Abstract
Alcohol use disorder is highly co-morbid with traumatic stress disorders in humans, and dually diagnosed individuals cite negative affective symptoms as a primary reason for drinking alcohol. Therefore, it is reasonable to hypothesize that traumatic stress history increases the rewarding properties and/or blunts the aversive properties of alcohol. We used a place conditioning procedure to test the rewarding/aversive properties of alcohol in adult male Wistar rats with or without a traumatic stress (i.e., predator odor exposure) history, and with or without an alcohol drinking history. Because extended amygdala regions have documented roles in stress, reward, and stress-induced changes in reward, we also tested the effect of acute alcohol on CREB phosphorylation (pCREB) and striatal-enriched protein tyrosine phosphatase (STEP) expression in central amygdala (CeA) and bed nucleus of stria terminalis (BNST). Our results show that a moderate alcohol dose (1.0 g/kg) produces conditioned place aversion (CPA) that is blunted by stress history but is not affected by alcohol drinking history, and this effect differed in pair-housed versus single-housed rats. Stress history reduced pCREB expression in BNST of rats with and without an alcohol drinking history. Finally, acute alcohol effects on pCREB and STEP expression in CeA were positively associated with preference for the alcohol-paired chamber. These data suggest that stress history reduces the aversive properties of moderate alcohol doses, and that alcohol aversion is associated with acute alcohol effects on pCREB and STEP expression in the extended amygdala.
Keywords: Post-traumatic stress disorder (PTSD), Alcohol reward, Place conditioning
1. Introduction
Post-traumatic stress disorder (PTSD) is a mental health condition affecting many millions of Americans (Harvard Medical School, 2007). Recent estimates suggest that about one-third of people with lifetime PTSD have lifetime alcohol use disorder (AUD; Kessler et al. 1995). People with PTSD cite their PTSD symptoms as a reason for their alcohol consumption, and total PTSD symptoms are positively associated with craving and quantity of alcohol consumption (Heinz et al., 2016; Nishith et al., 2001).
Place conditioning can be used to test the rewarding or aversive properties of a drug and is based on the premise that animals spend more time in a context paired with a rewarding drug dose, and less time in a context paired with an aversive drug dose (Reid et al., 1985). In general, alcohol does not produce strong conditioned place preference (CPP) in rats (Reid et al., 1985; Bozarth, 1990), and can even produce a conditioned place aversion (CPA; Asin et al., 1985; Stewart & Grupp, 1986; van der Kooy et al., 1983). Whether alcohol produces a CPP or CPA in rats is dictated by several factors that include 1) route of alcohol administration; oral alcohol self-administration leads to stronger CPP than i.p. alcohol injections (Bain & Kornetsky, 1989), 2) the dose of alcohol used; lower alcohol doses (0.25 – 0.5 g/kg) produce stronger CPP than higher alcohol doses (≥1.0 g/kg), and 3) the number of alcohol and environmental pairings; more alcohol-context pairings lead to more alcohol CPP (Bozarth, 1990). Furthermore, rats with a history of alcohol drinking exhibit stronger preference for a context paired with a moderate dose of alcohol (Reid et al., 1985). Alcohol CPP is also facilitated by stress (i.e., footshock) in mice and rats (Sperling et al., 2010; Matsuzawa et al., 1998), an effect that may be attributable to increased sensitivity to the anxiolytic effects of alcohol, and which may drive subsequent alcohol-seeking behavior (Sperling et al., 2010).
Many years of work have implicated extended amygdala regions in mediating stress effects, as well as alcohol and drug use/abuse. The extended amygdala is a conceptual macrostructure composed of the central amygdala (CeA), bed nucleus of the stria terminalis (BNST), and nucleus accumbens shell, and is dysregulated by traumatic stress and chronic alcohol (Daniel & Rainnie, 2006; Calhoon & Tye, 2015; Gilpin et al. 2015; Silberman & Winder, 2015). Here, we tested the effect of acute alcohol on CREB phosphorylation (pCREB) and striatal-enriched protein tyrosine phosphatase (STEP) expression in CeA and BNST of rats with different stress and alcohol drinking histories.
Our model of predator odor stress utilizes bobcat urine as an innately stressful stimulus. The urine of carnivorous species contains 2-phenylethylamine, a trace amine produced by the breakdown of the amino acid phenylalanine (Ferrero et al. 2011). Specifically, 2-phenylethylamine activates trace amine-associated receptor 4 (TAAR4) in the rodent olfactory cortex, is found in high concentrations in bobcat urine relative to the urine of other carnivorous species, and it elicits avoidance behavior in rats and mice (Ferrero et al. 2011). Detection of bobcat urine by the dorsal olfactory cortex mediates behavioral responses to odor (Ferrero et al. 2011), whereas detection of bobcat urine by the amygdalo-piriform transition area mediates increases in circulating stress hormones after exposure to bobcat urine or 2,3,5-trimethyl-3-thiazoline (TMT; Kondoh et al. 2016). More specifically, bobcat urine activates CRF cells in the paraventricular nucleus of the hypothalamus (PVN), increases circulating adrenocorticotrophic hormone (ACTH) and corticosterone levels in rats, and increases anxiety-like behavior in the elevated plus maze and open field test (Kondoh et al. 2016; Whitaker & Gilpin 2015).
Both stress and alcohol alter pCREB expression in BNST and CeA. Cat exposure reduces pCREB expression level in BNST of rats (Blundell & Adamec, 2007), whereas cat exposure and forced swim stress each acutely increase pCREB in CeA of rats (Adamec et al., 2006; Shen et al., 2004). Similarly, in mice, fear conditioning and foot shock each acutely increase pCREB in the CeA (Stanciu et al., 2001). Alcohol-preferring (P) rats drink more alcohol and exhibit higher basal anxiety-like behavior than non-alcohol-preferring (NP) rats, and P rats also have innately lower levels of pCREB in the CeA than NP rats (Pandey et al., 2005). Furthermore, acute alcohol reduces anxiety-like behavior and increases pCREB in the CeA (but not other amygdaloid nuclei) of P rats (but not NP rats), perhaps through effects on transcription of neuropeptide Y (NPY), an abundant and potent anti-anxiety peptide in the mammalian CNS (Pandey et al., 2005). Therefore, we hypothesized that acute alcohol would increase pCREB in the CeA and BNST of animals that exhibit more conditioned place preference (or at least less aversion) for an alcohol-paired chamber.
The CeA and BNST contain high amounts of corticotropin-releasing factor (CRF), a pro-stress neuropeptide implicated in alcohol reward, binge-like alcohol consumption, and alcohol dependence (Schreiber & Gilpin, 2018). CRF pools in the BNST and CeA project to many similar downstream regions related to reward, aversion, pain, and anxiety; these regions include the ventral tegmental area (VTA), lateral hypothalamus (LH), periaqueductal grey (PAG), and dorsal raphe nucleus (DRN; Dabrowska et al., 2016; Pomrenze et al., 2015). Striatal-enriched protein tyrosine phosphatase (STEP) is expressed in ≥98% of CRF neurons in the BNST and ≥94% of CRF neurons in the CeA (Dabrowska et al. 2013), is a negative regulator of ERK signaling (via de-phosphorylation) and glutamatergic transmission, and it has been used as a proxy for CRF neuron activation in BNST after stress; more specifically, chronic restraint stress reduces STEP expression in the BNST of rats (Dabrowska et al., 2013). Stress effects on STEP expression in CeA were not measured in the Dabrowska et al., 2013 study, and it is not known how alcohol or stress-alcohol interactions might affect STEP expression in CeA and BNST. We hypothesized that after acute alcohol injection, STEP expression would be higher (i.e., CRF neuron activation would be lower) in the CeA and BNST of animals that exhibit a conditioned place preference (or at least reduced aversion) for the alcohol-paired chamber.
