Abstract
Background:
The aim of this study was to determine whether long-term patterns of change in adiposity throughout young adulthood are associated with systolic and diastolic function in midlife.
Methods:
Participants in the Coronary Artery Risk Development in Young Adults study, a multicenter, population-based cohort, underwent repeated anthropometric assessment (body mass index [BMI], waist circumference, and waist-to-hip ratio) from examination years 0 to 25. At year 25, longitudinal, circumferential, and radial strain and tissue Doppler velocities were assessed by echocardiography. Group-based trajectory modeling was used to identify 25-year trajectories of change in anthropometric measures and to examine associations between trajectories of adiposity change and indices of cardiac mechanics.
Results:
Among 3,310 participants, four distinct trajectories of BMI change were identified: stable BMI (36% of the cohort; mean εBMI, 1.6 kg/m2), mild increase (40%; mean ABMI, 6.0 kg/m2), moderate increase (18%; mean ΔBMI, 10.8 kg/m2), and major increase (6%; mean ΔBMI, 15.5 kg/m2). Trajectories of greater BMI increase were associated with lower adjusted e′ velocity and higher E/e′ ratio compared with the stable BMI group, independent of year 0 or year 25 BMI. Participants in increasing BMI trajectory groups compared with the stable BMI group had lower absolute longitudinal strain and greater odds of diastolic dysfunction, independent of year 0 BMI but not year 25 BMI. Similar patterns were observed for change in waist circumference and waist-to-hip ratio trajectory groups.
Conclusions:
Steeper trajectories of BMI increase from young adulthood to middle age, a vulnerable period for weight gain, are independently associated with lower E/e′ velocity and higher E/e′ ratio, but not systolic dysfunction, in midlife. (J Am Soc Echocardiogr 2018;■:■-■.)
Keywords: Echocardiography, Heart failure with preserved ejection fraction, Epidemiology
Obesity has reached epidemic proportions in the United States and worldwide and is a well-established risk factor for heart failure.1,2 Obesity has been demonstrated to have many adverse effects on the cardiovascular system and has been linked to cardiac structural remodeling.3 Several studies have investigated the association of body mass index (BMI) with indices of cardiac structure and function, such as left ventricular (LV) mass, LV filling pressures, and systolic and diastolic function. In these investigations, higher BMI has been consistently associated with LV hypertrophy and dilatation, which are important intermediate phenotypes and precursors of clinical heart failure.4-6 However, available studies have been limited by cross-sectional associations of weight or BMI in middle age or older age and did not consider the potential effect of changes in weight during early adult-hood.7,8
Weight gain represents a major modifiable risk factor that is linked with adverse cardiovascular outcomes and decreased odds of healthy aging.9,10 Among patients with coronary artery disease, weight fluctuation was associated with a higher rate of cardiovascular events, supporting the independent contribution of weight change on adverse cardiovascular outcomes.11 Whereas obesity has been associated with adverse cardiac mechanics, data are limited regarding the association of long-term patterns of weight change and cardiac structure and function in midlife independent of obesity status. Therefore, we sought to (1) describe the patterns of percentage change in BMI occurring throughout early adulthood to middle age and (2) examine the association between patterns of percentage change in BMI and cardiac mechanics. We hypothesized that multiple different trajectories of change in BMI exist within the Coronary Artery Risk Development in Young Adults (CARDIA) study and that those groups with steeper increases in BMI from young adulthood to middle age would have evidence of greater degree of LV systolic and diastolic dysfunction in middle age.
METHODS
Study Population
The CARDIA study is a multicenter, community-based longitudinal cohort study designed to investigate the risk factors for the development of cardiovascular disease. In 1985 and 1986, 5,115 black and white men and women aged 18 to 30 years were recruited from four urban sites across the United States (Birmingham, Alabama; Chicago, Illinois; Minneapolis, Minnesota; and Oakland, California). Detailed descriptions of the study design and conduct have been previously published (see Supplemental Methods).12
Anthropometric Measures
Standardized protocols for data collection were used across study centers, and measurements have previously been described (see Supplemental Methods).12 Weight and height were measured with participants wearing light clothes and no shoes at each of the eight CARDIA examination cycles. BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference (WC) and hip circumference were measured in duplicate by trained personnel.
