Abstract
Management of heart failure is a major health care challenge. Healthcare providers are expected to use best practices described in clinical practice guidelines, which typically consist of a long series of complex rules. For heart failure management, the relevant guidelines are nearly 80 pages long. Due to their complexity, the guidelines are often difficult to fully comply with, which can result in suboptimal medical practices. In this paper, we describe a heart failure treatment adviser system that automates the entire set of rules in the guidelines for heart failure management. The system is based on answer set programming, a form of declarative programming suited for simulating human-style reasoning. Given a patient’s information, the system is able to generate a set of guideline-compliant recommendations. We conducted a pilot study of the system on 21 real and 10 simulated patients with heart failure. The results show that the system can give treatment recommendations compliant with the guidelines. Out of 187 total recommendations made by the system, 176 were agreed upon by the expert cardiologists. Also, the system missed eight valid recommendations. The reason for the missed and discordant recommendations seems to be insufficient information, differing style, experience, and knowledge of experts in decision-making that were not captured in the system at this time. The system can serve as a point-of-care tool for clinics. Also, it can be used as an educational tool for training physicians and an assessment tool to measure the quality metrics of heart failure care of an institution.
Keywords: Automated reasoning, knowledge representation, guideline automation, heart failure management
This paper reports an artificial intelligence-based implementation of the American Heart Association's guidelines for heart failure.
I. Introduction
Management of chronic diseases such as heart failure (HF) is a major public health challenge. To facilitate managing HF, national professional societies have developed clinical practice guidelines that all physicians should follow. Due to their complexity, these guidelines are difficult to implement and are adopted slowly by the medical community at large. Numerous works have shown that inadequate compliance with clinical guidelines, such as those for HF, occurs in most disciplines and countries [15]. In this paper we present a heart failure treatment adviser system that is capable of giving correct treatment recommendations for HF patients with respect to the 2013 and 2016 ACCF/AHA Guidelines for the Management of Heart Failure [5], [6] (referred to here as the 2013/2016 Guidelines). The system is based on a novel and powerful knowledge representation technique called Answer Set Programming (ASP) [1]. ASP is a declarative programming paradigm for solving hard problems in AI. It can model sophisticated human reasoning mechanisms like default reasoning and abductive reasoning. In ASP, problem specification is described by a non-monotonic logic program, where non-monotonicity means that new evidence may invalidate the earlier conclusions that were incorrectly drawn due to lack of information. We formulated the rules in the 2013/2016 Guidelines using ASP with the help of knowledge patterns [2]. We did a pilot study on 31 patients’ data to evaluate the efficacy of the system. The results of the study are discussed in detail in this paper.
Heart failure is the inability of the heart to perfuse target organs appropriately at normal filling pressures. Elevated left atrial filling pressures can cause edema in the lungs, abdomen, and legs, as well as symptoms of exercise intolerance. Half the people diagnosed with HF die within five years [3]. Statistics show that there are 11 million physician visits and 875,000 hospitalizations per year due to HF in U.S. alone. About 20% of patients with HF are readmitted to a hospital or visit an emergency room within thirty days of treatment [19].
Optimal management of HF requires adherence to evidence-based clinical practice guidelines. 2013/2016 Guidelines for the Management of Heart Failure have been created by a multi-disciplinary committee of experts and are based on thorough reviews of best available clinical evidence on the management of heart failure. They represent a consensus among experts on the appropriate treatment and management of HF [4].
The 2013/2016 Guidelines [5], [6] are intended to assist healthcare providers in clinical decision making by describing a range of generally acceptable approaches for the management of HF. The guideline is based on four progressive stages of heart failure. Stage A includes patients at risk of heart failure who are asymptomatic and do not have structural heart disease. Stage B describes asymptomatic patients with structural heart diseases. Stage C describes patients with structural heart disease who have prior or current symptoms of heart failure. Stage D describes patients with refractory heart failure who require specialized interventions. Interventions at each stage are aimed at reducing risk factors (stage A), treating structural heart disease (stage B) and reducing morbidity and mortality (stages C and D).
Though evidence-based guidelines should be the basis for all disease management [7], physicians’ adherence to guidelines is often poor [8]. The major reasons for the failure of guideline implementation are lack of awareness, lack of familiarity, lack of motivation, and external barriers. For 78% of all the clinical practice guidelines in medicine, more than 10% of physicians are not aware of their existence at all. Even when the guidelines are readily accessible, the physicians are not familiar enough with the guidelines to apply them correctly. In all the physician surveys conducted, the lack of familiarity was more common than the lack of awareness [8].
Another reason for unsatisfactory compliance is that guidelines can be quite complex, as is the case for HF management. For instance, more than 100 variables have been associated with mortality and re-hospitalization related to heart failure. In the 2013/2016 Guideline for the Management of Heart Failure, the variables range from simple information like age and sex to sophisticated data like electrocardiogram results and history of HF-related symptoms and diseases. Additionally, there are more than 60 rules to integrate all these data. Such complexity can lead to delays in adoption and missed opportunities at giving effective recommendations for even the most experienced healthcare providers [9]. To get an idea of the complexity of the rules, it is helpful to look at one example rule from the 2013/2016 Guidelines for Heart Failure Management [5], [6]:
“Aldosterone receptor antagonists (or mineralocorticoid receptor antagonists) are recommended in patients with NYHA class II–IV HF and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II HF should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L. Careful monitoring of potassium, renal function, and diuretic dosing should be performed at initiation and closely followed thereafter to minimize risk of hyperkalemia and renal insufficiency.”
II. Methods and Procedures
To overcome the difficulties that physicians face in implementing these guidelines, we built a heart failure treatment adviser software system [2] that automates the 2013/2016 Guidelines for the Management of Heart Failure. The system is able to give recommendations with justifications like a real human physician who strictly follows the guidelines, even under the condition of incomplete information about the patient.