The purpose of these studies was to test 1) the effect of predator odor stress on conditioned place preference/aversion for a moderate dose of alcohol (1.0 g/kg) in animals with and without a history of chronic alcohol drinking, 2) the effect of predator odor stress on CREB phosphorylation and STEP expression in CeA and BNST, and 3) the association between these behavioral and biological effects of acute alcohol in stressed and unstressed rats. We hypothesized that 1) stressed rats would exhibit stronger preference (or less aversion) for the alcohol-paired chamber, 2) that chronic alcohol drinking history would increase preference (or reduce aversion) for the alcohol-paired chamber, 3) that stressed alcohol drinkers would exhibit the strongest preference (or least aversion) for the alcohol-paired chamber, and 4) that alcohol CPP would be associated with higher pCREB and STEP expression in the CeA and BNST after acute alcohol injection.
2. Methods and Materials
2.1. General Methods
2.1.1. Animals
Specific pathogen free adult male Wistar rats (N=64) (Charles River, Raleigh, NC) weighing 225-250 g at the time of arrival were pair-housed (Experiment 1) or single-housed (Experiment 2) in a humidity- and temperature-controlled (22 °C) vivarium on a 12 h light/ 12 h dark cycle (lights off at 8 a.m.). Animals had ad libitum access to food and water throughout the experiments and were handled daily prior to initiation of experimental protocols. Behavioral tests occurred during dark period. All procedures were approved by the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and were in accordance with the National Institute of Health Guidelines.
2.1.2. Predator Odor Exposure
In Experiments 1 and 2, adult male Wistar rats were transferred from the home cage to a clean cage and exposed to ambient air (control group) or bobcat urine (Maine Outdoor Solutions, Herman, MA) (stressed group) for 15 minutes. Bobcat urine (~3 ml) was added to a sponge that was placed beside the cage. Stressed rats were not able to contact the sponge or the urine. Following the 15-minute exposure, each rat was returned to the home cage.
2.1.3. Alcohol Place Conditioning
In Experiments 1 and 2, adult male Wistar rats underwent a place conditioning procedure. Briefly, on the first day, rats were allowed access to three conditioning chambers with distinct sets of tactile (circles vs. grid. vs. rod floor) and visual (circles vs. white vs. stripes) cues. Sessions were videotaped and scored by an observer. The chamber in which each rat spent the most deviant amount of time (highly preferred or highly avoided) compared to the other two was excluded as a conditioning chamber for that rat. On day 1, rats were allowed 5 min to explore the two non-excluded conditioning chambers. Assignment of alcohol or saline to each chamber was counterbalanced for baseline chamber preference for each rat. Within drug groups, alcohol administrations were paired with one of the two contexts for each rat in an unbiased counterbalanced design. On days 1, 3, and 5 post-stress, rats were administered sterile saline (6.7 ml/kg; i.p.) and immediately placed in one chamber for 15 min (neutral chamber). On days 2, 4, and 6 post-stress, rats were injected with alcohol (15% w/v; 1.0 g/kg; i.p.) or sterile saline for saline controls and placed in the opposite chamber for 15 min (alcohol-paired chamber). On post-stress day 7, rats underwent a 5 min video-recorded post-test to explore the two conditioning chambers. Preference was calculated as a difference score between postconditioning time spent in drug-paired context and pre-conditioning time spent in drug-paired context. Conditioning procedures were repeated for 3 weeks (i.e., CPP/CPA test once every 7 days for 21 days after stress).
2.1.4. Intermittent Access 2 Bottle Choice 20% Alcohol Homecage Drinking
In Experiment 2 only, prior to stress and alcohol place conditioning, adult male Wistar rats were singly housed and given access to alcohol and water (or two water bottles for controls) in three 24-hour sessions per week as previously described (Simms et al., 2008). Briefly, rats were given access to 1 bottle of 20% v/v alcohol and 1 bottle of water 15 minutes after the start of the dark cycle on Mondays, Wednesday, and Fridays. After 24 hours, the alcohol bottle was replaced with a second water bottle that was available for the next 24 hours. Over the weekends, rats had unlimited access to 2 water bottles after the alcohol bottle was removed on Saturday. Bottles were weighed 30 min and 24 hours after alcohol presentation. The position of the alcohol bottle was alternated across sessions to control for side preferences. Mean alcohol intake was determined by averaging alcohol intake across the last 3 alcohol drinking sessions. Controls always had two water bottles on the home cage (i.e., they never drank alcohol).
2.1.5. Alcohol Challenge and Western Blot Analysis
In Experiment 1, twenty-four hours after the last CPA test, rats were injected with either alcohol (15% w/v; 1.0 g/kg; i.p.) or sterile saline (6.7 ml/kg; i.p.). In Experiment 2, twenty-four hours after the last CPA test, all rats were injected with alcohol (15% w/v; 1.0 g/kg; i.p.). Fifteen minutes after the injection, rats were deeply anesthetized with isoflurane (5%) and decapitated. Brains were removed and snap frozen in isopentane (2-methylbutane; Fisher Scientific) on dry ice. Brains were stored at −80°C until further analysis. 500 μm-thick coronal sections were collected using a cryostat and tissue punches of the BNST were taken using a 14-gauge hollow needle. Tissue punches of the CeA were taken using a 17-gauge hollow needle. Tissues punches were stored at −80°C until further analysis.
Tissue samples were homogenized by sonication in lysis buffer (320 ml sucrose, 5 ml HEPES, 1 ml EGTA, 1 ml EDTA, and 1% SDS, with protease inhibitor cocktail and phosphate inhibitor cocktails Ii and III diluted 1:100; Sigma, St Louis, MO, USA), heated at 100°C for 5 min and were stored at −80°C until the determination of protein concentration by a detergent-compatible Lowry method (Bio-Rad, Hercules, CA). Samples of protein (10 μg) were subjected to SDS-polyacrylamide gel electrophoresis on 8% acrylamide gels by using a Tris/Glycine/SDS buffer system (Bio-Rad), followed by electrophorectic transfer to polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ). Membranes were blocked overnight in 5% non-fat milk at 4°C and were then incubated in primary antibody. Primary antibodies include STEP (1:500; Santa Cruz Biotechnology Inc, Santa Cruz, CA) and pCREB (1:10,000; Millipore, Burlington, MA). Membranes were washed and labeled with species-specific peroxidase-conjugated secondary antibody (1:10000; Bio-Rad) for 1 h at room temperature. Following chemiluminescence detection (SuperSignal West Pico; Thermo-Scientific, Rockford, IL), blots were stripped for 20 min at room temperature (Restore; Thermo-Scientific) and were re-probed for GAPDH (1:1,000,000; Cell Signaling Technology, Danvers, MA) and total CREB (1:20,000; Millipore, Burlington, MA). Immunoreactivity was quantified by densitometry (Image J 1.45S; Bethesda, MD) under linear exposure conditions. Densitized values were expressed as a percentage of mean control values for each gel to normal data across blots. Individual STEP levels were normalized to total GAPDH levels and pCREB levels were normalized to tCREB levels for statistical comparison.
