Abstract
Purpose
The number of individuals who have started a regimen for HIV pre-exposure prophylaxis (PrEP) in the US is not well characterized, but has been on the rise since 2012. This analysis assesses the distribution of PrEP use nationally and among subgroups.
Methods
A validated algorithm quantifying TDF/FTC for PrEP in the US was applied to a national prescription database to determine the quarterly prevalence of PrEP use. HIV diagnoses from 2016 were used as an epidemiological proxy for PrEP need. The PrEP-to-need ratio (PnR) was defined as the number of PrEP users divided by new HIV diagnoses.
Results
A total of 70,395 individuals used PrEP in Q4 2017: 67,166 males and 3,229 females. Nationally, prevalence of PrEP use was 26/100,000 (range across states per 100,000 [RAS/100k]: 4–73) and the PnR was 1.8 (RAS: 0.5–6.6). Prevalence of PrEP use among males and females, respectively, was 50/100,000 and 2/100,000 (RAS/100k: 7–143 and 0.3–7) and PnR was 2.1 and 0.4 (RAS: 0.6–7.1 and 0.1–4.0). Prevalence of PrEP use was lowest among individuals ≤24 and ≥55 (15/100,000 and 6/100,000, RAS/100k: 1–45 and 0.4–14), with PnR 0.9 and 1.5 (RAS: 0.2–5.6 and 0.3–7.0). The Northeast had the highest PnR (3.3); the South had the lowest (1.0). States with Medicaid expansion had more than double the PnR than states without expansion.
Conclusions
Available data suggest that females, individuals ≤24, and residents of the South had lower levels of PrEP use relative to epidemic need. These results are ecological, and misclassification may attenuate results. PnR is useful for future assessments of HIV prevention strategy uptake.
Keywords: Pre-exposure prophylaxis, HIV, prevention
Introduction
Pre-exposure prophylaxis (PrEP) for human immunodeficiency virus (HIV) in the form of daily tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) is highly effective in reducing HIV transmission. For men who have sex with men (MSM), PrEP reduced HIV transmission by 44% among those assigned to the PrEP arm in a clinical trial,1 with a 96% reduction in transmission among those estimated to be taking four doses per week and 99% reduction among those taking seven doses per week.2 A meta-analysis of seven clinical trials among women found a 36% reduction in HIV transmission, and this increased to a 61% reduction in transmission for those with 75% adherence or greater.3 In a clinical trial of injection drug users, PrEP reduced HIV transmission by 49% overall,4 with a 56% reduction in transmission for those with >70% adherence.5 PrEP has also been shown to provide substantial protection against HIV transmission in open-label study6 and in clinical practice settings.7,8
To determine the need for PrEP use in a particular group of individuals, the World Health Organization (WHO) uses a ‘substantial risk’ threshold at the group level, considering a threshold of HIV incidence greater than 3 per 100 persons annually to indicate all individuals in the group for PrEP.9 Although this is a simple and useful criterion, incidence data may not always be available for all relevant groups. The US Centers for Disease Control and Prevention (CDC) uses a ‘substantial risk’ threshold at the individual level, basing threshold guidance on sexual risk history data and laboratory tests that can be collected for individual patients.10 CDC criteria for PrEP indications were developed based on risk score indices, PrEP clinical trial data, and epidemiological study data.10 Application of these screening criteria led to a CDC estimate that 1.2 million individuals in the US are eligible for PrEP: 492,000 MSM, 468,000 female heterosexuals, 157,000 male heterosexuals, and 115,000 adults who inject drugs.11
Two previous studies have estimated the number of individuals on PrEP in the US using aggregated PrEP prescription data. Based on a definition of ≥ 1 TDF/FTC prescription for PrEP in a year, one study estimated that the national prevalence of prescriptions was 765 in 2012 and 9,375 in 2014.12 These estimates relied on prescription data covering 40 million commercially-insured individuals, and the assumption that PrEP uptake among those individuals in the database was representative of PrEP uptake nationally. Using a definition of ≥ 1 TDF/FTC prescription for PrEP by unique individuals, others have previously reported estimated total cumulative PrEP ‘starts’ as 5,853 in 2012, 28,517 in 2014, and 98,732 in 2016.13 By the end of 2017, the cumulative number of individuals starting PrEP prescriptions since 2012 was estimated to be 140,000.13 Data for these estimates came from a national data aggregator estimated to cover over 80% of PrEP prescriptions. Both of these studies identified lower levels of PrEP prescription among women and younger individuals.
A combination of the methods used in each of these two different studies could be optimal to estimate the number of individuals potentially receiving PrEP protection: using a national database that better represents the totality of individuals in the United States and a prevalence estimate reflective of current levels of PrEP prescriptions. This study provides a national estimate for PrEP use in a period, defined as ≥1 day of prescribed TDF/FTC for PrEP in the fourth quarter of 2017 (Q4 2017). Data for this analysis are from a prescription database with greater coverage than previous analyses,13 with the current database estimated to substantially represent population-level PrEP user data.14 We selected a three-month period to match the usual maximum length of a single PrEP prescription period. We explore these data by gender, age, region, district, and state. We assess the number of PrEP users per population and number of PrEP users divided by the number of individuals newly diagnosed with HIV, which we term the ‘PrEP-toneed’ ratio (PnR).
Methods
This is a cross-sectional study of population-level data to describe the distribution of PrEP users and PnR in the fourth quarter of year 2017 (Q4 2017) in the United States’ 50 states, District of Columbia, and Puerto Rico.
Data sources and definitions
Deidentified state-level data regarding the number of PrEP users were obtained from a national health data company that aggregates clinic, patient, and provider data (Source Healthcare Analytics, SHA). SHA patient-level prescription data provided for this analysis are from over 54,000 pharmacies, 1,500 hospitals, 800 outpatient facilities, and 80,000 physician practices, with payment types including commercial plans, Medicare Part D, cash, assistance programs, and Medicaid. Closed healthcare systems and those that chose not to share their data with SHA are not included in the database. All patient-level prescription data and medical insurance claims data were de-identified.
