Abstract
Background
Despite considerable efforts, few drugs are available for the treatment of alcohol (ethanol [EtOH]) use disorder (AUD). Ethanol directly or indirectly modulates several aspects of the central nervous system, including neurotransmitter/neuromodulator systems. Relapse vulnerability is a challenge for the treatment of EtOH addiction. Ethanol withdrawal symptoms create motivational states that lead to compulsive EtOH drinking and relapse even after long periods of abstinence. Among the therapeutics to treat AUD, naltrexone (NTX) is a pharmacological treatment for relapse. The present study evaluated the effect of NTX on EtOH drinking in male and female EtOH-dependent rats during abstinence.
Methods
Wistar rats (males and females) were first trained to orally self-administer 10% EtOH. Half of the rats were then made dependent by chronic intermittent EtOH (CIE) vapor exposure, and the other half were exposed to air. Using this model, rats exhibit somatic and motivational signs of withdrawal. At the end of EtOH vapor (or air) exposure, the rats were tested for the effects of NTX (10 mg/kg, p.o.) on EtOH self-administration at three abstinence time points: acute abstinence (8 h, A-Abst), late abstinence (2 weeks, L-Abst), and protracted abstinence (6 weeks, P-Abst).
Results
Naltrexone decreased EtOH intake in nondependent rats, regardless of sex and abstinence time point. In post-dependent rats, NTX decreased EtOH intake only at a delayed abstinence time point (P-Abst) in males, whereas it similarly reduced EtOH drinking in females at all abstinence time points.
Conclusions
The therapeutic efficacy of NTX depends on the time of intervention during abstinence and is different between males and females. The data further suggest that EtOH dependence causes different neuroadaptations in male and female rats, reflected by differential effects of NTX. The results underscore the significance of considering the duration of EtOH abstinence and sex as a biological variable as important factors when developing pharmacotherapies for AUD.
Keywords: alcohol, ethanol, intake, abstinence, naltrexone, dependence
Introduction
Ethanol (EtOH) produces its effects on the central nervous system through several mechanisms, one of which involves alterations of the endogenous opioid system. Opioid receptor subtypes (μ, δ, κ) have high affinity for three main classes of endogenous opioids (β-endorphin, enkephalins, and dynorphins, respectively). Acute EtOH stimulates the release of β-endorphin, enkephalins, and dynorphin in humans and rats (Gianoulakis et al., 1996b, Marinelli et al., 2003, Marinelli et al., 2004, Marinelli et al., 2005, Marinelli et al., 2006, Dai et al., 2005, Popp and Erickson, 1998, Rasmussen et al., 1998).
Naltrexone (NTX) is an approved medication for the treatment of alcoholism, based on its efficacy in reducing the craving for and consumption of EtOH (Volpicelli et al., 1992). It is a nonspecific opioid receptor antagonist. In nondependent subjects, the NTX-induced blockade of opioid receptors for these endogenous ligands suppresses EtOH consumption (Gonzales and Weiss, 1998, Coonfield et al., 2002, Shoemaker et al., 2002, Stromberg, 2004), and this beneficial effect is blunted in post-dependent subjects (Ji et al., 2008, Sabino et al., 2006, Sabino et al., 2013, Walker and Koob, 2008). Although clinical trials have demonstrated the efficacy of NTX in reducing the risk of relapse in heavy drinkers, many patients experience no benefit of treatment (Krystal et al., 2001). This suggests that several factors influence treatment success (e.g., the time of intervention during abstinence, gender, and changes in the endogenous opioid system). Numerous investigations have sought to characterize biochemical modifications of the endogenous opioid system that are closely associated with the incidence of alcoholism or alcohol use disorder (AUD). For example, both mice and humans with a high risk of alcoholism present greater hypothalamic β-endorphin activity (Gianoulakis et al., 1996). Repeated EtOH administration induces both short- and long-term alterations of opioid levels in brain regions that are associated with motivation and reward (e.g., nucleus accumbens; (Lindholm et al., 2000). In rodents, chronic EtOH intake and EtOH withdrawal have been shown to induce a wide range of perturbations of the opioid system, suggesting a putative role for the endogenous opioid system in the effects of EtOH, such as a decrease in μ-opioid receptor expression in the nucleus accumbens and dorsal striatum (Turchan et al., 1999), an increase in prodynorphin mRNA levels in the nucleus accumbens (Przewlocka et al., 1997), a decrease in κ-opioid receptor mRNA expression in the ventral tegmental area and nucleus accumbens (Rosin et al., 1999), an increase in preproenkephalin mRNA expression in the amygdala, and a decrease in preproenkephalin mRNA expression in the nucleus accumbens (Cowen and Lawrence, 2001).
Men have been consistently shown to drink more EtOH and have a greater propensity to develop AUD than women. Recent data, however, suggest that EtOH consumption in men and women has become more similar over the past two decades (Keyes et al., 2008, Slade et al., 2016). Several studies have reported sex differences in EtOH intake in rats, in which females drank more than males (Blanchard et al., 1993, Morales et al., 2015, Walker et al., 2008, Li and Lumeng, 1984, Priddy et al., 2017), whereas other studies found no sex differences (van Haaren and Anderson, 1994, Moore and Lynch, 2015, Schramm-Sapyta et al., 2014). The use of different rat strains (e.g., Wistar, Sprague-Dawley, and Long Evans) or different experimental designs (e.g., two-bottle choice, self-administration, and chronic intermittent EtOH [CIE] vapor exposure) may account for such discrepancies. To our knowledge, no study to date has compared the efficacy of NTX in preventing EtOH drinking between males and females. Such information is especially important when attempting to develop efficient pharmacotherapies for the treatment of AUD.
The resumption of excessive EtOH drinking (i.e., relapse) even long after physical signs of withdrawal have dissipated is a challenge for the successful treatment of AUD. Although NTX is an approved treatment for AUD, it is unclear why treatment efficacy varies between subjects. Therefore, to gain a better understanding of the efficacy of NTX in preventing the recurrence of EtOH drinking during abstinence, the present study systematically tested the effects of NTX at different time points during abstinence in male and female rats.
Materials and Methods
Rats
Forty-eight Wistar rats (24 males and 24 females; Charles River, Wilmington, MA, USA), weighing 150–175 g upon arrival, were housed two per cage in a temperature- and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. The animals were given at least 1 week to acclimate to the housing conditions and handling before testing. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Ethanol self-administration training (Fig. 1)
Figure 1.
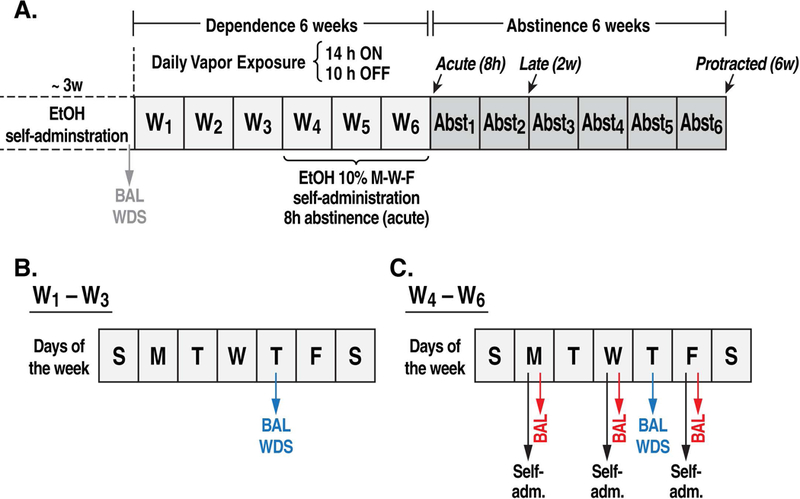
Experimental procedure. At the end of EtOH self-administration training (A), BALs were measured after the self-administration session during the last week of training. WDS scores were recorded upon the completion of training. (B) During weeks 1–3 of CIE vapor exposure, BALs were measured 15 min before the EtOH vapors were turned off, and the rats were scored for WDS 8 h after the EtOH vapor was turned off (on Thursdays). (C) During weeks 4–6 of CIE vapor exposure, BALs were measured an additional three times during the week immediately after the self-administration sessions (Monday, Wednesday, and Friday). BAL, blood alcohol level; WDS, somatic withdrawal signs; W, week.
