Abstract
Deep brain stimulation (DBS) is a highly efficacious treatment option for movement disorders and a growing number of other indications are investigated in clinical trials. To ensure optimal treatment outcome, exact electrode placement is required. Moreover, to analyze the relationship between electrode location and clinical results, a precise reconstruction of electrode placement is required, posing specific challenges to the field of neuroimaging. Since 2014 the open source toolbox Lead-DBS is available, which aims at facilitating this process. The tool has since become a popular platform for DBS imaging. With support of a broad community of researchers worldwide, methods have been continuously updated and complemented by new tools for tasks such as multispectral nonlinear registration, structural / functional connectivity analyses, brain shift correction, reconstruction of microelectrode recordings and orientation detection of segmented DBS leads. The rapid development and emergence of these methods in DBS data analysis require us to revisit and revise the pipelines introduced in the original methods publication. Here we demonstrate the updated DBS and connectome pipelines of Lead-DBS using a single patient example with state-of-the-art high-field imaging as well as a retrospective cohort of patients scanned in a typical clinical setting at 1.5T. Imaging data of the 3T example patient is co-registered using five algorithms and nonlinearly warped into template space using ten approaches for comparative purposes. After reconstruction of DBS electrodes (which is possible using three methods and a specific refinement tool), the volume of tissue activated is calculated for two DBS settings using four distinct models and various parameters. Finally, four whole-brain tractography algorithms are applied to the patient’s preoperative diffusion MRI data and structural as well as functional connectivity between the stimulation volume and other brain areas are estimated using a total of eight approaches and datasets. In addition, we demonstrate impact of selected preprocessing strategies on the retrospective sample of 51 PD patients. We compare the amount of variance in clinical improvement that can be explained by the computer model depending on the method of choice.
This work represents a multi-institutional collaborative effort to develop a comprehensive, open source pipeline for DBS imaging and connectomics, which has already empowered several studies, and may facilitate a variety of future studies in the field.
Introduction
In the field of deep brain stimulation (DBS), precise electrode placement is crucial for optimal treatment outcomes. Specifically, a direct relationship between electrode localization and clinical outcome has been shown in multiple studies (e.g. Butson et al., 2011; Dembek et al., 2017; Eisenstein et al., 2014; Garcia-Garcia et al., 2016;Horn et al., 2017c; Mosley et al., 2018b; also see fig. 1 A). To characterize this relationship in an objective manner, tools are required that facilitate the reconstruction of electrode placement such that comparisons between patients can be made. Group comparisons play a crucial role in identifying optimal electrode placement, providing both direct clinical and theoretical insights. Ideally, to fulfill reproducibility and transparency criteria needed for good scientific practice, these tools should be open source and publicly available. Finally, a specific challenge that differentiates the field of DBS imaging from most other neuroimaging domains is the need for absolute anatomical precision. A shift of two mm in electrode placement may represent a major change in clinical outcome, while in conventional fMRI studies, a change of an activity peak by two mm has little if no impact at all (figure 1).
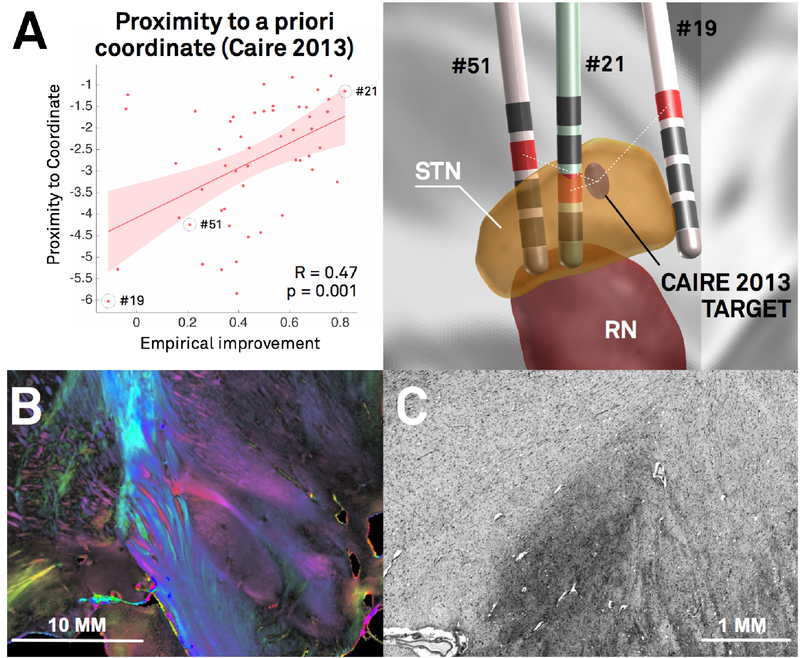
Figure 1:
In DBS imaging, “millimeters matter”, which poses specific methodological challenges. A) Re-analysis of the Berlin cohort described in (Horn et al., 2017c) shows that proximity of active contact centers to an optimal target is predictive of %-UPDRS-III improvement (left). The target was defined in a meta-analysis (Caire et al., 2013) and transformed to MNI space in a probabilistic fashion (Horn et al., 2017a). Active contacts between electrodes of patients 51 and 21 are a mere two mm away from each other, but result in largely different clinical results (right). The same distance (two mm) corresponds to the average image resolution of functional MRI for which many neuroimaging tools were initially developed. Thus, in the field of DBS imaging, the distance of two mm plays a crucial role, whereas it is often considered insignificant in common neuroimaging studies. B) Coronal polarized light imaging section of the human subthalamic nucleus with surrounding tracts. Image courtesy by Prof. Karl Zilles and Dr. Markus Axer, Forschungszentrum Jülich, INM-1. C) Coronal section of the BigBrain dataset (Amunts et al., 2013) as visualized in the microdraw online application (http://microdraw.pasteur.fr/). Cell sparser and denser subregions are discernible, potentially corresponding to functional zones of the nucleus (Marani et al., 2008). B) and C) demonstrate the tightly-packed anatomical complexity of the STN-DBS target region that is similarly reflected in clinical outcome (A). Of note, only a subregion of this small nucleus is considered an optimal DBS target. The combination of such small and complex DBS targets with a potentially huge impact of small misplacements poses extreme challenges to the field of DBS imaging and raise the need for high-precision pipelines.
In 2014, the software toolbox Lead-DBS was published that aimed at reconstructing DBS electrode placement based on pre- and postoperative imaging (Horn and Kühn, 2015; www.lead-dbs.org; RRID:SCR_002915). Using the toolbox, electrodes may be localized in relationship to surrounding brain anatomy. Since its initial publication, development efforts have continued at multiple institutions. Thus, over the years, numerous progress has been made and better alternatives for most steps described in the original pipeline are now provided (Ewert et al., 2018a; 2018b; Horn et al., 2017a; 2017b; 2017c). Moreover, several novel features that were not mentioned (or available) in the original publication have recently become crucial components of DBS imaging. These have now been integrated in the latest release. While other tools with similar aims have been introduced after publication of Lead-DBS (Bonmassar et al., 2014; da Silva et al., 2015; D’Albis et al., 2014; Husch et al., 2017; 2018; Lauro et al., 2015), the tool was recently described as the most established toolbox for electrode localizations (Husch et al., 2017) with over 6600 downloads and 75 citations. The aim of the project is to develop a scientific platform in a multi-institutional endeavor that is and remains available under an open license (GNU general public license v. 3) to ensure reproducibility and version control.
The growing user base of Lead-DBS as an academic toolbox and the divergence of the current methods and those described in the initial publication raise the need of an updated methodological pipeline description. In addition, we also use the opportunity to emphasize the latest default analysis options, pitfalls and methods throughout the pipeline.
Given the complexity of multiple processing stages (see figure 2 & tables 1-4), a thorough empirical evaluation of each stage exceeds the scope of this work. For instance, it would represent a study in itself to empirically probe which normalization method, which stimulation volume model or which fiber tracking approach could yield best results. Such studies have been conducted (Åström et al., 2014; Dembek et al., 2017; Fillard et al., 2011; Klein et al., 2009; Maier-Hein et al., 2017; McIntyre et al., 2004) and are currently underway in context of the Lead-DBS environment, as well (Ewert et al., 2018a). Instead, the aim of the present article is to give an overview of methods available in Lead-DBS. To make the processing stages concrete, the pipeline is described using a single patient example with state-of-the art high-field (3T) imaging as well as a retrospective sample of 51 PD patients imaged at 1.5T. The result is a focus on the methods section and a descriptive results section covering co-registration, normalization, electrode localization, VTA estimation, and structural-functional connectivity analyses. Finally, we demonstrate that more variance in clinical outcome may be explained when using the default pipeline in comparison to a more “standard neuroimaging” approach. The manuscript has a narrative prose with the aim of maximizing understandability while omitting unnecessary details where possible. Moreover, while the manuscript is still structured into conventional sections, the methods descriptions exceed the actual processing of the study with the aim of illustrating the multiple approaches implemented in Lead-DBS and providing notes about motivation and potential limitations.
Figure 2:
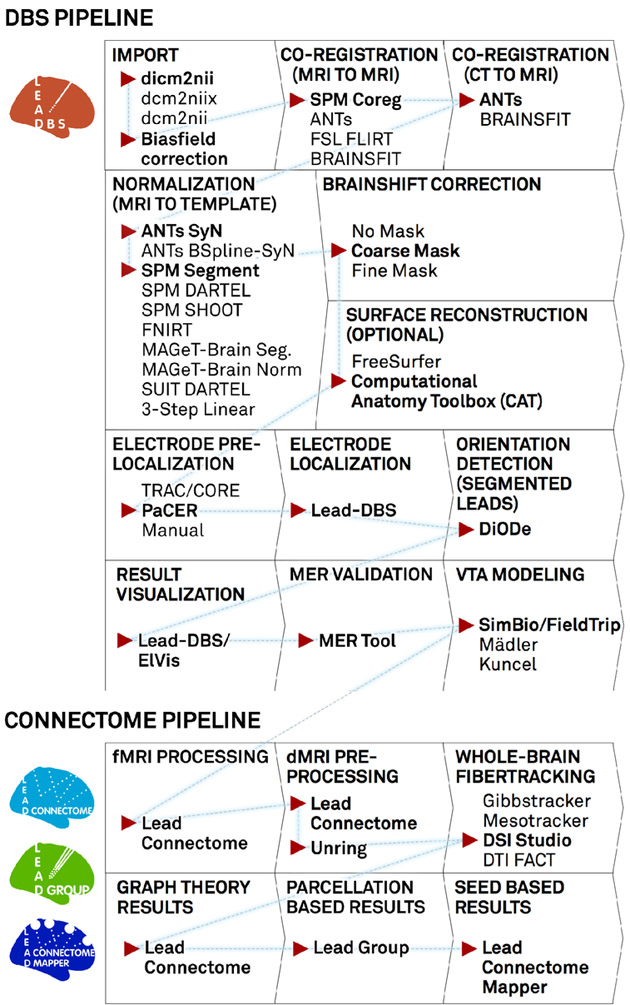
A “default pathway” through Lead-DBS. Processing stages are visualized in consecutive order, general choices are displayed for each step while default selections are marked with red arrow and bold text. For the normalization step, a larger evaluation showed both the ANTs SyN and SPM Segment approaches to perform equally optimal (Ewert et al., 2018a). The DBS and connectome pipelines work in parallel but seamlessly integrate via the order marked by the blue dashed line. After calculating results with Lead Connectome Mapper (last box), results may be used to predict clinical outcome using the Lead Predict tool (not shown) based on a model described in (Horn et al.,2017c).
Table 1:
Linear registration methods implemented in Lead-DBS.
| Software / Method Name | Used for MR to MR | Used for CT to MR |
Publication(s) |
|---|---|---|---|
| ANTs Rigid / Affine | + | + | Ashburner et al. 2007 |
| BRAINSFIT | + | + | Johnson et al. 2007 |
| SPM Co-register | + | − | Friston et al. 2004 |
| FSL FLIRT | + | − | Jenkinson et al. 2002 |
| Hybrid SPM & ANTs, Hybrid SPM & FSL, Hybrid SPM & BRAINSFIT |
+ | − | see above |
Table 4:
Whole-Brain fiber tracking methods implemented in Lead-DBS. Of note, the tensor based method is implemented for debugging purposes and not recommended for actual use.
| Tool name | Software | Approach | Support of Single/Multishell Data |
Publication(s) |
|---|---|---|---|---|
| Gibbs’ Tracker | DTI & Fibertools for SPM | Global Tractography | Single-Shell | Kreher et al. 2008, Reisert et al. 2011 |
| Model-Free Mesotracker | DTI & Fibertools for SPM | Mesoscopic Global Tractography | Both | Reisert et al. 2014, Konopleva et al. 2018 |
| Generalized q-Sampling Imaging | DSI Studio | Deterministic Tractography | Both | Yeh et al. 2010 |
| DTI / FACT Tracking | DTI & Fiber Tracking for Matlab | Tensor based Deterministic Tractography | Single-Shell | Dirk-Jan Kroon / Matlab File Exchange |
Methods
Patient Characteristics, Surgery & Imaging
Example patient
A male patient (65y) suffering from Parkinson’s Disease received two octopolar segmented DBS leads (Boston Scientific Vercise; BSci, Marlborough, Massachusetts, United States) targeting the subthalamic nucleus (STN). Surgery was done under general anaesthesia with two wakeful phases in which microelectrode recordings were obtained using a Neuro Omega drive (Alpha Omega Engineering, Nazareth, Israel) with a 45° rotated Ben Gun array. In the same session, test stimulations were performed. Recordings started 7.5 mm before reaching the target and were acquired in 15 consecutive steps of 0.5 mm. Recordings of cells with typical STN firing patterns were later transferred to the Lead-DBS session. Test stimulations were made at three mm dorsal to and at the surgical target. In a second surgery five days afterwards, a Boston Scientific Vercise Gevia Impulse Generator was implanted in the chest. Detailed imaging parameters can be found in supplementary material.
Retrospective patient cohort
Data from the patient described above embodies a state-of-the-art example dataset acquired at 3T including a specialized basal ganglia MR sequence and patient-specific diffusion MRI. To further illustrate the impact that different processing streams may have on typical clinical MRI data, we included data from a priorly published retrospective cohort that is described in detail elsewhere (Berlin cohort in Horn et al., 2017c). In brief, 51 patients received quadropolar electrodes (Medtronic type 3389) to the STN region to treat Parkinson’s Disease. Pre- and postoperative imaging was performed on a 1.5T MRI and included a preoperative T1 and T2 sequence as well as postoperative axial, coronal and sagittal T2 slabs of the basal ganglia. Six of 51 patients received a postoperative CT instead (for detailed imaging parameters see supplementary material and Horn et al., 2017c).
Linear (Within-Patient) Co-Registrations
When a patient folder is loaded in Lead-DBS, a bias-field correction step based on the N4 algorithm is automatically applied to all pre-operative MRI sequences (Tustison et al., 2010). Based on configuration preferences, Lead-DBS chooses one of the preoperative sequences as the anchor modality, i.e. the stationary sequence to which all other (preoperative and postoperative volumes) sequences are co-registered. By default, the T1-weighted sequence is used or if unavailable the T2-weighted sequence is substituted. This anchor modality is upsampled to isotropic 0.7 mm resolution to maintain high resolution in following steps. This step is common in similar pipelines (e.g. Gunalan et al., 2017). A reason is that (e.g. T2 weighted) acquisitions acquired in clinical routine often come in high in-plane resolution (e.g. 0.5 mm) but poor slice thickness (2-3 mm). If these images are resliced to a 1 mm isotropic MP-RAGE, much in-plane resolution is lost. Thus, our pipeline compromises on a 0.7 mm isotropic working space to which the anchor modality is resliced (images need to be resliced for multispectral normalizations). Several linear registration algorithms are included in Lead-DBS (see table 1). In the present example patient and retrospective cohort, all available preoperative acquisitions (i.e. T2, PD, FGATIR as well as the FA volume derived from the dMRI scan) were co-registered and resliced to the upsampled T1 using SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Similarly, the postoperative CT was co-registered using Advanced Normalization Tools (ANTs; http://stnava.github.io/ANTs/). Co-registration results were then manually checked using built-in tools that facilitate visual inspection which may be enhanced by automatic edge detection based wire-frame generation of the anchor modality, and false-color overlays.
Co-registration is a crucial step to achieve precise results since the preoperative data is used to define anatomy and the postoperative to define electrode locations. Thus, imprecise registrations lead to erroneous results in the end. In clinical settings, especially on MRI, postoperative volumes are often slabs (i.e. don’t cover the whole brain). Accurately registering these to preoperative data is especially challenging for registration algorithms and at times, postoperative data must be registered manually (e.g. using tools as 3D Slicer; www.slicer.org).
Nonlinear (Patient-to-Template) Co-Registrations
To relate electrode placement to anatomy and to make them comparable across patients and centers, it is useful to register individual patient anatomy to a template space. These template spaces often allow the most likely location of anatomical structures to be better defined, and can then be used to project subcortical atlases (table 5) or whole-brain parcellations (table 6) onto regions of interest.