Methods used to determine periods of unique persons’ exposure to medication (drug exposure period per person) have been described and validated elsewhere,15 and are similar to methods previously published.12 In brief, the duration of TDF/FTC course per PrEP user was determined by adding up subsequent prescription renewals. Using medical procedure and diagnosis codes in the deidentified claims database, drug exposure periods were classified as either: PrEP, post-exposure prophylaxis, HIV treatment, chronic hepatitis B management, or unclassified. Approximately 28% of TDF/FTC drug exposure periods were unclassified due to insufficient medical procedure or diagnosis code data. These were excluded from the present analysis. Demographic data for individuals in the unclassified group were not substantially different than demographic data for individuals with classified prescriptions included in the present analysis. A sensitivity analysis was conducted to explore the implications of excluding unclassified individuals from the present analysis. Patient-level data available in the dataset included age, gender, and state of residence. Race was not available in the commercial dataset.
HIV case surveillance data for 2016 were obtained from AIDSVu.org at the state-level by age and gender.16 Medicaid expansion under the Affordable Care Act was defined as states that have adopted the expansion of Medicaid eligibility to people with annual incomes below 138% of the federal poverty level; our analysis is from data available in 2018, with 33 states including DC having expanded Medicaid.17 States’ policy on Medicaid coverage of routine HIV screening versus coverage for medically necessary HIV testing only1 was based on a survey of state Medicaid coverage of adult preventive services published in 2014.18
Population estimates were obtained from National Historical Geographic Information System (NHGIS) based on the American Community surveys (ACS) provided by the US census Bureau.19 Data from the 2016 US Census were used to obtain state-level population estimates by age and gender, and to allow for division of states into quartiles of poverty, percent uninsured, and concentrations of African-American and Hispanic populations.20 To establish quartiles, the 50 states and District of Columbia and Puerto Rico were classified into four categories using quartile cut-offs corresponding to each of the variables of interest. Prevalence of PrEP use and new HIV diagnoses per 100,000 population and PnR were calculated for each of the quartiles (four state/area groups of n=13 each) for poverty, percent uninsured, and percent African American and Hispanic. For poverty we used 2016 US Census Bureau data, and its measure which defines poverty as three times the cost of a minimum food diet. For a single individual household under 65 years the poverty line was $12,486, and for a household of four the poverty line was $24,755.21 For percent uninsured, we used 2016 US Census Bureau data, and its measure that considers an individual insured if they were covered by any type of private or government insurance for any part of the previous year.22 PrEP clinic data were obtained from PrEP Locator, a national database of PrEP-prescribing clinics.23–25
Number of PrEP users, prevalence of PrEP use, and the PrEP-to-need ratio (PnR)
PrEP use period was defined as ≥1 day of TDF/FTC prescribed for PrEP for a unique person in a time period, which for this study is the three-month period from October 1, 2017 to December 31, 2017 (Q4 2017). For TDF/FTC to be classified as PrEP for an interval, an individual would need a TDF/FTC prescription for PrEP and to have at least one of those prescription days fall in the three-month period. Prevalence of PrEP use was defined as the number of PrEP users per 100,000 population aged over 13 years in a given geographic area. Epidemiological need, best estimated by HIV incidence, is instead estimated based on new HIV diagnoses because national incidence data is not available. To compare the relative levels of PrEP provision to the underlying epidemiological need, PnR was defined as the ratio of the number of PrEP users to the number of new HIV diagnoses for a given geographic area and/or population. PnR is limited in that it is an ecological construct that does not assess, on an individual level, whether PrEP is being appropriately targeted to those in need. Instead, the construct of PnR is used to explore disparities in provision across geographic areas and available demographic categories.
Analysis
Descriptive statistics were used to explore state-level data regarding PrEP use, an ecological analysis. The number of PrEP users per 100,000 population were estimated for each state, census region, gender, and age group. PnRs were calculated overall and for each age group to compare the number of PrEP users in Q4 of 2017 to the group-specific number of HIV diagnoses in 2016. Significance testing for differences was not conducted because the data are not based on a sample, but instead approximate population-data, which violates variance and confidence interval assumptions. Lorenz curves were used to display disparities in PrEP uptake at the national level. All data analysis was conducted in SAS 9.4 and R 3.4.3. (Cary, NC).
Results
A total of 70,395 unique individuals used TDF/FTC for PrEP in the fourth quarter of 2017 (Table 1). Nationally, the prevalence of PrEP use was 25.8/100,000 (range across states per 100,000 [RAS/100k]: 3.7–72.6) and the PnR was 1.8 (RAS: 0.5–6.6). This PnR can be interpreted as follows: for each one new HIV diagnosis, there were 1.8 PrEP users. By region, the Northeast accounted for 20,881 (30%) of PrEP use with a PnR of 3.3, the South 21,430 (30%) with a PnR of 1.0, the West 16,091 (23%) with a PnR of 2.1, and the Midwest 11,963 (17%) with a PnR of 2.4. Prevalence of PrEP use at the division level ranged from 14.2/100,000 population in the East South Central division to 47.3/100,000 in the Mid-Atlantic division. PnRs were similar among divisions within each region, indicating consistency in the ratio of PrEP use to epidemic need at the region level.
Table 1.