2.2. Experimental Protocols
2.2.1. Experiment 1. Effect of predator odor stress on alcohol place conditioning in non-drinkers.
Rats (n=32) were given free access to two conditioning chambers and the baseline time spent in each was recorded. To avoid potential stress effects on initial chamber preference, the pre-test occurred prior to predator odor exposure; it is important to note that predator odor exposure was not paired with any conditioning chambers. Three hours after the pre-test, rats were exposed to bobcat urine (as described in General Methods). Starting the next day, rats were then conditioned with either saline (6.7 ml/kg; i.p; n=16) or alcohol (15% w/v; 1 g/6.7 ml/kg; i.p.; n=16) over a period of 3 weeks (as described in Section 2.1.3; Fig 1A). On day 22 post-stress, rats were injected with saline or alcohol as described above, sacrificed by decapitation 15 minutes later, then brains were excised, frozen, and cut into 500-μm sections, and the BNST and CeA were collected for Western blot analysis of pCREB and STEP expression.
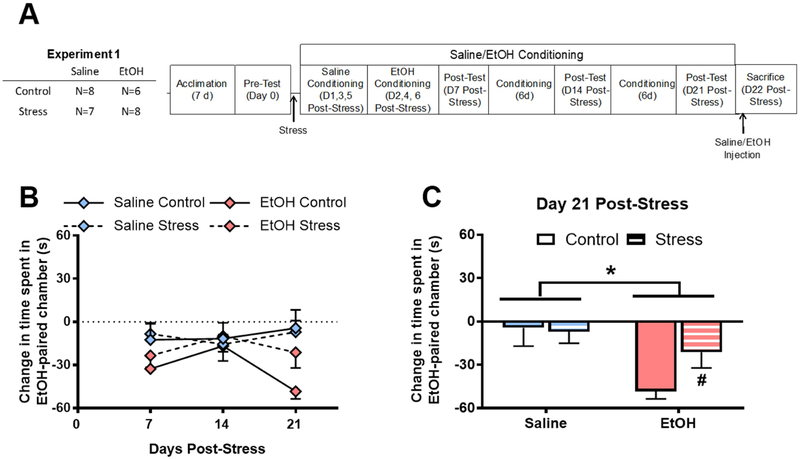
Figure 1. Stress reduces alcohol conditioned place aversion in pair-housed rats.
A) Experimental Timeline; B) Alcohol produces a conditioned aversion to the alcohol-paired chamber (p=0.06); C) Alcohol produces a conditioned aversion of alcohol-paired chamber (*p<0.01), which is blunted by a history of stress before conditioning (#p=0.051). Data are represented as mean ± SEM. Saline, blue; Alcohol, pink; Control, solid; Stress, striped.
2.2.2. Experiment 2. Effect of predator odor stress on alcohol place conditioning in alcohol drinkers.
Rats (n=28) were singly housed for the duration of the experiment (~9 weeks total) in order to measure homecage drinking in individual rats over time (Fig 4A). Rats drank alcohol or water in the intermittent access 2-bottle choice 20% alcohol homecage drinking paradigm (as described in Section 2.1.4) for 5 weeks before undergoing alcohol place conditioning (Fig 4A). Briefly, rats underwent a pre-test, then were exposed to predator odor 3 hours later (as described in General Methods). Starting the day after stress exposure, all rats were conditioned with alcohol (15% w/v; 1 g/6.7 ml/kg; i.p.) over a period of 3 weeks (as described in Section 2.1.3). On day 22 post-stress, rats were injected with alcohol as described above, sacrificed by decapitation 15 minutes later, then brains were excised, frozen, and cut into 500-μm sections, and the BNST and CeA were collected for Western blot analysis of pCREB and STEP expression.
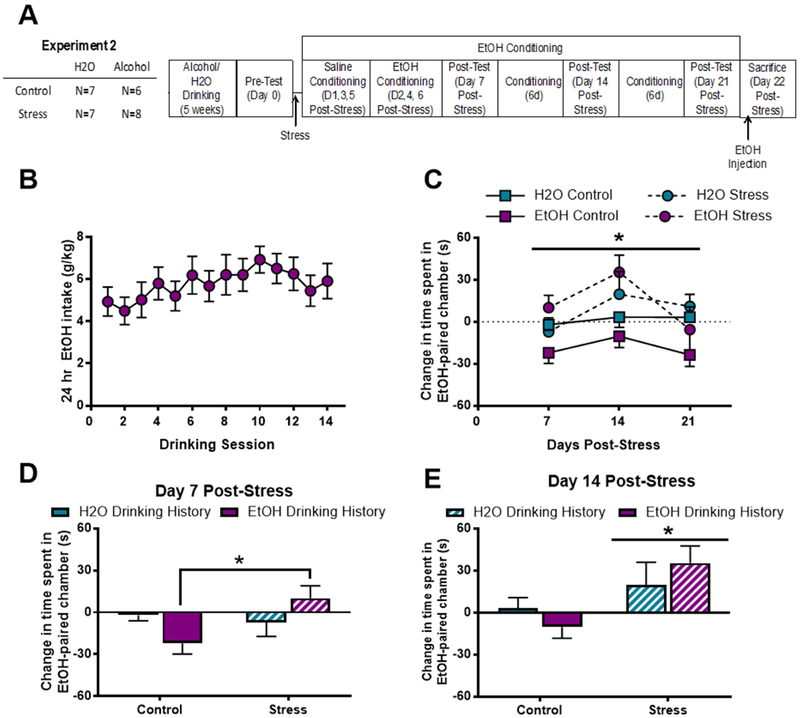
Figure 4. Stress increases conditioned preference for alcohol regardless of alcohol drinking history.
A) Experimental Timeline; B) Rats escalate alcohol consumption over 5 weeks. C) Stress increases preference for alcohol-paired chamber across test days (*p<0.01); D) 7 days post-stress, stressed rats with an alcohol drinking history display increased preference for alcohol paired chamber compared to unstressed rats with alcohol drinking history (*p=0.03); ) 14 days post-stress, all stressed rats significantly prefer the alcohol-paired chamber, regardless of alcohol drinking history (*p=0.01). Data are represented as mean ± SEM. H2O drinking history, turquoise; alcohol drinking history, purple; Control, solid; Stress, diagonal stripes.
2.3. Statistical Analysis
Data are shown as mean ± SEM with the number of animals indicated in the timeline figure for each experiment (Figures 1A & 4A). Behavioral results were analyzed using a 3-way repeated measures ANOVA: in Experiment 1, stress and alcohol conditioning solution were between-subjects factors and time was a within-subjects factor; in Experiment 2, stress and alcohol drinking history were between-subjects factors and time was a within-subjects factor. Each weekly CPA test was separately analyzed by a 2-way ANOVA with stress and alcohol conditioning (Experiment 1) or stress and alcohol drinking history (Experiment 2) as the between-subjects factors. Molecular results were analyzed using 2-way ANOVA with stress and alcohol conditioning (Experiment 1) or stress and alcohol drinking history (Experiment 2) as the between-subjects factors. In addition, relationships between molecular data and behavioral data were analyzed using linear regression analysis. Post-hoc analysis with Tukey’s HSD was used when appropriate. Statistical significance was set at p≤0.05.