Table 5:
Subcortical Atlases suitable for / available within Lead-DBS.
| Atlas Name | Pre- installe d in Lead- DBS |
Source(s) of Information | Structures of Focus / Specialities |
Publication(s) |
|---|---|---|---|---|
| “Made for” Lead-DBS | ||||
| DISTAL Atlas | + | Histology, MRI, Tractography | STN, GPi | Ewert et al. 2018,Chakravarty et al. 2006, Chakravarty et al. 2008 |
| Human Motor Thalamus | + | Histology | Motor domains of thalamus, projection from Cb, SN, BG | Ilinsky et al. 2018 |
| Focus on DBS Relevant Structures | ||||
| CIT168 Reinforcement Learning Atlas | + | MRI | Based on High Precision MRI | Pauli et al. 2017 |
| ATAG-Atlas | + | MRI | 7T MRI based segmentations | Keuken et al. 2014 |
| MNI PD25 Atlas | + | MRI, Histology | Multimodal MR sequences, histology | Xiao et al. 2017, Chakravarty et al. 2008, Chakravarty et al. 2006 |
| Ultra-high Field Atlas for DBS Planning | + | MRI | 7T MRI based segmentations | Wang et al. 2016 |
| DBS targets Atlas | + | Literature results | Literature informed DBS targets mapped to MNI space | Horn et al. 2017 |
| BGHAT Atlas | + | MRI | Basal Ganglia Segmentations | Prodoehl et al. 2008 |
| Basal Ganglia Atlas | − | MRI | Basal Ganglia Segmentations | Ahsan et al. 2007 |
| PPN Histological Atlas | + | Histology | Definition of the PPN based on histology | Alho et al. 2017 |
| GPi Probabilistic Parcellation Atlas | + | Tractography | Tractography based parcellation of the GPi | da Silva et al. 2016 |
| Nigral Organization Atlas | + | Tractography, rs-fMRI | Functional zones of substantia nigra segmented by connectivity | Zhang et al. 2017 |
| STN Only | ||||
| STN Functional Zones Atlas | + | Tractography | Tractography-based segmentation of STN into functional zones | Accolla et al. 2014 |
| ATAG-Atlas: STN Young–Middle-Aged–Elderly | + | MRI | 7T MRI based segmentations, Three age groups | Keuken et al. 2013 |
| Also see DISTAL, Human Motor Thalamus, CIT168, Ultra-High Field DBS, BGHAT & MNI PD25 atlases for definitions of the STN | ||||
| Focus on Thalamus | ||||
| Morel Atlas | − | Histology | Precise histological atlas of the subcortex | Jakab et al. 2012, Krauth et al. 2010, Morel et al. 2013 |
| Thalamic DBS Connectivity Atlas | + | Tractography | Tractography based parcellation of the thalamus | Akram et al. 2018 |
| Oxford Thalamic Connectivity Atlas | + | Tractography | Tractography based parcellation of the thalamus | Behrens et al. 2003 |
| Thalamic Connectivity Atlas | + | Tractography | Tractography based parcellation of the thalamus | Horn & Blankenburg 2016 |
| Thalamic Connectivity Atlas | + | Tractography, rs-fMRI | Structural-functional connectivity based parcellation of the thalamus | Zhang et al. 2008 |
| Also see DISTAL, Human Motor Thalamus & MNI PD25 atlases for thalamic structures | ||||
| Electrophysiological Data | ||||
| Electrophysiological Atlas of STN Activity | + | Electrophysiology | Beta Power mapped to STN | Horn et al. 2017 |
| Electrophysiological Atlas of GPi Activity | + | Electrophysiology | Theta Power mapped to GPi | Neumann et al. 2017 |
| Other / Brainstem | ||||
| Brainstem Connectome | + | Tractography | Population based fiber tracts of brainstem (HCP data) | Meola et al. 2016 |
| Macroscale Human Connectome Atlas | + | Tractography | Population based fiber tracts of the whole brain (HCP data) | Yeh et al. 2018 |
| BigBrain | − | Histology | Whole-Brain histological stacks registered to MNI space | Amunts et al. 2013 |
| Harvard Ascending Arousal Network Atlas | + | Tractography, MRI | Atlas of Brainstem Structures | Edlow et al. 2012 |
| Whole-Brain Parcellations with subcortical components useful for DBS | ||||
| Functional Striatum Parcellation Atlas | + | rs-fMRI | Parcellation of the Striatum into functional zones | Choi et al. 2012 |
| AICHA subcortical regions | + | rs-fMRI | Parcellation of the subcortical structures into functional zones | Joliot et al. 2015 |
| Harvard-Oxford Atlas | + | MRI | Whole-Brain Atlas with subcortical definitions | Frazier et al. 2005 |
| For a list of further whole-brain parcellations, see http://www.lead-dbs.org/?page_id=1004 | ||||
Table 6:
Brain parcellations (of use for connectomic analyses) suitable for / available within Lead-DBS.
| Atlas Name | Pre- installed in Lead- DBS |
Source(s) of Information |
Structures of Focus / Specialities | Publication(s) |
|---|---|---|---|---|
| Whole Brain | ||||
| Harvard-Oxford cortical/subcortical atlases | + | Manual segmentations | Whole Brain | Makris et al. 2006, Frazier et al. 2005, Desikan et al. 2006, Goldstein et al. 2007 |
| MICCAI 2012 Multi-Atlas Labeling Workshop and Challenge (Neuromorphometrics) | + | Manual segmentations | Whole Brain | neuromorphometrics.com |
| Brainnetome Atlas parcellation | + | fMRI, dMRI | Whole Brain | Fan et al. 2016 |
| Automated Anatomical Labeling (v2) | + | MRI | Whole Brain | Tzourio-Mazoyer et al. 2002 |
| AICHA: An atlas of intrinsic connectivity of homotopic areas | + | fMRI | Whole Brain | Joliot et al. 2015 |
| Hammers_mith Atlas | + | MRI | Whole Brain, special focus on temporal lobe | Hammers et al. 2003, Gousias et al. 2008 |
| PrAGMATiC | − | fMRI | Functional atlas based on task fMRI | Huth et al. 2016, Huth et al. 2015 |
| fMRI-based random parcellations | + | fMRI | Fine-grained random parcellations informed by rs-fMRI data | Craddock et al. 2012 |
| Voxelwise parcellations | + | MRI | Whole Brain | Horn & Kühn 2015 |
| Cortex Focus | ||||
| Mindboggle 101 | + | Manual segmentations | Cortex / Desikan protocol | Klein et al. 2012 |
| Yeo functional parcellations | + | fMRI | Cortex | Yeo et al. 2011 |
| Local-Global Parcellation of the Human Cerebral Cortex | + | fMRI | Cortex, refining Yeo 2011 parcellations | Schaefer et al. 2017 |
| Cortical Area Parcellation from Resting-State Correlations | + | rs-fMRI | Cortex | Gordon et al. 2016 |
| HCP MMP 1.0 | − | Multimodal | Cortex, surface maps | Glasser et al. 2016 |
| Desikan-Killiany Atlas | − | Gyrification related ROI | Cortex, surface maps | Desikan et al. 2006 |
| Destrieux Atlas | − | Gyrification related ROI | Cortex, surface maps | Destrieux et al. 2010, Fischl et al. 2004 |
| MarsAtlas | − | Gyrification related ROI | Cortex, surface maps | Auzias et al. 2016, Auzias et al. 2013 |
| Specific Subregions | ||||
| JuBrain / Juelich histological atlas | + | Histology | Specific regions | Zilles et al. 2010, Amunts et al. 2007, Eickhoff et al. 2005, Eickhoff et al. 2010, Eickhoff et al. 2006bp |
| Human Motor Area Template | + | fMRI / Metaanalysis | (Pre-) motor cortex | Mayka et al. 2006 |
| Sensorimotor Area Tract Template | + | dMRI | Corticospinal tract subregions | Archer et al. 2017 |
| SUIT cerebellar parcellation | + | MRI | Cerebellum |
Diedrichsen et al. 2006, Diedrichsen et al. 2011 |
| Buckner functional cerebellar parcellation | + | fMRI | Same networks as in Yeo 2011 cortical parcellations defined in cerebellum | Buckner et al. 2011 |
In the original Lead-DBS publication, all registrations between patient and template space were performed in a linear way following the approach introduced by Schönecker and colleagues (Schönecker et al., 2009). This approach was especially developed for DBS and followed a three-step registration with incremental focus on the subcortical region of interest. In the revised version of Lead-DBS, multiple nonlinear options have been added (table 2). Most of the included approaches were adapted with their parameters tuned for optimal results in the DBS context. Most refinements were performed based on experience in the daily use of the pipeline across multiple institutions. Recently, a study systematically analyzed results of various methods in which over 11,000 nonlinear deformations were solved and compared (Ewert et al., 2018a). Results of this study led to the present default presets implemented in Lead-DBS (figure 2). It is beyond the scope of the present work to describe every modification in each method in detail but the ability to explain clinical improvement using some examples was estimated for the retrospective cohort analyzed here and some details about modifications in regard to subcortical optimization are mentioned in the supplementary material.
Table 2:
Normalization Methods implemented in Lead-DBS:
| Software / Method Name | Adaptations in Lead-DBS | Supports Multispectral Warps |
Publication(s) |
|---|---|---|---|
| Statistic Parametric Mapping (SPM) | |||
| Unified Segmentation | Based on Tissue Probability Maps calculated from multispectral ICBM 2009b NLIN AsYM Space templates | + | Ashburner and Friston 2005 |
| DARTEL | Pair-wise instead of group-wise workflow (mapping directly from patient into template space) based on similarly generated DARTEL templates | + | Ashburner et al. 2007 |
| SHOOT | See DARTEL | + | Ashburner and Friston 2011 |
| SUIT based DARTEL | Specialized method if area of interest is in the brainstem (e.g. PPN); Use of SUIT Toolbox based registration to a brainstem/cerebellum template that was registered to ICBM 2009b | + | Diedrichsen et al. 2006, Ashburner et al. 2007 |
| FMRIB Software Library (FSL) | |||
| FNIRT | Standard presets | − | Andersson and Smith 2010 |
| Advanced Normalization Tools (ANTs) | |||
| SyN | Four-Stage preset with subcortical refinement, multiple presets developed for use in DBS | + | Avants et al. 2008 |
| BSpline-SyN | See SyN | + | Tustison & Avants 2013 |
| Other / Specialized | |||
| Linear Three-Step Normalization | Suited for use directly on postoperative MRI (or on preoperative MRI); Implemented using ANTs whereas original code was implemented with use of FSL | + | Schonecker et al. 2009 |
| MAGeT Brain-like Segmentation / Normalization | Multi-Subject/Template implementation inspired by the MAGeT-Brain approach but with strong differences. | + | None / see Chakravarty et al. 2012 for MAGeT-Brain approach |
| MAGeT Brain-like Normalization | Inversing the idea of MAGeT-Brain by directly averaging deformation fields learned from multiple indirect warps | + | None / see Chakravarty et al. 2012 for MAGeT-Brain approach |
Brain Shift Correction
During surgery, air may enter the skull after it is opened. This leads to a nonlinear deformation of the brain in relation to the bone which is called brain shift and typically pushes the forebrain into occipital direction (due to supine position of the patient). Especially when the pneumocephalus is still present during postoperative imaging, it introduces a bias between electrode (postoperative image) placement and anatomical structures on preoperative acquisitions. Whilst brain-shift introduces non-linear transforms, applying non-linear registration techniques to correct this would also deform the electrodes projections and corrupt the corresponding anatomical overlap. To avoid this, our brain-shift correction method uses the threefold linear registration described above (see Schönecker et al., 2009 for validation), which is stored internally and applied to DBS electrode placement afterwards (figure S1).
Electrode Localization
In Lead-DBS, the process of reconstructing electrode placement is divided into an automated (“pre-localization”) and a manual (“localization”) step. A wide range of electrodes from five manufacturers are readily implemented in Lead-DBS (table 7) and it is straight forward to implement custom models.
Table 7:
Electrode models included in Lead-DBS.
| Manufacturer | Type | # Contacts | Contact Spacing | Omnidirectional/Segm ented |
|---|---|---|---|---|
| DBS | ||||
| Medtronic | 3389 | 4 | 2 mm | O |
| 3387 | 4 | 3 mm | O | |
| 3391 | 4 | 7 mm | O | |
| Boston Scientific | Vercise | 8 | 2 mm | O |
| Vercise Directed | 8 | 2 mm / Segmented | S | |
| Abbott / St. Jude Medical | Active Tip 6146-6149 | 4 | 2 mm | O |
| Active Tip 6142-6145 | 4 | 3 mm | O | |
| Infinity Directed 6172 | 8 | 2 mm / Segmented | S | |
| Infinity Directed 6173 | 8 | 2 mm / Segmented | S | |
| PINS Medical | L301 | 4 | 2 mm | O |
| L302 | 4 | 3 mm | O | |
| L303 | 4 | 6 mm | O | |
| iEEG | ||||
| SDE | 08 S8 | 8 | 3.5 mm | O |
| 08 S10 | 10 | 3.5 mm | O | |
| 08 S12 | 12 | 3.5 mm | O | |
| 08 S16 | 16 | 3.5 mm | O | |
Automated pre-Localization
For the pre-localization part, four methods are available:
Manual click-and-point tool
Integration with 3D Slicer in which fiducial points are placed manually.
TRAC / CORE approach (Horn and Kühn, 2015)
The PaCER toolbox (https://adhusch.github.io/PaCER/; Husch et al., 2017).
In practice, PaCER usually requires very little to no manual refinement compared to the TRAC/CORE algorithm, but requires a postoperative CT acquisition in contrast to the other methods.
Manual Localization
The user-interface of this crucial processing step was specifically designed to allow for highly precise electrode reconstructions. At all times, the postoperative volume is visualized along planes that are cut orthogonally to the electrode (when moving the lead reconstruction in space, these cuts are updated in real time). A specialized “x-ray mode” can be activated in which the same view is enhanced by averaged stacks of orthogonal slices surrounding the lead. This visualization mode is helpful to reconstruct electrodes in poor resolution acquisitions where partial volume effects may blur or even shift the electrode artifact in space.
Estimating the Local Volume of Tissue Activated
The Volume of Tissue Activated (VTA) is a conceptual volume that is thought to elicit additional action potentials due to the electrical stimulations of axons (McIntyre and Grill, 2002). Much work has been done in this regard and models with increasing sophistication were introduced over the years (e.g. Åström et al., 2014; Butson and McIntyre, 2008; Chaturvedi et al., 2013). In contrast, some more clinically oriented papers aimed at finding fast heuristics to determine the rough extent of the VTA based on the stimulation parameters without actually creating a spatial model (Dembek et al., 2017; Kuncel et al., 2008; Lauro et al., 2015; Mädler and Coenen, 2012). Three such simple heuristic models are included in Lead-DBS (Dembek et al., 2017; Kuncel et al., 2008; Mädler and Coenen, 2012), and a more sophisticated, finite element method based approach was added in 2017 (Horn et al., 2017c; table 3). On a spectrum between simple heuristical and highly sophisticated models, it falls in the middle. This model was briefly described in the aforementioned publication and follows the overall concept described in (Åström et al., 2014). However, a full methods description of the specific model has not been published and can be found in the supplementary material.
Table 3:
VTA models included in Lead-DBS.
| Method Publication | Type |
|---|---|
| Horn et al. 2017 | FEM Based, 4-Compartment Model, Tetrahedral Mesh |
| Mädler & Coenen 2012 | Heuristic |
| Kuncel et al. 2008 | Heuristic |
| Dembek et al. 2017 | Heuristic |
Connectivity Estimation
In the revised version of Lead-DBS, several possibilities to estimate structural and functional connectivity exist. These are accessible via the submodules Lead Connectome, Lead Group and Lead Connectome Mapper. Moreover, single-patient connectivity metrics may be visually explored in the general 3D viewer module of the software (EIVis).
Methods may be divided into approaches that utilize patient-specific vs. normative/group-level resting-state functional MRI (rs-fMRI) or diffusion-weighted imaging based tractography (dMRI). Moreover, they can be subdivided in voxel-wise / data-driven or parcellation-/ROI-based methods.
Patient Specific Connectivity Estimates
A structural-functional processing pipeline was implemented into the Lead Connectome submodule based on the pipeline described in (Horn, 2015; Horn and Blankenburg, 2016; Horn et al., 2014). For rs-fMRI, the pipeline follows the recommendations given in (Weissenbacher et al., 2009). Briefly, this includes motion-correction (SPM based), detrending, regression of white matter and CSF-signals as well as motion parameters and bandpass-filtering (cutoff-values: 0.009-0.08 Hz) of time series. In dMRI, a Gibbs’ ringing removal step (Kellner et al., 2015) is performed before passing the diffusion data into either of four tools subsequently used to estimate a whole-brain tractogram (for a list of fiber-tracking methods implemented in Lead-DBS, see table 4). The Gibbs’ tracking algorithm was superior to nine competing algorithms in the 2009 Fiber Cup (Fillard et al., 2011) and was added as the first method. Its successor, a model-free version (Konopleva et al., 2018) of the Mesotracker algorithm (Reisert et al., 2014) was subsequently added to equally investigate mesoscopic properties of fiber tracts and support multi-shell diffusion data. Recently, the Generalized q-Sampling Imaging approach (Yeh et al., 2010) implemented in DSI Studio (http://dsi-studio.labsolver.org/) achieved the highest “valid connection” score in an open competition among 96 methods submitted from 20 different research groups around the world (Maier-Hein et al., 2017). As a result, this method was included into Lead-DBS as well. Finally, a dated simple tensor based deterministic method is also available for debugging or testing purposes.
Normative or population based Connectomes
In the DBS field, large cohorts exist in which patient-specific connectivity data is lacking. In such datasets, a novel technique that combines normative group connectome data with single-patient imaging results may be used. These group connectomes were informed by large cohorts of subjects or patients (e.g. N = 1000 in case of the Yeo 2011 normative connectome) that were often acquired on specialized MR hardware (such as the human connectome scanner at the Athinoula A. Martinos Center, Boston, MA; Setsompop et al., 2013). The utility of such normative connectomes in a clinical context was first demonstrated by mapping various neurological or psychiatric symptoms to networks influenced by stroke lesions (Boes et al., 2015; Darby et al., 2016; 2017; Fasano et al., 2017; Fischer et al., 2016; Laganiere et al., 2016). Recently, the approach was adapted to the field of DBS in first studies (Horn et al., 2017c; 2017b; 2017a) and, in order, to predict clinical outcome of transcranial magnetic stimulation treatment (Weigand et al., 2017).
A natural limitation of the approach is that the normative connectome data does not account for patient-specific differences in brain connectivity. However, despite its potential shortcomings in individualized connectivity, the use of normative connectomes has a major practical advantage, since larger cohorts of DBS patients with individualized connectivity data are not available and connectivity sequences in DBS patients are difficult to acquire postoperatively. As such, this approach is able to utilize large DBS cohorts collected across different centers, while studies using patient-specific connectivity are based on small cohorts (typically N < 25; e.g. Accolla et al., 2016; Akram et al., 2017; Vanegas Arroyave et al., 2016). Along the same lines, the approach may prove particularly valuable for emerging DBS indications in which only a limited number of patients are implanted world-wide. Thus, the ability to retrospectively analyze such DBS datasets despite the lack of patient-specific connectivity data represents a genuine window into understanding the role of brain connectivity in mediating DBS outcome.
The methods utilizing normative connectomes are implemented in the Lead Connectome Mapper, Lead Group and ElVis tools (figure 2). For an overview of normative connectomes available within Lead-DBS, see table 8.
