Abstract
Background
The delivery of monetary incentives contingent on verified abstinence is an effective treatment for alcohol use disorder. However, technological barriers to accurate, frequent biochemical verification of alcohol abstinence have limited the dissemination of this technique.
Methods
In the present randomized parallel trial, we employed a breathalyzer that allows remote, user‐verified collection of a breath alcohol sample, text messaging, and reloadable debit cards for remote delivery of incentives to evaluate a contingency management treatment for alcohol use disorder that can be delivered with no in‐person contact. Treatment‐seeking participants with alcohol use disorder (n = 40) were recruited from the community and randomized to either a contingent or a noncontingent group (n = 20 each). The contingent group received nearly immediate monetary incentives each day they remotely provided negative breathalyzer samples. The noncontingent group received matched monetary payments each day they successfully provided samples independent of alcohol content. Groups were not masked as awareness of group contingencies was an essential intervention component.
Results
The primary outcome of the intent‐to‐treat analyses (analyzed n = 40) was percent days abstinent as measured by the remote breathalyzer samples. Abstinence rates in the contingent group were 85%, which was significantly higher than the 38% recorded in the noncontingent group, corresponding to an odds ratio of 9.4 (95% CI = 4.0 to 22.2). Breathalyzer collection adherence rates were over 95%, and participant ratings of acceptability were also high.
Conclusions
These results support the efficacy, acceptability, and feasibility of this remotely deliverable abstinence reinforcement incentive intervention for the initiation and near‐term maintenance of abstinence from alcohol in adults with alcohol use disorder. Due to low provider and participant burden, this procedure has the potential for broad dissemination.
Keywords: Alcohol Use Disorder, Ecological Momentary Assessment, Contingency Management, Breathalyzer, Incentives
One of every 8 U.S. adults meets criteria for alcohol use disorder at some point in their lifetime, but only 20 to 24% of those individuals ever receive treatment of any kind (Grant et al., 2015; Hasin et al., 2007). Of the top 10 most common reasons cited for this group not seeking treatment, 7 of them (i.e., should be strong enough to handle it alone, was too embarrassed, could not afford it, did not want to go to treatment, hated answering questions, did not think anyone could help, and did not know any place to go) relate to an inability or unwillingness to attend traditional in‐person treatment (Cohen et al., 2007). The pervasiveness of alcohol use disorder indicates a need for continued development of high‐impact treatments that are effective, acceptable to the untreated, and easily disseminated widely.
Contingency management is a highly efficacious treatment approach that reduces drug and alcohol use (Davis et al., 2016; Higgins et al., 2008). In contingency management treatments, tangible incentives (e.g., money, privileges, or prizes) are provided contingent upon verified abstinence from the target drug or drugs. Among adults with alcohol use disorder (Bobova et al., 2009; Mitchell et al., 2005; Petry, 2001), delayed outcomes have relatively little control over behavior. Therefore, immediately available rewards such as those arranged in contingency management interventions are much more likely to promote positive behavior than the health or social gains associated with long durations of abstinence. In a strong set of experiments from multiple laboratories in a variety of settings, contingency management treatments are effective at reducing drug use (Higgins et al., 2008). Under more limited conditions, contingency management has shown effectiveness for alcohol use disorder as well (Alessi and Petry, 2013; Barnett et al., 2011, 2017; Koffarnus et al., 2011; Petry et al., 2000). Contingency management has been shown to be a cost‐effective addition to standard treatment (Sindelar et al., 2007) and has received funding support from governments such as New York City (Riccio et al., 2010). Recently, in response to the evidence showing it as an effective treatment, the Veterans Administration in the United States implemented contingency management treatment for substance use nationwide (Petry et al., 2014). These recent developments show that the growing evidence base for such treatments is persuading governments and healthcare providers to fund and implement these interventions.
Despite the proven effectiveness of contingency management interventions to promote abstinence from alcohol, significant logistical barriers have prevented this procedure from being disseminated. Contingency management interventions require verified biochemical measures of abstinence. The most reliable, noninvasive, and readily accessible biochemical measure of recent alcohol use has been the breathalyzer. However, the rapid elimination rate of alcohol necessitates multiple daily breathalyzer assessments to accurately capture most instances of alcohol use. Outside of an inpatient setting, the requirement for multiple assessments per day presents a significant barrier to treatment delivery. For example, in our past contingency management intervention among adults with alcohol use disorder (Koffarnus et al., 2011), breathalyzer assessments were obtained in the community multiple times per day by study personnel traveling to participants. While this treatment demonstrated a significant decrease in alcohol use, the financial and labor costs of these frequent breathalyzer assessments were substantial; despite great effort, only 60% of the breathalyzer assessments were successfully collected.
Breathalyzers with features that are ideal for remotely monitoring alcohol abstinence while verifying the identity of the user have been developed. For instance, the Soberlink SL2 breathalyzer (Soberlink Healthcare, LLC, Huntington Beach, CA) used in this research contains an integrated cellular radio for data transmission over cellular networks and an integrated camera to photograph the user as s/he supplies the sample. Initial work on the effectiveness and feasibility of remotely delivered contingency management with smartphone‐connected breathalyzers found high acceptance and reduced drinking in the active group compared to participants who were only monitored (Alessi and Petry, 2013). Additionally, research on remote carbon monoxide monitoring to incentivize reductions in cigarette smoking has found that this procedure is effective and well liked by participants (Dallery and Raiff, 2011; Dallery et al., 2013). In these studies, participants used a computer and webcam to upload videos of themselves using a carbon monoxide monitor, which were then used to reinforce abstinence. This allowed the researchers to deliver an effective smoking cessation intervention to rural participants who would have otherwise found it difficult to participate in an intervention of this type (Stoops et al., 2009).