Echocardiography
Echocardiography was performed at the year 25 examination using an Artida cardiac ultrasound machine (Toshiba Medical Systems, Tokyo, Japan) and a previously described protocol.13 Briefly, sonographers at each field center underwent initial centralized training followed by quality assurance and control procedures to assess intra- and intersonographer reproducibility. Measures of LV subclinical function (myocardial deformation) were acquired from two-dimensional images obtained from optimized apical four-chamber and parasternal short-axis views, specifically longitudinal strain (LS), radial strain (RS), and circumferential strain (CS). Studies were electronically transmitted to a core reading laboratory at Johns Hopkins University. Ejection fraction was calculated as the ratio of stroke volume to end-diastolic volume. Diastolic function was quantitated using early diastolic (E) and lateral tissue (e′) velocities measured from pulsed Doppler echocardiographic recordings of transmitral flow at the mitral valve. The lateral E/e′ ratio was calculated as an index of LV filling pressure. Speckle-tracking echocardiography was used to calculate strain measurements using Wall Motion 2D Tracking software (Toshiba Medical Systems). Three cardiac cycles from each view were recorded for offline analysis. LS was assessed from the four-chamber views. RS and CS were assessed from the short-axis view at the midventricular level. LS and CS were reported as absolute values, whereby worse strain is a lower value and better strain is a higher value. Quality control procedures performed included participant reexamination and blind re-reading to allow assessment of intra-and intersonographer variability and intra- and interreader variability with high rates of reproducibility.13
Statistical Analysis
Trajectories in percentage change in BMI between years 0 and 25 relative to baseline (year 0), modeled among all eligible participants in CARDIA with BMI measured at four or more examinations, were included after exclusion for missing baseline BMI (n = 24) and bariatric surgery (n = 73), resulting in a final sample of 4,350 for patterns of change identification (Supplemental Figure 1). Of note, BMI from specific visits at time of pregnancy were excluded. To evaluate the association between patterns of BMI change and cardiac mechanics, the analyses were then conducted in the 3,310 participants who completed the year 25 follow-up examination with echocardiography after exclusion for those who were missing key covariates of interest (n = 23).
We used latent class models to identify subgroups that shared similar trajectories in change in BMI, WC, and waist-to-hip ratio (WHR). These models were fit using SAS PROC TRAJ using available repeated measures of BMI, WC, and WHR.14 Given that latent group modeling assigns individuals to groups in a probabilistic fashion, we determined the optimal number of underlying trajectories by a composite criteria consisting of (1) confirming visually distinct trajectories, (2) ensuring that no group had <5% of the population, and (3) observing improvement in the Bayesian information criterion. The Bayesian information criterion provides a measure of model fit when comparing competing models and imposes a “penalty” for including more parameters in a model. The mean posterior probabilities of group membership were high (0.90, 0.90, and 0.87 for BMI, WC, and WHR trajectory groups, respectively). Participant characteristics were described using means, medians, and proportions (as appropriate) according to trajectory groups.
We estimated the relationship between trajectory group membership and indices of cardiac mechanics reflecting systolic and diastolic function using multivariate linear regression models. Models were adjusted for demographics (baseline age, sex, race, education, center, echocardiographic quality score, and analyst), cumulative burden of cardiovascular risk factors (cumulative systolic and diastolic blood pressure, number of visits with blood pressure medications, cumulative cholesterol, cumulative years of diabetes, and pack-years of cigarette smoking exposure), and BMI at baseline (year 0) and at time of echocardiography (year 25). For secondary analyses, systolic dysfunction was defined as ejection fraction < 50%, and diastolic dysfunction was defined using two approaches on the basis of the American Society of Echocardiography guidelines: (1) more than half of available diastolic indicators abnormal among e′ (septal e′ <7 cm/sec or lateral e′ <10 cm/sec), E/e′ ratio (average E/e′ > 14 or lateral E/e’ > 13 or septal E/e′ > 15), LA size (anteroposterior diameter > 4.0 cm in men or > 3.8 cm in women, diameter/height > 2.61 cm/m,15 or volume/body surface area >34 mL/m2), and TR velocity (>2.8 m/sec) and (2) average E/e′ > 14 or lateral E/e′ > 13 or septal E/e′ > 15 (modified American Society of Echocardiography criteria).16
We completed sensitivity analyses after exclusion of participants with cardiovascular events from the trajectory analysis, including nonfatal myocardial infarction or congestive heart failure (n = 97). For each event, medical records were obtained and adjudicated as previously described.17 In addition, we performed a sensitivity analysis using inverse probability of treatment weighting using the propensity score, as described in our previous work.13 The propensity score for inclusion in the analysis was based on a logistic regression model containing age, race, sex, systolic blood pressure, education, cigarette smoking, cholesterol, diabetes, fasting glucose, and weighted life-events score as baseline predictors. The inverse propensity probability of inclusion in the analysis was used to perform a weighted regression analysis of the outcomes, without significant change in results. All analyses were completed using SAS version 9.4 (SAS Institute, Cary, NC). Two-sided P values < .05 was considered to indicate statistical significance.
RESULTS
Patterns of Change in BMI from Young Adulthood to Midlife
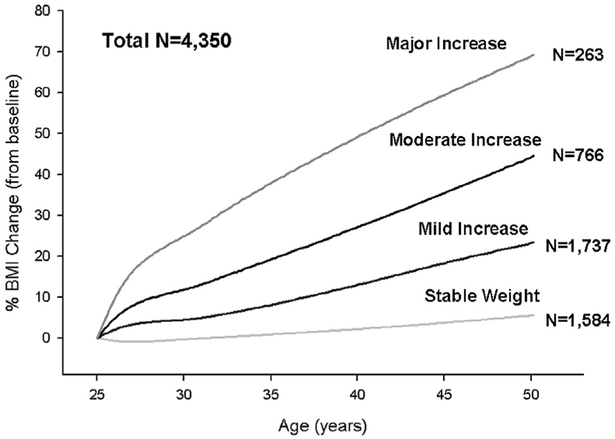
Four discrete trajectory groups or different patterns of weight change were identified using latent class trajectory modeling from young adulthood to midlife describing percentage change in BMI relative to the year 0 examination in the eligible 4,350 participants (Figure 1). Overall, BMI increased in all trajectory groups to some extent: 36% of the cohort (n = 1,584) maintained fairly stable BMI throughout follow-up (25-year mean ΔBMI, 1.6 α 2.5 kg/m2), 40% (n = 1,737) had mild increases in BMI (mean ΔBMI, 6.0 ± 2.6 kg/m2), 18% (1 = 766) had moderate increases in BMI (mean ΔBMI, 10.8 ± 3.9 kg/m2), and 6% (n = 263) had major increases in BMI (mean ΔBMI, 15.5 ± 5.0 kg/m2). Percentages of missing BMI values for the four trajectory groups were overall similar and did not affect trajectory group assignment (Supplemental Table 1).
