Abstract
Stress can precipitate or worsen symptoms of many psychiatric illnesses. Dysregulation of the prefrontal cortex (PFC) glutamate system may underlie these disruptions and restoring PFC glutamate signaling has emerged as a promising avenue for the treatment of stress disorders. Recently, we demonstrated that activation of metabotropic glutamate receptor subtype 3 (mGlu3) induces a postsynaptic form of long-term depression (LTD) that is dependent on the activity of another subtype, mGlu5. Stress exposure disrupted this plasticity, but the underlying signaling mechanisms and involvement in higher-order cognition have not yet been investigated. Acute stress was applied by 20-minutes restraint and early reversal learning was evaluated in an operant-based food-seeking task. We employed whole-cell patch-clamp recordings of layer 5 prelimbic (PL)-PFC pyramidal cells to examine mGlu3-LTD and several mechanistically distinct mGlu5-dependent functions. Acute stress impaired both mGlu3-LTD and early reversal learning. Interestingly, potentiating mGlu5 signaling with the mGlu5 positive allosteric modulator (PAM) VU0409551 rescued stress-induced deficits in both mGlu3-LTD and reversal learning. Other aspects of PL-PFC mGlu5 function were not disrupted following stress; however, signaling downstream of mGlu5-Homer interactions, phosphoinositide-3-kinase (PI3K), Akt, and glycogen synthase kinase 3β was implicated in these phenomena. These findings demonstrate that acute stress disrupts early reversal learning and PL-PFC-dependent synaptic plasticity and that potentiating mGlu5 function can restore these impairments. These findings provide a framework through which modulating coordinated mGlu3/mGlu5 signaling may confer benefits for the treatment of stress-related psychiatric disorders.
Keywords: stress, prelimbic prefrontal cortex, mGlu3, mGlu5, synaptic plasticity, reversal learning
1. Introduction
Stress-related psychiatric disorders pervade modern society. Acute stressors precipitate adaptive and maladaptive behavioral responses, the latter of which include the generation or exacerbation of symptoms of many diseases, including major depressive disorder, schizophrenia, and substance use disorders (Arnsten et al., 2017). While stress exerts pleiotropic actions throughout the central nervous system, many changes in motivation, decision-making, and executive function are thought to stem from disruptions in the function of the prefrontal cortex (PFC) (Holmes and Wellman, 2009).
The strength of excitatory synaptic transmission in the neocortex is dynamically regulated by multiple mechanistically and functionally distinct forms of synaptic plasticity (Bear and Malenka, 1994). We have recently characterized one form of plasticity whereby prelimbic (PL)-PFC synaptic strength undergoes a long-term depression (LTD) of excitatory transmission following activation of metabotropic glutamate (mGlu) receptor subtype 3 (mGlu3) (Joffe et al., 2017; Walker et al., 2015). mGlu3 is a promising target for the treatment of psychiatric disorders with underlying cognitive deficits, and modulating mGlu3 function can confer pro-cognitive and antidepressant-like effects in animal models (Engers et al., 2017; Jin et al., 2018). Moreover, polymorphisms in the gene encoding mGlu3 (GRM3) have been linked to poor cognitive performance in both schizophrenia patients and neurotypical controls (Egan et al., 2004; Harrison et al., 2008). Unlike canonical forms of plasticity mediated by mGlu3 or the closely related subtype mGlu2, PL-PFC mGlu3-LTD proceeds through a postsynaptic mechanism involving the internalization of AMPA receptors (Joffe et al., 2017). Remarkably, this LTD of excitatory synaptic transmission is impaired after a single exposure to restraint stress. Therefore, manipulations that restore this pathophysiological change in plasticity could provide insight towards novel approaches to ameliorating stress-induced deficits in cognition.
One potential therapeutic target to mitigate stress-induced impairments in mGlu3 signaling is the mGlu receptor subtype 5 (mGlu5), as we recently demonstrated that mGlu3 potentiates the function of mGlu5 in the PL-PFC, hippocampus, and striatum (Di Menna et al., 2018). In addition, we found that PL-PFC mGlu3-LTD requires co-activation of mGlu3 and mGlu5 and is blocked by the mGlu5 negative allosteric modulator (NAM) MTEP. Based on these findings, we reasoned that disruptions in the crosstalk between mGlu3 and mGlu5 might underlie stress-induced deficits in this unique form of mGlu3/mGlu5-dependent LTD in the PL-PFC, and this could participate in some stress-induced cognitive deficits. Here, we tested this hypothesis and further investigated the molecular mechanisms required for PL-PFC mGlu3/mGlu5-dependent LTD.
As reported previously, exposure to a single restraint stress episode impaired the induction of PL-PFC mGlu3/mGlu5-LTD. We now report the surprising finding that a highly selective mGlu5 positive allosteric modulator (PAM) completely rescues the deficit in mGlu3/mGlu5-dependent LTD observed following exposure to restraint stress. mGlu5 can signal through both mobilization of intracellular Ca2+ and activation of the phosphoinositide-3-kinase (PI3K)/Akt signaling pathway. We therefore performed additional studies to interrogate the mechanisms of mGlu3/mGlu5-LTD to identify candidate players underlying the stress-induced disruptions. Our studies suggest that mGlu3/mGlu5-LTD is not dependent on mobilization of intracellular Ca2+ but does require activation of the PI3K/Akt signaling pathway. Furthermore, we identified a role for Homer-mGlu5 interactions and glycogen synthase kinase 3β (GSK3β) in mGlu3/mGlu5-LTD. Finally, we assessed the behavioral ramifications of this stress exposure and observed an impairment in early reversal learning in an operant task. Interestingly, in addition to reversing the stress-induced deficit in mGlu3/mGlu5 LTD, the mGlu5 PAM rescued the effect of stress on reversal learning. Together, these data illuminate a novel behavior and molecular pathway involved in mGlu3 signaling and provide a framework in which modulating the function of mGlu3-mGlu5 crosstalk may provide a therapeutic benefit for the treatment of stress-related psychiatric disorders.
2. Material and Methods
2.1. Animals.
Adult (>8-week), male, C57Bl6/J mice (Jackson, Bar Harbor, ME, U.S.A.) were used for all experiments. Mice were group-housed (2–5 per cage) on a 12-hour light cycle (lights on at 6:00 am). Food and water were available ad libitum. All experimental protocols were approved by the Vanderbilt Institutional Animal Care and Use Committee. Some animals underwent a single, 20-minute exposure to restraint stress on the day of experimentation. Stress was induced by 20-minutes restraint in a soft plastic cone. For behavioral studies, mice underwent restraint stress simultaneously 60 minutes prior to the experiment, in the testing room. For electrophysiology studies, mice were individually stressed 30 minutes prior to sacrifice, the timepoint of mGlu5 PAM administration in the reversal learning experiment, in a separate room near the slice preparation area.
2.2. Whole-cell electrophysiology.
Brain slices were prepared and recordings were made as described (Di Menna et al., 2018; Joffe and Grueter, 2016). Briefly, mice were anesthetized with isoflurane and decapitated for acute slice preparation. Coronal slices (300 μm) were prepared using an NMDG-based cutting/recovery solution. The artificial cerebrospinal fluid (aCSF) contained (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgCl2, 1 NaH2PO4, 11 glucose, and 26 NaHCO3. The recording chamber was perfused with warm (30 ± 1 °C), oxygenated (95% O2 / 5% CO2) aCSF at 2 mL/min. Layer 5 PL-PFC neurons were filled with a K-based solution (in mM): 125 K-gluconate, 4 NaCl, 10 HEPES, 4 MgATP, 0.3 NaGTP, 10 Trisphosphocreatine. Local glutamate release was evoked with electrical stimulation (0.1 Hz, 0.1–0.15 ms, and 5–50 μA) via a concentric bipolar electrode placed in layer 5. Recordings were made at −70 mV unless otherwise noted. mGlu3-dependent LTD was induced by applying LY379268 to the bath for 3 (threshold) or 10 (maximal) minutes (Di Menna et al., 2018). Inhibitors were bath-applied for 5–10 minutes prior to LY379268 wash-on and were removed 5 minutes after wash-out. For postsynaptic loading experiments, cells were dialyzed for 20–30 minutes prior to LY379268 wash-on. mGlu5-dependent muscarinic LTD was induced by applying 100 μM carbachol for 5 minutes (Ghoshal et al., 2017). mGlu5-mediated inward currents were obtained using DHPG in the presence of tetrodotoxin (TTX) and the mGlu1 NAM VU0469650 (Lovell et al., 2013). The function of small conductance Ca2+-activated potassium (SK) channels was evaluated in TTX and VU0469650 by measuring the charge transfer of the afterhyperpolarization (QAHP) – the area under the curve following a 400-ms depolarizing step from −50 mV to (−40, −20, 0, or +20 mV) and then back to −50 mV. mGlu5-mediated inhibition of (SK) channels was assessed after 5-minute bath application of DHPG by measuring the relative change in charge transfer after a voltage step to +20 mV.