3. Results
3.1. Predator odor stress reduces alcohol conditioned place aversion in pair-housed non-drinkers
In Experiment 1, rats were stressed, then conditioned with either saline or alcohol (1 g/kg; i.p.) in an alcohol place conditioning procedure. A 3-way repeated measures ANOVA revealed no significant effect of stress on place conditioning over time, but there was a trend toward an alcohol CPA (F[1,25]=3.76; p=0.06; Fig. 1B). A 2-way ANOVA of day 7 conditioned preference revealed that rats conditioned with alcohol spent significantly less time in the alcohol-paired chamber than rats conditioned with saline (F[1,25]=4.34; p=0.047), but there was no main effect of stress (F[1,25]=0.62; p=0.44), nor was there a significant stress × alcohol interaction (F[1,25]=0.09; p=0.76). Analysis of day 14 conditioned preference by 2-way ANOVA revealed no main effect of alcohol (F[1,25]<0.01; p=0.98), stress F[1,25]=0.02; p=0.89), or a stress × alcohol interaction (F[1,25]=0.27; p=0.61) on time spent in the alcohol-paired chamber. A separate, 2-way ANOVA of day 21 conditioned preference (i.e., after all conditioning days were complete) revealed that rats conditioned with alcohol spent significantly less time in the alcohol-paired chamber than rats conditioned with saline (F[1,25]=8.11; p<0.01; Fig. 1C). There was no main effect of stress (F[1,25]=1.42; p=0.24), nor was there a significant stress × alcohol interaction (F[1,25]=2.16; p=0.16). Based on the a priori hypothesis that stress history would reduce alcohol aversion in alcohol-conditioned rats, we analyzed Day 21 data in alcohol-conditioned rats using a two-sample t-test where stress was the between-subjects factor. This analysis revealed that stressed rats conditioned with alcohol exhibited less conditioned aversion to an alcohol-paired chamber relative to unstressed rats conditioned with alcohol (t(13)=2.145; p=0.05; Fig. 1C).
3.2. Stress history and acute alcohol do not alter pCREB or STEP expression in the CeA of pair-housed non-drinkers
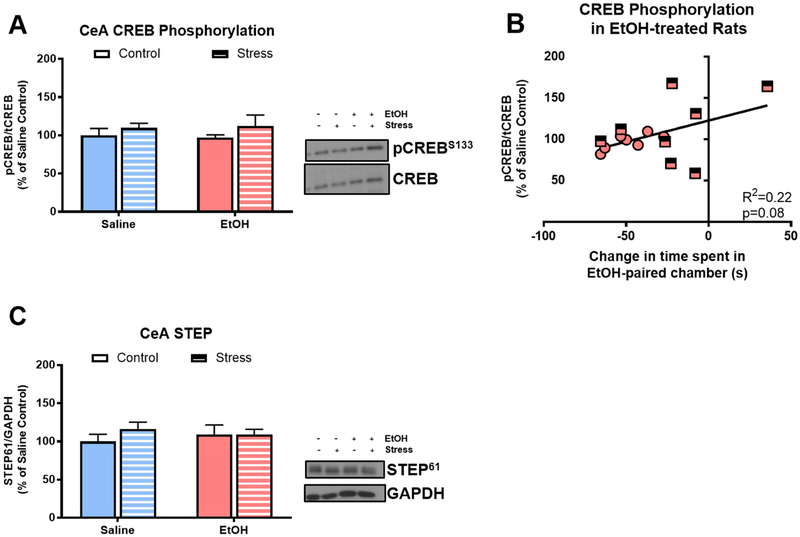
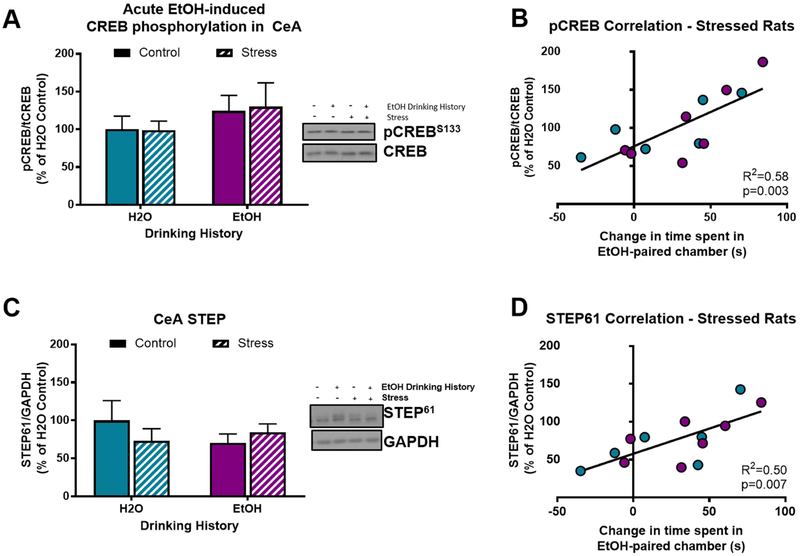
In Experiment 1, we tested the effect of acute alcohol on pCREB and STEP expression in CeA, and the association of pCREB and STEP levels with conditioned alcohol aversion. There was not a main effect of acute alcohol injection (F[1,25]=0.00; p=0.99) or stress (F[1,25]=1.59; p=0.22; Fig. 2A) on pCREB expression in CeA, but there was not a significant correlation of acute alcohol injection-induced pCREB expression and time spent in the alcohol-paired chamber in rats conditioned with alcohol (R2=0.22; p=0.08; Fig 2B), such that rats with less conditioned alcohol aversion exhibited higher levels of pCREB in the CeA following an alcohol injection, regardless of stress history. There was not a main effect of alcohol (F[1,25]=0.01; p=0.94) or stress (F[1,25]=0.72; p=0.40; Fig 2C) on STEP expression in the CeA, nor was there a significant correlation of STEP expression with conditioned aversion in any treatment group.
Figure 2. Stress and alcohol do not alter pCREB or STEP expression in CeA of pair-housed rats.
A) Neither stress history nor acute alcohol altered CREB phosphorylation in CeA 15 minutes after injection; B) CREB phosphorylation 15 minutes following an acute alcohol injection exhibited a trend toward a positive correlation with change in time spent in alcohol-paired chamber (R2=0.22; p=0.08); C) Neither stress history nor acute alcohol altered STEP expression in CeA 15 minutes after injection. Data are represented as mean ± SEM; Saline, blue; alcohol, pink; Control, solid; Stress, striped.