Table 8:
Normative Connectomes available in Lead-DBS format.
| Name | Repository | N | Population | Publication – Dataset |
Processing Methods |
Publication – Processing |
|---|---|---|---|---|---|---|
| rs-fMRI based “functional connectivity” | ||||||
| Functional group connectome 1000 healthy subjects GSP | Harvard Genomic Superstruct Project (GSP) | 1000 | Controls | Yeo et al. 2011 | Lead Connectome fMRI pipeline | Horn et al. 2017 |
| Functional group connectome 74 PPMI PD-patients, 15 controls | Parkinson’s Disease Progression Marker Initiative (PPMI) | 74 / 15 | PD patients, controls | Marek et al. 2011 | Lead Connectome fMRI pipeline | Horn et al. 2017 |
| dMRI based “structural connectivity” | ||||||
| Structural group connectome 20 subjects Gibbs-tracker | Horn 2014 study | 20 | Controls | Horn et al. 2014 | Lead Connectome, Gibbs’ tracker | Horn et al. 2014 |
| Structural group connectome 169 NKI subjects Gibbs-tracker | Horn 2016 study | 169 | Controls | Nooner et al. 2012 | Lead Connectome, Gibbs’ tracker | Horn et al. 2016 |
| Structural group connectome 32 Adult Diffusion HCP subjects GQI | HCP MGH Adult Diffusion dataset | 30 | Controls | Setsompop etal. 2013 | Lead Connectome, DSI Studio (GQI) | Horn et al. 2017 |
| Structural group connectome 90 PPMNI PD-patients GQI | Parkinson’s Disease Progression Marker Initiative (PPMI) | 90 | PD patients | Marek et al. 2011 | Lead Connectome, DSI Studio (GQI) | Ewert et al. 2018 |
Explaining variance in clinical outcome within the retrospective 1.5T cohort
DBS-Electrodes of the retrospective cohort were localized using Lead-DBS (see fig. 2) after several normalization and registration strategies were performed. Specifically, preoperative acquisitions were registered into template space using the two default approaches (SPM New Segment and ANTs SyN) identified in (Ewert et al. 2018a). In addition, the ANTs SyN approach without the subcortical refinement step was applied. Finally, a T1-only monospectral approach (FSL FNIRT) was added to compare results with a more typical “standard procedure” used in the neuroimaging field. Electrode localizations (performed in native space) where then registered to template space using the deformation fields obtained by the various approaches and VTAs were calculated using default parameters in template space. Overlaps between VTAs and the subthalamic nucleus as defined by the DISTAL atlas (Ewert et al. 2018b) were calculated for both hemispheres and summed up. In addition, overlap between the E-field and the STN were calculated in a weighted fashion by multiplying the binary STN image with the non-binary E-Field and summing up voxels. Finally, streamlines were isolated from a priorly published normative connectome based on the Parkinson’s Progression Marker Inititative (PPMI) data (Marek et al. 2011; Ewert et al. 2018) using the E-Field of the SPM New Segment method as a weighted seed.
These imaging based metrics (VTA-STN overlap, E-Field-STN weighted overlap, weighted streamlines seeding from E-Field connected to SMA) were correlated with empirical % improvement on the Unified Parkinson’s Disease Rating Scale (UPDRS) III. In a second step, they were fed into general linear models (GLM) that additionally included seven additional clinical covariates of the sample (age, sex, percent improvement in Levodopa response, disease duration until surgery, Levodopa Equivalent Daily Dosage (LEDD) ON and OFF DBS as well as %LEDD reduction by DBS). From these GLMs, root-mean-square error (RSME), R2-Statistic and p-value of the F-statistic as well as significance predictors are reported.
Methods summary
A list of tools to which interfaces exist or that form a native (preinstalled) part of Lead-DBS is given in table 10. To make deliberate choices regarding which option to choose for each processing step, users require a high methodological level of understanding. To account for this, figure 2 gives an overview of the “default pathway” through Lead-DBS which is further demonstrated in detail in a walkthrough-video available online (http://www.lead-dbs.org/?page_id=192).
Table 10:
Interfaces between Lead-DBS and other neuroimaging tools. Except for FreeSurfer, all tools are available on Windows, macOS and Linux. Necessary binaries for FSL tools were adapted to work on Windows although there is no official Windows FSL release.
| Tool Name | Tool Website | Tool Task | Type of Interface | Main Tool Publication |
|---|---|---|---|---|
| Statistic Parametric Mapping (SPM) | http://www.fil.ion.ucl.ac.uk/spm/software/spm12/ | Image Registration & Manipulation | Dependency (Matlab Path) | Friston etal. 2004 |
| FMRIB Software Library (FSL) | https://fsl.fmrib.ox.ac.uk/ | Image Registration | Necessary tools included | Woolrich et al. 2009 |
| Advanced Normalization Tools (ANTs) http://stnava.github.io/ANTs/ | Image Registration | Included | Avants etal. 2008 | |
| 3D Slicer | https://www.slicer.org/ | Image Visualization | Dependency, direct uplink via Python calls (System Path) | Fedorov etal. 2012 |
| SurfIce | https://www.nitrc.org/projects/surfice/ | Surface Visualization | Included | – |
| BRAINSFit | https://www.nitrc.org/projects/multimodereg/ | Image Registration | Included | Johnson etal. 2007 |
| FieldTrip | http://www.fieldtriptoolbox.org/ | Mesh Generation | Necessary tools adapted / included | Oostenvel et al. 2010 |
| SimBio | https://www.mrt.uni-jena.de/simbio/ | FEM Modeling | Necessary tools adapted / included | Vorwerk etal. 2018 |
| SUIT | http://www.diedrichsenlab.org/imaging/suit.htm | Specialized Registration (Brainstem) | Dependency (Matlab Path) | Diedrichsen et al. 2006 |
| FreeSurfer | https://surfer.nmr.mgh.harvard.edu/ | Surface Reconstruction (ECoG Electrodes) | Dependency (system calls) | Dale et al. 1999 |
| Computational Anatomy Toolbox (CAT) | http://www.neuro.uni-jena.de/cat/ | Surface Reconstruction (ECoG Electrodes) | Dependency (Matlab Path) | Gaser and Dahnke 2016 |
| PaCER | https://adhusch.github.io/PaCER/ | Electrode Localization | Included | Husch etal. 2017 |
| dcm2nii, dcm2niix, dicm2nii | https://www.nitrc.org/projects/dcm2nii/ | DICOM import | Included | Li et al. 2016 |
| Gibbs’ Tracker | https://www.uniklinik-freiburg.de/mr-en/research-groups/diffperf/fibertools.html | Fiber tracking | Included | Reisert etal. 2011 |
| Model-Free Mesotracker | https://bitbucket.org/reisert/mesoft_v1 | Fiber tracking | Included | Konopleva et al. 2018 |
| DSI Studio | http://dsi-studio.labsolver.org | Fiber tracking | Included | Yeh et al. 2010 |
| Gibbs’ Ringing Artifact Removal Tool | https://bitbucket.org/reisert/unring | dMRI preprocessing | Included | Kellner etal. 2015 |
| Network Based Statistics (NBS) | https://sites.google.com/site/bctnet/comparison/nbs | Graph-Theory Level Statistics | Included | Zalesky etal. 2010 |
Results
Patient Outcome
3T example patient:
Before surgery, the 64 year male patient had an UPDRS-III score of 64 points (OFF dopamine replacement therapy; Hoehn-Yahr stage IV). Seven days post-surgery, the patient was discharged with an appreciable stun effect and a subjective improvement of gait. Stimulation was set to 0.5 mA bilateral on the lower segmented contacts (ring mode). Under stimulation and medication, a UPDRS-III of four points was taken. At the time of writing, no score under dopaminergic withdrawal and under stimulation is available but will be taken during the three-month postoperative visit.
Retrospective 1.5T cohort:
The 51 (18 female) patients were 60 ± 7.9 years old at time of surgery and UPRDS-III improved by 45.3 ± 23.0% from a baseline points (postoperative OFF at 12 months) of 38.6 ± 12.9 to 21.1 ± 8.8 (DBS ON, Med OFF at same day). Disease duration at time of surgery was 10.4 ± 3.9 years and LEDD reduction was 52.8% (1072.72 in baseline vs. 484.57 at 12 months post surgery).
Preoperative baseline of the sample had been 32 ± 11 UPRDS-III points in Med OFF vs. 12 ± 5 points in Med ON conditions (53.5 ± 17.2% percent improvement in levodopa response).
Image Registration
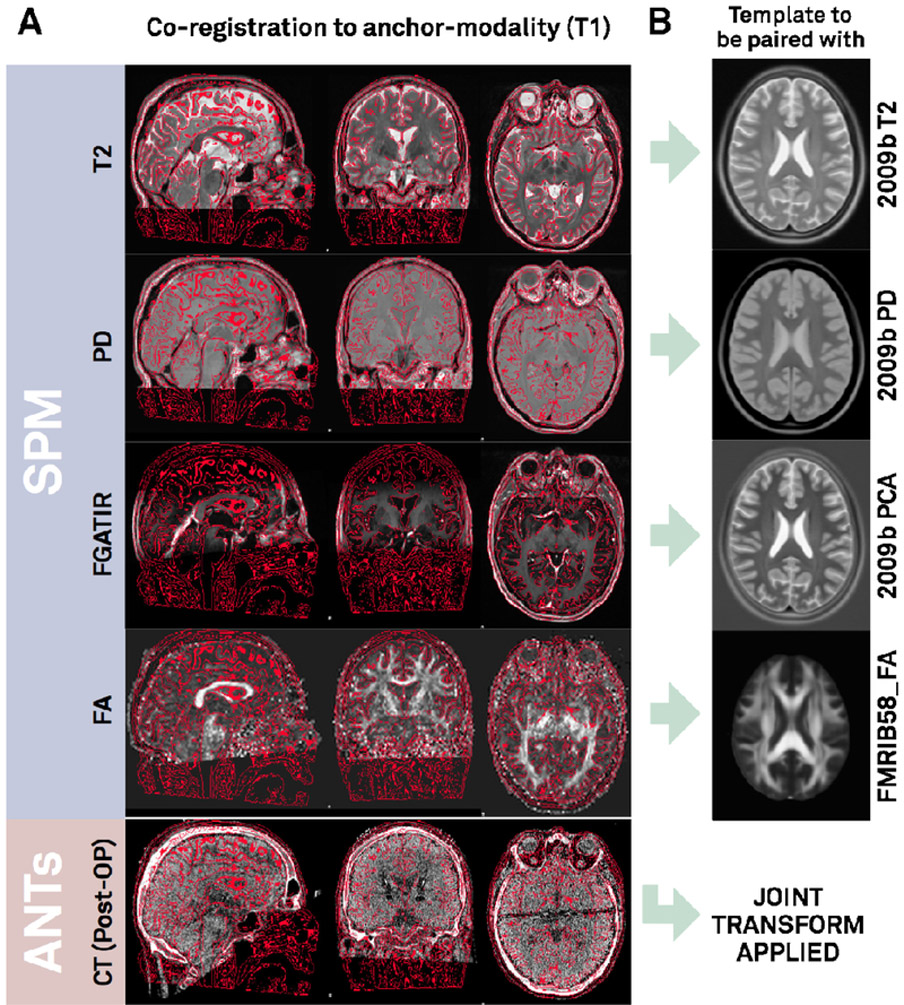
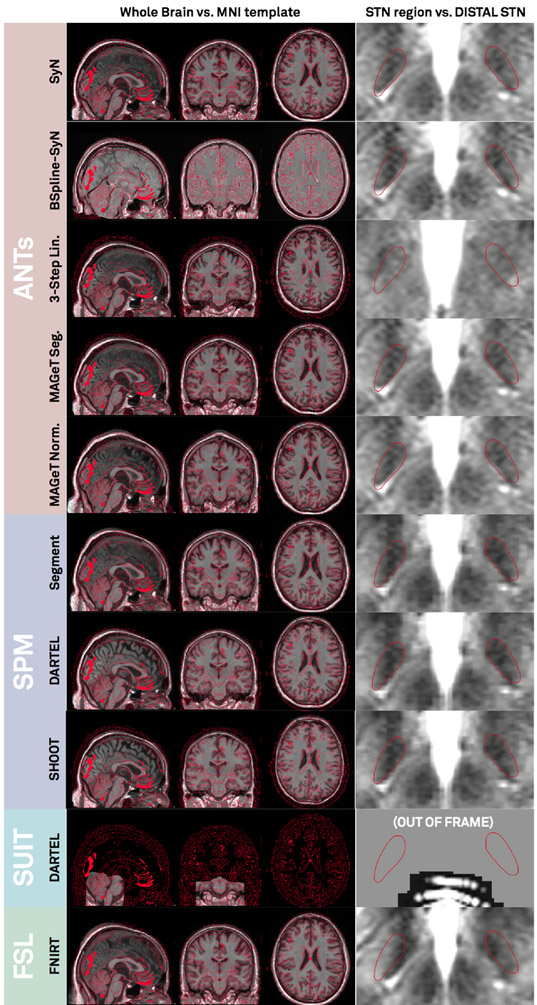
Co-registration results of T2- and if available PD- and FGATIR sequences as well as postoperative MR / CT to the anchor-modality (T1) were done using default presets (figure 2) and were accurate upon visual inspection. Results of the 3T example patient are shown in figure 3A. Similarly, fractional anisotropy (FA) computed from the preoperative dMRI acquisition in the 3T example patient was registered to T1. All preoperative sequences (including FA if available) were used for nonlinear registrations to template space in the ANTs-based approaches (shown in rows 1-3 in figure 4 for the 3T example). In these multispectral warps, the T1-scan was mapped to the T1 template, T2 to T2, PD to PD (figure 3B). The FA volume was instead paired with the FMRIB58 FA template (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FMRIB58_FA) that had been registered to 2009b space using an MNI-152 6th-gen to 2009b space transform (Horn, 2016). Since no FGATIR-template exists in 2009b space, Lead-DBS automatically paired this scan with an aggregated PCA template (Horn, 2017). The linear three-step registration was included mainly for reproducibility purposes (Schönecker et al., 2009) but equally supports multispectral registrations. In the MAGeT Brain-like approaches (rows 4-5 in figure 4), only T1-, T2- and PD-weighted acquisitions were used given these sequences were available in the IXI database of age-matched peer-brains (i.e. “templates” in MAGeT nomenclature). SPM-based approaches (rows 6-7 in figure 4) used all preoperative acquisitions except the FA volume. Here, volumes were not paired with a specific template as in the ANTs-based registrations. Instead, tissue priors were used to learn posterior segmentations using voxel intensities across image modalities (Ashburner and Friston, 2005). All methods except the FNIRT and Linear Three-Step registrations were able to precisely segment the STN target region based on manual inspection. Note that the FNIRT method does not support multispectral warps and estimated the warp based on the T1 volume only (on which the STN is not visible). This may explain the mismatch in template vs. subject STN target regions.
Figure 3:
A) Co-registration results of the 3T example patient. Lead-DBS linearly registers preoperative T2, PD, FGATIR and FA volumes to the T1 anchor modality (visualized as red edge contours) using SPM. Similarly, by default, postoperative CT is linearly mapped to T1 using ANTs. A tone mapped version of the CT is shown (equally displaying brain- and bone-windows). B) In the following ANTs-based normalization step, the T1 volume will be registered to the T1-weighted MNI template (2009b NLIN Asym space; not shown). Likewise, T2 and PD volumes will be mapped to T2-/PD-templates. FGATIR volume by default is mapped to a synthetic PCA template while FA to a registered version of the FMRIB58_FA template. These five transforms result in a joint nonlinear deformation field that is equally applied to pre- and postoperative acquisitions.
Figure 4:
Normalization results of the 3T example patient. Based on the preoperative multimodal MRI (T1, T2, PD, FGATIR) of the patient, individual anatomy was registered into ICBM 2009b NLIN Asym (“MNI”) space using various methods. Left column: MNI space (red wireframes) overlaid to normalized T1 acquisition. Right column: DISTAL atlas STN (red wireframes) overlaid to normalized T2 acquisition. Note that the SUIT registration uses SPM methods too, but is based on a toolbox focusing on brainstem and cerebellum anatomy. Thus, normalizing the STN region with this preset is not possible, the method is still displayed for the sake of completeness. It is applicable for brainstem targets such as the Pedunculopontine Nucleus (PPN).
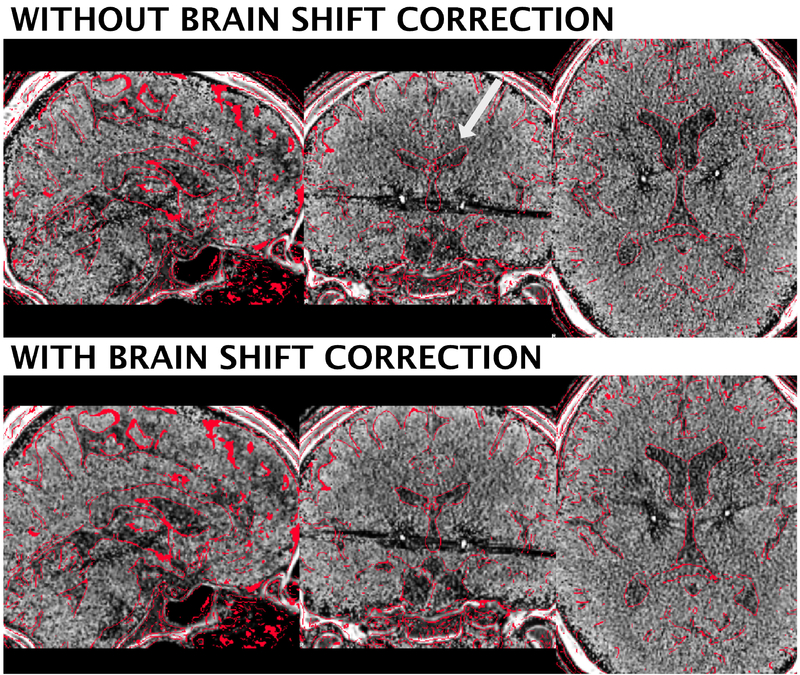
Finally, in the 3T example patient, brain shift correction led to a refined registration between postoperative CT and preoperative anchor modality (T1). A shift of 0.17 mm to the left, 0.9 mm to anterior and 1.66 mm in dorsal direction was introduced (figure 5). In the present case, not much pneumocephalus was present and the example may rather demonstrate the introduction of higher robustness and precision with an additional subcortical refinement transform. An example of the tool in case of prevalent pneumocephalus may be seen in the methods figure S1.
Figure 5:
Brain shift correction results of the 3T example patient. The approach serves as a refinement registration step between post- and preoperative acquisitions and is able to minimize nonlinear registration errors due to pneumocephalus (figure S1). In the present example, the postoperative CT was shifted by 1.66 mm in z-, 0.9 mm in y- and 0.17 mm in x-direction. A better registration can best be seen in the area of the ventricles (white arrow). Postoperative CT was tone mapped to show contrast in both brain and skull windows.
