Abstract
Individuals with ASD have increased rates of depression compared to the general population. Repetitive cognition is a core feature of ASD; in typically developing adults, repetitive cognition has been associated with attentional biases to negative emotional material and increased prospective depression risk. We compared adults with ASD to typically developing adults with depression and never-depressed controls, using a paired preference paradigm sensitive to affective biases in the context of repetitive cognition. Both clinical cohorts oriented faster to negative social-emotional material and spent less time overall on positive material, compared to healthy controls. Exploratory analyses within ASD revealed specific influences of repetitive behavior on patterns of affective bias. Findings help pinpoint susceptibilities in ASD that may confer increased risk for depression.
Keywords: autism spectrum disorder, mood, rumination, repetitive thinking, negativity bias, eye-tracking
Individuals with ASD reportedly experience depression at higher rates than the typically developing population (Buck et al., 2014; Kerns, Roux, Connell, & Shattuck, 2016; Lugnegard, Hallerbäck, & Gillberg, 2011; Mattila et al., 2010; Mazefsky et al., 2012) and tend to endorse high levels of depressive symptoms, even among those who do not meet clinical criteria for comorbid depression diagnoses (Gotham, Unruh, & Lord, 2015). Reports of increased prevalence of depression, coupled with overlap between autistic and depressive symptomology (e.g., social withdrawal, perseverative rumination), has opened the door to an emerging literature exploring mechanisms that may be common to both disorders.
Depression prevalence in ASD is intuitive from both psychosocial and biological standpoints. Individuals with ASD specifically show impairments in navigating and maintaining reciprocal social relationships (Howlin, Goode, Hutton, & Rutter, 2004; Lord et al., 2000). Such challenges leave these individuals particularly susceptible to loneliness and lack of social connectedness, both of which are reported at increased rates in ASD (Bauminger & Kasari, 2000; White & Roberson-Nay, 2009; Lasgaard et al., 2010) and are prospective predictors of depressive symptomology in typically developing populations (Cacioppo, Hughes, Waite, Hawkley, & Thisted, 2006; Qualter, Brown, Munn, & Rotenberg, 2010). Negative interactions with or feedback from peers may also affect cognitive attributions about self-worth in the ASD population (Gotham, Unruh, & Lord, 2015; Mazefsky et al., 2012). Therefore, the presence of autism likely increases psychosocial risk factors for development of depression (in line with Caron & Rutter, 1991). Evidence suggests that family members of people with ASD also have elevated rates of mood disorders even prior to the birth of a child with developmental disability, implicating potentially increased genetic risk for psychiatric comorbidities in individuals with ASD (Bolton, Pickles, Murphy, & Rutter, 1998; DeLong, 2004; Piven & Palmer, 1999).
Repetitive thinking is common to the behavioral presentations of both ASD and depression. In depression, repetitive thought patterns are often focused on negative mood state and labeled rumination. Rumination has been shown to prospectively predict depression in typically developing populations (Nolen-Hoeksema, 2000) and to perpetuate negative mood states, therefore prolonging and/or increasing the severity of depressive episodes (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). Repetitive thinking is also evident in the core features of ASD, such as circumscribed interests and insistence on sameness (American Psychiatric Association, 2013). In the framework of NIH Research Domain Criteria (RDoC), rumination is classified as a “loss” behavioral construct within Negative Valence Systems, whereas the repetitive symptoms of autism are classified as “habit” behavioral constructs within the Positive Valence Systems matrix. Although uniquely identified by valence within the RDoC framework, both loss behavior and habitual behavior are thought to share common neural mechanisms, including the dorsal and ventral striatum and subregions of the medial prefrontal cortex (e.g., de Wit et al., 2012; Drevets, 1999; Naranjo, Tremblay, & Busto, 2001). Thus repetitive thinking is one promising endophenotype for investigating shared neurobiology in autism and depression, and in turn, for identifying intervention targets for this comorbidity.
In a 2014 study of psychosocial correlates of depression in ASD, participants who perceived greater autism-related impairment in themselves also exhibited higher levels of depressive symptoms (Gotham et al., 2014). Of note, this association was most robust in individuals who displayed high levels of rumination (defined as passive, perseverative thinking about one’s own distress; Nolen-Hoeksema & Morrow, 1991). Further, rumination scores were significantly associated with insistence on sameness, a core and repetitive feature of ASD, in this adult sample. These findings lend support to the idea that repetitive thinking may be a moderating factor in the intersection between autism and depression.