The EtOH self-administration training procedure was a modification of early studies by Wise (Wise, 1973) and revised by Simms et al. (Simms et al., 2008). The procedure did not require any fading (saccharin or sucrose) procedure to initiate voluntary EtOH drinking. On the Monday (Day 1) following the end of the housing acclimatization period, the rats were single-housed and presented with two bottles: one bottle contained H2O, and one bottle contained 10% (w/v) EtOH (prepared in tap water from 95% w/v EtOH) for 3 days (Monday, Tuesday, Wednesday; Days 1–3). On Thursday and Friday (Days 4 and 5), the rats were grouped-housed (two per cage) and given only H2O in their home cage. On Saturday (Day 6), the rats were given access to EtOH self-administration for 12 h in standard operant conditioning chambers (29 cm × 24 cm × 19.5 cm; Med Associates, St. Albans, VT, USA) on a fixed-ratio 1 schedule of reinforcement, in which each response at the right active lever (i.e., the only lever available at that time) resulted in the delivery of 0.1 ml of fluid and brief illumination of a cue light (0.5 s) above the lever. Food and water were available in the operant chambers during this self-administration session to avoid food or water deprivation because of the extensive length of the operant procedure. Following the 12 h session, the rats were returned to their home cage. On Sunday (Day 7), they were left undisturbed. On Monday and Tuesday (Days 8 and 9), the rats were given access to EtOH self-administration for 2 h/day and on Wednesday and Thursday (Day 10 and 11) for 1 h/day. Starting on Friday (Day 12) and for the rest of the self-administration days, the rats underwent 30 min self-administration sessions with the introduction of a second (left) inactive lever at which responses were recorded but had no programmed consequences. Tail bleeds were performed after each self-administration session during the last 5 days of training, and blood alcohol levels (BALs) were measured (Fig. 1A). The rats were scored for somatic withdrawal signs (WDS) upon the completion of training (Fig. 1A).
Chronic intermittent EtOH vapor exposure (Fig. 1)
At the end of EtOH self-administration training, two rats (one male and one female) did not acquire EtOH self-administration, thus reducing the number of animals to 46. After 21 sessions of EtOH self-administration, half of the rats (n = 22; 11 males and 11 females) were then made dependent (EtOH post-dependent [EtOHPD]) by CIE vapor exposure. The other half (n = 24; 12 males and 12 females) comprised the nondependent group (EtOHND) that was exposed only to air. During 6-week dependence induction, the rats underwent daily cycles of 14 h ON (BALs during vapor exposure ranged between 150 and 250 mg%, measured with a blood analyzer [GC-headspace, Agilent Technologies, Santa Clara, CA, USA]) and 10 h OFF and were left undisturbed for 3 weeks except to control their BALs (measured during the last 15 min of vapor exposure) and to score WDS (at 8 h of abstinence) once per week. Behavioral signs of withdrawal were measured by a laboratory assistant who was blind to the experimental conditions using a rating scale that was adapted from an original study by Macey et al. (Macey et al., 1996) and included ventromedial limb retraction, vocalization (i.e., irritability to touch), tail rigidity, abnormal gait, and body tremors. Each sign was given a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, and 2 = severe. The sum of the 5 scores (0–10) was used as a quantitative measure of withdrawal severity and to confirm dependence. In this model, rats exhibit somatic and motivational signs of withdrawal (Vendruscolo and Roberts, 2014). Starting at the beginning of the fourth week of CIE vapor exposure, the rats were subjected to 30 min FR1 EtOH self-administration sessions when acute abstinence occurred (i.e., 8 h after the vapor was turned off when brain and blood alcohol levels were negligible), three times per week (Monday, Wednesday, and Friday), in which a gradual increase in EtOH intake should be measured (Vendruscolo and Roberts, 2014). The air-exposed rats underwent the same procedure.
Effects of naltrexone on EtOH self-administration during abstinence (Fig. 1)
Following 6 weeks of CIE vapor or air exposure, the animals began the abstinence phase and were tested for the effect of NTX (naltrexone hydrochloride, Sigma, St. Louis, MO, USA) on EtOH self-administration at three abstinence points: acute abstinence (A-Abst, 8 h), late abstinence (L-Abst, 2 weeks), and protracted abstinence (P-Abst, 6 weeks). At each time point of abstinence, the rats were first scored for behavioral signs of withdrawal and then received oral NTX (10 mg/kg in a volume of 3 ml/kg; n = 6) or vehicle (water, n = 5/6 animals) administration. Sixty minutes later, they were placed in the operant chambers and allowed to self-administer 10% EtOH on an FR1 schedule for 30 min. Naltrexone (or vehicle) was administered according to a within-subjects design, such that the same group of rats received three NTX (or vehicle) treatments: one at A-Abst, one at L-Abst, and one at P-Abst.
It is known that the plasma concentration of NTX 60 minutes following an oral administration is ~ 10 times lower when compared to the subcutaneous route (see Hussain et al., 1987 and Ciccocioppo et al., 2003 for comparison). The rationale for using a moderate 10 mg/kg oral dose of NTX was based on the literature and specifically on a recent study that reported a significant reduction of EtOH drinking in alcohol-preferring (P) rats following oral administration of 10 mg/kg NTX (Rorick-Kehn et al., 2014).The oral bioavailability of NTX needs to be taken in account when comparing the present data with other studies that used other routes of administration (Walker and Koob, 2008; Ciccocioppo et al., 2003).
Statistical analysis
Two-way analysis of variance (ANOVA) was used to compare EtOH intake and inactive lever responses between males and females during the last 5 days of training. Two-way ANOVA was used to divide male and female rats into two subgroups to obtain a similar baseline (BSL) of EtOH intake based on the last 3 days of training pre-CIE vapor exposure. Two-way ANOVA was used to analyze differences in BALs between males and females that were caused by CIE vapor exposure. Three-way ANOVA was used to analyze differences in EtOH intake, inactive lever responses, BALs, and WDS during training and CIE vapor exposure between males and females. Three-way ANOVA was used to analyze differences in EtOH intake, inactive lever responses, BALs, and WDS related to NTX (or VEH) treatment at different time points of abstinence. Although WDS were measured prior to NTX and VEH treatment, the treatment factor (NTX vs. VEH) was retained as a variable to maintain the group subdivision at each abstinence point and consistency among the data presentation. When no significant three-way interaction was found, two-way ANOVA was used to analyze significant two-way interactions. Significant effects in the ANOVAs were followed by the Tukey post hoc test. WDS values were transformed into log10 for statistical analysis and back-transformed for graphical representation. The results are expressed as mean ± SEM. Differences were considered significant at p < 0.05. The statistical analysis was performed using GraphPad Prism 7 and Statistica 7.0 software.
Results
Two animals were excluded from the study, one in the male EtOHPD group and one in the female EtOHPD group, because they did not acquire EtOH self-administration, thus reducing the number of animals to 46 (n = 12 EtOHND males and n = 11 EtOHPD males during training and CIE exposure; n = 6 EtOHND males treated with NTX or vehicle, n = 6 EtOHPD males treated with NTX, and n = 5 EtOHPD males treated with vehicle; n = 12 EtOHND females and n = 11 EtOHPD females during training and CIE rocedure; n = 6 EtOHND females treated with NTX or vehicle, n = 6 EtOHPD females treated with NTX, and n = 5 EtOHPD females treated with vehicle).
Ethanol self-administration training
During the last 5 days of EtOH self-administration training (Fig. 2), both groups of animals (males and females) presented stable and comparable EtOH intake in a 30-min daily session (two-way ANOVA: sex [male vs. female], F1,44 = 0.1, p > 0.05; time [days of self-administration], F4,176 = 1.3, p > 0.05; sex × time interaction, F4,176 = 1.1, p > 0.05). Responses at the inactive lever were low and constant across the last five days of training (two-way ANOVA: sex [male vs. female], F1,44 = 5.8, p < 0.05; time [days of self-administration], F4,176 = 0.3, p > 0.05; sex × time interaction, F4,176 = 0.3, p > 0.05; Fig. 2, bottom panel). The rats were then divided into four groups: EtOHND males, EtOHPD males, EtOHND females, and EtOHPD females.
Figure 2.
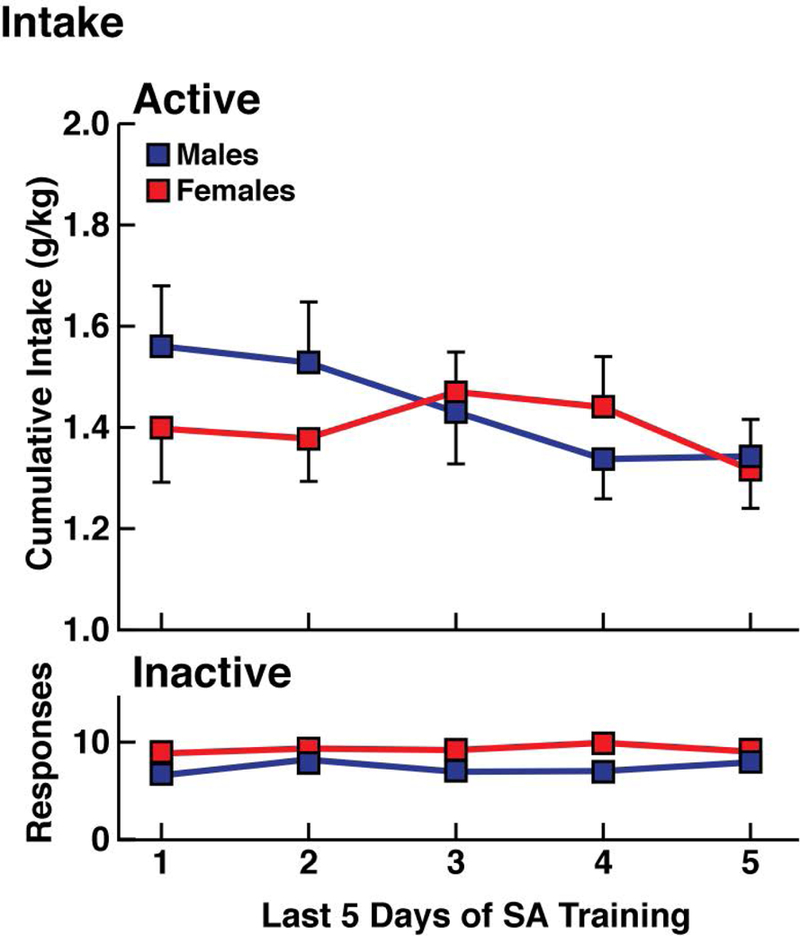
Ethanol intake (upper panel) over the last 5 days of self-administration in daily 30 min sessions in males and females. Responses at the inactive lever are represented in the lower panel. n = 23 rats/group.