Figure 1.
Changes in BMI trajectories by age in the CARDIA study. Four discrete trajectory groups were identified from young adulthood to middle age describing percentage change in BMI relative to the year 0 examination in the 4,350 participants. Overall, BMI increased in all trajectory groups to some extent; 36% of the cohort (n = 1,584) maintained fairly stable BMI throughout follow-up (mean ΔBMI, 1.6 ± 2.5 kg/m2), 40% (n = 1,737) had mild increases in weight (mean ΔBMI, 6.0 ± 2.6 kg/m2), 18% (n = 766) had moderate increases in weight (mean ΔBMI, 10.8 ± 3.9 kg/m2), and 6% (n = 263) had major increases in weight (mean Δ 15.5 ±BMI, 5.0 kg/m2).
Participant characteristics according to BMI trajectory group at baseline are presented in Table 1. Individuals in the stable BMI group were more likely to be white men and more highly educated, compared with trajectory groups with greater BMI changes. African Americans were more likely to belong to trajectory groups with BMI increases, with the major increase group having the largest proportion of African American women. Hypertension prevalence at year 25 was higher in increasing BMI change groups and was present in 26% (n = 313), 34% (n = 444), 42% (n = 244), and 46% (n = 84) in the stable BMI, mild increase, moderate increase, and major increase groups, respectively (Supplemental Table 2). In addition, groups with greater increases in BMI had higher glucose and triglycerides and lower high-density lipoprotein cholesterol levels.
Table 1.
Baseline clinical characteristics at year 0 by change in BMI trajectory groups
| Variable | Stable BMI (n = 1,225) | Mild increase (n = 1,324) | Moderate increase (n = 576) | Major increase (n = 185) | P* |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age (y) | 26.3 ± 3.2 | 25.3 ± 3.4 | 23.4 ± 3.6 | 21.4 ± 3.1 | <.0001 |
| Race | |||||
| Black | 419 (34.2) | 613(46.3) | 360 (62.5) | 131 (70.8) | <.0001 |
| Women | 646 (52.7) | 698 (52.7) | 364 (63.2) | 136 (73.5) | <.0001 |
| Education | <.0001 | ||||
| High school or less | 337 (27.5) | 455 (34.4) | 244 (42.4) | 92 (49.7) | |
| Some college | 347 (28.3) | 448 (33.8) | 237(41.2) | 79 (42.7) | |
| College graduate | 345 (28.2) | 277 (20.9) | 69 (12.0) | 10(5.4) | |
| Graduate school | 196 (16.0) | 144 (10.9) | 26 (4.5) | 4 (2.2) | |
| Cardiovascular risk factors at year 0 | |||||
| Current smoker | 314 (25.8) | 349 (26.5) | 151 (26.4) | 42 (22.7) | .731 |
| BMI (kg/m2) | 24.7 ± 5.1 | 24.1 ± 4.4 | 24.0 ± 4.3 | 23.2 ± 3.5 | <.0001 |
| WC (cm) | 78.8 ± 11.9 | 77.3 ± 10.2 | 75.5 ± 9.9 | 73.6 ± 8.3 | <.0001 |
| WHR | 0.78 ± 0.07 | 0.78 ± 0.07 | 0.76 ± 0.06 | 0.75 ± 0.07 | <.0001 |
| Systolic BP (mm Hg) | 110 ± 11 | 110 ± 11 | 110 ± 10 | 107 ± 11 | .004 |
| Diastolic BP (mm Hg) | 69 ± 10 | 69 ± 9 | 68 ± 9 | 66 ± 9 | <.0001 |
| Hypertension | 32 (2.6) | 26 (2.0) | 10 (1.7) | 4 (2.2) | .594 |
| Total cholesterol (mg/dL) | 180 ± 34 | 178 ± 32 | 173 ± 30 | 169 ± 33 | <.0001 |
| Fasting glucose (mg/dL) | 83 ± 11 | 82 ± 9 | 80 ± 8 | 80 ± 10 | <.0001 |
| HDL cholesterol (mg/dL) | 53 ± 13 | 53 ± 12 | 54 ± 13 | 54 ± 12 | .031 |
| Triglyceride (mg/dL) | 77 ± 56 | 71 ± 39 | 64 ± 35 | 60 ± 28 | <.0001 |
BP, Blood pressure; HDL, high-density lipoprotein.
Data are expressed as mean ± SD or as number (percentage).
Chi-square test for categorical variables and analysis of variance for continuous variables.