In the present parallel trial, we developed and tested the effectiveness and acceptability of a remotely delivered contingency management intervention to reduce alcohol use with a randomized controlled trial. Multiple technological enhancements were made to standard contingency management protocols to facilitate the treatment approach. We hypothesized that (i) contingent incentives would effectively reduce alcohol use when compared to a control condition balanced for total incentives received, and (ii) the approach would be acceptable to participants.
Materials and Methods
Study Design
This randomized parallel trial was split into 2 phases: a 6‐day monitoring only phase and a 21‐day treatment phase. Participants also completed 3 in‐laboratory assessment sessions which occurred immediately prior to the monitoring only phase, immediately after the treatment phase, and 1 month following the end of the treatment phase.
Participants
Participants were recruited from the community surrounding Roanoke, VA, primarily with postings and advertisements in public places. Eligible participants were at least 18 years of age, met DSM 5 criteria for alcohol use disorder (American Psychiatric Association, 2013), not meet DSM criteria for other substance use disorder (excluding caffeine and nicotine), scored below 23 on the Alcohol Withdrawal Symptom Checklist (Pittman et al., 2007), and expressed a desire to cut down or quit drinking. All participants provided written informed consent, and this protocol was monitored by the Virginia Tech Institutional Review Board and registered with clinicaltrials.gov (NCT03507075).
Monitoring Only Phase
During this phase, participants were asked to provide daily self‐report ecological momentary assessments of previous‐day drinking and current withdrawal symptoms via cell phone text message. This occurred for 6 days with no other study intervention taking place. The purpose for this baseline period was to verify that the alcohol use patterns meet patterns of heavy drinking. At the end of this phase, participants who indicated patterns of at least 2 heavy‐drinking episodes (≥4 drinks in 1 day for women, ≥5 drinks for men) and successfully reported their level of drinking as requested on 5 of the 6 days were invited to continue in the study. Each day, participants were asked to report how many alcoholic drinks they consumed the previous day and their current alcohol withdrawal symptom severity. We asked them about the previous‐day alcohol use instead of the current day to best capture all drinks consumed each day without inconsistent response times complicating the measurement. Participants were allowed to report this information at any time throughout the day but were encouraged through prompts to do so in the morning to increase the likelihood of accurate recall of previous‐day use. Participants received a reminder to report their previous‐day drinking with text messages, followed by a phone call if they did not contact us by early evening. For completing this daily self‐report, participants received a $1 adherence incentive. If a participant reported any withdrawal symptoms (≥2 on a scale from 0 [no symptoms] to 9 [severe symptoms]), research staff called them and administered the Alcohol Withdrawal Symptom Checklist (Pittman et al., 2007). If their score on this assessment indicated clinically significant withdrawal symptoms, they were put in contact with a physician to determine whether any medical intervention is necessary.
Randomization and Masking
At the end of the monitoring only phase, eligible participants were randomly assigned to either the contingent or the noncontingent group (even allocation ratio between groups) with a computerized algorithm that biased the random assignment to balance the groups on current alcohol use (average drinks per day during baseline period), alcohol use history (years of self‐reported heavy drinking), and current use of outside treatment resources (measured with the Treatment Services Review; McLellan et al., 1992a). The first 2 participants to enroll in the study were assigned to the contingent group to accommodate the yoking procedure (see group descriptions below). Participants and research staff were blinded to group assignment during the monitoring only phase (group assignment had not yet occurred at this point), but because knowledge of abstinence reinforcement contingencies and incentive schedule was an integral part of the intervention, they were not masked during the treatment phase. Additionally, at the point of randomization, participants completed a second consent session where they were provided with a detailed explanation of the contingencies relevant to their own group. Participants were not made aware of contingencies for the other group or that another group existed. All other information provided to participants was the same for both groups, which included instruction to consider the first day of the treatment phase as their “quit date.”
Treatment Phase
Both groups were exposed to the same treatment events, but the consequence for breathalyzer screens differed by group. The treatment period consisted of 21 consecutive days with 3 remote breathalyzer screens per day. During this 21‐day period as they did during the baseline period, participants self‐reported their previous‐day alcohol use and current withdrawal symptoms daily in response to a text message and/or phone call. Participants were provided with a prepaid cell phone (if necessary) at the beginning of the study and a breathalyzer at the beginning of the treatment phase.
Breathalyzer Monitoring
Alcohol use during the treatment phase was monitored remotely with thrice‐daily breathalyzer screens with a Soberlink SL2 breathalyzer. During breathalyzer assessments, a picture was automatically taken of the user, which was compared to a reference picture taken at the onset of the treatment phase. The breathalyzer automatically uploaded the breathalyzer results and the picture of the user to a centralized, secure web site where the data were available to research staff. The Soberlink system uses automatic facial recognition technology to verify that the uploaded picture matched a reference picture for that person, which successfully approved 68% of submissions in this study (SD = 20%, minimum = 12%, maximum = 97%). Submissions that included poor lighting or eyeglasses were less likely to be automatically approved. Research staff monitored these results, verified that the picture matched a reference picture for that participant if not approved by automatic facial recognition, and informed the participant via text message of the consequences of the breathalyzer screen. No images were unable to be recognized by either automatic facial recognition or manual recognition by research staff, and text message feedback was sent as soon as possible after submissions were received and typically within a 2‐hour window postsubmission.
