SUMMARY
The mitochondrial respiratory chain is organized in a dynamic set of supercomplexes (SCs). The COX7A2L protein is essential for mammalian SC III2+IV assembly. However, its function in respirasome (SCs I+III2+IVn) biogenesis remains controversial. To unambiguously determine the COX7A2L role, we generated COX7A2L-knockout (COX7A2L-KO) HEK293T and U87 cells. COX7A2L-KO cells lack SC III2+IV but have enhanced complex III steady-state levels, activity, and assembly rate, normal de novo complex IV biogenesis, and delayed respirasome formation. Nonetheless, the KOs have normal respire some steady-state levels, and only larger structures (SCs I1–2+III2+IV2-n or megacomplexes) were undetected. Functional substrate-driven competition assays showed normal mitochondrial respiration in COX7A2L-KO cells in standard and nutritional-, environmental-, and oxidative-stress-challenging conditions. We conclude that COX7A2L establishes a regulatory checkpoint for the biogenesis of CIII2 and specific SCs, but the COX7A2L-dependent MRC remodeling is essential neither to maintain mitochondrial bioenergetics nor to cope with acute cellular stresses.
Graphical Abstract
In Brief
The role of COX7A2L in mitochondrial respiratory chain supercomplex biogenesis and function remains controversial. By analyzing COX7A2L- knockout human cells, Lobo-Jarne et al. report that this protein promotes specific respiratory chain complex assembly and organization remodeling but does not affect mitochondrial bioenergetics in physiological, nutritional, or oxidative stress conditions.
INTRODUCTION
The mitochondrial respiratory chain (MRC) consists of four enzymatic multimeric complexes (CI to CIV) and two mobile electron carriers (coenzyme Q and cytochrome c), which catalyze electron transfer from reducing equivalents (NADH and FADH2) to molecular oxygen. The process is coupled to the generation of a proton gradient that drives ATP synthesis by the ATP synthase through oxidative phosphorylation (OXPHOS). The proton-pumping complexes (CI, CIII, and CIV) can assemble into higher supramolecular structures known as supercomplexes (SCs) (Cruciat et al., 2000; Schägger and Pfeiffer, 2000). Mammalian CI is primarily found assembled in SCs, either interacting with the CIII dimer (CIII2) to form SC I+III2, or with both CIII2 and CIV to form SC I+III2+IV1, to which additional CIV monomers can be added. These structures are known as the respirasomes (Schägger and Pfeiffer, 2000), since they were initially proposed to contain all the components required to transfer electrons from NADH to molecular oxygen (Ac´ın-Pérez et al., 2008). In addition, CIII2 and CIV form the scarce SC III2+IV that coexists with the relatively abundant SCs I+III2+IV0–4, as well as with CIII2, CIV2, and monomeric CIV (Lobo-Jarne and Ugalde, 2018). The structural and functional organization of the MRC complexes is currently considered to be dynamic, where the proportion of free complexes and SCs is possibly modulated to adapt ATP production to changing cellular metabolic demands and environmental conditions (Acin-Perez and Enriquez, 2014). However, the functional roles of the SCs remain intriguing. The general arrangement of the MRC in SCs was initially suggested to confer catalytic advantages to the system, as it was proposed to enhance the electron flux through substrate channeling (Bian-chi et al., 2004), and to allow optimization of the available metabolic substrates via partitioned coenzyme Q and cyto- chrome c pools (Lapuente-Brun et al., 2013). However, direct spectroscopic studies argued in favor of single coenzyme Q and cytochrome c pools (Blaza et al., 2014; Trouillard et al., 2011), and a definitive demonstration that quinone and quinol diffuse freely in and out of SCs has recently come from studies that incorporated an alternative quinol oxidase into mammalian heart mitochondrial membranes and showed to establish a competing pathway for quinol oxidation (Fedor and Hirst, 2018). Therefore, the catalytic relevance of the SCs remains questioned (Lobo-Jarne and Ugalde, 2018; Milenkovic et al., 2017). In addition, the I+III2+IVn respirasomes have been proposed to stabilize CI (Moreno-Lastres et al., 2012; Scha¨ gger et al., 2004) and to prevent the production of CI-derived reactive oxygen species (ROS) (Maranzana et al., 2013). The recently defined high-resolution cryoelectron microscopy (cryo-EM) reconstructions of the mammalian respirasome (Gu et al., 2016; Letts et al., 2016; Sousa et al., 2016; Wu et al., 2016) are expected to be instrumental to unveil the potential functional properties of the SCs. Structural studies of the SC I+III2+IV1 from bovine, ovine, and porcine heart mitochondria showed that CIV is positioned at the distal end of the membrane arm of CI and adjacent to CIII2. In addition, structural analyses in HEK293 cells showed the first architecture of the human respirasome, as well as the arrangement of the MRC complexes in a novel circular structure termed the megacomplex I2+III2+IV2 (Guo et al., 2017), where CIII2 forms a central core surrounded by two copies each of CI and CIV. The availability of these structures has only reinforced the intrigue about the biogenetic mechanisms and players that trigger the formation of the SCs. Besides the presence of the phospholipid cardiolipin (Mileykovskaya and Dowhan, 2014), SCs formation requires the action of specific assembly factors both in yeast (Chen et al., 2012; Singhal et al., 2017; Strogolova et al., 2012; Vukotic et al., 2012) and mammals (Chen et al., 2012; Davoudi et al., 2016; Ikeda et al., 2013; Lapuente-Brun et al., 2013; Mourier et al., 2014; Pérez-Pérez et al., 2016), although their specific functions remain under debate.
A controversial case involves the role of the protein COX7A2- like (COX7A2L, SCAFI, or COX7RP), highly homologous to the CIV subunit COX7A. COX7A2L was initially proposed to be central for the inclusion of CIV in SCs III2+IV and I+III2+IV1–4 (Lapuente-Brun et al., 2013). Two COX7A2L variants were detected in commonly used laboratory mouse strains: a full-length 113-amino acids (long) protein present in CD1 mice, and a 111- amino acids (short) protein present in the C57BL/6 and BALB/c strains that missed two conserved residues (V72-P73) important for its stability and function in SCs formation (Lapuente-Brun et al., 2013). However, several groups reported the presence of respirasomes in C57BL/6 mice (Barrientos and Ugalde, 2013; Ikeda et al., 2013; Lapuente-Brun et al., 2013; Mourier et al., 2014; Williams et al., 2016), and cultured human cells silenced for COX7A2L expression also showed a specific requirement of COX7A2L for SC III2+IV assembly but not for respirasomes accumulation (Pérez-Pérez et al., 2016). These studies highlighted the preferential interaction of COX7A2L with CIII2 and to a minor extent with monomeric CIV (Pérez-Pérez et al., 2016), which suggested an additional CIII-related mechanism of action for COX7A2L. There is currently a consensus that the full-length COX7A2L is required to promote SC III2+IV formation and that it does so by binding independently to both CIII2 and CIV (Cogliati et al., 2016; Pérez-Pérez et al., 2016; Zhang et al., 2016). It is also agreed that COX7A2L depletion does not affect the accumulation of the respirasomes I+III2+IV1–4 in some mouse tissues such as heart, as well as in COX7A2L- silenced human cells (Mourier et al., 2014; Pérez-Pérez et al., 2016; Williams et al., 2016). Interestingly, the short COX7A2L iso- form in C57BL/6 mice induced tissue-specific differences in the levels of the larger respirasomes I+III2+IV2–4, which were less abundant in liver than in heart mitochondria (Williams et al., 2016). Even the respirasome I+III2+IV1 was shown to be unstable in some tissues according to some reports (Cogliati et al., 2016), but not to others (Davoudi et al., 2016; Mourier et al., 2014; Sun et al., 2016; Williams et al., 2016). Based on proteomics analyses of the SCs subunit composition, it was hypothesized that the expression of tissue-specific isoforms of CIV subunits (e.g heart-muscle/liver COX7A1/COX7A2) could substitute COX7A2L in the respirasomes, inducing slight structural alterations in the CIV holocomplex that would differentially affect the assembly of the SCs in the presence of a specific COX7A variant (Cogliati et al., 2016), a possibility that remains to be experimentally demonstrated.
To unambiguously determine the function of COX7A2L in the organization of the human respiratory chain, we have used transcription activator-like effector nucleases (TALENs) technology to generate stable human COX7A2L-knockout (COX7A2L-KO) lines in HEK293T and in glioblastoma U87 cells, which were complemented either with the wild-type (WT) human COX7A2L, or with a mutant variant carrying an in-frame 6-bp deletion similar to that previously identified in the C57BL/6 mouse strain. Our results confirm that WT (long) COX7A2L is specifically required for the assembly of SC III2+IV and the accumulation of megacomplexes, but dispensable for respirasome (or SCs I+III2+IV1) biogenesis, and reconcile an array of previous observations. De novo assembly studies show that COX7A2L regulates the assembly kinetics of the respirasomes probably through the modulation of CIII2 levels. Biochemical data also demonstrate that the mutant COX7A2L variant does not support SC III2+IV assembly because, despite being able to bind to both CIII2 and SC I+III2, it cannot interact with CIV or CIV-containing SCs. Functional substrate competition assays showed no differences in mitochondrial respiration between control and COX7A2L-KO cells, even under conditions of nutritional, environmental, or oxidative cellular stress. We conclude that by preventing the formation of SC III2+IV, COX7A2L establishes a checkpoint for the regulation of CIII2 levels and its incorporation into specific SCs. Most importantly, COX7A2L promotes MRC remodeling without affecting mitochondrial bioenergetics.
RESULTS
TALEN-Mediated Generation of COX7A2L-KO Cell Lines
To determine the requirement of human COX7A2L for mitochondrial SCs formation, and specifically for the assembly of the respirasomes, we used the TALEN gene-editing approach (Christian et al., 2010; Li et al., 2011) to create stable human COX7A2L-KO lines in HEK293T (human embryonic kidney) cells. A TALEN pair was designed to target a region within the first exon of the COX7A2L gene immediately downstream the start codon (Figure 1A). We co-transfected HEK293T cells with the TALEN pair, and subsequently, single clones were isolated by size cell sorting and analyzed for mutations leading to COX7A2L protein loss. More than 100 clones were screened by immunoblotting from cell extracts using a specific anti- COX7A2L antibody. We selected five promising candidates that completely lack the COX7A2L protein (Figure 1B). The COX7A2L gene was sequenced in two of these clones and found to carry KO mutations. Clone KO1 (C2E11) is homozygous and clone KO2 (C1C3) is compound heterozygous, both carrying COX7A2L alleles with short deletions involving the start codon and leading to the complete absence of COX7A2L (Figure 1C).
Figure 1. TALEN-Mediated Generation of COX7A2L-KO Clones in HEK293T Cells.
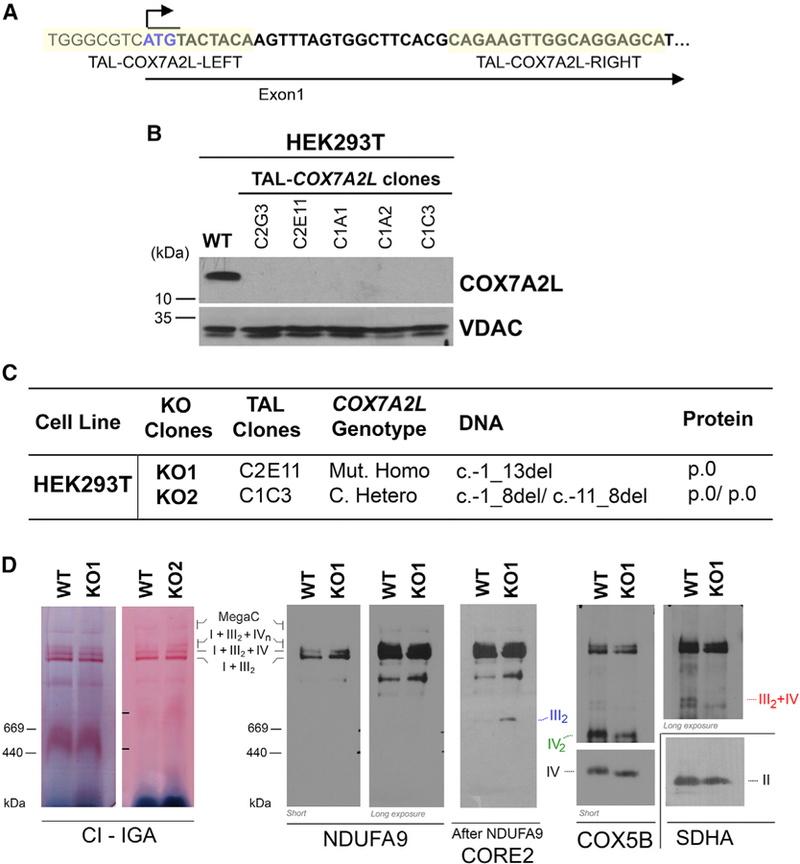
(A) Schematic representation of the first exon of the COX7A2L locus and the sequences of recognition sites of the two TALEN pairs.