Overall patterns of change over young adulthood to middle age were similar when we examined the trajectories of percentage changes in WC or WHR separately as another measure of adiposity (Supplemental Figure 2). Participant characteristics at year 0 according to WC and WHR trajectory group are presented in Supplemental Tables 3 and 4. Changes in WC and WHR trajectory groups were highly to moderately correlated with percentage change in BMI trajectory groups (Spearman correlation coefficients of 0.82 for WC and 0.39 for WHR, P < .0001 for both).
Association of Adiposity Trajectories with Measures of Cardiac Structure and Function
Unadjusted analysis of echocardiographic parameters revealed grossly preserved systolic function as measured by LVejection fraction in all four BMI trajectory groups at year 25 (Table 2). Small but statistically significant differences were observed in structural parameters indicating the presence of cardiac remodeling, including LV end-diastolic volume, LV mass index, and LV mass/volume ratio, with less favorable values in groups with greater BMI increase. Finally, several parameters, which represent surrogate measures of diastolic function, had worse values in groups with steeper BMI increases, including left atrial dimension and E/A ratio. Similar patterns were seen in conventional echocardiographic parameters by WC and WHR trajectory groups (Supplemental Tables 5–7).
Table 2.
Echocardiographic characteristics at year 25 by BMI Trajectory groups
| Variable | Stable BMI (n = 1,225) |
Mild increase (n = 1,324) |
Moderate increase (n = 576) |
Major increase (n = 185) |
P* |
|---|---|---|---|---|---|
| LV ejection fraction (%) | 61.4 ± 0.2 | 61.4 ± 0.2 | 61.1 ± 0.3 | 61.2 ± 0.6 | .856 |
| LV end-systolic volume (mL) | 43.2 ± 0.5 | 43.9 ± 0.5 | 45.5 ± 1.0 | 45.3 ± 1.4 | .066 |
| LV end-diastolic volume (mL) | 110.2 ± 0.9 | 112.2 ± 0.8 | 114.6 ± 1.4 | 114.9 ± 2.4 | .023 |
| LV mass index (g/m2.7) | 37.18 ± 0.31 | 40.29 ± 0.34 | 42.79 ± 0.54 | 43.66 ± 0.91 | <.0001 |
| LV mass/volume ratio | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | <.0001 |
| Left atrial dimension (cm) | 3.61 ± 0.01 | 3.72 ± 0.01 | 3.83 ± 0.02 | 3.84 ± 0.04 | <.0001 |
| Stroke volume (mL) | 86.1 ± 0.7 | 88.9 ± 0.6 | 90.6 ± 1.0 | 89.2 ± 1.8 | .0004 |
| E velocity (cm/sec) | 77.4 ± 0.4 | 78.6 ± 0.4 | 80.7 ± 0.7 | 83.3 ± 1.2 | <.0001 |
| A velocity (cm/sec) | 60.7 ± 0.5 | 63.9 ± 0.5 | 67.2 ± 0.7 | 69.2 ± 1.1 | <.0001 |
| E/A ratio | 1.34 ± 0.01 | 1.29 ± 0.01 | 1.26 ± 0.02 | 1.25 ± 0.02 | <.0001 |
| e′ velocity | 12.1 ± 0.1 | 11.6 ± 0.1 | 11.4 ± 0.1 | 11.3 ± 0.2 | <.0001 |
| E/e′ ratio | 6.8 ± 0.1 | 7.2 ± 0.1 | 7.4 ± 0.1 | 7.8 ± 0.2 | <.0001 |
| Absolute LS (%) | 15.4 ± 0.1 | 15.0 ± 0.1 | 14.6 ± 0.1 | 14.8 ± 0.2 | <.0001 |
| RS (%) | 37.2 ± 0.4 | 37.3 ± 0.4 | 36.8 ± 0.6 | 37.2 ± 1.0 | .867 |
| Absolute CS (%) | 15.5 ± 0.1 | 15.2 ± 0.1 | 15.1 ± 0.1 | 15.3 ± 0.3 | .006 |
Data are expressed as mean ± SE.
Analysis of variance.
Indices of cardiac mechanics and tissue Doppler velocities demonstrated worse subclinical LV systolic function, impaired LV relaxation, and higher filling pressures in groups with greater increases in BMI through young adulthood, after adjusting for cumulative exposure to key comorbidities and cardiovascular risk factors (Supplemental Table 8). Associations of BMI trajectory with absolute LS, e′ velocity, and E/e′ ratio remained significant after adjustment for baseline BMI, demonstrating the association of patterns of weight change with systolic and diastolic mechanics independent of the starting BMI (Figure 2, filled circle plots). In a separate multivariate model, association of BMI trajectory group, only e′ velocity and the E/e′ ratio remained significant after adjustment for final or year 25 BMI, demonstrating that the pattern of change in weight was independently associated with parameters of diastolic dysfunction (Figure 2, open circle plots). No significant differences in CS and RS were observed across trajectory groups. In secondary analyses, participants in greater BMI change trajectory groups had greater odds of overt systolic (ejection fraction < 50%) and diastolic dysfunction, even after adjustment for cumulative risk factors and baseline (year 0) BMI. This association was attenuated when adjusting for current (year 25) BMI. Sensitivity analyses excluding participants with interim nonfatal myocardial infarction or congestive heart failure demonstrated similar trends across trajectory groups (Supplemental Table 9). As expected, associations with measures of central fat distribution trajectory groups and cardiac measures of subclinical LV systolic function and diastolic function demonstrated similar trends after adjustment for year 25 BMI but were statistically significant only for E/e′ ratio in the WC trajectory groups (Table 3).