Electrode Reconstructions
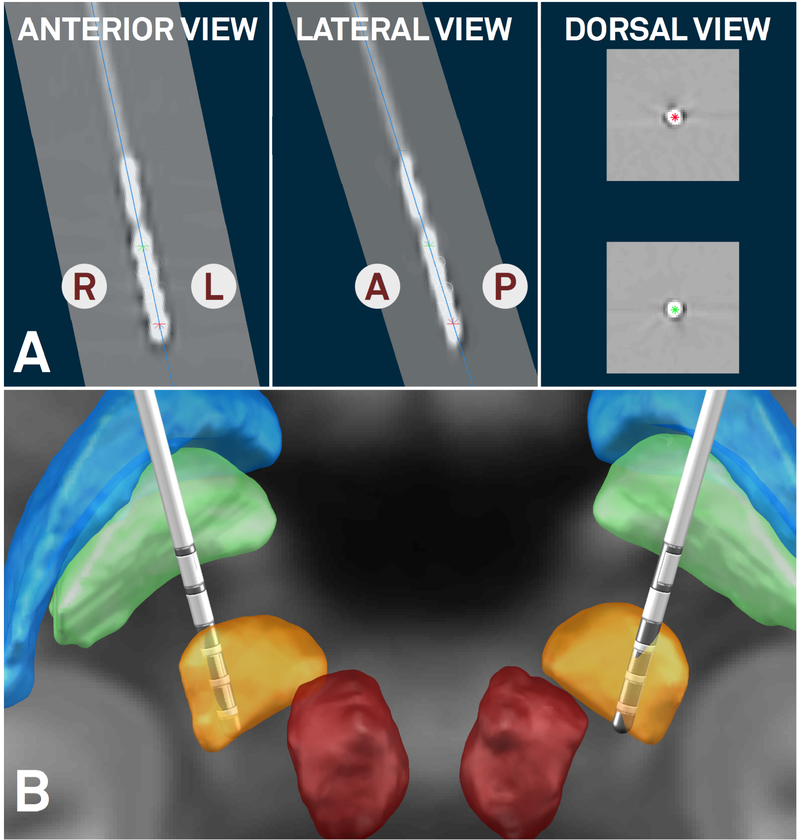
In the 3T example patient, the PaCER method found an optimal solution including location of electrode contacts in a fully automated manner whereas the TRAC/CORE method robustly reconstructed the trajectory but contacts had to be adjusted manually. Final fully automatic PaCER reconstruction is shown in figure 6. In the subsequent step, orientation of the segmented electrode was reconstructed using the Directional Orientation Detection (DiODe) algorithm, an updated version of the approach described in (Sitz et al., 2017). Relative to a marker position pointing strictly to anterior, rotation of the electrodes was corrected by 65° (right lead) and 30° (left lead) clockwise as seen from the tip, respectively. Final MNI coordinates of planning (lowermost) contacts were x = 11.6, y = −16.2, z = −9.1 on the right and x = −12.7, y = −15.1, z = −10.7 mm on the left. Relative to the midcommissural point, in stereotactic coordinates, these corresponded to x = 11.5, y = −4.4, z = −5.9 (right) and x = −12.7, y = −3.9, z = −7.0 mm (left).
Figure 6:
Fully automated electrode reconstruction results (PaCER method contributed by Husch and colleagues) of the 3T example patient. Orientation of lead reconstructed using the method by Sitz and colleagues. A) Postoperative CT is shown orthogonal to reconstructed trajectory (right hemisphere, blue line) in anterior, lateral and dorsal views. Ventral- and dorsalmost contacts marked by red and green asterisks, respectively. Using this view, users can fine-tune electrode reconstructions in a very precise way. B) Final 3D rendering of results in synopsis with key structures defined by the DISTAL atlas. Both electrodes placed in dorsolateral STN which corresponds to the sensorimotor functional zone of the STN. Right lead resides minimally more medial than left (in respect to atlas STN) which can be accounted for by field steering (figure 8).
Microelectrode Recordings
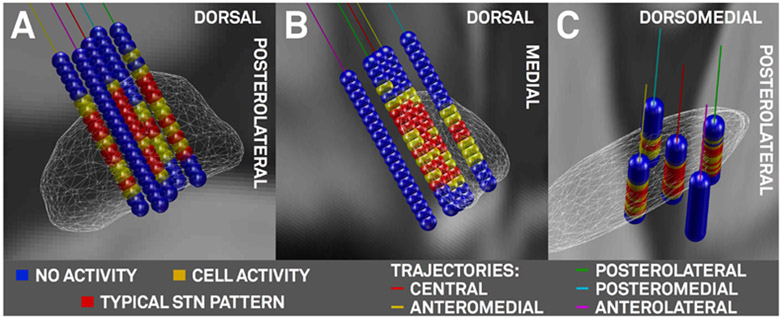
After electrode reconstruction, classifications (no cell activity, unspecific cell activity, clear STN typic activity pattern) recorded in the 3T patient were mapped to the subcortex and are shown in figure 7 for the right hemisphere. On both sides, boundaries of firing patterns on the top and bottom of the STN corresponded well to the atlas-/imaging-defined STN. For instance, in the left lateral trajectory, no clear STN activity was reported and in agreement to that, the trajectory traverses outside (lateral) to the imaging-defined STN throughout its whole course.
Figure 7:
Left-Hemispheric microelectrode recording results of the 3T example patient. A) oblique view orthogonal to the lateral surface of the STN (white wireframes). B) view from posterior and C) dorsal. Markers in blue (no cell activity), yellow (cell activity), red (typical STN firing pattern) placed on 45° rotated Ben Gun (X-) array of microelectrodes between 7.5 and −1.5 mm distance to surgical target in 0.5 mm steps. Trajectories: central (red), lateral (magenta), medial (cyan), posterior (green) and anterior (yellow) trajectories.
VTA Calculation
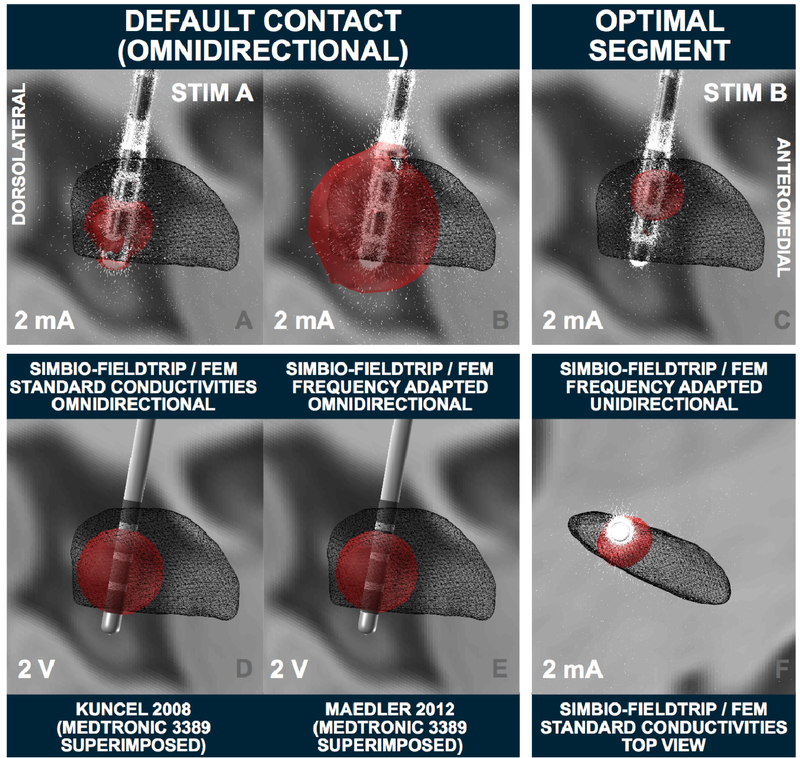
Stimulation parameters of 2 mA on the planning contact (ventral segmented level in ring mode) were calculated using the FEM-model and standard vs. frequency adapted conductivity values (figure 8 A, B). Here, the “heuristic” electric-field (E-field) threshold of 0.2 V/mm was used. The E-Field is the first derivative of the voltage distribution across the tissue surrounding the electrode. To demonstrate the possibilities of segmented electrodes, an additional unidirectional setting was calculated (figure 8 C, F). Finally, to allow for comparison with heuristic VTA models implemented in Lead-DBS, 2 V estimates on a ring electrode (Medtronic model 3389) are shown in figure 8 D, E using the Mädler or Kuncel models, respectively.
Figure 8:
VTA modeling (right hemispheric lead) in the 3T example patient. Left two columns: several omnidirectional stimulations at the default contact. Top row demonstrates the strong impact on standard conductivities vs. frequency-adapted conductivities in the resulting FEM-based VTA. Bottom row shows two heuristic voltage driven models implemented in Lead-DBS. These models are not validated for directional leads, thus, a Medtronic 3389 electrode is visualized instead. Right column: The “optimal” segment on the top (K13) is used as cathode, steering the field anterolaterally to reach a good coverage of the dorsolateral STN. Simulations marked with Stim A and B are used as connectivity seeds in subsequent figures.
Connectivity from VTA to other Brain Regions
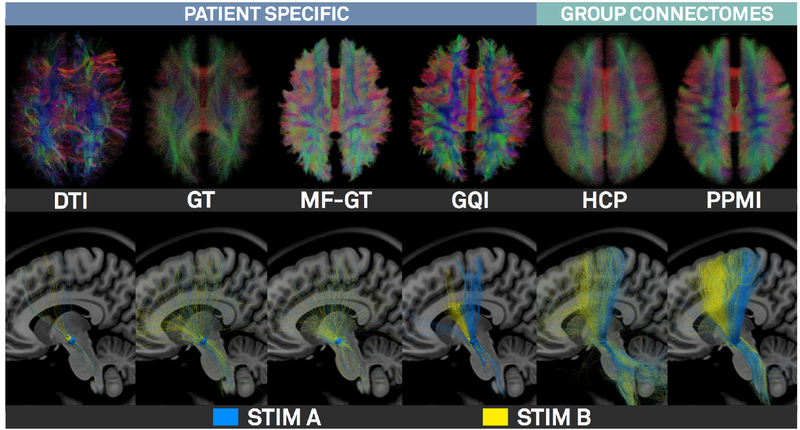
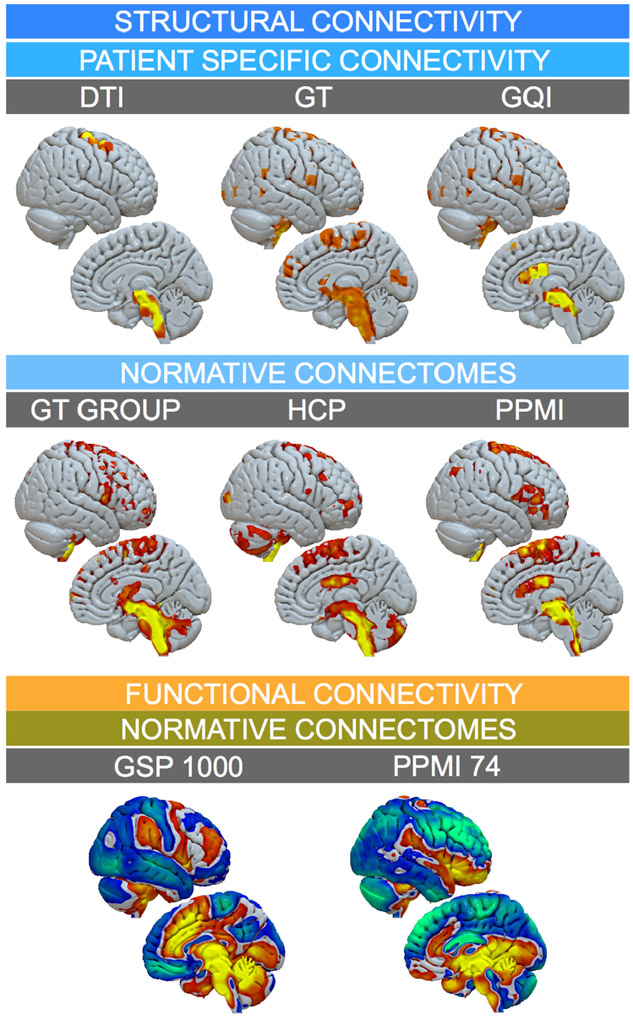
Top row of figure 9 shows results of the (3T patient’s) patient-specific deterministic DTI tractography, Gibbs’-tracking (GT), Mesotracking (MF-GT) and Generalized Q-sampling Imaging (GQI) approaches (whole connectome) as well as Human Connectome Project (HCP; Setsompop et al., 2013; Horn et al., 2017a) and PPMI (Marek et al. 2011; Ewert et al. 2017) based normative connectomes. In the bottom row of figure 9, fiber tracts running through the VTA defined in figure 8A and C are shown. Figure 10 shows connectivity profiles from the VTA defined in figure 8A projected to the cortex using various structural and functional connectomes.
Figure 9:
Fiber tracking results of the 3T example patient. First four columns show results of the patient-specific DTI, Gibbs’-tracking, Modelfree Meso global tracker and GQI approaches (top: whole connectome, bottom, tracts seeding from STIM A & B; see figure 8). Last two columns show the same views on HCP and PPMI based normative connectomes.
Figure 10:
Connectivity from VTA defined by “STIM A” (figure 8) projected on the right hemispheric surface as defined by various connectomes. Top row: Patient specific structural connectivity using DTI, Gibbs’ Tracking and GQI methods. Mid row: Connections defined by the structural Gibbsconnectome, HCP Adult Diffusion and PPMI PD connectomes. Bottom row: Functional connectivity between VTA and other brain regions as defined by normative GSP 1000 and PPMI 74 PD connectomes. This figure demonstrates a multitude of options to analyze VTA connectivity in Lead-DBS, but also highlights challenges of the process, since different methods/datasets yield different results.
Explaining clinical improvement in retrospective cohort
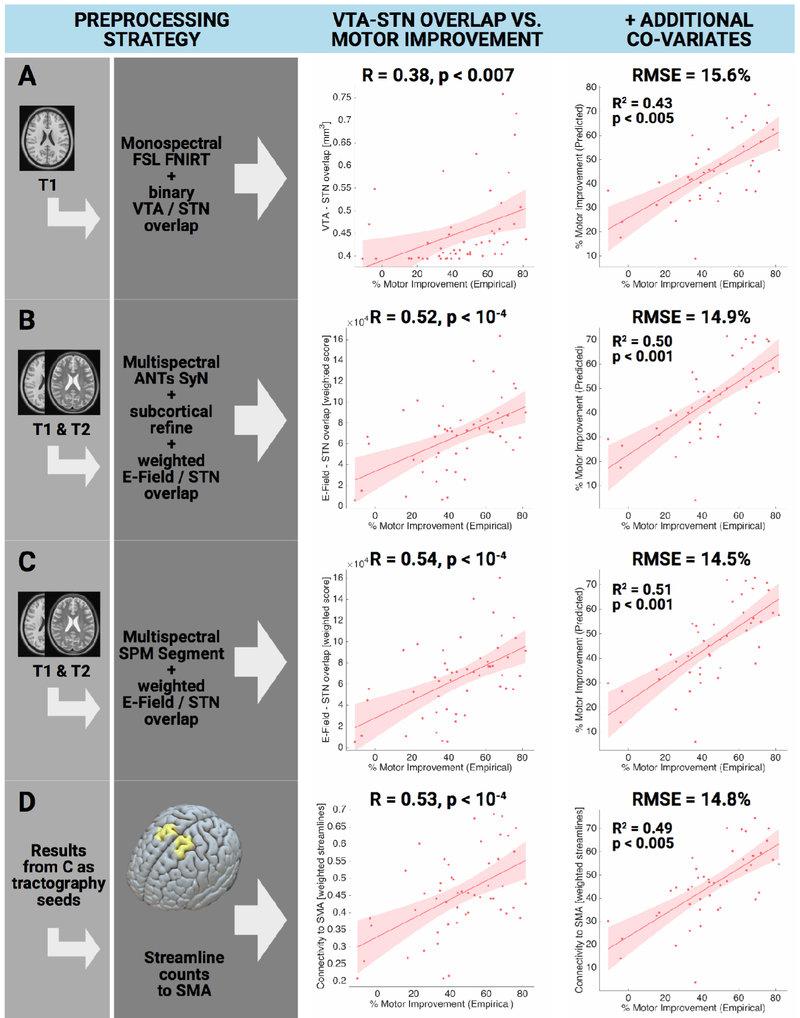
Results are summarized in table 9. Briefly, volumes of overlap correlated significantly with the empirical clinical outcome (FSL FNIRT: R = 0.38 at p = 0.007, ANTs SyN without / with subcortical refinement: R = 0.47 / 0.49 at p < 0.001; SPM New Segment: R = 0.52 at p < 0.001). Weighted overlaps between E-Field and STN correlated higher with clinical improvement for all normalization methods (FSL FNIRT: R = 0.46 at p < 0.001, ANTs SyN without / with subcortical refinement: R = 0.47 / 0.52 at p < 0.001 / < 10−4, SPM New Segment: R = 0.54 at p < 10−4). Mid column of figure 11 summarizes these findings. When adding additional clinical co-variates to a GLM to explain motor improvement (right column in figure 11), RMSE was comparable between methods (~14-16%) but explained variance was 8% higher between best (SPM New Segment with E-Field) vs. worst (FSL FNIRT with binary VTA) methods (table 9).
Table 9:
Preprocessing strategies used to explain variance in 1.5T retrospective cohort.
| VTA strategy | MR volumes used |
Shown in fig. 11 |
Correlation (image metric vs. motor improvement) |
GLM (+ additional covariates) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Normalization strategy | R | p | RSME [%] | R2 | Overall p | Significant predictors (p < 0.05) | |||
| Binary VTA overlap | |||||||||
| Monospectral FSL FNIRT | T1 | Panel A | 0.38 | < 0.007 | 15.1 | 0.43 | < 0.005 | Sex | |
| Multispectral ANTs SyN | w/o subcortical refine | T1 & T2 | − | 0.46 | < 0.001 | 15.3 | 0.45 | < 0.005 | Sex |
| with subcortical refine | T1 & T2 | − | 0.49 | < 0.001 | 14.8 | 0.48 | < 0.005 | Sex, VTA overlap | |
| Multispectral SPM New Segment | T1 & T2 | − | 0.51 | < 10−4 | 14.7 | 0.49 | < 0.001 | Sex, VTA overlap | |
| Weighted E-field VTA / STN overlap | |||||||||
| Monospectral FSL FNIRT | T1 | − | 0.46 | < 0.001 | 15.1 | 0.46 | < 0.005 | Sex | |
| Multispectral ANTs SyN | w/o subcortical refine | T1 & T2 | − | 0.47 | < 0.001 | 15.3 | 0.46 | < 0.005 | Sex |
| with subcortical refine | T1 & T2 | Panel B | 0.52 | < 10−4 | 14.9 | 0.48 | < 0.005 | Sex, E-Field overlap | |
| Multispectral SPM New Segment | T1 & T2 | Panel C | 0.54 | < 10−4 | 14.5 | 0.51 | < 0.001 | Sex, E-Field overlap | |
| Weighted streamline counts to SMA | |||||||||
| Weighted streamline counts to SMA seeding from E-Field (based method one row above) | T1 & T2 | Panel D | 0.53 | < 10−4 | 14.8 | 0.49 | < 0.005 | Sex, Streamlines to SMA | |
Figure 11:
Amount of variance in clinical outcome explained when applying various preprocessing options (retrospective 1.5T cohort). A) an exemplary “standard neuroimaging” approach with a monospectral (T1 only) FSL FNIRT based registration and a binary VTA. B & C) Default pathways of Lead-DBS, registering preoperative data into template space in multispectral fashion according to the most optimal method as delineated in (Ewert et al. 2018a). In these approaches, the overlap sum between the E-Field gradient magnitude inside the STN was calculated. D) Using results from C as weighted seeds to isolate fibers from the normative PPMI connectome (table 8), correlating weighted numbers of streamlines to SMA to explain clinical motor improvement.
