Abstract
Purpose
Dabrafenib plus trametinib improved relapse-free survival (RFS) versus placebo (hazard ratio [HR], 0.47; P < .001) in patients with resected BRAF V600–mutant stage III melanoma (BRF115532; COMBI-AD; ClinicalTrials.gov identifier: NCT01682083). We present an updated RFS analysis on the basis of extended study follow-up and a cure-rate model analysis to estimate the fraction of patients expected to remain relapse free long term.
Methods
In this phase III trial, patients with resected BRAF V600–mutant stage III melanoma were randomly assigned to 12 months of adjuvant dabrafenib plus trametinib versus placebo. We report updated RFS (primary end point) and distant metastasis–free survival. RFS was also analyzed by subgroups defined by baseline disease stage (American Joint Committee on Cancer 7th and 8th editions), nodal metastatic burden, and ulceration status. The fraction of patients who remained relapse free long term was estimated using a Weibull mixture cure-rate model.
Results
At median follow-up of 44 months (dabrafenib plus trametinib) and 42 months (placebo), 3- and 4-year RFS rates were 59% (95% CI, 55% to 64%) and 54% (95% CI, 49% to 59%) in the dabrafenib plus trametinib arm and 40% (95% CI, 35% to 45%) and 38% (95% CI, 34% to 44%) in the placebo arm, respectively (HR, 0.49; 95% CI, 0.40 to 0.59). Distant metastasis–free survival also favored dabrafenib plus trametinib (HR, 0.53; 95% CI, 0.42 to 0.67). The estimated cure rate was 54% (95% CI, 49% to 59%) in the dabrafenib plus trametinib arm compared with 37% (95% CI, 32% to 42%) in the placebo arm. Subgroup analysis of RFS demonstrated similar treatment benefit regardless of baseline factors, including disease stage, nodal metastatic burden, and ulceration.
Conclusion
Longer follow-up confirmed RFS benefit with dabrafenib plus trametinib. Subgroup analysis suggested that dabrafenib plus trametinib benefited patients regardless of baseline factors.
INTRODUCTION
The development of targeted therapies and immune checkpoint inhibitors has led to substantial improvements in outcomes for patients with unresectable or metastatic melanoma.1 Recently, advances in systemic therapy in the metastatic setting have translated to effective adjuvant therapy for patients with resected, regionally advanced disease who have a high risk of relapse.2-6 The anti–cytotoxic T-cell lymphocyte-antigen 4 antibody ipilimumab, anti–programmed death-1 antibodies nivolumab and pembrolizumab, and the combination of dabrafenib and trametinib have demonstrated significant relapse-free survival (RFS) benefit as adjuvant therapies in patients with resected melanoma.2-6
The COMBI-AD trial was the first prospective phase III trial evaluating BRAF inhibitor and MEK inhibitor combination therapy as adjuvant treatment in patients with completely resected BRAF V600 mutation–positive stage III melanoma6; 12 months of adjuvant dabrafenib plus trametinib significantly improved RFS compared with placebo. At the primary analysis—median follow-up of 34 months (dabrafenib plus trametinib) and 33 months (placebo)—estimated 3-year RFS was 58% in the combination therapy arm versus 39% in the placebo arm (hazard ratio [HR], 0.47; P < .001). An interim analysis of overall survival (OS) demonstrated an improvement in the combination therapy arm, with a 3-year OS of 86% versus 77% in the placebo group (HR, 0.57; 95% CI, 0.42 to 0.79; P = .0006), but this improvement did not cross the prespecified interim analysis significance threshold of P = .000019. Adjuvant dabrafenib plus trametinib also reduced the risk of distant metastatic relapse or death (HR, 0.51; P < .001) and was associated with no new toxicities compared with those observed in the metastatic melanoma setting.7,8 On the basis of these results, the combination of dabrafenib plus trametinib has been approved by the US Food and Drug Administration and the European Commission as adjuvant therapy for patients with completely resected BRAF V600E/K–mutant stage III melanoma.
Here, we present updated RFS and distant metastasis–free survival (DMFS) analyses resulting from an extended follow-up of patients in the COMBI-AD trial. An updated OS analysis was not performed because the prespecified number of events has not been reached. The data from this analysis were then also used to generate a statistical Weibull mixture cure-rate model to provide an estimate of the fraction of patients who will remain relapse free long term. To our knowledge, this is the first use of this methodology in a trial that evaluated adjuvant therapy in patients with resected melanoma. Finally, we present results of RFS analyses from clinically relevant subgroups, including by baseline disease stage (per American Joint Committee on Cancer [AJCC] 7th9 and 8th10 editions), baseline nodal metastatic burden (micro- or macrometastasis), and ulceration status, to evaluate whether any baseline factors may be predictive of response to adjuvant dabrafenib plus trametinib therapy.
METHODS
Study Design and Participants
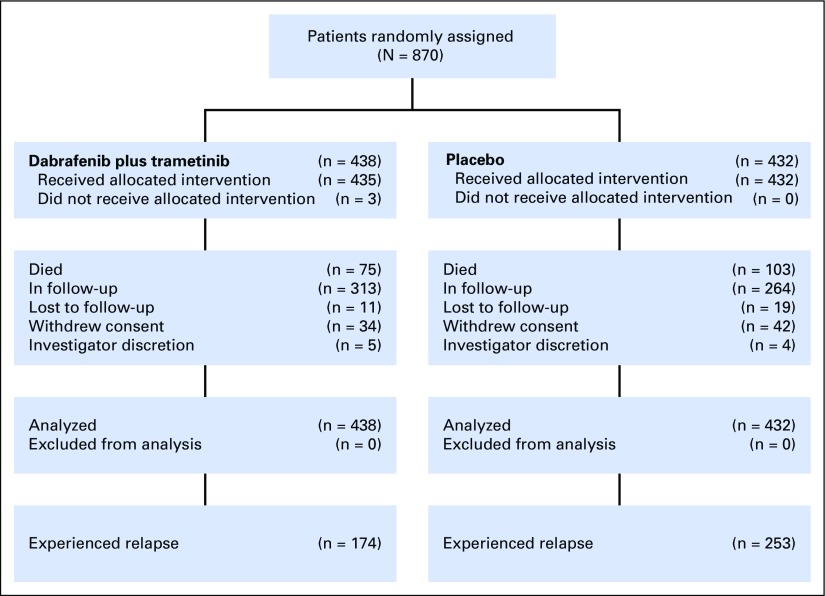
COMBI-AD was a randomized, double-blind, placebo-controlled, phase III trial comparing dabrafenib 150 mg twice per day plus trametinib 2 mg once per day versus two matched placebos (Fig 1). This trial enrolled patients from 169 sites across 25 countries from January 2013 to December 2014. Full eligibility criteria have been reported previously.6 Eligible patients (age ≥ 18 years) underwent complete resection of histologically confirmed stage IIIA (lymph node metastasis > 1 mm), IIIB, or IIIC cutaneous melanoma (per AJCC 7th edition) that was positive for a BRAF V600E or V600K mutation confirmed in primary tumor or lymph node tissue by a central reference laboratory. Patients were required to have undergone and recovered from a complete lymphadenectomy without clinical or radiographic indication of regional nodal disease within 12 weeks of random assignment and to have an Eastern Cooperative Oncology Group performance status of 0 or 1.