Figure 2.
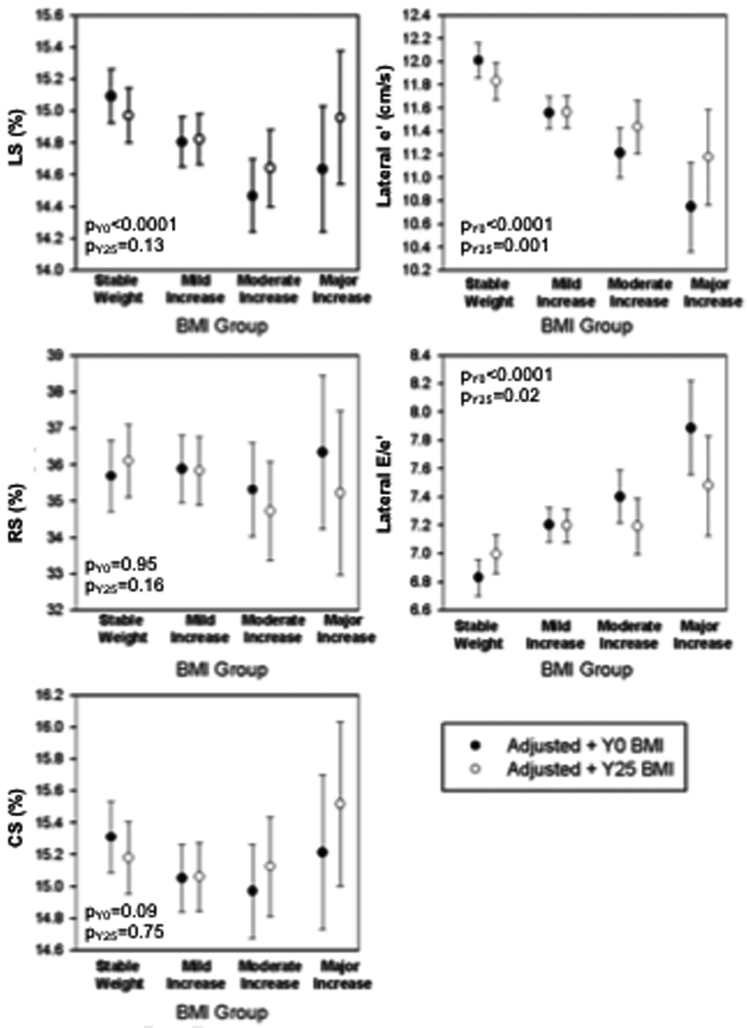
Adjusted least square means (95% CIs) for BMI trajectory groups associated with strain percentages and tissue Doppler velocity measures. Indices of cardiac mechanics and tissue Doppler velocities (absolute LS, e′ velocity, and E/e′ ratio) were significantly worse in groups with greater increases in BMI through young adulthood, even after adjusting for cumulative exposure to key comorbidities, cardiovascular risk factors, and baseline BMI. In addition, association of BMI trajectory group and e′ velocity and E/e′ ratio remained significant after adjustment for year 25 follow-up BMI. No significant differences in CS and RS were observed across trajectory groups.
Table 3.
Comparison of adjusted least square means (95% CIs) for percentage change trajectories in various measures of overall and central adiposity associated with strain percentages and tissue Doppler velocity adjusted for year 25 BMI
| Variable | % Change in BMI* | % Change in WC | % Change in WHR | |
|---|---|---|---|---|
| Absolute LS (%) | ||||
| Stable | 15.0 (14.8–15.1) | 14.9 (14.7–15.1) | Stable | 15.0 (14.8–15.1) |
| Mild increase | 14.8 (14.7–15.0) | 14.9 (14.7–15.0) | Mild to moderate | 14.9 (14.7–15.0) |
| Moderate increase | 14.6 (14.4–14.9)† | 14.8 (14.6–15.1) | Increase | |
| Major increase | 15.0 (14.5–15.4) | 14.8 (14.4–15.2) | Major increase | 14.7 (14.4–15.0) |
| P for trend | .131 | .588 | .136 | |
| RS (%) | ||||
| Stable | 36.1 (35.1–37.1) | 35.9 (34.8–36.9) | Stable | 35.2 (34.1–36.4) |
| Mild increase | 35.8 (34.9–36.8) | 36.0 (35.1–36.9) | Mild to moderate | 36.0 (35.2–36.9) |
| Moderate increase | 34.7 (33.4–36.1) | 35.1 (33.9–36.4) | Increase | |
| Major increase | 35.2 (33.0–37.5) | 35.2 (33.0–37.5) | Major increase | 35.7 (34.2–37.2) |
| P for trend | .157 | .406 | .371 | |
| Absolute CS (%) | ||||
| Stable | 15.2 (15.0–15.4) | 15.1 (14.9–15.3) | Stable | 15.1 (14.9–15.4) |
| Mild increase | 15.1 (14.8–15.3) | 15.1 (14.9–15.3) | Mild to moderate | 15.1 (14.9–15.3) |
| Moderate increase | 15.1 (14.8–15.4) | 15.1 (14.8–15.4) | Increase | |
| Major increase | 15.5 (15.0–16.0) | 15.8 (15.3–16.3)† | Major increase | 15.3 (14.9–15.6) |
| P for trend | .753 | .208 | .706 | |
| e′ velocity, cm/sec | ||||
| Stable | 11.8 (11.7–12.0) | 11.8 (11.6–12.0) | Stable | 11.8 (11.6–12.0) |
| Mild increase | 11.6 (11.4–11.7)† | 11.6 (11.4–11.7)† | Mild to moderate | 11.6 (11.5–11.7) |
| Moderate increase | 11.4 (11.2–11.7)† | 11.6 (11.4–11.8) | Increase | |
| Major increase | 11.2 (10.8–11.6)† | 11.4 (11.0–11.8) | Major increase | 11.6 (11.3–11.8) |
| P for trend | .001 | .109 | .129 | |
| E/e′ ratio | ||||
| Stable weight | 7.0 (6.9–7.1) | 7.0 (6.8–7.1) | Stable weight | 7.1 (6.9–7.2) |
| Mild increase | 7.2 (7.1–7.3)† | 7.2 (7.1–7.3)† | Mild to moderate | 7.1 (7.0–7.2) |
| Moderate increase | 7.2 (7.0–7.4) | 7.2 (7.0–7.4) | Increase | |
| Major increase | 7.5 (7.1–7.8)† | 7.3 (7.0–7.7) | Major increase | 7.3 (7.0–7.5) |
| P for trend | .016 | .035 | .311 |
Using model 3 which included adjustment for age, gender, race, education, center, echocardiography analyst, quality scores (for strain percentages only; apical for LS and short axis for RS and CS), cumulative systolic and diastolic blood pressure, number of visits with blood pressure medication, cumulative cholesterol, years with diabetes, cigarette smoking, and respective year 25 anthropometric measure (e.g., BMI, WC, WHR).
Significantly different from stable weight group at the P < .05 level.