Ethanol self-administration during CIE vapor exposure
Before CIE or air exposure, male and female rats were divided into two subgroups to obtain a similar baseline (BSL) of EtOH intake based on the last 3 days of training pre-CIE vapor exposure (BSL; two-way ANOVA: sex [male vs. female], F1,42 = 0.04, p > 0.05; group [EtOHND vs. EtOHPD], F1,42 = 0.06, p > 0.05; sex × group interaction, F1,42 = 0.54, p > 0.05; Fig. 3A). At the end of CIE exposure (W4-W6), an increase in EtOH intake (calculated by averaging the measures that were obtained Monday, Wednesday, and Friday of each week; Fig. 3A) was detected in EtOHPD rats, with an overall difference between males and females (three-way ANOVA: sex [male vs. female], F1,168 = 5.4, p < 0.05; group [EtOHND vs. EtOHPD], F1,168 = 98.2, p < 0.001; time [BSL, week 4, week 5, and week 6], F3,168 = 8.0, p < 0.001; sex × group × time interaction, F3,168 = 0.3, p > 0.05; Fig. 3A, B). Although no significant three-way interaction was detected, a significant two-way group × time interaction was found (F3,168 = 11.8, p < 0.001). Separate two-way ANOVAs by sex confirmed an increase in EtOH intake at weeks 4, 5, and 6 in males and females (males: group [EtOHND vs. EtOHPD], F1,21 = 36.2, p < 0.001; time [BSL, week 4, week 5, and week 6], F3,63 = 4.9, p < 0.01; group × time interaction, F3,63 = 19.7, p < 0.001; females: group [EtOHND vs. EtOHPD], F1,21 = 18.7, p < 0.05; time [BSL, week 4, week 5, and week 6], F3,63 = 7.0, p < 0.001; group × time interaction, F3,63 = 4.6, p < 0.01). Tukey post hoc tests confirmed that EtOHPD rats drank significantly more EtOH from week 4 to week 6 of CIE vapor exposure (p < 0.001) vs. baseline and the EtOHND group. Inactive lever responses remained low and unaffected in both males and females (three-way ANOVA: sex [male vs. female], F1,168 = 24.7, p < 0.001; group [EtOHND vs. EtOHPD], F1,168 = 2.1, p > 0.05; time [BSL, week 4, week 5, and week 6], F3,168 = 2.4, p > 0.05; sex × group × time interaction, F3,168 = 0.2, p > 0.05; Fig. 3A).
Figure 3.
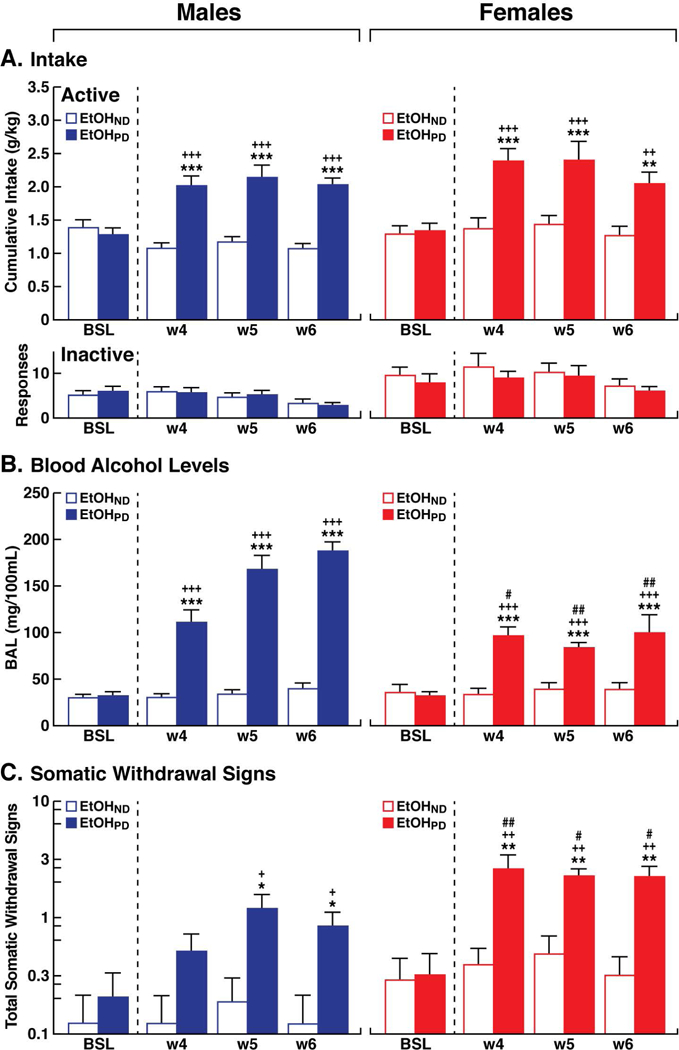
(A) Upper panel. Cumulative EtOH intake was higher in EtOHPD animals compared with EtOHND animals and compared with the training condition in both males and females. Lower panel. Responses at the inactive lever remained low and stable in both sexes. (B) Blood alcohol levels (BALs) that were measured immediately after the self-administration session during CIE vapor exposure were higher in EtOHPD animals compared with EtOHND animals in males and females, with females having lower BALs compared with males. (C) Somatic withdrawal signs (WDS) that were recorded prior to the EtOH self-administration sessions during CIE vapor exposure at 8 h of abstinence were higher in EtOHPD animals than in EtOHND animals in males and females, with females exhibiting higher WDS compared than males at weeks 4, 5, and 6 of CIE exposure. The Y-axis for WDS is expressed as log10. +p < 0.05, ++p < 0.01, +++p < 0.001, vs. respective BSL (Tukey post hoc test); *p < 0.05, **p < 0.01, ***p < 0.001, vs. EtOHND (Tukey post hoc test);, #p < 0.05, ##p < 0.01, vs. respective time point in males (Tukey post hoc test). n = 11/12 animals/group. BSL, baseline during training.
Blood alcohol levels
During weeks 1, 2, and 3 of CIE exposure, the EtOH delivery was adjusted to reach and maintain an average BAL of 150–250 mg%. During weeks 4, 5, and 6 of CIE, BALs remained stable within the required range (150–250 mg%). Blood was collected once per week (on Thursdays; Fig. 1B) 15 min before the EtOH vapor was turned off: week 4 (male: 171.9 ± 14.3 mg/100 ml; female: 154.0 ± 13.9 mg/100 ml), week 5 (male: 170.4 ± 22.6 mg/100 ml; female: 154.4 ± 8.8 mg/100 ml), and week 6 (male: 184.9 ± 18.9 mg/100 ml; female: 159.3 ± 6.0 mg/100 ml). No differences in BALs were found between males and females over the last 3 weeks of CIE vapor exposure (two-way ANOVA: sex, F1,20 = 3.4, p > 0.05; time, F2,40 = 0.5, p > 0.05; sex × time interaction, F2,40 = 0.5, p > 0.05).
Blood alcohol levels that were measured after each self-administration session during the CIE vapor exposure were higher in EtOHPD rats (both males and females) compared with their respective pre-CIE vapor exposure (BSL). Post self-administration BALs were higher in EtOHPD animals vs. EtOHND for both sexes, with EtOHPD females having lower BALs than EtOHPD males across weeks 4, 5, and 6 of CIE vapor exposure (p < 0.05, Tukey post hoc test following three-way ANOVA: sex [male vs. female], F1,168 = 26.8, p < 0.001; group [EtOHND vs. EtOHPD], F1,168 = 261.3, p < 0.001; time [BSL, week 4, week 5, and week 6], F3,168 = 39.8, p < 0.001; sex × group × time interaction, F3,168 = 7.2, p < 0.001; Fig. 3B).
Withdrawal scores
In males, during weeks 5, and 6 of CIE vapor exposure, somatic withdrawal signs that were measured before the self-administration session were higher in EtOHPD animals compared with EtOHND animals and compared with BSL. In females, somatic withdrawal signs that were measured before the self-administration session were higher in EtOHPD animals compared with EtOHND animals during weeks 4, 5, and 6 of CIE vapor exposure and compared with BSL. Compared with males, EtOHPD females exhibited significantly higher WDS (p < 0.05, Tukey post hoc test following three-way ANOVA: sex, F1,168 = 29.9, p < 0.001; group [EtOHND and EtOHPD], F1,168 = 57.6, p < 0.001; time [BSL, weeks 4, 5, and 6], F3,168 = 8.3, p < 0.001; sex × group × time interaction, F3,168 = 5.2, p < 0.05; Fig. 3C).