All participants completed breathalyzer assessments 3 times per day for 21 days. Participants chose these times each day with guidance from research staff. Participants were asked to choose an assessment time shortly after they usually awaken, shortly before they go to bed at night, and once throughout the day. Chosen times could be between 5:00 am and midnight and had to be separated by at least 6 hours. This ensured that the first and last screens each day were at least 12 hours apart, and the screens were distributed throughout the waking hours. Participants were reminded via text message when a sample was to be collected, and samples were accepted up to 15 minutes before the scheduled time and 60 minutes after the scheduled time. Both groups received a $1 adherence incentive payment for each breathalyzer result submitted within the allowed 75‐minute submission period, regardless of the result of that test. This payment was to encourage participants to complete screens, even if they had consumed alcohol that day.
Contingent Group
Participants in the contingent group earned incentive payments based on the results of the breathalyzer screens. Any breath alcohol concentration reading ≥0.02% or a missed submission was considered a positive indicator of alcohol use. A participant that submitted 3 negative samples in a given day earned an abstinence incentive payment that escalated in value with each day of negative results. The first day a participant recorded all negative samples, s/he received an abstinence incentive payment of $5. For each subsequent day of negative samples, this daily payment increased by $1 to a maximum daily payment of $25 if no alcohol use was recorded for all 21 days. In addition, the participant received a $5 bonus for every third consecutive day of negative samples. A participant who never recorded a positive sample and never missed a screen earned $350 in abstinence incentive payments over 3 weeks. If a positive sample was recorded, the participant received no breath‐sample payments that day other than the $1 adherence incentive for each submitted sample and their escalating pay schedule was reset to the base rate of $5. If, after a positive sample, the participant recorded 3 consecutive days of negative samples, their contingency payment reverted back to the value it was before being reset. This escalating system of payments with bonuses and pay resets for positive samples was based on previous contingency management interventions where it was shown to be more highly effective (Roll and Higgins, 2000; Roll et al., 1996), including studies of alcohol use (Barnett et al., 2011) and remote monitoring of smoking (Dallery and Raiff, 2011).
Noncontingent Group
Like the contingent group, the noncontingent group was required to submit 3 breathalyzer samples per day and received a $1 adherence incentive for each submitted sample. They also received incentive payments, but the payments received were not contingent on their breathalyzer results. Instead, noncontingent participants were yoked to a completed participant in the contingent group and received a payment equal to the payment the contingent participant would have received on that study day if s/he submitted negative samples. This way, both groups experienced the same payment schedule with the same likelihood of pay increases, bonuses, and pay resets, isolating the contingency tying the payments to breathalyzer results for comparison between the groups.
Remote Delivery of Payments
To allow for incentive payments that were both convenient and rapidly available, we delivered payments to participants with reloadable prepaid debit cards through Greephire ClinCard (https://greenphire.com/clincard/). As payments were earned in the course of the study, additional funds were added to the account for that participant. Funds were immediately available when added and research staff sent participants a text message notifying them of payments when they were added.
Assessment Sessions
Assessment sessions included various behavioral assessments and questionnaires related to alcohol use and associated cognitive processes. The Timeline Followback (TLFB) assessment (Sobell and Sobell, 1992) was used to assess daily drinking quantity for the 30 days preceding each assessment session. The Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993) was used to assess alcohol use disorder risk factors, the Treatment Services Review (McLellan et al., 1992a) assessed use of treatment resources including professional counseling and attendance at groups such as Alcoholics Anonymous, and the Addiction Severity Index‐Lite (ASI‐Lite) (McLellan et al., 1992b) assessed medical, legal, employment, psychiatric, and social factors related to substance use.
Outcome Variables and Data Analyses
The primary measure of alcohol use during the intervention period was alcohol use measured by the Soberlink breathalyzer 3 times per day. This outcome was coded as a dichotomous variable once per day as either positive (at least 1 positive screen throughout the day), negative (all 3 screens submitted on time and negative), or missing (at least 1 missing screen with any submitted screens recorded as negative). This variable was also analyzed with any missing samples treated as positive. Data were analyzed in a generalized logistic model with main effects of group and study day and an autoregressive(1) working correlation matrix. Generalized estimating equations (GEE) were used to accommodate intrasubject correlation inherent in repeated measurements (Liang and Zeger, 1986). As a secondary measure of alcohol use, we also analyzed the daily self‐reports of previous‐day drinks. These data were analyzed as above with generalized linear regression. Additional variables measured with generalized linear or logistic regression as above include breathalyzer adherence (missing vs. submitted) and daily self‐reported withdrawal symptoms on a 0 to 9 scale.
Additional measures of alcohol use were collected during the 3 assessment sessions. The TLFB assessment (mean drinks per day) and AUDIT score were each compared across assessment session, group, and for a session by group interaction in generalized linear or logistic regression using GEE. Measures of acceptability and effectiveness at the posttreatment assessment session were compared between groups with multivariate general linear regression, and participant characteristics were compared between groups with independent t‐tests or Fisher's exact tests as appropriate. All analyses were conducted in SPSS 24 (IBM Analytics, Armonk, NY) and Prism 7 (GraphPad Software, La Jolla, CA) with alpha set at 0.05.
Results
A total of 69 participants were recruited from the community from November 10, 2014 to January 31, 2017. Eighteen were excluded after the initial assessment session, most for failing to meet inclusion criteria (Fig. 1). An additional 11 participants were excluded for failing to meet drinking criteria during the monitoring phase (see below), leaving 40 participants randomized into a study arm and included in final data analyses. This sample size was determined with a power analysis based on previous work (80% power) (Koffarnus et al., 2011). Participant characteristics for randomized participants are shown in Table 1. No group differences were detected on any characteristic variable including gender, race, age, monthly income, AUDIT score, drinks per day as measure by the TLFB, Treatment Services Review score, or years of heavy drinking as assessed by the ASI‐Lite. Along with drinks per day during the monitoring only phase (see below), Treatment Services Review score and years of heavy drinking were used as group stratification variables.