2.3. Reversal learning task.
Mice were trained to respond for a liquid reinforcer (33% Strawberry Ensure, 20 μL) in a standard operant conditioning chamber with two holepokes and a feeding device as described (Gould et al., 2015). Mice were trained to respond on a single holepoke operandi, counterbalanced across subjects, on a continuous schedule of reinforcement. The liquid reinforcer was available for 5 seconds, during which the house lights were turned off and holepoke responses were recorded but had no consequence. The task reset after this period. No cue lights were ever used to denote the active operandi. Sessions were terminated after one hour or 50 reinforcers. Mice underwent food-restriction for <3 days of training, after which they were fed ad libitum for the remainder of the study. After obtaining stable performance (≥35 reinforcers/session and ≥80% active holepoke responding) for 3–4 consecutive days, mice underwent a reversal session, where the active holepoke was switched to the opposite side. Correction trials were not included and there was no punishment for incorrect responses. The average training required to initially reach criteria was 12.5 ± 0.9 sessions, N = 35. Training history was counterbalanced across treatment groups. Stressed mice underwent 20-minutes restraint, once, one-hour prior to the reversal session, and VU0409551 (30 mg/kg, intraperitoneal, 10% v/v Tween-80/saline vehicle at 10 μL/g) was administered 30-min prior to the session start. The route of administration and dose was selected to engage central mGlu5 throughout the duration of the reversal session (tmax = 30 mins, t1/2 = 1.6 hr) (Conde-Ceide et al., 2015). Treatment assignments were counterbalanced across cohorts.
2.4. Drugs.
LY379268 (200 nM), (S)-DHPG (100 μM), and TTX (500 nM) were purchased from Abcam (Cambridge, U.K.). EGTA (20 mM) and BAPTA (20 mM) were purchased from Sigma. Carbachol (100 μM), apamin (100 nM), LY294002 (20 μM), Akti-1/2 (10 μM), KU-0063794 (1 μM), anisomycin (20 μM), and CHIR 99021 (2 μM) were purchased from Tocris (Bristol, U.K.). VU0409551 (10 μM) and VU0469650 (10 μM) were synthesized in-house. The control, mutated peptide mGlu5-mut (YGRKKRRQRRRALTPLSPRR) and the active, dominant negative mGlu5-C-ter (YGRKKRRQRRRALTPPSPFR) (Mao et al., 2005; Ronesi and Huber, 2008) were prepared by Bio-Synthesis (Lewisville, TX, U.S.A.) and added to the internal solution at 20 nM.
2.5. Statistics.
The number of mice in each experiment is denoted by “N” and the cells by “n”. Data are presented as mean ± standard error of the mean. Analyses were performed using GraphPad Prism (La Jolla, CA, U.S.A.). Two-tailed Student’s t-test, Mantel-Cox test, and one/two-way ANOVA with Bonferonni post-test were used as appropriate. Results of statistical analyses are presented in the figure legends.
3. Results
3.1. Potentiating mGlu5 function rescues stress-induced deficit in PL-PFC plasticity.
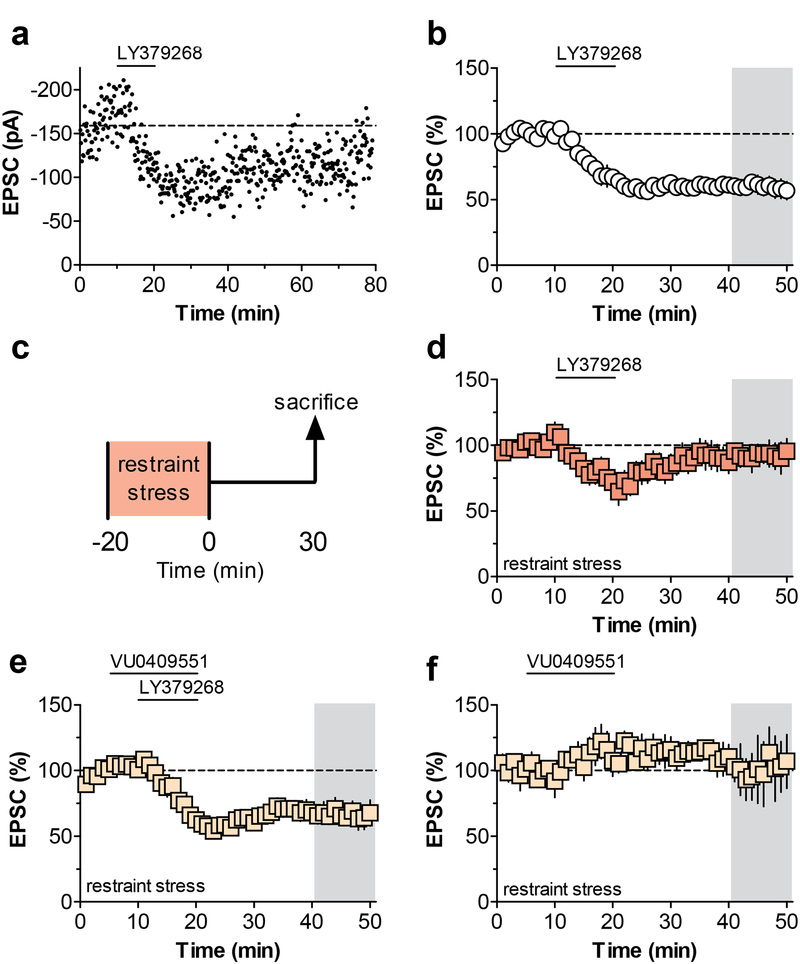
Our previous findings demonstrating that mGlu3 potentiates mGlu5 signaling in the PL-PFC (Di Menna et al., 2018), raises the interesting possibility that potentiating mGlu5 signaling could rescue deficits in mGlu3/mGlu5-LTD observed after stress exposure (Joffe et al., 2017). Thus, we set out to test the hypothesis that stress-induced impairments in mGlu3-LTD and cognition could be ameliorated using selective mGlu5 PAMs. Consistent with our previous findings (Joffe et al., 2017; Walker et al., 2015), perfusion of PL-PFC slices with the mGlu3 agonist, LY379268, induced robust LTD in acute PL-PFC slices (Figure 1a & 1b), a response that depends on co-activation of mGlu3 and mGlu5 (Di Menna et al., 2018). Furthermore, as we reported previously (Joffe et al., 2017), exposure to a single session of restraint stress abolished the expression of PL-PFC mGlu3/mGlu5-LTD (Figure 1c & 1d). Interestingly, perfusion with the selective mGlu5 PAM, VU0409551 (Rook et al., 2015), restored mGlu3/mGlu5-LTD (Figure 1e) in slices of animals exposed to acute stress, without affecting basal synaptic transmission on its own (Figure 1f). These data indicate that selectively enhancing the function of mGlu5 using an mGlu5 PAM can restore the expression of mGlu3/mGlu5-LTD following acute restraint stress.
Figure 1. Potentiating mGlu5 function rescues stress-induced deficits in mGlu3/mGlu5 plasticity.
(a) Representative experiment showing that LY379268, an orthosteric agonist for metabotropic glutamate (mGlu) receptor subtype 3 (mGlu3), induces long-term depression (LTD) of excitatory transmission onto pyramidal cells in the prelimbic prefrontal cortex (PL-PFC). (b) Summary of all control PL-PFC mGlu3-LTD recordings (60.5 ± 4.9% baseline, n/N = 9/8 cells/mice). (c) Schematic, acute restraint stress was applied 30 minutes before mice were sacrificed for electrophysiology. (d) Acute restraint impairs the induction of ex vivo LTD (93.4 ± 8.0% baseline, n/N = 7/6). (e) In slices from stress-exposed mice, application of an mGlu5 positive allosteric modulator, VU0409551, restores the expression of LTD (69.4 ± 5.6% baseline, n/N = 6/5). A one-way ANOVA revealed a main effect of treatment (F1,2 = 8.337; p = 0.0025) and Bonferonni post-tests confirmed the stress-induced impairment and rescue with VU0409551 bath application. (f) VU0409551 does not alter excitatory transmission by itself in slices from stress-exposed mice. (100.6 ± 13.8% baseline, n/N = 5/4). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3 and mGlu5, metabotropic glutamate receptor subtype 3 and 5; PL-PFC, prelimbic prefrontal cortex.