Fig 1.
CONSORT diagram.
Patients were stratified by BRAF mutation status (V600E or V600K) and disease stage (IIIA, IIIB, or IIIC according to AJCC 7th edition). Treatment continued for 12 months unless preceded by disease relapse, unacceptable toxicity, withdrawal of consent, or death, whichever occurred first. Patients were observed for disease relapse until first relapse was observed and were then observed for survival. Disease assessments via imaging using computed tomography, magnetic resonance imaging, or both were performed every 3 months for the initial 24 months, then every 6 months until study completion or disease relapse. Dermatologic evaluations were performed every 2 months during treatment, every 3 months during follow-up for the first 24 months, and every 6 months thereafter. Dose modifications or interruptions were allowed.6 To date, two RFS analyses have been conducted: the primary analysis previously reported and the extended follow-up reported here. The trial is ongoing. An interim analysis of OS was reported with the primary RFS analysis.6 The next OS analysis will be conducted when 50% of events have occurred.
End Points
The primary end point was RFS, defined as the time from random assignment until disease relapse (or new primary melanoma) or death from any cause. DMFS was defined as the time from random assignment to the date of first distant metastasis or date of death. Other secondary end points, including OS, freedom from relapse, and safety, were defined previously.6 Assessment of RFS in subgroups on the basis of baseline disease stage (per AJCC 7th edition) and nodal tumor burden were prespecified exploratory analyses. Evaluation of RFS on the basis of AJCC 8th edition disease stage at baseline and tumor ulceration status were post hoc analyses. All disease-relapse analyses were based on investigator assessment in the intent-to-treat population (all randomly assigned patients).
Statistical Analysis
We used the Kaplan-Meier method to estimate RFS and DMFS. A stratified log-rank test to compare differences between treatment groups was used for the primary analysis. HRs and 95% CIs were calculated using the Pike estimator.11,12 A mixed Weibull cure-rate model was used to estimate long-term relapse–free fractions of patients in each treatment arm. Cure-rate models represent a statistical modeling approach that was developed to model time-to-event data in situations in which it is reasonable to assume that a subset of patients will remain event free long term and are therefore cured.13,14
Trial Oversight
The trial was originally sponsored by GlaxoSmithKline; dabrafenib and trametinib were designated as assets of Novartis on March 2, 2015, after which Novartis took over sponsorship of the trial. The trial was conducted in accordance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by the institutional review board or independent ethics committee at each trial center. All patients provided written informed consent for data collection supporting these analyses. All authors developed the initial draft of the manuscript and made the decision to submit it for publication. All authors contributed to subsequent drafts. The authors affirm the accuracy and completeness of the data and adherence of the trial to the protocol.
RESULTS
A total of 870 patients (dabrafenib plus trametinib, n = 438; placebo, n = 432) were randomly assigned to receive 12 months of adjuvant treatment. Baseline characteristics were well balanced between treatment arms (Appendix Table A1, online only). At the time of this analysis (April 30, 2018), no patients remained on treatment—last dose of study drug was administered to the last patient in December 2015. Median patient follow-up was 44 months in the dabrafenib plus trametinib arm and 42 months in the placebo arm (minimum study follow-up, 40 months; Appendix Table A2, online only).
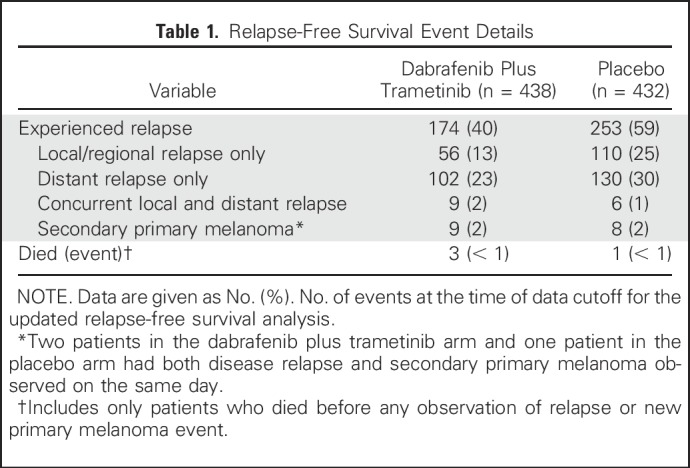
During the additional follow-up from the primary analysis,6 RFS events were reported in 11 patients—two patients with new primary melanoma—in the dabrafenib plus trametinib arm and in six patients in the placebo arm. Overall, 174 patients (40%) in the dabrafenib plus trametinib arm and 253 (59%) in the placebo arm experienced relapse, and the majority in both arms experienced distant relapse (Table 1). The most common sites of distant relapse were lung (8%), liver (5%), and CNS (5%) in the dabrafenib plus trametinib arm and lung (14%), lymph node (7%), and liver (5%) in the placebo arm.
Table 1.