Distribution of number PrEP users and PrEP need in the United States by geographic region and state-level demographic characteristics, Q4 2017
| Total | |||||
|---|---|---|---|---|---|
|
| |||||
| Number of PrEP users | New HIV Diagnoses | PrEP-to-need ratio (PnR) | |||
|
|
|
||||
| N (%) | per 100,000 population° | N (%) | per 100,000 population° | ||
|
| |||||
| Totalº | 70,395 | 25.8 | 40,183 | 14.7 | 1.8 |
|
| |||||
| Census Region and Division | |||||
|
|
|||||
| Midwest | |||||
|
|
|||||
| East North Central | 8,858 (13) | 22.6 | 3,807 (10) | 9.7 | 2.3 |
|
|
|||||
| West North Central | 3,105 (4) | 17.7 | 1,232 (3) | 7.0 | 2.5 |
|
| |||||
| Midwest, Total | 11,963 (17) | 21.0 | 5,039 (13) | 8.9 | 2.4 |
|
| |||||
| Northeast | |||||
|
|
|||||
| Mid-Atlantic | 16,628 (24) | 47.3 | 5,168 (13) | 14.7 | 3.2 |
|
|
|||||
| New England | 4,253 (6) | 33.6 | 1,131 (3) | 8.9 | 3.8 |
|
| |||||
| Northeast, Total | 20,881 (30) | 43.7 | 6,299 (16) | 13.2 | 3.3 |
|
| |||||
| South | |||||
|
|
|||||
| East South Central | 2,253 (3) | 14.2 | 1,991 (5) | 12.6 | 1.1 |
|
|
|||||
| West South Central | 5,880 (8) | 18.3 | 6,222 (16) | 19.3 | 0.9 |
|
|
|||||
| South Atlantic | 13,297 (19) | 24.6 | 12,309 (31) | 22.8 | 1.1 |
|
| |||||
| South, total | 21,430 (30) | 21.0 | 20,522 (52) | 20.1 | 1.0 |
|
| |||||
| West | |||||
|
|
|||||
| Mountain | 3,546 (5) | 18.0 | 2,067 (5) | 10.5 | 1.7 |
|
|
|||||
| Pacific | 12,545 (18) | 28.4 | 5,733 (14) | 13.0 | 2.2 |
|
| |||||
| West, Total | 16,091 (23) | 25.2 | 7,800 (20) | 12.2 | 2.1 |
|
| |||||
| Status of State Medicaid expansion¶ | |||||
|
|
|||||
| Adopted | 50,876 (72) | 30.3 | 21,338 (54) | 12.7 | 2.4 |
|
|
|||||
| Not adopted | 19,489 (28) | 19.0 | 18,322 (46) | 17.8 | 1.1 |
|
| |||||
| State medicaid coverage of routine HIV screeningɤ | |||||
|
|
|||||
| Covered | 54,367 (77) | 28.5 | 25,552 (64) | 13.4 | 2.1 |
|
|
|||||
| Not covered | 15,998 (23) | 20.1 | 14,108 (36) | 17.7 | 1.1 |
|
| |||||
| By Stateº | |||||
|
| |||||
| Poverty (by state) | |||||
|
| |||||
| Less than 11.9% poverty | 10,145 (14) | 22.0 | 4,750 (12) | 10.3 | 2.1 |
|
| |||||
| 11.9% – <14.9% poverty | 12,288 (17) | 22.2 | 4,256 (11) | 7.7 | 2.9 |
|
| |||||
| 14.9% – <17.0% poverty | 39,508 (56) | 25.0 | 22,437 (56) | 14.2 | 1.8 |
|
| |||||
| 17.0% or more poverty | 8,454 (12) | 15.4 | 8,740 (22) | 16.0 | 1.0 |
|
| |||||
| Percent Uninsured (by state) | |||||
|
| |||||
| Less than 5.6% uninsured | 11,896 (17) | 21.4 | 4,191 (10) | 7.5 | 2.9 |
|
| |||||
| 5.6% – <7.9% uninsured | 34,081 (48) | 29.5 | 13,585 (34) | 11.8 | 2.5 |
|
| |||||
| 7.9% – <9.6% uninsured | 7,858 (11) | 14.6 | 4,861 (12) | 9.0 | 1.6 |
|
| |||||
| 9.6% or more uninsured | 16,560 (24) | 17.1 | 17,546 (44) | 18.2 | 0.9 |
|
| |||||
| African American Concentration (by state) | |||||
|
| |||||
| Less than 3.3% African American | 2,553 (4) | 15.7 | 866 (2) | 5.3 | 3.0 |
|
| |||||
| 3.3% – <7.6% African American | 19,794 (28) | 25.8 | 8,593 (21) | 11.2 | 2.3 |
|
| |||||
| 7.6% – <15.6% African American | 20,179 (29) | 21.2 | 12,783 (32) | 13.4 | 1.6 |
|
| |||||
| 15.6% or more African American | 27,869 (40) | 32.7 | 17,941 (45) | 21.1 | 1.5 |
|
| |||||
| Hispanic Concentration (by state) | |||||
|
| |||||
| Less than 4.8% Hispanic | 6,518 (9) | 16.2 | 3,771 (9) | 9.4 | 1.7 |
|
| |||||
| 4.8% – <9.0% Hispanic | 10,889 (15) | 17.7 | 7,664 (19) | 12.4 | 1.4 |
|
| |||||
| 9.0% – <13.8% Hispanic | 12,240 (17) | 29.0 | 6,286 (16) | 14.9 | 1.9 |
|
| |||||
| 13.8% or more Hispanic | 40,748 (58) | 31.5 | 22,462 (56) | 17.3 | 1.8 |
source: Kaiser Commision on Status of State Action on the Medicaid Expansion Decision: 2018.
source: Kaiser Commission on State Medicaid Coverage of Routine HIV Screening: 2014.
population aged 13 and above in each of the corresponding categories
New HIV diagnoses are considered as an ecological proxy for PrEP need per geographic area; states with more new HIV diagnoses have greater need for PrEP use
including District of Columbia and Puerto Rico
The PnR was higher in states that have expanded Medicaid under the Affordable Care Act (PnR=2.4) compared to states without expansion (PnR=1.1) (Table 1). Similarly, states in which Medicaid covered routine HIV screening costs had higher PnRs (2.1) than states without coverage (1.1). States in the highest quartile of proportion of residents living in poverty had lower prevalence of PrEP use (15.4/100,000) and PnR (1.0) than states in the lowest quartile (22.0/100,000 and 2.1). Similarly, states in the highest quartile of proportion of residents without insurance had lower prevalence of PrEP use (17.1/100,000) and PnR (0.9) than states in the lowest quartile (21.4/100,000 and 2.9). States with the highest quartiles of African American resident concentration had higher prevalence of PrEP use (32.7/100,000) but lower PnRs (1.5) than states in the lowest quartile (15.7/100,000 and 3.0).
There were 3,229 female PrEP users and 67,166 male PrEP users (Table 2). Prevalence of PrEP use was substantially lower for females (2.3/100,000) than for males (50.3/100,000) (RAS/100k: 0.3–7.3 and 7.4–143.2, respectively). The PnR was more than 5 times lower for females (0.4) than males (2.1) (RAS: 0.1–4.0 and 0.6–7.1, respectively). Lower levels of prevalence of PrEP use and PnRs for females were observed across all regions. Nationally, by age group, there were 7,990 PrEP users aged ≤24 years; 27,556 PrEP users aged 25–34; 16,991 PrEP users aged 35–44; 12,121 PrEP users aged 45–54; and 5,774 PrEP users aged ≥ 55 (Table 3). The prevalence of PrEP use was lowest among individuals aged ≤24 (15.2/100,000) and ≥55 (6.3/100,000) (RAS/100k: 1.1–45.3 and 0.4–14.0, respectively) and the PnR was 0.9 and 1.5, respectively (RAS: 0.2–5.6 and 0.3–7.0). The prevalence of PrEP use was highest among individuals aged 24–35 i.e. 61.5/100,000 (RAS/100k: 6.2–184.1) and PnR=2.0 (RAS: 0.5–6.7)
Table 2.