3.2. Several mGlu5-dependent functions remain intact following acute stress.
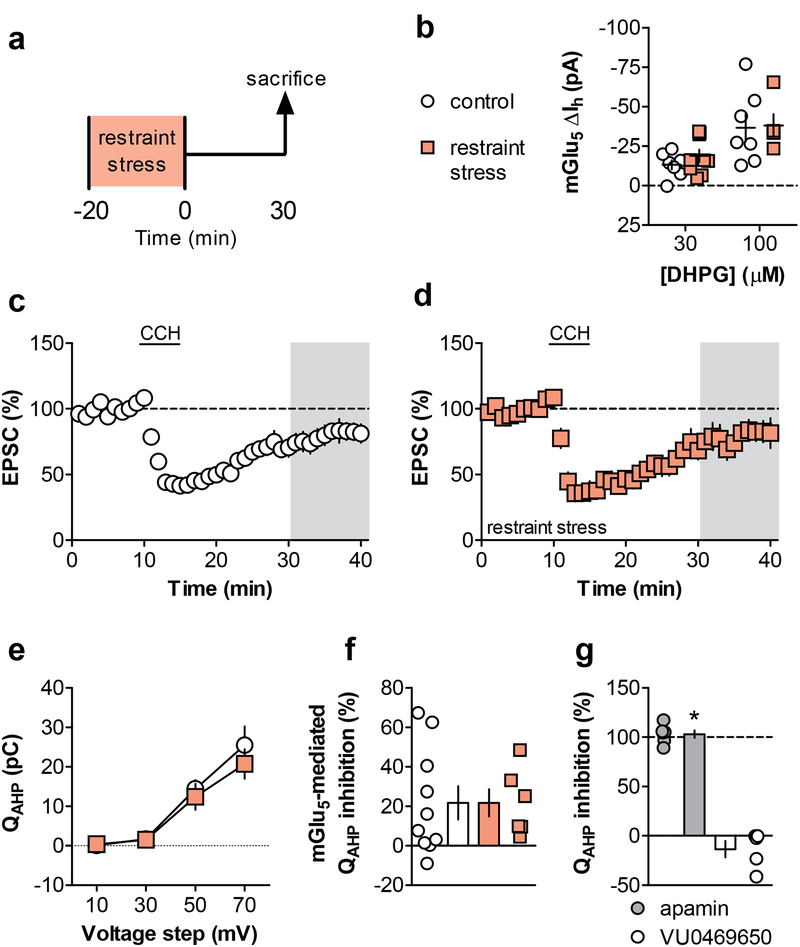
The finding that acute stress disrupts mGlu3/mGlu5-LTD and that this is restored using an mGlu5-selective PAM raises the possibility that stress induces a general reduction in multiple mGlu5-mediated responses. Alternatively, acute stress may have more selective actions on the mechanisms required for LTD, without inducing a general disruption of mGlu5 signaling. We took several approaches to assess the function of mGlu5 in the PL-PFC following acute restraint stress (Figure 2a). At two concentrations of the orthosteric agonist DHPG, we observed no difference in mGlu5-mediated inward current in restraint stress mice relative to controls (Figure 2b). Next, we proceeded to assess another form of synaptic plasticity that involves mGlu5 function. Bath application of the muscarinic agonist carbachol induces LTD that is dependent on the M1 muscarinic acetylcholine receptor and also mGlu5 (Ghoshal et al., 2017) (Figure 2c). Following restraint stress, this M1/mGlu5-dependent LTD remained intact (Figure 2d). Finally, we assessed the ability of mGlu5 activation to inhibit the function of SK channels (Cannady et al., 2017). We evaluated SK channel function by measuring QAHP and found no basal difference following restraint stress (Figure 2e). We then corroborated that mGlu5 inhibits SK channel function and observed no difference between the control and restraint stress group (Figure 2f). Control experiments confirmed that QAHP reflects SK channel function as the selective inhibitor apamin inhibited this measurement below detectable levels (Figure 2g). Taken together, these data suggest that acute restraint does not impair overall mGlu5 function in PL-PFC pyramidal cells. Instead, these data pointed towards specific alterations in downstream mGlu3-mGlu5 signaling underlying the stress-induced impairment, therefore we aimed to gain a better understanding of the signaling mechanisms required for mGlu3/mGlu5-LTD.
Figure 2. Several mGlu5-dependent functions remain intact following acute stress.
(a) Schematic displaying acute stress exposure paradigm. Mice underwent 20 minutes of restraint stress and were sacrificed for electrophysiology 30 minutes later. (b) Inward currents induced by a threshold and high concentration of the orthosteric agonist, DHPG, were acquired in the presence of the metabotropic glutamate (mGlu) receptor subtype 1 (mGlu1) negative allosteric modulator VU0469650 and tetrodotoxin. These mGlu5-mediated currents were not different between controls (13.1 ± 3.0, 36.7 ± 8.7 pA, n/N = 7/3 cells/mice) and the restraint stress group (18.8 ± 3.9, 38.0 ± 7.1 pA, n/N = 5/3, 9/5). (c/d) mGlu5-dependent LTD was elicited with the muscarinic agonist carbachol (CCH). CCH-LTD was comparable between control (80.8 ± 6.5% baseline, n/N = 8/5) and restraint stress groups (78.6 ± 7.8% baseline, n/N = 6/3). (e) The charge transfer through small conductance potassium (SK) channels was determined as the charge transfer of the medium afterhyperpolarization (QAHP) following increasing voltage steps (F3,33 = 45.63, p < 0.0001). Baseline SK channel function was not altered following restraint stress (F1,11 = 0.36, n.s.). (f) mGlu5 activation inhibited SK channel function to a similar degree in control (21.7 ± 7.8% inhibition, n/N = 10/4) and restraint stress mice (21.7 ± 6.9% inhibition, n/N = 6/2). (g) Control experiments displaying that the selective SK channel inhibitor apamin completely blocked QAHP (103.1 ± 3.9% inhibition, n/N = 6/3, *: p < 0.0001 one-sample t-test vs. 0% inhibition), while VU0469650 exerted no effect by itself (−13.5 ± 8.2% inhibition, n/N = 5/3). CCH, carbachol; EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu1 and mGlu5, metabotropic glutamate receptor subtype 1 and 5; SK, small conductance potassium; QAHP, charge transfer of the medium afterhyperpolarization.
3.3. mGlu3/mGlu5-LTD proceeds through PI3K/Akt signaling pathway.
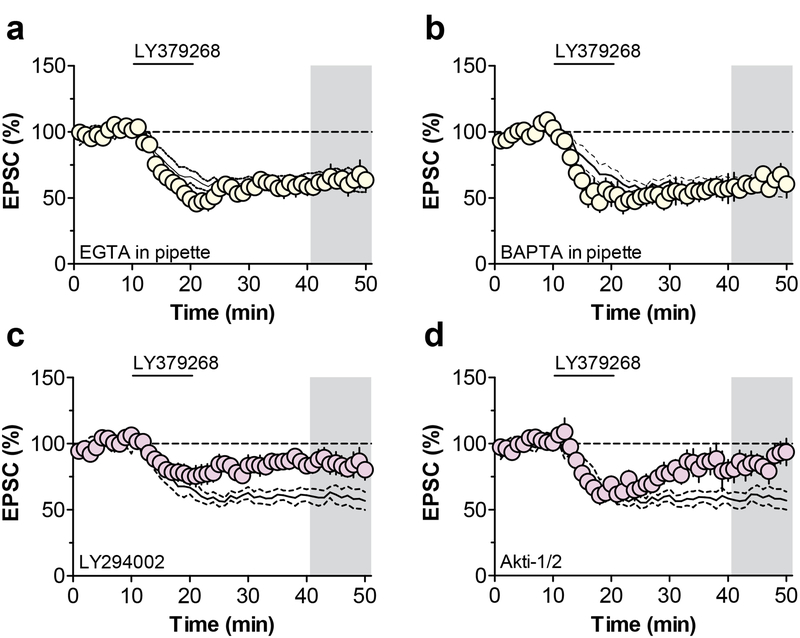
In contrast to mGlu3 functions in other brain regions, we previously demonstrated that PL-PFC LTD involves postsynaptic mechanisms and the internalization of AMPA receptors (Joffe et al., 2017). In some brain regions, mGlu5 can induce a similar form of LTD through the activation of Gq proteins and the mobilization of intracellular Ca2+ (Grueter et al., 2010; Kelly et al., 2009). Moreover, we had previously demonstrated that activation of PL-PFC mGlu3 potentiates mGlu5-mediated Ca2+ mobilization (Di Menna et al., 2018); thus, we aimed to test whether increases in postsynaptic Ca2+ are required to drive mGlu3-LTD. Chelators of intracellular divalent ions are commonly used to sequester postsynaptic Ca2+ in single neurons. To our surprise, inclusion of EGTA or BAPTA did not block LTD (Figure 3a & 3b), suggesting that intracellular Ca2+ mobilization is not necessary for mGlu3/mGlu5-LTD. Hippocampal mGlu5-LTD requires the activation of PI3K (Hou and Klann, 2004), so we tested whether the kinase inhibitor LY294002 inhibits PL-PFC mGlu3-LTD. Bath application of LY294002 blocked the induction of mGlu3/mGlu5-LTD (Figure 3c), corroborating a PI3K-dependent mechanism. Furthermore, Akti-1/2, an Akt inhibitor structurally distinct from LY294002, also blocked LTD (Figure 3d). These data are consistent with a Ca2+-independent, PI3K-dependent pathway for the induction of PLPFC mGlu3/mGlu5-LTD, similar to the mechanisms required for mGlu5-LTD at the Schaffer collateral-CA1 synapse in the hippocampus (Luscher and Huber, 2010).