Relapse-Free Survival Event Details
Updated RFS and DMFS
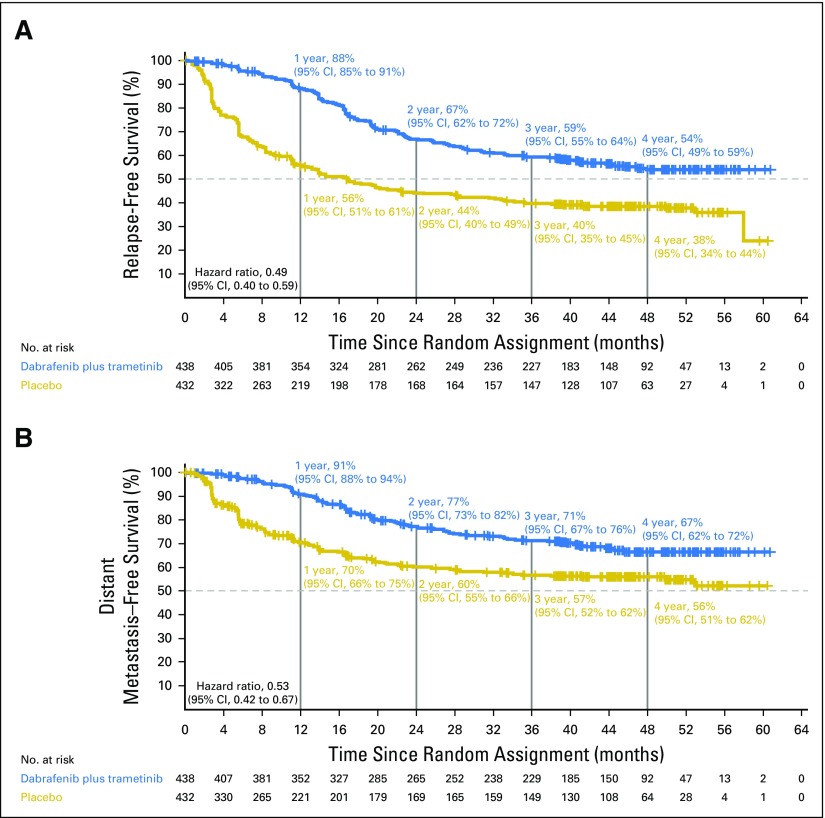
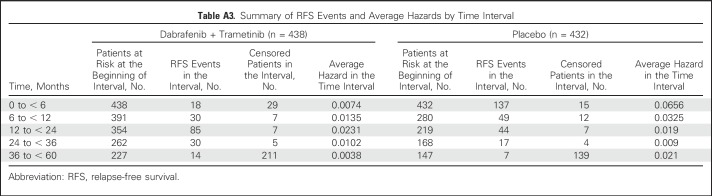
Investigator-assessed median RFS was not reached (95% CI, 46.9 months to not reached) in the dabrafenib plus trametinib arm compared with 16.6 months (95% CI, 12.7 months to 22.1 months) in the placebo arm (HR, 0.49; 95% CI, 0.40 to 0.59; Fig 2A). Three- and 4-year RFS rates were 59% (95% CI, 55% to 64%) and 54% (95% CI, 49% to 59%) in the dabrafenib plus trametinib group and 40% (95% CI, 35% to 45%) and 38% (95% CI, 34% to 44%) in the placebo group, respectively. Few RFS events occurred after 3 years of follow-up in both treatment arms (Appendix Table A3, online only).
Fig 2.
Relapse-free survival and distant metastasis–free survival. (A) Kaplan-Meier estimates of relapse-free survival and (B) distant metastasis–free survival from the intent-to-treat population at the data cutoff of April 30, 2018.
Updated analysis of DMFS yielded an HR of 0.53 (95% CI, 0.42 to 0.67), which indicated a 47% reduction in the risk of developing distant metastases or death when patients were treated with dabrafenib plus trametinib (Fig 2B).
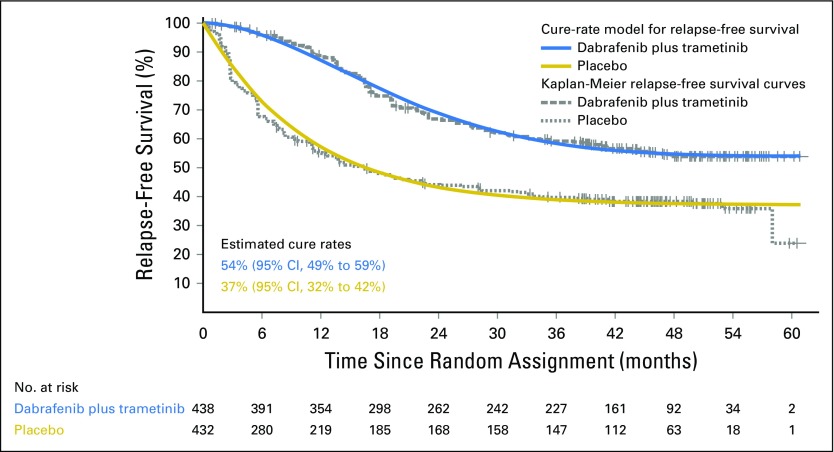
Cure-Rate Model
On the basis of a Weibull mixture cure-rate model for RFS, the estimated fraction of patients who may never experience relapse was 54% (95% CI, 49% to 59%) in the dabrafenib plus trametinib arm versus 37% (95% CI, 32% to 42%) in the placebo arm (Fig 3).
Fig 3.
Weibull mixture cure-rate model. Curves fitted to the Weibull mixture cure-rate analysis (solid lines) are overlaid with Kaplan-Meier curves of relapse-free survival (dashed lines) using the data cutoff of April 30, 2018.