First, we give a brief introduction to answer set programming (ASP), which is the crucial technique used in our system. ASP is a declarative programming paradigm for automated reasoning [10]. ASP is well-suited for knowledge-intensive applications thanks to its non-monotonic reasoning capabilities [11]. An example of ASP can be seen in Fig. 1. The solution, or the stable model, of this program is {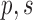 }. In other word, given the program in Fig. 1, the query
}. In other word, given the program in Fig. 1, the query 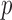 will succeed because the program includes
will succeed because the program includes 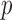 as a fact. The query
as a fact. The query 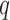 will fail because no rule has it as the head. The query
will fail because no rule has it as the head. The query 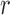 will fail because the query
will fail because the query 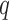 fails. Finally, the query
fails. Finally, the query 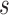 succeeds because both the query
succeeds because both the query 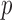 and not
and not
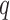 succeed.
succeed.
FIGURE 1.
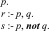
An example of ASP.
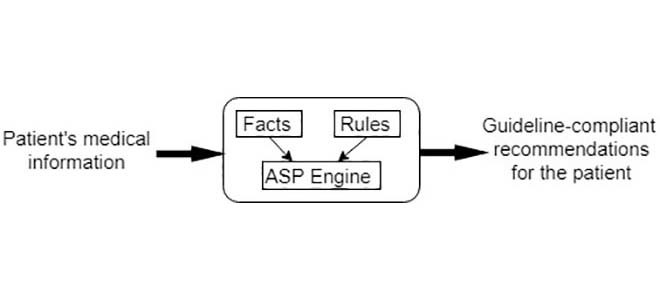
The heart failure treatment adviser system consists of a rule database and a fact table. The rule database covers all the knowledge in the 2013/2016 Guidelines for the Management of Heart Failure. The fact table contains the relevant information of the patient with heart failure. The system’s input includes 3 pieces of demographic information, 10 measurements and 25 types of HF-related diseases and symptoms. The treatments for heart failure are 13 pharmacological treatments, 9 management objectives and 4 device/surgical therapies. The complete list of input parameters and output recommendations of the heart failure treatment adviser system can be found in TABLE 1 and TABLE 2, respectively.
TABLE 1. Input Parameters of the HF Adviser System.
| Category | Data entry |
|---|---|
| Demographics | gender; age; race |
| Measurements | weight; creatinine; potassium; left bundle branch block; non-left bundle branch block; QRS duration; ejection fraction; NYHA class; ACCF/AHA stage; sinus rhythm |
| Diseases and symptoms | sleep apnea, acute coronary syndrome; myocardial infarction; diabetes; stroke; fluid retention; angioedema; ischemic attack; thromboembolism; elevated plasma natriuretic peptide level; asymptomatic ischemic cardiomyopathy; atrial fibrillation; myocardial ischemia; lipid disorders; acute profound hemodynamic compromise; obesity; angina; threatened end organ dysfunction; ischemic heart disease; structural cardiac abnormalities; atrioventricular block; hypertension; dilated cardiomyopathy; volume overload; coronary artery disease; |
| Other | expectation of survival; pregnancy; history of standard neurohumoral antagonist therapy; risk of cardioembolic stroke; ischemic etiology of HF; eligibility of mechanical circulatory support; dependence of continuous parenteral inotropic; requirement of ventricular pacing; history of cardiovascular hospitalization; eligibility of significant ventricular pacing; |
TABLE 2. Output Recommendations of the HF Adviser System.
| Category | Data entry |
|---|---|
| Pharmacological treatments | ACE inhibitors; ARBs; Beta blockers; statin; diuretics; aldosterone receptor antagonists; digoxin; inotropes; anticoagulants; Omega-3 fatty acids; hydralazine and isosorbide dinitrate; ARNI; ivabradine |
| Management objectives | systolic blood pressure control; diastolic blood pressure control; obesity control; diabetes control; tobacco avoidance; cardiotoxic agents avoidance; atrial fibrillation control; sodium restriction; water restriction; |
| Device/surgical therapies | implantable cardioverter-defibrillator; cardiac resynchronization therapy; mechanical circulatory support; coronary revascularization |
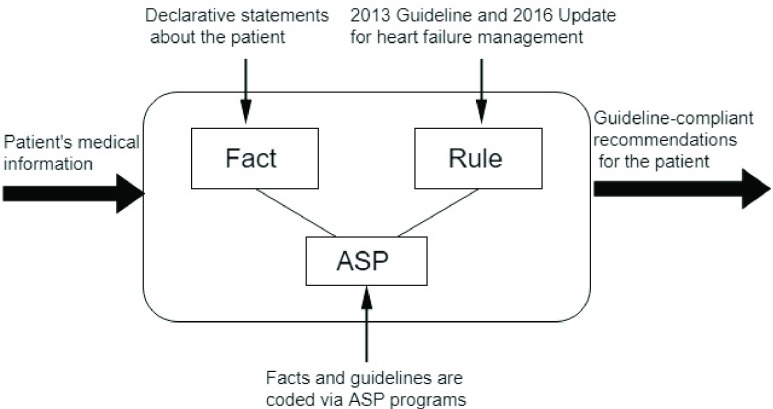
Note that co-morbidities such as MI and diabetes play significant roles in the management of HF. However, these conditions have their own dedicated clinical guidelines which are not covered by the heart failure treatment adviser system developed here. In this paper, we limit the knowledge base of the system to the context of 2013/2016 Guidelines for the Management of Heart Failure. Fig. 2 displays the architecture of the system.
FIGURE 2.
The architecture of the heart failure treatment adviser system.
The rules and facts can be loaded into s(ASP), a predicate ASP system [12], and the query ?- recommendation(Treatment, Recommendation_class) can be posed to find possible treatments for a patient. Note that there may be more than one potential treatment that can be discovered. The s(ASP) system also has a justification feature, which allows a user to examine the justification (essentially a proof trace) for each recommendation.
The heart failure treatment adviser system is built based on two core concepts: recommendation and contraindication. A recommendation can be translated into an action or activity that can be implemented or measured. A contraindication is a specific situation in which a drug, procedure, or surgery should not be used because it may be harmful to the person. Four facts were taken into consideration when the system was developed. Below is the list of the facts as well as the corresponding example rules.