Studies have found associations between rumination and biased processing of negative emotional information in the general population (Joormann, Dkane, & Gotlib, 2006). Specifically, in individuals with higher levels of rumination, attention is more often biased toward negative information and away from task-relevant cues during attentional tasks (e.g., Donaldson, Lam, & Mathews, 2007; Gotlib et al., 2004; Gotlib & Cane, 1987; Segal, Gemar, Truchon, Guirguis, & Horowitz, 1995). Paired viewing paradigms are commonly used to assess biases in attention. In affective adaptations of this method, biased attentional orienting and attention maintenance can be measured by pairing an emotional face with an identical face of neutral valence. This paradigm has been used to demonstrate attentional differences specific to dysphoric (i.e., sad content) stimuli, such that typically developing individuals with depression displayed longer initial fixation duration and longer total fixation duration to sad images, compared to non-depressed controls (Duque & Vázquez, 2015). In that sample, increased attention to sad faces was significantly correlated with the severity of depressive symptoms.
The purpose of this paper was to examine affective bias in adults with ASD (a) in comparison to typically developing adults with and without depression, and (b) in relation to repetitive thinking and depressive symptoms within ASD. Thus, the goals of the current study were to explore:
Affective attentional biases in autism: We hypothesized that individuals with ASD would attend to emotional stimuli in patterns more similar to typically developing participants with depression (TD-Dep), reflected as increased attention to sad images and decreased attention to happy images, compared to never-depressed TD controls (TD-Con);
Repetitive thinking in autism: We hypothesized that individuals with ASD who exhibit greater intensity in behaviors likely associated with repetitive cognition (circumscribed interests, CI; insistence on sameness, IS) would also demonstrate higher scores on measures of rumination and depression;
The influence of repetitive thinking and depressive symptoms on affective bias in autism: Finally, we hypothesized that measures of repetitive and depressive symptoms would be positively associated with increased attention to negative emotional images (angry, sad) among participants with ASD.
This study provides one of the first explorations of key behavioral similarities between individuals with ASD and those with depression in a novel direct comparison, with the goal of outlining potential susceptibilities in ASD that may be co-opted to result in the onset of depression.
Methods
Participants
Three diagnostic cohorts were recruited for this study: Those with an autism spectrum disorder (ASD; n = 28), typically developing adults with a current depressive disorder (TD-Dep; n = 24), and typically developing adults with no history of anxiety, depression, or family history of ASD (TD-Con; n = 24). Participants were recruited from national and local (Vanderbilt Kennedy Center Study Finder) resources. Eligibility criteria included verbal IQ>=80; verbal fluency per Autism Diagnostic Observation Schedule, 2nd edition (ADOS-2; Lord et al., 2012) module selection criteria; reading level >= 5th grade; at least 20/30 vision at 80 cm on the Snellen eye chart; and no history or concerns of psychotic or bipolar disorders, current substance use disorders, or uncorrected vision problems or ocular abnormalities. Participants in the autism cohort had existing diagnoses of Asperger syndrome, autism, or ASD, which were confirmed with the ADOS-2. Participants in the depression cohort had existing diagnoses of a current unipolar depressive disorder, which were confirmed with the Structured Clinical Interview for DSM Disorders (SCID-5; First, Gibbon, Spitzer, Benjamin, & Williams, 1997). Table 1 provides demographic information by cohort. Our sample was not sex-matched in order to better represent the respective male and female skewing that is present in the autism and depression populations. However, as our sex distributions reflect, we placed specific emphasis on recruitment of females with ASD and males with depression in an effort to balance these samples. Procedures included a telephone screening, followed by 1-2 lab visits, in which the current task was one in a larger study.
Table 1.