Figure 3. mGlu3-LTD proceeds through PI3K-Akt signaling pathway.
(a) The divalent ion chelator EGTA was added to the patch pipette to quench Ca2+ signaling in the postsynaptic cell. This manipulation had no effect on the expression of metabotropic glutamate receptor subtype 3 (mGlu3)-dependent long-term depression (LTD) (62.1 ± 6.9% baseline, n/N = 7/4 cells/mice). Black lines in panels 3a-4d represent data from control experiments displayed in panel 1b. (b) The divalent ion chelator BAPTA was added to the patch pipette and did not affect mGlu3-LTD (59.9 ± 4.9% baseline, n/N = 5/3). (c) The phosphoinositide 3-kinase (PI3K) inhibitor LY294002 impaired the expression of mGlu3-LTD (85.7 ± 5.1% baseline, n/N = 8/6). (d) The Akt inhibitor Akti-1/2 blocked mGlu3-LTD (89.6 ± 11% baseline, n/N = 5/4). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu3, metabotropic glutamate receptor subtype 3; PI3K, phosphoinositide 3-kinase.
3.4. PL-PFC mGlu3/mGlu5-LTD is fundamentally distinct from classical hippocampal mGlu5-LTD.
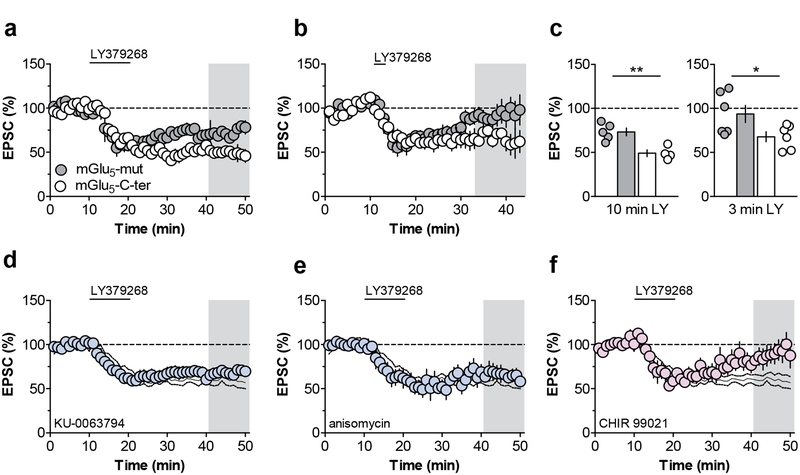
While PL-PFC mGlu3/mGlu5-LTD is unique in that it requires activation of both mGlu3 and mGlu5, the finding that this LTD requires activation of PI3K suggests that the downstream pathways may be mechanistically similar to postsynaptic mGlu5- LTD at the hippocampal Schaffer collateral-CA1 synapse. In CA1 pyramidal cells, Homer proteins act as scaffolds to link mGlu5 with effectors such as the inositol triphosphate receptor and PI3K enhancer (Tu et al., 1999; Xiao et al., 1998), and the interaction between mGlu5 and Homer proteins is essential for the expression of hippocampal mGlu5-LTD (Ronesi and Huber, 2008). One means to disrupt this interaction is by introducing a dominant negative peptide containing 15 residues of the mGlu5 C-terminal tail (mGlu5-C-ter) (Mao et al., 2005; Ronesi and Huber, 2008). As a control, we used a peptide with two residues mutated in the enabled/VASP homology 1 domain such that the peptide does not interact with Homers (mGlu5-mut). Interestingly, including mGlu5-C-ter in the patch pipette did not block mGlu3-LTD and, in fact, slightly enhanced LTD relative to cells dialyzed with mGlu5-mut (Figure 4a & 4c). To further test whether disrupting the mGlu5-Homer interaction might enhance mGlu3/mGlu5-LTD, we assessed threshold LTD in separate cells. While no LTD was observed in cells dialyzed with mGlu5-mut, the threshold protocol elicited LTD when mGlu5-C-ter was included in the internal solution (Figure 4b & 4c). These data suggest that mGlu5-Homer interactions are not necessary for mGlu3-LTD and may even tonically inhibit its induction.
Figure 4. mGlu3-LTD is modulated by mGlu5-Homer interactions and Glycogen synthase kinase 3.
(a) Inclusion of a peptide that blocks the interaction between metabotropic glutamate receptor subtype 5 (mGlu5) and Homer proteins (mGlu5-C-ter) in the patch pipette enhances mGlu3-LTD (49.0 ± 7.5% baseline, n/N = 4/2 cells/mice) relative to a control peptide (mGlu5-mut) that does not impair the mGlu5-Homer interaction (73.2 ± 4.3% baseline, n/N = 5/4). (b) Whole-cell infusion of mGlu5-C-ter generates LTD following threshold application of LY379268 (67.8 ± 5.6% baseline, n/N = 6/4), whereas inclusion of mGlu5-mut in the pipette has no effect (93.6 ± 9.8% baseline, n/N = 6/4). (c) Summary of the last 10 minutes of the recordings from the timecourse experiments (*: p < 0.05, **: p < 0.01, t-tests). (d) The mechanistic target of rapamycin inhibitor KU-0063794 does not affect mGlu3-LTD (69.2 ± 5.2% baseline, n/N = 7/5). Black lines in panels 4d-5f denote control experiments from figure 1b. (e) Blocking protein translation with anisomycin does not impair mGlu3-LTD (62.7 ± 6.4% baseline, n/N = 6/6). (f) The glycogen synthase kinase 3 inhibitor CHIR 99203 blocks the induction of LTD (89.6 ± 11.0% baseline, n/N = 5/5). EPSC, excitatory postsynaptic current; LTD, long-term depression; mGlu5, metabotropic glutamate receptor subtype 5.
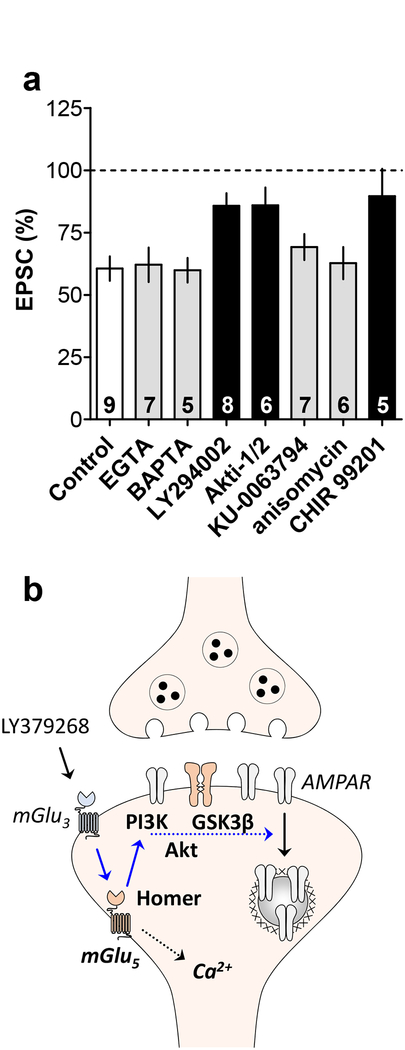
In the hippocampus, mGlu5-LTD requires the activation of the mammalian target of rapamycin (mTOR) and the rapid initiation of protein translation (Huber et al., 2000), both of which can be activated downstream of PI3K and Akt. In contrast to that mechanism, the mTOR inhibitor KU-0063794 and the translation inhibitor anisomycin each exerted no effect on the expression of PL-PFC mGlu3-LTD (Figure 4d & 4e). Akt signaling also proceeds through multiple downstream targets not dependent on mTOR activation (Manning and Toker, 2017). In particular, dysfunction of GSK3β has been implicated in several stress-related psychiatric disorders (Chen et al., 2015; Jope, 2011), so we sought to examine whether its function is involved in mGlu3/mGlu5-LTD. The GSK3 inhibitor CHIR 99021 blocked mGlu3/mGlu5-LTD (Figure 4f), providing an alternative pathway by which Akt activation leads to PL-PFC LTD autonomous from mTOR signaling. Taken together these studies illustrate that, while still dependent on mGlu5 and the PI3K pathway, PL-PFC mGlu3/mGlu5-LTD proceeds through a different mechanism than hippocampal mGlu5-LTD (Figure 5).