Subgroup Analysis of RFS
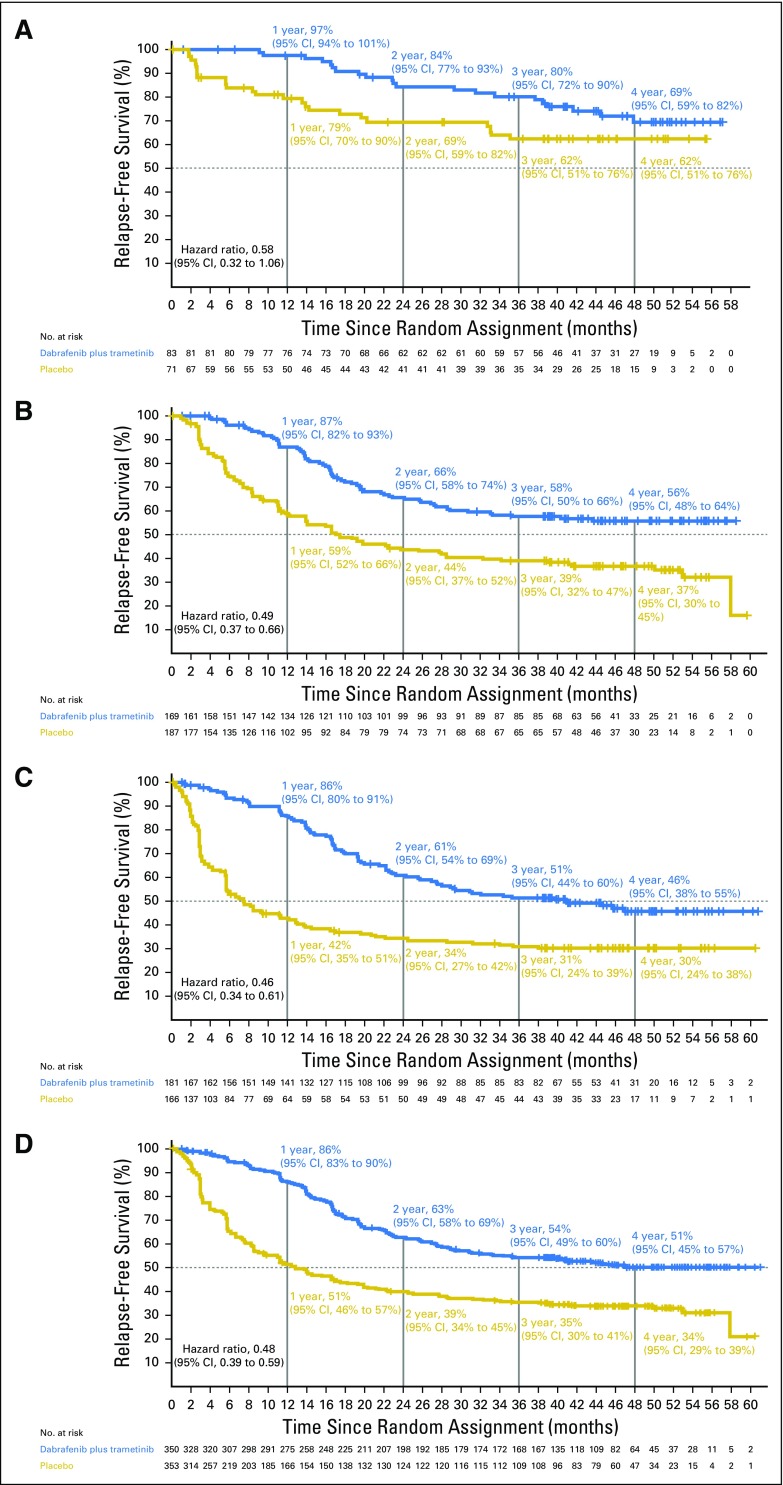
RFS on the basis of disease stage (AJCC 7th and 8th editions).
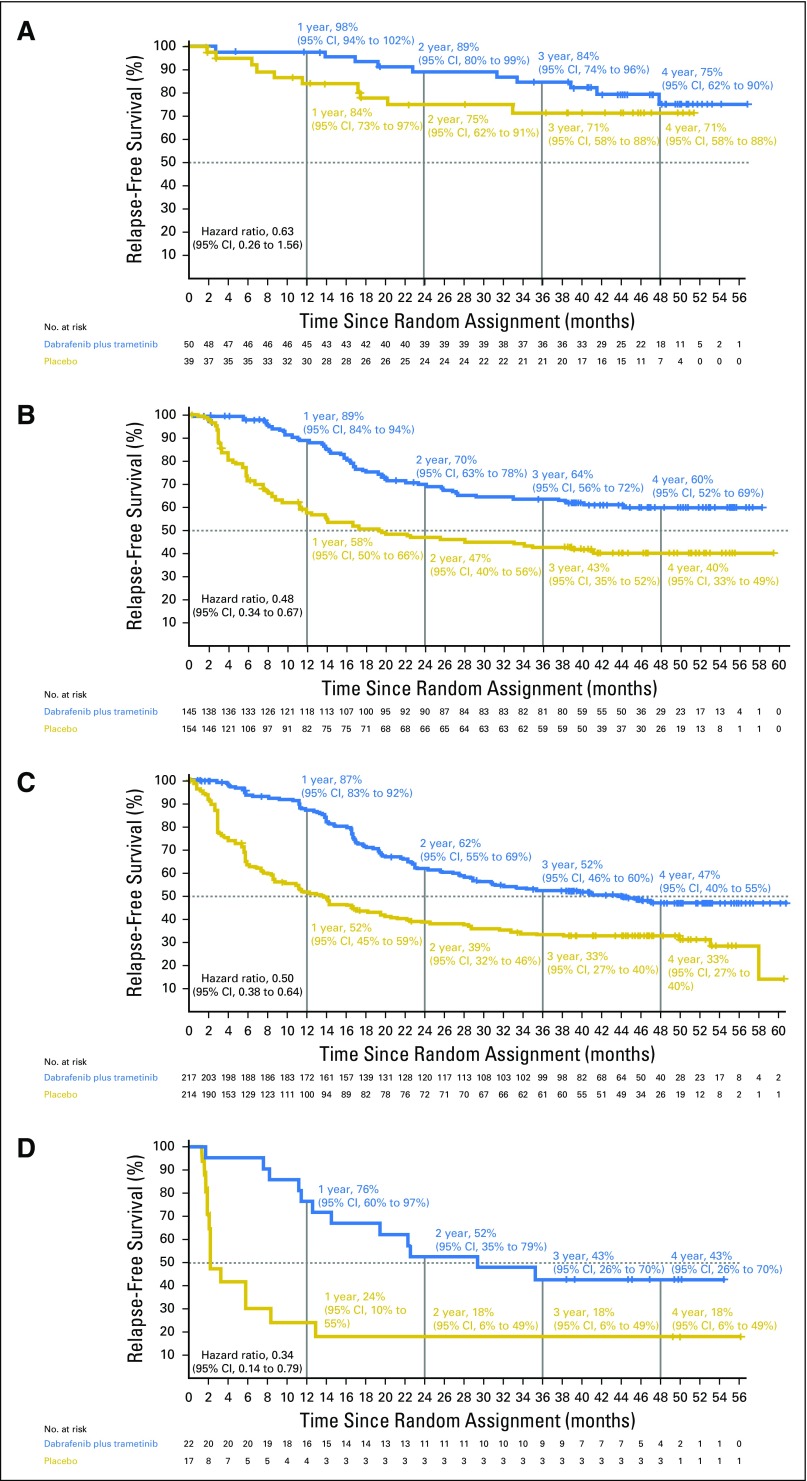
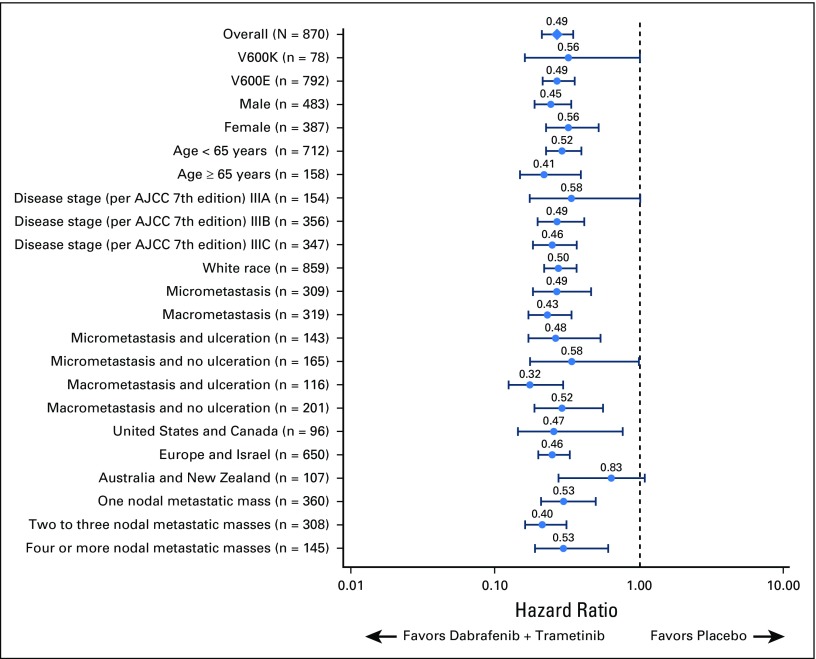
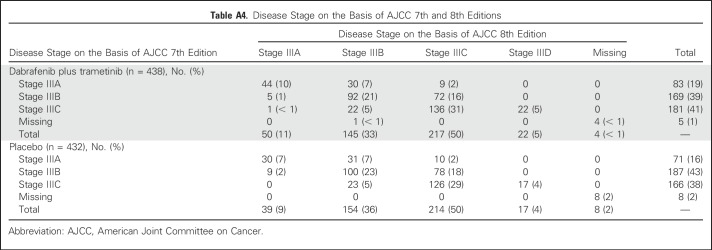
Disease stage on the basis of AJCC 7th edition—stage IIIA, IIIB, and IIIC—was a stratification factor in the trial (Appendix Table A4, online only). Subgroup analysis on the basis of disease stage per AJCC 7th edition demonstrated that dabrafenib plus trametinib improved RFS across all disease stage subgroups compared with placebo (stage IIIA: HR, 0.58; 95% CI, 0.32 to 1.06; stage IIIB: HR, 0.49; 95% CI, 0.37 to 0.66; stage IIIC: HR, 0.46; 95% CI, 0.34 to 0.61; Fig 4). Of note, when patients with stage IIIB or IIIC—those at highest risk for disease relapse—were combined, a 52% reduction in the risk of relapse or death was observed that favored the dabrafenib plus trametinib arm (HR, 0.48; 95% CI, 0.39 to 0.59; Fig 4). Because COMBI-AD was initiated before the release of the recent AJCC 8th edition, stage grouping was based on AJCC 7th edition. Post hoc analysis of RFS was conducted on the basis of baseline disease stage according to AJCC 8th edition, which, unlike AJCC 7th edition, includes T stage in addition to N stage (stage IIIA, IIIB, and IIIC, as well as the new stage IIID category; Appendix Table A4). Similar to the analysis by AJCC 7th edition criteria, dabrafenib plus trametinib improved RFS across all AJCC 8th edition stage subgroups compared with placebo (stage IIIA: HR, 0.63; 95% CI, 0.26 to 1.56; stage IIIB: HR, 0.48; 95% CI, 0.34 to 0.67; stage IIIC: HR, 0.50; 95% CI, 0.38 to 0.64; stage IIID: HR, 0.34; 95% CI, 0.14 to 0.79; Fig 5).