First column of scatterplots shows direct correlation between VTA-STN overlap (weighted E-field overlap or weighted streamlines to SMA) with clinical improvement. Second column shows GLM with additional clinical covariates. Table 9 shows results for additional preprocessing strategies.
Panel A of figure 11 shows the FNIRT method (which does not work multispectrally and had only T1 MRIs as input, thus has poor if any information on the STN location) in combination with a binarized VTA. This approach could be seen as a “common neuroimaging” approach since most fMRI studies use T1 weighted images in their normalization step and binarized VTAs are common in the field. Panels B and C show the two multispectral Lead-DBS default pathways identified in (Ewert et al. 2018a) and apply a weighted VTA (E-Field magnitude). Both yield a similar outcome of ~R = 0.5, increasing the amount of variance explained by imaging alone from ~14% (FNIRT T1) to ~26% (Lead-DBS defaults). Their connectivity strength (number of weighted streamlines to the SMA as defined by the 6ma entry – the medial and anterior parcel of sensorimotor numbered results – of the Glasser et al. 2016 parcellation) was calculated using Lead Connectome Mapper. Resulting values were equally correlated with empirical motor improvement scores (fig. 11 panel D).
Discussion
We present a comprehensive and advanced processing pipeline to reconstruct, visualize and analyze DBS electrode placement based on neuroimaging data. Specific strengths in comparison to other tools are a seamless integration with a wide array of neuroimaging tools (table 11), a strong focus on precise spatial normalization and connection to a structural and functional connectome pipeline that facilitates connectivity analyses within the DBS context (figs. 9 & 10).
Table 11:
Software with aims comparable to Lead-DBS. Entries based on publicly available information at the time of writing.
| Name | Institution / Company |
Publicly available |
Open Source |
Free of cost |
CE / FDA | Website | Citation |
|---|---|---|---|---|---|---|---|
| Scientific Applications | |||||||
| DBSproc | NIH | + | + | + | − | afni.nimh.nih.gov | Lauro et al. 2015 |
| PyDBS | Univ. Rennes, Univ. Descartes Paris | − | − | acoustic.univ-rennes1.fr/doku.php/software | D’Albis et al. 2014 | ||
| PaCER | Université du Luxembourg | + | + | + | − | adhusch.github.io/PaCER | Husch et al. 2017 |
| DBSmapping | INESC TEC Porto | − | N/A | N/A | − | − | Da Silva et al. 2015 |
| “Virtual Patient” | MGH/HMS | − | N/A | N/A | − | − | Bonmassar et al. 2014 |
| CiceroneDBS | Case Western Reserve University | discont. | N/A | N/A | − | − | Miocinovic etal. 2007 |
| Lead-DBS | Charité – University Medicine Berlin | + | + | + | − | lead-dbs.org | Horn and Kühn 2015 |
| Commercial Applications | |||||||
| GUIDE | Boston Scientific | discont. | N/A | N/A | N/A | − | N/A |
| GUIDE XT | Brainlab / Boston Scientific | + | − | − | filed | brainlab.com | N/A |
| Optivize | Medtronic | discont. | N/A | N/A | N/A | medtonic.com | N/A |
| SureTune | Sapiens Steering Brain Stimulation | discont. | N/A | N/A | N/A | − | N/A |
| SureTune vs. 2 & 3 | Medtronic | + | − | − | + | medtonic.com | N/A |
| CranialCloud / CranialDrive | Neurotargeting LLC | + | − | − | + | app.cranialdrive.com | |
Contributions of the present paper are three-fold. First, an overview is provided regarding the novel neuroimaging methods that were added or updated over the course of four years since the initial publication of Lead-DBS. Second, a default pathway navigating through the multiple options in both DBS and connectome pipelines is outlined (figure 2). This pathway is motivated by both empirical data (Ewert et al., 2018a; Fillard et al., 2011; Klein et al., 2009; Maier-Hein et al., 2017) and by the experience of the Berlin DBS center where Lead-DBS or similar applications were used to localize roughly three thousand DBS electrodes since 2008. Third, results of various processing steps are visualized for a single patient and quantitatively analyzed in a retrospective analysis of 51 patients. In the latter, we do not only demonstrate that overlap between stimulation volumes and the subthalamic nucleus may explain clinical motor improvement, but we also show that the amount of variance explained may depend on the applied preprocessing strategy. Specifically, in the cohort investigated here, the advanced multispectral normalization pipelines implemented as defaults in Lead-DBS are able to explain more variance in clinical outcome than a “typical neuroimaging” pipeline.
In total, Lead-DBS includes five methods for linear co-registrations (table 1), ten normalization approaches (table 3), four approaches for electrode reconstructions, four VTA models (table 4) and four whole-brain tractography pipelines (table 6). Twenty-four subcortical atlases (table 2) and 17 brain parcellations (table 7) are pre-installed. Finally, two functional and four structural connectomes were converted into a format suitable for use in Lead-DBS (table 8). Taken together, these resources build a comprehensive toolbox for DBS electrode localization and the analysis of local (coordinate- or VTA-based) and global (structural and functional connectivity) features. While the number of these methods may introduce complexity, a user-friendly “default pathway” (figure 2) was established which works robustly and well for most applications. This pathway was established while working on several studies that were empowered by Lead-DBS with a variety of clinical and scientific aims. Some of these used Lead-DBS to integrate population based neural activity with anatomical structures (“Subcortical Electrophysiology Mapping” approach; Accolla et al., 2017; Geng et al., 2018; Horn et al., 2017b; Lofredi et al., 2018; Neumann et al., 2017; van Wijk et al., 2017). Other studies used connectivity profiles from DBS electrodes to predict clinical outcome (Horn et al., 2017c) or combined electrophysiological measures with DBS contact connectivity profiles (Accolla et al., 2016). In an effort to improve the safety profile of DBS implantations, some aimed at determining the relationship between electrode positions and clinical side effects or non-motor symptoms (Mosley et al., 2018b; 2018a). Finally, in other publications, the main aim was to ensure that the analyzed electrodes were indeed placed within the target region (Barow et al., 2014; Brücke et al., 2014; Ehlen et al., 2017; Hohlefeld et al., 2015; 2017; 2013; Krause et al., 2015; 2016; Kroneberg et al., 2017; Merkl et al., 2016; Neumann et al., 2015; 2016; Schroll et al., 2015; Tiedt et al., 2016).
The precision of Lead-DBS
Quantifying the precision of the processing pipeline is a difficult task but a frequently asked question and crucial to the widespread use of the tool. Unfortunately, without postmortem histological examination, no real ground truth exists. However, some indirect measures may help to address the question. First, it should be mentioned that errors may originate from several sources including i) MR-distortion artifacts, ii) within-patient co-registration including brain shift, iii) patient-to-template normalization and iv) electrode localization. Quantifying the first source falls under the domain of MR physics research and goes beyond the present scope. Still, it is advisable to apply distortion correction steps in each MR sequence if possible – even more so when high field magnets are involved. Second, errors in linear co-registration can be minimized by care- and skillful inspection of the data. The check co-registation and brain shift correction modules were specifically designed for the task at hand and iteratively improved to suit the needs and precision of DBS imaging. For instance, the brain shift correction step often sensibly corrects registrations on a submillimeter scale (figs 5 and S1). Normalization procedures were recently addressed in a comparative study (Ewert et al., 2018a). The study defined the default normalization pipeline which, depending on data quality, resulted in an average surface distance of STN/GPi boundaries between 0.38 and 0.75 mm, while inter-rater distance was between 0.41 and 0.82 mm. Based on these results, the pipeline is able to segment STN and GPi nuclei equally well as human experts. In this context, an anteroposterior iron gradient in the STN poses an additional problem for this specific target. The posterolateral sensorimotor part of the nucleus that is targeted to treat movement disorders contains less iron than its anteriomedial parts, often rendering it smaller on MRI than it actually is (Dormont et al. 2004, Richter et al. 2004, Schäfer et al. 2011, de Hollander et al. 2014, Massey et. al 2012). This makes the registration of this specific nucleus to a template space more error-prone than other targets and yet again raises the need for ultra high-field multispectral preoperative imaging (e.g. see Forstmann et al. 2017, Keuken et al. 2014). Fourth, in the electrode reconstruction step, prior studies have used phantoms to verify that electrode artifact centers in MRI (Pollo et al., 2004; Yelnik et al., 2003) and CT (Hemm et al., 2009; Husch et al., 2017) indeed correspond to electrode centers in the brain. To this end, the PaCER algorithm as the default reconstruction algorithm for postoperative CT yielded an average reconstruction error below 0.2 mm – again depending on data quality. On the other end of the spectrum, the “x-ray mode” of Lead-DBS was specifically designed to reduce errors introduced by partial volume effects in imaging data of suboptimal resolution. In summary, all sources of error can be minimized by using high-quality imaging data, distortion correction and careful inspection or registration and localization results. Based on the retrospective cohort analysed in the present study, we demonstrate that the specialized and elaborate default pipeline of Lead-DBS may add to the amount of variance explained in DBS imaging data.
Reproducibility, Open Science & Experimental Features
As stated above, a key mission of Lead-DBS development is to provide a platform for DBS imaging that is and remains i) free of use, ii) reproducible, open source & transparent, and iii) independent from commercial manufacturers. While this hinders the application in a clinical context (see below), within research, it has several advantages. First, the free software nature offers excellent worldwide accessibility, the possibility of fast skill dissemination in open workshops or courses within the academic field. Similarly, it is easy to script, automate and modify as permitted by the open source license while this is tedious or impossible in closed environments of clinical software. Second, transparent and open source code that is developed in a version controlled fashion (https://github.com/leaddbs/leaddbs) permits excellent reproducibility that is required for good scientific practice. In contrast, undocumented changes or discontinuity in the commercial applications may impose risks for producing consistent results. Discontinuation of commercial products has happened on multiple occasions in the field of DBS (table 10). Consequently, published studies that used discontinued software exist and are now hard if not impossible to reproduce. A slightly less obvious advantage of academic software is that its development is much more flexible. Commercial applications undergo highly involved and time-consuming certification processes to achieve CE-marks or FDA-approvals for safe use in clinical context. Needless to say, this is a great advantage or even requirement for clinicians but may drastically slow down software development. Furthermore, new research tools may not be easily integrated into commercial pipelines since these would require re-certification. In contrast, new tools can be integrated into academic software from idea and concept to end-user deployment within days. For instance, in 2009, the global fiber tracking approach (Reisert et al., 2011) won the Neurospin Fiber Cup evaluated as the best fiber tracking software compared to nine competitors (Fillard et al., 2011). With help from its developers, the Gibbs’ Tracker was integrated into the Lead Connectome pipeline within weeks. Recently, a newer comparative study found that the generalized q-sampling algorithm implemented in DSI studio yielded the highest "valid connection" score (Maier-Hein et al., 2017). Again, with kind support and permission of the developer, this method was integrated into Lead-DBS. A last example is the brain shift correction feature that was developed from idea to published code during a three day “brainhack global” event in 2017 at MIT (Craddock et al., 2016). Finally, a strong focus of clinical applications lies on their usability and processing speed. This is important since tools are used by medical personnel working under stressful circumstances where introduction into various complex software tools and long processing times are not tolerable. In contrast, in a research setting, search for innovative application, and development of new features outweighs the burden of computational time. Often, high performance compute clusters are available or jobs are run overnight. Thus, processes with high computational cost will be optimized for speed and standard applications in the former and for development and precision in the latter context. These thoughts illustrate that both types of tools – i.e. clinical vs. academic software – are needed. Given contradictory demands, a one-stop solution serving all purposes is hard if not impossible to create.
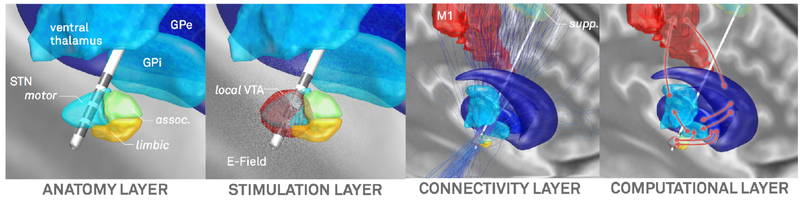
Limitations and Future Directions
In comparison to the first release, version 2 represents a major update and a drastically enhanced pipeline for DBS imaging. However, further development is planned to address remaining limitations and further maximize precision of the pipeline. To this end, the pipeline and resulting models may be broken down into four layers, each of which could be further improved as technology and methodology advance (figure 12). First, an anatomy layer describes the local surroundings of the electrode and helps to define electrode placement initially. This layer is presently defined by imaging and brain atlases (of which some may be informed by histology or other sources of information, table 2). It was mentioned multiple times that naturally, overall precision drastically depends on imaging quality (Ewert et al., 2018a; Husch et al., 2017). Crucially, the MR protocol of the 3T example patient represents a state-of-the art pipeline achievable in typical hospital settings and comprises a specialized basal ganglia sequence (FGATIR; Sudhyadhom et al., 2009). However, the diffusion-MRI acquired here may not be optimally suited to investigate the fine and complex details around DBS targets but was possible to scan within clinical routine. An example of a more optimal scan protocol can be found in (Akram et al., 2017). Moreover, as discussed in our original article (Horn & Kühn 2015), the use of postoperative CT or MRI each has specific advantages (higher signal to noise of the electrode on CT but direct visibility of surrounding anatomical structures on MRI, no radiation). It is hard to tell on empirical grounds which is better but the visibility of structures on the MRI generates a strong argument in favor for postoperative MRI – with the possibility to much more deliberately control for accuracy of post- to pre-co-registration around the target region. Similarly specialized methods like quantitative susceptibility mapping (Wang and Liu, 2015) or the use of ultra-highfield MRI (Forstmann et al., 2017; 2014) are other potential ways of increasing anatomical precision. As figure 1 illustrates, DBS target regions are typically small in size and reside in complex surroundings with a multitude of fiber tracts and functional segregations. Thus, sources above and beyond MRI may be needed to refine definition on the anatomy layer. To this end, techniques like polarized light imaging (Axer et al., 2011) or anisotropic scattering imaging (Shin et al., 2014) as well as the registration of histological stacks into template space (Alho et al., 2017; Amunts et al., 2013; Chakravarty et al., 2006; Ewert et al., 2018b; Forstmann et al., 2016; Jakab et al., 2012; Yelnik et al., 2007) are already applied increasingly.
Figure 12:
Four layers in a DBS imaging pipeline that may need continuous refinement as technology and methodology advance.
The second layer deals with modeling the local stimulation effects which are often represented by an E-Field or VTA. The anatomy layer directly informs these computations given distinct and even anisotropic conductivity values present in gray or white matter (Butson et al., 2006; Horn et al., 2017c). As mentioned above, to this end, other groups have created much more elaborate models over the last twenty years. Among others, pioneering work by the McIntyre, Butson, Grill, van Rienen and Wårdell groups should be mentioned (e.g. Åström et al., 2014; 2009; Butson et al., 2006; Butson and McIntyre, 2008; Chaturvedi et al., 2013; Gunalan et al., 2017; Schmidt et al., 2013; Schmidt and van Rienen, 2012). A practical disadvantage of these models is that they require manual interventions at multiple stages and use of a multitude of software applications (some of which are expensive commercial solutions; e.g. see Gunalan et al., 2017). On the other end of the spectrum, even simpler models exist that were successfully employed in clinical context (Dembek et al., 2017; Kuncel et al., 2008; Lauro et al., 2015; Mädler and Coenen, 2012; Vanegas Arroyave et al., 2016). Still, while it remains to be shown that more clinical variance may be explained when applying more sophisticated models, the stimulation layer is definitely one where Lead-DBS has yet much room for improvement.
The third layer deals with the transition from a local VTA to a global volume of modulation by applying brain connectivity. Using tractography or functional connectivity to estimate which other brain areas could potentially be modulated by DBS is a powerful technique that was already used to predict clinical outcome in PD patients (Horn et al., 2017c). However, a big challenge is that both methods are highly indirect. As recently demonstrated, tractography results are dominated by false positive connections (Maier-Hein et al., 2017). On the other hand, resting-state functional MRI is only able to give rough statistical dependencies between an indirect measure of brain activity that operates on a very slow temporal scale. Thus, the conclusions drawn from these measures need careful interpretation and benefit from validation via anatomical or electrophysiological work. For instance, the use of combined LFP-MEG recordings (Litvak et al., 2011; Neumann et al., 2015; Oswal et al., 2016) may validate fMRI findings and vice versa, while animal, tracer or gross-dissection studies may be used to interpret tractography results (e.g. Forel, 1877; Iwahori, 1978; T. Kita and H. Kita, 2012; Marburg, 1904). With these limitations in mind, it should be mentioned that the two main tractography algorithms included in Lead-DBS were each best performers in large open competitions (Fillard et al., 2011; Maier-Hein et al., 2017) and a specific advantage of the GQI method in clinical context lies in its low false positive score (Maier-Hein et al., 2017).
Finally, a fourth layer could be seen as modeling dynamics or connectivity changes induced by DBS. This layer is not touched upon here, but computational modeling based on empirical data seems the only way to investigate how brain activity and connectivity responds to stimulation of a specific target. Already, Lead-DBS was used in such basal ganglia modeling studies (Schroll et al., 2015) and an aim of future versions is to incorporate or interface with modeling software – steadily working toward a “virtual patient” model that facilitates a better understanding of DBS.