-
Fact #1:
Multiple rules can trigger a recommendation. An instance of this fact can be found in the following two rules recommending Implantable Cardioverter Defibrillator (ICD):
“Class I: ICD therapy is recommended for primary prevention of SCD to reduce total mortality in selected patients with nonischemic DCM or ischemic heart disease at least 40 days post-MI with LVEF of 35% or less and NYHA class II or III symptoms on chronic GDMT, who have reasonable expectation of meaningful survival for more than 1 year.”
“Class I: ICD therapy is recommended for primary prevention of SCD to reduce total mortality in selected patients at least 40 days post-MI with LVEF of 30% or less, and NYHA class I symptoms while receiving GDMT, who have reasonable expectation of meaningful survival for more than 1 year.”
-
Fact #2:
Multiple rules can lead to a contraindication. An instance of this fact can be found in the following two rules forbidding ACE inhibitors:
“Patients should not be given an ACE inhibitor if they have experienced life-threatening adverse reactions (i.e., angioedema) during previous medication exposure or if they are pregnant or plan to become pregnant.”
“Class III: Routine combined use of an ACE inhibitor, ARB and aldosterone antagonist is potentially harmful for patients with HFrEF.”
-
Fact #3:
The recommendation of a treatment can’t be made if at least one contraindication for that treatment is present. The following rule states the condition in which digoxin is inappropriate:
“Patients should not be given digoxin if they have significant sinus or atrioventricular block unless the block has been addressed with a permanent pacemaker.”
-
Fact #4:
The recommendation/contraindication of one treatment can impact other treatments. An example of this fact can be found in the following rule recommending ARBs:
“Class I: ARBs are recommended in patients with HFrEF with current or prior symptoms who are ACE inhibitor intolerant, unless contraindicated, to reduce morbidity and mortality.”
The guideline rules are fairly complex and require the use of negation as failure, non-monotonic reasoning and reasoning with incomplete information [14]. The ability of answer set programming to model defaults, exceptions, weak exceptions, preferences, etc. [14], makes it ideally suited for coding these guidelines. The methodology we adopted when we were building the heart failure treatment adviser system was to identify a set of knowledge patterns that represent the logical relations between treatment recommendations. These patterns are quite universal and serve as solid building blocks for systematically translating the specifications written in English to ASP code. TABLE 3 summarizes all the knowledge patterns that we have identified from the 2013/2016 Guidelines. The formalizations of these patterns can be found in [2].
TABLE 3. Knowledge Patterns in the Guidelines for the Management of Heart Failure.
| Pattern name | Pattern meaning |
|---|---|
| Aggressive reasoning | Make a recommendation if the conditions are met; no evidence of contraindication means there is no contraindication. |
| Conservative reasoning | Make a recommendation if the conditions are met and the explicit proof of no contraindication is available. |
| Anti-recommendation | A recommendation is prohibited if the evidence of at least one contraindication is present. |
| Preference | Make the first-line recommendation whenever possible. If not, use the second-line recommendation |
| Concomitant choice | If a recommendation is made, some other recommendations are automatically in effect unless they are contraindicated. |
| Indispensable choice | If a recommendation is made, some other choices must also be made; if those choices can’t be made, then the first choice is revoked. |
| Incompatible choice | Certain recommendations can’t be in effect at the same time. |
With the help of knowledge patterns, all rules for the treatment of ACCF/AHA stages A to D are coded in ASP to run on the heart failure treatment adviser system. For instance, consider one rule from ACCF/AHA stage C [5], [6]:
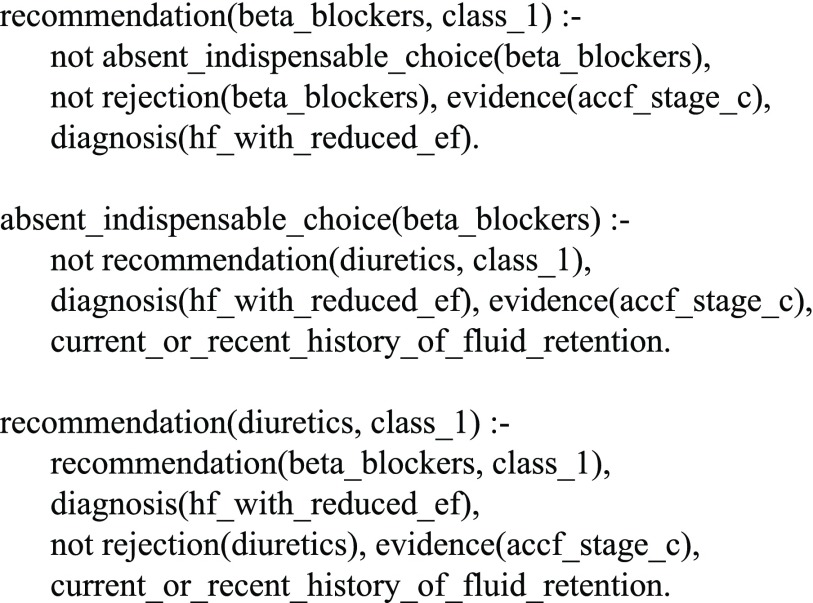
“In patients with a current or recent history of fluid retention, beta blockers should not be prescribed without diuretics.”
In the heart failure treatment adviser system, this rule is coded as in Fig. 3. It is worth mentioning that the rule is written by simply populating the code template of the knowledge pattern indispensable choice [2].
FIGURE 3.
The ASP code for a rule regarding beta blockers and diuretics.
To better understand what the code snippet does, let us suppose that we have a patient who has heart failure with reduced ejection (HFrEF) and is in ACCF/AHA stage C. According to the 2013/2016 Guidelines, our system would recommend beta blockers. If we add the information that the patient has a history of fluid retention, then the system would try to add diuretics. However, if diuretics are contraindicated for any reason, the system would not recommend beta blockers either.