Figure 1.
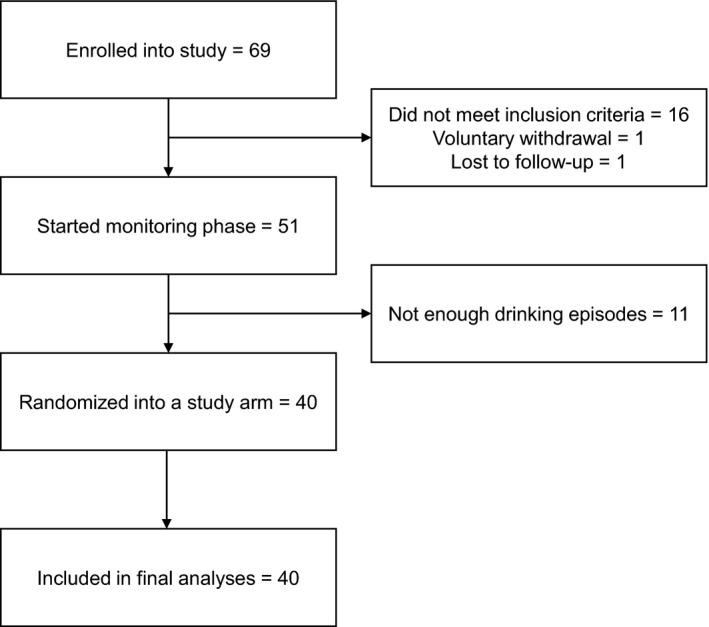
Reasons that participants were excluded or dropped out after initial enrollment.
Table 1.
Participant Characteristics
| Contingent (n = 20) | Noncontingent (n = 20) | Statistic | |
|---|---|---|---|
| Gender | 7 female, 13 male | 5 female, 15 male | Fisher's exact p = 0.7 |
| Race | 4 African American, 16 White | 1 African American, 19 White | Fisher's exact p = 0.3 |
| Age | 46.6 (SD = 12.5) | 45.2 (SD = 11.5) | t(38) = 0.37, p = 0.7 |
| Monthly Income | US$2,790 (SD = US$2,260; median = US$2,250; IQR = US$763 to US$4,375) | US$2,694 (SD = US$2,337; median = US$2,125; IQR = US$1,400 to US$2,725) | t(38) = 0.13, p = 0.9 |
| AUDIT | 24.2 (SD = 7.3) | 24.1 (SD = 5.9) | t(38) = 0.05, p > 0.9 |
| Drinks per day (TLFB) | 6.5 (SD = 2.8) | 5.8 (SD = 4.0) | t(38) = 0.59, p = 0.5 |
| Treatment Services Review alcohol score | 0.9 (SD = 1.0) | 1.0 (SD = 2.7) | t(38) = 0.39, p = 0.9 |
| Years of heavy drinking (ASI‐Lite) | 21.1 (SD = 10.0) | 20.7 (SD = 10.9) | t(38) = 0.38, p = 0.9 |
Drinks per day is a 30‐day average preceding the consent session from the Timeline Follow‐Back (TLFB) assessment. Years of heavy drinking was assessed within the Addiction Severity Index‐Lite (ASI‐Lite).
Throughout the 21‐day treatment phase, participants were asked to submit 3 remote breathalyzer assessments per day. The primary abstinence outcome was the percent days abstinent from these breathalyzer submissions (i.e., all 3 samples were submitted on time and were negative for alcohol). Mean percent days abstinent reached 85% in the contingent group, which was significantly higher than the 38% in the noncontingent group (Fig. 2 top; χ2 = 26.34, p < 0.0001). This difference corresponds to an odds ratio of 9.4 (95% CI = 4.0 to 22.2). No main effect of treatment day on daily abstinence was observed (χ2 = 0.89, p = 0.4). Results were similar if all missing samples were considered positive, with a significant effect of group (χ2 = 22.63, p < 0.0001) and no significant effect of treatment day (χ2 = 2.42, p = 0.1).
Figure 2.
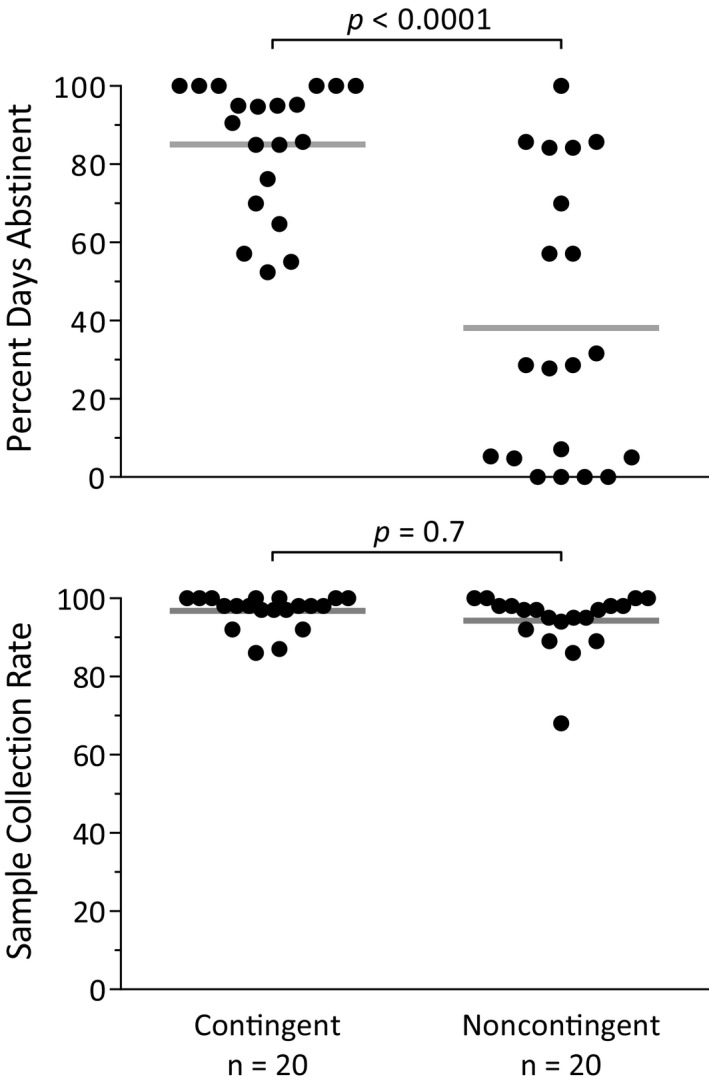
Abstinence and collection rate results from the thrice‐daily remote breathalyzer assessments during the treatment phase. Percent days abstinent was significantly higher in the contingent group (top), and the collection rate was similarly high in both groups (bottom).