Distribution of number of PrEP users and PrEP need in the United States by gender and census region, Q4 2017
| Females | Males | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of PrEP users | New HIV Diagnoses | PrEP-to-need ratio (PnR) | Number of PrEP users | New HIV Diagnoses | PnR | |||||
| N (%) | per 100,000 population° | N (%) | per 100,000 population° | N (%) | per 100,000 population° | N (%) | per 100,000 population° | |||
| Total* | 3,229 | 2.3 | 7,638 | 5.5 | 0.4 | 67,166 | 50.3 | 32,545 | 24.4 | 2.1 |
| Census Region and Division | ||||||||||
| Midwest | 594 (18) | 2.0 | 919 (12) | 3.2 | 0.6 | 11,369 (17) | 40.8 | 4,120 (13) | 14.8 | 2.8 |
| Northeast | 1,106 (34) | 4.5 | 1,475 (20) | 6.0 | 0.7 | 19,775 (30) | 85.5 | 4,824 (15) | 20.9 | 4.1 |
| South | 1,004 (31) | 1.9 | 4,157 (55) | 7.9 | 0.2 | 20,426 (30) | 41.2 | 16,365 (51) | 33.0 | 1.2 |
| West | 524 (16) | 1.6 | 978 (13) | 3.0 | 0.5 | 15,567 (23) | 49.2 | 6,822 (21) | 21.6 | 2.3 |
including District of Columbia and Puerto Rico
Table 3.
Distribution of number of PrEP users and PrEP need in the United States by age categories and census region, Q4 2017
| Number of PrEP users | New HIV Diagnoses | PrEP-to-need ratio (PnR) | |||
|---|---|---|---|---|---|
| N (%) | per 100,000 population° | N (%) | per 100,000 population° | ||
| Age groups | |||||
| Less than 24 years | |||||
| Midwest | 1,601 (20) | 14.5 | 1,304 (15.4) | 11.8 | 1.2 |
| Northeast | 2,680 (34) | 30.5 | 1,117 (13.2) | 12.7 | 2.4 |
| South | 2,203 (28) | 11.2 | 4,550 (53.8) | 23.1 | 0.5 |
| West | 1,503 (19) | 12.1 | 1,480 (17.5) | 11.9 | 1.0 |
| Less than 24, Total* | 7,990 | 15.2 | 8,531 | 16.2 | 0.9 |
| 25 to 34 years | |||||
| Midwest | 4,763 (17) | 53.5 | 1,667 (12.2) | 18.7 | 2.9 |
| Northeast | 8,730 (32) | 114.3 | 2,144 (15.7) | 28.1 | 4.1 |
| South | 8,110 (29) | 48.8 | 7,091 (51.9) | 42.6 | 1.1 |
| West | 5,939 (22) | 52.9 | 2,763 (20.2) | 24.6 | 2.1 |
| 25 to 35, Total | 27,556 | 61.5 | 13,791 | 30.8 | 2.0 |
| 35 to 44 years | |||||
| Midwest | 2,804 (17) | 34.0 | 900 (11.9) | 10.9 | 3.1 |
| Northeast | 4,919 (29) | 71.9 | 1,227 (16.2) | 17.9 | 4.0 |
| South | 5,255 (31) | 33.7 | 3,830 (50.6) | 24.5 | 1.4 |
| West | 4,008 (24) | 40.2 | 1,619 (21.4) | 16.3 | 2.5 |
| 35 to 44, Total | 16,991 | 41.3 | 7,700 | 18.7 | 2.2 |
| 45 to 54 years | |||||
| Midwest | 1,994 (16) | 22.3 | 724 (11.8) | 8.1 | 2.8 |
| Northeast | 3,066 (25) | 39.0 | 1,082 (17.7) | 13.8 | 2.8 |
| South | 3,989 (33) | 24.8 | 3,044 (49.8) | 18.9 | 1.3 |
| West | 3,066 (25) | 31.1 | 1,265 (20.7) | 12.8 | 2.4 |
| 45 to 54, Total | 12,121 | 28.1 | 6,221 | 14.4 | 2.0 |
| 55 years and older | |||||
| Midwest | 814 (14) | 4.1 | 444 (11.5) | 2.3 | 1.8 |
| Northeast | 1,496 (26) | 9.0 | 729 (18.9) | 4.4 | 2.0 |
| South | 1,878 (33) | 5.5 | 2,007 (52.1) | 5.9 | 0.9 |
| West | 1,584 (27) | 7.8 | 673 (17.5) | 3.3 | 2.4 |
| 55 and older, Total | 5,774 | 6.3 | 3,940 | 4.3 | 1.5 |
including District of Columbia and Puerto Rico
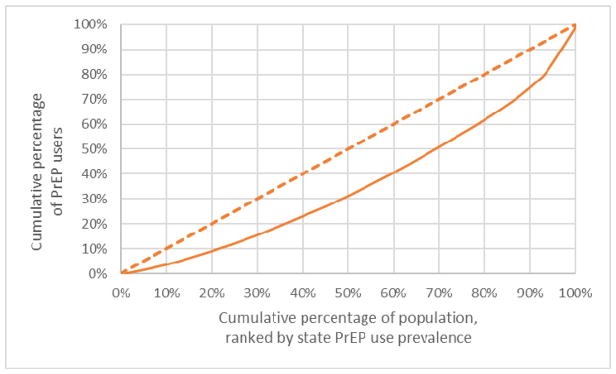
Lorenz curves, a technique to assess the distribution of health-related outcomes in a population,26–28 were used to illustrate disparities in PrEP use (Figures 1 and 2). Lorenz curves are compared to a 45-degree ‘line of equality’ that indicates perfectly equal distribution of the outcome. The level of the Lorenz curve’s deviation from the line of equality indicates the degree of inequality, which is frequently described in a single ‘gini’ coefficient that ranges from 0 (perfect equality) to 1 (perfect inequality).29 In Figure 1, the Lorenz curve represents the distribution of the cumulative percentage of PrEP users (the health outcome of interest, y-axis) as a function of the cumulative percentage of the total US population (x-axis). The total US population is ordered by state ranking, with states arranged from the lowest prevalence of PrEP use to the highest prevalence of PrEP use. Low PrEP use prevalence states that comprise 20% of the US population accounted for only 9% of PrEP users. Lower- and moderate-PrEP use prevalence states that comprise 60% of the US population accounted for 40% of PrEP users. The gini coefficient was 0.29.