(B) Immunoblot analysis of the steady-state levels of COX7A2L in HEK293T (WT) and TALEN-transfected HEK293T cell lines. VDAC was used as a loading control.
(C) COX7A2L alleles in TAL-COX7A2L clones. The DNA numbering refers to the coding sequence (c.) and the protein (p.) number to the predicted full polypeptide (den Dunnen and Antonarakis, 2000). C, compound; Mut, mutant; Hetero, heterozygous; Homo, homozygous; del, deletion; -, position before starting ATG.
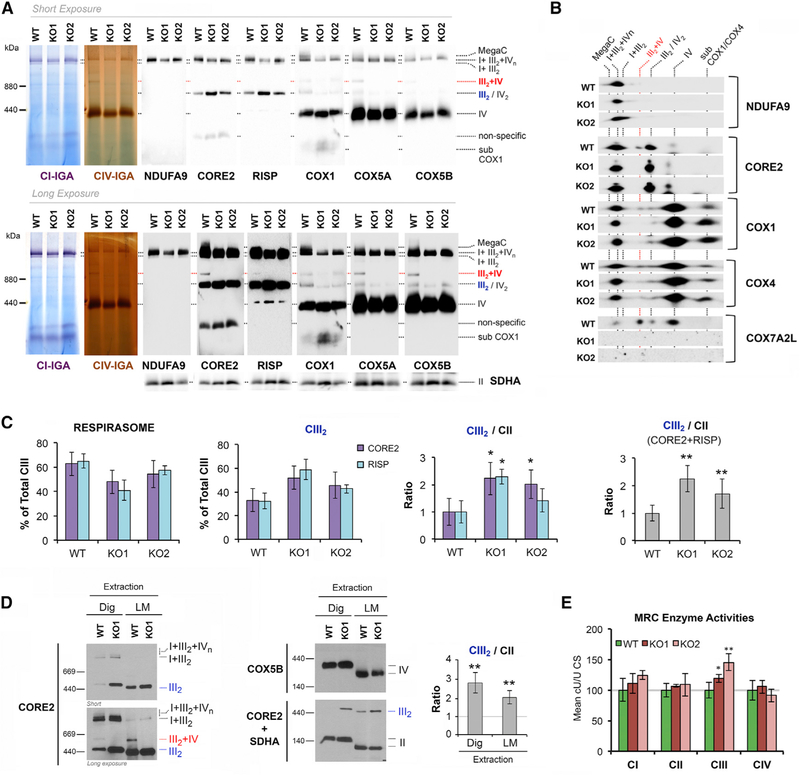
(D) BN-PAGE analysis of whole cells extracted with digitonin (detergent/protein ratio, 4:1) separated in a 4%–8% linear gradient polyacrylamide gel, followed by CI in-gel activity (IGA) or immunoblotting with the indicated antibodies. The identity of MRC complexes and SCs is indicated in the margins. MegaC, megacomplexes probably containing more than one copy of CI, CIII2, and CIV. See also Figure S1.
COX7A2L-KO Cells Display Absence of SC III2+IV with Normal Respirasome Levels and Increased CIII2 Levels and Activity
To analyze the pattern of supramolecular assemblies of MRC complexes resulting from the total absence of COX7A2L in human cells, we performed a first exploration in digitonin-solubilized cell extracts from WT and COX7A2L-KO cells by blue native (BN)-PAGE followed by CI-in gel activity (IGA) and immunoblotting. Our results unambiguously show that human COX7A2L is essential for the formation of SC III2+IV, but its loss does not affect the basic respirasome (or SCs I+III2+IV1) that accumulates equally in WT and KO cells (Figure 1D). Similar results were obtained when using mitochondria-enriched fractions from WT HEK293T, KO1, and KO2 clones (Figure 2A). However, some scarce SCs larger than the basic respirasome I+III2+IV1, occasionally appeared to be at lower levels in some experiments with HEK293T cells. These SCs could contain multiple CIV units (I+III2+IV2–4) but are also compatible with the recently described respiratory megacomplex I2+III2+IV2 (Guo et al., 2017). For simplification, in the figures we have labeled them as MegaC (megacomplexes). The loss of these larger SCs was clearly re-produced in a different model of human COX7A2L-KO in U87 glioblastoma cells (see below), suggesting that these MRC structures fail to assemble in the absence of functional COX7A2L, but might also be more labile than in WT mitochondria.
Figure 2. COX7A2L-KO Cells Display Absence of SC III2+IV with Normal Respirasome Levels and Altered Accumulation of CIII2.
The effect of COX7A2L absence on MRC complex assembly was investigated in two COX7A2L-KO clones, clone 1 (KO1) and clone 2 (KO2), compared with the control HEK293T cells (WT).
(A) Mitochondria extracted with a digitonin/protein ratio of 4:1 (g/g) and analyzed by BN-PAGE, followed by CI- and CIV-IGA assays, or alternatively, by immunoblotting using the indicated antibodies.
(B) Subsequent 2D-BN/SDS-PAGE and immunoblot analyses were performed with antibodies against COX7A2L and the indicated OXPHOS subunits.
(C) To address the relative amount of CIII2 in COX7A2L-KO cells, the signals from the CORE2 antibody from four BN-PAGE experiments were quantified by densitometry, normalized by CII, and indicated as mean ± SD.
(D) BN-PAGE analyses in whole-cell extracts prepared in the presence of digitonin (detergent/protein ratio, 4:1) or 1% lauryl maltoside (LM). The CIII2 signals were quantified and normalized by CII using the histogram function of the Adobe Photoshop program on digitalized images, and the values were expressed relative to the control. Error bars represent the mean ± SD of four independent experiments.
(E) Spectrophotometric measurements of the individual activities of MRC complexes I to IV (CI–CIV) in WT and COX7A2L-KO cells. Enzyme activities are expressed as cU/U citrate synthase (CS). Error bars represent the mean ± SD of four repetitions. *p < 0.05; **p < 0.01. MegaC, megacomplexes probably containing more than one copy of CI, CIII2, and CIV. I+III2+IVn, SCs containing CI, CIII2, and CIV. I+III2, SC containing CI and CIII2. III2+IV, SC containing CIII2 and CIV. III2, complex III dimer (CIII2). IV, complex IV; IV2, complex IV dimer (CIV2). II, complex II. Subcomplexes that contain COX1 and COX4 are indicated. Apparent subcomplexes that contain CORE2 are antibody artifacts that disappear in 2D-BN/SDS-PAGE gels. See also Figure S2.
To assess whether the stability of the respirasomes depends on COX7A2L, we exposed mitochondria-enriched fractions from WT and COX7A2L-KO cells to varying concentrations of digitonin and analyzed the extracts by BN-PAGE followed by CI- and CIV-IGAs or immunoblotting (Figure S1). Stringent SCs extraction conditions using increasing (4–40 mg/mg) digitonin-to-protein ratios (Figure S1A) led to a parallel disintegration of the respirasomes (SCs I+III2+IVn) and consequent accumulation of SC I+III2 and free CI in both cell types. Only when we used an extremely harsh 40 mg/mg digitonin:protein ratio were the respirasomes slightly more unstable in COX7A2L-KO than in WT cells. Milder SCs extraction conditions using decreasing (4–1 mg/mg) digitonin-to-protein ratios, revealed no differences in the levels of SC I+III2+IV1 between COX7A2L-KO and WT cells (Figure S1B), but a clear decrease in the abundance of larger SCs (indicated with an asterisk) in COX7A2L-KO cells (Figures 2A and 2B), further suggesting that COX7A2L may be required for the normal assembly or stability of these megastructures. On the contrary, the abundance and stability of monomeric and dimeric CIV were not affected by the absence of COX7A2L (Figures 2A and 2B).
We next used a two-dimensional (2D)-BN/SDS-PAGE system to analyze in detail the pattern of SCs in COX7A2L-KO cells and the co-localization of COX7A2L with all MRC structures in WT cells. Although we had previously reported that COX7A2L associates with pre-CIII2 before the incorporation of the RISP subunit(Pérez-Pérez et al., 2016), RISP assembly is not affected in COX7A2L-KO cells as it was detected in CIII2, and in SCs I+III2 and I+III2+IV1-n (Figure 2A). However, the accumulation of CIII2 was significantly increased by ~2-fold in the absence of COX7A2L (Figures 1D, 2A–2D, S1A, and S1B). The increase in CIII2 levels was not only a consequence of redistribution from the unassembled SC III2+IV. A boost in CIII2 levels was detected when using COX7A2L-KO mitochondrial extracts prepared not only in the presence of digitonin but also in the presence of lauryl maltoside (Figure 2D), which disrupts SC integrity, indicating that the total amount of assembled CIII2 is increased. In agreement, spectrophotometric measurements of MRC enzyme activities showed that CIII activity was specifically enhanced by ~40% in the KOs (Figure 2E), consistent with the accumulation of CIII2 in these cells.
All of the phenotypes described in COX7A2L-KO cells in this section, particularly the absence of SC III2+IV and the accumulation of CIII2, were specific since they were restored by the over- expression of recombinant COX7A2L-Myc-DDK in both KOs (Figures S2A–S2C), thus eliminating the possibility of off-target effects that could have arisen during the COX7A2L gene disruption. Tagged-COX7A2L incorporated into the same MRC structures as the endogenous COX7A2L (Figure S2B), and its mild overexpression (2- to 3-fold) did not induce any aberrant pheno- type (Figure S2).