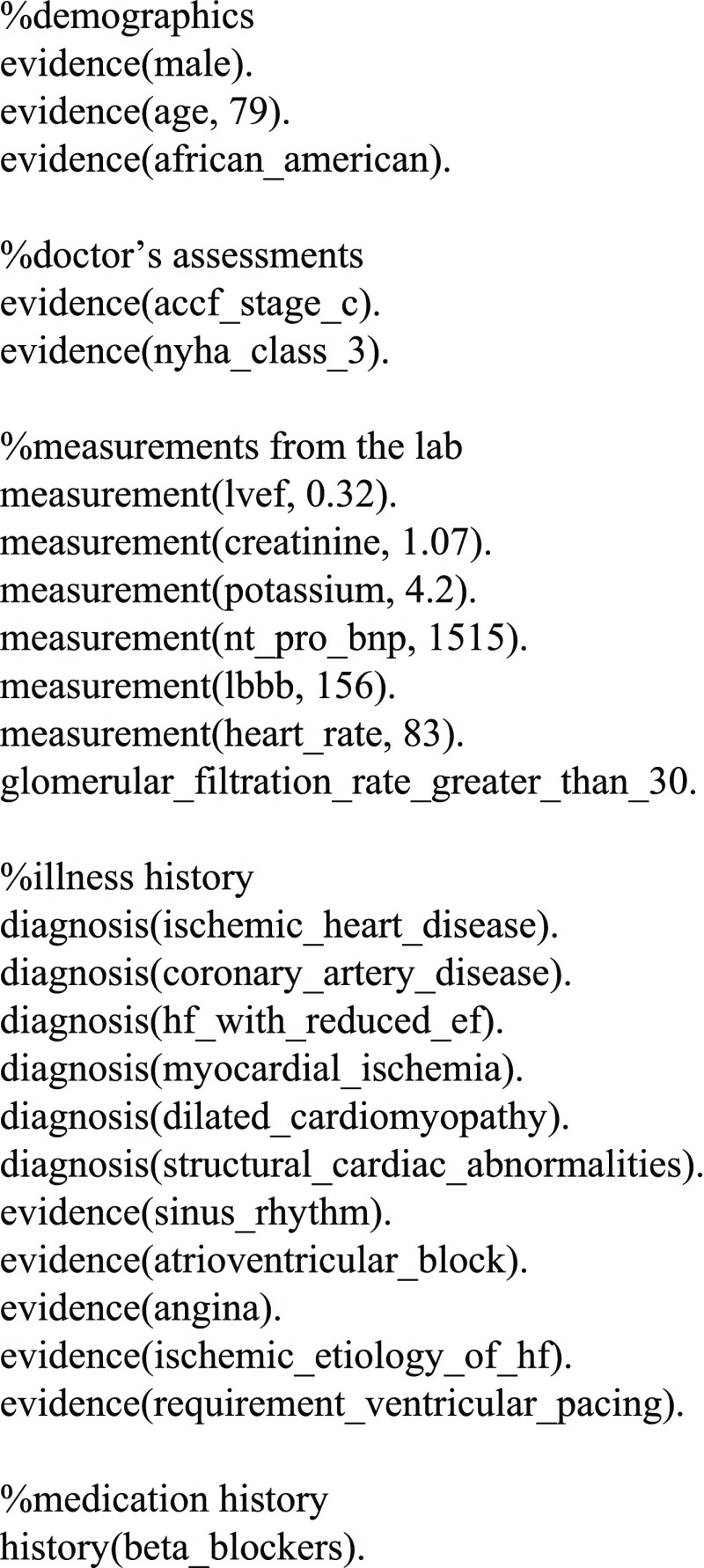
The input data for the system were extracted from the patient’s profile by a computer scientist, a co-author of this paper, who has limited knowledge of clinical medicine but is familiar with the 2013/2016 ACCF/AHA Guidelines for the Management of Heart Failure. He was able to identify useful information from a patient’s profile and encode them into ASP programs that the heart failure treatment adviser system could execute. Figure 4 is an example of a fact table distilled from the profile of one of the real patients (No. 30).
FIGURE 4.
Representation of patient No. 30’s information in ASP.
The 10 simulated patients consist of 4 women and 6 men. The youngest patient is 32 and the oldest is 73. Among them are 6 African Americans and 4 Caucasians. The 21 real patients consist of 6 women and 14 men; one patient’s gender is not shown in the record. The youngest one is 36 and the oldest one is 82. There are 5 Caucasians and the ethnicity of the rest is not clear. We have IRB approval for retrospective review of the anonymous patient data.
III. Results and Discussions
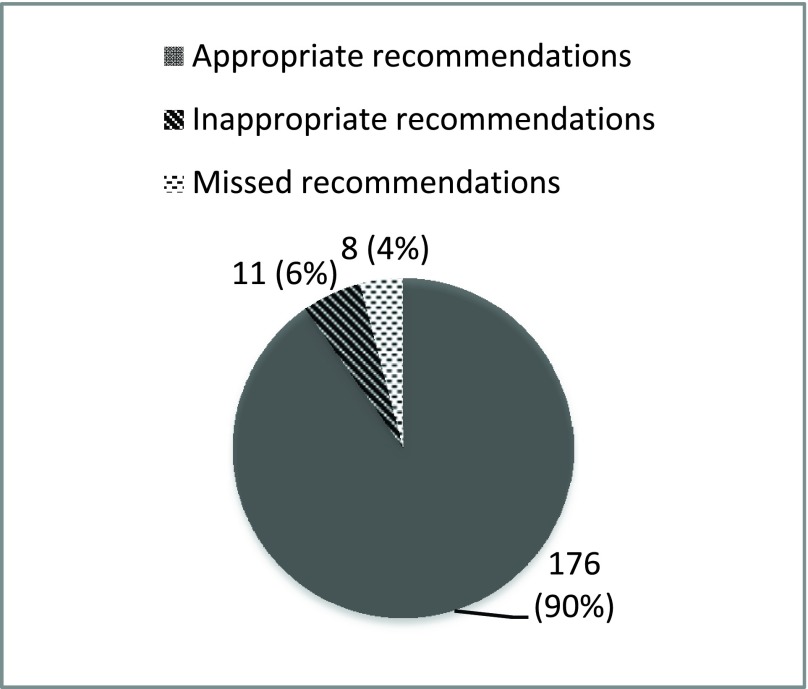
To validate the efficacy of the heart failure treatment adviser system for HF management, we ran the system using data of 10 simulated patients and 21 real patients with HF. The patients’ profiles were provided by our cardiologist co-authors from the University of Texas Southwestern Medical Center. The idea is to let the system give its recommendations independently for those patients and compare the system’s recommendations with the ones from the cardiologists. Figure 5 displays the results of the study we performed on 10 simulated patients and 21 real patients.