Effects of NTX on EtOH self-administration during abstinence
Ethanol intake.
Separate analyses of the differential behavioral effects of NTX across abstinence time points were performed between males and females. In males (Fig. 4A), EtOHND rats responded differently to NTX than EtOHPD rats over the different abstinence time points (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 6.7, p < 0.01; treatment [VEH vs. NTX], F1,54 = 43.26, p < 0.001; dependence [EtOHND vs. EtOHPD], F1,54 = 32.17, p < 0.001; time × treatment × dependence interaction, F2,54 = 1.4, p > 0.05). No significant three-way interaction was detected, but a significant two-way time × treatment interaction was found (F2,54 = 4.6, p < 0.05). In EtOHND males, the overall effect of NTX treatment was significant (two-way ANOVA: treatment, F1,9 = 26.99, p < 0.001). In EtOHPD males, significant effects of time and NTX treatment were found (two-way ANOVA: time, F2,18 = 9.6, p < 0.01; treatment, F1,9 = 6.0, p < 0.05). Tukey post hoc tests confirmed that NTX reduced EtOH intake in EtOHND rats at all abstinence time points (p < 0.05). In EtOHPD rats, NTX reduced EtOH intake only at P-Abst (p < 0.05). Inactive lever responses remained low and unaffected by NTX treatment (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 1.7, p > 0.05; treatment [VEH vs. NTX], F1,54 = 0.2, p > 0.05; dependence [EtOHND vs. EtOHPD], F1,54 = 0.7, p > 0.05; time × treatment × dependence interaction, F2,54 = 0.4, p > 0.05; Fig. 4A).
Figure 4.
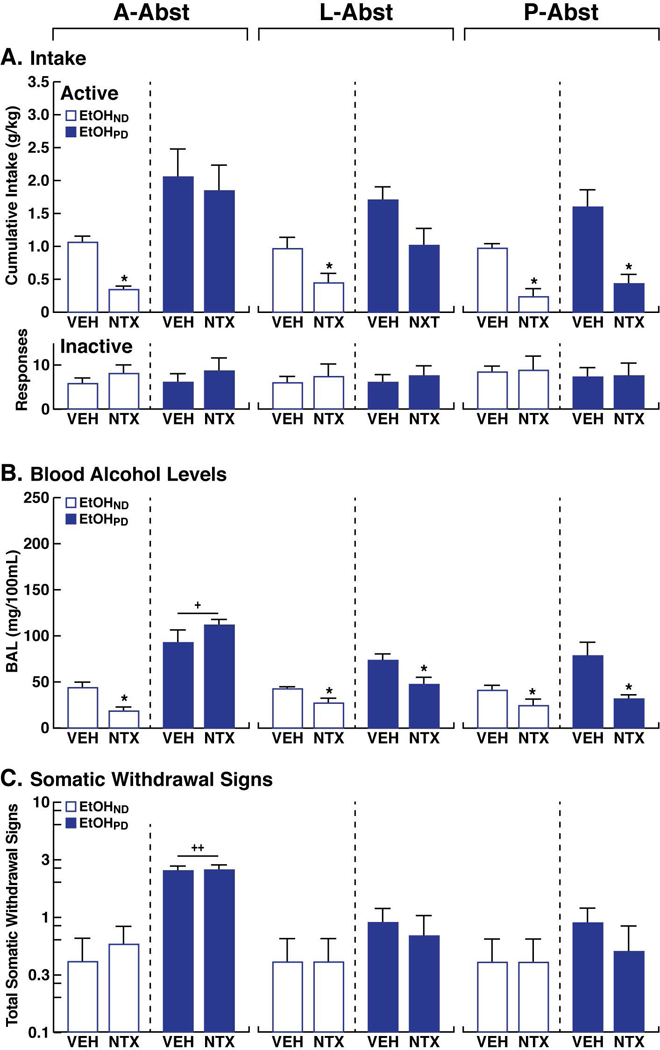
Effects of NTX in males. (A) Upper panel. Cumulative EtOH intake after NTX or VEH administration in EtOHPD animals and EtOHND animals at A-Abst, L-Abst, and P-Abst. Lower panel. Responses at the inactive lever. (B) Blood alcohol levels (BAL) were measured immediately after self-administration following NTX or vehicle (VEH) administration in EtOHPD and EtOHND animals at A-Abst, L-Abst, and P-Abst. (C) Somatic withdrawal signs (WDS) were recorded prior to EtOH self-administration before NTX or VEH administration in EtOHPD and EtOHND animals at A-Abst, L-Abst, and P-Abst. The Y-axis for WDS is expressed as log10. *p < 0.05, vs. respective VEH (Tukey post hoc test); +p < 0.05, ++p < 0.001, vs. EtOHND (Tukey post hoc test). n = 5/6 animals/group.
In females (Fig. 5A), NTX decreased EtOH intake in both the EtOHND and EtOHPD groups at all abstinence time points (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 0.9, p > 0.05; treatment [VEH vs. NTX], F1,54 = 38.6, p < 0.001; dependence [EtOHND vs. EtOHPD], F1,54 = 22.5, p < 0.001; time × treatment × dependence interaction, F2,54 = 0.2, p > 0.05). No significant three-way interaction was detected, but a significant two-way treatment × dependence interaction was found (F1,54 = 4.6, p < 0.05). Naltrexone reduced EtOH intake at A-Abst (two-way ANOVA: treatment, F1,18 = 9.1, p < 0.05; dependence, F1,18 = 4.744, p < 0.05; treatment × dependence interaction, F1,18 = 0.4, p > 0.05), L-Abst (two-way ANOVA: treatment, F1,18 = 16.3, p < 0.001; dependence, F1,18 = 13.1, p < 0.01; treatment × dependence interaction, F1,18 = 3.4, p > 0.05), and P-Abst (two-way ANOVA: treatment, F1,18 = 16.9, p < 0.001; dependence, F1,18 = 9.3, p < 0.01; treatment × dependence interaction, F1,18 = 1.9, p > 0.05) in both EtOHND and EtOHPD rats. Tukey post hoc tests confirmed that NTX reduced EtOH intake in EtOHND and EtOHPD rats at all abstinence time points (p < 0.05). Inactive lever responses remained low and unaffected (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 0.9, p > 0.05; treatment [VEH vs. NTX], F1,54 = 1.1, p > 0.05; dependence [EtOHND vs. EtOHPD], F1,54 = 1.0, p > 0.05; time × treatment × dependence interaction, F2,54 = 0.3, p > 0.05; Fig. 5A).
Figure 5.
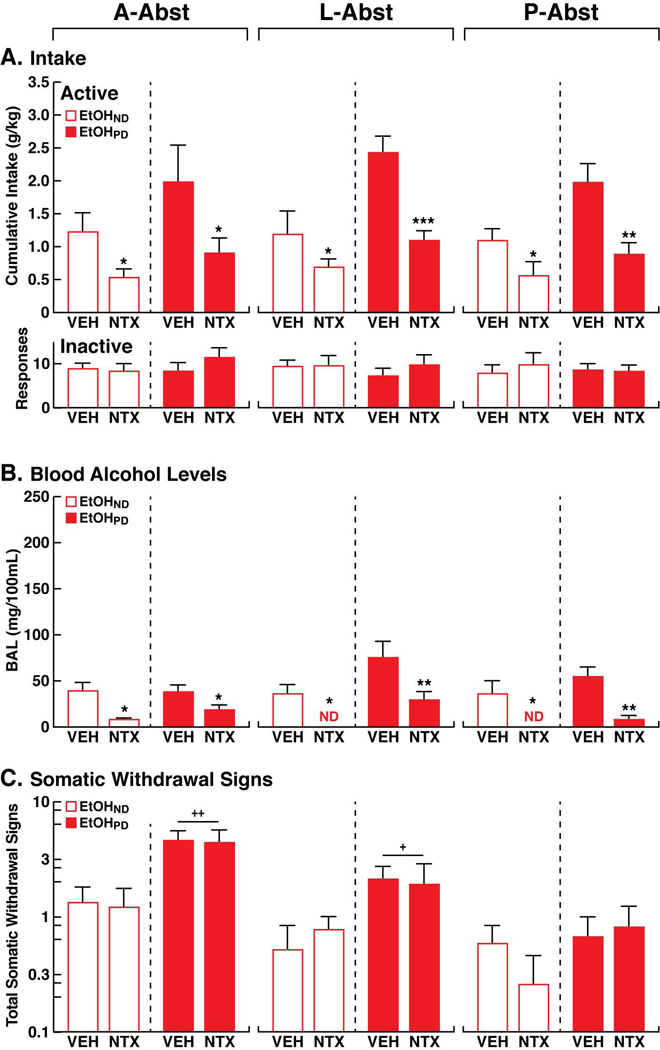
(A) Upper panel. Effects of NTX in females. Cumulative EtOH intake following NTX or vehicle (VEH) administration in EtOHPD animals and EtOHND animals at A-Abst, L-Abst, and P-Abst. Lower panel. Responses at the inactive lever. (B) Blood alcohol levels (BALs) were measured immediately after self-administration following NTX or VEH administration in EtOHPD and EtOHND animals at A-Abst, L-Abst, and P-Abst. ND, not detectable. (C) Somatic withdrawal signs (WDS) were recorded immediately prior to self-administration before NTX or VEH administration in EtOHPD and EtOHND animals at A-Abst, L-Abst, and P-Abst. The Y-axis for WDS is expressed as log10. *p < 0.05, **p < 0.01, ***p < 0.001, vs. respective VEH (Tukey post hoc test); +p < 0.05, ++p < 0.01, vs. EtOHND (Tukey post hoc test). n = 5/6 animals/group.