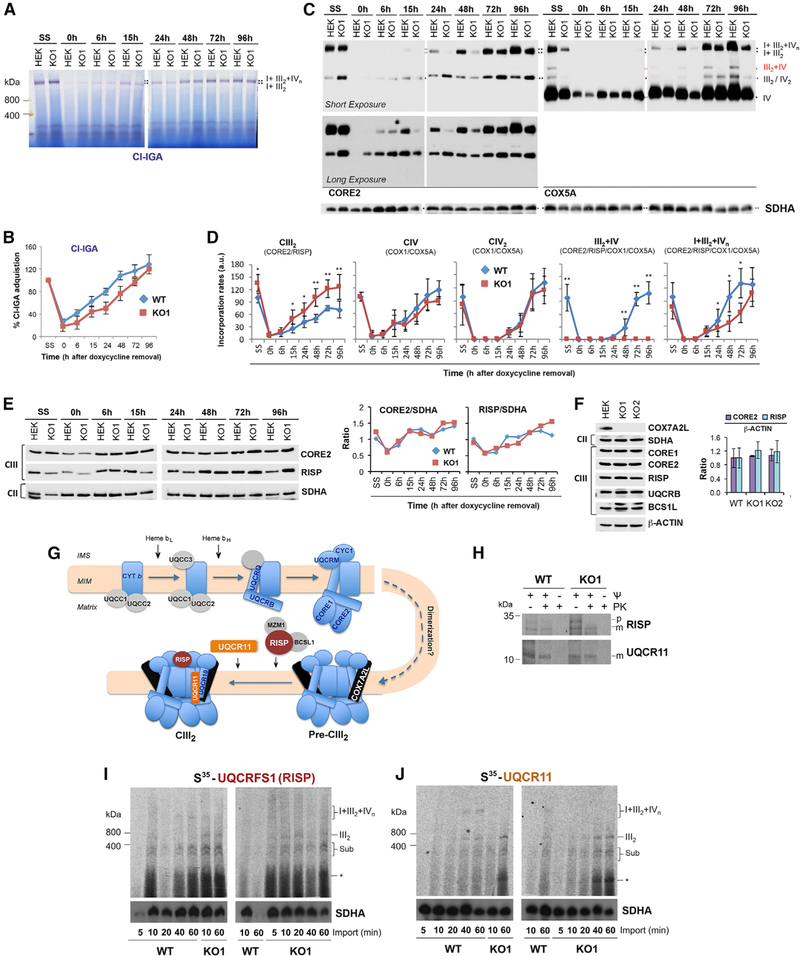
COX7A2L-KO Cells Display a Boost in CIII2 Biogenesis in Parallel with Slower Respirasome Assembly Kinetics
We next analyzed the assembly kinetics of MRC complexes and SCs by doxycycline-induced reversible inhibition of mitochondrial translation in WT and COX7A2L-KO cells. Doxycycline was removed from the cell culture media after 6 days of treatment, and samples were collected at different time points (0, 6, 15, 24, 48, 72, and 96 hr). To follow the reappearance of newly assembled CIII2, CIV, CIV2, and SCs, digitonin-solubilized mitochondria were separated by BN-PAGE and subsequently analyzed by either CI-IGA assays (Figures 3A and 3B) or immunoblot using antibodies that recognize CORE2 (CIII), COX1 (CIV; not shown), and COX5A (CIV) (Figures 3C and 3D). Following 6 days of doxycycline treatment (time 0 hr), only residual levels of CI, CIV, and CIII2 (5%–10% of untreated cells) were detected in either control or KO cells (Figures 3A–3D). The treatment did not affect CII levels, as expected, since CII lacks mtDNA-encoded subunits. Once mitochondrial translation resumed (times 6–96 hr), the SC III2+IV formed de novo only in the WT cells (Figures 3C and 3D). The levels of CIV and CIV2 increased at similar rates in WT and COX7A2L-KO cells (Figures 3C and 3D), ruling out a role for COX7A2L in the biogenesis of this complex. On the contrary, the rate of CIII2 biogenesis, as shown for its steady-state levels in Figure 2, was markedly faster in the KOs than in the WT cells (Figures 3C and 3D). These data indicate a deregulation of CIII2 levels in the absence of COX7A2L, suggesting that this protein establishes a regulatory checkpoint in CIII2 biogenesis. Immunoblot analysis of CIII subunit levels during the recovery from the doxycycline treatment (Figure 3E) and in the steady state (Figure 3F) showed no significant differences in the steady-state levels of CIII subunits between WT and KOs, which indicate that a larger pool of unassembled sub- units may exist in the WT cells. Therefore, we propose that the CIII2 increase in the KOs is a consequence of a more efficient CIII2 assembly/stability rather than increased de novo synthesis of CIII subunits. Strikingly, the reappearance of the respirasomes I+III2+IVn was clearly delayed in the COX7A2L-KO cells (Figures 3A–3D), but it reached levels comparable to the WT at later time points (78–96 hr; Figures 3A–3D) and in the steady state (Figure 2). These results suggest that, although the lack of COX7A2L does not prevent the formation of the respirasomes, it hampers their assembly efficiency perhaps by increasing the threshold of CIII2 levels required to start the process (Moreno-Lastres et al., 2012).
Figure 3. COX7A2L-KO Cells Have Enhanced Rate of De Novo CIII2 Biogenesis and Delayed Respirasomes Formation.
(A–E) HEK293T (WT) and COX7A2L-KO clone 1 (KO1) cells were cultured for 8 days in the presence of 15 mg/mL doxycycline and collected at different time points (0, 6, 15, 24, 48, 72, and 96 hr) after doxycycline removal. Mitochondria prepared from these samples were extracted with a digitonin/protein ratio of 4 g/g and analyzed by BN-PAGE in combination with (A) CI-IGA assays or (C) immunoblotting with the indicated antibodies. (B) Mean CI activity recovery after doxycycline removal quantified from (A).
(C) Mean incorporation rates of CORE2 subunit in CIII2, and of COX1 (not shown) and COX5A subunits in CIV and CIV2, or their assembly kinetics in the I+III2+IV1-n respirasomes.
(D) The signals from three independent experiments (as in C) for WT and KO cells were quantified and normalized by CII. Time point values are expressed as percentages of the untreated cells (SS) and indicated as means ± SD. *p < 0.05, **p < 0.01. I+III2+IVn, SCs containing CI, CIII2, and CIV. III2+IV, SC containing CIII2 and CIV; III2, complex III dimer (CIII2); IV, complex IV; IV2, complex IV dimer (CIV2); II, complex II.
(E) For two doxycycline experiments, samples were analyzed by SDS-PAGE for the steady-state levels of CIII subunits CORE2 and RISP. On the right panel, the signals were quantified and plotted as ratio of SDHA. The values for the two independent experiments did not differ by more than 5%.
(F) Steady-state levels of the indicated CIII subunits in HEK293T WT, KO1, and KO2 cell lines. On the right panel, the signals of CORE2 and RISP were quantified and expressed as ratio of the signal of ACTIN, used as a loading control. Error bars represent the mean ± SD of three independent experiments.
(G) Simplified current model of CIII assembly depicting the order of subunit incorporation and time of dimerization, modified from Ferna´ ndez-Vizarra and Zeviani (2015).
(H) In organello import of the indicated recombinant proteins synthesized in a reticulocyte system in the presence of 35S-methionine. The import assays were performed for 30 min in the absence or presence of the uncoupler CCCP to disrupt the mitochondrial membrane potential (J). Following import, an aliquot was treated with proteinase K to digest non-imported precursor proteins. M, mature; p, precursor.
(I and J) BN-PAGE analysis of the incorporation of the indicated radiolabeled recombinant proteins into CIII assembly intermediates, dimer, and SCs in HEK293T WT and KO1 cells during increasing times from 5 to 60 min. Import assays were performed in duplicates with similar results. Sub, subassemblies. The asterisk indicates small subassemblies that may correspond to the protein being imported bound to specific chaperones.
To validate the hypotheses raised from the doxycycline experiments, we took into account the current model for CIII2 assembly (Figure 3G), which includes the binding of COX7A2L to pre-CIII2 prior to the incorporation of subunits UQCRFS1 and UQCR11 (Ferna´ ndez-Vizarra and Zeviani, 2015; Pérez-Pérez et al., 2016). The process of CIII2 biogenesis, however, is not known in detail. For example, it remains unclear at which stage CIII2 dimerization occurs. Here, we performed import/assembly assays in isolated WT and COX7A2L-KO mitochondria with specific 35S-methionine-radiolabeled CIII precursors synthesized in vitro, in the presence and absence of mitochondrial membrane potential (Figure 3H). We chose to synthesize and import RISP (UQCRFS1; Figure 3I) and UQCR11 (Figure 3J), two late assembly proteins that are known to incorporate after the binding of COX7A2L to pre-CIII2. In WT mitochondria, UQCRFS1 and UQCR11 incorporated into several subcomplexes up to the CIII2 and followed by later incorporation into SCs (I+III2 and I+III2+IVn). In COX7A2L-KO mitochondria, UQCRFS1 and UQCR11 incorporated more efficiently into the same intermediates as well as into CIII2 than in WT mitochondria. On the contrary, the incorporation signals of these two radiolabeled proteins into SCs were less marked in the KO than in WT cells. Together, our import data show enhanced incorporation of newly imported proteins into CIII2 assembly intermediates and attenuated formation of CIII2-containing SCs in the COX7A2L-KO, suggesting that endogenous CIII2 biogenesis is preferentially boosted and assembly intermediates are more abundant in COX7A2L-KO than in WT mitochondria.
Mitochondrial Bioenergetics in COX7A2L-KO Cells Is Indistinguishable from WT in Normal Physiological Conditions or under CI Deficiency
To ascertain the functional consequences of COX7A2L and SC III2+IV depletion, we performed high-resolution endogenous oxygraphy to measure coupled cell respiration in intact cells, which is unaffected in the COX7A2L-KO clones (not shown).
Furthermore, substrate-driven competition was determined by treating digitonin-permeabilized cells supplemented with NADH- linked substrates (pyruvate, glutamate, malate) and FADH2-linked substrates (succinate, glycerol-3-phosphate), with specific inhibitors of CI (piericidin A or rotenone) or CII (malonate). This experimental procedure allowed us to determine the respective contribution of CI and CII in feeding the respiratory chain (RC) with electrons during uncoupled respiration (Figures 4A–4D). In contrast with previous results in liver mitochondria from C57BL/6 mice (Lapuente-Brun et al., 2013) and in line with results obtained by some of us in liver and heart mitochondria from C57BL/6J and C57BL/6N mice (Mourier et al., 2014), substrate-driven competition was not affected in the COX7A2L-KO clones. Also, no effect on respiration resulted from COX7A2L overexpression (Figures 4A–4D). These results indicate that, in standard cell culture conditions, neither COX7A2L nor SC III2+IV have a sub- stantial functional effect on electron flow through the MRC.
Figure 4. COX7A2L-KO Cells Are Capable of Normal OXPHOS Performance.
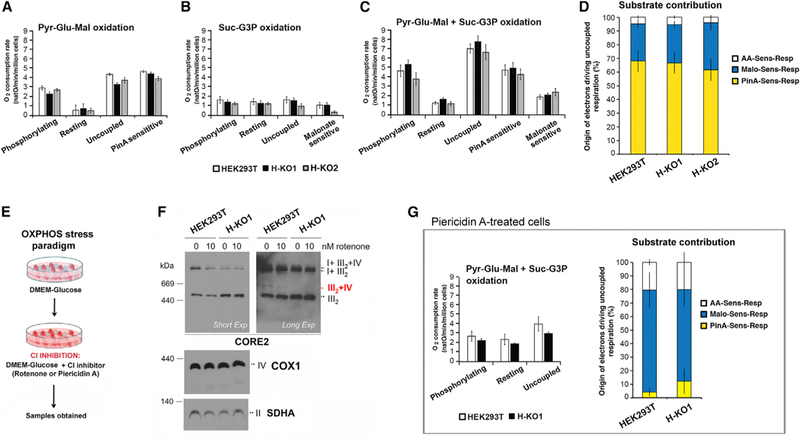
Respiration of permeabilized COX7A2L KO and control HEK cells cultivated on DMEM high glucose (A–D) or DMEM high glucose supplemented with 1.2 mM piericidin A, a CI inhibitor (G).
(A) Respiration of digitonin-permeabilized HEK293T cells assessed in the presence of Pyr-Glu-Mal: pyruvate (10 mM), glutamate (10 mM), and malate (5 mM) under different respiratory states (phosphorylating, resting, and uncoupled) and piericidin A-sensitive respiration (PinA sens).
(B) Respiration of digitonin-permeabilized HEK293T cells assessed in the presence of Suc-G3P: succinate (10 mM) and glycerol-3-phosphate (5 mM) under different respiratory states (phosphorylating, resting, and uncoupled) and malonate-sensitive respiration (Malo sens).
(C) Respiration of digitonin-permeabilized HEK cells incubated with Pyr-Glu-Mal and Suc-G3P. The Piericidin A- (PinA sens) and Malonate-sensitive (Malo sens) respiration are determined under uncoupled conditions.
(D) Respective contribution of NADH and succinate dehydrogenases in providing electrons to sustain uncoupled respiration assessed with all substrates under uncoupled state.
(E) OXPHOS stress paradigm based on in cello CI inhibition with rotenone or piericidin A.