Figure 1.
Lorenz curve of PrEP use by population by state, Q4 2017 United States
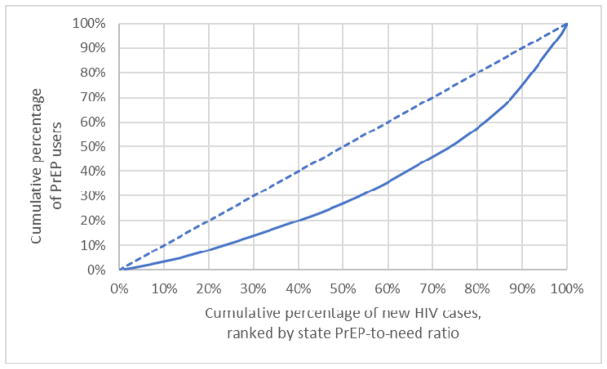
Figure 2.
Lorenz curve of PrEP use by new HIV cases by state, Q4 2017 United States
Figure 2 presents PrEP users as a function of the cumulative percentage of persons diagnosed with HIV in 2016, ordered by state ranking of PnR. Low PnR states that accounted for 20% of new HIV diagnoses in 2016 had only 8% of PrEP users. Lower- and moderate-PnR states that accounted for 60% of new HIV diagnoses in 2016 accounted for only 35% of PrEP users. The gini coefficient was 0.33.
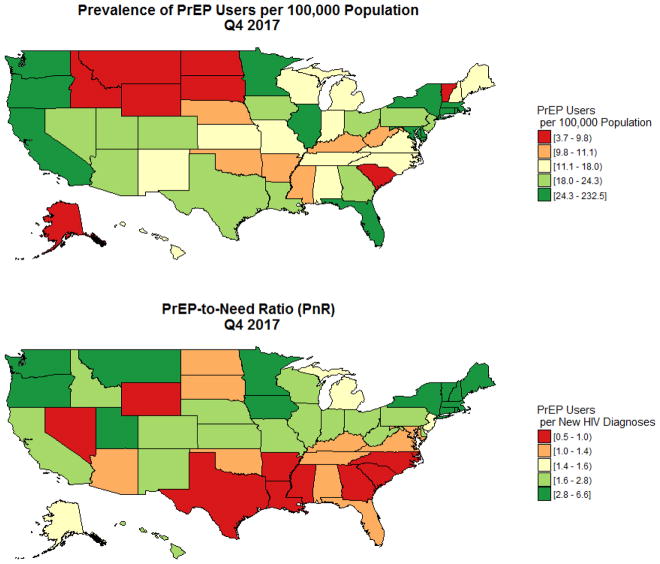
State-level data regarding prevalence of PrEP use and PnRs are presented in Table 4, classified by census division and region. Prevalence of PrEP use ranged from a low of 3.7/100,000 in Wyoming to a high of 72.6/100,000 in New York. The median number of PrEP users per publicly-listed PrEP clinics among states was 45.0, with a range of 3.5 to 372. PnRs ranged from a low of 0.5 in South Carolina to a high of 6.6 in Vermont. Puerto Rico had low prevalence of PrEP use and PnRs. Maps of state-level data (Figure 3) show lowest levels of prevalence of PrEP use in the center of the country. However, the lowest PnRs were concentrated in Southern states.
Table 4.
Distribution of number of PrEP users by total population, publicly-listed PrEP clinics, and PrEP need by state, Q4 2017
| Region | Division | State | PrEP users Total |
PrEP users N per 100,000 people* |
PrEP users N per publicly-listed PrEP clinics |
PrEP users PrEP-to-need ratio (PnR) |
|---|---|---|---|---|---|---|
| Midwest | East North Central | Illinois | 3,782 | 35.3 | 46.1 | 2.7 |
| Indiana | 852 | 15.5 | 65.5 | 1.8 | ||
| Michigan | 1,218 | 14.5 | 33.8 | 1.6 | ||
| Ohio | 2,376 | 24.3 | 54.0 | 2.5 | ||
| Wisconsin | 630 | 12.9 | 33.2 | 2.8 | ||
| West North Central | Iowa | 502 | 19.2 | 62.8 | 3.8 | |
| Kansas | 274 | 11.5 | 91.3 | 1.9 | ||
| Minnesota | 1,143 | 24.9 | 127.0 | 4.0 | ||
| Missouri | 916 | 18.0 | 65.4 | 1.8 | ||
| Nebraska | 165 | 10.6 | 41.3 | 2.2 | ||
| North Dakota | 60 | 9.6 | 12.0 | 1.3 | ||
| South Dakota | 45 | 6.4 | 45.0 | 1.2 | ||
| Northeast | Mid-atlantic | New Jersey | 1,822 | 24.2 | 50.6 | 1.6 |
| New York | 12,160 | 72.6 | 55.3 | 4.2 | ||
| Pennsylvania | 2,646 | 24.3 | 49.0 | 2.3 | ||
| New England | Connecticut | 831 | 27.2 | 29.7 | 3.3 | |
| Maine | 153 | 13.3 | 6.4 | 3.1 | ||
| Massachusetts | 2,666 | 45.6 | 72.1 | 3.8 | ||
| New Hampshire | 178 | 15.4 | 22.3 | 4.2 | ||
| Rhode Island | 372 | 40.8 | 372.0 | 5.3 | ||
| Vermont | 53 | 9.8 | 3.5 | 6.6 | ||
| South | East South Central | Alabama | 629 | 15.4 | 125.8 | 1.2 |
| Kentucky | 409 | 11.0 | 68.2 | 1.3 | ||
| Mississippi | 255 | 10.3 | 31.9 | 0.6 | ||
| Tennessee | 960 | 17.2 | 64.0 | 1.3 | ||
| South Atlantic | Delaware | 213 | 26.4 | 35.5 | 1.8 | |
| District of Columbia | 1,364 | 232.5 | 113.7 | 4.2 | ||
| Florida | 5,393 | 30.5 | 68.3 | 1.1 | ||
| Georgia | 1,848 | 21.7 | 92.4 | 0.7 | ||
| Maryland | 1,438 | 28.5 | 47.9 | 1.3 | ||
| North Carolina | 1,248 | 14.7 | 32.0 | 0.9 | ||
| South Carolina | 404 | 9.7 | 36.7 | 0.5 | ||
| Virginia | 1,224 | 17.3 | 58.3 | 1.4 | ||
| West Virginia | 165 | 10.6 | 165.0 | 2.5 | ||
| West South Central | Arkansas | 275 | 11.1 | 39.3 | 0.9 | |
| Louisiana | 733 | 18.9 | 26.2 | 0.6 | ||
| Oklahoma | 347 | 10.8 | 69.4 | 1.2 | ||
| Texas | 4,525 | 20.0 | 92.3 | 1.0 | ||
| West | Mountain | Arizona | 1,111 | 19.3 | 58.5 | 1.4 |
| Colorado | 980 | 21.2 | 12.6 | 2.3 | ||
| Idaho | 122 | 8.9 | 17.4 | 2.8 | ||
| Montana | 61 | 6.9 | 15.3 | 3.6 | ||
| Nevada | 497 | 20.3 | 62.1 | 0.9 | ||
| New Mexico | 271 | 15.6 | 16.9 | 2.2 | ||
| Utah | 486 | 20.4 | 54.0 | 3.6 | ||
| Wyoming | 18 | 3.7 | 9.0 | 0.9 | ||
| Pacific | Alaska | 59 | 9.8 | 7.4 | 1.6 | |
| California | 8,974 | 27.4 | 41.0 | 1.8 | ||
| Hawaii | 167 | 13.9 | 27.8 | 2.0 | ||
| Oregon | 880 | 25.3 | 44.0 | 4.0 | ||
| Washington | 2,465 | 40.4 | 19.4 | 5.7 | ||
| Puerto Rico | 30 | 1.0 | 7.5 | 0.1 | ||
denominator used here is total population aged 13 and above in each of the corresponding states
Figure 3.