Sample demographics and self-report descriptives
| Mean (SD) Range |
TD-Con (N=24) |
ASD (N=28) |
TD-Dep (N=24) |
Group differences* |
|---|---|---|---|---|
| N | 24 | 28 | 24 | |
| Age in Years | 25.22 (5.07); 18-35 | 23.44 (4.73); 18-33 | 25.43 (4.76); 19-34 | n.s. |
| Verbal IQ | 115.61 (12.90); 90-147 |
104.16 (10.81); 81-120 | 112.13 (8.22); 84-124 | F(2, 68)=7.124, p=.001; TD-Con>ASD, p<.001 TD-Dep>ASD, p=.034 |
| Nonverbal IQ | 108.35 (13.91); 79-136 | 104.00 (15.46); 71-141 | 107.61 (9.79); 86-126 | n.s. |
| Gender (% F) | 61% | 19% | 54 % | χ2(1)=6.4, p=.01(ASD<TD-con) χ2(1)=6.9, p=.03(ASD<TD-dep) |
|
| ||||
| BDI-II raw score | 2.18 (2.17); 0-8 | 13.08 (10.43); 0-34 | 26.70 (6.94); 17-45 | F(2, 68)=62.566, p<.001; ASD>TD-Con, p<.001 TD-Dep>TD-Con, p<.001 TD-Dep>ASD, p<.001 |
| RRS total score | 30.96 (7.65); 23-53 | 43.80 (13.81); 23-74 | 54.57 (7.15); 39-68 | F(2, 68)=31.287, p<.001; ASD>TD-Con, p<.001 TD-Dep>TD-Con, p <.001 TD-Dep>ASD, p=.001 |
| RBS-R total score | 3.33(4.58); 0-15 |
21.4(23.40); 0-79 |
12.1(15.0); 0-56 |
F(2, 68)=8.896, p<.001; ASD>TD-Con, p<.001 |
| RBSR-IS severity score | .78 (1.38); 0-5 | 6.44 (6.85); 0-27 | 4.43 (7.51); 0-30 | F(2, 68)=5.523, p=.006; ASD>TD-Con, p=.004 |
| CI severity score | 8.09 (1.81); 5-12 | 11.88 (3.54); 7-20 | 9.52 (2.19); 7-16 | F(2, 68)=12.577, p<.001; ASD>TD-Con, p<.001 ASD>TD-Dep, p=.008 |
| SRS T-score | 43.00 (4.08); 36-54 | 65.36 (11.04); 45-83 | 55.48 (9.20); 43-76 | F(2, 68)=39.569, p<.001; ASD>TD-Con, p<.001 ASD>TD-Dep, p=.001 TD-Dep>TD-Con, p=.001 |
Group differences reported only when Fisher’s LSD post-hoc or Chi-square tests of association significant at p < .05.
Stimuli and task
The preferential viewing task used for this study was comprised of paired arrays containing both an emotional and neutral facial expression made by the same actor. Arrays were characterized by the presence of a sad, angry, or happy face, with 28 arrays per condition. Arrays were balanced by gender (14 males and 14 females per condition) and laterality of emotional image (right vs. left balanced). The stimuli described here were used, with permission, as an exact replica of the original version of the task developed by Duque and colleagues (Duque & Vázquez, 2015). Face images were modified from the Karolinska Directed Emotional Faces (KDEF) database (Lundqvist, Flykt, & Öhman, 1998), by using an oval window frame to remove salient aspects such as hair. Emotional intensity for these images has been validated in previous studies (Schaefer, Nils, Sanchez, & Philippot, 2010). Images were presented in grayscale to better account for low-level stimulus properties. Refer to Figure 1 for task schematic.
Figure 1.
The affective bias task (a) Sample task schematic using Karolinska Directed Emotional Faces (KDEF) images. (b) Sample KDEF images from each affective category represented in the task
The passive viewing task was comprised of 84 trials (28 images per category), with affective category randomized across trials. Each trial began with a presentation of a white fixation cross in the center of the screen, displayed at a variable duration of 1-4 seconds followed by the presentation of an affective array for 5 seconds. Participants were instructed to keep their eyes on the screen at all times and to focus on the fixation cross when it was present, but were given no further instructions.
Eye-tracking procedures
Testing occurred in a research laboratory. Participants sat approximately 60 cm from a 1,024 horizontal × 768 vertical 17-inch display and viewed stimuli subtending a visual angle of 16.1 degrees. Eye movements were recorded with a Tobii X2-60 eye tracker (Tobii Technology, Stockholm, Sweden). The system uses an infrared light to produce reflection patterns on the corneas of the eye and monitors these reflections relative to the eye’s position. This system samples at a rate of 60 Hz. This eye tracking system is mounted on the computer monitor, and therefore does not interfere with data collection. The system allows for head movement within a cubic space of 30×15×20 cm from a distance of 60 cm, allowing the participants to view in a naturalistic manner.
Self-report measures
The Beck Depression Inventory, second edition (BDI-II; Beck, et al., 1996) is a widely used scale of depressive symptoms for adolescents and adults. This self-report measure is designed to capture depression-related emotions, physical and psycho-somatic symptoms, and lifestyle changes. This measure has been found to have high internal consistency and strong convergent validity (Dozois, Dobson, & Ahnberg, 1998), and is commonly used in adult ASD samples (Cederlund, Hagberg, & Gillberg, 2010; Crane, Goddard, & Pring, 2013; Gotham et al., 2014).
The Ruminative Response Scale (RRS; Nolen-Hoeksema & Morrow, 1991) is a frequently used measure of self- and symptom-focused thoughts that may persist as patterns of rumination, including brooding and reflective pondering. This scale was developed to assess rumination that is related to, but not confounded by depression (Treynor, Gonzalez, & Nolen-Hoeksema, 2003).