(F) Effect of 24-hr incubation in the presence of 50 nM rotenone in HEK293T WT and KO1 cells on SC stability, analyzed in digitonized cell extracts by BN-PAGE and immunoblotting with the indicated antibodies.
(G) Respiration analysis of HEK cells cultivated during 48 hr in presence of CI inhibitor (piericidin A). The respiration of digitonin-permeabilized cells incubated with Pyr-Glu-Mal and Suc-G3P assessed under different respiratory states (phosphorylating, resting, and uncoupled). The piericidin A (PinA sens)-, malonate (Malo sens)-, and remaining antimycin A (AA)-sensitive respirations are determined under uncoupled conditions. The graph on the right side represents the respective contribution of CI and CII in providing electrons to sustain uncoupled respiration. In all the panels, error bars represent the mean ± SD of four biological repetitions. See also Figure S3.
In human cells, more than 90% of CI is present in the respirasomes and the SC III2+IV only constitutes ~5% of the total amount of MRC structures (Moreno-Lastres et al., 2012). Taking this MRC organization into account, we contemplated that, by limiting the enzymatic activity of CI, we could discern whether the absence of SC III2+IV and the accumulation of CIII2 in the COX7A2L-KO clones affect substrate competitive oxidation. To induce a partial inhibition of CI activity, we first treated the cells with 10 nM rotenone, 1.2 mM piericidin A, or the control vehicle (0.05% or 8.5 mM ethanol) for 24 hr (Figure 4E). CI inhibition did not significantly alter the distribution of MRC complexes and SCs in WT or KO cells (Figure 4F). Piericidin A treatment resulted in a comparable ~50% decrease in pyruvate-glutamate-malate + succinate-glycerol-3-phosphate (G3P) oxidation and similar substrate contribution in WT and KO digitonin-per-meabilized cells (Figure 4G).
Mitochondrial Bioenergetics in COX7A2L-KO Cells Is Indistinguishable from WT under Nutritional or Environmental Stress
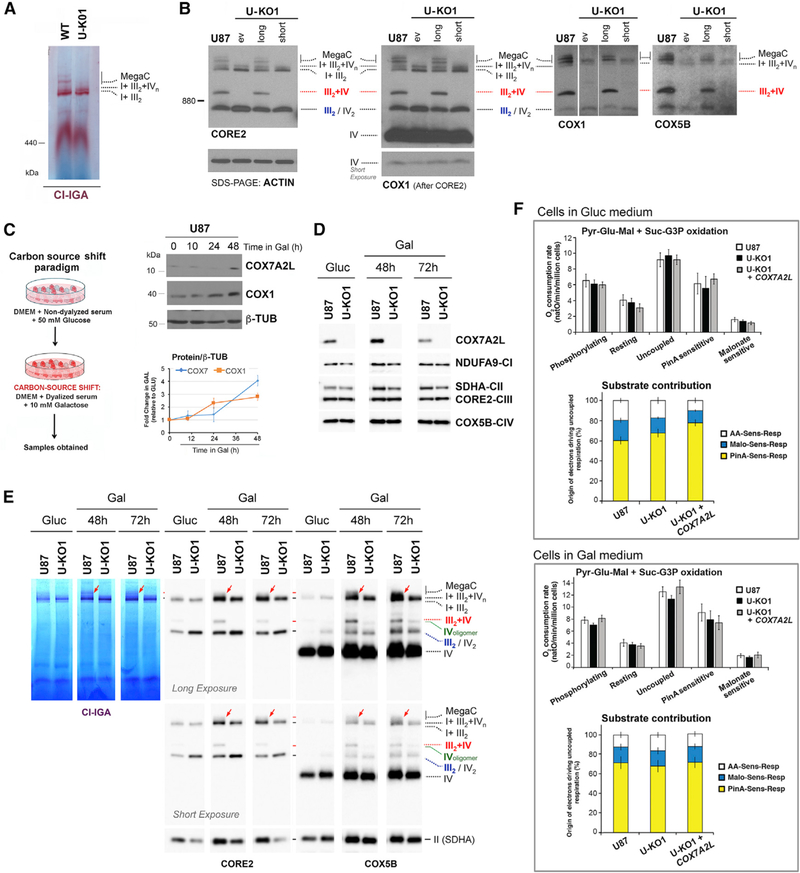
The faster CIII2 biogenesis and slower respirasome assembly rate observed in COX7A2L-KO cells would be consistent with a role for COX7A2L in accelerating SCs assembly to attend physiological needs or to recover from insults that could damage MRC complexes. In fact, it has been proposed that COX7A2L is induced, incorporated into CIV, and then enhances CIV activity under cellular stress conditions such as endoplasmic reticulum (ER) stress or ischemia (Zhang et al., 2016). Therefore, we assessed whether stressed HEK293T COX7A2L-KO cells could reveal a bioenergetics role for COX7A2L. Preliminary tests informed us that HEK293T cells do not exhibit a robust response to general stresses such as heat shock (Figure S3A), and although they respond to oxidative stress (Figures S3D and S3E), COX7A2L is not induced in the conditions tested (Figure S3B). Among the several cell lines examined, glioblastoma U-87 exhibited the most robust stress response (Figures S3A and S3B) and was used to generate a new COX7A2L-KO (U- KO). Two U-KO clones were obtained (Figures S4A and S4B). Since they exhibited a similar phenotype that was fully complemented by COX7A2L reconstitution (Figures 5B, S3C, and S3D), U-KO1 was used for subsequent experiments.
Figure 5. A Nutritional Challenge Induced by Switching the Carbon Source in the Media from Glucose to Galactose Does Equally Enhance Mitochondrial Bioenergetics Parameters in WT and COX7A2L-KO Cells.
(A and B) Characterization of glioblastoma U87 WT and COX7A2L-KO (U-KO1) cells. The KO cells were stably transfected with an empty vector (ev) or constructs to express the long or short versions of COX7A2L. (A) and (B) show the BN-PAGE analysis of whole cells extracted with digitonin (detergent/protein ratio, 4:1) separated in a 4%–8% (A) or a 3%–12% (B) linear gradient polyacrylamide gel, followed by CI in-gel activity (IGA) (A) or immunoblotting with the indicated antibodies (B). The identity of MRC complexes and SCs is indicated in the margins. MegaC, megacomplexes probably containing more than one copy of CI, CIII2, and CIV.
(C) Scheme depicting the carbon source switch paradigm used in this study.
(D) Time course quantification of COX7A2L induction by galactose in U87 WT cells. The graphs represent a quantification of the signals in three independent experiments, with error bars representing the mean ± SD.
(E) Mitochondria extracted with a digitonin/protein ratio of 4:1 (g/g) from cells grown in either glucose-containing (Gluc) or galactose-containing (Gal) medium and analyzed by BN-PAGE, followed by CI-IGA assays or, alternatively, by immunoblotting using the indicated antibodies. The red arrows indicate MegaCs exclusively detected in WT cells.
(F) Respiration of glioblastoma U87 cells assessed under different respiratory states (phosphorylating, resting, and uncoupled) and respective contribution of NADH and succinate dehydrogenases to the uncoupled respiration cultured in DMEM-glucose (upper panel), or DMEM-galactose (lower panel). Error bars represent the mean ± SD of four biological repetitions. See also Figure S4.
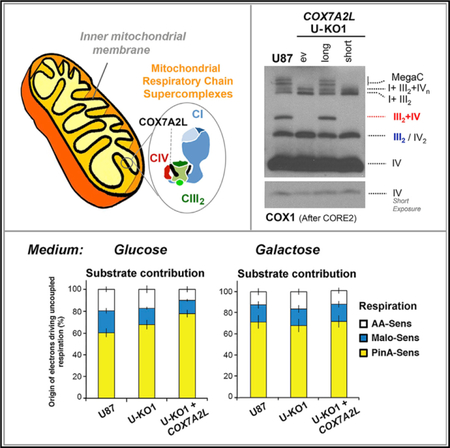
The pattern of MRC complexes and SCs was similar in HEK293T and U87 WT cells. However, SCs and MegaCs seemed more abundant in U87 (Figures 5A and 5B). The absence of COX7A2L in U87 cells prevented the assembly of SC III2+IV and enhanced CIII2 levels (Figures 5A and 5B) and delayed the assembly of the respirasomes (Figure S4C) as seen in HEK293T cells. It further limited the accumulation of SCs larger than the basic respirasome SC I+III2+IV1, although the detection of some traces of these SCs in U-KO cells could reflect their instability rather than failed assembly (Figures 5A, 5B, S4C).
In U87 WT cells, COX7A2L protein levels were enhanced up to ~4 fold following 48 hr under nutritional stress induced by carbon source switch from glucose to galactose (Figures 5C and 5D), a paradigm known to stimulate mitochondrial energy metabolism (Rossignol et al., 2004). The existing MRC complexes and SCs in WT and U-KO cells were equally induced after 48 hr in galactose and remained high at 72 hr (Figure 5E), when COX7A2L levels in WT cells had already attenuated (Figure 5D). As expected, coupled and uncoupled oxidation of pyruvate- glutamate-malate + succinate-G3P in permeabilized cells was stimulated in WT cells, but also in U-KO cells, and no differences were observed in substrate contribution (Figure 5F). Therefore, COX7A2L does not have any evident impact in glucose-togalactose nutritional-stress-induced MRC biogenesis and mitochondrial bioenergetics.
Environmental stresses in U87 cells such as acute heat shock (1 hr at 42○C; Figures S3A–S3C) or exogenous oxidative stress (1 hr in the presence of 100 mM H2O2; Figures S3D–S3F) also induced COX7A2L 2–4 hr after the insult. Yet, these stresses and subsequent recovery did not modify the MRC organization significantly (Figures S3C and S3F). The exposition to H2O2 promoted the equal accumulation of a CORE2-containing sub-SC in U87 WT and U-KO cells (Figure S3F), which could be a SC degradation product. As a minor difference, a proportion of U-KO cells higher than that of WT died during the acute oxidative stress, but no differences were detected in the endogenous respiration of the surviving cells (Figure S3G). We conclude that the MRC remodeling induced by COX7A2L does not influence mitochondrial bioenergetics during acute cellular stress.
A Mutant COX7A2L Variant Carrying a 6-bp Deletion Present in C57BL/6 Mice Retains the Ability to Bind CIII2 but Does Not Rescue SC III2+IV Assembly
In humans, only one COX7A2L protein of 114 amino acids has been reported (https://www.uniprot.org/uniprot/O14548) that is homolog to the long COX7A2L variant present in CD1 mice (Pérez-Pérez et al., 2016). To explore the functionality in human cells of the short COX7A2L isoform present in C57BL/6 and BALB/c mouse strains (Lapuente-Brun et al., 2013; Mourier et al., 2014), we generated cell lines constitutively expressing either FLAG-tagged COX7A2L (long) or a mutant version (short) carrying a deletion of amino acids V72 and P73 of human COX7A2L (Figure 6A). Both variants were stably expressed in WT HEK293T and COX7A2L-KO cells, which yielded no major differences in the steady-state levels of RC subunits (Figure 6A). MRC organization analysis by 2D-BN/SDS-PAGE revealed that the short COX7A2L variant was imported into mitochondria, where it colocalized with CIII2 and SC I+III2, but not with any CIV-containing structure (Figure 6B), as previously reported (Pérez-Pérez et al., 2016). Whereas expression of the long COX7A2L variant in COX7A2L-KO cells restored normal levels of CIII2 and SC III2+IV, the short variant did not (Figures 6B–6D). Our analyses further disclosed that the expression of either the long or the short COX7A2L variants did not affect the steady-state levels of the respirasomes (Figures 6C and 6D). Similar observations were made in U87 and U-KO cells (Figure 5B). Together, these results support our view (Pérez-Pérez et al., 2016) that the 2-amino acid deletion present in the short COX7A2L isoform prevents its association with CIV but does not affect its binding to CIII2, an interaction that is not sufficient to promote the formation of the SC III2+IV.