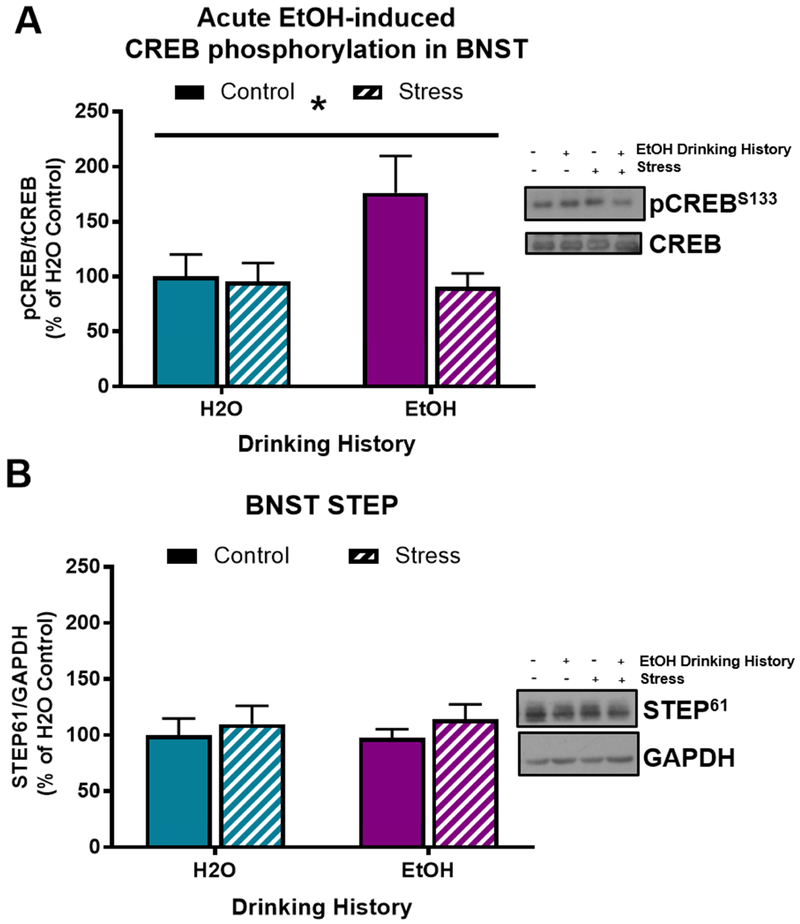
3.3. Stress history reduces CREB phosphorylation in the BNST of pair-housed non-drinkers
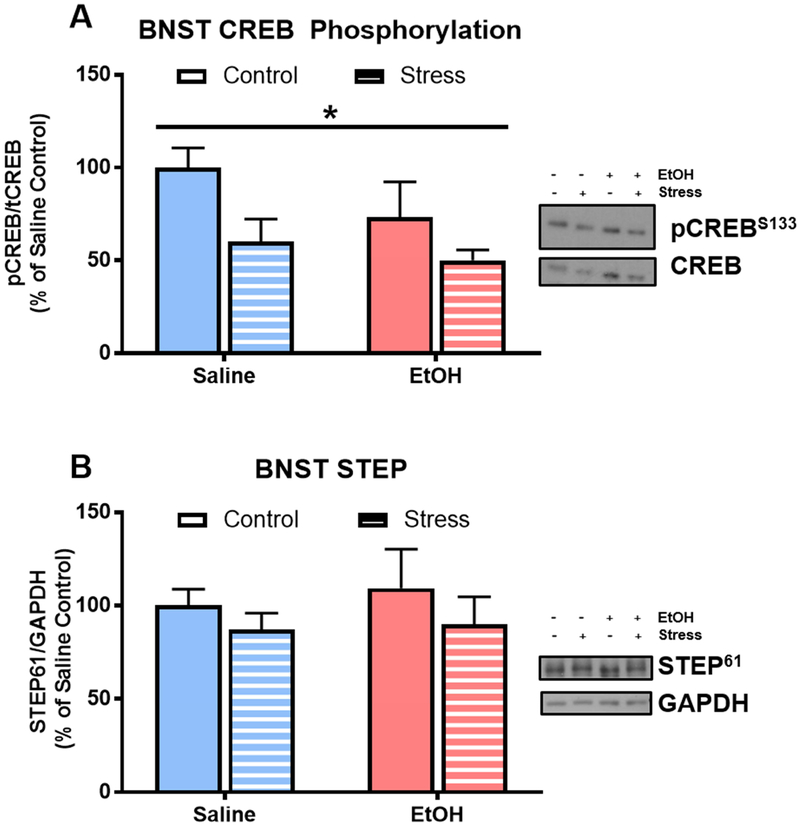
In Experiment 1, stress history significantly reduced CREB phosphorylation in the BNST of non-drinkers (F[1,21]=6.77; p=0.02; Fig 3A). However, there was not a main effect of acute alcohol injection on pCREB in the BNST of non-drinkers (F[1,21]=2.29; p=0.15), nor was there a stress history × acute alcohol interaction effect (F[1,21]=0.46; p=0.51). There was also not a main effect of acute alcohol injection (F[1,25]=0.01; p=0.94) or stress (F[1,25]=0.72; p=0.40; Fig 2C) on STEP expression in the BNST. There was not a significant correlation of pCREB or STEP with conditioned alcohol aversion in any of the treatment groups.
Figure 3. Stress history reduces CREB phosphorylation in BNST of pair-housed rats.
A) Stress decreases CREB phosphorylation in the BNST 15 minutes after an acute saline or alcohol injection (*p=0.02); B) Neither stress or alcohol affect STEP in the BNST. Data are represented as mean ± SEM; Saline, blue; alcohol, pink; Control, solid; Stress, striped.
3.4. Stress history increases time spent in alcohol-paired context in single-housed animals with and without an alcohol drinking history
In Experiment 2, rats were singly housed and allowed to consume alcohol or water in an intermittent access 2-bottle choice homecage drinking procedure for five weeks. In this paradigm, rats modestly escalated alcohol consumption over time such that at the end of the 5 weeks (14th drinking session), rats were consuming an average of 5.91 ± 0.86 g/kg alcohol per 24-hour period (Fig 4B). Rats were then stressed or not stressed, and all rats underwent alcohol place conditioning. A 3-way (stress history × alcohol history × test) RM ANOVA revealed that stress history (F[2,23]=5.66; p<0.01) significantly increased the amount of time spent in the alcohol-paired chamber (Fig 4C). A separate, 2-way ANOVA of day 7 place conditioning data revealed no significant effect of stress (F[1,23]=3.08; p=0.09) or alcohol drinking history (F[1,23]=0.03; p=0.86) on time spent in alcohol-paired chamber. However, there was a significant interaction effect (F[1,23]=5.79; p=0.03), such that stressed alcohol drinkers spent significantly more time in the alcohol-paired chamber relative to unstressed alcohol drinkers (Tukey’s, p=0.03; Fig. 4D); this effect of stress was not observed in alcohol-naïve rats. For day 14 place conditioning data, a 2-way ANOVA revealed that stress history increased acute alcohol-conditioned place preference (F[1,23]=7.86; p=0.01), but there was no main effect of alcohol drinking history (F[1,23]=0.01; p=0.93) nor was there a stress history × alcohol drinking history interaction effect in alcohol place conditioning (F[1,23]=1.75; p=0.19; Fig. 4E). For day 21 place conditioning data, a 2-way ANOVA revealed no effect of stress history (F[1,20]=9.169; p=0.35), alcohol drinking history (F[1,20]=2.584; p=0.12), or a stress history × alcohol drinking history interaction effect in alcohol place conditioning (F[1,20]=0.148; p=0.70). There was no significant correlation between amount of alcohol consumed during homecage drinking and amount of time spent in the alcohol-paired chamber at any of the time points examined. However, there was a trend toward a positive correlation between average amount of alcohol consumed during the 5th week of homecage drinking and amount of time spent in the alcohol-paired chamber 14 days post-stress only in stressed rats (R2=0.44; p=0.07, data not shown). Interestingly, at the same timepoint, there was a weak negative correlation between average alcohol consumption and time spent in the alcohol-paired chamber in control rats (R2=0.22; p=0.29; data not shown).