In conclusion, we present an updated, advanced and integrative platform for DBS imaging research that is openly available with the aim to further elucidate the mechanisms of DBS and improve therapeutic outcome for DBS patients worldwide.
Supplementary Material
Acknowledgements
This study was supported by Deutsche Forschungsgesellschaft (grants KFO 247, SPP 2041) to AAK, Stiftung Charité, Berlin Institute of Health and Prof. Klaus Thiemann Foundation to AH. TMH has received research support from the American Brain Foundation / American Academy of Neurology and NINDS grant K23NS099380. QF was supported by NIH R01-GM114365 (from NIGMS) and R01-CA204443 (from NCI). TP was supported by the Victorian Government’s Operational Infrastructure Support Programme, the Colonial Foundation and the St. Vincent’s Hospital Melbourne Research Endowment Fund. JV was supported by the National Science Foundation (NSF): US IGNITE - 10037840. NL, AK, CB, SE, AT, AH, TP, MR, HS, RO, CR, FCY, QF, TMH and JV have nothing to disclose. AAK reports personal fees and non-financial support from Medtronic, personal fees from Boston Scientific and personal fees from St. Jude Medical outside the submitted work. AH and TAD received speaker honoraria from Medtronic outside the submitted work. WJN received travel grants from Medtronic and St. Jude medical outside of the submitted work. Data collection and sharing for this project was provided by the Human Connectome Project (HCP; Principal Investigators: Bruce Rosen, M.D., Ph.D., Arthur W. Toga, Ph.D., Van J. Weeden, MD). HCP funding was provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH), and the National Institute of Neurological Disorders and Stroke (NINDS). HCP data are disseminated by the Laboratory of Neuro Imaging at the University of Southern California. The HCP project (Principal Investigators: Bruce Rosen, M.D., Ph.D., Martinos Center at Massachusetts General Hospital; Arthur W. Toga, Ph.D., University of Southern California, Van J. Weeden, MD, Martinos Center at Massachusetts General Hospital) is supported by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute of Mental Health (NIMH) and the National Institute of Neurological Disorders and Stroke (NINDS). HCP is the result of efforts of co-investigators from the University of Southern California, Martinos Center for Biomedical Imaging at Massachusetts General Hospital (MGH), Washington University, and the University of Minnesota. Data used in the preparation of this article were obtained from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org. PPMI - a public-private partnership - is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, see www.ppmi-info.org/fundingpartners.
List of abbreviations
- ANTs
Advanced Normalization Tools, see http://stnava.github.io/ANTs/
- BSpline-SyN
ANTs normalization method; Explicit B-spline regularization in symmetric diffeomorphic image registration
- CORE
Algorithm for “reconstruction of electrode contact positions” defined in Horn & Kühn 2014
- DARTEL
SPM normalization method; A fast diffeomorphic image registration algorithm.
- DiODe
Directional Orientation Detection (Sitz et al. 2017)
- dMRI
Diffusion-weighted MRI – in the context of diffusion-imaging based tractography.
- FA
Fractional Anisotropy
- FGATIR
Fast Grey Matter Acquisition T1 Inversion Recovery
- FLIRT
FMRIB's Linear Image Registration Tool
- FNIRT
FSL normalization method; FMRIB's Nonlinear Image Registration Tool
- FSL
FMRIB Software Library, see https://fsl.fmrib.ox.ac.uk/
- GNU
Recursive acronym for “GNU's Not Unix.”; GNU GPL is a popular open license supported by the Free Software Foundation (see http://www.gnu.org). The Free Software Foundation (FSF) is a nonprofit with a worldwide mission to promote computer user freedom.
- GPi
internal segment of the globus pallidus
- GPe
external segment of the globus pallidus
- GQI
Generalized q-sampling imaging, dMRI processing method implemented in DSI studio
- HCP
Human Connectome Project
- LEDD
Levodopa Equivalent Daily Dosage
- MAGeT Brain
Multiple Automatically Generated Templates brain segmentation algorithm, see https://github.com/CobraLab/MAGeTbrain
- PaCER
Precise and Convenient Electrode Reconstruction for DBS, see https://adhusch.github.io/PaCER/
- PCA
Principal Component Analysis
- PPMI
Parkinson’s Disease Progression Marker Initiative
- PPN
Pedunculopontine nucleus
- RN
red nucleus
- ROI
Region of Interest
- RSME
Root-mean-square error
- QSM
Quantitative Susceptibility Mapping
- rs-fMRI
Resting-state functional MRI
- SHOOT
SPM normalization method; Diffeomorphic registration using geodesic shooting and Gauss–Newton optimisation
- SPM
Statistic Parametric Mapping, see http://www.fil.ion.ucl.ac.uk/spm/software/spm12/
- STN
subthalamic nucleus
- SyN
ANTs normalization method; Symmetric diffeomorphic image registration / symmetric image normalization
- TRAC
Algorithm for “trajectory reconstruction” defined in Horn & Kühn 2014
- UPDRS
Unified Parkinson’s Disease Rating Scale
- VTA
Volume of Tissue Activated
References
- Accolla EA, Herrojo Ruiz M, Horn A, Schneider G-H, Schmitz-Hubsch T, Draganski B, Kühn AA, 2016. Brain networks modulated by subthalamic nucleus deep brain stimulation. Brain 139, 2503–2515. doi:10.1093/brain/aww182 [DOI] [PubMed] [Google Scholar]
- Accolla EA, Horn A, Herrojo Ruiz M, Neumann W-J, kühn AA, 2017. Reply: Oscillatory coupling of the subthalamic nucleus in obsessive compulsive disorder. Brain 140, e57–e57. doi:10.1093/brain/awx165 [DOI] [PubMed] [Google Scholar]
- Ahsan RL, Allom R, Gousias IS, Habib H, Turkheimer FE, Free S, Lemieux L, Myers R, Duncan JS, Brooks DJ, 2007. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus 38, 261–270. doi: 10.1016/j.neuroimage.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Akram H, Dayal V, Mahlknecht P, Georgiev D, Hyam J, Foltynie T, Limousin P, De Vita E, Jahanshahi M, Ashburner J, Behrens T, Hariz M, Zrinzo L, 2018. Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin 18, 130–142. doi:10.1016/j.nicl.2018.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram H, Sotiropoulos SN, Jbabdi S, Georgiev D, Mahlknecht P, Hyam J, Foltynie T, Limousin P, De Vita E, Jahanshahi M, Hariz M, Ashburner J, Behrens T, Zrinzo L, 2017. Subthalamic deep brain stimulation sweet spots and hyperdirect cortical connectivity in Parkinson's disease. Neuroimage 158, 332–345. doi:10.1016/j.neuroimage.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho ATDL, Hamani C, Alho EJL, da Silva RE, Santos GAB, Neves RC, Carreira LL, Araújo CMM, Magalhães G, Coelho DB, Alegro MC, Martin MGM, Grinberg LT, Pasqualucci CA, Heinsen H, Fonoff ET, Amaro E, 2017. Magnetic resonance diffusion tensor imaging for the pedunculopontine nucleus: proof of concept and histological correlation. Brain Structure and Function 12, 26–12. doi: 10.1007/s00429-016-1356-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alho EJL, Di Lorenzo Alho AT, Grinberg L, Amaro E, Santos GAB, Silva RE, Neves RC, Alegro M, Coelho DB, Teixeira MJ, Fonoff ET, Heinsen H, 2017. High thickness histological sections as alternative to study the three-dimensional microscopic human sub-cortical neuroanatomy Eduardo Joaquim Lopes Alho, Ana Tereza Di Lorenzo Alho, Lea Grinberg, Edson Amaro, Gláucia Aparecida Bento dos Santos, et al. Brain Structure and Function 1–14. doi: 10.1007/s00429-017-1548-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Lepage C, Borgeat L, Mohlberg H, Dickscheid T, Rousseau M-É, Bludau S, Bazin P-L, Lewis LB, Oros-Peusquens A-M, Shah NJ, Lippert T, Zilles K, Evans AC, 2013. BigBrain: an ultrahigh-resolution 3D human brain model. Science 340, 1472–1475. doi:10.1126/science.1235381 [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Zilles K, 2007. Cytoarchitecture of the cerebral cortex--more than localization. Neuroimage 37, 1061–5– discussion 1066–8. doi: 10.1016/j.neuroimage.2007.02.037 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Smith S, 2010. Non-linear registration, aka spatial normalisation. FMRIB technical report 1–22. [Google Scholar]
- Archer DB, Vaillancourt DE, Coombes SA, 2017. A Template and Probabilistic Atlas of the Human Sensorimotor Tracts using Diffusion MRI. Cerebral Cortex 1–15. doi: 10.1093/cercor/bhx066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, 2007. A fast diffeomorphic image registration algorithm. 38, 95–113. doi: 10.1016/j.neuroimage.2007.07.007 [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2011. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. NeuroImage 55, 954–967. doi:10.1016/j.neuroimage.2010.12.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. 26, 839–851. doi: 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Auzias G, Coulon O, Brovelli A, 2016. MarsAtlas: A cortical parcellation atlas for functional mapping. Hum Brain Mapp. doi:10.1002/hbm.23121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auzias G, Lefèvre J, Le Troter A, Fischer C, Perrot M, Régis J, Coulon O, 2013. Model-Driven Harmonic Parameterization of the Cortical Surface: HIP-HOP. IEEE Trans Med Imaging 32, 873–887. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12, 26–41. doi:10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axer M, Amunts K, Grässel D, Palm C, Dammers J, Axer H, Pietrzyk U, Zilles K, 2011. A novel approach to the human connectome: ultra-high resolution mapping of fiber tracts in the brain. 54, 1091–1101. doi:10.1016/j.neuroimage.2010.08.075 [DOI] [PubMed] [Google Scholar]
- Åström M, Diczfalusy E, Martens H, Wårdell K, 2014. Relationship between Neural Activation and Electric Field Distribution during Deep Brain Stimulation. IEEE Trans. Biomed. Eng. 1–1. doi:10.1109/TBME.2014.2363494 [DOI] [PubMed] [Google Scholar]
- Åström M, Zrinzo LU, Tisch S, Tripoliti E, Hariz MI, Wårdell K, 2009. Method for patient-specific finite element modeling and simulation of deep brain stimulation. Med Biol Eng Comput 47, 21–28. doi:10.1007/s11517-008-0411-2 [DOI] [PubMed] [Google Scholar]
- Baldermann JC, Kohl S, Visser-Vandewalle V, Klehr M, Huys D, Kuhn J, 2016. Deep Brain Stimulation of the Ventral Capsule/Ventral Striatum Reproducibly Improves Symptoms of Body Dysmorphic Disorder. Brain Stimul. doi:10.1016/j.brs.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Barow E, Neumann W-J, Brücke C, Huebl J, Horn A, Brown P, Krauss JK, Schneider G-H, Kühn AA, 2014. Deep brain stimulation suppresses pallidal low frequency activity in patients with phasic dystonic movements. Brain 137, 3012–3024. doi:10.1093/brain/awu258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM, 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. 6, 750–757. doi:10.1038/nn1075 [DOI] [PubMed] [Google Scholar]
- Boes AD, Prasad S, Liu H, Liu Q, Pascual-Leone A, Caviness VS Jr, Fox MD, 2015. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075. doi:10.1093/brain/awv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonmassar G, Angelone LM, Makris N, 2014. A Virtual Patient Simulator Based on Human Connectome and 7 T MRI for Deep Brain Stimulation. International journal on advances in life sciences 6, 364–372. [PMC free article] [PubMed] [Google Scholar]
- Brücke C, Horn A, Huppke P, Kupsch A, Schneider G-H, Kühn AA, 2014. Failure of Pallidal Deep Brain Stimulation in a Case of Rapid- Onset Dystonia Parkinsonism (DYT12). Movement Disorders Clinical Practice n/a–n/a. doi: 10.1002/mdc3.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT, 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106, 2322–2345. doi:10.1152/jn.00339.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, McIntyre CC, 2006. Predicting the effects of deep brain stimulation with diffusion tensor based electric field models. Med Image Comput Comput Assist Interv 9, 429–437. [DOI] [PubMed] [Google Scholar]
- Butson CR, Cooper SE, Henderson JM, Wolgamuth B, McIntyre CC, 2011. Probabilistic analysis of activation volumes generated during deep brain stimulation. 54, 2096–2104. doi:10.1016/j.neuroimage.2010.10.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butson CR, McIntyre CC, 2008. Current steering to control the volume of tissue activated during deep brain stimulation. Brain Stimul 1, 7–15. doi:10.1016/j.brs.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, 2006. Rhythms of the Brain. Oxford University Press. [Google Scholar]
- Caire F, Ranoux D, Guehl D, Burbaud P, Cuny E, 2013. A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson's disease. Acta Neurochir (Wien) 155, 1647–54–discussion 1654. doi: 10.1007/s00701-013-1782-1 [DOI] [PubMed] [Google Scholar]
- Cameron Craddock R, S Margulies D, Bellec P, Nolan Nichols B, Alcauter S, A Barrios F, Burnod Y, J Cannistraci C, Cohen-Adad J, De Leener B, Dery S, Downar J, Dunlop K, R Franco A, Seligman Froehlich C, J Gerber A, S Ghosh S, J Grabowski T, Hill S, Sólon Heinsfeld A, Matthew Hutchison R, Kundu P, R Laird A, Liew S-L, J Lurie D, G McLaren D, Meneguzzi F, Mennes M, Mesmoudi S, O’Connor D, H Pasaye E, Peltier S, Poline J-B, Prasad G, Fraga Pereira R, Quirion P-O, Rokem A, S Saad Z, Shi Y, C Strother S, Toro R, Q Uddin L, D Van Horn J, W Van Meter J, C Welsh R, Xu T, 2016. Brainhack: a collaborative workshop for the open neuroscience community. Gigascience 5, 16. doi:10.1186/s13742-016-0121-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty MM, Bertrand G, Hodge CP, Sadikot AF, Collins DL, 2006. The creation of a brain atlas for image guided neurosurgery using serial histological data. Neuroimage 30, 359–376. doi:10.1016/j.neuroimage.2005.09.041 [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Sadikot AF, Germann J, Bertrand G, Collins DL, 2008. Towards a validation of atlas warping techniques. Med Image Anal 12, 713–726. doi:10.1016/j.media.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Chakravarty MM, Steadman P, van Eede MC, Calcott RD, Gu V, Shaw P, Raznahan A, Collins DL, Lerch JP, 2012. Performing label-fusion-based segmentation using multiple automatically generated templates. Hum Brain Mapp 34, 2635–2654. doi:10.1002/hbm.22092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi A, Luján JL, McIntyre CC, 2013. Artificial neural network based characterization of the volume of tissue activated during deep brain stimulation. J Neural Eng 10, 056023. doi:10.1088/1741-2560/10/5/056023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi EY, Yeo BTT, Buckner RL, 2012. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol 108, 2242–2263. doi: 10.1152/jn.00270.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, Hu XP, Mayberg HS, 2012. A whole brain fMRI atlas generated via spatially constrained spectral clustering. Hum Brain Mapp 33, 1914–1928. doi:10.1002/hbm.21333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva NM, Ahmadi S-A, Tafula SN, Cunha JPS, Bötzel K, Vollmar C, Rozanski VE, 2016. A diffusion-based connectivity map of the GPi for optimised stereotactic targeting in DBS. NeuroImage 1–9. doi:10.1016/j.neuroimage.2016.06.018 [DOI] [PubMed] [Google Scholar]
- da Silva NM, Rozanski VE, Cunha JPS, 2015. A 3D multimodal approach to precisely locate DBS electrodes in the basal ganglia brain region, in:. Presented at the 2015 7th International IEEE/EMBS Conference on Neural Engineering (NER), pp. 292–295. doi:10.1109/NER.2015.7146617 [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9, 179–194. doi:10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- Darby R, Laganiere S, Pascual-Leone A, Prasad S, Fox MD, 2016. Finding the imposter: brain connectivity of lesions causing delusional misidentifications. Brain 1–11. doi:10.1093/brain/aww288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby RR, Horn A, Cushman F, Fox MD, 2017. Lesion network localization of criminal behavior. Proc Natl Acad Sci USA 56, 201706587–6. doi: 10.1073/pnas.1706587115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembek TA, Barbe MT, Åström M, Hoevels M, Visser-Vandewalle V, Fink GR, Timmermann L, 2017. Probabilistic mapping of deep brain stimulation effects in essential tremor. Neuroimage Clin 13, 164–173. doi:10.1016/j.nicl.2016.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ, 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–980. doi:10.1016/j.neuroimage.2006.01.021 [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E, 2010. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53, 1–15. doi:10.1016/j.neuroimage.2010.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, 2006. A spatially unbiased atlas template of the human cerebellum. Neuroimage 33, 127–138. doi:10.1016/j.neuroimage.2006.05.056 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Maderwald S, Küper M, Thürling M, Rabe K, Gizewski ER, Ladd ME, Timmann D, 2011. Imaging the deep cerebellar nuclei: a probabilistic atlas and normalization procedure. Neuroimage 54, 1786–1794. doi: 10.1016/j.neuroimage.2010.10.035 [DOI] [PubMed] [Google Scholar]
- Dormont D, Seidenwurm D, Galanaud D, Cornu P, Yelnik J, & Bardinet E, 2010. Neuroimaging and Deep Brain Stimulation. AJNR American Journal of Neuroradiology, 31(1), 15–23. http://doi.org/10.3174/ajnr.A1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Albis T, Haegelen C, Essert C, Fernandez-Vidal S, Lalys F, Jannin P, 2014. PyDBS: an automated image processing workflow for deep brain stimulation surgery. - PubMed - NCBI. Int J CARS 10, 117–128. [DOI] [PubMed] [Google Scholar]