FIGURE 5.
Statistics of the results of the pilot study of HF adviser system.
Eleven of the 21 real patients are provided by UTSW Heart Failure Specialty Clinic and the rest 10 by UTSW Clinical Heart Center General Cardiology Clinic. We choose to present the results only for ACCF/AHA Stage C recommendations for which the 2013/2016 Guidelines has the most complicated rules. Stage A and Stage B rules are quite universal and are very small in number. There is almost no mismatch between the system’s output and cardiologists’ treatment plan. To save space we omit the results for Stage A and B. Stage D recommendations are either palliative care like inotropes or advanced therapy like cardiac transplantation. We decided that our system may not be the best fit for end-of-life care, which has to be far more individualized to patient preferences and is much less amenable to being fully captured in guidelines. Note that the 2013/2016 Guidelines do not have enough details covering advanced therapies. However, our method would be applicable for advanced therapies if the dedicated guidelines for them were available. One thing worth mentioning is that the system’s rule database does include recommendation rules for both heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF). None of the 31 cases considered here fall into the category of HFpEF. The reason that we validated our system only on HFrEF is that there are only two simple Class I recommendations for HFpEF (blood pressure control and diuretics) in the 2013/2016 Guidelines and generating those recommendations for patients with HFpEF is very straightforward.
Let’s take the case presented in Fig. 4 for example. Its Stage C recommendations and their corresponding rules used are listed in TABLE 4:
TABLE 4. Recommendation List for Patient No. 30.
| Recommendation | Justification rule [5] |
|---|---|
| Ace inhibitor – Class I | “ACE inhibitors are recommended in patients with HFrEF and current or prior symptoms, unless contraindicated, to reduce morbidity and mortality.” |
| Beta blocker – Class I | “Use of 1 of the 3 beta blockers proven to reduce mortality (eg, bisoprolol, carvedilol, and sustained release metoprolol succinate) is recommended for all patients with current or prior symptoms of HFrEF, unless contraindicated, to reduce morbidity and mortality” |
| Aldosterone antagonist – Class I | “Aldosterone receptor antagonists are recommended in patients with NYHA class II–IV HF and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II HF should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L.” |
| Hydralazine and isosorbide dinitrate – Class I | “The combination of hydralazine and isosorbide dinitrate is recommended to reduce morbidity and mortality for patients self-described as African Americans with NYHA class III–IV HFrEF receiving optimal therapy with ACE inhibitors and beta blockers, unless contraindicated.” |
| Cardiac resynchronization therapy – Class I | “CRT is indicated for patients who have LVEF of 35% or less, sinus rhythm, left bundle-branch block (LBBB) with a QRS duration of 150 ms or greater, and NYHA class II, III, or ambulatory IV symptoms on GDMT.” |
| Sodium restriction – Class IIa | “Sodium restriction is reasonable for patients with symptomatic HF to reduce congestive symptoms.” |
| Omega 3 fatty acids – Class IIa | “Omega-3 polyunsaturated fatty acid (PUFA) supplementation is reasonable to use as adjunctive therapy in patients with NYHA class II–IV symptoms and HFrEF or HFpEF, unless contraindicated, to reduce mortality and cardiovascular hospitalizations.” |
From the fifth row of TABLE 4 we know that cardiac resynchronization therapy (CRT) is recommended for this patient. The rationale for recommending CRT is listed in the same row. Examining the patient’s information in Fig. 4 tells us the exact facts which triggered this recommendation: the LVEF is 32%; sinus rhythm; left bundle-branch block with a QRS duration of 156ms; ACCF Stage C and NYHA Class III. The system found no sign of contraindication for CRT in this case. In fact, the 2013/2016 Guidelines does not mention any explicit rule regarding the contraindication of CRT. However, our approach can easily incorporate such a rule in case it is added to the guidelines in the future. It is worth noting that the system did not include implantable cardioverter defibrillator (ICD) in its recommendation list. The patient’s information in Fig.4 does not give the system enough evidence to meet the conditions stated by either of the two rules:
“ICD therapy is recommended for primary prevention of SCD in selected patients with HFrEF at least 40 days post-MI with LVEF ≤30% and NYHA class I symptoms while receiving GDMT, who are expected to live >1 year.”
“ICD therapy is recommended for primary prevention of SCD in selected patients with HFrEF at least 40 days post-MI with LVEF ≤35% and NYHA class II or III symptoms on chronic GDMT, who are expected to live >1 year.”
To activate the first rule, the system would need to see that the patient is in NYHA class I with at least 40 days of post-MI and has LVEF ≤30%. Also, the expected survival duration should be more than 1 year. Similarly, the second rule requires that the patient has an expected survival duration of more than 1 year and also has 40 days of post-MI. Given the information in Fig. 4, the system decided to recommend CRT without ICD, i.e., CRT-P, to the patient No. 30.
In cases where both CRT and ICD are included in the system’s recommendation list, the physician can choose either one or both using her discretion. Note that the 2013/2016 Guidelines does not use the term “CRT-D” in its rule set. Instead, it has independent rules for CRT and ICD. As an example, consider a real case (patient No. 31) in which both CRT and ICD are applicable (CRT-D). Figure 6 shows the information of this patient obtained from one of our collaborators’ clinic and TABLE 5 shows Stage C recommendations given by the system for this patient.
FIGURE 6.
Representation of patient No. 31’s information in ASP.