Fig 4.
Kaplan-Meier curve of relapse-free survival by disease stage per American Joint Committee on Cancer (AJCC) 7th edition. Data from patients with baseline stage (A) IIIA, (B) IIIB, (C) IIIC, and (D) IIIB/C disease per AJCC 7th edition are shown with a data cutoff of April 30, 2018.
Fig 5.
Post hoc analysis of relapse-free survival by disease stage on the basis of American Joint Committee on Cancer (AJCC) 8th edition. Data from patients with baseline stage (A) IIIA, (B) IIIB, (C) IIIC, and (D) IIID disease per AJCC 8th edition are shown with a data cutoff of April 30, 2018.
RFS on the basis of nodal metastatic burden and ulceration.
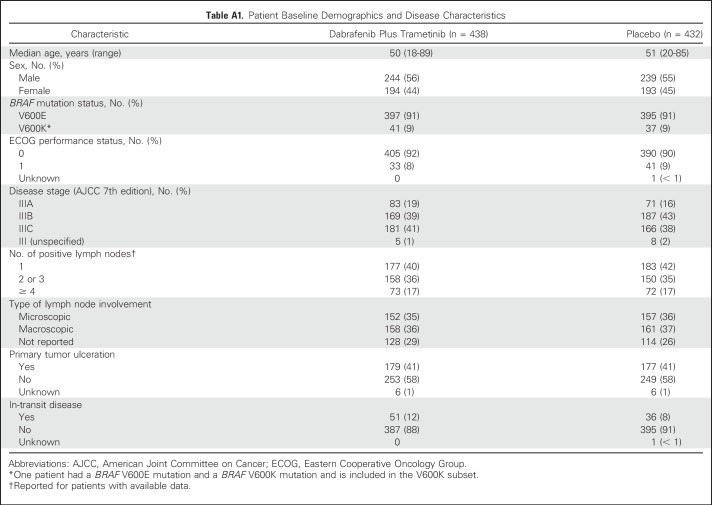
Additional analyses demonstrated that RFS favored dabrafenib plus trametinib across all prespecified subgroups (Fig 6). Treatment with dabrafenib plus trametinib led to a similar reduction in the risk of relapse or death in patients with baseline micrometastases (HR, 0.49; 95% CI, 0.34 to 0.70) and in those with baseline macrometastases (HR, 0.43; 95% CI, 0.31 to 0.58) compared with treatment with placebo. Post hoc analysis of RFS by baseline tumor ulceration status—ulcerated versus nonulcerated—also showed similar benefit that favored dabrafenib plus trametinib compared with placebo regardless of status. In patients without baseline tumor ulceration, HR for RFS was 0.53 (95% CI, 0.41 to 0.69). Among patients with tumor ulceration at baseline, HR was 0.45 (95% CI, 0.34 to 0.60). Across all baseline factors, consistent benefit favoring dabrafenib plus trametinib versus placebo was observed.