DISCUSSION
In this population-based study of adults recruited and followed in parallel with the US obesity epidemic, we identified four unique patterns of change in BMI over a 25-year span. BMI trajectory groups were significantly and independently associated with indices of diastolic function (e′ velocity and E/e′ ratio), highlighting the importance of cumulative weight gain through midlife, independent of baseline (year 0) or final (year 25) BMI. In addition, BMI trajectories were significantly associated with LS, overt systolic dysfunction (defined as ejection fraction < 50%), and diastolic dysfunction, but this finding was not independent of year 25 BMI. Although the absolute differences in parameters of cardiac mechanics were small, our cohort is among the youngest studied to date and therefore may have milder changes in echocardiographic parameters. In addition, the overall prevalence of diastolic dysfunction was quite low, ranging from 2% to 7%. To our knowledge, our study is one of the largest investigations to date evaluating the association of changes in measures of adiposity beginning from young adulthood (mean age, 25 years) and indices of cardiac mechanics in midlife (mean age, 50 years).
The findings of the present study provide unique insights into long-term patterns of change in anthropometric measures during early adulthood and highlight the heterogeneous trajectories of change in adiposity as assessed by BMI, WC, and WHR during early adulthood to midlife. Importantly, participants at baseline had similar average BMI values across all trajectory groups, with the greatest increase occurring during the first 10 years of follow-up. Of note, the participants with the greatest BMI change constituted only 6% of the cohort and therefore represent small numbers resulting in wide error bars in Figure 2. Prior work in the National Health and Nutrition Examination Survey I and the first 10-year follow-up of CARDIA identified young adulthood (early 20s) as the peak age for greatest average weight gain.15,18 Latent class modeling, as used in the present analysis, has allowed identification of different patterns of adiposity change as separate trajectory groups and thus provides a more realistic understanding of lifetime trends in weight and highlighting the period of transition from adolescence to young adulthood as a prime target for health promotion, primordial prevention, and long-term morbidity and disease prevention.
The present study extends prior findings by classifying participants by trajectories of BMI changes and demonstrates that steeper BMI change trajectories are associated with markers of LV relaxation and filling pressure (e′ velocity, E/e′ ratio), independent of year 25 BMI, measured at the time of echocardiography, suggesting a cumulative response to weight exposure over time on diastolic dysfunction. Among CARDIA participants, Reis et al19 previously demonstrated that a longer duration of obesity was independently associated with greater LV mass, and a greater degree of adiposity at year 25 was associated with greater LV mass, LV end-diastolic volume, LV volume-to-mass ratio, and left atrial dimension. In this study, trajectory groups of BMI change showed only a modest correlation with cumulative BMI (Spearman correlation coefficient = 0.40, P < .001), demonstrating that trajectories offer additional unique information and allow objective categorization of weight change categories so not to rely on subjective cut points.
