Solid tumors account for the overwhelming majority of cancer-related deaths, reflecting our inability to cure metastatic disease in these patients.1 Many of these cancers are diagnosed at a locoregional stage, when there is still the potential of cure, but they often recur as a result of undetected micrometastatic disease.1,2 After regional therapies with curative intent, these patients undergo further adjuvant systemic therapies to improve survival through eradication of these micrometastases. Adjuvant therapies have shown improvement in long-term outcomes and have arguably contributed more to the improvement of survival than palliative therapies, albeit with varying degrees of success across tumor types.2 Despite this, drug development is overwhelmingly focused on the metastatic setting where advances are most often incremental; only in a minority of cases are the advances substantial. The pace of improvements in adjuvant therapies has unfortunately been critically slow. For example, in non–small-cell lung cancer, two decades of research have resulted in an absolute increase in 5-year survival of 4% to 5%, and the 5-year survival rates for patients with stage II and IIIA disease continue to be dismally low at 30% to 40%.3 In colon cancer, the 5-year survival rate of high-risk subgroups such as patients with stage IIIC disease treated with the current standard of care is as low as 53%.4 Similarly, for breast cancer, the 5-year survival rate of patients with stage III disease is only approximately 50%.5 The case of resectable pancreatic adenocarcinoma is particularly dismal, with 5-year survival rates for these patients of 10% to 20%.6 Clearly, the status quo for adjuvant therapy for solid tumors is not adequate and needs a major overhaul.
What are the obstacles in the development of novel adjuvant therapies? Perhaps the most critical one is that any current adjuvant trial design mandates large sample sizes and long periods of follow-up. These requirements stem from two main factors. First is the inability to definitively identify patients at risk for recurrence, which leaves us with the current paradigm of “treat all to save a few.” As a result, the absolute risk reduction is low and the number needed to treat is high for adjuvant trials. Second, we lack validated surrogate end points for survival in this setting, and therefore, most adjuvant trials are designed with the primary end point of either disease-free or overall survival, which both require long follow-up. The net effect is a high barrier to the conduct of adjuvant studies as a result of the associated costs and risks. A key limitation is our inability to establish proof of concept in the adjuvant space (analogous to phase IB or II studies in the metastatic setting) before embarking on these large, expensive randomized studies. As a result, adjuvant studies commonly test drugs that are active in the metastatic setting, although extensive fundamental work suggests important differences between clinically overt and micrometastatic disease. For instance, in colorectal cancer, agents active in the advanced setting have failed in adjuvant trials cumulatively including more than 15,000 patients.7-14 Here, we propose that circulating tumor DNA (ctDNA) has the potential to radically change this approach and accelerate adjuvant drug development.
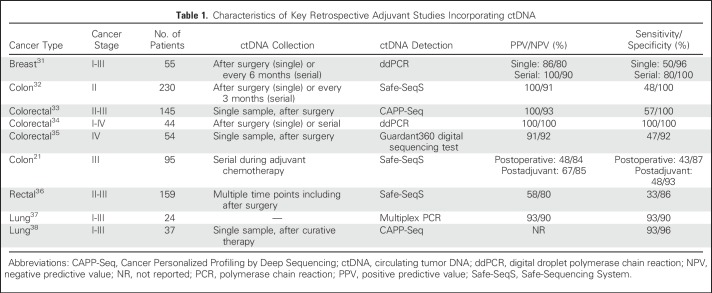
A rapidly increasing body of work has established that ctDNA drawn after completion of curative therapies can identify patients for whom there remains evidence of residual, radiographically occult cancer (Table 1). This state of minimal residual disease (MRD) can be considered analogous to persistent detection of myeloma cells by flow cytometry or polymerase chain reaction (PCR) techniques (below the limit detected by cytomorphology) or to patients with testicular cancer who fail to normalize their tumor markers despite radiographic complete response. These data consistently show that ctDNA has a remarkably high positive predictive value (ie, almost all patients with positive ctDNA assay eventually develop clinical recurrences). Studies evaluating serial samples also suggest that although pretreatment ctDNA levels are not prognostic, serial sampling in the adjuvant setting may improve overall sensitivity. Assays for ctDNA detection include targeted methods based on PCR for known variants such as digital PCR and beads in emulsions and