One of the primary goals of this study was to establish the feasibility of remotely assessing abstinence from alcohol, with our primary measure of this being adherence to the scheduled breathalyzer assessments. Adherence was high in both groups, with an overall sample collection rate of 95.6% and all but 1 participant having greater than an 80% collection rate (Fig. 2 bottom). Collection rate did not differ between groups (χ2 = 0.20, p = 0.7), but a significant tendency for collection rate to decrease as the study progressed was detected (χ2 = 10.91, p = 0.001; odds ratio = 1.07 [95% CI = 1.03 to 1.11]).
Previous‐day drinks per day and withdrawal symptoms rated on a 0 to 9 scale were collected daily via text message prompts sent to each participant. The effects of treatment group (contingent vs. noncontingent), phase (monitoring only vs. treatment), study day, and the interaction of group and phase were assessed with each of these self‐reported measures with general linear regression and GEE to control for repeated measurements. On drinks per day (Fig. 3 top), an overall main effect of group (χ2 = 6.93, p = 0.008), a main effect of phase (χ2 = 31.15, p < 0.0001), as well as a significant group by phase interaction (χ2 = 8.23, p = 0.004) were observed. Pairwise comparisons revealed a significant difference between the groups in the treatment phase only (p = 0.001) with no difference in the monitoring phase (p = 0.6) and no main effect of study day (χ2 = 0.08, p = 0.9).
Figure 3.
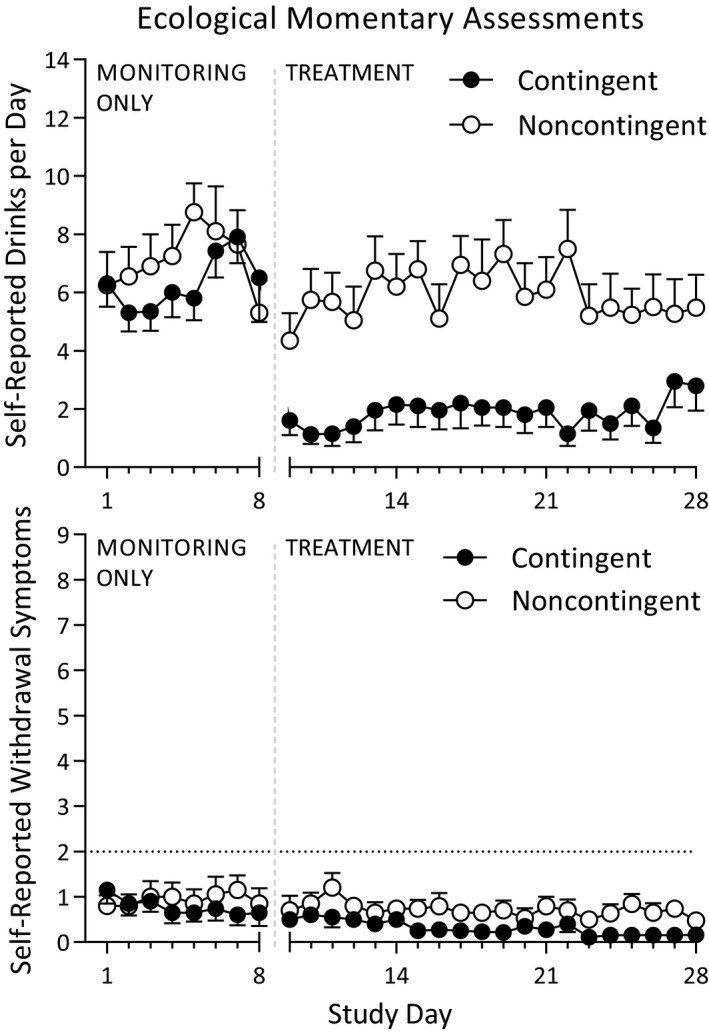
Ecological momentary assessments of drinks per day and withdrawal symptoms collected throughout the monitoring only and treatment phases. Drinks per day were significantly lower in the contingent group during the treatment phase only (top), and withdrawal symptoms were similarly low throughout the study in both groups (bottom).