Fig 6.
Forest plot of relapse-free survival by subgroup. Number of patients are included in parentheses. Only subgroups with ≥ 20 patients are included. AJCC, American Joint Committee on Cancer.
DISCUSSION
In this updated analysis, we confirmed the clinically relevant RFS benefit favoring dabrafenib plus trametinib versus placebo. RFS Kaplan-Meier curves continued to be separated after the 12-month treatment phase. With extended follow-up, RFS Kaplan-Meier curves are fully mature up to approximately 40 months and thus provide stable 3-year RFS rates that demonstrate a nearly 20% absolute difference between arms. Moreover, the number of RFS events in both arms decreased over time, with few events observed after 3 years of follow-up, which is consistent with what has been reported in other studies.15
RFS Kaplan-Meier curves generated in this updated analysis suggest the potential formation of an RFS plateau; however, additional follow-up is needed to confirm this. Of note, a similar observation was made in the EORTC 18071 trial that evaluated adjuvant ipilimumab versus placebo in patients with resected stage III melanoma.2,16 In that trial, improved RFS in patients who were treated with ipilimumab compared with placebo observed at the primary analysis (median follow-up, 2.7 years; HR, 0.75) was also observed with extended follow-up to 5.3 years, with a lower rate of RFS events reported over time. Anti–programmed death-1 therapies nivolumab and pembrolizumab have also demonstrated significant RFS benefit compared with ipilimumab in patients with resected stage IIIB/C or IV disease3,4 and compared with placebo in patients with resected stage IIIA/B/C disease,5 respectively. Thus far, follow-up in these trials has been limited to 24 months for the CheckMate-238 trial and a 15-month median follow-up in the KEYNOTE-054 trial.4,5 Additional follow-up is necessary to understand whether the number of RFS events diminishes over time with these therapies as well.
Among patients with relapsed disease, distant relapses were the most common sites in patients from both the dabrafenib plus trametinib (64% of patients with relapse) and placebo arms (54%). These rates are consistent with reports from other adjuvant trials with rates of distant relapse of 57% and 58% among patients who experienced a relapse event in the CheckMate-238 (with nivolumab) and KEYNOTE-054 (with pembrolizumab) trials, respectively.4,5 Lower rates of distant relapse (51%) have been reported in a retrospective analysis of patients with stage III disease15; however, this discrepancy may be explained, at least in part, by enhanced screening in a trial setting.
OS in the EORTC 18071 trial at the primary RFS analysis was immature at a median follow-up of 2.7 years16; however, extended follow-up in the EORTC study2 has demonstrated significant OS benefit. Updated OS analysis from the COMBI-AD trial is not reported here because the number of events needed for the next prespecified interim analysis of OS has not been reached. Statistical modeling using a Weibull mixture cure-rate analysis that allows for the estimation of the fraction of patients who may never experience relapse provides a means of assessing long-term outcomes in the absence of direct OS data. Curves fitted using the cure-rate model closely match Kaplan-Meier curves of RFS, with estimated cure rates of 54% versus 37% in the dabrafenib plus trametinib versus placebo arms, respectively. The difference between those two estimated rates indicate the magnitude of RFS effect that is preserved long term. In general, cure-rate modeling in cancer trial settings has complemented standard survival analysis and has been used in situations in which it is reasonable to assume that a fraction of patients will never experience an event of interest—the model allows for estimation of this fraction.13 These models have been used successfully in multiple disease settings, including early-stage cutaneous melanoma, gastric cancer, acute myeloid leukemia, and multiple myeloma.14,17-19 In a population-based study of patients with cutaneous melanoma, cure-rate modeling demonstrated significant differences in cure rate on the basis of disease stage at diagnosis.17 In patients with stage III melanoma, 10-year RFS rates of approximately 35% have been reported with a decreasing frequency of RFS events over time, which indicates that a subset of patients experienced long-term RFS.15,20 Additional validation of the utility of this approach with extended follow-up in patients receiving adjuvant therapy for melanoma is warranted, including correlation with OS data.
The COMBI-AD trial was designed to evaluate 12 months of adjuvant therapy with dabrafenib plus trametinib. No patients were receiving treatment—last dose of study drug was administered to the last patient in December 2015—during this extended follow-up period. Because no additional drug-related toxicities were anticipated, safety results were not updated at this data cutoff. At the primary analysis,6 the most common adverse events reported in the combination therapy arm were pyrexia (any grade, 63%; grade 3 and 4, 5%), fatigue (47%; 4%), and nausea (40%; 1%). These adverse events are similar to those reported in phase III trials of dabrafenib plus trametinib in patients with stage IIIC unresectable melanoma or stage IV metastatic melanoma with BRAF V600E or V600K mutations.7,8 Health-related quality-of-life data demonstrated that adjuvant treatment with dabrafenib plus trametinib did not negatively affect patient-reported quality of life during the treatment phase or in long-term follow-up.21
Patients with high-risk resectable melanoma represent a broad and diverse group; therefore, there is great clinical interest in understanding outcomes according to baseline characteristics. Subgroup analysis of RFS in this study demonstrated that the RFS benefit observed with dabrafenib plus trametinib versus placebo was similar regardless of baseline disease stage, metastatic load, or tumor ulceration status. This is comparable to results from subgroup analyses of data from the CheckMate-238 and KEYNOTE-054 trials, which demonstrated improved RFS with nivolumab and pembrolizumab, respectively, across subgroups.4,5 Although comparisons between clinical trials should be interpreted cautiously, 2-year RFS rates in patients with stage IIIB/C disease (per AJCC 7th edition) were similar in the nivolumab arm of the CheckMate-238 trial (64%)4 and in the dabrafenib plus trametinib arm of the COMBI-AD trial (63%). All patients in COMBI-AD had BRAF-mutant disease compared with 41% of patients in the nivolumab arm of CheckMate-238 (62% 2-year RFS rate among patients with BRAF-mutant melanoma). To date, RFS on the basis of AJCC 8th edition10 criteria for the CheckMate-238 and KEYNOTE-054 trials has not been published, and it remains to be seen whether results from these trials will be similar when patient disease-stage subgroups are categorized according to these new criteria. In the COMBI-AD trial, we observed a consistent benefit favoring dabrafenib plus trametinib across subgroups according to AJCC 8th edition criteria.