The prevalence of PrEP users and the PrEP-to-need ratio by state, Q4 2017
To explore the impact of limitations in the dataset, we conducted a sensitivity analysis for the number of national PrEP users in the fourth quarter of 2017 (Table 5). We did this by varying plausible ranges of values for the proportion of unclassified Truvada monotherapy in the SHA database that are used for PrEP and the proportion of Truvada prescriptions that are not captured in the SHA database. This analysis yielded a range of 70,395–140,309 individuals using PrEP in the quarter, with a best estimate of 118,249.
Table 5.
Sensitivity analysis on number of PrEP users nationally in Q4 2017, United States
| Percent of Truvada prescriptions not captured by SHA* | ||||||
|---|---|---|---|---|---|---|
| 0% | 10% | 20% | 25% | 30% | ||
| Percent of unclassified Truvada monotherapy used for PrEP† | 0% | 70,395 | 78217 | 87994 | 93860 | 100564 |
| 25% | 77350 | 85945 | 96688 | 103134 | 110500 | |
| 50% | 84306 | 93673 | 105382 | 112407 | 120436 | |
| 75% | 91261 | 101401 | 114076 | 121681 | 130373 | |
| 87% | 94599 | 105110 | 118249 | 126132 | 135142 | |
| 100% | 98216 | 109129 | 122770 | 130955 | 140309 | |
SHA estimates that 20% of all prescriptions are not included in their database.
In two validaon studies, esmates of Truvada mono therapy that were not used for PrEP were 4% for Chronic Hepatitis B off label use and 9% for Post-Exposure Prophylaxis. The remainder (87%) was used for PrEP.
Bolded values indicate best estimates.
Discussion
This study is the first to present the number of individuals using PrEP in a quarter, defined as ≥ 1 day of prescribed TDF/FTC for PrEP in a three-month period. The national estimate of the number of PrEP users was 70,395 in the fourth quarter of 2017. Our sensitivity analysis had a best point estimate of 118,249. Both figures are less than the estimated 140,000 cumulative PrEP starts since 201213 and considerably higher than the estimated 9,375 total PrEP users in 2014.12 This difference is meaningful because, assuming that individuals on PrEP are CDC-indicated for PrEP, the best estimate resulting from our sensitivity analysis suggests that less than 10% of the 1.2 million individuals indicated for PrEP are potentially receiving protection. The estimates are imperfect: some known limitations in the dataset lead to underestimation, such as the exclusion of some closed health systems, and other limitations lead to overestimation, such as the assumption that individuals prescribed PrEP are those who are indicated for PrEP.
The difference in PrEP use presented here, versus cumulative PrEP starts presented previously, are expected because individuals may discontinue PrEP care over time. Substantial discontinuation of PrEP care has been observed in a number of studies, with differential levels of discontinuation by age, gender, and race.30–35 Data from the present study indicate a need to continue research into discontinuation of PrEP care, including development of a better understanding of potential determinants of discontinuation. It is important to understand whether or not the cessation of PrEP generally corresponds with cessation of periods of epidemiological risk. Further, there is a need to characterize when, and for what reason, individuals cycle into and out of PrEP care.
The prevalence of PrEP use was over an order of magnitude lower for women than for men. Although the HIV epidemic in the US is concentrated among MSM, the PnR is standardized to levels of new HIV diagnoses, and for women is less than one-fifth the PnR for men. This may indicate unmet need in HIV prevention in women, and further analyses exploring this finding in light of PrEP indication estimates will yield useful information. PnRs were also lower among youth aged ≤24 years; addressing PrEP in this group is particularly important because those aged 13–24 accounted for 21% of new HIV diagnoses in 2016.16 Research and programs to effectively bring PrEP to scale among younger and female populations are therefore especially critical.
The South region accounted for nearly 50% of new HIV diagnoses in the US in 2016, but the region had lower prevalence of PrEP use than the Northeast and West and a similar level of prevalence of PrEP use to the Midwest. This results in a PnR for the South that is half of the next lowest region. States in the highest quartiles of the percent of the population living in poverty, percent uninsured, and percent of residents being African American had lower PnR. Viewed through more traditional metrics such as prevalence of PrEP use, the disparity in care access is less clear, indicating utility of the ratio. Multilevel interventions that address areas along the HIV prevention continuum are needed to address disparities in PrEP access,36 particularly in states with less advantaged or more at-risk populations such as those in the South region.