Daily reports of previous‐day withdrawal symptoms indicated minimal withdrawal symptoms throughout the study in both groups (Fig. 3 bottom). No main effect of group (χ2 = 1.18, p = 0.3) or phase (χ2 = 1.18, p = 0.3) was detected, nor was there a group by phase interaction (χ2 = 2.53, p = 0.1). A significant effect of study day indicated that self‐reported withdrawal symptoms lessened over the course of the study independent of groups and phases (χ2 = 10.93, p = 0.0009).
Three measures of alcohol use and dependence were collected at each of the 3 monthly assessment sessions. The TLFB (Fig. 4 left) contained retrospective recall of alcohol use prior to the study consent session, during the 21 active‐intervention days (collected at the end of treatment assessment session) and for 1 month following the intervention (collected at a 1‐month follow‐up session). Average drinks per day were similar between the groups prior to study consent, but diverged during the study and throughout the 1‐month follow‐up period. This pattern resulted in an overall effect of treatment group on average drinks per day (χ2 = 4.05, p = 0.04), a significant main effect of session (χ2 = 43.50, p < 0.0001), and a significant group by session interaction (χ2 = 14.24, p = 0.0008). Holm–Šidák post hoc tests revealed no difference prior to consent (p = 0.5), but fewer drinks per day in the contingent group both during the study (p = 0.001) and posttreatment (p = 0.04).
Figure 4.
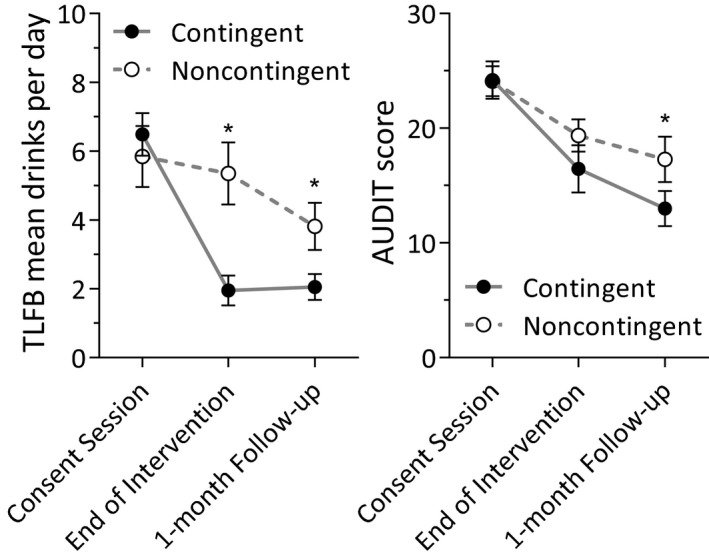
Drinks per day prior to, during, and after the treatment as assessed by Timeline Follow back assessments administered at the study consent session, immediately after the treatment phase, and at the 1‐month follow‐up session, respectively (left). Significant group differences emerged at the end of treatment and 1‐month follow‐up assessments. Alcohol use disorder severity was assessed with AUDIT scores at each assessment session (right). Significant differences emerged by the 1‐month follow‐up session. *Significant group difference (p < 0.05).
Symptoms of alcohol use disorder were assessed with the AUDIT at each assessment session (Fig. 4 right). AUDIT scores were similar between groups at the consent session, but differences emerged as the study progressed. This pattern resulted in an overall main effect of group that approached significance (χ2 = 3.73, p = 0.053), a main effect of assessment session (χ2 = 52.28, p < 0.0001), and a significant group by session interaction (χ2 = 8.28, p = 0.02). Group differences were not significant at the consent session (p > 0.9) and the end of intervention (p = 0.3), but reached significance at the 1‐month follow‐up (p = 0.003).
Participants completed a custom questionnaire with questions grouped into the categories of overall satisfaction, treatment effectiveness, and the ease of use of treatment components (Fig. 5). Both groups were satisfied overall with the approach, with the contingent group scoring rating the treatment significantly higher in their likelihood of recommending the treatment to someone else, F(1, 37) = 5.3, p = 0.03, and in the category overall, F(1, 37) = 4.5, p = 0.04. In the treatment effectiveness category of questions, the noncontingent group rated the components approximately “somewhat” effective, with the contingent group giving ratings significantly higher for payment helpfulness, F(1, 37) = 6.0, p = 0.02, and marginally higher for the overall category, F(1, 37) = 3.8, p = 0.06. Participants rated the various components of the treatment approach between “somewhat easy” and “very easy” with no significant differences between the groups for any question.
Figure 5.
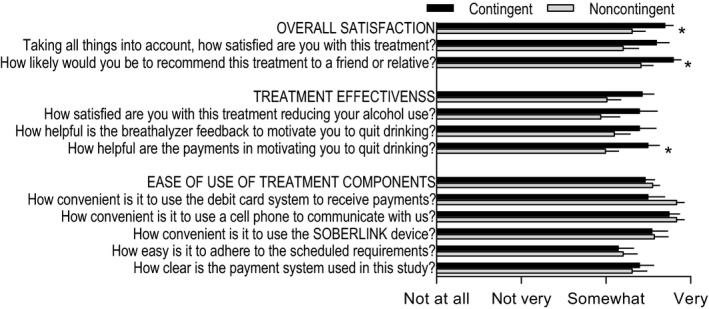
Ratings were measured across 3 general categories: overall satisfaction, treatment effectiveness, and ease of use of treatment components. *Significant group difference (p < 0.05).