TABLE 5. Recommendation List for Patient No. 31.
| Recommendation | Justification rule [5] |
|---|---|
| Ace inhibitor – Class I | “ACE inhibitors are recommended in patients with HFrEF and current or prior symptoms, unless contraindicated, to reduce morbidity and mortality.” |
| Beta blocker – Class I | “Use of 1 of the 3 beta blockers proven to reduce mortality (eg, bisoprolol, carvedilol, and sustained release metoprolol succinate) is recommended for all patients with current or prior symptoms of HFrEF, unless contraindicated, to reduce morbidity and mortality” |
| Aldosterone antagonist – Class I | “Aldosterone receptor antagonists are recommended in patients with NYHA class II–IV HF and who have LVEF of 35% or less, unless contraindicated, to reduce morbidity and mortality. Patients with NYHA class II HF should have a history of prior cardiovascular hospitalization or elevated plasma natriuretic peptide levels to be considered for aldosterone receptor antagonists. Creatinine should be 2.5 mg/dL or less in men or 2.0 mg/dL or less in women (or estimated glomerular filtration rate >30 mL/min/1.73 m2), and potassium should be less than 5.0 mEq/L.” |
| Implantable cardioverter-defibrillator – Class I | “ICD therapy is recommended for primary prevention of SCD to reduce total mortality in selected patients with nonischemic DCM or ischemic heart disease at least 40 days post-MI with LVEF of 35% or less and NYHA class II or III symptoms on chronic GDMT, who have reasonable expectation of meaningful survival for more than 1 year.” |
| Cardiac resynchronization therapy – Class I | “CRT is indicated for patients who have LVEF of 35% or less, sinus rhythm, left bundle-branch block (LBBB) with a QRS duration of 150 ms or greater, and NYHA class II, III, or ambulatory IV symptoms on GDMT.” |
| Sodium restriction – Class IIa | “Sodium restriction is reasonable for patients with symptomatic HF to reduce congestive symptoms.” |
| Digoxin – Class IIa | “Digoxin can be beneficial in patients with HFrEF, unless contraindicated, to decrease hospitalizations for HF.” |
| Omega 3 fatty acids – Class IIa | “Omega-3 polyunsaturated fatty acid (PUFA) supplementation is reasonable to use as adjunctive therapy in patients with NYHA class II–IV symptoms and HFrEF or HFpEF, unless contraindicated, to reduce mortality and cardiovascular hospitalizations.” |
ICD is recommended by the heart failure treatment adviser system because it finds the following information in Fig. 6: the patient has been diagnosed with ischemic heart disease 40 days post-MI; his left ventricular ejection fraction is 15%, which is less than 35%; he is in NYHA Class III and has expectation of survival duration for more than 1 year.
CRT is also recommended by the system due to the following facts found in Fig. 6: the patient has left ventricular ejection fraction of 15%, which is less than 35%; he has sinus rhythm; he has left bundle-branch block with a QRS duration of 170ms, which is greater than 150ms; he is in NYHA Class III.
In the rest of this section we discuss the validation results for the 31 cases. Figure 5 illustrates the statistics of the experimental results of the pilot study of using the heart failure treatment adviser system. The total in Fig. 5 is the number of all recommendations given by the system plus the number of legitimate recommendations missed by the system. Specifically, the system made 187 stage C recommendations for 31 patients. 11 of these recommendations were inconsistent with cardiologist’s opinion and 8 legitimate recommendations were missed. While not reflected in Fig. 5, the system managed to pick up 26 applicable recommendations missed by the original cardiologists providing the care, and left out 5 non-guideline-compliant recommendations given by the original cardiologists.
Next, we discuss the implications of these results. The heart failure treatment adviser system identified some appropriate treatments which were overlooked by the attending cardiologists. Similarly the system chose not to include certain medications on the recommendation list. For example, aldosterone antagonists are drugs which are sometimes inappropriately prescribed by a physician. From the guidelines we know that to ensure safety of the patients, the creatinine level, potassium level and glomerular filtration rate must be within certain ranges before prescribing this drug. However, in some cases in our study, the original attending physician recommended the drug without having obtained these three lab results. The cardiologists who treated the patients may have adopted a reasoning pattern called aggressive reasoning in this scenario as described in TABLE 3. In the case of aldosterone antagonists, this type of reasoning assumes all three missing lab results would be within safe range if there is no explicit mention of them as being abnormal. On the contrary, the system’s developer coded the relevant rules using a conservative reasoning pattern. As a result, the system would not decide aldosterone antagonists are valid choice unless it sees the evidence that all three labs results are present and within a safe range. We call this phenomenon the “double standard dilemma” in the implementation of clinical practice guideline. From the results it can be concluded that the cardiologists use aggressive reasoning for some rules but conservative reasoning for others. One way to resolve this dilemma is to parameterize both reasoning patterns and let the cardiologists decide which reasoning pattern applies for each medication.
The reason why the heart failure treatment adviser system did either make “wrong” recommendations or miss relevant treatments is well understood by us. There are mainly four reasons for this. First, the discordant recommendations happened because of the “double standard dilemma”. As we have discussed, parameterization of reasoning patterns will be the solution for this. Secondly, physicians use personal preference to interpret the guideline. For example, one of our cardiologist collaborators decided in one case that digoxin is not appropriate because the patient’s level of potassium is less than 4 mEq/L, which may pose some risk of arrhythmia. The 2013/2016 Guidelines, however, does not contain such a rule. The closest description the system’s developers have found in the 2013/2016 Guidelines is “the major adverse effects (of digoxin) include cardiac arrhythmias, gastrointestinal symptoms and neurological complaints.” It is not uncommon in practice that physicians have different interpretations of the guidelines. This phenomenon does not challenge the validity of our approach since it is not difficult to encode any individual or organizational preference. The third reason for the system’s discordant or missing treatment recommendations is the system developer’s ignorance of medical knowledge other than the 2013/2016 Guidelines. For instance, aldosterone antagonists, ARNI and digoxin are contraindicated in case of renal failure. The heart failure treatment adviser system did not contain this rule because the guidelines do not mention it. However, every cardiologist probably has this rule in their knowledge reserve. Another example is the system’s failure to recommend anticoagulants for a patient with LV thrombus. It should be a straightforward choice for any physician to indicate anticoagulants in such a case unless there is a contraindication for them. Since the 2013/2016 Guidelines do not have such a rule, the heart failure treatment adviser system was not recommending anticoagulants to the patient. This issue can be fixed by adding additional rules to the rule database of the system as situations arise. Due to the non-monotonic nature of ASP, the system is always friendly to the addition of rules and such an extension normally requires minimal change to existing code. The last cause for system’s less-than-ideal performance is insufficient information. In one case anticoagulants were recommended for the patient by the system. Our cardiologist collaborators disagreed with its recommendation due to intracranial bleeding. However, such an event was not documented in the notes given to the system. To address this issue, it would require that physicians always keep the patient record up to date, which is beyond the topic of this paper. That being said, the heart failure treatment adviser system is geared to give the guidelines-compliant treatment options even in the absence of complete information. Again, the parameterization of reasoning patterns can solve this problem since the system can be configured to reason aggressively or conservatively for each medication.