This analysis is subject to a number of limitations. Grouping PrEP users by state policy and state-level demographic quartiles is an ecological assessment, and does not indicate causation. In particular, PrEP may accrue to the ‘worried well;’ individuals with the highest epidemiological risk for HIV transmission may not be those receiving PrEP prescriptions.37–39 Due to the ecological nature of the data, comparisons for PrEP-to-need cannot account for variation across states in the proportion of prescriptions accruing to the ‘worried well,’ or conversely those accruing to individuals at highest risk of transmission. Data on race or other key variables associated with HIV transmission, although also ecological, would provide important information, as would individual-level data on payer. A limitation of PnR is that it is based on HIV diagnoses, and not PrEP indication. This could limit comparisons, such as those made between women and men for PrEP, due to confounding. CDC’s determination of PrEP indication is based on behavioral criteria previously associated with HIV transmission risk, and has been estimated at the national level for some high-risk populations.11 Future assessments should consider including both PnR and PrEP indication.
Estimates of prescribed PrEP may represent a lower-bound estimate for the overall number of PrEP users in the fourth quarter of 2017, and the sensitivity analysis we conducted could provide a more accurate national point estimate. Over one-quarter of all prescriptions in the SHA database for TDF/FTC had insufficient information in the medical claims to classify as either prescriptions for HIV PrEP or for other use, e.g. HIV treatment, and were excluded from this analysis. An assessment of demographic and location data for the unclassified and classified PrEP users’ groups found them to be similar, indicating that there is likely minimal bias for the scope of comparisons made within this analysis. The SHA database does not include closed healthcare systems or health systems that opt to not share their data. Our sensitivity analysis does not address sub-national estimation problems, such as areas with higher reliance on closed healthcare systems being more impacted by underestimation errors than areas without such reliance. For instance, in California where enrollment in closed healthcare systems such as Kaiser Permanente is common, one study combined self-report data and clinic records to estimate higher levels of PrEP prescription in 2016 in San Francisco (12,500)40 than our estimate in the fourth quarter of 2017 for the entire state of California (8,974). Conversely, some factors would result in inflation of the final estimate of PrEP users. Any patient prescribed a single day of PrEP in a quarter would be classified in our dataset as a PrEP user for the period, despite potentially having only a very short period of use. Moreover, classifying an individual filling a PrEP prescription as a ‘PrEP user’ requires an assumption of adherence that is unlikely to apply to all individuals. Despite these limitations, we believe that this analysis provides a meaningful assessment of the current state of PrEP use overall, and by geographical and demographic categories.
Data for PnR were based on new HIV diagnoses, which do not necessarily represent incident HIV cases. For the PnR, the numerator (number of PrEP users) could influence the denominator (new HIV diagnoses). Modeling data through the PRISM Health PrEP Guidelines web modeling tool41 indicate that this impact is likely limited; compared to a baseline scale-up scenario of 10% PrEP coverage, a scale-up scenario of 30% PrEP coverage reduces HIV incidence over a ten-year period by an estimated 25%. The more modest current provision of PrEP therefore has little substantial impact on new HIV diagnoses, but, if PrEP is brought to greater scale, the PnR calculation may benefit from further refinement. Another limitation is that we do not know the underlying HIV transmission risk profiles for individuals using PrEP, and these individuals may not be those at highest or even at substantial risk for HIV transmission.
This study estimates that less than 10% of those behaviorally indicated for PrEP by CDC are potentially receiving PrEP protection, demonstrating a need to scale-up PrEP among all groups and in all regions. PrEP is a relatively new intervention, receiving FDA approval in 2012, and while this estimate of targeted coverage is lower than some previous estimates, it is an encouraging start. To provide more substantive information regarding success of PrEP scale-up programs, future research should investigate PrEP provision at more detailed geographic levels, such as the county, and should include other key demographic variables, such as race/ethnicity, and more nuanced assessments of HIV risk. State-level policies, such as Medicaid expansion and the use of public programs to cover PrEP with minimal access barriers, should be considered as factors that influence TDF/FTC for PrEP uptake. As programs seek to continue the scale-up of PrEP, disparities in coverage documented in this study should be addressed. Specifically, PrEP programs should target young individuals at high risk for HIV transmission, at-risk women of all ages, and the Southern US.
Supplementary Material
Acknowledgments
Funding
This work was supported by the National Institute of Mental Health (R01MH114692), the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN, protocol 159) from the National Institutes of Health (U19HD089881), and by the Emory Center for AIDS Research (P30AI050409). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The 16 states in which Medicaid covers only medically necessary HIV testing (and not general screening) are: Alabama, Arizona, Arkansas, Florida, Georgia, Indiana, Iowa, Maine, Maryland, Michigan, Mississippi, Nebraska, South Carolina, South Dakota, Utah, and Virginia.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. The New England journal of medicine. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanscom B, Janes HE, Guarino PD, et al. Preventing HIV-1 Infection in Women using Oral Pre-Exposure Prophylaxis: A Meta-analysis of Current Evidence. Journal of acquired immune deficiency syndromes (1999) 2016;73(5):606–608. doi: 10.1097/QAI.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. The Lancet. 381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 5.Martin M, Vanichseni S, Suntharasamai P, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. Aids. 2015;29(7):819–824. doi: 10.1097/QAD.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 6.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus JL, Hurley LB, Hare CB, et al. Preexposure Prophylaxis for HIV Prevention in a Large Integrated Health Care System: Adherence, Renal Safety, and Discontinuation. J Acquir Immune Defic Syndr. 2016;73(5):540–546. doi: 10.1097/QAI.0000000000001129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Volk JE, Marcus JL, Phengrasamy T, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;61(10):1601–1603. doi: 10.1093/cid/civ778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. WHO; 2016. p. 429. Available at: http://www.who.int/iris/handle/10665/208825. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. US Public Health Service preexposure prophylaxis for the prevention of HIV infection in the United States–2014 clinical practice guideline. 2014 http://wwwcdcgov/hiv/pdf/prepguidelines2014pdf.