Figure 5. Mechanisms of mGlu3-LTD in the prelimbic prefrontal cortex.
(a) Long-term depression (LTD) of excitatory transmission in the prelimbic prefrontal cortex (PL-PFC) was induced by bath application of LY379268, an orthosteric agonist at metabotropic glutamate (mGlu) receptor subtype 3 (mGlu3). In previous publications (Di Menna et al, 2018; Joffe et al, 2017), we have demonstrated that this LTD requires the activity of mGlu3 and mGlu5. Here, we demonstrate that PL-PFC mGlu3-LTD is not impaired by manipulations that sequester intracellular Ca2+ (EGTA and BAPTA) or block the mechanistic target of rapamycin and protein translation (KU-0063794 and anisomycin). Instead, PL-PFC mGlu3-LTD is blocked by a Phosphoinositide-3-kinase (PI3K) inhibitor (LY294002), an Akt inhibitor (Akti-1/2), and a Glycogen synthase kinase 3 inhibitor (CHIR 99201). The number in each bar represents the number of cells per experiment. A one-way ANOVA revealed a main effect of treatment (F1,7 = 3.957; p = 0.0019). Grey bars denote no significant difference from Control. Black bars denote p < 0.05, Bonferonni post-test vs. Control. (b) Conclusions drawn from the present studies are displayed in bold and findings from two previous papers are italicized. While activation of mGlu3 potentiates mGlu5-mediated Ca2+ mobilization, the present findings suggest that is not related to LTD. Instead, mGlu3-LTD is modulated by interactions between mGlu5 and Homer proteins and requires signaling through PI3K, Akt, and GSK3. This signaling culminates with the dynamin-dependent internalization of AMPA receptors and a long-term decrease in postsynaptic quantal size and content. EPSC, excitatory postsynaptic current; GSK3β, glycogen synthase kinase 3β LTD, long-term depression; mGlu3 and mGlu5, metabotropic glutamate receptor subtype 3 and 5; PI3K, phosphoinositide 3-kinase; PL-PFC, prelimbic prefrontal cortex.
3.5. Acute restraint stress impairs early reversal learning.
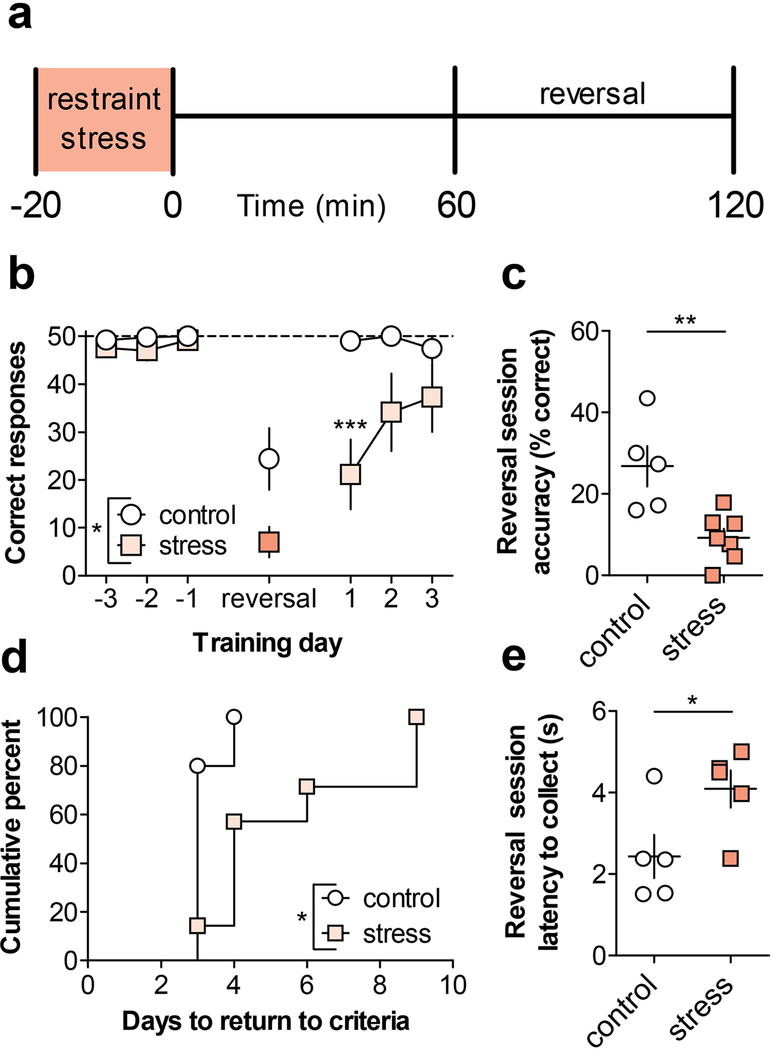
Recent findings suggest that deficits in mGlu-dependent LTD are associated with impairments in cognitive flexibility (Eales et al., 2014; Mills et al., 2014). Based on this, we postulated that the loss of mGlu3/mGlu5-LTD in response to acute stress exposure might be associated with an impairment in reversal learning in an operant food-seeking task. To test this hypothesis, mice were trained in an operant conditioning chamber to holepoke on one-of-two operandi for liquid food delivery under a continuous schedule of reinforcement. After acquiring stable performance, some mice underwent a single 20-minute restraint stressor, one hour prior to the instrumental reversal session (Figure 6a). Stressed mice exhibited a significant reduction in correct responses during the reversal session, and this deficit carried over onto the following training day as well (Figure 6b). Stressed mice also displayed a deficit in accuracy, or the percentage of correct responses, on the day of the reversal (Figure 6c), indicating a deficit in reversal learning. Furthermore, mice that received acute restraint during the first reversal session required more sessions to reach criteria (Figure 6d). Notably, however, stressed mice displayed a longer latency to retrieve the reward during the reversal session (Figure 6e), raising the possibility that a motivational deficit could contribute to the decrease in performance. We therefore performed control experiments in which mice were stressed prior to a normal conditioning session without instrumental reversal (Figure S1a). Under these conditions, stress did not impact the total number of correct responses, the response accuracy, or the latency to collect the reward (Figure S1b-S1d). Additionally, acute stress did not impact the any of these parameters when mice were trained on a progressive ratio schedule of reinforcement (Figure S1e-S1g).
Figure 6. Acute restraint stress impairs early reversal learning.
(a) Schematic. Mice were trained in an operant conditioning apparatus to holepoke on a continuous schedule of reinforcement for liquid food. On the day of testing only, some mice underwent a 20-minute restraint stress exposure. One hour after the stress terminated, mice underwent an operant test session where the active holepoke designation was reversed. (b) There was a main effect of stress (F1,10 = 7.07, p < 0.03), session (F6,60 = 18.62, p < 0.001), and an interaction (F6,60 = 3.3, p < 0.01) on performance across training, reversal, and follow-up sessions. Post-tests revealed a strong trend towards a decrease in the number of correct holepoke responses on the day of the reversal (7 ± 3 vs. 24 ± 7 holepokes, N = 5–7 mice, 95% confidence interval of difference: [−35.6, 0.8] Bonferonni post-test). In addition, this deficit carried over until the following day, when the mice were not stressed before the initiation of the task (21 ± 7 vs. 49 ± 1 holepokes, ***: p < 0.001, Bonferonni post-test). (c) Stressed mice exhibited decreased accuracy during the reversal session (9.3 ± 2.2% vs. 26.8 ± 5.0% correct holepokes, **: p<0.01, t-test). (d) Stressed mice required more days to reach criteria following the reversal than controls (χ2 = 5.4, df=1, p < 0.02, Mantel-Cox Test). (e) Stressed mice displayed a longer latency to retrieve the food reward than controls during the reversal session (2.4 ± 0.5 vs. 4.1 ± 0.5s, *: p<0.05, t-test).