flow cytometry. Although they are cost effective and relatively rapid without the need for bioinformatic analyses, they allow monitoring of only known aberrations. Other next-generation sequencing methods (targeted to a panel of genes as in AmpliSeq [Thermo Fisher Scientific, Waltham, MA], Safe-Sequencing System, and Cancer Personalized Profiling by Deep Sequencing) do not need prior knowledge of the molecular alterations for testing but require comparison with tumor sequencing results to reduce false-positive results.24 An overview of the different platforms and tumor types in which they have been evaluated is provided in Table 1 and detailed in prior reviews.25,26 Although these studies have been done with relatively small sample sizes and varying platforms and methodologies, systematic reviews and meta-analyses combining their data facilitate more reliable and categorical conclusions about the prognostic effects and clinical utility of ctDNA.27-29 They have also provided the preliminary data required to launch larger, prospective, and confirmatory trials to firmly establish the clinical utility and validity of ctDNA as a marker for MRD.
Table 1.
Characteristics of Key Retrospective Adjuvant Studies Incorporating ctDNA
Meanwhile, leveraging lessons we have learned from these trials, we propose that small, proof-of-principle, phase II adjuvant studies with novel therapies could be conducted in a population with detectable disease by ctDNA after curative therapies. These studies, which could be conducted with cohorts of less than 100 patients, would foster rapid progress in the adjuvant space by allowing a lower bar for drug entry. In this setting, evaluation of novel agents can be done efficiently in those at the highest risk of recurrence, while sparing toxicities in those who are not at high risk of recurrence.
As an extension of this logic, clearance of ctDNA may be used as a surrogate end point under the assumption that this is necessary (albeit insufficient as of now) for eventual cure. Although experience from developing biomarkers in the adjuvant setting thus far has shown that the majority of these biomarkers tend to be prognostic with limited predictive value, recent data from an adjuvant colon cancer trial suggest that, in contrast, evaluating serial ctDNA status may in fact serve as a real-time marker of adjuvant therapy efficacy. In this trial, in patients whose ctDNA cleared with adjuvant therapy, there was a trend toward a better 2-year relapse-free survival (hazard ratio, 3.6; P = .06).20 Conversely, patients who were ctDNA negative but turned positive at the end of adjuvant therapy had worse 2-year relapse-free survival (hazard ratio, 6.6; P < .001). It is also crucial that, simultaneously, a longer term goal of validation of clearance of ctDNA as a formal surrogate biomarker of survival be undertaken, similar to the development of pathologic complete response in breast cancer and complete response rate at 30 months in follicular lymphoma as surrogate end points.30-33
We envision that future adjuvant trials may adopt more novel study designs. There are two potential approaches. The first approach is to direct postoperative ctDNA-positive patients who were deemed low risk (by conventional approaches such as staging or histopathology) to adjuvant chemotherapy. The DYNAMIC study (Circulating Tumor DNA [ctDNA] Analysis Informing Adjuvant Chemotherapy in Stage II Colon Cancer; Australian New Zealand Clinical Trials Registry identifier: ACTRN12615000381583) is randomly assigning patients with stage II colon cancer to adjuvant therapy according to the standard-of-care approach versus based on ctDNA results. The primary outcome of the study is to evaluate whether the adjuvant therapy strategy based on ctDNA results affects the number of patients treated with chemotherapy and improves recurrence-free survival. An NRG/NCTN Oncology phase II and III study under development, (CR 1643) aims to enroll patients with resected stage II colon cancer deemed suitable for observation by their physicians; patients will be randomly assigned to observation or adjuvant therapy based on ctDNA results. The primary objective of the phase II portion is to evaluate the rate of ctDNA clearance with adjuvant therapy, whereas the primary objective of the phase III portion is to evaluate recurrence-free survival in ctDNA-positive patients who do and do not receive adjuvant chemotherapy.