- Ehlen F, Vonberg I, Tiedt HO, Horn A, Fromm O, Kühn AA, Klostermann F, 2017. Thalamic deep brain stimulation decelerates automatic lexical activation. Brain Cogn 111, 34–43. doi:10.1016/j.bandc.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Edlow BL, Takahashi E, Wu O, Benner T, Dai G, Bu L, Grant PE, Greer DM, Greenberg SM, Kinney HC, Folkerth RD, 2012. Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J. Neuropathol. Exp. Neurol. 71, 531–546. doi:10.1097/NEN.0b013e3182588293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K, 2006. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32, 570–582. doi: 10.1016/j.neuroimage.2006.04.204 [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TEJ, 2010. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. 30, 6409–6421. doi:10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K, 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. 25, 1325–1335. doi:10.1016/j.neuroimage.2004.12.034 [DOI] [PubMed] [Google Scholar]
- Eisenstein SA, Koller JM, Black KD, Campbell MC, Lugar HM, Ushe M, Tabbal SD, Karimi M, Hershey T, Perlmutter JS, Black KJ, 2014. Functional anatomy of subthalamic nucleus stimulation in Parkinson disease. Ann Neurol 76, 279–295. doi: 10.1002/ana.24204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewert S, Horn A, Finkel F, Li N, Kühn AA, Herrington TM, 2018a. Toward optimized nonlinear deformation algorithms for subcortical DBS target regions in MRI, in:. Presented at the Deutsche Gesellschaft für Klinische Neurophysiologie und Funktionelle Bildgebung. [Google Scholar]
- Ewert S, Plettig P, Li N, Chakravarty MM, Collins DL, Herrington TM, Kühn AA, Horn A, 2018b. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage 170, 271–282. doi:10.1016/j.neuroimage.2017.05.015 [DOI] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, Fox PT, Eickhoff SB, Yu C, Jiang T, 2016. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb. Cortex 26, 3508–3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, Laganiere SE, Lam S, Fox MD, 2017. Lesions causing freezing of gait localize to a cerebellar functional network. Ann Neurol 81, 129–141. doi: 10.1002/ana.24845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R, 2012. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30, 1323–1341. doi:10.1016/j.mri.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillard P, Descoteaux M, Goh A, Gouttard S, Jeurissen B, Malcolm J, Ramirez-Manzanares A, Reisert M, Sakaie K, Tensaouti F, Yo T, Mangin J-F, Poupon C, 2011. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom 56, 220–234. doi:10.1016/j.neuroimage.2011.01.032 [DOI] [PubMed] [Google Scholar]
- Fischer DB, Boes AD, Demertzi A, Evrard HC, Laureys S, Edlow BL, Liu H, Saper CB, Pascual-Leone A, Fox MD, Geerling JC, 2016. A human brain network derived from coma-causing brainstem lesions. 10.1212/WNL.0000000000003404–9. doi: 10.1212/WNL.0000000000003404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM, 2004. Automatically parcellating the human cerebral cortex. Cerebral Cortex 14, 11–22. doi:10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, Herbert MR, Bent EK, Koneru VK, Dieterich ME, Hodge SM, Rauch SL, Grant PE, Cohen BM, Seidman LJ, Caviness VS, Biederman J, 2005. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am J Psychiatry 1–10. doi:10.1176/appi.ajp.162.7.1256 [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ, 2004. Statistical parametric maps in functional imaging: A general linear approach. Hum Brain Mapp 2, 189–210. doi:10.1002/hbm.460020402 [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL, 2009. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage 47, S102. [Google Scholar]
- Forel A, 1877. Untersuchungen über die Haubenregion und ihre oberen verknüpfungen im Gehirne des Menschen und einiger Säugethiere. [Google Scholar]
- Forstmann BU, Isaacs BR, Temel Y, 2017. Ultra High Field MRI-Guided Deep Brain Stimulation. Trends in Biotechnology 35, 904–907. doi:10.1016/j.tibtech.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Alkemade A, 2016. The next step for imaging the subthalamic nucleus. Brain aww245–2. doi:10.1093/brain/aww245 [DOI] [PubMed] [Google Scholar]
- Forstmann BU, Keuken MC, Schäfer A, Bazin P-L, Alkemade A, Turner R, 2014. Multi-modal ultra-high resolution structural 7-Tesla MRI data repository. Sci. Data 1, 140050–8. doi: 10.1038/sdata.2014.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia D, Guridi J, Toledo JB, Alegre M, Obeso JA, Rodríguez-Oroz MC, 2016. Stimulation sites in the subthalamic nucleus and clinical improvement in Parkinson's disease: a new approach for active contact localization. http://dx.doi.org/10.3171/2015.9.JNS15868. 1–12 doi: 10.3171/2015.9.JNS15868 [DOI] [PubMed] [Google Scholar]
- Gaser C, 2016. CAT-a computational anatomy toolbox for the analysis of structural MRI data. Organization for Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng X, Xu X, Horn A, Li N, Ling Z, Brown P, Wang S, 2018. Intra-operative characterisation of subthalamic oscillations in Parkinson’s disease. Clinical Neurophysiology 0. doi: 10.1016/j.clinph.2018.01.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Uğurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC, 2016. A multi-modal parcellation of human cerebral cortex. Nature. doi: 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, Ahern T, O’Brien LM, Caviness VS, Kennedy DN, Faraone SV, Tsuang MT, 2007. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. BPS 61, 935–945. doi: 10.1016/j.biopsych.2006.06.027 [DOI] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, Petersen SE, 2016. Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cereb. Cortex 26, 288–303. doi:10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gousias IS, Rueckert D, Heckemann RA, Dyet LE, Boardman JP, Edwards AD, Hammers A, 2008. Automatic segmentation of brain MRIs of 2-year-olds into 83 regions of interest. NeuroImage 40, 672–684. doi: 10.1016/j.neuroimage.2007.11.034 [DOI] [PubMed] [Google Scholar]
- Gunalan K, Chaturvedi A, Howell B, Duchin Y, Lempka SF, Patriat R, Sapiro G, Harel N, McIntyre CC, 2017. Creating and parameterizing patient-specific deep brain stimulation pathway-activation models using the hyperdirect pathway as an example. PLoS ONE 12, e0176132. doi:10.1371/journal.pone.0176132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, Mitchell TN, Brooks DJ, Duncan JS, 2003. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp 19, 224–247. doi:10.1002/hbm.10123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasgall PA, Di Gennaro F, Baumgartner C, Neufeld E, Gosselin MC, Payne D, Klingenböck A, Kuster N, 2015. IT’IS Database for thermal and electromagnetic parameters of biological tissues. doi:DOI: 10.13099/VIP21000-03-0 [Google Scholar]
- Hell F, Taylor PCJ, Mehrkens JH, Bötzel K, 2018a. Subthalamic stimulation, oscillatory activity and connectivity reveal functional role of STN and network mechanisms during decision making under conflict. NeuroImage. doi:10.1016/j.neuroimage.2018.01.001 [DOI] [PubMed] [Google Scholar]
- Hell F, Plate A, Mehrkens JH, & Bötzel K, 2018b. Subthalamic oscillatory activity and connectivity during gait in Parkinson's disease. NeuroImage. Clinical, 19, 396–405. http://doi.org/10.1016/j.nicl.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemm S, Coste J, Gabrillargues J, Ouchchane L, Sarry L, Caire F, Vassal F, Nuti C, Derost P, Durif F, Lemaire J-J, 2009. Contact position analysis of deep brain stimulation electrodes on post-operative CT images. Acta Neurochir (Wien) 151, 823–829. doi:10.1007/s00701-009-0393-3 [DOI] [PubMed] [Google Scholar]
- Hemm S, Mennessier G, Vayssiere N, Cif L, Coubes P, 2005. Co-registration of stereotactic MRI and isofieldlines during deep brain stimulation. Brain Res Bull 68, 59–61. doi: 10.1016/j.brainresbull.2005.08.024 [DOI] [PubMed] [Google Scholar]
- Hohlefeld FU, Ehlen F, Tiedt HO, Krugel LK, Horn A, Kühn AA, Curio G, Klostermann F, Nikulin VV, 2015. Correlation between cortical and subcortical neural dynamics on multiple time scales in Parkinson's disease. Neuroscience 298, 145–160. doi: 10.1016/j.neuroscience.2015.04.013 [DOI] [PubMed] [Google Scholar]
- Hohlefeld FU, Ewald A, Ehlen F, Tiedt HO, Horn A, Kühn AA, Curio G, Klostermann F, Nikulin VV, 2017. Neural correlates of lexical decisions in Parkinson's disease revealed with multivariate extraction of cortico-subthalamic interactions. Clin Neurophysiol 128, 538–548. doi:10.1016/j.clinph.2016.12.026 [DOI] [PubMed] [Google Scholar]
- Hohlefeld FU, Huchzermeyer C, Huebl J, Schneider GH, Nolte G, Brücke C, Schönecker T, Kühn AA, Curio G, Nikulin VV, 2013. Functional and effective connectivity in subthalamic local field potential recordings of patients with Parkinson's disease. Neuroscience 250, 320–332. doi: 10.1016/j.neuroscience.2013.07.028 [DOI] [PubMed] [Google Scholar]
- de Hollander G, Keuken MC, Bazin P-L, Weiss M, Neumann J, Reimann K, et al. , 2014. A gradual increase of iron toward the medial-inferior tip of the subthalamic nucleus. Human Brain Mapping, 35(9), 4440–4449. http://doi.org/10.1002/hbm.22485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, 2017. PCA MNI 2009b NLIN template. doi:10.6084/m9.figshare.4644472.v2 [Google Scholar]
- Horn A, 2016. MNI T1 6thGen NLIN to MNI 2009b NLIN ANTs transform. doi: 10.6084/m9.figshare.3502238.v2 [Google Scholar]
- Horn A, 2015. A structural group-connectome in standard stereotactic (MNI) space. DiB 5, 292–296. doi:10.1016/j.dib.2015.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Blankenburg F, 2016. Toward a standardized structural-functional group connectome in MNI space. NeuroImage 124, 310–322. doi:10.1016/j.neuroimage.2015.08.048 [DOI] [PubMed] [Google Scholar]
- Horn A, Kühn AA, 2015. Lead-DBS: a toolbox for deep brain stimulation electrode localizations and visualizations. NeuroImage 107, 127–135. doi:10.1016/j.neuroimage.2014.12.002 [DOI] [PubMed] [Google Scholar]
- Horn A, Kühn AA, Merkl A, Shih L, Alterman R, Fox M, 2017a. Probabilistic conversion of neurosurgical DBS electrode coordinates into MNI space. NeuroImage 150, 395–404. doi:10.1016/j.neuroimage.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Neumann W-J, Degen K, Schneider G-H, Kühn AA, 2017b. Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. doi:10.1002/hbm.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A, Ostwald D, Reisert M, Blankenburg F, 2014. The structural-functional connectome and the default mode network of the human brain. NeuroImage 102 Pt 1, 142–151. doi:10.1016/j.neuroimage.2013.09.069 [DOI] [PubMed] [Google Scholar]
- Horn A, Reich M, Vorwerk J, Li N, Wenzel G, Fang Q, Schmitz-Hubsch T, Nickl R, Kupsch A, Volkmann J, Kühn AA, Fox MD, 2017c. Connectivity Predicts deep brain stimulation outcome in Parkinson disease. Ann Neurol 82, 67–78. doi: 10.1002/ana.24974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Petersen MV, Gemmar P, Goncalves J, Hertel F, 2017. PaCER - A fully automated method for electrode trajectory and contact reconstruction in deep brain stimulation. Neuroimage Clin 17, 80–89. doi:10.1016/j.nicl.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husch A, Petersen MV, Gemmar P, Goncalves J, Sunde N, Hertel F, 2018. Post-operative deep brain stimulation assessment: Automatic data integration and report generation. Brain Stimul 1–14. doi:10.1016/j.brs.2018.01.031 [DOI] [PubMed] [Google Scholar]
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, Gallant JL, 2016. Natural speech reveals the semantic maps that tile human cerebral cortex. Nature 532, 453–458. doi: 10.1038/nature17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth AG, Griffiths TL, Theunissen FE, Gallant JL, 2015. PrAGMATiC: a Probabilistic and Generative Model of Areas Tiling the Cortex. [Google Scholar]
- Ilinski I, Horn A, Paul-Gilloteaux P, Gressens P, Verney C, Kultas-Ilinsky K, 2018. Human Motor Thalamus Reconstructed in 3D from Continuous Sagittal Sections with Identified Subcortical Afferent Territories. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahori N, 1978. A Golgi study on the subthalamic nucleus of the cat. The Journal of comparative neurology 182, 383–397. doi:10.1002/cne.901820303 [DOI] [PubMed] [Google Scholar]
- Jakab A, Blanc R, Berenyi EL, Szekely G, 2012. Generation of Individualized Thalamus Target Maps by Using Statistical Shape Models and Thalamocortical Tractography. AJNR Am J Neuroradiol 33, 2110–2116. doi:10.3174/ajnr.A3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S, 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. 17, 825–841. doi: 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Johnson H, Harris G, Williams K, 2007. BRAINSFit: mutual information rigid registrations of whole-brain 3D images, using the insight toolkit. Insight J [Google Scholar]
- Joliot M, Jobard G, Naveau M, Delcroix N, Petit L, Zago L, Crivello F, Mellet E, Mazoyer B, Tzourio-Mazoyer N, 2015. AICHA: An atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 254, 46–59. doi:10.1016/j.jneumeth.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Kellner E, Dhital B, Kiselev VG, Reisert M, 2015. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine n/a-n/a. doi: 10.1002/mrm.26054 [DOI] [PubMed] [Google Scholar]
- Keuken MC, Bazin P-L, Schäfer A, Neumann J, Turner R, Forstmann BU, 2013. Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J Neurosci 33, 4896–4900. doi:10.1523/JNEUROSCI.3241-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuken MC, Bazin PL, Crown L, Hootsmans J, Laufer A, Müller-Axt C, Sier R, van der Putten EJ, Schäfer A, Turner R, Forstmann BU, 2014. Quantifying inter-individual anatomical variability in the subcortex using 7 T structural MRI. NeuroImage 94, 40–46. doi:10.1016/j.neuroimage.2014.03.032 [DOI] [PubMed] [Google Scholar]
- Kita T, Kita H, 2012. The subthalamic nucleus is one of multiple innervation sites for long-range corticofugal axons: a single-axon tracing study in the rat. J Neurosci 32, 5990–5999. doi:10.1523/JNEUROSCI.5717-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, Christensen GE, Collins DL, Gee J, Hellier P, Song JH, Jenkinson M, Lepage C, Rueckert D, Thompson P, Vercauteren T, Woods RP, Mann JJ, Parsey RV, 2009. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage 46, 786–802. doi: 10.1016/j.neuroimage.2008.12.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva L, Il'yasov KA, Skibbe H, Kiselev VG, Kellner E, Dhital B, Reisert M, 2018. Modelfree global tractography. NeuroImage. doi: 10.1016/j.neuroimage.2018.03.058 [DOI] [PubMed] [Google Scholar]
- Krause P, Brüggemann N, Völzmann S, Horn A, Kupsch A, Schneider GH, Lohmann K, Kühn A, 2015. Long-term effect on dystonia after pallidal deep brain stimulation (DBS) in three members of a family with a THAP1 mutation. J Neurol 262, 2739–2744. doi:10.1007/s00415-015-7908-z [DOI] [PubMed] [Google Scholar]
- Krause P, Lauritsch K, Lipp A, Horn A, Weschke B, Kupsch A, Kiening KL, Schneider GH, Kühn AA, 2016. Long-term results of deep brain stimulation in a cohort of eight children with isolated dystonia. J Neurol 263, 2319–2326. doi: 10.1007/s00415-016-8253-6 [DOI] [PubMed] [Google Scholar]
- Kroneberg D, Plettig P, Schneider G-H, Kühn AA, 2017. Motor Cortical Plasticity Relates to Symptom Severity and Clinical Benefit From Deep Brain Stimulation in Cervical Dystonia. Neuromodulation: Technology at the Neural Interface 27, 1789. doi: 10.1111/ner.12690 [DOI] [PubMed] [Google Scholar]
- Krauth A, Blanc R, Poveda A, Jeanmonod D, Morel A, Székely G, 2010. A mean three-dimensional atlas of the human thalamus: generation from multiple histological data. Neuroimage 49, 2053–2062. doi:10.1016/j.neuroimage.2009.10.042 [DOI] [PubMed] [Google Scholar]
- Kreher BW, Mader I, Kiselev VG, 2008. Gibbs tracking: a novel approach for the reconstruction of neuronal pathways. Magn Reson Med 60, 953–963. doi: 10.1002/mrm.21749 [DOI] [PubMed] [Google Scholar]
- Kuncel AM, Cooper SE, Grill WM, 2008. A method to estimate the spatial extent of activation in thalamic deep brain stimulation. Clin Neurophysiol 119, 2148–2158. doi:10.1016/j.clinph.2008.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laganiere S, Boes AD, Fox MD, 2016. Network localization of hemichorea-hemiballismus 86, 2187–2195. doi:10.1212/WNL.0000000000002741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauro PM, Vanegas Arroyave N, Huang L, Taylor PA, Zaghloul KA, Lungu C, Saad ZS, Horovitz SG, 2015. DBSproc: An open source process for DBS electrode localization and tractographic analysis. Hum Brain Mapp n/a–n/a. doi: 10.1002/hbm.23039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Morgan PS, Ashburner J, Smith J, Rorden C, 2016. The first step for neuroimaging data analysis: DICOM to NIfTI conversion. J. Neurosci. Methods 264, 47–56. doi: 10.1016/j.jneumeth.2016.03.001 [DOI] [PubMed] [Google Scholar]
- Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P, 2011. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson's disease. Brain 134, 359–374. doi: 10.1093/brain/awq332 [DOI] [PubMed] [Google Scholar]
- Lofredi R, Neumann W-J, Bock A, Horn A, Huebl J, Siegert S, Schneider G-H, Krauss JK, Kühn AA, 2018. Dopamine-dependent scaling of subthalamic gamma bursts with movement velocity in patients with Parkinson's disease. Elife 7, e31895. doi:10.7554/eLife.31895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio S, Fresard S, Adaszewski S, Kherif F, Chowdhury R, Frackowiak RS, Ashburner J, Helms G, Weiskopf N, Lutti A, Draganski B, 2016. New tissue priors for improved automated classification of subcortical brain structures on MRI. NeuroImage 130, 157–166. doi:10.1016/j.neuroimage.2016.01.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein KH, Neher PF, Houde J-C, Cote M-A, Garyfallidis E, Zhong J, Chamberland M, Yeh F-C, Lin YC, Ji Q, Reddick WE, Glass JO, Chen DQ, Feng Y, Gao C, Wu Y, Ma J, Renjie H, Li Q, Westin C-F, Deslauriers-Gauthier S, Gonzalez JOO, Paquette M, St-Jean S, Girard G, Rheault F, Sidhu J, Tax CMW, Guo F, Mesri HY, David S, Froeling M, Heemskerk AM, Leemans A, Bore A, Pinsard B, Bedetti C, Desrosiers M, Brambati S, Doyon J, Sarica A, Vasta R, Cerasa A, Quattrone A, Yeatman J, Khan AR, Hodges W, Alexander S, Romascano D, Barakovic M, Auria A, Esteban O, Lemkaddem A, Thiran J-P, Cetingul HE, Odry BL, Mailhe B, Nadar MS, Pizzagalli F, Prasad G, Villalon-Reina JE, Galvis J, Thompson PM, De Santiago Requejo F, Laguna PL, Lacerda LM, Barrett R, Dell'Acqua F, Catani M, Petit L, Caruyer E, Daducci A, Dyrby TB, Holland-Letz T, Hilgetag CC, Stieltjes B, Descoteaux M, 2017. The challenge of mapping the human connectome based on diffusion tractography. Nature Communications 2017. 8:1 8, 1349. doi:10.1038/s41467-017-01285-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, Hodge SM, Caviness VS, Faraone SV, Tsuang MT, Seidman LJ, 2006. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophrenia Research 83, 155–171. doi: 10.1016/j.schres.2005.11.020 [DOI] [PubMed] [Google Scholar]