3.5. Post-alcohol pCREB & STEP expression in CeA of single-housed stressed rats correlates with alcohol place conditoning.
In Experiment 2, we tested the association of alcohol place conditioning with pCREB and STEP expression in the CeA of stressed rats injected with acute alcohol prior to sacrifice. There was no main effect of alcohol drinking history (F[1,24=1.39; p=0.25) or stress history (F[1,24]=0.01; p=0.99; Fig. 5A) on CREB phosphorylation. However, similar to the trend observed in pair-housed rats in Experiment 1, after acute alcohol injection, pCREB expression was positively correlated with time spent in the alcohol-paired chamber, but only in stressed rats (R2=0.58; p<0.01; Fig 5B). That is, stressed rats that exhibited stronger alcohol CPP also exhibited increased CREB phosphorylation in the CeA following an acute alcohol injection, regardless of alcohol drinking history.
Figure 5. Alcohol CPP positively correlates with pCREB and STEP expression in CeA of single-housed stressed rats.
A) Neither stress history nor acute alcohol alter CREB phosphorylation in the CeA 15 minutes after an acute alcohol injection (1 g/kg; i.p.); B) in stressed rats, CREB phosphorylation 15 minutes following an acute alcohol injection positively correlates with change in time spent in alcohol-paired chamber (R2=0.58; p<0.01); C) neither stress history nor acute alcohol alter STEP expression in the CeA; D) in stressed rats, STEP expression 15 minutes following an acute alcohol injection positively correlates with change in time spent in alcohol-paired chamber (R2=0.50; p=0.01); Data are represented as mean ± SEM. H2O drinking history, turquoise; alcohol drinking history, purple; Control, solid; Stress, diagonal stripes.
Neither alcohol drinking history (F[1,24]=0.45; p=0.51) nor stress history (F[1,24]=0.25; p=0.62; Fig 5C) produced main effects on STEP expression in CeA after acute alcohol injection. However, after acute alcohol injection, STEP expression was positively correlated with time spent in the alcohol-paired chamber, again only in stressed rats (R2=0.50; p=0.01; Fig 5D). That is, stressed rats that exhibited stronger alcohol CPP also exhibited higher STEP expression in the CeA following an acute alcohol injection, regardless of alcohol drinking history.
3.6. Stress history reduces pCREB expression in BNST of single-housed alcohol drinkers after an acute alcohol injection
In Experiment 2, stress history significantly reduced pCREB expression in BNST (F[1,21]=4.81; p=0.04), and there was no main effect of alcohol drinking history on pCREB expression in BNST (F[1,21]=3.081; p=0.09). There was a marginally non-significant trend toward a stress history × alcohol drinking history interaction effect on pCREB expression after acute alcohol injection (F[1,21]=3.88; p=0.06; Fig 6A). These data suggest that stress history reduced CREB phosphorylation in the BNST of alcohol drinkers after an acute alcohol injection (Alcohol Stress vs. Alcohol Control, Tukey’s p=0.04). There was not a main effect of alcohol drinking history (F[1,24]=0.01; p=0.96) or stress history (F[1,24]=0.90; p=0.35; Fig 5B), nor was there an alcohol drinking history × stress history interaction effect (F[1,24]=0.06; p=0.81) on STEP expression in the BNST after acute alcohol injection. There was not a significant correlation of pCREB or STEP expression in BNST with time spent in the alcohol-paired chamber in any of the treatment groups.
Figure 6. Stress blunts alcohol-induced increase in pCREB expression in BNST of single-housed rats.
A) Stress significantly blunts acute-alcohol induced increase in pCREB expression in BNST (*p=0.04); B) Neither stress nor alcohol affect STEP in the BNST. Data are represented as mean ± SEM. H2O drinking history, turquoise; alcohol drinking history, purple.
4. Discussion
The main results of these studies demonstrate that 1) stress blunts alcohol aversion and may increase the rewarding effects of a moderate alcohol dose, 2) in rats with a stress history, increased alcohol-conditioned reward is associated with higher pCREB and higher STEP expression (i.e., reduced CRF cell activation) in the CeA after an acute alcohol injection, 3) stress history reduces CREB phosphorylation in BNST after an acute alcohol injection, and 4) the effects of acute alcohol on conditioned reward/aversion and CREB phosphorylation in BNST are different in single-housed and pair-housed rats.
We tested the effect of predator odor (i.e., bobcat urine) stress on alcohol place conditioning in rats with and without a history of alcohol drinking, as well as the effect of acute alcohol injection on STEP expression (as a proxy for CRF cell activation) and CREB phosphorylation in the CeA and BNST. Stress history reduced conditioned place aversion to 1.0 g/kg alcohol in rats without a history of alcohol drinking that were pair-housed. In addition, stress history produced a conditioned place preference for 1.0 g/kg alcohol in single-housed rats, regardless of alcohol drinking history. The difference in housing conditions between the two studies may contribute to the difference in place conditioning times for the same alcohol dose, especially since alcohol drinking history did not have a significant main effect on alcohol place conditioning. Single-housed animals (water drinkers and alcohol drinkers) may be more innately stressed or more primed to be stressed by predator odor exposure, and one or both of these stress effects may make alcohol less aversive/more rewarding. Prior work by other labs shows that social isolation does not increase alcohol drinking in adult rats (Thorsell et al. 2005; Chappell et al. 2013), but social isolation produces long-term increases in alcohol drinking in adolescent male (but not female) rats (Chappell et al. 2013). Based on those data, we hypothesize that either 1) single-housed rats in our experiment were more primed for predator odor stress effects, or 2) there are strain differences (Wistars vs. Long-Evans) in the effects of single housing on alcohol reward/drinking in adult rats. We conclude that social isolation in Experiment 2 may have been innately stressful or may have primed animals for predator odor stress effects, and may have thereby increased time spent in the alcohol-paired context relative to pair-housed rats in Experiment 1.
This study examined the effect of stress on alcohol conditioned place aversion/preference over time. Alcohol place conditoning is influenced by several factors including the dose of alcohol used, the number of alcohol and environmental pairings, history of alcohol drinking, and stress. In this study, after place conditioning with a dose of alcohol that is not strongly rewarding or aversive (1 g/kg), pair-housed stressed rats exhibited less aversion for the alcohol-paired chamber 21 days post-stress (after 9 alcohol-context pairings) relative to pair-housed unstressed rats, while single-housed stressed rats spent more time in the alcohol-paired context than single-housed unstressed rats both 7 and 14 days after stress (i.e., after 3 and 6 alcohol-context pairings), but this effect was not observed 21 days post-stress. Therefore, stress-induced blunting of conditioned alcohol aversion emerged slowly over time (and conditioning sessions) in pair-housed rats, whereas stress-induced increases in time spent in the alcohol-paired context emerged and dissipated more quickly in single-housed rats. It is not entirely clear why the emergence and dissipation of stress effects on acute alcohol place conditioning exhibited a different time course in pair-housed and single-housed rats, but again, it is possible that single housing may “prime” animals for experimental stress effects or mitigate those effects depending on timing and outcome measure. At the very least, we conclude that stress effects on alcohol conditioned reward/aversion is a dynamic temporal process.