Absolute mean differences in tissue Doppler velocity (e′) and filling pressures (E/e′ ratio) between trajectory groups in our study reflected overall normal filling pressures, which is not surprising in an asymptomatic predominantly young cohort. Tissue Doppler indices of impaired relaxation (e′) and increased filling pressures (E/e′ ratio) are sensitive indicators of diastolic dysfunction and may represent onset of adverse decline before onset of diastolic dysfunction and overt heart failure. In fact, the overall prevalence of diastolic dysfunction in the CARDIA cohort as defined by 2016 ASE guidelines was low, ranging between 2.9% (full criteria) and 4.7% (modified criteria). The prevalence of diastolic dysfunction increases with age and is clinically relevant, as even mild diastolic dysfunction is significantly associated with all-cause mortality in asymptomatic individuals.20
Increasing BMI trajectory groups are associated with overt systolic dysfunction (ejection fraction < 50%) and subclinical LV dysfunction (LS), independent of year 0 BMI. The presence of lower ejection fraction and lower absolute LS, specifically, in those with steeper BMI trajectories is likely explained by the vulnerability of the subendocardium, which is composed mainly of longitudinal myocardial fibers and is the most sensitive and vulnerable to myocardial disease processes and may precede global systolic dysfunction and overt reduction in ejection fraction.21 Current hypotheses posit that the presence of excess weight may induce a systemic proinflammatory state and subsequent coronary microvascular endothelial dysfunction resulting in subendocardial dysfunction reflected by global LS.22 Our findings are also clinically relevant on the basis of prior studies relating measures of cardiac mechanics to outcomes. Although the absolute differences measured in LS adjusted for year 0 BMI are small between the stable BMI and major increase groups, prior studies have demonstrated a continuous dose-dependent association with relative increases in risk for adverse outcomes by 18% to 28% per 1% worse absolute global LS.23,24 Participants with even mildly reduced parameters of subclinical LV systolic function and presence of diastolic dysfunction at such a young age may be at greater lifetime risk for development of clinical heart failure as they age, as abnormal cardiac mechanics have been reported to predict and precede development of heart failure.25,26
Our study has several strengths. The longitudinal nature of CARDIA and detailed phenotyping at each of the eight examinations provides detailed long-term data with which to analyze patterns of BMI change during younger adulthood into middle age. In addition to our BMI findings, we saw similar trajectory patterns with measures of central fat distribution with greater degree of systolic and diastolic dysfunction in groups with greatest increase. We used sophisticated echocardiographic speckle-tracking indices of cardiac mechanics as a measure of subclinical LV dysfunction at year 25, and we applied innovative statistical methods to examine patterns of changes in several anthropometric measures (BMI, WC, and WHR) in a large, well-characterized cohort of black and white Americans. Although changes in weight are strongly associated with hypertension, diabetes, and metabolic syndrome, our findings are independent of the known contributions from these key comorbidities to LV systolic and diastolic function.7,27
Several limitations should also be considered when interpreting our study results. The absolute event rate for the clinical outcome of heart failure remains quite low at this time in CARDIA, thereby limiting our ability to examine associations between BMI trajectories and clinical outcomes. However, this is not unexpected given the participants’ current age. LS was measured from only the apical four- chamber view. However, as the cohort is young and predominantly healthy, this likely did not alter our overall findings. In addition, we performed sensitivity analyses after excluding participants with cardiovascular events (myocardial infarction or congestive heart failure, n = 97) with similar findings. Not all CARDIA participants had weight and height information available at all examination periods, but missing values are unlikely to have altered our findings, as missing BMI data at each visit was similar for all trajectory groups and similar to prior trajectory analyses in CARDIA.28 Participants who did not attend more recent examinations were more likely to be African American and of lower socioeconomic status and were more likely to have a greater burden of cardiovascular risk factors; nevertheless these subgroups are well represented in CARDIA participants who did attend the year 25 examination.
CONCLUSION
The findings of our study suggest that the trajectory of change in BMI throughout early adulthood to middle age (mean age 25 to 50 years), independent of baseline and current BMI, may provide additional information about the cumulative burden of weight gain and risk for development of abnormal cardiac mechanics. This novel characterization identifying trajectories of change in several anthropometric measures across a vulnerable time period for significant weight gain highlights young adulthood as a potential important target for behavior and lifestyle interventions for primordial prevention.
Supplementary Material
HIGHLIGHTS.
In this population-based cohort study, four groups of BMI change were identified over 25 years of follow-up, including stable BMI and mild, moderate, and major increases.
Groups with greater increases in BMI had significantly worse measures of diastolic function (lower e′, higher E/e′) in midlife.
Young adulthood may be a critical period in life for behavior and lifestyle interventions for primordial prevention to prevent the development of adverse cardiac mechanics before the onset of clinical heart failure.
ACKNOWLEDGMENTS
We thank the participants of the CARDIA study for their long-term commitment and important contributions to the study.
The Coronary Artery Risk Development in Young Adults (CARDIA) study is conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham (HHSN268201300025C and HHSN268201300025C), Northwestern University (HHSN268201300027C), the University of Minnesota (HHSN268201300028C), the Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging and an intra-agency agreement between the National Institute on Aging and the National Heart, Lung, and Blood Institute (AG0005). This report has been reviewed by CARDIA for scientific content. Dr. Khan issupported by a research grantfrom the National Institutes of Health (F32HL129695 and KL2TR001424).
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant KL2TR001424 to S.S.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, or the US Department of Health and Human Services.