Blood alcohol levels
In EtOHND males, BALs after self-administration following NTX treatment at A-Abst, L-Abst, and P-Abst were lower compared with VEH-treated animals (p < 0.05, Tukey post hoc test). In EtOHPD rats, lower BALs in NTX-treated animals vs. vehicle-treated animals were observed at L-Abst and P-Abst (p < 0.05, Tukey post hoc test) but not at A-Abst (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 12.5, p < 0.001; treatment [VEH vs. NTX], F1,54 = 21.6, p < 0.001; dependence [EtOHND vs. EtOHPD], F1,54 = 101.4, p < 0.001; time × treatment × dependence interaction, F2,54 = 8.0, p < 0.001; Fig. 4B). EtOHND rats exhibited lower BALs vs. EtOHPD rats at A-Abst (p < 0.05, Tukey post hoc test; Fig. 4B).
In EtOHND and EtOHPD females, BALs after self-administration following NTX treatment at A-Abst, L-Abst, and P-Abst were lower compared with VEH-treated animals (p < 0.05, Tukey post hoc test following three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 3.23, p < 0.05; treatment [VEH vs. NTX], F1,54 = 38.9, p < 0.001; dependence [EtOHND vs. EtOHPD], F1,54 = 19.9, p < 0.001; time × treatment × dependence interaction, F2,54 = 6.5, p < 0.01; Fig. 5B).
Somatic withdrawal signs
In males (Fig. 4C), overall effects of time of abstinence and dependence were found when scoring WDS (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 9.4, p < 0.001; treatment [VEH vs. NTX], F1,54 = 0.2, p > 0.05; dependence [EtOHND vs. EtOHPD], F1,54 = 26.0, p < 0.001; time × treatment × dependence interaction, F2,54 = 0.1, p > 0.05). No significant three-way interaction was detected, but a significant two-way time × dependence interaction was found (F2,54 = 15.7, p < 0.001). EtOHPD rats had higher WDS scores compared with EtOHND rats only at A-Abst in both VEH-treated animals (p < 0.01, Tukey post hoc test following two-way ANOVA: time, F2,18 = 7.7, p < 0.01; dependence, F1,9 = 20.2, p < 0.001; time × dependence interaction, F2,18 = 6.4, p < 0.05) and NTX-treated animals (p < 0.01, Tukey post hoc test following two-way ANOVA: time, F2,18 = 9.1, p < 0.01; dependence, F1,9 = 11.7, p < 0.01; time × dependence interaction, F2,18 = 6.2, p < 0.05). Further scrutiny of WDS scores between A-Abst and weeks 4–6 of CIE exposure suggested an increase in WDS scores at A-Abst, but this was not supported by the statistical analysis (one-way ANOVA: F4,39 = 1.6, p > 0.05; Fig. 3C, 4C).
In females (Fig. 5C), similar to males, overall effects of time of abstinence and dependence were found when scoring WDS (three-way ANOVA: time [A-Abst, L-Abst, P-Abst], F2,54 = 19.5, p < 0.001; treatment [VEH vs. NTX], F1,54 = 0.05, p > 0.05; dependence [EtOHND vs. EtOHPD], F1,54 = 28.5, p < 0.001; time × treatment × dependence interaction, F2,54 = 0.1, p > 0.05). No significant three-way interaction was detected, but a significant two-way time × dependence interaction was found (F2,54 = 6.9, p < 0.01). EtOHPD rats had higher WDS scores compared with EtOHND rats at A-Abst and L-Abst, whereas no difference in WDS scores was observed at P-Abst between ETOHND and EtOHPD rats in both VEH-treated animals (p < 0.05, Tukey post hoc test following two-way ANOVA: time, F2,18 = 11.5, p < 0.01; dependence, F1,9 = 18.2, p < 0.01; time × dependence interaction, F2,18 = 4.8, p < 0.05) and NTX-treated animals (p < 0.05, Tukey post hoc test following two-way ANOVA: time, F2,18 = 14.6, p < 0.001; dependence, F1,9 = 5.7, p < 0.05; time × dependence interaction, F2,18 = 3.9, p < 0.05). Further scrutiny of WDS scores between A-Abst and weeks 4–6 of CIE exposure suggested an increase in WDS scores at A-Abst, but this was not support by the statistical analysis (one-way ANOVA: F4,39 = 1.6, p > 0.05; Fig. 3C, 5C).
Discussion
One characteristic of AUD in humans is that dependent subjects will consume EtOH to relieve or avoid withdrawal symptoms (Peer et al., 2013). Similarly, in preclinical studies, EtOH post-dependent rats exhibit an EtOH-dependence syndrome that is characterized by both somatic and motivational withdrawal symptoms that usually begin after 6–8 h of abstinence and engage in excessive drinking when EtOH is made available again (Roberts et al., 1996, Vendruscolo and Roberts, 2014). The present study strongly supported the validity of the CIE vapor exposure model as a valid procedure to induce and study EtOH dependence in both male and female rats. Indeed, EtOHPD rats exhibited an enhancement (i.e., escalation) of EtOH drinking during dependence (week 4 to week 6), suggesting a transition from controlled to excessive EtOH intake, most likely in an effort to alleviate negative withdrawal states (for review, see (Koob, 2014). These behavioral changes are characteristic of dependence and reflect neuroadaptive changes that are induced by chronic intermittent EtOH that in turn disrupt brain function (e.g., reward), physiological processes, and cognitive processes (Koob, 2008, Becker and Mulholland, 2014) and could account for the changes in the efficacy of NTX in reducing EtOH drinking in the present study.
Although an overall significant effect of sex on EtOH drinking was observed during CIE vapor exposure (Fig. 3A), the possible increase in EtOH intake in females from week 4 to week 6 (suggested by further scrutiny of Fig. 3A) was not confirmed statistically. However, compared with females, males had higher BALs (Fig. 3B) and significantly lower WDS scores at weeks 4, 5, and 6 of CIE vapor exposure (Fig. 3C). The EtOH intake that was observed in males and females is difficult to reconcile with previous studies that reported a range of contradictory results. Several studies reported sex differences in EtOH intake, in which female rats drank more than males (Blanchard et al., 1993, Morales et al., 2015, Walker et al., 2008, Li and Lumeng, 1984), whereas other studies found no differences (van Haaren and Anderson, 1994, Moore and Lynch, 2015, Schramm-Sapyta et al., 2014). The use of different strains (e.g., Wistar, Sprague-Dawley, and Long Evans) or different experimental designs (e.g., two-bottle choice, self-administration, and CIE vapor exposure) may account for such discrepancies. For example, female Long Evans and Wistar rats consumed more EtOH than their male counterparts when given either continuous or intermittent access to EtOH in their home cages (Priddy et al., 2017). Under operant conditions, no sex or strain differences were found in drinking prior to the development of EtOH dependence (Priddy et al., 2017). Consistent with the present results, upon dependence induction by CIE exposure, Wistar rats of both sexes substantially escalated their EtOH intake compared with their nondependent drinking levels, whereas Long Evans rats only exhibited a moderate escalation of drinking, without showing any sex difference (Priddy et al., 2017). Thus, strain, sex, and drinking condition may interact to modulate EtOH drinking and are important factors to consider when exploring individual differences in EtOH drinking and dependence.
