall during middle childhood. Journal of Experimental Child Psychology, 146, 95–105. https://doi.org/10.1016/j.jecp.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry, 66(12), 1075–1082. https://doi.org/10.1016/j.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, & Rauch SL (2005). Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America, 102(2004), 10706–10711. https://doi.org/10.1073/pnas.0502441102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, & Rauch SL (2007). A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biological Psychiatry, 62(10), 1191–1194. https://doi.org/10.1016/j.biopsych.2007.04.032 [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, & Rauch SL (2007). Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry, 62(5), 446–454. https://doi.org/10.1016/j.biopsych.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Mineka S, Mystkowski JL, Hladek D, & Rodriguez BI (1999). The Effects of Changing Contexts on Return of Fear Following Exposure Therapy for Spider Fear. Journal of Consulting and Clinical Psychology, 67(4), 599–604. https://doi.org/10.1037/0022-006X.67.4.599 [DOI] [PubMed] [Google Scholar]
- Molapour T, Golkar A, Navarrete CD, Haaker J, & Olsson A (2015). Neural correlates of biased social fear learning and interaction in an intergroup context. NeuroImage, 121, 171–183. https://doi.org/10.1016/j.neuroimage.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olman CA, Davachi L, & Inati S (2009). Distortion and signal loss in medial temporal lobe. PLoS ONE, 4(12). https://doi.org/10.1371/journal.pone.0008160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini C. a., Kim JH, Knapska E, & Maren S (2011). Hippocampal and Prefrontal Projections to the Basal Amygdala Mediate Contextual Regulation of Fear after Extinction. Journal of Neuroscience, 31(47), 17269–17277. https://doi.org/10.1523/JNEUROSCI.4095-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Bath KG, Casey BJ, Ninan I, & Lee FS (2011). Selective early-acquired fear memories undergo temporary suppression during adolescence. Proceedings of the National Academy of Sciences of the United States of America, 108(3), 1182–1187. https://doi.org/10.1073/pnas.1012975108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, & Mueller D (2008). Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology, 33(1), 56–72. https://doi.org/10.1038/sj.npp.1301555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Lyons M, Mori S, Milad MR, Liberzon I, & Luan Phan K (2014). Cannabinoid modulation of prefrontal-limbic activation during fear extinction learning and recall in humans. Neurobiology of Learning and Memory, 113, 125–134. https://doi.org/10.1016/j.nlm.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, & Porter JT (2008). Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience, 28(15), 4028–36. https://doi.org/10.1523/JNEUROSCI.2623-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiele MA, Reinhard J, Reif A, Domschke K, Romanos M, Deckert J, & Pauli P (2016). Developmental aspects of fear: Comparing the acquisition and generalization of conditioned fear in children and adults. Developmental Psychobiology, 58(4), 471–481. https://doi.org/10.1002/dev.21393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. Journal of Neuroscience, 27(9), 2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Ronkin EG, Jarcho JM, Mash JA, Michalska KJ, … Pine DS (2015). Fear conditioning and extinction in anxious and nonanxious youth and adults: Examining a novel developmentally appropriate fear-conditioning task. Depression and Anxiety, 32(4), 277–288. https://doi.org/10.1002/da.22318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Hong M, Britton JC, Pine DS, & Fox NA (2014). Fear conditioning and extinction across development: Evidence from human studies and animal models. Biological Psychology. https://doi.org/10.1016/j.biopsycho.2014.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman LE, Rudie JD, Pfeifer JH, Masten CL, McNealy K, & Dapretto M (2014). Development of the Default Mode and Central Executive Networks across early adolescence: A longitudinal study. Developmental Cognitive Neuroscience, 10, 148–159. https://doi.org/10.1016/j.dcn.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, … Joliot M (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage, 15(1), 273–289. https://doi.org/10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, & Nishijo H (2012). Developmental Trajectories of Amygdala and Hippocampus from Infancy to Early Adulthood in Healthy Individuals. PLoS ONE, 7(10). https://doi.org/10.1371/journal.pone.0046970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, & Eelen P (2005). Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behaviour Research and Therapy, 43(3), 323–336. https://doi.org/10.1016/j.brat.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Zbukvic IC, Park CHJ, Ganella DE, Lawrence AJ, & Kim JH (2017). Prefrontal Dopaminergic Mechanisms of Extinction in Adolescence Compared to Adulthood in Rats. Frontiers in Behavioral Neuroscience, 11(February), 32 https://doi.org/10.3389/fnbeh.2017.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.