Figure 6. The Short-COX7A2L Variant Binds to CIII2 but Does Not Rescue SC III2+IV Assembly in COX7A2L-KO Cells.
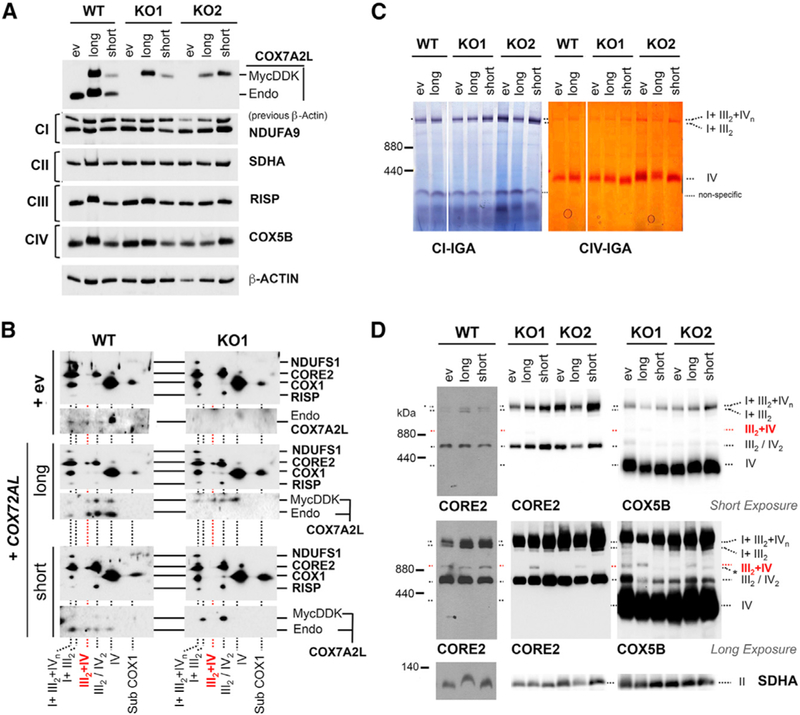
Control HEK293T cells (WT) and both COX7A2L- KO (KO1 and KO2) clones carrying an empty vector (ev) or constructs to overexpress COX7A2L-Myc- DDK (long) or short-COX7A2L-Myc-DDK (short) were used in the following experiments.
(A) SDS-PAGE followed by immunoblotting to estimate steady-state levels of endogenous COX7A2L (~12.6 kDa) from exogenous COX7A2L-Myc-DDK (~16.2 kDa). Membranes were also incubated with antibodies that recognize the indicated OXPHOS subunits.
(B). 2D-BN/SDS-PAGE and immunoblotting using digitonin-solubilized mitochondrial extracts (detergent/protein ratio, 4:1) and the indicated antibodies. (C and D) BN-PAGE followed by immunoblotting in digitonized whole-cell extracts (C) or by CI-IGA and CIV-IGA assays and/or immunoblotting in digitonized isolated mitochondria (D). Membranes were incubated with the indicated antibodies. I+III2+IVn, SCs containing CI, CIII2, and CIV. I+III2, SC containing CI and CIII2. III2+IV, SC containing CIII2 and CIV. III2, complex III dimer (CIII2). IV, complex IV; IV2, complex IV dimer (CIV2); II, complex II. Subcomplexes that contain COX1 are indicated as subCOX1. In (D), an unidentified band running a bit faster than the SC III2+IV cross-reacting with the COX5B antibody (Ab) (but not with the CORE2 Ab) is indicated with an asterisk.
Overexpression of CIV-Subunit Tissue-Specific Isoforms in COX7A2L-KO Cells Neither Rescues SC III2+IV Assembly nor Enhances Respirasome Levels
Studies in mice suggested that COX7A2L could regulate the formation or stability of the CIV-containing SCs III2+IV and I+III2+IV2-n in a tissue-specific manner (Williams et al., 2016). The relative levels of these SCs were lower in liver than in heart mitochondria from C57BL/6 mice, carrying the short COX7A2L variant, which could be explained by the occurrence of tissue- specific CIV subunit isoforms. Six isoforms have been so far described for the nucleus-encoded COX subunits in mammals: three liver/heart-type pairs of subunits (COX6A1/COX6A2, COX7A2/COX7A1, and COX8–1/COX8–2), the lung-specific iso- form COX4–2, and two testes-specific isoforms, COX6B and COX8–3 (Pierron et al., 2012). While heart (or muscle) isoforms are expressed in tissues with high aerobic capacity and abundant mitochondria, liver (or non-muscle) isoforms are found in tissues like brain, liver, and kidney that generally contain fewer mitochondria (Pierron et al., 2012).
An attractive hypothesis suggests that COX7A2L could be replaced by the CIV subunit COX7A2 in the respirasomes, thereby supporting the co-existence of alternative SCs in different tissues (Cogliati et al., 2016; Letts et al., 2016; Letts and Sazanov, 2017). To demonstrate whether the expression of tissue-specific isoforms of CIV subunits differentially affects the assembly of the SCs in the absence of COX7A2L, we attempted to induce the formation of ‘‘tissue-specific’’ CIV in WT HEK293T and COX7A2L-KO cells, which constitutively express the CIV liver isoforms. To induce the formation of a ‘‘heart-type’’ CIV, we overexpressed either FLAG-tagged COX6A2 or COX7A1, and to induce a ‘‘lung-type’’ CIV, we overexpressed COX4i2 in both cell types (Figures S5A–S5C). We also analyzed the effect of COX7A2 overexpression in COX7A2L-KO cells (Figures S5A– S5C). None of the isoforms rescued the formation of SC III2+IV or clearly altered the levels of the respirasomes (Figure S5D).
DISCUSSION
The biochemical and functional characterization of COX7A2L- KO human cell lines presented in this manuscript provide significant insights into the regulation of the MRC structural organization and respiratory metabolism by human COX7A2L. Our data help to clarify conflicting results on COX7A2L function that were primarily obtained in mouse models (Davoudi et al., 2016; Ikeda et al., 2013; Lapuente-Brun et al., 2013; Mourier et al., 2014; Pérez-Pérez et al., 2016; Williams et al., 2016). In this work, we demonstrate the role of COX7A2L in the coordinated regulation of CIII2 and SCs biogenesis. We unambiguously show that COX7A2L is essential to promote the assembly of SC III2+IV in human cells, and also the accumulation of large SCs compatible with either respirasomes containing several copies of CIV (I+III2+IV2-n) or with the recently described MegaC (I2+III2+IV2) (Guo et al., 2017). In contrast, COX7A2L is dispensable for the formation of the basic respirasomes (SCs I+III2 and I+III2+IV1), although its absence hampers the assembly efficiency of these MRC structures, thus providing an explanation to the previous controversy. Importantly, COX7A2L establishes a regulatory checkpoint that specifically limits the accumulation of CIII2. Physiologically, this adaptation of MRC organization and abundance promoted by COX7A2L is not essential to cope with nutritional, metabolic, or environmental cellular stresses and does not offer an obvious bioenergetics advantage, in contrast with a previous report (Lapuente-Brun et al., 2013). These concepts are depicted in a model presented in Figure 7 and are discussed below.
Figure 7. Structural and Functional Rearrangements of the RC in the Absence of COX7A2L.
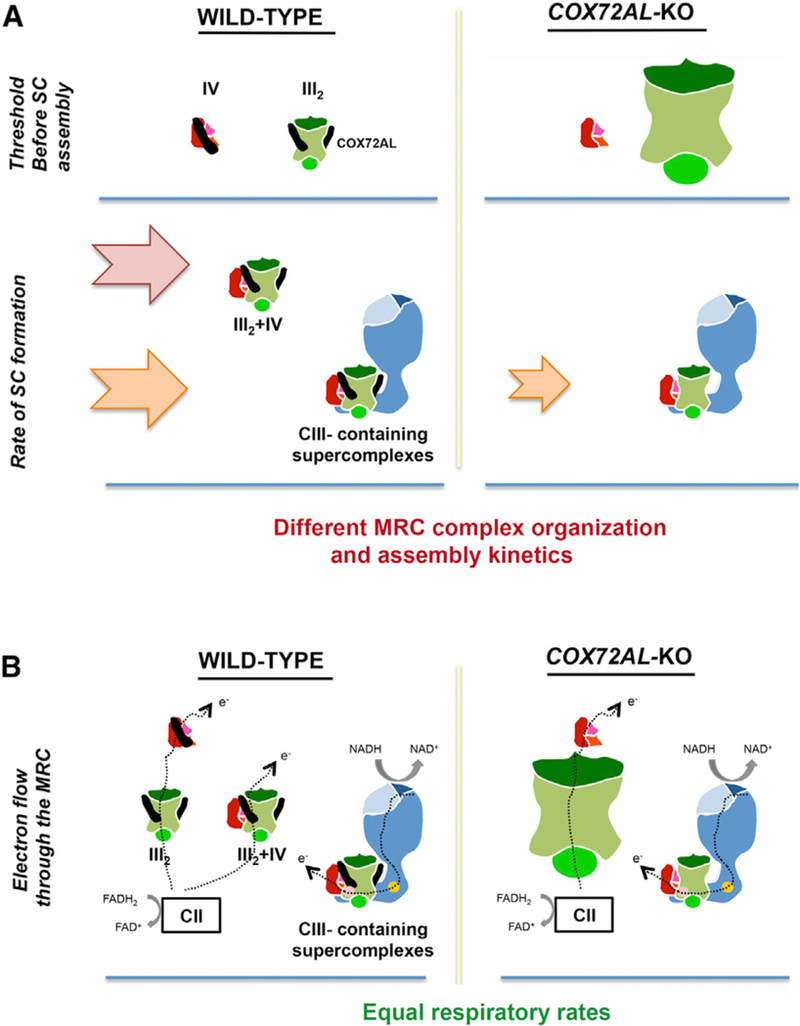
(A) Fully assembled CIII2 and CIV accumulate until they reach a threshold that ignites SC III2+IV and respirasome assembly at rates arbitrarily indicated by arrows. In the absence of COX7A2L, CIII2 levels are ~2–3-fold, SC III2+IV is not formed, and the assembly kinetics of CIII-containing SCs is slower.
(B) In physiological, nutritional, and environmental stress conditions, individual and simultaneous oxidation of NADH- and FAD-linked substrates is similar in WT and COX7A2L-KO cells. Mitochondrial CI, CIII2, and CIV are represented in blue, green, and red, respectively. COX7A2L is represented as a black stick.
COX7A2L was originally presented as a SC assembly factor essential for the incorporation of CIV into SCs III2+IV and I+III2+IV1-n in mice (Lapuente-Brun et al., 2013). In C57BL/6 and BALB/c mouse strains, the lack of residues V72-P73 in a short COX7A2L variant was found to impact COX7A2L stability and its role in the assembly of CIV-containing SCs, including SC III2+CIV and the respirasomes (Lapuente-Brun et al., 2013). In contrast, the results presented here show that, whereas the SC III2+IV is not formed in human COX7A2L-KO cells, the respirasomes I+III2+IV1-n accumulate to normal levels, consistent with previous data (Davoudi et al., 2016; Mourier et al., 2014; Pérez-Pérez et al., 2016; Williams et al., 2016), despite that they are assembled at a slower rate. However, SCs larger than I+III2+IV1 are unstable, particularly in U87 cells, in which MegaCs may fail to assemble. In vivo, the effect of COX7A2L in the SCs assembly rates may vary from tissue to tissue, which could be a contributing factor to explain why large respirasomes containing more than one CIV unit (I+III2+IV2-n) seem to be less abundant in liver than in heart from C57BL/6 mice (Williams et al., 2016).