We measured CREB phosphorylation, an upstream transcription event that leads to production of many proteins including the anti-anxiety peptide NPY, and we also measured STEP expression, used as a proxy for CRF cell activation, in CeA after an acute alcohol injection in stressed and unstressed rats with and without alcohol drinking history. In general, we hypothesized that higher pCREB and STEP expression in CeA after acute alcohol injection would associate with lower alcohol aversion and/or higher alcohol reward. While there were no significant main effects of stress history or alcohol drinking history on CREB phosphorylation in CeA after acute alcohol injection, stressed rats (regardless of alcohol drinking history) that exhibited higher preference for an alcohol-paired chamber also exhibited increased CREB phosphorylation in CeA after an acute alcohol injection, suggesting that increased alcohol reward is associated with increased engagement by alcohol of CREB transcription targets in CeA. This result exhibits striking similarity to results from the Pandey group showing that in alcohol-preferring P rats, a rat line with innately high anxiety-like and alcohol drinking behaviors, but not NP rats, acute alcohol increases CREB phosphorylation in CeA and this effect is associated with lower anxiety-like behavior (Pandey et al., 2005), perhaps by triggering increased NPY production in CeA (Zhang et al., 2010). This suggests that acute alcohol-induced increases in CREB phosphorylation in CeA 1) predict higher alcohol reward (or lower alcohol aversion), and/or 2) occur only in rats that are stressed or have high innately high anxiety-like behavior.
Although there was no main effect of acute alcohol, stress history, or alcohol drinking history on STEP expression in CeA, increased preference for the alcohol-paired chamber was positively correlated with STEP expression in CeA only in stressed rats. Because STEP is a negative regulator of glutamatergic activity and multiple signaling cascades (e.g., ERK) in CRF cells in the CeA (Dabrowska et al., 2013), this suggests that higher preference for an alcohol-paired chamber is correlated with lower activation of putative CRF cells in CeA (i.e., lower pro-stress signaling in CeA) after an acute alcohol injection. In support of the notion that low CRF cell activation is associated with increases in alcohol reward (and/or decreases in alcohol aversion), infusions of exogenous CRF into the CeA produce conditioned aversion (Itoga et al. 2016), whereas CRFR1 antagonism in CeA reduces escalated alcohol drinking in alcohol-dependent rats (Roberto et al. 2010; Funk et al. 2006) and escalated alcohol drinking in mice stressed using social defeat (Newman et al. 2018). That said, it is important to note that our study used STEP expression as a proxy for CRF cell activation but did not directly measure CeA CRF cell activity after acute alcohol. Future work will use slice electrophysiology and fiber-based photometry to test the effect of stress and alcohol on in vitro and in vivo CRF cell activity.
We also measured pCREB and STEP expression in the BNST after an acute alcohol injection in stressed and unstressed rats with and without an alcohol drinking history. In both experiments, stress reduced CREB phosphorylation in the BNST regardless of acutely injected solution or alcohol drinking history. These data corroborate past findings that cat exposure reduces CREB phosphorylation in the BNST in an NMDA-dependent fashion (Blundell & Adamec, 2007). Furthermore, rats that display high fear-potentiated startle have less CREB phosphorylation in the BNST than rats with low fear-potentiated startle (Meloni et al. 2006). These data collectively suggest that stress-induced reductions in CREB phosphorylation in BNST may reflect high stress reactivity; an alternative possibility is that pCREB in BNST is associated with different stress coping strategies although this has not been tested. We did not observe an association between pCREB in BNST and preference for the alcohol-paired chamber, suggesting that alcohol and stress may have different interaction effects in BNST versus CeA. CREB is expressed in multiple cell types in the BNST and CeA, and CREB is a transcriptional regulator of multiple genes including CRF and NPY (Kasagi et al., 2002; Nestler, 2004; Zhang et al., 2010); as such, future work will measure stress- and alcohol-induced activation of specific cell types and synthesis of specific pCREB transcriptional targets in BNST and CeA. Unlike our results in the CeA, STEP expression in the BNST was not correlated with time spent in the alcohol-paired chamber in stressed or unstressed rats.
Overall, our main findings demonstrate that predator odor stress increases alcohol reward and/or reduces alcohol aversion, and that this effect may be impacted by housing conditions. We also report that, in stressed rats only, acute alcohol effects on CREB phosphorylation and STEP expression in CeA are positively associated with preference for an alcohol-paired chamber. In general, these and past results support the hypothesis that alcohol acts as a negative reinforcer by engaging anti-stress systems and suppressing pro-stress systems in the extended amygdala of rats that are stressed or exhibit innately high anxiety. Future work will examine Avoiders and Non-Avoiders in our bobcat urine conditioned avoidance model of traumatic stress (Edwards et al., 2013), test stress-alcohol interaction effects on the activity of specific CeA and BNST cell types and circuits, and will test the role of CeA and BNST in mediating stress effects on escalated alcohol drinking in this model.
Highlights.
Predator odor stress history reduces alcohol aversion
Housing conditions affect alcohol aversion and extended amygdala protein expression
Alcohol aversion is associated with CREB and STEP expression in central amygdala
Acknowledgments
Funding and Disclosures
This work was supported by NIH grants AA023696 (ALS), AA023305 (NWG), and AA026531 (NWG). NWG is a consultant for Glauser Life Sciences. All other authors report no biomedical financial interests of potential conflicts of interest.