Overall, the updated results with longer follow-up confirm an RFS benefit in patients who are treated with dabrafenib plus trametinib compared with placebo-treated patients. Subgroup analysis supports the use of dabrafenib plus trametinib regardless of clinical and pathologic factors at initiation of therapy on the basis of similar RFS benefit.
ACKNOWLEDGMENT
We thank the patients and their families for participating in this study. We also thank Maurizio Voi (Novartis Pharmaceuticals Corporation) for guidance and critical review of the report. Editorial assistance was provided by Jorge J. Moreno-Cantu (Novartis Pharmaceuticals Corporation). Medical writing assistance was provided by Michael Demars (ArticulateScience) and funded by Novartis Pharmaceuticals Corporation.
Appendix
Table A1.
Patient Baseline Demographics and Disease Characteristics
Table A2.
Patient Disposition
Table A3.
Summary of RFS Events and Average Hazards by Time Interval
Table A4.
Disease Stage on the Basis of AJCC 7th and 8th Editions
Footnotes
Processed as a Rapid Communication manuscript.
COMBI-AD was originally sponsored by GlaxoSmithKline; dabrafenib and trametinib were designated as assets of Novartis on March 2, 2015, after which Novartis took over sponsorship of the trial.
AUTHOR CONTRIBUTIONS
Conception and design: Axel Hauschild, Reinhard Dummer, Caroline Robert, Mark Shilkrut, John M. Kirkwood, Georgina V. Long
Provision of study materials or patients: Axel Hauschild, Dirk Schadendorf, Mario Santinami, Mario Mandalà, Vanna Chiarion-Sileni, Andrew Haydon, Caroline Robert, Thierry Lesimple, Ruth Plummer, Richard Kefford, Georgina V. Long
Collection and assembly of data: Axel Hauschild, Reinhard Dummer, Dirk Schadendorf, Mario Santinami, Victoria Atkinson, Mario Mandalà, Vanna Chiarion-Sileni, James Larkin, Marta Nyakas, Caroline Dutriaux, Andrew Haydon, Caroline Robert, Jacob Schachter, Ruth Plummer, Kohinoor Dasgupta, Mark Shilkrut, Eduard Gasal, Georgina V. Long
Data analysis and interpretation: Axel Hauschild, Reinhard Dummer, Dirk Schadendorf, Mario Santinami, Victoria Atkinson, Mario Mandalà, Vanna Chiarion-Sileni, James Larkin, Andrew Haydon, Caroline Robert, Laurent Mortier, Jacob Schachter, Thierry Lesimple, Ruth Plummer, Kohinoor Dasgupta, Tomas Haas, Mark Shilkrut, Eduard Gasal, Richard Kefford, Georgina V. Long
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Longer Follow-Up Confirms Relapse-Free Survival Benefit With Adjuvant Dabrafenib Plus Trametinib in Patients With Resected BRAF V600–Mutant Stage III Melanoma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Axel Hauschild
Honoraria: Amgen, Bristol-Myers Squibb, MedImmune, Merck Serono, Merck Sharp & Dohme, Novartis, OncoSec, Roche, Nektar, Philogen, Provectus, Regeneron
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, MedImmune, Merck Serono, Merck Sharp & Dohme, Novartis, OncoSec, Roche, Nektar, Philogen, Provectus, Regeneron
Research Funding: Amgen, Bristol-Myers Squibb, Celgene, Eisai, GlaxoSmithKline, Merck Serono, Merck Sharp & Dohme, Novartis, Roche
Travel, Accommodations, Expenses: Amgen, Bristol-Myers Squibb, MedImmune, Merck Serono, Merck Sharp & Dohme, Novartis, OncoSec, Roche, Nektar, Philogen, Provectus, Regeneron
Reinhard Dummer
Honoraria: Roche, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Amgen, Takeda, Pierre Fabre, Sun Pharma
Consulting or Advisory Role: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst), Novartis (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst)
Dirk Schadendorf
Honoraria: Roche/Genentech, Novartis, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Sysmex, Immunocore, Grünenthal Group, Merck Serono, Agenus, Array BioPharma, AstraZeneca, LEO Pharma, Incyte, Pfizer, Pierre Fabre, Philogen, Regeneron, 4SC, Mologen
Consulting or Advisory Role: Roche/Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Mologen, Sanofi, Regeneron
Speakers' Bureau: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen, Incyte, Pierre Fabre
Research Funding: Bristol-Myers Squibb (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Bristol-Myers Squibb, Amgen, Merck, Merck Serono, Novartis
Mario Santinami
No relationship to disclose
Victoria Atkinson
Honoraria: Bristol-Myers Squibb, Novartis
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Merck Serono, Pierre Fabre
Speakers' Bureau: Roche/Genentech, Bristol-Myers Squibb, Novartis, Merck Sharp & Dohme
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Mario Mandalà
Honoraria: Merck Oncology, Novartis, Roche/Genentech, Bristol-Myers Squibb, Pierre Fabre
Consulting or Advisory Role: Novartis, Bristol-Myers Squibb, Pierre Fabre, Roche/Genentech, Merck Oncology
Research Funding: Novartis (Inst), Roche/Genentech (Inst)
Vanna Chiarion-Sileni
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Pierre Fabre, Merck Oncology
Speakers' Bureau: Bristol-Myers Squibb, Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb
James Larkin
Honoraria: Eisai, Bristol-Myers Squibb, Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Sectra, Pierre Fabre, EUSA Pharma
Consulting or Advisory Role: Eisai, Bristol-Myers Squibb, Merck Sharp & Dohme, GlaxoSmithKline, Pfizer, Novartis, Roche/Genentech, Sectra, Pierre Fabre, EUSA Pharma
Research Funding: Pfizer (Inst), Novartis (Inst), Merck Sharp & Dohme (Inst), Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Pfizer, Novartis, Genentech
Marta Nyakas
Consulting or Advisory Role: Novartis (Inst), Incyte (Inst), Merck Oncology (Inst)
Caroline Dutriaux
Consulting or Advisory Role: Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Roche
Andrew Haydon
Honoraria: Novartis, Merck
Consulting or Advisory Role: Novartis
Speakers' Bureau: Novartis, Merck
Caroline Robert
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, Merck, Amgen, Novartis, GlaxoSmithKline, Pierre Fabre, Merck Serono
Laurent Mortier