IV. Technology Translation
On the translational aspect of our system, we have developed a roadmap. The work presented in this paper marks the completion of the pre-clinical research (T1 Translation [20]) where we have tested our system on 31 cases (21 real and 10 simulated). For T2 Translation [20] we plan to do a clinical trial that aims to establish the efficacy of the system’s ability to improve heart failure management guideline compliance among physicians. The trial will be open-label and randomized. The idea is to compare the degree of guideline compliance among physicians with and without the usage of the heart failure treatment adviser system. We will evaluate the guideline compliance for both the experimental group (with the system) and control group (without the system). After we have completed the T2 Translation, we will be focusing on clinical implementation of the system. Extensive interviews with potential users and iterative system development will be done to ensure the maximal integration of the system with physician’s everyday workflow. Popular medical data exchange standards such as HL7 and FHIR will be used to interface the system with EHRs. We will be working with the University of Texas Southwestern Medical School in designing the system and they will also be the first testing ground for this system. We expect the system to be deployed in the U.S. first since the system we have developed is compliant with 2013/2016 Guidelines. Subsequently, guidelines from other countries will be implemented and deployed in other parts of the world.
At the time of writing there are no official regulatory requirements for clinical decision support (CDS) systems such as our heart failure treatment adviser system from the U.S. Food and Drug Administration (FDA). However, FDA has published guidance for clinical and patient decision support software systems [18]. The current thinking of FDA on this topic, as we infer, is that the CDS systems such as the heart failure treatment adviser system does not meet the definition of ‘device’ and is exempted from FDA regulations [18] provided it meets all of the four following criteria: 1) “Not intended to acquire, process, or analyze a medical image or a signal from an in vitro diagnostic device or a pattern or signal from a signal acquisition system”; 2) “Intended for the purpose of displaying, analyzing, or printing medical information about a patient or other medical information”; 3) “Intended for the purpose of supporting or providing recommendations to a health care professional about prevention, diagnosis, or treatment of a disease or condition”; 4) “Intended for the purpose of enabling such health care professional to independently review the basis for such recommendations that such software presents so that it is not the intent that such health care professional relies primarily on any of such recommendations to make a clinical diagnosis or treatment decision regarding an individual patient”. The heart failure treatment adviser system clearly satisfies all the criteria, especially, when the system is extended to produce the rules justifying each recommendation of the system as in TABLEs 4 and 5. This extension is feasible and will be done when the prototype is productized. One interesting note is that since our system is logic-based, it has greater algorithmic transparency than any statistics-based CDS software. For example, given a patient, we can tell why a drug is recommended (or rejected) by which rule(s). In fact, the FDA guidance gives an example of CDS system that is not a ‘device’ [18] and thus exempted from FDA regulations: “software that uses rule-based tools that compare patient-specific signs, symptoms, or results with available practice guidelines (institutions-based or academic/clinical society-based) to recommend condition specific diagnostic tests, investigations or therapy”. The heart failure treatment adviser system fits exactly with such a definition.
V. Conclusion
In this paper we presented the heart failure treatment adviser system. This system is designed to automate the 2013/2016 Guidelines for the Management of Heart Failure. We discussed the results of our pilot study with 10 simulated patients and 21 real patients. The system made 187 stage C recommendations for those patients, 176 of which are guideline-compliant recommendations. The discordant 11 and the 8 missing recommendations can be easily fixed with finer granularity of modeling and rule database expansion. The system managed to point out 26 legitimate recommendations missed by the attending cardiologists and avoided 5 inconsistent care options that were inappropriately recommended by the attending cardiologists. The experimental results are very promising and prove the efficacy of the AI-based heart failure treatment adviser system and the feasibility of turning it into a viable product usable in clinical settings.
Note that the right columns of TABLE 4 and TABLE 5 were populated by post hoc analyses. Automatic notification of relevant guideline rules used by the system in giving a particular recommendation will be part of our future work. Thanks to the goal-driven nature of the s(ASP) reasoning engine used here, such an extension is very practical. We are also planning to augment the system in the future with a feature that automatically evaluates the cardiologists’ recommendations and gives real-time clues to help them correct their non-guideline-compliant recommendations. A preliminary study of this feature is presented in our work [13], where we used abductive reasoning to accomplish this task. The heart failure treatment adviser system that we have developed here can serve as a valuable point-of-care tool for physicians to use during a clinical visit.
Our system received positive feedback from most of the cardiologists we are working with. One cardiologist said, “the system beats the most experienced physicians in some cases.” Another cardiologist however remained skeptical about the practicality of the system. He argued that, “a cardiologist’s job is more than following the guideline  There are lots of nuances involved”. In response to this comment, we would like to stress that the heart failure treatment adviser system won’t and can’t replace human cardiologists. What it does is more like what a competent assistant would do. It automates the clinical guidelines and thus gives user a solid foundation from which he/she can deal with the “nuances” more easily. Another physician suggested the addition of an interface to the existing electronic health records (EHR) systems. Such an interface would help physician users reconcile their inconsistent recommendations detected by our system using the latest data from EHR.
There are lots of nuances involved”. In response to this comment, we would like to stress that the heart failure treatment adviser system won’t and can’t replace human cardiologists. What it does is more like what a competent assistant would do. It automates the clinical guidelines and thus gives user a solid foundation from which he/she can deal with the “nuances” more easily. Another physician suggested the addition of an interface to the existing electronic health records (EHR) systems. Such an interface would help physician users reconcile their inconsistent recommendations detected by our system using the latest data from EHR.