The Interest Scale (Turner-Brown et al., 2011) is used to collect detailed information on the presence and severity of circumscribed interests (CI). The severity score characterizes an individual’s strongest interest, including the degree to which this interest is shared with other people (social involvement), and the flexibility, frequency, intensity, interference, and accommodation of that specific interest. These clinical aspects of CI are known to be associated with cognitive-affective processes, including enhanced reward processing (Cascio et al., 2014) as well as repetitive engagement and/or discussion of these interests (South, Ozonoff, & McMahon, 2005). For these reasons, we included this measure as a reasonable proxy for assessing repetitive cognitive patterns in ASD.
The Repetitive Behavior Scale-Revised (RBS-R; Lam & Aman, 2007) identifies the frequency and severity of five categories of repetitive behavior, including motor stereotypy, repetitive self-injury, compulsions, insistence on sameness, and restricted interests. The self-report version of this questionnaire was used for this study, with analyses focusing on the insistence on sameness domain raw total score (RBSR-IS).
Analysis of task performance
The intent of the paired preference task is that the participants will look at each slide for a sufficient amount of time to observe both images. Therefore, we developed a method to exclude participants based on insufficient total look time per slide, so as to eliminate potential bias from the data. Total viewing time was calculated for each trial; any trial with less than 70% total viewing time (3.5 seconds) was excluded from analyses. Further, any participant with fewer than 60% of trials included per condition was excluded from group means due to insufficient data. Applying these criteria resulted in exclusion of 2 participants in the TD-Con cohort, 8 participants in the ASD cohort, and 2 participants in the TD-Dep cohort. Analyses revealed that excluded participants did not differ from the included participants on IQ (t = −.094, p = .926), age (t = −.672, p = .532), autism severity (ADOS; t = .486, p = .643) or depression severity (BDI; t = .361, p = .719).
Eye-tracking variables
Gaze patterns were analyzed as a result of conducting fixation analyses. Fixations were classified using the Tobii Studio I-VT filter, which defines fixations as gaze moving at a velocity slower than 30 degrees per second for at least 60 milliseconds. Three dependent variables were extracted from the data collected: (a) Prioritization: the latency to first fixation on each stimulus type, which measures attention capture and orienting; (b) Disengagement: The latency between first fixation on the emotional stimulus and subsequent fixation on the neutral stimulus, indicating duration/maintenance of initial attention to the emotional stimulus; and (c) Preference: the proportion of onscreen fixation time devoted to each image type, relative to total time spent on the stimulus array, indicating overall attention maintenance.
Statistical analysis
Repeated measures analysis of variance (RM-ANOVA) was conducted on each of the primary variables, with emotion type (neutral, sad, angry, happy) as the within-subjects variable and group (ASD, TD-Dep, TD-Con) as the between-groups variable. Analyses were performed with sex (male, female) included as a covariate in the model. However, as this covariate was nonsignificant in all analyses, with small effect sizes (all ps > .05 and partial eta squared < .025) and gender differences were not part of a priori hypotheses, sex was excluded from the model reported in Results. Post-hoc univariate ANOVA analyses also were performed to assess single condition between-group differences, and paired samples t-tests were conducted for within-group comparisons. Omnibus F statistics were interpreted using a threshold of p < .05 for statistical significance. Follow-up analyses were corrected for multiple comparisons using Tukey’s HSD.
Two additional sets of analyses were performed on participants with ASD only. In the first set of analyses, a series of Pearson correlations was performed between measures of depression symptomology (BDI-II), ruminative thinking (RRS), insistence on sameness (RBSR-IS), and circumscribed interest (CI) severity on the Interests Scale. In the second set of analyses, deemed exploratory because of sample size, participants with ASD were grouped by median split on these psychometric measurements. Analogous RM-ANOVAs were performed to compare eye-tracking variables between emotions (sad, angry, happy) for “high” and “low” participant groups and were interpreted using reported effect sizes (Cohen’s d) rather than significance values from F statistics.
Results
Aim 1: Affective bias in ASD
Prioritization
To determine whether participants differed in latency to view a specific category of emotional faces, we conducted a series of 2 × 3 RM-ANOVAs, each comparing two emotions (one always neutral), by three groups (ASD, TD-Dep, TD-Con). Group means for latency in all pairs of emotion comparisons are displayed in Figure 2.
Figure 2.