Travel, Accommodations, Expenses: Genentech, Novartis, Bristol-Myers Squibb
Jacob Schachter
Honoraria: Bristol-Myers Squibb, Merck Sharp & Dohme
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Thierry Lesimple
Consulting or Advisory Role: Roche, Novartis, Merck Sharp & Dohme, Incyte
Speakers' Bureau: Merck Sharp & Dohme, Bristol-Myers Squibb, Roche, Novartis
Research Funding: Roche (Inst)
Travel, Accommodations, Expenses: Roche, Merck Sharp & Dohme
Ruth Plummer
Honoraria: Roche/Genentech, Bristol-Myers Squibb, Pfizer (I)
Consulting or Advisory Role: Roche/Genentech, Clovis Oncology, Merck Oncology, Novartis, Astex Pharmaceuticals, Pierre Fabre, Bayer, Octimet, Biosceptre, Ellipses Pharma, Karus Therapeutics
Speakers' Bureau: Novartis
Research Funding: AstraZeneca/MedImmune (Inst)
Patents, Royalties, Other Intellectual Property: Patent for use of PARP inhibitor rucaparib (Inst)
Travel, Accommodations, Expenses: Merck Oncology, Bristol-Myers Squibb
Kohinoor Dasgupta
Employment: Novartis
Tomas Haas
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Travel, Accommodations, Expenses: Novartis
Mark Shilkrut
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Eduard Gasal
Employment: Novartis
Stock and Other Ownership Interests: Novartis
Richard Kefford
Consulting or Advisory Role: Amgen (Inst), Teva Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Amgen
John M. Kirkwood
Consulting or Advisory Role: Bristol-Myers Squibb, Novartis, Array BioPharma, Merck, Roche, Amgen, Immunocore
Research Funding: Merck (Inst), Prometheus Laboratories (Inst)
Georgina V. Long
Honoraria: Bristol-Myers Squibb, Merck, Roche, Novartis, Incyte
Consulting or Advisory Role: Bristol-Myers Squibb, Roche/Genentech, Amgen, Merck, Novartis, Array BioPharma, Pierre Fabre, Incyte
REFERENCES
- 1.Ugurel S, Röhmel J, Ascierto PA, et al. : Survival of patients with advanced metastatic melanoma: The impact of novel therapies-update 2017. Eur J Cancer 83:247-257, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. : Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375:1845-1855, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber J, Mandala M, Del Vecchio M, et al. : Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824-1835, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, Mandalà M, Del Vecchio M, et al. : Adjuvant therapy with nivolumab (NIVO) versus ipilimumab (IPI) after complete resection of stage III/IV melanoma: Updated results from a phase III trial (CheckMate 238). J Clin Oncol 36:18s, 2018. (suppl; abstr 9502) [Google Scholar]
- 5.Eggermont AMM, Blank CU, Mandala M, et al. : Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med 378:1789-1801, 2018 [DOI] [PubMed] [Google Scholar]
- 6.Long GV, Hauschild A, Santinami M, et al. : Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813-1823, 2017 [DOI] [PubMed] [Google Scholar]
- 7.Long GV, Stroyakovskiy D, Gogas H, et al. : Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 386:444-451, 2015 [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Karaszewska B, Schachter J, et al. : Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 372:30-39, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Edge S, Byrd DR, Compton CC, et al. : AJCC Cancer Staging Manual (ed 7). New York, NY, Springer International Publishing, 2010 [Google Scholar]
- 10.Amin MB, Edge S, Greene F, et al. : AJCC Cancer Staging Manual (ed 8). New York, NY, Springer International Publishing, 2017 [Google Scholar]
- 11.Bernstein L, Anderson J, Pike MC: Estimation of the proportional hazard in two-treatment-group clinical trials. Biometrics 37:513-519, 1981 [PubMed] [Google Scholar]
- 12.Berry G, Kitchin RM, Mock PA: A comparison of two simple hazard ratio estimators based on the logrank test. Stat Med 10:749-755, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Lambert PC, Thompson JR, Weston CL, et al. : Estimating and modeling the cure fraction in population-based cancer survival analysis. Biostatistics 8:576-594, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Othus M, Barlogie B, Leblanc ML, et al. : Cure models as a useful statistical tool for analyzing survival. Clin Cancer Res 18:3731-3736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romano E, Scordo M, Dusza SW, et al. : Site and timing of first relapse in stage III melanoma patients: Implications for follow-up guidelines. J Clin Oncol 28:3042-3047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. : Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol 16:522-530, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Andersson TM, Eriksson H, Hansson J, et al. : Estimating the cure proportion of malignant melanoma, an alternative approach to assess long term survival: A population-based study. Cancer Epidemiol 38:93-99, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Martinez EZ, Achcar JA, Jácome AA, et al. : Mixture and non-mixture cure fraction models based on the generalized modified Weibull distribution with an application to gastric cancer data. Comput Methods Programs Biomed 112:343-355, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Bower H, Andersson TM, Björkholm M, et al. : Continued improvement in survival of acute myeloid leukemia patients: An application of the loss in expectation of life. Blood Cancer J 6:e390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiter U, Buettner PG, Eigentler TK, et al. : Hazard rates for recurrent and secondary cutaneous melanoma: An analysis of 33,384 patients in the German Central Malignant Melanoma Registry. J Am Acad Dermatol 66:37-45, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Schadendorf D, Hauschild A, Santinami M, et al. : Effect on health-related quality of life (HRQOL) of adjuvant treatment (tx) with dabrafenib plus trametinib (D + T) in patients (pts) with resected stage III BRAF-mutant melanoma. J Clin Oncol 36:18s, 2018. (suppl; abstr 9590) [Google Scholar]