Blood alcohol levels following EtOH self-administration at weeks 4 to 6 of CIE vapor exposure were higher in males than in females (Fig. 3B). Biological (sex-related) factors, including differences in EtOH metabolism (for review, see (Cederbaum, 2012) and its effect on brain function and the levels of sex hormones, may contribute to some of these differences. For example, male and female mice differed in their sensitivity to changes in γ-aminobutyric acid-ergic neurosteroid levels with regard to EtOH exposure (Finn et al., 2010) and an interactive effect of sex and chronic EtOH consumption on blood glucose homeostasis was observed in Wistar rats (Sumida et al., 2004). Another explanation for such differences is that females may have failed to drink the EtOH that was dispensed compared with males. Dependent females exhibited the same EtOH intake at week 6 of CIE and at A-Abst after vehicle treatment, but they had different BALs after the self-administration sessions. This discrepancy is difficult to explain. One possibility could be that females metabolize EtOH differently across the estrous cycle. For example, mesocortical dopaminergic pathways are differentially sensitive to acute EtOH administration across the estrous cycle (Dazzi et al., 2007). Additionally, as mentioned above, an alternative explanation could be that females simply did not actually drink the EtOH that was delivered at A-Abst. Females not only had lower BALs than males; they also had higher WDS scores than males (Fig. 3C). These results are difficult to reconcile with previous studies that usually reported an increase in WDS in males but not in females (for review, see (Becker and Koob, 2016). However, several studies showed that females may be more sensitive to the degenerative effects of EtOH dependence (i.e., brain area volume reductions), and women seek treatment earlier in their drinking history than males (for review, see (Sharrett-Field et al., 2013), strongly suggesting a differential effect of chronic EtOH exposure in females vs. males. One explanation for the greater severity of WDS in females in the present study could be the experimental design (i.e., the duration of CIE vapor exposure to which females may be more sensitive). The observation that EtOHND females had WDS scores that were comparable to EtOHPD males was also unexpected but may have resulted from increases in stress or anxiety responses in females (for review, see (Bangasser and Valentino, 2014). For example, vocalization, one of the WDS measures that was scored, is a known stress response and could have accounted for an overall higher WDS score in EtOHND females (Meyer, 2015). Such a possibility, however, requires further investigation.
The neurobiological basis for sex differences in AUD is largely unknown, partially because most studies of EtOH drinking are conducted only in males. Gender differences in responses to AUD treatments are currently considered clinically, but the number of female subjects is usually limited and not always sufficient to establish the efficacy of treatment (Agabio et al., 2016). The present results showed that both male and female Wistar rats acquired EtOH self-administration and consumed the same amount of EtOH during the training phase. During the final 3 weeks of the CIE procedure (week 4 to week 6), both males and females exhibited significant escalation of EtOH intake compared with their respective air-exposed control groups. However, males and females responded differently to NTX. In EtOHND animals (both males and females), NTX significantly reduced EtOH intake at all abstinence time points (A-Abst, L-Abst, and P-Abst), and a significant change in the efficacy of NTX was observed in EtOHPD. Naltrexone was effective in EtOHPD males only at P-Abst but reduced EtOH drinking in EtOHPD females at all three abstinence time points.
Nondependent males and nondependent and post-dependent females presented a NTX-induced decrease in EtOH drinking, regardless of the duration of abstinence. However, post-dependent males were responsive to NTX after a longer period of abstinence. This variability in the efficacy of NTX is consistent with earlier studies that showed that NTX suppressed EtOH consumption in nondependent subjects (Gonzales and Weiss, 1998, Coonfield et al., 2002, Shoemaker et al., 2002, Stromberg, 2004), and this effect was blunted in post-dependent subjects (Ji et al., 2008, Sabino et al., 2006, Sabino et al., 2013, Walker and Koob, 2008). The efficacy of NTX in reducing EtOH intake may be affected by several factors, such as the model that is used (e.g., two-bottle choice vs. self-administration), the duration of EtOH self-administration, the severity of dependence, timing of the pharmacological intervention, sex, and changes in the endogenous opioid system. For example, acute NTX administration suppressed binge drinking in rats (Ji et al., 2008), and NTX was also highly effective in nondependent rats that were selectively bred for high alcohol preference (Sardinian alcohol-preferring [sP] rats; (Sabino et al., 2006). The lack of an effect of NTX at A-Abst in males was surprising when considering an earlier study by Walker and Koob (2008) that showed that a subcutaneous injection of NTX at lower doses than the one that was used in the present study reduced EtOH self-administration in nondependent and post-dependent animals during acute abstinence (Walker and Koob, 2008). One tentative explanation for this discrepant finding could be the lower bioavailability of NTX following oral administration (e.g., (Hussain et al., 1987). Chronic NTX administration blocked the increase in EtOH consumption after a 5-day period of forced abstinence (Heyser et al., 2003). Changes in the endogenous opioid system are observed in response to chronic exposure to EtOH and after its removal, and these changes may be responsible for the effect of NTX or lack thereof. For example, chronic EtOH administration has been reported to increase activity of the hypothalamic β-endorphin system (Schulz et al., 1980, Angelogianni and Gianoulakis, 1993), cause no change (Seizinger et al., 1983), or even decrease activity (Scanlon et al., 1992). One hypothesis could be that the greater β-endorphin release that is induced by acute exposure to EtOH induces drinking, whereas a decrease in β-endorphin activity that is induced by chronic EtOH intake may promote and maintain EtOH consumption because of negative reinforcement rather than positive reinforcement. However, the reason why NTX was ineffective in post-dependent males at A-Abst and L-Abst in the present study is unclear and will need further exploration.
One tentative explanation for the differential effect of NTX between males and females may be differential changes in the endogenous opioid system in response to chronic EtOH. Overall, acute EtOH has been shown to stimulate the release of β-endorphin, enkephalins, and dynorphin in humans and rats (Gianoulakis et al., 1996a, Marinelli et al., 2003, Marinelli et al., 2005, Marinelli et al., 2006, Dai et al., 2005). Chronic EtOH exposure induced changes in opioid peptide systems that involve alterations at the levels of the peptides themselves (Gianoulakis et al., 1996b, Lindholm et al., 2000), changes in receptor densities and effector systems (Turchan et al., 1999, Chen and Lawrence, 2000), and modifications of mRNA that encode both opioid peptides and receptors (Przewlocka et al., 1997, Rosin et al., 1999). Therefore, the differential effects of NTX in males and females may reflect sexual dimorphism of the endogenous opioid system and different effects of chronic EtOH use in males and females. Sexual dimorphism in the endogenous opioid system has been described in the control of pain. For example, variable responses to the analgesic effects of opioids have been attributable to sex (Dahan et al., 2008), but the mechanisms that underlie sex differences in opioid analgesia remain elusive. Sex differences in opioid analgesia are also not likely related to differences in opioid receptor density because no differences were found between male and female rats in brain μ- or δ-opioid receptor populations (Kepler et al., 1991). In humans, nonselective κ and μ ligands have been reported to produce stronger analgesic effects in women than in men (Rasakham and Liu-Chen, 2011). In animals, selective κ receptor agonists have been found to produce greater antinociceptive effects in males than in females (Rasakham and Liu-Chen, 2011). These observations suggest that the extent of sex differences in κ-, μ-, and δ-mediated analgesia is related to species, strain, ligand, and pain model. For example, the selective κ-opioid receptor agonist spiradoline was more potent in female than in male Wistar rats in the tail-withdrawal test (Terner et al., 2003). The exact nature of the changes in the endogenous opioid system during EtOH dependence and withdrawal that may explain the higher sensitivity to NTX in EtOHPD females remains to be established.
Clinical studies have shown that NTX treatment is more effective in patients who continue to drink EtOH during the course of treatment (Heinala et al., 2001, Killeen et al., 2004). One could argue that the efficacy of NTX treatment at P-Abst in EtOHPD males was the result of prior exposure to NTX with EtOH during the earlier tests (A-Abst and L-Abst). Therefore, in contrast to females, repeated NTX dosing in EtOHPD males may be necessary to elicit efficacy and thus be independent of the time of abstinence. Another explanation for the NTX-induced decrease in EtOH drinking could be related to an interaction between NTX and the palatability (taste) of EtOH. Supporting this hypothesis, previous studies found that NTX induced a conditioned taste aversion to EtOH in rats, monkeys, and humans and palatable food and sweet solutions (Ferraro et al., 2002, Coonfield et al., 2002, Williams and Woods, 1999b, Williams and Woods, 1999a, Mitchell et al., 2009, Stromberg et al., 2002, Beczkowska et al., 1992), consequently reducing EtOH intake. Sex differences in the palatability of EtOH could also contribute to the differential effect of NTX in reducing EtOH intake in EtOHPD rats. For example, women have been found to be more sensitive to the aversive effects of NTX (O’Malley et al., 2007, Garbutt et al., 2005, Agabio et al., 2016). Therefore, one can argue that the treatment efficacy of NTX in the present study might be correlated with the level of NTX-induced aversive side effects (Mitchell et al., 2009).
In conclusion, the present results further support targeting the endogenous opioid system to prevent excessive drinking that is characteristic of AUD, even after long periods of abstinence. The present study extends our knowledge of the efficacy of NTX treatment by showing that it varies between sexes and different time points of abstinence. This could explain some of the contradictory findings that described various effects or lack of effects of NTX in some cases (Kakidani et al., 1982, Bell and Reisine, 1993). In males, NTX was ineffective in reducing EtOH drinking during A-Abst, whereas NTX decreased EtOH drinking at A-Abst, L-Abst, and P-Abst in females. These findings suggest that NTX might modulate long-lasting craving-induced EtOH drinking in males and not acute EtOH withdrawal-induced (e.g., corticotropin-releasing factor-induced) EtOH drinking (Koob, 2015). This hypothesis will need further investigation. Overall, the present study has important implications because it points toward individual differences in the endogenous opioid system that should be considered when developing new pharmacotherapies to treat AUD.