3.6. Potentiating mGlu5 function rescues stress-induced deficit in cognition.
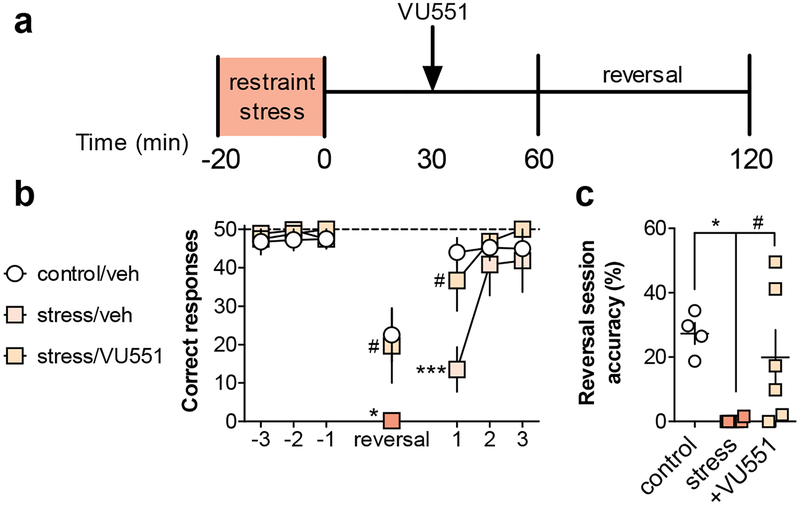
Previous studies have shown that mGlu5 PAMs can enhance multiple aspects of cognitive function and correct deficits in reversal learning in rodent models of schizophrenia (Gastambide et al., 2012; Stansley and Conn, 2018; Stefani and Moghaddam, 2010). Based on this, and the current finding that VU0409551 reverses stress-induced deficits in mGlu3/mGlu5-LTD, we posited that the mGlu5 PAM might also restore the stress-induced impairment in reversal learning. Mice were administered vehicle or VU0409551 30-minutes after acute restraint stress and before the instrumental reversal session (Figure 7a). Vehicle-treated stressed mice recapitulated the deficit in performance relative to vehicle-treated controls (Figure 7b & 7c). Acute treatment with VU0409551 rescued the stress-induced deficit, both in the total number of correct responses and the accuracy during the instrumental reversal session. We performed additional experiments in non-stressed mice and found that VU0409551 did not enhance performance on the reversal task in control mice (Figure S2a-S2d), nor did VU0409551 alter food-seeking on a standard, no reversal, session in either control or stressed mice (Figure S2e-S2g). Taken together, these data suggest potentiating mGlu5 function can ameliorate acute stress-induced deficits in PL-PFC plasticity and also concomitant impairments in cognition.
Figure 7. Potentiating mGlu5 function rescues stress-induced deficit in early reversal learning.
(a) Schematic for behavioral experiments. Mice were trained in an operant conditioning apparatus to holepoke on a continuous schedule of reinforcement for liquid food. On the day of testing only, some mice underwent a 20-minute restraint stress exposure. One hour after the stress terminated, mice underwent an operant test session where the active holepoke designation was reversed. VU0409551 or vehicle was administered 30-minutes after the stress and before the reversal test session. (b) There was a trend effect of treatment (F2,13 = 2.9, p < 0.1), a significant main effect of session (F6,78 = 26.03, p < 0.001), and a significant interaction (F12,78 = 2.5, p < 0.01) on performance across training, reversal, and post-test sessions. Post-tests revealed that mice exposed to acute restraint exhibited a decrease in the number of correct holepoke responses on the day of the holepoke reversal (0 ± 1 vs. 23 ± 7 holepokes, N = 4–6 mice, *: p<0.05, Bonferonni post-test vs. control/veh). In addition, this deficit carried over until the following day, when the mice were not stressed before the initiation of the task (14 ± 6 vs. 44 ± 4 holepokes, ***: p<0.001, Bonferonni post-test vs. control/veh). VU0409551 administration rescued the stress-induced deficit in reversal learning on both the day of reversal and the following day (20 ± 10 holepokes, N = 6, #: p<0.05, Bonferonni post-test vs. stress/veh). (c) Stressed mice exhibited decreased accuracy during the reversal session (0 ± 0.3% vs. 27.4 ± 3.3% correct holepokes, N = 4–6, *: p<0.05, Bonferonni post-test vs. control) that was rescued by pretreatment with VU0409551 (20 ± 8.5% correct holepokes, N = 6, #: p<0.05, Bonferonni post-test vs. stress). mGlu3 and mGlu5, metabotropic glutamate receptor subtype 3 and 5; veh, vehicle; VU551, VU0409551.
4. Discussion
In the present studies, we demonstrated that potentiating mGlu5 function rescues stress-induced deficits to PL-PFC synaptic plasticity and cognition. Moreover, we thoroughly investigated the signaling mechanisms involved in mGlu3/mGlu5-dependent plasticity and have identified PI3K/Akt signaling molecules as key players. These findings provide insight into the basic biology of mGlu receptor crosstalk, the actions of acute stress on PL-PFC function, and the signaling mechanisms involved in restoring cognitive function. Our previous studies demonstrated that prophylactic treatment with an mGlu3 NAM prevented stress-induced deficits in PL-PFC function (Joffe et al., 2017). However, that approach necessitates intervention before or immediately after the stress exposure, limiting its therapeutic utility. Our current findings provide an alternative therapeutic strategy that could be applied at later time points, when the stress-induced physiological changes have already begun. Specifically, positive modulation of mGlu5 proved to be one such approach as VU0409551 treatment after stress rescued the impairments in reversal learning and the induction of mGlu3/mGlu5-dependent synaptic plasticity.
PL-PFC-dependent functions are dynamically modulated by acute stress (Arnsten et al., 2017; Holmes and Wellman, 2009). For example, acute stress impairs attentional set-shifting in rodents alongside synaptic changes in the PL-PFC glutamate system (Izquierdo et al., 2006). Here, we demonstrated that acute stress also impairs early reversal learning, a simplified model of executive function. At face value, this finding seems to conflict with studies demonstrating that acute stress does not affect early reversal learning (Bryce and Howland, 2015; Graybeal et al., 2011; Izquierdo et al., 2017; Thai et al., 2013), however, several important methodological differences should be considered. In previous studies, rodents underwent acute swim stress within 30 minutes or immediately prior to the reversal learning sessions. Our previous work demonstrated that acute restraint stress applied immediately before an operant conditioning session decreased the breakpoint and reinforcers earned on a progressive ratio schedule (Joffe et al., 2017). Based on this, we assessed reversal learning one hour after restraint stress termination, a time at which we did not observe deficits in motivation. Differences in the timing of the stressors, or between swim and restraint stress, could play a part in the divergence between the literature and current findings. Another major difference is that mice in the present study were fed ad libitum during all test sessions. In contrast, previous studies employed food restriction, a process that can alter the central processing of rewarding stimuli (Cabeza de Vaca and Carr, 1998). We believe the discrepant internal states likely contribute to the apparent divergence from previous findings. In addition, previous studies finding no effect of stress on early reversal learning were conducted in tasks with visual cue components, such as touchscreen-based chambers (Bryce and Howland, 2015; Graybeal et al., 2011). In contrast, no light cues were present for any training or trials in the present study, effectively isolating the spatial component of the task. These technical differences aside, the current results provide evidence that, under some circumstances, acute stress impairs reversal learning, and, as observed in another reversal learning study (Gastambide et al., 2012), this impairment can be rescued through positive modulation of mGlu5.
Layer 5 of the PL-PFC is thought to provide a major excitatory input to the ventral striatum and other components of the limbic system. As such, this area represents a key node at the interface between stress and motivated behaviors. While the present series of physiological studies were limited to this specific PL-PFC subregion, stress-induced dysfunction to mGlu3 and mGlu5 function is likely to extend to layer 2/3 and other cortical areas such as, the infralimbic PFC (Graybeal et al., 2011) and orbitofrontal cortex (OFC) (Dalton et al., 2016; Gourley et al., 2010; Graybeal et al., 2011). The OFC, in particular, is thought to be heavily involved in several types of reversal learning, and remarkably little is known about mGlu receptor function within that brain region. Perhaps the strong reciprocal connections between the PL-PFC and the medial divisions of the OFC (Hoover and Vertes, 2011; Vertes, 2004) provide an anatomical substrate for the phenomena observed in the present study. In addition, reversal learning tasks also require proper function of many striatal areas, and the dorsomedial region in particular (Castane et al., 2010; Ragozzino, 2007). Portions of the ventral and dorsomedial striatum receive dense excitatory input from the PL-PFC (Hart et al., 2018; Vertes, 2004), and future studies should be directed at testing whether specific PL-PFC corticostriatal projections regulate reversal learning. Finally, it should be noted that several studies using lesions or other forms of inactivation did not detect a role for the PL-PFC in reversal learning tasks (Izquierdo et al., 2017). In our studies, we have found that acute stress impairs LTD, which would be predicted to cause excessive activation of the PL-PFC. To the best of our knowledge, no studies have directly assessed whether such excessive activation of the prelimbic and/or other cortical subregions can modulate reversal learning. Our current findings suggest that transient hyperactivity in the PL-PFC, or maladaptive integration of synaptic inputs, might impair early phases of reversal learning.