Regarding the short COX7A2L variant, expression of a human version in COX7A2L-KO cells does not support SC III2+IV assembly, as seen in C57BL/6 mice (Lapuente-Brun et al., 2013),and it does not affect the steady-state levels of SCs I+III2+IV1-n. Importantly, whereas the short variant retains the ability to bind both CIII2 and SC I+III2, it does not interact with CIV or CIV-containing respirasomes. In contrast, WT (long) COX7A2L preferentially co-segregates with monomeric CIV, with SCs III2+IV and the respirasomes, and, to a minor extent, with CIII2 in human HEK293T cells, as it occurs in mouse heart mitochondria and in human 143B osteosarcoma cells (Pérez-Pérez et al., 2016). These data are consistent with the views that COX7A2L associates with CIII2 and CIV through independent domains to form the SC III2+IV (Cogliati et al., 2016; Pérez-Pérez et al., 2016; Zhang et al., 2016), and that COX7A2L binding to CIV requires the correct orientation of a histidine residue at position 73 (Cogliati et al., 2016). Altogether, these results suggest that COX7A2L promotes specific interactions between CIII2 and CIV that are essential for their association into SC III2+IV, which are probably different to their interactions in the respirasomes, where CI is recruited into the macrostructure (Gu et al., 2016; Letts et al., 2016; Sousa et al., 2016; Wu et al., 2016). This supports the hypothesis that different pathways may operate to assemble the different SCs (Letts and Sazanov, 2017). SC III2+IV and MegaCs could be assembled by the coming together of the individual complexes, which requires COX7A2L. In contrast, SC I+III2+IV1 would be assembled in a COX7A2L-independent manner, compatible with the incorporation of newly synthesized sub- units/subassemblies from CIII2 and CIV that accumulate once these fully assembled complexes have reached their steady- state levels, into larger structures containing CI intermediates, as we previously proposed (Moreno-Lastres et al., 2012).
In our model, the deregulation in the CIII2 steady-state levels provoked by the absence of COX7A2L would delay the formation of the basic I+III2+IV1 respirasome, as well as the further incorporation of additional fully assembled CI or CIV units to generate larger respirasomes or MegaCs. The significant accumulation of CIII2 levels was restored to normal by complementation with the long-COX7A2L variant, but not with the short variant, despite its retention of the ability to bind the complex. In this vein, we previously reported that, in cybrid cell lines lacking CIII2, the stability of COX7A2L is largely compromised (Pérez-Pérez et al., 2016). Furthermore, de novo assembly studies in control 143B cells indicated that COX7A2L assembles into a pre-CIII2 before the incorporation of the catalytic RISP subunit, whereas COX7A2L only binds CIV once this complex is fully assembled (Pérez-Pérez et al., 2016). These negative genetic interactions initially suggested an indirect regulatory role for COX7A2L in regulating CIII2 assembly or stability, most probably through the biogenesis of SC III2+IV. In this work, we have further demonstrated that the loss of COX7A2L enhances the de novo synthesis of CIII2 in detriment of the respirasomes, and therefore COX7A2L establishes a threshold to the accumulation of CIII2 in the steady state required to ignite respirasome assembly (Moreno-Lastres et al., 2012).
Finally, the impact of the MRC structural remodeling promoted by COX7A2L on mitochondrial physiology has also been a source of controversy. It was proposed that COX7A2L-dependent SC organization remodeling provides a mechanism for the physiological regulation of energy metabolism in mammals by providing alternate paths for electrons derived from the catabolism of specific substrates (Lapuente-Brun et al., 2013). In this model, electron flux from CI to CIII2 (carried by NADH) would proceed essentially within the CI-containing SCs, whereas electron flow from CII (carried by FAD) would preferentially occur through free CIII2 and SC III2+IV (Lapuente-Brun et al., 2013). ATP production and respiration rates were found higher in mouse liver mitochondria and permeabilized fibroblasts with the unstable short-COX7A2L variant, both in the presence of pyruvate + malate (NADH-linked substrates) or succinate (FAD-linked substrate), whereas maximal respiration and ATP production in cells expressing long-COX7A2L required substrates for both electron transfer paths (Lapuente-Brun et al., 2013). However, other groups reported lower mitochondrial respiration and ATP synthesis in muscle (Ikeda et al., 2013) and liver (Shiba et al., 2017) from COX7A2L-KO mice or no effect of short-COX7A2L on mouse heart mitochondrial respiration (Mourier et al., 2014). Our human COX7A2L-KO cellular models displayed no differences with WT cells in coupled endogenous cell respiration, or in single and combined substrate (pyruvate- glutamate-malate or succinate-G3P) oxidation, indicating a minor functional role of COX7A2L in normal cultured cell physiological conditions. Similar results were obtained when the cultures were exposed to several nutritional, oxidative, and environmental cellular stresses, even though they actually induce COX7A2L protein by 4- to 10-fold. Altogether, our results contest the highly controversial role of COX7A2L in MRC SCs organization that has been hypothesized to maximize mitochondrial bioenergetics efficiency (Bianchi et al., 2004; Lapuente-Brun et al., 2013).
A potential caveat of our studies is the use of cell culture models, which are maintained in conditions that could differ in vivo, particularly regarding tissue oxygen and nutrient availabilities. However, similar results were obtained in two different cell models and an array of culture conditions. Our data serve to clarify some of the current discrepancies regarding the impact of COX7A2L on the organization and function of the MRC complexes. We conclude that the role of COX7A2L in SC III2+IV assembly, which is well established in the literature, and in the assembly/stability of higher SCs or MegaCs, has no impact in mitochondrial bioenergetics in any of the conditions tested. One could claim that the excess of free CIII2 that accumulates when SC III2+IV is absent could compensate for the instability of MegaCs and the slower SC assembly kinetics observed in COX7A2L-KO cells, but if this occurs, it is not via preferential substrate utilization, as also supported by other groups (Blaza et al., 2014; Fedor and Hirst, 2018; Trouillard et al., 2011). Alternatively, COX7A2L could be part of a response to accelerate CIII- containing SCs assembly when needed to preserve stability of individual complexes (Acı´n-Pérez et al., 2004) or to minimize ROS production (Maranzana et al., 2013), possibilities that warrant future research efforts.
STAR★METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| ATP5A | Abcam | Cat# ab14748 RRID: AB_301447 |
| β-ACTIN | Abcam | Cat# ab8227 RRID: AB_2305186 |
| BCS1-L | Abcam | Cat# ab102808 RRID: AB_10859410 |
| CORE1 | Abcam | Cat# ab110252 RRID: AB_10863633 |
| CORE2 | Abcam | Cat# ab14745 RRID: AB_2213640 |
| COX1 | Abcam | Cat# ab14705 RRID: AB_2084810 |
| COX2 | Abcam | Cat# ab110258 RRID: AB_10887758 |
| COX4I1 | Abcam | Cat# ab14744 RRID: AB_301443 |
| COX4I2 | Abcam | Cat# ab70112 RRID: AB_2085283 |
| COX5A | Sigma | Cat# HPA027525 |
| COX5B | Santa Cruz | Cat# sc-374417 RRID: AB_10988066 |
| COX6A1 | Sigma | Cat# HPA062394 RRID: AB_2684749 |
| COX6A2 | Abcam | Cat# ab103139 RRID: AB_10710958 |
| COX7A1 | Abcam | Cat# ab134989 |
| COX7A2L | ProteinTech | Cat#11416–1-AP RRID: AB_2245402 |
| FLAG-tag | Sigma | Cat# F3165 RRID: AB_259529 |
| HSP70 (HSPA6) | Origene | Cat# TA501950 RRID: AB_11124627 |
| NDUFA9 | Abcam | Cat# ab14713 RRID: AB_301431 |
| NDUFS1 | Abcam | Cat# ab52690 RRID: AB_2151096 |
| RISP | Abcam | Cat# ab14746 RRID: AB_301445 |
| SDHA | Abcam | Cat# ab14715 RRID: AB_301433 |
| SOD2 | Sigma | Cat# HPA001814 RRID: AB_1080134 |
| TIM50 | Abcam | Cat# ab109527 RRID: AB_10858241 |
| TOM20 | Santa Cruz | Cat# sc-11415 RRID: AB_2207533 |
| b-TUBULIN | Sigma | Cat# C4585 RRID: AB_258868 |
| UQCRB | Abcam | Cat# ab190360 |
| VDAC1 | Abcam | Cat# ab14734 RRID: AB_443084 |
| 2○ Ab-mouse | Rockland Immunochemicals | Cat# 610–103-121 RRID: AB_218457 |
| 2○ Ab-rabbit | Rockland Immunochemicals | Cat# 611–1302 RRID: AB_219720 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Dulbecco’s Modified Eagle Medium (DMEM) | Invitrogen | Cat# 31966–047 |
| Fetal bovine serum (FBS) | Sigma | Cat# 12303C |
| Lipofectamine 2000 | Invitrogen | Cat# 1168019 |
| Opti-MEM I Reduced Serum Medium | ThermoFisher # | Cat# 31985062 |
| n-dodecyl-b-d-maltoside (DDM) | Sigma | Cat# 5172 |
| Digitonin, High Purity | Calbiochem | Cat# 300410 |
| Native PAGE 20X Running Buffer | Novex-Life Technologies | Cat# BN2001 |
| Native PAGE 20X Cathode Buffer Additive | Novex-Life Technologies | Cat# BN2002 |
| Critical Commercial Assays | ||
| TNT T7 Quick Coupled Transcription/Translation System | Promega | Cat# L1170 |
| SuperSignal West Femto Maximum Sensitivity Substrate | ThermoFisher | Cat# 34095 |
| Experimental Models: Cell Lines | ||
| HEK293T | ATCC | Cat# CRL-3216 |
| Glioblastoma U87 | ATCC | Cat# HTB-14 |
| Oligonucleotides | ||
| COX7A2L-short-Forward: GTTTTTCCAGAAAGCTGATGGT | Sigma | This paper |
| COX7A2L-short-Reverse: GCCTCGTTTCAGGTAGAC | Sigma | This paper |
| COX7A2L-KO-Forward: AAGTTAGGCGATCTTCGGGC | Sigma | This paper |
| COX7A2L-KO-Reverse: GCTCGGACATGAGAAGTGGC | Sigma | This paper |
| SAS1_Hs01: | Sigma | Cat# oligo 3019431149–000060 |
| SAS1_Hs02: | Sigma | Cat# oligo 3019431149–000070 |
| Recombinant DNA | ||
| TAL Effector (F)- COX7A2L: TGGGCGTCATGTACTACAA | Invitrogen | N/A |
| TAL Effector (R)- COX7A2L: GCAGAAGTTGGCAGGAGCA | Invitrogen | N/A |
| UQCR11 in pReceiver-B31 | GeneCopoeia | Cat# EX-I0287-B31 |
| UQCRB in pReceiver-B31 | GeneCopoeia | Cat# EX-F0223-B31 |
| UQCRFS1 or RISP in pReceiver-B31 | GeneCopoeia | Cat# EX-A3744-B31 |
| COX7A2L- Myc-DDK in pCMV6-Entry | Origene | Cat# RC202697 |
| COX7A1- Myc-DDK in pCMV6-Entry | Origene | Cat# RC201154 |
| COX4I1- Myc-DDK in pCMV6-Entry | Origene | Cat# RC209374 |
| COX4I2- Myc-DDK in pCMV6-Entry | Origene | Cat# RC209204 |
| COX6A1- Myc-DDK in pCMV6-Entry | Origene | Cat# RC210485 |
| COX6A2- Myc-DDK in pCMV6-Entry | Origene | Cat# RC206539 |
| pCMV6-A-Entry-Hygro | Origene | Cat# PS100024 |
| COX7A2L-Myc-DDK in pCMV6-A-Entry-Hygro | This paper | N/A |
| Software and Algorithms | ||
| SPSS | IBM, v 21.0 | N/A |
| ImageJ | NIH | https://imagej.nih.gov/ij/ |
| GraphPad Prism | GraphPad Software v.5.0a | N/A |
| ImageLab | BioRad, v 6.0.1 | N/A |
| Other | ||
| Pre-cast NuPAGE 4%–12% Bis-Tris gels | Invitrogen | Cat# NP0321BOX |
| Pre-cast NativePAGE 3%–12% Bis-Tris gels | Invitrogen | Cat# BN2011BX10 |
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Antoni Barrientos, Ph.D. (abarrientos@med.miami.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Human cell lines, transfection and cell culture
HEK293T (CRL-3216) and glioblastoma U-87 (HTB-14) cells were obtained from ATCC and cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate, 50 mg/ml uridine and antibiotics at 37○C under 5% CO2. Analysis for mycoplasma contamination was routinely performed.