Discussion
The first goal of this feasibility study was to assess the efficacy of this adaptation of the contingency management approach for remote delivery. Alcohol use as measured by thrice‐daily remote breathalyzer assessments (Fig. 2), daily ecological momentary assessment self‐reports of drinks per day (Fig. 3), and TLFB assessments completed at monthly intervals (Fig. 4 left) all showed a large treatment effect of the contingent incentives. Additionally, contingent incentives also produced a significant treatment effect on AUDIT scores at the 1‐month follow‐up session, indicating a reduction in alcohol‐associated problems in addition to the measured reductions in drinking. We imposed minimal exclusion criteria to increase the representativeness of a community population interested in reducing their alcohol use and therefore increase the generalizability of these results. These results corroborate and expand upon previous findings with remotely monitoring of alcohol coupled with contingent incentives (Alessi and Petry, 2013).
Breathalyzer assessment adherence rate and incidence of withdrawal symptoms constituted our primary measure of feasibility as the successful dissemination of this procedure would require assessments to be valid and safe. Feasibility data were encouraging with high breathalyzer assessment adherence in both groups (Fig. 2) and incidences of withdrawal symptoms (Fig. 3) were rare and well managed. The obtained adherence rate of >95% compares favorably to a 60% rate in our previous work (Koffarnus et al., 2011) that relied on community‐based in‐person breathalyzer assessments.
Participant ratings of acceptability were assessed because successful dissemination of this procedure will rely on potential patients agreeing to comply with the procedures. Acceptability ratings were assessed with a custom questionnaire containing questions split into 3 broad categories (Fig. 5). Ratings of satisfaction, effectiveness, and ease of use were high in both groups, indicating that this treatment was acceptable among the sample obtained for this experiment.
This combination of high efficacy, high adherence rate, and positive ratings of acceptability implies a strong clinical benefit of this approach among adults in the community with alcohol use disorder. However, the high rate of biochemically verified abstinence we observed also has implications for the use of this procedure in experimental contexts. Experimental questions that necessitate the production of a period of abstinence from alcohol may use this procedure to manipulate level of abstinence with a high degree of experimental control.
A modest sample size may limit the generalizability of the present work to dissimilar groups of alcohol users. This may be most acutely true for participants who are less compliant with instructions, as we only randomized participants who were compliant with daily text message requests on 5 of 7 days in the monitoring only phase. However, this limitation is mitigated by the robust effect size that corroborates decades of work demonstrating that contingent incentives, when delivered in a timely manner and contingently on biochemical measures of abstinence, effectively reduce substance use (Davis et al., 2016; Higgins et al., 2008; Lussier et al., 2006; Prendergast et al., 2006). Furthermore, a large effect size in a smaller number of participants may be more useful when making clinical recommendations to individual patients than a small effect size in a large sample (Williams, 2010). The major step forward with this work is the verification that this beneficial effect holds true for alcohol use when technological tools allow for the frequent, unobtrusive measurement of alcohol use in the community and the rapid delivery of incentive payments with reloadable debit cards.
In this study, we chose to use static timing of breathalyzer assessment to increase adherence, even though this predictability increased the possibility of using small amounts of alcohol undetected. Some participants reported using some alcohol undetected by the breathalyzer assessments, even though we scheduled the assessments throughout the waking hours and included 1 breathalyzer assessment late in the evening just prior to the participant's typical bedtime. Furthermore, reductions in self‐report measures of drinking and AUDIT scores corresponded to a greater incidence of negative breathalyzer screens in the contingent group, suggesting that any undetected drinking that occurred still represented a significant and clinically relevant reduction in the contingent group compared to the noncontingent group.
Transdermal alcohol sensing devices have been piloted in a contingency management framework to facilitate the measurement of alcohol use (Barnett et al., 2011, 2017; Dougherty et al., 2014). This class of devices holds great potential, but there are several limitations of the currently available devices. To our knowledge, the only transdermal device to be evaluated in a contingency management framework is the Secure Continuous Remote Alcohol Monitor bracelet. While many individuals endorse willingness to use this device for therapeutic purposes, high cost and other barriers to adoption do exist (Alessi et al., 2017). Technological barriers include the need to tie into either a landline or a hardwired Ethernet port. Additionally, some participants are reluctant to use the devices due to social stigma associated with the constant wearing of an alcohol detection anklet associated with criminal justice, due to prohibitive work environments that preclude the operation of the device, or physical discomfort. Rapid improvements in technology hold promise as less obtrusive devices are under development that more closely resemble that of a FitBit or activity‐tracking device (Leffingwell et al., 2013). The effectiveness and acceptability of the current protocol suggest that an incentive intervention with transdermal alcohol sensors could be a promising approach with even less participant burden.
Contingent incentives are highly effective at promoting behavior change but were difficult to conduct with alcohol use as a target due to difficulty in monitoring and verifying abstinence from alcohol use. Through the use of a technologically advanced breathalyzer and remote incentive payments, the present study has provided a framework for effective delivery of a contingency management intervention to promote alcohol abstinence in adults with alcohol use disorder. This intervention has the potential for wide dissemination due to low participant and provider burden.
Authors Contributions
MNK designed the experiment, collected the data, analyzed the data, interpreted the data, and drafted the manuscript. WKB and ASK assisted with study design and data collection. All authors read and approved the final version of the manuscript.
Declaration of Interests
MNK and ASK have nothing to disclose. WKB is a principal of HealthSim, LLC and Notifius, LLC; a scientific advisory board member of Sober Grid, Inc. and DxRx, Inc.; and a consultant for ProPhase, LLC and Teva Branded Pharmaceutical Products R&D, Inc. None of these companies or their products were involved in the research presented here.
Acknowledgments
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under award number R21 AA022727 to MNK. 100% of this research was supported by federal money with no financial or nonfinancial support from nongovernmental sources. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funding source did not have a role in writing this manuscript or in the decision to submit it for publication. All authors had full access to the data in this study, and the corresponding author had final responsibility for the decision to submit these data for publication.