The second approach is to escalate therapy for patients with ctDNA despite completion of adjuvant chemotherapy. As discussed earlier, current data show that this would provide a lead time to diagnosis of recurrence of several months over conventional surveillance. Is there a survival benefit in initiating therapy early, before imaging scans show definite evidence of relapse? One could argue, on the basis of conventional wisdom, that because MRD represents lower volume metastatic disease it would be better to proceed with early initiation of therapy rather than waiting until disease is radiographically evident. However, the persistence or reappearance of MRD could also represent a fundamental inability to completely eradicate disease as a result of the intrinsic disease biology, and perhaps one would simply diagnose these patients earlier without effect on long-term outcome.31 One potential option would be to consider a clinical trial where ctDNA-positive patients who have completed adjuvant therapy are randomly assigned to immediate initiation of novel therapy versus a delay in treatment until radiographic progression. The c-TRAK TN trial (ClinicalTrials.gov identifier: NCT03145961) is evaluating this concept by randomly assigning patients with triple-negative breast cancer who are ctDNA positive after completion of primary treatment based on serial ctDNA screening done every 3 months 2:1 to pembrolizumab versus observation.
Several other questions remain unanswered that future trials will also need to focus on. What are the best time points and diagnostic platforms to maximize sensitivity of ctDNA detection? What are the patterns of recurrence (oligometastatic vs not) in ctDNA-positive patients? Is there a role for preemptive locoregional therapies in patients in whom radiographic recurrence is not yet apparent? Can the genomic profiling data provided by ctDNA be used to guide therapies in this setting? What proportion of aberrations detected by ctDNA are driver versus passenger alterations? Most, if not all, of these questions can be tested quickly in the previously described ctDNA-based adjuvant phase II trials before confirmatory phase III trials as needed. As is abundantly clear from prior experience, it is rare that one solution fits all in oncology, and each tumor type will likely require different study designs, novel therapies, and/or different end points.
Although ctDNA holds immense potential, it is important to acknowledge its limitations. Current ctDNA assays have modest sensitivity in the adjuvant setting, ranging from 50% to 60% (Table 1). This is not surprising given that ctDNA constitutes only a small portion of the total circulating free DNA. Furthermore, concentrations of ctDNA correlate strongly with tumor volumes, which are low after resection of all visible disease.34,35 Further technologic advances or combination with orthogonal methodologies may improve this to a certain extent; perhaps the most practical way for now would be to consider serial monitoring.35 As sensitivity increases with future assays, the possibility of false-positive results will also need to be considered, especially age-related somatic mutations leading to hematopoietic clonal expansions.34,36,37 In addition, most mutations are not cancer specific, and therefore, ctDNA may not always accurately identify the primary site. These issues are mitigated to a large extent in the adjuvant setting where patient-specific analyses based on sequencing of the resected primary tumor are typically used. Finally, tumor DNA may not enter the bloodstream from sanctuary sites such as the brain and the testes, and thus, tumors involving these areas could be missed. Looking beyond these logistical issues, several key methodologic questions remain unanswered. For example, should ctDNA measurement be a binary (positive vs negative) or a continuous (volume) variable? The multitude of ctDNA assays available and the pace at which they continue to evolve unfortunately make standardization of data across studies difficult. This highlights the urgent need to develop preanalytic standards and rigor in establishing references, as done by the International Myeloma Working Group for the evaluation of MRD in myeloma.38
In summary, ctDNA shows great promise in adjuvant therapy. The time is now to launch collaborative efforts involving researchers, pharmaceuticals, the National Institutes of Health and the U.S. Food and Drug Administration to incorporate new ideas and novel approaches to harness the full potential of ctDNA for the betterment of our patients.