- 11.Smith DK, Van Handel M, Wolitski RJ, et al. Vital Signs: Estimated Percentages and Numbers of Adults with Indications for Preexposure Prophylaxis to Prevent HIV Acquisition--United States, 2015. MMWR Morbidity and mortality weekly report. 2015;64(46):1291–1295. doi: 10.15585/mmwr.mm6446a4. [DOI] [PubMed] [Google Scholar]
- 12.Wu H, Mendoza MCB, Huang Y-lA, Hayes T, Smith DK, Hoover KW. Uptake of HIV Preexposure Prophylaxis Among Commercially Insured Persons—United States, 2010–2014. Clinical Infectious Diseases. 2017;64(2):144–149. doi: 10.1093/cid/ciw701. [DOI] [PubMed] [Google Scholar]
- 13.Giller R, Trevor H, Bush S, Rawlings K, McCallister S. Changes in Truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilisation in the United States:(2012–2016). Paper presented at: International AIDS Society Conference; 2017. [Google Scholar]
- 14.Sullivan PS, Mera Giler R, Mouhanna F, et al. Trends in active prescriptions of emtricitabine/tenofovir disoproxil fumarate for pre-exposure prophylaxis against HIV infections, United States, 2012–2016. Annals of epidemiology. 2018 doi: 10.1016/j.annepidem.2018.06.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mera Giler R, Trevor H, Bush S, Rawlings K, McCallister S. Changes in truvada (TVD) for HIV pre-exposure prophylaxis (PrEP) utilization in the United States:(2012–2016). Paper presented at: 9th International AIDS Society Conference on HIV Science; 2017. [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed February 2, 2018];NCHHSTP AtlasPlus. Updated 2017; https://www.cdc.gov/nchhstp/atlas/index.htm.
- 17.Kaiser Family Foundation. [Accessed February 10, 2018];Current Status of State Medicaid Expansion Decisions. 2018 https://www.kff.org/health-reform/slide/current-status-of-the-medicaid-expansion-decision/
- 18.Foundation. KF. [Accessed February 15, 2018];State Medicaid Coverage of Routine HIV Screening. 2014 https://www.kff.org/hivaids/fact-sheet/state-medicaid-coverage-of-routine-hiv-screening/
- 19.Manson S, Schroeder J, Van Riper D, Ruggles S. IPUMS National Historical Geographic Information System: Version 12.0 [Database] Minneapolis: University of Minnesota; 2017. [Google Scholar]
- 20.United States Census Bureau. [Accessed October 23, 2017];Geography: Centers of population. https://www.census.gov/geo/reference/centersofpop.html.
- 21.Semega JL, Fontenot KR, Kollar MA. Income and poverty in the United States: 2016. Current Population Reports. 2017:10–11. [Google Scholar]
- 22.Barnett JC, Berchick ER. Health insurance coverage in the United States: 2016. US Government Printing Office; 2017. [Google Scholar]
- 23.Siegler AJ, Wirtz S, Weber S, Sullivan PS. Developing a Web-Based Geolocated Directory of HIV Pre-Exposure Prophylaxis-Providing Clinics: The PrEP Locator Protocol and Operating Procedures. JMIR Public Health Surveill. 2017;3(3):e58. doi: 10.2196/publichealth.7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Accessed January 26, 2018];PrEP Locator: Find Your Provider. https://preplocator.org/
- 25.Bratcher A, Wirtz SS, Siegler AJ. Users of a National Directory of PrEP Service Providers: Beliefs, Self-Efficacy, and Progress toward Prescription. J Acquir Immune Defic Syndr. 2018 doi: 10.1097/QAI.0000000000001706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerani RP, Handcock MS, Handsfield HH, Holmes KK. Comparative geographic concentrations of 4 sexually transmitted infections. Am J Public Health. 2005;95(2):324–330. doi: 10.2105/AJPH.2003.029413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chesson HW, Sternberg M, Leichliter JS, Aral SO. Changes in the state-level distribution of primary and secondary syphilis in the USA, 1985–2007. Sex Transm Infect. 2010;86(Suppl 3):iii58–62. doi: 10.1136/sti.2009.040865. [DOI] [PubMed] [Google Scholar]
- 28.Mauguen A, Begg CB. Using the Lorenz Curve to Characterize Risk Predictiveness and Etiologic Heterogeneity. Epidemiology (Cambridge, Mass) 2016;27(4):531–537. doi: 10.1097/EDE.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenz MO. Methods of measuring the concentration of wealth. Publications of the American statistical association. 1905;9(70):209–219. [Google Scholar]
- 30.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. doi: 10.7448/IAS.19.1.20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montgomery MC, Oldenburg CE, Nunn AS, et al. Adherence to Pre-Exposure Prophylaxis for HIV Prevention in a Clinical Setting. PLoS One. 2016;11(6):e0157742. doi: 10.1371/journal.pone.0157742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med. 2016;176(1):75–84. doi: 10.1001/jamainternmed.2015.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosek S, Landovitz R, Rudy B, et al. An HIV pre-exposure prophylaxis (PrEP) demonstration project and safety study for adolescent MSM ages 15–17 in the United States (ATN 113). International AIDS Conference (IAS); 2016; Durban, South Africa. 2016. [Google Scholar]
- 34.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav. 2017 doi: 10.1007/s10461-017-2009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackstock OJ, Patel VV, Felsen U, Park C, Jain S. Pre-exposure prophylaxis prescribing and retention in care among heterosexual women at a community-based comprehensive sexual health clinic. AIDS Care. 2017;29(7):866–869. doi: 10.1080/09540121.2017.1286287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hargreaves JR, Delany-Moretlwe S, Hallett TB, et al. The HIV prevention cascade: integrating theories of epidemiological, behavioural, and social science into programme design and monitoring. The Lancet HIV. 2016;3(7):e318–e322. doi: 10.1016/S2352-3018(16)30063-7. [DOI] [PubMed] [Google Scholar]
- 37.Calabrese SK, Magnus M, Mayer KH, et al. Putting PrEP into practice: lessons learned from early-adopting US providers’ firsthand experiences providing HIV pre-exposure prophylaxis and associated care. PLoS One. 2016;11(6):e0157324. doi: 10.1371/journal.pone.0157324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golub SA, Gamarel KE, Surace A. Demographic differences in PrEP-related stereotypes: implications for implementation. AIDS and Behavior. 2017;21(5):1229–1235. doi: 10.1007/s10461-015-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold EA, Hazelton P, Lane T, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PloS one. 2012;7(7):e40603. doi: 10.1371/journal.pone.0040603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scheer S, Scott H, Marcus J, et al. PrEP Uptake and Demographic Characteristics of PrEP Users in Two Large Medical Systems in San Francisco. Paper presented at: R4P; 2016; Chicago, IL. [Google Scholar]
- 41.Jenness SM, Goodreau SM, Rosenberg E, et al. Impact of the Centers for Disease Control’s HIV Preexposure Prophylaxis Guidelines for Men Who Have Sex With Men in the United States. The Journal of infectious diseases. 2016;214(12):1800–1807. doi: 10.1093/infdis/jiw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.