Footnotes
Conflict of Interest Statement
The data presented are the original work of the authors and there are no competing financial interests in relation to the work described in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Asin KE, Wirtshafter D, Tabakoff. 1985. Failure to establish a conditioned place preference with ethanol in rats. Pharmacol Biochem Behav. 22(2): 169–73. [DOI] [PubMed] [Google Scholar]
- 2.Bain GT, Kornetsky C. 1989. Ethanol oral self-administration and rewarding brain stimulation. Alcohol. 6(6):499–503. [DOI] [PubMed] [Google Scholar]
- 3.Blundell J, Adamec R. 2007. The NMDA receptor antagonist CPP blocks the effects of predator stress on pCREB in brain regions involved in fearful and anxious behavior. Brain Res. 1136(1):59–76. [DOI] [PubMed] [Google Scholar]
- 4.Bozarth MA. 1990. Evidence for the rewarding effects of ethanol using the conditioned place preference model. Pharmacol Biochehm Behav. 35(2):485–7. [DOI] [PubMed] [Google Scholar]
- 5.Calhoon GG, Tye KM. 2015. Resolving the neural circuits of anxiety. Nature Neuroscience. 18(10):1394–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chappell AM, Carter E, McCool BA, Weiner JL. 2013. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking male long evans rats. ACER 37(S1):E394–E403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabrowska J, Hazra R, Guo Jd, Li C, Dewitt S, Xu J, Lombroso PJ, Rainnie DG. 2013. Striatal-enriched protein tyrosine phosphatase-STEPs toward understanding chronic stress-induced activation of corticotrophin releasing factor neurons in the rat bed nucleus of the stria terminalis. Biol Psychiatry 74 (11):817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dabrowska J, Martinon D, Moaddab M, Rainnie DG. 2016. Targeting Corticotropin-Releasing Factor Projections from the Oval Nucleus of the Bed Nucleus of the Stria Terminalis Using Cell-Type Specific Neuronal Tracing Studies in Mouse and Rat Brain. J Neuroendocrinol. 28 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daniel SE, Rainnie DG. 2016. Stress modulation of opposing circuits in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 41(1):103.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. 2013. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 3:e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrero DM, Lemon JK, Fluegge D, Pashkovski SL, Korzan WJ, Datta SR, Spehr M, Fendt M, Liberies SD. 2011. Detection and avoidance of a carnivore odor by prey. Proc Natl Acad Sci U S A. 108(27):11235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk CK, O’Dell LE, Crawford EF, Koob GF. 2006. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-withdrawn rats. J Neurosci. 26(44):11324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilpin NW, Herman MA, Roberto M. 2015. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psych. 77(10):859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez JL, Lewis MJ, Sebastian V, Serrano P, Luine VN. 2013. Alcohol administration blocks stress-induced impairments in memory and anxiety, and alters hippocampal neurotransmitter receptor expression in male rats. Horm Behav. 63(4)659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harvard Medical School. 2007. National Comorbidity Survey. (2018, March 5). Retrieved from https://www.hcp.med.harvard.edu/ncs/index.php. Data Table 2: 12-month prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort. [Google Scholar]
- 16.Heinz AJ, Pennington DL, Cohen N, Schemling B, Lasher BA, Schrodek E, Batki SL. 2016. Relations between cognitive functioning and alcohol use, craving, post-traumatic stress: An examination among trauma-exposed military veterans with alcohol use disorder. Mil Med. 181(7):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hershon HI. 1977. Alcohol withdrawal symptoms and drinking behavior. J Stud Alcohol. 38 (5):953–971. [DOI] [PubMed] [Google Scholar]
- 18.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. 1995. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 52(12):1048–60. [DOI] [PubMed] [Google Scholar]
- 19.Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu YL, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN, Gilpin NW. 2016. Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology. 41(10):2463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondoh K, Lu Z, Ye X, Olson DP, Lowell BB, Buck LB. 2016. A specific area of olfactory cortex involved in stress hormone responses to predator odours. Nature. 532(7597):103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuzawa S, Suzuki T, Misawa M. 1998. Conditioned fear stress induces ethanol-associated place preference in rats. Eur J Pharmacol. 341(2-3):127–30. [DOI] [PubMed] [Google Scholar]
- 22.Meloni EG, Jackson A, Gerety LP< Cohen BM, Carlezon WA Jr. 2006. Role of the bed nucleus of the stria terminalis (BST) in the expression of conditioned fear. Ann N Y Acad Sci. 1071:538–41. [DOI] [PubMed] [Google Scholar]
- 23.Nestler EJ. 2004. Molecular mechanisms of drug addiction. Neuropharmacology. 47(1):24–32. [DOI] [PubMed] [Google Scholar]
- 24.Newman EL, Albrechet-Souza L, Andrew PM, Auld JG, Burk KC, Hwa LS, Zhang EY, DeBould JF, Miczek KA. 2018. Persistent escalation of alcohol consumption by mice exposed to brief episodes of social defeat stress; suppression by CRF-R1 antagonism. Psychopharm (Berl). 235(6):1807–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishith P, Resick PA, Meuser KT. 2001. Sleep difficulties and alcohol use motives in female rape victims with posttraumatic stress disorder. J Trauma Stress. 14(3):469–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey Sc, Zhang H, Roy A, Xu T. 2005. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 115(10):2762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. 2008. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: A model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 105(14):5567–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pomrenze MB, Millan EZ, Hopf FW, Keiflin R, Maiya R, Blasio A, Dadgar J, Kharazia V, De Guglielmo G, Crawford E, Janak PH, George O, Rice KC, Messing RO. 2015. A Transgenic Rat for Investigating the Anatomy and Function of Corticotrophin Releasing Factor Circuits. Front Neurosci. 9:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reid LD, Hunter GA, Beaman CM, Hubbel CL. 1985. Toward understanding ethanol’s capacity to be reinforcing: a conditioned place preference following injections of ethanol. Pharmacol Biochem Behav. 22(3):483–7. [DOI] [PubMed] [Google Scholar]
- 30.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cotton P, Madamba SG, Stouffer DG, Zorrilla EP, Koob FG, Siggins GR, Parsons LH. 2010. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psych. 67(9):831–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roltsch-Hellard EA, Impastato RA, Gilpin NW. 2016. Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol. 22(3):692–701. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber AL, Gilpin NW. 2018. Corticotropin-Releasing Factor (CRF) Neurocircuitry and and Neuropharmacology in Alcohol Drinking. Handb Exp Pharmacol. doi:10.1007/164_2017_86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen CP, Tsimberg Y, Salvadore C, Meller E. 2004. Activation of Erk and JNK MAPK pathways by acute swim stress in rat brain regions. BMC Neurosci. 5:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silberman Y, Winder DG. 2013. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Frontiers in Psychiatry. 4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinha R 2012. How does stress lead to risk of alcohol relapse? Alcohol Res. 34(4):432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sperling RE, Gomes SM, Sypek EI, Carey AN, McLaughlin JP. 2010. Endogenous kappa-opioid mediation of stress-induced potentiation of ethanol-conditioned place preference and self-administration. Psychopharmacology (Berl). 210(2):199–209. [DOI] [PubMed] [Google Scholar]
- 37.Stanciu M, Radulovic J, Spiess J. 2001. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 94(1-2):15–24. [DOI] [PubMed] [Google Scholar]
- 38.Stewart RB, Grupp LA. 1986. Conditioned place aversion mediated by orally self-administered ethanol in the rat. Pharmacol Biochem Behav. 24(5):1369–75. [DOI] [PubMed] [Google Scholar]
- 39.Thorsell A, Slawecki CJ, Khoury A, Mathe AA, Ehlers CL. 2005. Effect of social isolation on ethanol consumption and substance P/neurokinin expression in Wistar rats. Alcohol. 36(2):91–7. [DOI] [PubMed] [Google Scholar]
- 40.van der Kooy D, O’Shaughnessy M, Mucha RF, Kalant H. 1983. Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav. 19(3):441–5. [DOI] [PubMed] [Google Scholar]
- 41.Vazquez-Leon P, Martinez-Mota L, Quevedo-Corona L, Miranda-Paez A. 2017. Isolation stress and chronic mild stress induced immobility in the defensive burying behavior and a transient increased ethanol intake in Wistar rats. Alcohol. 63:43–51. [DOI] [PubMed] [Google Scholar]
- 42.Whitaker AM, Gilpin NW. 2015. Blunted hypothalamo-pituitary adrenal axis response to predator odor predict high stress reactivity. Physiology and Behavior. 147:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Sakharkar AJ, Shi G, Ugale R, Prakash A, Pandey SC. 2010. Neuropeptide Y signaling in the central nucleus of amygdala regulates alcohol drinking and anxiety-like behaviors of alcohol-preferring rats. Alcohol Clin Exp Res. 34(3):451–61. [DOI] [PMC free article] [PubMed] [Google Scholar]