Abbreviations
- BMI
Body mass index
- CARDIA
Coronary Artery Risk Development in Young Adults
- CS
Circumferential strain
- LS
Longitudinal strain
- LV
Left ventricular
- RS
Radial strain
- WC
Waist circumference
- WHR
Waist-to-hip ratio
Footnotes
Conflicts of Interest: None.
SUPPLEMENTARY DATA
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.echo.2018.07.014.
Contributor Information
Sadiya S. Khan, Division of Cardiology, Department of Medicine; Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Sanjiv J. Shah, Division of Cardiology, Department of Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Laura A. Colangelo, Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Anita Panjwani, Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Kiang Liu, Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Cora E. Lewis, Division of Preventive Medicine, University of Alabama at Birmingham, Birmingham, Alabama.
Christina M. Shay, Department of Nutrition, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
Jr. David C. Goff, Department of Epidemiology, Colorado School of Public Health, Aurora, Colorado.
Jared Reis, Division of Cardiovascular Sciences, National Heart, Lung, and Blood Institute, Bethesda, Maryland.
Henrique Doria De Vasconcellos, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, Maryland.
Joao A. C. Lima, Division of Cardiology, Department of Medicine, Johns Hopkins University, Baltimore, Maryland.
Donald Lloyd-Jones, Division of Cardiology, Department of Medicine; Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
Norrina B. Allen, Department of Preventive Medicine; Northwestern University Feinberg School of Medicine, Chicago, Illinois.
REFERENCES
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 2014;129:e28–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States 2005 to 2014. JAMA 2016; 315:2284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation 2017;136:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardin JM, McClelland R, Kitzman D, Lima JA, Bommer W, Klopfenstein HS, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol 2001;87:1051–7 [DOI] [PubMed] [Google Scholar]
- 5.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–13. [DOI] [PubMed] [Google Scholar]
- 6.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. JAMA 1991;266:231–6. [PubMed] [Google Scholar]
- 7.Selvaraj S, Aguilar FG, Martinez EE, Beussink L, Kim KY, Peng J, et al. Association of comorbidity burden with abnormal cardiac mechanics: findings from the HyperGEN study. J Am Heart Assoc 2014;3:e000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhana K, van Rosmalen J, Vistisen D, Ikram MA, Hofman A, Franco OH, et al. Trajectories of body mass index before the diagnosis of cardiovascular disease: a latent class trajectory analysis. Eur J Epidemiol 2016;31: 583–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpert MA. Obesity cardiomyopathy: pathophysiology and evolution of the clinical syndrome. Am J Med Sci 2001;321:225–36. [DOI] [PubMed] [Google Scholar]
- 10.Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 2017;318:255–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangalore S, Fayyad R, Laskey R, DeMicco DA, Messerli FH, Waters DD. Body-weight fluctuations and outcomes in coronary disease. N Engl J Med 2017;376:1332–40. [DOI] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 1988;41:1105–16. [DOI] [PubMed] [Google Scholar]
- 13.Armstrong AC, Ricketts EP, Cox C, Adler P, Arynchyn A, Liu K, et al. Quality control and reproducibility in M-mode, two-dimensional, and speckle tracking echocardiography acquisition and analysis: the CARDIA study, year 25 examination experience. Echocardiography 2015;32: 1233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagin DS, Odgers CL. Group-based trajectory modeling (nearly) two decades later. J Quant Criminol 2010;26:445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis CE, Jacobs DR Jr., McCreath H, Kiefe CI, Schreiner PJ, Smith DE, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol 2000;151:1172–81. [DOI] [PubMed] [Google Scholar]
- 16.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 17.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third universal definition of myocardial infarction. Nat Rev Cardiol 2012;9:620–33. [DOI] [PubMed] [Google Scholar]
- 18.Williamson DF, Kahn HS, Remington PL, Anda RF. The 10-year incidence of overweight and major weight gain in US adults. Arch Intern Med 1990; 150:665–72. [PubMed] [Google Scholar]
- 19.Reis JP, Allen N, Gibbs BB, Gidding SS, Lee JM, Lewis CE, et al. Association of the degree of adiposity and duration of obesity with measures of cardiac structure and function: the CARDIA study. Obesity (Silver Spring) 2014; 22:2434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Redfield MM, Jacobsen SJ, Burnett JC Jr., Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202. [DOI] [PubMed] [Google Scholar]
- 21.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr 2010;23:351–69. [DOI] [PubMed] [Google Scholar]
- 22.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 23.Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, et al. Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. Eur Heart J Cardiovasc Imaging 2012; 13:827–33. [DOI] [PubMed] [Google Scholar]
- 24.Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging 2012;5:719–25. [DOI] [PubMed] [Google Scholar]
- 25.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC Jr, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi EY, Rosen BD, Fernandes VR, Yan RT, Yoneyama K, Donekal S, et al. Prognostic value of myocardial circumferential strain for incident heart failure and cardiovascular events in asymptomatic individuals: the Multi-Ethnic Study of Atherosclerosis. Eur Heart J 2013;34:2354–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloyd-Jones DM, Liu K, Colangelo LA, Yan LL, Klein L, Loria CM, et al. Consistently stable or decreased body mass index in young adulthood and longitudinal changes in metabolic syndrome components: the Coronary Artery Risk Development in Young Adults Study. Circulation 2007;115:1004–11. [DOI] [PubMed] [Google Scholar]
- 28.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, et al. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA 2014;311:490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.