Of interest to the mechanisms uncovered here, VU0409551 has been characterized as displaying stimulus bias downstream of mGlu5 activation (Rook et al., 2015). Specifically, the PAM potentiates Ca2+ mobilization and hippocampal LTD without directly enhancing N-methyl-D-aspartate (NMDA) receptor function. Therefore, although mGlu5 has been intimately linked with NMDA receptor-dependent plasticity (Joffe et al., 2018), the molecular pharmacology suggests those signaling pathways are likely not involved in the effects observed here. In addition, Balu et al. (Balu et al., 2016) reported that VU0409551 treatment enhances PL-PFC Akt phosphorylation in a genetic model of schizophrenia-like deficits as well as in control mice. These results and the present findings are consistent with VU0409551 restoring deficits in mGlu3-LTD and reversal learning through actions on the PI3K/Akt signaling pathway. Downstream from Akt activation, we identified a role for GSK3β in this PL-PFC synaptic plasticity. GSK3β has been suggested to be a therapeutic target of lithium and valproate (De Sarno et al., 2002), two mood stabilizing medications used for the treatment of bipolar disorder. Moreover, like GRM3 (Egan et al., 2004; Harrison et al., 2008), polymorphisms in the coding gene GSK3B have been linked to deficits in cognition and greater risk for the development of stress-related psychiatric disorders (Chen et al., 2015). The present data suggest that positive modulation of mGlu3 and/or mGlu5 may provide therapeutic benefits in patient populations with genetic or functional disruptions in GSK3β.
Similar to the plasticity described in the present studies, canonical hippocampal mGlu5- dependent LTD proceeds through PI3K and Akt; however, that LTD also requires physical interactions between mGlu5 and Homer proteins (Luscher and Huber, 2010). In that system, Homer proteins scaffold PI3K enhancer and PI3K itself, and they may also be involved in the recruitment of mTOR downstream of Akt activation. In stark contrast to the hippocampal literature, we found that mGlu5-Homer interactions are not required for PL-PFC LTD, and instead may even inhibit its induction. In some cases, Homer interactions can regulate constitutive activity of mGlu5 (Ango et al., 2001; Tronson et al., 2010), and acute stress has been shown to dysregulate such an interaction in the hippocampus (Tronson et al., 2010). Perhaps the basally expressed PL-PFC Homer proteins throttle mGlu5 signaling and prevent LTD from occurring without coincidental mGlu3 activation. These interactions may have great implications for the development of mGlu-directed therapeutics and potential comorbidities between stress-related psychiatric disorders. Overall, further investigation into the mechanisms underlying stress-induced deficits to PL-PFC plasticity provides an avenue towards the discovery and development of novel means to treat stress-related cognitive disorders. These current findings suggest that potentiating mGlu3 and/or mGlu5 function may deliver one such approach.
Supplementary Material
(a) Schematic. Control experiments were performed where mice underwent restraint stress prior to a standard (no reversal) operant test session. (b) Stress does not decrease the number of correct (active) holepokes on a continuous schedule of reinforcement (F6,6 = 1.13; n.s.) (N = 7 mice). (c) Stress does not affect response accuracy in control test sessions. (d) Stress does not alter the latency to collect the food reward. (e) Stress does not alter the number of holepokes on a progressive ratio (PR) schedule of reinforcement (F3,11 = 0.17; n.s.) (N = 12). (f) Stress does not affect the number of rewards earned on a PR schedule (F3,11 = 0.32; n.s.). (g) Stress does not alter the latency to collect the food reward (F3,11 = 0.24; n.s.). PR, progressive ratio.
(a) Schematic for behavioral experiments. On the day of testing only, mice underwent an operant test session where the active holepoke designation was reversed and VU0409551 or vehicle was administered 30-minutes beforehand. (b) VU0409551 administration did not alter the number of correct responses across reversal learning sessions (main effect: F1,6 = 0.02, n.s; interaction: F6,36 = 0.37, n.s.) (N = 4 mice/group). (c) VU0409551 did not affect the percent of correct responses elicited during the reversal test session. (d) VU0409551 did not alter the days to reach criteria following the reversal session. (e) Control experiments were performed where VU0409551 was administered to control or stressed mice prior to a standard (no reversal) operant test session (N = 6). There was no main effect of session (F6,60 = 0.67; n.s.) or stress (F1,10 = 0.03; n.s.) on food-seeking behavior under these standard conditions. (f) VU0409551 did not alter the ability to discriminate between active and inactive operandi. (g) VU0409551 did not alter the latency to collect the food reward. VU551, VU0409551.
Highlights:
PL-PFC mGlu3/mGlu5-LTD requires PI3K-Akt signaling, and not Ca2+ mobilization
Acute stress disrupts mGlu3/mGlu5-LTD without a gross impairment to mGlu5 function
Potentiating mGlu5 rescues LTD deficit following acute restraint stress
Potentiating mGlu5 ameliorates reversal learning deficit following acute stress
Acknowledgements
We thank members of the Conn lab for helpful discussions surrounding the contents of this manuscript. Behavioral experiments were performed through the Murine Neurobehavior Core lab at the Vanderbilt University Medical Center.
Funding and disclosures
This work was supported by National Institutes of Health (NIH) grants R01MH062646 and R37NS031373 (P.J.C.). M.E.J. was supported by NIH grant T32MH093366 and a postdoctoral fellowship through the Pharmaceutical Research and Manufacturers of America Foundation. C.I.S. was supported by the Searle Undergraduate Research Program at Vanderbilt University. R.G.G. was supported by NIH grant K01MH112983. C.W.L. has been funded by the NIH, Johnson and Johnson, Bristol-Myers Squibb, AstraZeneca, Michael J. Fox Foundation, as well as Seaside Therapeutics. He has consulted for AbbVie and received compensation. P.J.C. has been funded by NIH, AstraZeneca, Bristol-Myers Squibb, Michael J. Fox Foundation, Dystonia Medical Research Foundation, CHDI Foundation, and Thome Memorial Foundation. Over the past three years he has served on the Scientific Advisory Boards for Michael J. Fox Foundation, Stanley Center for Psychiatric Research Broad Institute, Karuna Pharmaceuticals, Lieber Institute for Brain Development, Clinical Mechanism and Proof of Concept Consortium, and Neurobiology Foundation for Schizophrenia and Bipolar Disorder. C.W.L. and P.J.C. are inventors on patents that protect different classes of metabotropic glutamate allosteric modulators. M.E.J., C.I.S., B.J.S., J.M., R.G.G, J.L.E., and F.N. declare no potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L, 2001. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature 411, 962–965. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Lee D, Pittenger C, 2017. Risky Business: The Circuits that Impact Stress-Induced Decision-Making. Cell 171, 992–993. [DOI] [PubMed] [Google Scholar]
- Balu DT, Li Y, Takagi S, Presti KT, Ramikie TS, Rook JM, Jones CK, Lindsley CW, Conn PJ, Bolshakov VY, Coyle JT, 2016. An mGlu5-Positive Allosteric Modulator Rescues the Neuroplasticity Deficits in a Genetic Model of NMDA Receptor Hypofunction in Schizophrenia. Neuropsychopharmacology 41, 2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Malenka RC, 1994. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol 4, 389–399. [DOI] [PubMed] [Google Scholar]
- Bryce CA, Howland JG, 2015. Stress facilitates late reversal learning using a touchscreen-based visual discrimination procedure in male Long Evans rats. Behav Brain Res 278, 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD, 1998. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci 18, 7502–7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannady R, McGonigal JT, Newsom RJ, Woodward JJ, Mulholland PJ, Gass JT, 2017. Prefrontal Cortex KCa2 Channels Regulate mGlu5-Dependent Plasticity and Extinction of Alcohol-Seeking Behavior. J Neurosci 37, 4359–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castane A, Theobald DE, Robbins TW, 2010. Selective lesions of the dorsomedial striatum impair serial spatial reversal learning in rats. Behav Brain Res 210, 74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Wang M, Waheed Khan RA, He K, Wang Q, Li Z, Shen J, Song Z, Li W, Wen Z, Jiang Y, Xu Y, Shi Y, Ji W, 2015. The GSK3B gene confers risk for both major depressive disorder and schizophrenia in the Han Chinese population. J Affect Disord 185, 149–155. [DOI] [PubMed] [Google Scholar]
- Conde-Ceide S, Martinez-Viturro CM, Alcazar J, Garcia-Barrantes PM, Lavreysen H, Mackie C, Vinson PN, Rook JM, Bridges TM, Daniels JS, Megens A, Langlois X, Drinkenburg WH, Ahnaou A, Niswender CM, Jones CK, Macdonald GJ, Steckler T, Conn PJ, Stauffer SR, Bartolome-Nebreda JM, Lindsley CW, 2015. Discovery of VU0409551/JNJ-46778212: An mGlu5 Positive Allosteric Modulator Clinical Candidate Targeting Schizophrenia. ACS Med Chem Lett 6, 716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton GL, Wang NY, Phillips AG, Floresco SB, 2016. Multifaceted Contributions by Different Regions of the Orbitofrontal and Medial Prefrontal Cortex to Probabilistic Reversal Learning. J Neurosci 36, 1996–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Li X, Jope RS, 2002. Regulation of Akt and glycogen synthase kinase-3 beta phosphorylation by sodium valproate and lithium. Neuropharmacology 43, 1158–1164. [DOI] [PubMed] [Google Scholar]
- Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressens P, Cannella M, Caraci F, Copani A, Bruno V, Battaglia G, Conn PJ, Nicoletti F, 2018. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eales KL, Palygin O, O’Loughlin T, Rasooli-Nejad S, Gaestel M, Muller J, Collins DR, Pankratov Y, Correa SA, 2014. The MK2/3 cascade regulates AMPAR trafficking and cognitive flexibility. Nat Commun 5, 4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Straub RE, Goldberg TE, Yakub I, Callicott JH, Hariri AR, Mattay VS, Bertolino A, Hyde TM, Shannon-Weickert C, Akil M, Crook J, Vakkalanka RK, Balkissoon R, Gibbs RA, Kleinman JE, Weinberger DR, 2004. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc Natl Acad Sci U S A 101, 12604–12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engers JL, Bollinger KA, Weiner RL, Rodriguez AL, Long MF, Breiner MM, Chang S, Bollinger SR, Bubser M, Jones CK, Morrison RD, Bridges TM, Blobaum AL, Niswender CM, Conn PJ, Emmitte KA, Lindsley CW, 2017. Design and Synthesis of N-Aryl Phenoxyethoxy Pyridinones as Highly Selective and CNS Penetrant mGlu3 NAMs. ACS Med Chem Lett 8, 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Cotel MC, Gilmour G, O’Neill MJ, Robbins TW, Tricklebank MD, 2012. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology 37, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal A, Moran SP, Dickerson JW, Joffe ME, Grueter BA, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2017. Role of mGlu5 Receptors and Inhibitory Neurotransmission in M1 Dependent Muscarinic LTD in the Prefrontal Cortex: Implications in Schizophrenia. ACS Chem Neurosci 8, 2254–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Dencker D, Grannan M, Bubser M, Zhan X, Wess J, Xiang Z, Locuson C, Lindsley CW, Conn PJ, Jones CK, 2015. Role for the M1 Muscarinic Acetylcholine Receptor in Top-Down Cognitive Processing Using a Touchscreen Visual Discrimination Task in Mice. ACS Chem Neurosci 6, 1683–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR, 2010. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur J Neurosci 32, 1726–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybeal C, Feyder M, Schulman E, Saksida LM, Bussey TJ, Brigman JL, Holmes A, 2011. Paradoxical reversal learning enhancement by stress or prefrontal cortical damage: rescue with BDNF. Nat Neurosci 14, 1507–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC, 2010. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci 13, 1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Lyon L, Sartorius LJ, Burnet PW, Lane TA, 2008. The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J Psychopharmacol 22, 308–322. [DOI] [PubMed] [Google Scholar]
- Hart G, Bradfield LA, Balleine BW, 2018. Prefrontal Corticostriatal Disconnection Blocks the Acquisition of Goal-Directed Action. J Neurosci 38, 1311–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL, 2009. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci Biobehav Rev 33, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP, 2011. Projections of the medial orbital and ventral orbital cortex in the rat. J Comp Neurol 519, 3766–3801. [DOI] [PubMed] [Google Scholar]
- Hou L, Klann E, 2004. Activation of the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin signaling pathway is required for metabotropic glutamate receptor-dependent long-term depression. J Neurosci 24, 6352–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF, 2000. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 288, 1254–1257. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Brigman JL, Radke AK, Rudebeck PH, Holmes A, 2017. The neural basis of reversal learning: An updated perspective. Neuroscience 345, 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A, 2006. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J Neurosci 26, 5733–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, Paspalas CD, 2018. mGluR2 versus mGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks. Cereb Cortex 28, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Centanni SW, Jaramillo AA, Winder DG, Conn PJ, 2018. Metabotropic Glutamate Receptors in Alcohol Use Disorder: Physiology, Plasticity, and Promising Pharmacotherapies. ACS Chem Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Grueter BA, 2016. Cocaine Experience Enhances Thalamo-Accumbens N-Methyl-D-Aspartate Receptor Function. Biol Psychiatry 80, 671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Santiago CI, Engers JL, Lindsley CW, Conn PJ, 2017. Metabotropic glutamate receptor subtype 3 gates acute stress-induced dysregulation of amygdalo-cortical function. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS, 2011. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly L, Farrant M, Cull-Candy SG, 2009. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci 12, 593–601. [DOI] [PubMed] [Google Scholar]
- Lovell KM, Felts AS, Rodriguez AL, Venable DF, Cho HP, Morrison RD, Byers FW, Daniels JS, Niswender CM, Conn PJ, Lindsley CW, Emmitte KA, 2013. N-Acyl-N’-arylpiperazines as negative allosteric modulators of mGlu1: identification of VU0469650, a potent and selective tool compound with CNS exposure in rats. Bioorg Med Chem Lett 23, 3713–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Huber KM, 2010. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Toker A, 2017. AKT/PKB Signaling: Navigating the Network. Cell 169, 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ, 2005. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci 25, 2741–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills F, Bartlett TE, Dissing-Olesen L, Wisniewska MB, Kuznicki J, Macvicar BA, Wang YT, Bamji SX, 2014. Cognitive flexibility and long-term depression (LTD) are impaired following beta-catenin stabilization in vivo. Proc Natl Acad Sci U S A 111, 8631–8636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, 2007. The contribution of the medial prefrontal cortex, orbitofrontal cortex, and dorsomedial striatum to behavioral flexibility. Ann N Y Acad Sci 1121, 355–375. [DOI] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM, 2008. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci 28, 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, Thompson AD, Byun N, Collier RL, Bubser M, Nedelcovych MT, Gould RW, Stauffer SR, Daniels JS, Niswender CM, Lavreysen H, Mackie C, Conde-Ceide S, Alcazar J, Bartolome-Nebreda JM, Macdonald GJ, Talpos JC, Steckler T, Jones CK, Lindsley CW, Conn PJ, 2015. Biased mGlu5-Positive Allosteric Modulators Provide In Vivo Efficacy without Potentiating mGlu5 Modulation of NMDAR Currents. Neuron 86, 1029–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansley BJ, Conn PJ, 2018. The therapeutic potential of metabotropic glutamate receptor modulation for schizophrenia. Curr Opin Pharmacol 38, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B, 2010. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol 639, 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai CA, Zhang Y, Howland JG, 2013. Effects of acute restraint stress on set-shifting and reversal learning in male rats. Cogn Affect Behav Neurosci 13, 164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Guzman YF, Guedea AL, Huh KH, Gao C, Schwarz MK, Radulovic J, 2010. Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biol Psychiatry 68, 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF, 1999. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron 23, 583–592. [DOI] [PubMed] [Google Scholar]
- Vertes RP, 2004. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. [DOI] [PubMed] [Google Scholar]
- Walker AG, Wenthur CJ, Xiang Z, Rook JM, Emmitte KA, Niswender CM, Lindsley CW, Conn PJ, 2015. Metabotropic glutamate receptor 3 activation is required for long-term depression in medial prefrontal cortex and fear extinction. Proc Natl Acad Sci U S A 112, 1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF, 1998. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron 21, 707–716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Schematic. Control experiments were performed where mice underwent restraint stress prior to a standard (no reversal) operant test session. (b) Stress does not decrease the number of correct (active) holepokes on a continuous schedule of reinforcement (F6,6 = 1.13; n.s.) (N = 7 mice). (c) Stress does not affect response accuracy in control test sessions. (d) Stress does not alter the latency to collect the food reward. (e) Stress does not alter the number of holepokes on a progressive ratio (PR) schedule of reinforcement (F3,11 = 0.17; n.s.) (N = 12). (f) Stress does not affect the number of rewards earned on a PR schedule (F3,11 = 0.32; n.s.). (g) Stress does not alter the latency to collect the food reward (F3,11 = 0.24; n.s.). PR, progressive ratio.
(a) Schematic for behavioral experiments. On the day of testing only, mice underwent an operant test session where the active holepoke designation was reversed and VU0409551 or vehicle was administered 30-minutes beforehand. (b) VU0409551 administration did not alter the number of correct responses across reversal learning sessions (main effect: F1,6 = 0.02, n.s; interaction: F6,36 = 0.37, n.s.) (N = 4 mice/group). (c) VU0409551 did not affect the percent of correct responses elicited during the reversal test session. (d) VU0409551 did not alter the days to reach criteria following the reversal session. (e) Control experiments were performed where VU0409551 was administered to control or stressed mice prior to a standard (no reversal) operant test session (N = 6). There was no main effect of session (F6,60 = 0.67; n.s.) or stress (F1,10 = 0.03; n.s.) on food-seeking behavior under these standard conditions. (f) VU0409551 did not alter the ability to discriminate between active and inactive operandi. (g) VU0409551 did not alter the latency to collect the food reward. VU551, VU0409551.