Acknowledgements
This is publication number 29664 from The Scripps Research Institute. The authors thank Dr. Eric Zorrilla for assistance with the statistical analysis and Michael Arends for assistance with manuscript preparation.
Source of support: This work was supported by the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism (grant no. DA033344, AA024146, AA006420, AA022249, and AA026999 to RM-F).
Footnotes
The authors have no conflict of interest.
References
- AGABIO R, PANI PP, PRETI A, GESSA GL & FRANCONI F 2016. Efficacy of Medications Approved for the Treatment of Alcohol Dependence and Alcohol Withdrawal Syndrome in Female Patients: A Descriptive Review. Eur Addict Res, 22, 1–16. [DOI] [PubMed] [Google Scholar]
- ANGELOGIANNI P & GIANOULAKIS C 1993. Chronic ethanol increases proopiomelanocortin gene expression in the rat hypothalamus. Neuroendocrinology, 57, 106–14. [DOI] [PubMed] [Google Scholar]
- BANGASSER DA & VALENTINO RJ 2014. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol, 35, 303–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER HC & MULHOLLAND PJ 2014. Neurochemical mechanisms of alcohol withdrawal. Handb Clin Neurol, 125, 133–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECKER JB & KOOB GF 2016. Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev, 68, 242–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECZKOWSKA IW, BOWEN WD & BODNAR RJ 1992. Central opioid receptor subtype antagonists differentially alter sucrose and deprivation-induced water intake in rats. Brain Res, 589, 291–301. [DOI] [PubMed] [Google Scholar]
- BELL GI & REISINE T 1993. Molecular biology of somatostatin receptors. Trends Neurosci, 16, 34–8. [DOI] [PubMed] [Google Scholar]
- BLANCHARD BA, STEINDORF S, WANG S, LEFEVRE R, MANKES RF & GLICK SD 1993. Prenatal ethanol exposure alters ethanol-induced dopamine release in nucleus accumbens and striatum in male and female rats. Alcohol Clin Exp Res, 17, 974–81. [DOI] [PubMed] [Google Scholar]
- CEDERBAUM AI 2012. Alcohol metabolism. Clin Liver Dis, 16, 667–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN F & LAWRENCE AJ 2000. Effect of chronic ethanol and withdrawal on the mu-opioid receptor- and 5-Hydroxytryptamine(1A) receptor-stimulated binding of [(35)S]Guanosine-5’-O-(3-thio)triphosphate in the fawn-hooded rat brain: A quantitative autoradiography study. J Pharmacol Exp Ther, 293, 159–65. [PubMed] [Google Scholar]
- COONFIELD DL, HILL KG, KACZMAREK HJ, FERRARO FM 3RD & KIEFER SW 2002. Low doses of naltrexone reduce palatability and consumption of ethanol in outbred rats. Alcohol, 26, 43–7. [DOI] [PubMed] [Google Scholar]
- COWEN MS & LAWRENCE AJ 2001. Alterations in central preproenkephalin mRNA expression after chronic free-choice ethanol consumption by fawn-hooded rats. Alcohol Clin Exp Res, 25, 1126–33. [PubMed] [Google Scholar]
- DAHAN A, KEST B, WAXMAN AR & SARTON E 2008. Sex-specific responses to opiates: animal and human studies. Anesth Analg, 107, 83–95. [DOI] [PubMed] [Google Scholar]
- DAI X, THAVUNDAYIL J & GIANOULAKIS C 2005. Differences in the peripheral levels of beta-endorphin in response to alcohol and stress as a function of alcohol dependence and family history of alcoholism. Alcohol Clin Exp Res, 29, 1965–75. [DOI] [PubMed] [Google Scholar]
- DAZZI L, SEU E, CHERCHI G, BARBIERI PP, MATZEU A & BIGGIO G 2007. Estrous cycle-dependent changes in basal and ethanol-induced activity of cortical dopaminergic neurons in the rat. Neuropsychopharmacology, 32, 892–901. [DOI] [PubMed] [Google Scholar]
- FERRARO FM 3RD, HILL KG, KACZMAREK HJ, COONFIELD DL & KIEFER SW 2002. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol, 27, 107–14. [DOI] [PubMed] [Google Scholar]
- FINN DA, BECKLEY EH, KAUFMAN KR & FORD MM 2010. Manipulation of GABAergic steroids: Sex differences in the effects on alcohol drinking- and withdrawal-related behaviors. Horm Behav, 57, 12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARBUTT JC, KRANZLER HR, O’MALLEY SS, GASTFRIEND DR, PETTINATI HM, SILVERMAN BL, LOEWY JW, EHRICH EW & VIVITREX STUDY G 2005. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA, 293, 1617–25. [DOI] [PubMed] [Google Scholar]
- GIANOULAKIS C, DE WAELE JP & THAVUNDAYIL J 1996a. Implication of the endogenous opioid system in excessive ethanol consumption. Alcohol, 13, 19–23. [DOI] [PubMed] [Google Scholar]
- GIANOULAKIS C, KRISHNAN B & THAVUNDAYIL J 1996b. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry, 53, 250–7. [DOI] [PubMed] [Google Scholar]
- GONZALES RA & WEISS F 1998. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci, 18, 10663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINALA P, ALHO H, KIIANMAA K, LONNQVIST J, KUOPPASALMI K & SINCLAIR JD 2001. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol, 21, 287–92. [DOI] [PubMed] [Google Scholar]
- HEYSER CJ, MOC K & KOOB GF 2003. Effects of naltrexone alone and in combination with acamprosate on the alcohol deprivation effect in rats. Neuropsychopharmacology, 28, 1463–71. [DOI] [PubMed] [Google Scholar]
- HUSSAIN MAA, B J; KEARNEY A; & SHEFTER E 1987. Buccal and oral bioavailability of naloxone and naltrexone in rats. international Journal of Pharmaceutics, 36, 127–130. [Google Scholar]
- JI D, GILPIN NW, RICHARDSON HN, RIVIER CL & KOOB GF 2008. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol, 19, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKIDANI H, FURUTANI Y, TAKAHASHI H, NODA M, MORIMOTO Y, HIROSE T, ASAI M, INAYAMA S, NAKANISHI S & NUMA S 1982. Cloning and sequence analysis of cDNA for porcine beta-neo-endorphin/dynorphin precursor. Nature, 298, 245–9. [DOI] [PubMed] [Google Scholar]
- KEPLER KL, STANDIFER KM, PAUL D, KEST B, PASTERNAK GW & BODNAR RJ 1991. Gender effects and central opioid analgesia. Pain, 45, 87–94. [DOI] [PubMed] [Google Scholar]
- KEYES KM, GRANT BF & HASIN DS 2008. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug Alcohol Depend, 93, 21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLEEN TK, BRADY KT, GOLD PB, SIMPSON KN, FALDOWSKI RA, TYSON C & ANTON RF 2004. Effectiveness of naltrexone in a community treatment program. Alcohol Clin Exp Res, 28, 1710–7. [DOI] [PubMed] [Google Scholar]
- KOOB GF 2008. A role for brain stress systems in addiction. Neuron, 59, 11–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB GF 2014. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol, 125, 33–54. [DOI] [PubMed] [Google Scholar]
- KOOB GF 2015. The dark side of emotion: the addiction perspective. Eur J Pharmacol, 753, 73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRYSTAL JH, CRAMER JA, KROL WF, KIRK GF, ROSENHECK RA & VETERANS AFFAIRS NALTREXONE COOPERATIVE STUDY, G. 2001. Naltrexone in the treatment of alcohol dependence. N Engl J Med, 345, 1734–9. [DOI] [PubMed] [Google Scholar]
- LI TK & LUMENG L 1984. Alcohol preference and voluntary alcohol intakes of inbred rat strains and the National Institutes of Health heterogeneous stock of rats. Alcohol Clin Exp Res, 8, 485–6. [DOI] [PubMed] [Google Scholar]
- LINDHOLM S, PLOJ K, FRANCK J & NYLANDER I 2000. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol, 22, 165–71. [DOI] [PubMed] [Google Scholar]
- MACEY DJ, SCHULTEIS G, HEINRICHS SC & KOOB GF 1996. Time-dependent quantifiable withdrawal from ethanol in the rat: effect of method of dependence induction. Alcohol, 13, 163–70. [DOI] [PubMed] [Google Scholar]
- MARINELLI PW, BAI L, QUIRION R & GIANOULAKIS C 2005. A microdialysis profile of Met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res, 29, 1821–8. [DOI] [PubMed] [Google Scholar]
- MARINELLI PW, LAM M, BAI L, QUIRION R & GIANOULAKIS C 2006. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res, 30, 982–90. [DOI] [PubMed] [Google Scholar]
- MARINELLI PW, QUIRION R & GIANOULAKIS C 2003. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacology (Berl), 169, 60–7. [DOI] [PubMed] [Google Scholar]
- MARINELLI PW, QUIRION R & GIANOULAKIS C 2004. An in vivo profile of beta-endorphin release in the arcuate nucleus and nucleus accumbens following exposure to stress or alcohol. Neuroscience, 127, 777–84. [DOI] [PubMed] [Google Scholar]