- Marani E, Heida T, Lakke EAJF, Usunoff KG, 2008. The Subthalamic Nucleus Springer Science & Business Media, Berlin, Heidelberg. doi:10.1007/978-3-540-79462-2 [Google Scholar]
- Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, Flagg E, Chowdhury S, Poewe W, Mollenhauer B, Klinik P-E, Sherer T, Frasier M, Meunier C, Rudolph A, Casaceli C, Seibyl J, Mendick S, Schuff N, Zhang Y, Toga A, Crawford K, Ansbach A, De Blasio P, Piovella M, Trojanowski J, Shaw L, Singleton A, Hawkins K, Eberling J, Brooks D, Russell D, Leary L, Factor S, Sommerfeld B, Hogarth P, Pighetti E, Williams K, Standaert D, Guthrie S, Hauser R, Delgado H, Jankovic J, Hunter C, Stern M, Tran B, Leverenz J, Baca M, Frank S, Thomas C-A, Richard I, Deeley C, Rees L, Sprenger F, Lang E, Shill H, Obradov S, Fernandez H, Winters A, Berg D, Gauss K, Galasko D, Fontaine D, Mari Z, Gerstenhaber M, Brooks D, Malloy S, Barone P, Longo K, Comery T, Ravina B, Grachev I, Gallagher K, Collins M, Widnell KL, Ostrowizki S, Fontoura P, Ho T, Luthman J, Brug MVD, Reith AD, Taylor P, 2011. The Parkinson Progression Marker Initiative (PPMI). Prog. Neurobiol. 95, 629–635. doi: 10.1016/j.pneurobio.2011.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey LA, Miranda MA, Zrinzo L, Al-Helli O, Parkes HG, Thornton JS, et al. , 2012. High resolution MR anatomy of the subthalamic nucleus: Imaging at 9.4T with histological validation, 59(3), 2035–2044. http://doi.org/10.1016/j.neuroimage.2011.10.016 [DOI] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE, 2006. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta-analysis. NeuroImage 31, 1453–1474. doi: 10.1016/j.neuroimage.2006.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marburg O, 1904. Mikroskopisch-topographischer Atlas des menschlichen Zentralnervensystems mit begleitendem Texte. [Google Scholar]
- Mädler B, Coenen VA, 2012. Explaining Clinical Effects of Deep Brain Stimulation through Simplified Target-Specific Modeling of the Volume of Activated Tissue. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A2906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola A, Yeh F-C, Fellows-Mayle W, Weed J, Fernandez-Miranda JC, 2016. Human Connectome-Based Tractographic Atlas of the Brainstem Connections and Surgical Approaches. Neurosurgery 1–18. doi:10.1227/NEU.0000000000001224 [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, 2002. Extracellular Stimulation of Central Neurons: Influence of Stimulus Waveform and Frequency on Neuronal Output. J Neurophysiol 88, 1592–1604. doi: 10.1016/S0165-0173(99)00008-9 [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV, 2004. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J Neurophysiol 91, 1457–1469. doi:10.1152/jn.00989.2003 [DOI] [PubMed] [Google Scholar]
- Merkl A, Neumann W-J, Huebl J, Aust S, Horn A, Krauss JK, Dziobek I, Kuhn J, Schneider G-H, Bajbouj M, Kün AA, 2016. Modulation of Beta-Band Activity in the Subgenual Anterior Cingulate Cortex during Emotional Empathy in Treatment-Resistant Depression. Cereb. Cortex 26, 2626–2638. doi: 10.1093/cercor/bhv100 [DOI] [PubMed] [Google Scholar]
- Middlebrooks EH, Tuna IS, Grewal SS, Almeida L, Heckman MG, Lesser ER, et al. , 2018. Segmentation of the Globus Pallidus Internus Using Probabilistic Diffusion Tractography for Deep Brain Stimulation Targeting in Parkinson Disease. AJNR Am J Neuroradiol, 107, 64 http://doi.org/10.3174/ajnr.A5641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miocinovic S, Noecker AM, Maks CB, Butson CR, McIntyre CC, 2007. Cicerone: stereotactic neurophysiological recording and deep brain stimulation electrode placement software system. Acta Neurochir. Suppl. 97, 561–567. [DOI] [PubMed] [Google Scholar]
- Morel A, 2013. Stereotactic Atlas of the Human Thalamus and Basal Ganglia. CRC Press. [Google Scholar]
- Mosley PE, Marsh R, Perry A, Coyne T, Silburn P, 2018a. Persistence of Mania After Cessation of Stimulation Following Subthalamic Deep Brain Stimulation. J Neuropsychiatry Clin Neurosci appi.neuropsych. doi: 10.1176/appi.neuropsych.17060129 [DOI] [PubMed] [Google Scholar]
- Mosley PE, Smith D, Coyne T, Silburn P, Breakspear M, Perry A, 2018b. The site of stimulation moderates neuropsychiatric symptoms after subthalamic deep brain stimulation for Parkinson's disease. Neuroimage Clin. doi:10.1016/j.nicl.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann W-J, Horn A, Ewert S, Huebl J, Brücke C, Slentz C, Schneider G-H, Kühn AA, 2017. A localized pallidal physiomarker in cervical dystonia. Ann Neurol. doi:10.1002/ana.25095 [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Jha A, Bock A, Huebl J, Horn A, Schneider G-H, Sander TH, Litvak V, Kühn AA, 2015. Cortico-pallidal oscillatory connectivity in patients with dystonia. Brain 138, 1894–1906. doi:10.1093/brain/awv109 [DOI] [PubMed] [Google Scholar]
- Neumann W-J, Staub F, Horn A, Schanda J, Mueller J, Schneider G-H, Brown P, Kühn AA, 2016. Deep Brain Recordings Using an Implanted Pulse Generator in Parkinson's Disease. Neuromodulation: Technology at the Neural Interface 19, 20–24. doi: 10.1111/ner.12348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nooner KB, Colcombe SJ, Tobe RH, Mennes M, Benedict MM, Moreno AL, et al. 2012. The NKI-Rockland sample: a model for accelerating the pace of discovery science in psychiatry. Front. Neurosci 6 (152). http://doi.org/10.3389/fnins.2012.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen J-M, 2010. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput Intell Neurosci 2011, 1–9. doi: 10.1155/2011/156869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A, Jha A, Neal S, Reid A, Bradbury D, Aston P, Limousin P, Foltynie T, Zrinzo L, Brown P, Litvak V, 2016. Analysis of simultaneous MEG and intracranial LFP recordings during Deep Brain Stimulation: a protocol and experimental validation. J. Neurosci. Methods 261, 29–46. doi: 10.1016/j.jneumeth.2015.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli WM, Nili AN, Tyszka JM, 2017. A High-Resolution Probabilistic In Vivo Atlas of Human Subcortical Brain Nuclei. bioRxiv 211201. doi:10.1101/211201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisker C, Schüller T, Peters J, Wagner B, Schilbach L, Müller U, Visser-Vandewalle V, Kuhn J, 2018. Nucleus Accumbens Deep Brain Stimulation in Patients with Substance Use Disorders and Delay Discounting. Brain Sciences 8, 21–15. doi: 10.3390/brainsci8020021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipitone J, Park MTM, Winterburn J, Lett TA, Lerch JP, Pruessner JC, Lepage M, Voineskos AN, Chakravarty MM, Alzheimer's Disease Neuroimaging Initiative, 2014. Multi-atlas segmentation of the whole hippocampus and subfields using multiple automatically generated templates. NeuroImage 101, 494–512. doi: 10.1016/j.neuroimage.2014.04.054 [DOI] [PubMed] [Google Scholar]
- Pollo C, Villemure JG, Vingerhoets F, Ghika J, Maeder P, Meuli R, 2004. Magnetic resonance artifact induced by the electrode Activa 3389: an in vitro and in vivo study. Acta Neurochir (Wien) 146, 161–164. doi:10.1007/s00701-003-0181-4 [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Yu H, Little DM, Abraham I, Vaillancourt DE, 2008. Region of interest template for the human basal ganglia: Comparing EPI and standardized space approaches 39, 956–965. doi:10.1016/j.neuroimage.2007.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert M, Kiselev VG, Dihtal B, Kellner E, Novikov DS, 2014. MesoFT: Unifying Diffusion Modeling and Fiber Tracking, in: Medical Image Computing and Computer-Assisted Intervention – MICCAI 2014, Lecture Notes in Computer Science. Springer International Publishing, Cham, pp. 201–208. doi:10.1007/978-3-319-10443-0_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert M, Mader I, Anastasopoulos C, Weigel M, Schnell S, Kiselev V, 2011. Global fiber reconstruction becomes practical. NeuroImage 54, 955–962. doi:10.1016/j.neuroimage.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Richter EO, Hoque T, Halliday W, Lozano AM, & Saint-Cyr JA, 2004. Determining the position and size of the subthalamic nucleus based on magnetic resonance imaging results in patients with advanced Parkinson disease. Journal of Neurosurgery, 100(3), 541–546. http://doi.org/10.3171/jns.2004.100.3.0541 [DOI] [PubMed] [Google Scholar]
- Ruiz MH, Bin Hong S, Hennig H, Altenmüller E, Kühn AA, 2014. Long-range correlation properties in timing of skilled piano performance: the influence of auditory feedback and deep brain stimulation. Front Psychol 5, 377. doi: 10.3389/fpsyg.2014.01030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Yeo BTT, 2017. Local-Global Parcellation of the Human Cerebral Cortex from Intrinsic Functional Connectivity MRI. Cerebral Cortex 1–20. doi: 10.1093/cercor/bhx179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer A, Forstmann BU, Neumann J, Wharton S, Mietke A, Bowtell R, & Turner R, 2012. Direct visualization of the subthalamic nucleus and its iron distribution using high-resolution susceptibility mapping. Human Brain Mapping, 33(12), 2831–2842. http://doi.org/10.1002/hbm.21404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Grant P, Lowery M, van Rienen U, 2013. Influence of uncertainties in the material properties of brain tissue on the probabilistic volume of tissue activated. IEEE Trans. Biomed. Eng. 60, 1378–1387. doi:10.1109/TBME.2012.2235835 [DOI] [PubMed] [Google Scholar]
- Schmidt C, van Rienen U, 2012. Modeling the Field Distribution in Deep Brain Stimulation: The Influence of Anisotropy of Brain Tissue. Biomedical Engineering, IEEE Transactions on 59, 1583–1592. doi:10.1109/TBME.2012.2189885 [DOI] [PubMed] [Google Scholar]
- Schönecker T, Kupsch A, Kühn AA, Schneider G-H, Hoffmann KT, 2009. Automated Optimization of Subcortical Cerebral MR Imaging-Atlas Coregistration for Improved Postoperative Electrode Localization in Deep Brain Stimulation. AJNR Am J Neuroradiol 30, 1914–1921. doi:10.3174/ajnr.A1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroll H, Horn A, Gröschel C, Brücke C, Lütjens G, Schneider G-H, Krauss JK, Kühn AA, Hamker FH, 2015. Differential contributions of the globus pallidus and ventral thalamus to stimulus-response learning in humans. NeuroImage 122, 233–245. doi:10.1016/j.neuroimage.2015.07.061 [DOI] [PubMed] [Google Scholar]
- Setsompop K, Kimmlingen R, Eberlein E, Witzel T, Cohen-Adad J, McNab JA, Keil B, Tisdall MD, Hoecht P, Dietz P, Cauley SF, Tountcheva V, Matschl V, Lenz VH, Heberlein K, Potthast A, Thein H, Van Horn J, Toga A, Schmitt F, Lehne D, Rosen BR, Wedeen V, Wald LL, 2013. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage 80, 220–233. doi: 10.1016/j.neuroimage.2013.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin RK, Qureshi RA, Harris NR, Bakar D, Li TP, Jafri MS, Tang C-M, 2014. Wilbrand knee. Neurology 82, 459–460. doi:10.1212/WNL.0000000000000084 [DOI] [PubMed] [Google Scholar]
- Sitz A, Hoevels M, Hellerbach A, Gierich A, Luyken K, Dembek TA, Klehr M, Wirths J, Visser-Vandewalle V, Treuer H, 2017. Determining the orientation angle of directional leads for deep brain stimulation using computed tomography and digital x-ray imaging: A phantom study. Med. Phys. 44, 4463–4473. doi: 10.1002/mp.12424 [DOI] [PubMed] [Google Scholar]
- Sudhyadhom A, Haq IU, Foote KD, Okun MS, Bova FJ, 2009. A high resolution and high contrast MRI for differentiation of subcortical structures for DBS targeting: the Fast Gray Matter Acquisition T1 Inversion Recovery (FGATIR). NeuroImage 47 Suppl 2, T44–52. doi:10.1016/j.neuroimage.2009.04.018 [DOI] [PubMed] [Google Scholar]
- Tiedt HO, Ehlen F, Krugel LK, Horn A, Kühn AA, Klostermann F, 2016. Subcortical roles in lexical task processing: Inferences from thalamic and subthalamic event-related potentials. Hum Brain Mapp 38, 370–383. doi: 10.1002/hbm.23366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, Gee JC, 2010. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29, 1310–1320. doi: 10.1109/TMI.2010.2046908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, & Avants BB, 2013. Explicit B-spline regularization in diffeomorphic image registration. Frontiers in Neuroinformatics, 7, 39 http://doi.org/10.3389/fninf.2013.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M, 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. 15, 273–289. doi:10.1006/nimg.2001.0978 [DOI] [PubMed] [Google Scholar]
- van Wijk BCM, Pogosyan A, Hariz MI, Akram H, Foltynie T, Limousin P, Horn A, Ewert S, Brown P, Litvak V, 2017. Localization of beta and high-frequency oscillations within the subthalamic nucleus region. Neuroimage Clin 16, 175–183. doi: 10.1016/j.nicl.2017.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanegas Arroyave N, Lauro PM, Huang L, Hallett M, Horovitz SG, Zaghloul KA, Lungu C, 2016. Tractography patterns of subthalamic nucleus deep brain stimulation. Brain 139, 1200–1210. doi:10.1093/brain/aww020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasques X, Cif L, Hess O, Gavarini S, Mennessier G, Coubes P, 2009. Stereotactic model of the electrical distribution within the internal globus pallidus during deep brain stimulation. Journal of computational neuroscience 26, 109–118. doi: 10.1007/s10827-008-0101-y [DOI] [PubMed] [Google Scholar]
- Vorwerk J, Cho J-H, Rampp S, Hamer H, Knösche TR, Wolters CH, 2014. A guideline for head volume conductor modeling in EEG and MEG. NeuroImage 100, 590–607. doi: 10.1016/j.neuroimage.2014.06.040 [DOI] [PubMed] [Google Scholar]
- Vorwerk J, Oostenveld R, Piastra MC, Magyari L, Wolters CH, 2018. The FieldTrip-SimBio pipeline for EEG forward solutions. - PubMed - NCBI. BioMed Eng OnLine 17, 413. doi: 10.1186/s12938-018-0463-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BT, Poirier S, Guo T, Parrent AG, Peters TM, Khan AR, 2016. Generation and evaluation of an ultra-high-field atlas with applications in DBS planning. SPIE Medical Imaging 9784, 97840H–97840H–10. doi: 10.1117/12.2217126 [Google Scholar]
- Wang Y, Liu T, 2015. Quantitative susceptibility mapping (QSM): Decoding MRI data for a tissue magnetic biomarker. Magnetic Resonance in Medicine 73, 82–101. doi: 10.1002/mrm.25358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand A, Horn A, Caballero R, Cooke D, Stern AP, Taylor SF, Press D, Pascual-Leone A, Fox MD, 2017. Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. BPS 0, 888–894. doi:10.1016/j.biopsych.2017.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Weissenbacher C, 2009. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. 47, 1408–1416. doi: 10.1016/j.neuroimage.2009.05.005 [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM, 2009. Bayesian analysis of neuroimaging data in FSL. NeuroImage 45, S173–86. doi: 10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- Xiao Y, Fonov V, Chakravarty MM, Bériault S, Al-Subaie F, Sadikot A, Pike GB, Bertrand G, Collins DL, 2017. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson’s disease cohort. NeuroImage 12, 370–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh F-C, Wedeen VJ, Tseng W-YI, 2010. Generalized q-Sampling Imaging. IEEE Trans Med Imaging 29, 1626–1635. doi:10.1109/TMI.2010.2045126 [DOI] [PubMed] [Google Scholar]
- Yeh F-C, Panesar S, Fernandes D, Meola A, Yoshino M, Fernandez-Miranda JC, et al. 2018. Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57–68. http://doi.org/10.1016/j.neuroimage.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BTT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zollei L, Polimeni JR, Fischl B, Liu H, Buckner RL, 2011. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106, 1125–1165. doi:10.1152/jn.00338.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelnik J, Bardinet E, Dormont D, Malandain G, Ourselin S, Tandé D, Karachi C, Ayache N, Cornu P, Agid Y, 2007. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. NeuroImage 34, 618–638. doi: 10.1016/j.neuroimage.2006.09.026 [DOI] [PubMed] [Google Scholar]
- Yelnik J, Damier P, Demeret S, Gervais D, Bardinet E, Bejjani B-P, François C, Houeto J-L, Arnule I, Dormont D, Galanaud D, Pidoux B, Cornu P, Agid Y, 2003. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg 99, 89–99. doi:10.3171/jns.2003.99.1.0089 [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET, 2010. Network-based statistic: identifying differences in brain networks. 53, 1197–1207. doi: 10.1016/j.neuroimage.2010.06.041 [DOI] [PubMed] [Google Scholar]
- Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME, 2008. Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol 100, 1740–1748. doi:10.1152/jn.90463.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Larcher KM-H, Misic B, Dagher A, 2017. Anatomical and functional organization of the human substantia nigra and its connections. Elife 6, e26653. doi: 10.7554/eLife.26653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Amunts K, 2010. Centenary of Brodmann's map--conception and fate., Nature reviews. Neuroscience. doi:10.1038/nrn2776 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.