Nutritional and environmental stress conditions
For some experiments cells were exposed to nutritional, OXPHOS bioenergetics an environmental stressors. Nutritional stress was induced by transferring cells from glucose-containing to galactose-containing media and samples were analyzed over increasing times (12 to 48 hours). OXPHOS bioenergetics stress was induced with MRC complex I inhibitors: cells were exposed to 10 nM rote- none, 1.2 mM piericidin A or the control vehicle (0,05% or 8,5 mM ethanol) for 24 hours. Heat stress was induced by exposing cultures to 42○C for 1 hour and oxidative stress by supplementing the media with 100 mM H2O2 for 1 hour. Following heat or oxidative stress, the media was changed and the cultures incubated in non-stress conditions before samples were collected after increasing times of recovery (0 to 8 hours).
METHOD DETAILS
Key reagents
Tables presenting the list of antibodies, recombinant DNAs, oligonucleotides and siRNA oligoribonucleotides used in this study are included in the supplementary material.
Plasmid transfection
To create stable human COX7A2L knockout (KO) lines in HEK293T and U87 cells, we used a pair of TALEN constructs obtained from Thermo-Invitrogen. The left and right TALEN of the pair were designed to target the TGGGCGTCATGTACTACAA and the GCAGAAGTTGGCAGGAGCA DNA sequences, respectively, at the COX7A2L locus (see key reagents tables). HEK293T or U87 cells grown on a 6-well plate at 30% confluency were transfected with 4 mg of the right and left TALEN plasmids as a pair using 5 mL of Lipofectamine 2000 (Thermo Fisher) pre-incubated in 300 mL of Opti-MEM (ThermoFisher). After 4 hours of incubation, the media were changed to complete DMEM medium. After 3–6 times of repetitive transfections every three days, cells were collected, diluted in complete DMEM medium and seeded as single cells in multiple 96 well plates. In some repetitions, single cells were isolated using Fluorescence Activated Cell Sorting (FACS). The surviving colonies screened by immunoblotting against COX7A2L and by genotyping, as reported (Bourens et al., 2014). For genotyping, COX7A2L was sequenced using oligonucleotides COX7A2L-KO-Forward and COX7A2L-KO-Reverse (see Key resources table)
The COX7A2L-KO cell lines were reconstituted with Myc-DDK-tagged variants (long or short) of COX7A2L. COX7A2L-Myc-DDK was cloned under the control of a CMV promoter in the pCMV6-A-Entry-Hygro plasmid (Origene, PS100024) using SfaAI and MssI sites. The short version of mouse COX7A2L comprises an in-frame 6 base pair deletion that led to the absence of 2 amino acids (V72 and P73) (Lapuente-Brun et al., 2013). To generate the short variant of human COX7A2L, we used the Q5® Site-Directed Mutagenesis Kit from NEB. ~20 ng of template DNA extracted from HEK293T cells were used, along with the primers COX7A2L-short-F: 50-GTTTTTCCAGAAAGCTGATGGT-30 (forward) and COX7A2L-short-R: 50-GCCTCGTTTCAGGTAGAC-30 (reverse), designed to flank the region to be deleted. After exponential amplification and the treatment with kinase and ligase, 5 ml of the reaction were transformed NEB 5-alpha competent E.coli cells using the pCMV6-A-Entry-Hygro plasmid (Origene).
For transfection of COX7A2L-Myc-DDK-Hygro constructs, we used 10 mL of Lipofectamine™ (Thermo Fisher) mixed with 4 mg of vector DNA in OPTIMEM-I media (GIBCO) according to the manufacturer’s instructions. Two days after transfection, the media was supplemented with 200 mg/ml of hygromycin and drug selection was maintained for at least 21 days.
Whole Cell extracts and Mitochondrial isolation
Whole cell extracts were obtained by solubilization in RIPA buffer (25 mM Tris-HCl pH 7.6; 150 mM NaCl; 1% NP-40; 1% sodium deoxycholate and 0.1% SDS) with 1 mM PMSF and 1x mammalian protease inhibitor cocktail (Sigma). Extracts were cleared by 5 minutes centrifugation at 10,000 g at 4○C.
Mitochondria-enriched fractions were isolated from at least ten 80% confluent 175 cm2 flasks as described previously (Bourens et al., 2014; Ferna´ ndez-Vizarra et al., 2010; Moreno-Lastres et al., 2012). To extract mitochondrial proteins in native conditions, mito- chondria were pelleted and solubilized in 200 mL buffer containing 1.5 M aminocaproic acid and 50 mM Bis-Tris (pH 7.0). After optimizing solubilization conditions, we decided to use digitonin at a concentration of 4 g/g protein. In some experiments, lauryl maltoside (LM) was used at 1%. Solubilized samples were incubated on ice for 15 min and centrifuged for 30 min at 10,000 xg at 4○C, and the supernatant was combined with 20 mL of sample buffer (750 mM aminocaproic acid, 50 mM Bis-Tris, 0.5 mM EDTA, 5% Serva Blue G-250) prior to loading.
Blue Native Electrophoresis and In-Gel Activity Assays
Native PAGE Novex® 3%–12% Bis-Tris Protein Gels (Life Technologies) gels were loaded with 60–80 mg of mitochondrial protein or 400 mg of total cell extracts prepared in the presence of either lauryl maltoside (LM) or digitonin at the protein-detergent ratios indicated in the figure legends. After electrophoresis, proteins were transferred to PDVF or nitrocellulose membranes and used for immunoblotting. Duplicate gels were further used for in-gel activity (IGA) assays and for second-dimension (2D) 10% SDS-PAGE gels.
SDS-PAGE and immunoblotting
Protein concentration was measured with the BCA reagent (Thermo Scientific). 20–60 mg of mitochondrial protein extract was separated by SDS–PAGE in the Laemmli buffer system (Laemmli, 1970). Then, proteins were transferred to nitrocellulose membranes at 40 V overnight and probed with specific primary antibodies listed in the Key reagents Table. Peroxidase-conjugated anti-mouse and anti-rabbit IgGs were used as secondary antibodies (Molecular Probes). Immunoreactive bands were detected with an ECL prime Western Blotting Detection Reagent (Amersham) in a ChemiDoc MP Imager (Biorad) or by exposition to X-ray films. Optical densities of the immunoreactive bands were measured using the ImageLab (Biorad) software or the ImageJ software in digitalized images.
Characterization of the mitochondrial respiratory chain and oxidative phosphorylation system
Mitochondrial respiratory chain enzyme activities were performed according to established methods (Medja et al., 2009), and expressed relative to the citrate synthase activity.
Endogenous cell respiration was measured polarographically at 37○C using a Clark-type electrode from Hansatech Instruments (Norfolk, United Kingdom). Substrate-driven respiration was assayed in digitonin-permeabilized cultured cells as reported (Barrien-tos et al., 2009). Briefly, trypsinized cells were washed with permeabilized-cell respiration buffer (PRB) containing 0.3 M mannitol, 10 mM KCl, 5 mM MgCl2, 0.5 mM EDTA, 0.5 mM EGTA, 1 mg/ml BSA and 10 mM KH3PO4 (pH 7.4). The cells were resuspended at ~4 3 106 cells/ml in 1.5 mL of the same buffer air-equilibrated at 37 ○C supplemented 10 units of hexokinase and 2 mM ADP. One ml of cell suspension was immediately placed into the polarographic chamber to measure endogenous respiration.
High-resolution oxygen consumption rate of digitonin-permeabilized cells was performed as described previously (Silva Ramos et al., 2016) at 37○C, using 1 million cells diluted in 2 mL of respiratory buffer (120 mM sucrose, 50 mM KCl, 20 mM Tris-Base, 4 mM KH2PO4, 2 mM MgCl2, 1 mM EDTA, pH 7.2) using an Oxygraph-2K (Oroboros). Briefly, cells were permeabilized with 0.02 mg/ml digitonin. The oxygen consumption rate under phosphorylating condition was assessed using either NADH-linked substrates (10 mM glutamate, 10 mM pyruvate and 5 mM malate), or FADH-linked substrates (10 mM succinate plus 5 mM glycerol-3-phosphate) in the presence of 2.5 mM ADP. The non-phosphorylating state was obtained after ATP synthesis inhibition using 0.75 mg/ml oligomycin. Mitochondrial respiration was uncoupled by successive addition of up to 0.4 mM CCCP to reach maximal oxygen consumption. To assess the contribution of NADH-linked and FADH-linked substrates to total oxygen consumption, respiration was assessed in the presence of both kinds of substrates. Subsequently, complex I and complex II activities were sequentially inhibited using respectively 1.2 mM piericidin A and 3 mM malonate. Afterward, 5mM of antimycin A was added to validate the mitochondrial origin of the oxygen consumption measured.
De novo mitochondrial respiratory chain complex and supercomplex assembly
To follow the assembly kinetics of MRC complexes and supercomplexes, we depleted cells of the structures containing mtDNA- encoded subunits by treating the cultures with doxycycline, a reversible inhibitor of mitochondrial translation as reported (Moreno-Lastres et al., 2012). We cultured WT (HEK293 or U87) control and COX7A2L-KO cells for 6 days in the presence of 15 mg/ml doxycycline. To follow the accumulation of newly synthesized MRC complexes and their further association into super complexes, samples were collected at different time points (0, 6, 15, 24, 48, 72, and 96 hours) after doxycycline removal. Digitonin- solubilized mitochondrial particles were separated by BN-PAGE and analyzed by immunoblotting.
Overexpression of Complex IV tissue-specific (liver and heart) subunit isoforms in COX7A2L-KO cells
Genes coding for selected liver and heart Complex IV subunit isoforms (COX4I1. COX4I2, COX6A1, COX6A2, COX7A1 and COX7A2) were cloned in frame with a Myc-DDK-tag into pCMV6 plasmid carrying a hygromycin resistance cassette. Each construct was transfected into the COX7A2L-KO cell line, by using 10 mL of Lipofectamine™ (Thermo Fisher) mixed with 4 mg of vector DNA in OPTIMEM-I media (GIBCO), according to the manufacturer’s instructions. Two days after transfection, the media was supplemented with 200 mg/ml hygromycin and drug selection was maintained for at least 21 days.
In organello import of radiolabelled recombinant proteins
Plasmids with CIII subunit ORFs for in organello import were obtained from GeneCopoeia™. pReceiver-B31 vectors have UQCR11 (EX-I0287-B31) and UQCRFS1 or RISP (EX-A3744-B31) ORFs under the control of the T7 promoter. Radiolabeled UQCR11 and UQCRFS1 proteins were synthesized in the presence of [35S]-methionine, using the TNT T7 Quick Coupled Transcription/Translation System (Promega). Import experiments were performed by incubating radiolabeled precursor subunits with 200 mg of freshly isolated mitochondria prepared as reported (Ferna´ ndez-Vizarra et al., 2010) in presence 33 mL of import buffer (20 mM HEPES-KOH pH 7.4, 600mM mannitol, 16 mM MgCl2, 5 mM ATP, 225 mM KCl, 0.1 mg/ml pyruvate kinase, 5 mM methionine and 3% (w/v) fatty acid-free bovine serum albumin) in a total volume of 200 ml in STE buffer (0.32 M sucrose, 1 mM EDTA, 10 mM Tris-HCl pH 7.4), at 37○C for increasing times (5 to 60 min). To assess import dependence of mitochondrial membrane potential, control samples were incubated with 10 mM of CCCP. For SDS-PAGE analysis, samples were split into two aliquots and treated with or without 100 mg/ml proteinase K (Sigma) for 20 min on ice, before stopping the reaction with 1 mM PMSF for 10 min. Mitochondria were pelleted at 8,000 xg for 8 min at 4○C and resuspended in 100 ml of STE buffer for their further analysis. For BN-PAGE analysis, mitochondria samples were subjected to protease treatment and pelleted before undergoing the analysis as described above.