- MEYER RE 2015. Physiologic Measures of Animal Stress during Transitional States of Consciousness. Animals (Basel), 5, 702–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL JM, BERGREN LJ, CHEN KS, ROWBOTHAM MC & FIELDS HL 2009. Naltrexone aversion and treatment efficacy are greatest in humans and rats that actively consume high levels of alcohol. Neurobiol Dis, 33, 72–80. [DOI] [PubMed] [Google Scholar]
- MOORE CF & LYNCH WJ 2015. Alcohol preferring (P) rats as a model for examining sex differences in alcohol use disorder and its treatment. Pharmacol Biochem Behav, 132, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORALES M, MCGINNIS MM & MCCOOL BA 2015. Chronic ethanol exposure increases voluntary home cage intake in adult male, but not female, Long-Evans rats. Pharmacol Biochem Behav, 139, 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’MALLEY SS, SINHA R, GRILO CM, CAPONE C, FARREN CK, MCKEE SA, ROUNSAVILLE BJ & WU R 2007. Naltrexone and cognitive behavioral coping skills therapy for the treatment of alcohol drinking and eating disorder features in alcohol-dependent women: a randomized controlled trial. Alcohol Clin Exp Res, 31, 625–34. [DOI] [PubMed] [Google Scholar]
- PEER K, RENNERT L, LYNCH KG, FARRER L, GELERNTER J & KRANZLER HR 2013. Prevalence of DSM-IV and DSM-5 alcohol, cocaine, opioid, and cannabis use disorders in a largely substance dependent sample. Drug Alcohol Depend, 127, 215–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POPP RL & ERICKSON CK 1998. The effect of an acute ethanol exposure on the rat brain POMC opiopeptide system. Alcohol, 16, 139–48. [DOI] [PubMed] [Google Scholar]
- PRIDDY BM, CARMACK SA, THOMAS LC, VENDRUSCOLO JC, KOOB GF & VENDRUSCOLO LF 2017. Sex, strain, and estrous cycle influences on alcohol drinking in rats. Pharmacol Biochem Behav, 152, 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRZEWLOCKA B, TURCHAN J, LASON W & PRZEWLOCKI R 1997. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett, 238, 13–6. [DOI] [PubMed] [Google Scholar]
- RASAKHAM K & LIU-CHEN LY 2011. Sex differences in kappa opioid pharmacology. Life Sci, 88, 2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RASMUSSEN DD, BRYANT CA, BOLDT BM, COLASURDO EA, LEVIN N & WILKINSON CW 1998. Acute alcohol effects on opiomelanocortinergic regulation. Alcohol Clin Exp Res, 22, 789–801. [PubMed] [Google Scholar]
- ROBERTS AJ, COLE M & KOOB GF 1996. Intra-amygdala muscimol decreases operant ethanol self-administration in dependent rats. Alcohol Clin Exp Res, 20, 1289–98. [DOI] [PubMed] [Google Scholar]
- RORICK-KEHN LM, WITKIN JM, STATNICK MA, EBERLE EL, MCKINZIE JH, KAHL SD, FORSTER BM, WONG CJ, LI X, CRILE RS, SHAW DB, SAHR AE, ADAMS BL, QUIMBY SJ, DIAZ N, JIMENEZ A, PEDREGAL C, MITCH CH, KNOPP KL, ANDERSON WH, CRAMER JW & MCKINZIE DL. 2014. LY2456302 is a novel, potent, orally-bioavailable small molecule kappa-selective antagonist with activity in animal models predictive of efficacy in mood and addictive disorders. Neuropharmacology, 77, 131–44. [DOI] [PubMed] [Google Scholar]
- ROSIN A, LINDHOLM S, FRANCK J & GEORGIEVA J 1999. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett, 275, 1–4. [DOI] [PubMed] [Google Scholar]
- SABINO V, COTTONE P, KOOB GF, STEARDO L, LEE MJ, RICE KC & ZORRILLA EP 2006. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl), 189, 175–86. [DOI] [PubMed] [Google Scholar]
- SABINO V, KWAK J, RICE KC & COTTONE P 2013. Pharmacological characterization of the 20% alcohol intermittent access model in Sardinian alcohol-preferring rats: a model of binge-like drinking. Alcohol Clin Exp Res, 37, 635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCANLON MN, LAZAR-WESLEY E, GRANT KA & KUNOS G 1992. Proopiomelanocortin messenger RNA is decreased in the mediobasal hypothalamus of rats made dependent on ethanol. Alcohol Clin Exp Res, 16, 1147–51. [DOI] [PubMed] [Google Scholar]
- SCHRAMM-SAPYTA NL, FRANCIS R, MACDONALD A, KEISTLER C, O’NEILL L & KUHN CM 2014. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology (Berl), 231, 1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZ R, WUSTER M, DUKA T & HERZ A 1980. Acute and chronic ethanol treatment changes endorphin levels in brain and pituitary. Psychopharmacology (Berl), 68, 221–7. [DOI] [PubMed] [Google Scholar]
- SEIZINGER BR, BOVERMANN K, MAYSINGER D, HOLLT V & HERZ A 1983. Differential effects of acute and chronic ethanol treatment on particular opioid peptide systems in discrete regions of rat brain and pituitary. Pharmacol Biochem Behav, 18 Suppl 1, 361–9. [DOI] [PubMed] [Google Scholar]
- SHARRETT-FIELD L, BUTLER TR, REYNOLDS AR, BERRY JN & PRENDERGAST MA 2013. Sex differences in neuroadaptation to alcohol and withdrawal neurotoxicity. Pflugers Arch, 465, 643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHOEMAKER WJ, VAVROUSEK-JAKUBA E, ARONS CD & KWOK FC 2002. The acquisition and maintenance of voluntary ethanol drinking in the rat: effects of dopaminergic lesions and naloxone. Behav Brain Res, 137, 139–48. [DOI] [PubMed] [Google Scholar]
- SIMMS JA, STEENSLAND P, MEDINA B, ABERNATHY KE, CHANDLER LJ, WISE R & BARTLETT SE 2008. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32, 1816–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE T, CHAPMAN C, SWIFT W, KEYES K, TONKS Z & TEESSON M 2016. Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: systematic review and metaregression. BMJ Open, 6, e011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROMBERG MF 2004. The effect of baclofen alone and in combination with naltrexone on ethanol consumption in the rat. Pharmacol Biochem Behav, 78, 743–50. [DOI] [PubMed] [Google Scholar]
- STROMBERG MF, RUKSTALIS MR, MACKLER SA, VOLPICELLI JR & O’BRIEN CP 2002. A comparison of the effects of 6-beta naltrexol and naltrexone on the consumption of ethanol or sucrose using a limited-access procedure in rats. Pharmacol Biochem Behav, 72, 483–90. [DOI] [PubMed] [Google Scholar]
- SUMIDA KD, QURESHI T, CATANZARO MJ, ARIMOTO SM & HILL JM 2004. Chronic alcohol consumption yields sex differences in whole-body glucose production in rats. Alcohol Alcohol, 39, 418–26. [DOI] [PubMed] [Google Scholar]
- TERNER JM, BARRETT AC, COOK CD & PICKER MJ 2003. Sex differences in (−)-pentazocine antinociception: comparison to morphine and spiradoline in four rat strains using a thermal nociceptive assay. Behav Pharmacol, 14, 77–85. [DOI] [PubMed] [Google Scholar]
- TURCHAN J, PRZEWLOCKA B, TOTH G, LASON W, BORSODI A & PRZEWLOCKI R 1999. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience, 91, 971–7. [DOI] [PubMed] [Google Scholar]
- VAN HAAREN F & ANDERSON K 1994. Sex differences in schedule-induced alcohol consumption. Alcohol, 11, 35–40. [DOI] [PubMed] [Google Scholar]
- VENDRUSCOLO LF & ROBERTS AJ 2014. Operant alcohol self-administration in dependent rats: focus on the vapor model. Alcohol, 48, 277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLPICELLI JR, ALTERMAN AI, HAYASHIDA M & O’BRIEN CP 1992. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry, 49, 876–80. [DOI] [PubMed] [Google Scholar]
- WALKER BM & KOOB GF 2008. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology, 33, 643–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER BM, WALKER JL & EHLERS CL 2008. Dissociable effects of ethanol consumption during the light and dark phase in adolescent and adult Wistar rats. Alcohol, 42, 83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS KL & WOODS JH 1999a. Conditioned effects produced by naltrexone doses that reduce ethanol-reinforced responding in rhesus monkeys. Alcohol Clin Exp Res, 23, 708–15. [PubMed] [Google Scholar]
- WILLIAMS KL & WOODS JH 1999b. Naltrexone reduces ethanol- and/or water-reinforced responding in rhesus monkeys: effect depends upon ethanol concentration. Alcohol Clin Exp Res, 23, 1462–7. [PubMed] [Google Scholar]
- WISE RA 1973. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia, 29, 203–10. [DOI] [PubMed] [Google Scholar]