ACKNOWLEDGMENT
Supported by the National Cancer Institute at the National Institutes of Health (Grants No. P30CA016672 [S.K. and A.D.] and R01CA184843 [S.K.]) and The University of Texas MD Anderson Cancer Center Moonshots Program (S.K.).
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Circulating Tumor DNA–Defined Minimal Residual Disease in Solid Tumors: Opportunities to Accelerate the Development of Adjuvant Therapies
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Arvind Dasari
No relationship to disclose
Axel Grothey
Consulting or Advisory Role: Genentech (Inst), Bayer (Inst), Bristol-Myers Squibb (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Amgen (Inst), Array BioPharma (Inst), Guardant Health (Inst)
Research Funding: Genentech (Inst), Bayer (Inst), Pfizer (Inst), Eisai (Inst), Sanofi (Inst), Eli Lilly (Inst), Boston Biomedical (Inst), Boehringer Ingelheim (Inst)
Travel, Accommodations, Expenses: Genentech, Bayer, Bristol-Myers Squibb, Boston Biomedical, Amgen, Boehringer Ingelheim, Merck Sharp & Dohme
Scott Kopetz
Stock or Other Ownership: MolecularMatch, Navire
Consulting or Advisory Role: Amgen, Roche, Bayer, Array BioPharma, Genentech, Symphogen, EMD Serono, Merck, Karyopharm Therapeutics
Research Funding: Amgen (Inst), Sanofi (Inst), Biocartis (Inst), Guardant Health (Inst), Array BioPharma (Inst), Genentech (Inst), EMD Serono (Inst), MedImmune (Inst), Novartis (Inst)
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival. J Natl Cancer Inst. 2017;109:djx030-djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, III, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370–398. doi: 10.6004/jnccn.2017.0036. [DOI] [PubMed] [Google Scholar]
- 5.Gradishar WJ, Anderson BO, Balassanian R, et al. NCCN guidelines insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw. 2017;15:433–451. doi: 10.6004/jnccn.2017.0044. [DOI] [PubMed] [Google Scholar]
- 6.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. doi: 10.6004/jnccn.2017.0131. [DOI] [PubMed] [Google Scholar]
- 7.Dasari A, Messersmith WA. Should we perform a new adjuvant trial with bevacizumab? Curr Colorectal Cancer Rep. 2011;7:218–226. [Google Scholar]
- 8.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Labianca R, Bodoky G, et al. Randomized phase III trial comparing biweekly infusional fluorouracil/leucovorin alone or with irinotecan in the adjuvant treatment of stage III colon cancer: PETACC-3. J Clin Oncol. 2009;27:3117–3125. doi: 10.1200/JCO.2008.21.6663. [DOI] [PubMed] [Google Scholar]
- 10.Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802) Ann Oncol. 2009;20:674–680. doi: 10.1093/annonc/mdn680. [DOI] [PubMed] [Google Scholar]
- 11.Alberts SR, Sargent DJ, Nair S, et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: A randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taieb J, Tabernero J, Mini E, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:862–873. doi: 10.1016/S1470-2045(14)70227-X. [DOI] [PubMed] [Google Scholar]
- 13.Allegra CJ, Yothers G, O’Connell MJ, et al. Bevacizumab in stage II-III colon cancer: 5-year update of the National Surgical Adjuvant Breast and Bowel Project C-08 trial. J Clin Oncol. 2013;31:359–364. doi: 10.1200/JCO.2012.44.4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Gramont A, Van Cutsem E, Schmoll H-J, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): A phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 15.Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–238. doi: 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 17.Merker JD, Oxnard GR, Compton C, et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists joint review. J Clin Oncol. 2018;36:1631–1641. doi: 10.1200/JCO.2017.76.8671. [DOI] [PubMed] [Google Scholar]