QUANTIFICATION AND STATISTICAL ANALYSIS
Unless indicated, all experiments were performed at least in triplicate and results were presented as mean ± standard deviation (SD) of absolute values or percentages of control. Statistical p values were obtained by application of the Mann-Whitney U test using the SPSS v21.0 program. p < 0.05 was considered significant test (*p < 0.05; **p < 0.01; ***p < 0.001). Information on biological and technical replicates and statistical significance is included in the figure legends.
Supplementary Material
Highlights.
COX7A2L-knockout human cells lack SC III2+IV and some megacomplexes
COX7A2L-KO cells have enhanced CIII2 steady-state levels and assembly rate
COX7A2L-KO cells have slower respirasome assembly but normal steady-state levels
COX7A2L-dependent MRC remodeling does not affect mitochondrial bioenergetics
ACKNOWLEDGMENTS
We thank Carlos T. Moraes and Erika Fernandez-Vizarra for critical reading of the manuscript. This research was supported by NIH R01 Grants GM105781 (to A.B. and C.U.) and GM112179 (to A.B.), NIH R35 Grant GM118141 (to A.B.), MDA Grant MDA-381828 (to A.B.), AHA Development Grant 14SDG20040003 (to F.F.), and Instituto de Salud Carlos III-MINECO/European FEDER Funds Grants PI14–00209 and PI17–00048 (to C.U.). A.M. receives support from the AFM-Telethon (Trampoline Grant 19613) and ANR JCJC (ANR-16-CE14–0013).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Acin-Perez R, and Enriquez JA (2014). The function of the respiratory super- complexes: the plasticity model. Biochim. Biophys. Acta 1837, 444–450. [DOI] [PubMed] [Google Scholar]
- Acı´n-Pérez R, Bayona-Bafaluy MP, Ferna´ ndez-Silva P, Moreno-Loshuertos R, Pérez-Martos A, Bruno C, Moraes CT, and Enrı´quez JA (2004). Respiratory complex III is required to maintain complex I in mammalian mito-chondria. Mol. Cell 13, 805–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acı´n-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, and Enriquez JA (2008). Respiratory active mitochondrial supercomplexes. Mol. Cell 32, 529–539. [DOI] [PubMed] [Google Scholar]
- Barrientos A, and Ugalde C (2013). I function, therefore I am: overcoming skepticism about mitochondrial supercomplexes. Cell Metab 18, 147–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos A, Fontanesi F, and Diaz F (2009). Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet Chapter 19, Unit 19.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi C, Genova ML, Parenti Castelli G, and Lenaz G (2004). The mitochondrial respiratory chain is partially organized in a supercomplex assembly: kinetic evidence using flux control analysis. J. Biol. Chem 279, 36562–36569. [DOI] [PubMed] [Google Scholar]
- Blaza JN, Serreli R, Jones AJ, Mohammed K, and Hirst J (2014). Kinetic evidence against partitioning of the ubiquinone pool and the catalytic relevance of respiratory-chain supercomplexes. Proc. Natl. Acad. Sci. USA 111, 15735–15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourens M, Boulet A, Leary SC, and Barrientos A (2014). Human COX20 cooperates with SCO1 and SCO2 to mature COX2 and promote the assembly of cytochrome c oxidase. Hum. Mol. Genet 23, 2901–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Taylor EB, Dephoure N, Heo JM, Tonhato A, Papandreou I, Nath N, Denko NC, Gygi SP, and Rutter J (2012). Identification of a protein mediating respiratory supercomplex stability. Cell Metab 15, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Cermak T, Doyle EL, Schmidt C, Zhang F, Hummel A, Bogdanove AJ, and Voytas DF (2010). Targeting DNA double-strand breaks with TAL effector nucleases. Genetics 186, 757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliati S, Calvo E, Loureiro M, Guaras AM, Nieto-Arellano R, Garcia-Poyatos C, Ezkurdia I, Mercader N, Vázquez J, and Enriquez JA (2016). Mechanism of super-assembly of respiratory complexes III and IV. Nature 539, 579–582. [DOI] [PubMed] [Google Scholar]
- Cruciat CM, Brunner S, Baumann F, Neupert W, and Stuart RA (2000). The cytochrome bc1 and cytochrome c oxidase complexes associate to form a single supracomplex in yeast mitochondria. J. Biol. Chem 275, 18093–18098. [DOI] [PubMed] [Google Scholar]
- Davoudi M, Kotarsky H, Hansson E, Kallija¨ rvi J, and Fellman V (2016). COX7A2L/SCAFI and pre-Complex III modify respiratory chain supercomplex formation in different mouse strains with a Bcs1l mutation. PLoS One 11, e0168774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen JT, and Antonarakis SE (2000). Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum. Mutat 15, 7–12. [DOI] [PubMed] [Google Scholar]
- Fedor JG, and Hirst J (2018). Mitochondrial Supercomplexes do not enhance catalysis by quinone channeling. Cell Metab 28, 525–531.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Vizarra E, and Zeviani M (2015). Nuclear gene mutations as the cause of mitochondrial complex III deficiency. Front. Genet 6, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Vizarra E, Ferrı´n G, Pérez-Martos A, Fernández-Silva P, Zeviani M, and Enrı´quez JA (2010). Isolation of mitochondria for biogenetical studies: an update. Mitochondrion 10, 253–262. [DOI] [PubMed] [Google Scholar]
- Gu J, Wu M, Guo R, Yan K, Lei J, Gao N, and Yang M (2016). The architecture of the mammalian respirasome. Nature 537, 639–643. [DOI] [PubMed] [Google Scholar]
- Guo R, Zong S, Wu M, Gu J, and Yang M (2017). Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell 170, 1247–1257.e12. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Shiba S, Horie-Inoue K, Shimokata K, and Inoue S (2013). A stabilizing factor for mitochondrial respiratory supercomplex assembly regulates energy metabolism in muscle. Nat. Commun 4, 2147. [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lapuente-Brun E, Moreno-Loshuertos R, Acı´n-Pérez R, Latorre-Pellicer A, Colás C, Balsa E, Perales-Clemente E, Quirós PM, Calvo E, Rodr´ıguez-Hernández MA, et al. (2013). Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340, 1567–1570. [DOI] [PubMed] [Google Scholar]
- Letts JA, and Sazanov LA (2017). Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat. Struct. Mol. Biol 24, 800–808. [DOI] [PubMed] [Google Scholar]
- Letts JA, Fiedorczuk K, and Sazanov LA (2016). The architecture of respiratory supercomplexes. Nature 537, 644–648. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, Weeks DP, and Yang B (2011). Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res 39, 6315–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo-Jarne T, and Ugalde C (2018). Respiratory chain supercomplexes: structures, function and biogenesis. Semin. Cell Dev. Biol 76, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranzana E, Barbero G, Falasca AI, Lenaz G, and Genova ML (2013). Mitochondrial respiratory supercomplex association limits produc tion of reactive oxygen species from complex I. Antioxid. Redox Signal 19, 1469–1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medja F, Allouche S, Frachon P, Jardel C, Malgat M, Mousson de Camaret B, Slama A, Lunardi J, Mazat JP, and Lombe` s A (2009). Development and implementation of standardized respiratory chain spectrophoto-metric assays for clinical diagnosis. Mitochondrion 9, 331–339. [DOI] [PubMed] [Google Scholar]
- Milenkovic D, Blaza JN, Larsson NG, and Hirst J (2017). The enigma of the respiratory chain supercomplex. Cell Metab 25, 765–776. [DOI] [PubMed] [Google Scholar]
- Mileykovskaya E, and Dowhan W (2014). Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem. Phys. Lipids 179, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lastres D, Fontanesi F, Garcı´a-Consuegra I, Martı´n MA, Arenas J, Barrientos A, and Ugalde C (2012). Mitochondrial complex I plays an essential role in human respirasome assembly. Cell Metab 15, 324–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourier A, Matic S, Ruzzenente B, Larsson NG, and Milenkovic D (2014). The respiratory chain supercomplex organization is independent of COX7a2l isoforms. Cell Metab 20, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Pérez R, Lobo-Jarne T, Milenkovic D, Mourier A, Bratic A, Garcı´a-Bartolomé A, Fernández-Vizarra E, Cadenas S, Delmiro A, Garcı´a-Consuegra I, et al. (2016). COX7A2L Is a mitochondrial complex III binding protein that stabilizes the III2+IV supercomplex without affecting respirasome formation. Cell Rep 16, 2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierron D, Wildman DE, Hu€ttemann M, Markondapatnaikuni GC, Aras S, and Grossman LI (2012). Cytochrome c oxidase: evolution of control via nuclear subunit addition. Biochim. Biophys. Acta 1817, 590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol R, Gilkerson R, Aggeler R, Yamagata K, Remington SJ, and Capaldi RA (2004). Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res 64, 985–993. [DOI] [PubMed] [Google Scholar]
- Schägger H, and Pfeiffer K (2000). Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J 19, 1777–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H, de Coo R, Bauer MF, Hofmann S, Godinot C, and Brandt U (2004). Significance of respirasomes for the assembly/stability of human respiratory chain complex I. J. Biol. Chem 279, 36349–36353. [DOI] [PubMed] [Google Scholar]
- Shiba S, Ikeda K, Horie-Inoue K, Nakayama A, Tanaka T, and Inoue S (2017). Deficiency of COX7RP, a mitochondrial supercomplex assembly promoting factor, lowers blood glucose level in mice. Sci. Rep 7, 7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Ramos E, Larsson NG, and Mourier A (2016). Bioenergetic roles of mitochondrial fusion. Biochim. Biophys. Acta 1857, 1277–1283. [DOI] [PubMed] [Google Scholar]
- Singhal RK, Kruse C, Heidler J, Strecker V, Zwicker K, Dusterwald L, Westermann B, Herrmann JM, Wittig I, and Rapaport D (2017). Coi1 is a novel assembly factor of the yeast complex III-complex IV supercomplex. Mol. Biol. Cell 9, Published online August 9, 2018. https://doi.org/10.1091/mbc.E17-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa JS, Mills DJ, Vonck J, and Ku€hlbrandt W (2016). Functional asymmetry and electron flow in the bovine respirasome. eLife 5, e21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strogolova V, Furness A, Robb-McGrath M, Garlich J, and Stuart RA (2012). Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cyto-chrome c oxidase supercomplex. Mol. Cell. Biol 32, 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Li B, Qiu R, Fang H, and Lyu J (2016). Cell type-specific modulation of respiratory chain supercomplex organization. Int. J. Mol. Sci 17, E926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillard M, Meunier B, and Rappaport F (2011). Questioning the functional relevance of mitochondrial supercomplexes by time-resolved analysis of the respiratory chain. Proc. Natl. Acad. Sci. USA 108, E1027–E1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukotic M, Oeljeklaus S, Wiese S, Vögtle FN, Meisinger C, Meyer HE, Zieseniss A, Katschinski DM, Jans DC, Jakobs S, et al. (2012). Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab 15, 336–347. [DOI] [PubMed] [Google Scholar]
- Williams EG, Wu Y, Jha P, Dubuis S, Blattmann P, Argmann CA, Houten SM, Amariuta T, Wolski W, Zamboni N, et al. (2016). Systems proteomics of liver mitochondria function. Science 352, aad0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Gu J, Guo R, Huang Y, and Yang M (2016). Structure of mammalian respiratory supercomplex I1III2IV1. Cell 167, 1598–1609.e10. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang G, Zhang X, Hu€ttemann PP, Qiu Y, Liu J, Mitchell A, Lee I, Zhang C, Lee JS, et al. (2016). COX7AR is a stress-inducible mitochondrial cox subunit that promotes breast cancer malignancy. Sci. Rep 6, 31742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.