One potential use of our system is to give guidance to primary care physicians treating patients who have no access to qualified cardiologists. It can enable primary care physicians to manage patients with CHF with relatively minimal supervision from a heart failure specialist. This is potentially valuable in underserved areas and in developing countries where patients lack adequate specialist medical care. Currently the system gives only recommendations that are compliant with the 2013/2016 Guidelines for the Management of Heart Failure. For users in countries other than the United States such as Europe, they may find the output of the system does not agree with the guideline in their countries. That being said, the methodology described in this paper is generic enough to be used to automate clinical guidelines of different countries and disciplines. For example, one rule from the European version of the guideline for heart failure management is [17]:
“If a patient is scheduled to receive an ICD and is in sinus rhythm with a QRS duration ≥ 30 ms, CRT-D should be considered if QRS is between 130 and 149 ms and is recommended if QRS is ≥ 150 ms.”
The above rule can be easily coded using concomitant choice pattern [2] in which ICD is the trigger recommendation and CRT is the concomitant recommendation. Once we have rolled out the system in U.S. we will work on extending our system to deployment in other countries.
In addition to being a point-of-care tool for use by general physicians and cardiologists, the heart failure treatment adviser system can be used as an analysis engine that gives feedback to clinical leadership and the healthcare system administration regarding the overall quality of their heart failure care. Another application of this system is the use of it as an educational tool to train physicians. The approach taken in building the heart failure treatment adviser system can also be extended to many other diseases that have guidelines in place.
Acknowledgment
K. Marple was with the Computer Science Department, The University of Texas at Dallas, Richardson, TX, USA.
Funding Statement
This work was supported in part by NSF under Grant 1718945 and the Texas Medical Research Collaborative.
References
- [1].Eiter T., Ianni G., and Krennwallner T., “Answer set programming: A primer,” Reasoning Web. Semantic Technologies for Information Systems. New York, NY, USA: Springer, 2009, pp. 40–110. [Google Scholar]
- [2].Chen Z., Marple K., Salazar E., Gupta G., and Tamil L., “A physician advisory system for chronic heart failure management based on knowledge patterns,” Theory Pract. Log. Program., vol. 16, nos. 5–6, pp. 604–618, 2016. [Google Scholar]
- [3].Go A. S.et al. , “Heart disease and stroke statistics—2013 update: A report from the American Heart Association,” Circulation, vol. 127, no. 1, pp. e6–e245, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jacobs A. K.et al. , “ACCF/AHA Clinical practice guideline methodology summit report: A report of the american college of cardiology foundation/american heart association task force on practice guidelines,” J. Amer. College Cardiol., vol. 61, no. 2, pp. 213–265, 2013. [DOI] [PubMed] [Google Scholar]
- [5].Yancy C. W.et al. , “2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines,” J. Amer. College Cardiol., vol. 62, no. 16, pp. e147–e239, 2013. [DOI] [PubMed] [Google Scholar]
- [6].Yancy C. W.et al. , “2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure,” J. Amer. College Cardiol., vol. 68, no. 13, pp. 1476–1488, 2016. [DOI] [PubMed] [Google Scholar]
- [7].Faxon D.et al. , “Improving quality of care through disease management: Principles and recommendations from the American Heart Association’s Expert Panel on Disease Management,” Stroke, vol. 35, no. 6, pp. 1527–1530, 2004. [DOI] [PubMed] [Google Scholar]
- [8].Cabana M. D.et al. , “Why don’t physicians follow clinical practice guidelines?: A framework for improvement,” JAMA, vol. 282, no. 15, pp. 1458–1465, 1999. [DOI] [PubMed] [Google Scholar]
- [9].Group M. N., “Enhancing the use of clinical guidelines: A social norms perspective,” Amer. College Surgeons, vol. 202, no. 5, pp. 826–836, 2006. [DOI] [PubMed] [Google Scholar]
- [10].Lifschitz V., “What is answer set programming?” in Proc. 23rd Nat. Conf. Artif. Intell. (AAAI), vol. 3, 2008, pp. 1594–1597. [Google Scholar]
- [11].Brewka G., Nonmonotonic Reasoning: Logical Foundations of Commonsense. New York, NY, USA: Cambridge Univ. Press, 1991. [Google Scholar]
- [12].Marple K., Salazar E., and Gupta G. (2017). “Computing stable models of normal logic programs without grounding.” Accessed: Oct. 17, 2018. [Online]. Available: https://arxiv.org/abs/1709.00501 [Google Scholar]
- [13].Chen Z.et al. , “Improving adherence to heart failure management guidelines via abductive reasoning,” Theory Pract. Log. Program., vol. 17, nos. 5–6, pp. 764–779, 2017. [Google Scholar]
- [14].Gelfond M. and Kahl Y., Knowledge Representation, Reasoning and the Design of Intelligent Agents: The Answer-Set Programing Approach. New York, NY, USA: Cambridge Univ. Press, 2014. [Google Scholar]
- [15].Barth J. H.et al. , “Why are clinical practice guidelines not followed?” Clin. Chem. Lab. Med., vol. 54, no. 7, pp. 1133–1139, 2016. [DOI] [PubMed] [Google Scholar]
- [16].Trochim W., Kane C., Graham M. J., and Pincus H. A., “Evaluating translational research: A process marker model,” Clin. Transl. Sci., vol. 4, no. 3, pp. 153–162, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ponikowski P.et al. , “2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC,” Eur. J. Heart Failure, vol. 18, no. 8, pp. 891–975, 2016. [DOI] [PubMed] [Google Scholar]
- [18].U.S. Food and Drug Administration. Clinical and Patient Decision Support Software: Draft Guidance for Industry and Food and Drug Administration Staff. Accessed: Oct. 3, 2018. [Online]. Available: https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm587819.pdf
- [19].Bergethon K. E.et al. , “Trends in 30-day readmission rates for patients hospitalized with heart failure: Findings from the Get With the Guidelines-Heart Failure Registry,” Circulation: Heart Failure, vol. 9, no. 6, p. e002594, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gannon F., “The steps from translatable to translational research,” EMBO Rep., vol. 15, no. 11, pp. 1107–1108, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]