- 18.Cargnin S, Canonico PL, Genazzani AA, et al. Quantitative analysis of circulating cell-free DNA for correlation with lung cancer survival: A systematic review and meta-analysis. J Thorac Oncol. 2017;12:43–53. doi: 10.1016/j.jtho.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Fan G, Zhang K, Yang X, et al. Prognostic value of circulating tumor DNA in patients with colon cancer: Systematic review. PLoS One. 2017;12:e0171991. doi: 10.1371/journal.pone.0171991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan G, Chu C, Gui X, et al. The prognostic value of circulating cell-free DNA in breast cancer: A meta-analysis. Medicine (Baltimore) 2018;97:e0197. doi: 10.1097/MD.0000000000010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tie J, Cohen J, Wang Y, et al. Serial circulating tumor DNA analysis as a prognostic marker and a real-time indicator of adjuvant chemotherapy efficacy in stage III colon cancer J Clin Oncol 362018supplabstr 3516) [Google Scholar]
- 22.Gormley NJ, Farrell AT, Pazdur R. Minimal residual disease as a potential surrogate end point: Lingering questions. JAMA Oncol. 2017;3:18–20. doi: 10.1001/jamaoncol.2016.3112. [DOI] [PubMed] [Google Scholar]
- 23.Anderson KC, Auclair D, Kelloff GJ, et al. The role of minimal residual disease testing in myeloma treatment selection and drug development: Current value and future applications. Clin Cancer Res. 2017;23:3980–3993. doi: 10.1158/1078-0432.CCR-16-2895. [DOI] [PubMed] [Google Scholar]
- 24.Shi Q, Flowers CR, Hiddemann W, et al. Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: An individual patient-level analysis of multiple randomized trials. J Clin Oncol. 2017;35:552–560. doi: 10.1200/JCO.2016.70.8651. [DOI] [PubMed] [Google Scholar]
- 25.Berruti A, Amoroso V, Gallo F, et al. Pathologic complete response as a potential surrogate for the clinical outcome in patients with breast cancer after neoadjuvant therapy: A meta-regression of 29 randomized prospective studies. J Clin Oncol. 2014;32:3883–3891. doi: 10.1200/JCO.2014.55.2836. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich MJ. Going with the flow: The promise and challenge of liquid biopsies. JAMA. 2017;318:1095–1097. doi: 10.1001/jama.2017.10203. [DOI] [PubMed] [Google Scholar]
- 27.Donaldson J, Park BH. Circulating tumor DNA: Measurement and clinical utility. Annu Rev Med. 2018;69:223–234. doi: 10.1146/annurev-med-041316-085721. [DOI] [PubMed] [Google Scholar]
- 28.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genovese G, Kähler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–2487. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7:302ra133. doi: 10.1126/scitranslmed.aab0021. [DOI] [PubMed] [Google Scholar]
- 32.Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Diehn M, Alizadeh AA, Adams H-P, et al. Early prediction of clinical outcomes in resected stage II and III colorectal cancer (CRC) through deep sequencing of circulating tumor DNA (ctDNA) J Clin Oncol. 2017;35(suppl) abstr 3591. [Google Scholar]
- 34.Schøler LV, Reinert T, Ørntoft MW, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23:5437–5445. doi: 10.1158/1078-0432.CCR-17-0510. [DOI] [PubMed] [Google Scholar]
- 35.Overman MJ, Vauthey J-N, Aloia TA, et al. Circulating tumor DNA (ctDNA) utilizing a high-sensitivity panel to detect minimal residual disease post liver hepatectomy and predict disease recurrence J Clin Oncol 352017supplabstr 3522) [Google Scholar]
- 36.Tie J, Cohen JD, Wang Y, et al. Serial circulating tumour DNA analysis during multimodality treatment of locally advanced rectal cancer: A prospective biomarker study. Gut. doi: 10.1136/gutjnl-2017-315852. 10.1136/gutjnl-2017-315852 [epub ahead of print on February 2, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbosh C, Birkbak NJ, Wilson GA, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–451. doi: 10.1038/nature22364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]