References
- Alessi SM, Barnett NP, Petry NM (2017) Experiences with SCRAMx alcohol monitoring technology in 100 alcohol treatment outpatients. Drug Alcohol Depend 178:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi SM, Petry NM (2013) A randomized study of cellphone technology to reinforce alcohol abstinence in the natural environment. Addiction 108:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn American Psychiatric Publishing, Arlington, VA. [Google Scholar]
- Barnett NP, Celio MA, Tidey JW, Murphy JG, Colby SM, Swift RM (2017) A preliminary randomized controlled trial of contingency management for alcohol use reduction using a transdermal alcohol sensor. Addiction 112:1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett NP, Tidey J, Murphy JG, Swift R, Colby SM (2011) Contingency management for alcohol use reduction: a pilot study using a transdermal alcohol sensor. Drug Alcohol Depend 118:391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J (2009) Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol 17:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E, Feinn R, Arias A, Kranzler HR (2007) Alcohol treatment utilization: findings from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug Alcohol Depend 86:214–221. [DOI] [PubMed] [Google Scholar]
- Dallery J, Raiff BR (2011) Contingency management in the 21st century: technological innovations to promote smoking cessation. Subst Use Misuse 46:10–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallery J, Raiff BR, Grabinski MJ (2013) Internet‐based contingency management to promote smoking cessation: a randomized controlled study. J Appl Behav Anal 46:750–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST (2016) A review of the literature on contingency management in the treatment of substance use disorders, 2009–2014. Prev Med 92:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Hill‐Kapturczak N, Liang Y, Karns TE, Cates SE, Lake SL, Mullen J, Roache JD (2014) Use of continuous transdermal alcohol monitoring during a contingency management procedure to reduce excessive alcohol use. Drug Alcohol Depend 142:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B (2015) Epidemiology of DSM‐5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry 72:757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF (2007) Prevalence, correlates, disability, and comorbidity of DSM‐IV alcohol abuse and dependence in the United States – Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 64:830–842. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH (2008) Contingency Management in Substance Abuse Treatment Guilford Press, New York, NY. [Google Scholar]
- Koffarnus MN, Wong CJ, Diemer K, Needham M, Hampton J, Fingerhood M, Svikis DS, Bigelow GE, Silverman K (2011) A randomized clinical trial of a Therapeutic Workplace for chronically unemployed, homeless, alcohol‐dependent adults. Alcohol Alcohol 46:561–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffingwell TR, Cooney NJ, Murphy JG, Luczak S, Rosen G, Dougherty DM, Barnett NP (2013) Continuous objective monitoring of alcohol use: twenty‐first century measurement using transdermal sensors. Alcohol Clin Exp Res 37:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KY, Zeger SL (1986) Longitudinal data‐analysis using generalized linear‐models. Biometrika 73:13–22. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST (2006) A meta‐analysis of voucher‐based reinforcement therapy for substance use disorders. Addiction 101:192–203. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, Obrien CP (1992a) A new measure of substance‐abuse treatment – initial studies of the treatment services review. J Nerv Ment Dis 180:101–110. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M (1992b) The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat 9:199–213. [DOI] [PubMed] [Google Scholar]
- Mitchell JM, Fields HL, D'Esposito M, Boettiger CA (2005) Impulsive responding in alcoholics. Alcohol Clin Exp Res 29:2158–2169. [DOI] [PubMed] [Google Scholar]
- Petry NM (2001) Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology 154:243–250. [DOI] [PubMed] [Google Scholar]
- Petry NM, DePhilippis D, Rash CJ, Drapkin M, McKay JR (2014) Nationwide dissemination of contingency management: the Veterans Administration initiative. Am J Addict 23:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Martin B, Cooney JL, Kranzler HR (2000) Give them prizes, and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol 68:250–257. [DOI] [PubMed] [Google Scholar]
- Pittman B, Gueorguieva R, Krupitsky E, Rudenko AA, Flannery BA, Krystal JH (2007) Multidimensionality of the alcohol withdrawal symptom checklist: a factor analysis of the alcohol withdrawal symptom checklist and CIWA‐Ar. Alcohol Clin Exp Res 31:612–618. [DOI] [PubMed] [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J (2006) Contingency management for treatment of substance use disorders: a meta‐analysis. Addiction 101:1546–1560. [DOI] [PubMed] [Google Scholar]
- Riccio JA, Dechausay N, Greenberg DM, Miller C, Rucks Z, Verma N (2010) Toward reduced poverty across generations: early findings from New York City's conditional cash transfer program. MDRC, March 2010. Available at: https://ssrn.com/abstract=1786981. Accessed March 16, 2011. [Google Scholar]
- Roll JM, Higgins ST (2000) A within‐subject comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. Drug Alcohol Depend 58:103–109. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Badger GJ (1996) An experimental comparison of three different schedules of reinforcement of drug abstinence using cigarette smoking as an exemplar. J Appl Behav Anal 29:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption–II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Sindelar J, Elbel B, Petry NM (2007) What do we get for our money? Cost‐effectiveness of adding contingency management. Addiction 102:309–316. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) Timeline follow‐back: a technique for assessing self‐reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods (Litten RZ, Allen JP. eds), pp 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Stoops WW, Dallery J, Fields NM, Nuzzo PA, Schoenberg NE, Martin CA, Casey B, Wong CJ (2009) An Internet‐based abstinence reinforcement smoking cessation intervention in rural smokers. Drug Alcohol Depend 105:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BA (2010) Perils of evidence‐based medicine. Perspect Biol Med 53:106–120. [DOI] [PubMed] [Google Scholar]