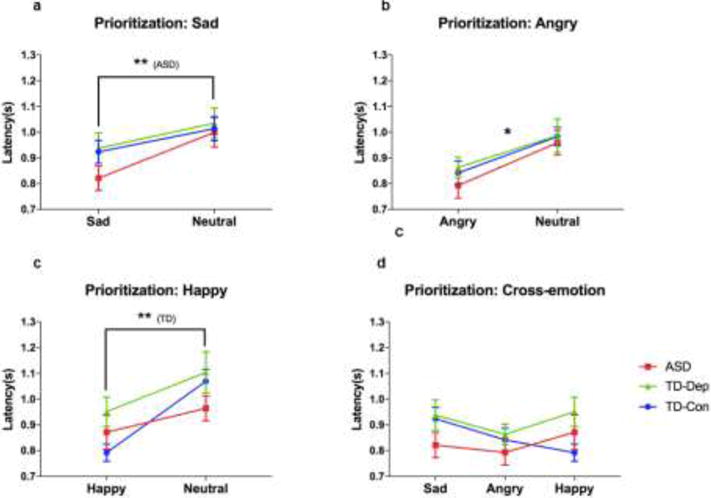
Prioritization of affective stimuli for participants with ASD, TD-Dep, and TDCon (a) Participants with ASD looked significantly faster to sad faces than neutral faces. (b) Across groups, participants looked faster to angry faces than neutral faces. (b) TDCon participants looked more quickly to happy faces than neutral faces. (d) TD-Con participants looked most quickly to happy faces, while participants with ASD and TDDep looked most quickly to angry faces. Error bars indicate standard error of the mean. *, p < .05; **, p < .01; ASD, autism spectrum disorder; TD-Con, typically developing controls; TD-Dep, typically developing with depression
For comparisons between Sad and Neutral, there was no significant emotion × group interaction (F(2, 62)= 1.07, p = .348) or main effect of group (F(1, 62) = .703, p = .499). There was a main effect of emotion (F(1, 62) = 20.398, p < .001), indicating that across groups, participants were faster to fixate on sad faces than neutral faces. Follow-up paired-samples t-tests revealed that this effect was driven by participants in the ASD group, who displayed significantly faster fixations to sad than neutral faces (t = -3.960, p = .001), while this effect did not reach significance in TD-Con (t = -1.981, p = .061) or TD-Dep participants (t = -1.972, p = .062).
For comparisons between Angry and Neutral faces, there was no significant emotion × group interaction (F(2, 62)= .185, p = .831) or main effect of group (F(1, 62) = .374, p = .689). Again there was a main effect of emotion (F(1, 62) = 25.177, p < .001), indicating that across groups, participants were faster to fixate on angry faces than neutral faces. Follow-up paired-samples t-tests revealed that this effect was present regardless of diagnostic status.
Similarly, for happy faces, there was no emotion × group interaction (F(2, 62)= 2.218, p = .117) or main effect of group (F(1, 62) = 1.582, p = .214). There was a main effect of emotion (F(1, 62) = 22.714, p < .001), indicating that across groups, participants were faster to fixate on happy faces than neutral faces. Follow-up paired-samples t-tests revealed that this effect was driven by participants in the TD-Con group, who displayed significantly faster fixations to happy than neutral faces (t = -7.332, p < .001), while this effect did not reach significance for participants in the TD-Dep (t = -1.941, p = .066) or ASD groups (t = -1.292, p = .212).
Prioritization: Post-hoc analyses across emotion conditions
Participants always saw emotional faces paired with a neutral face from the same actor. However, we conducted a 3 (emotion: sad, angry, happy) × 3 (group: ASD, TD-Dep, TD-Con) RM-ANOVA to determine whether participants differed in absolute latency scores across emotional faces. Group means are displayed in Figure 2. There was a significant main effect of emotion (F(1, 62) = 3.322, p = .039), in which post-hoc analyses indicated latencies to angry faces were significantly shorter than latencies to sad faces (p = .010). There was also a significant emotion × group interaction (F(2, 62)= 3.641, p = .008), although there was no significant main effect of group (F(1, 62) = 1.172, p = .317). Follow-up ANOVA revealed that this significant interaction was driven by opposite patterns of latency to Happy in TD-Con versus the two clinical groups.
Put together, these results indicate that participants in clinical groups (ASD, TD-Dep) oriented most quickly to negative emotional images (angry faces), and were slowest to orient to positive emotional images (happy faces). In contrast, never-depressed TD controls oriented most quickly to happy faces, compared to negative emotional images (angry and sad faces).
Disengagement
A 3 (emotion: sad, angry, happy) × 3 (group: ASD, TD-Dep, TD-Con) RM-ANOVA was conducted to determine whether participants differed in duration to stimulus disengagement across emotional stimuli. Group means are displayed in Figure 3. There was no significant emotion × group interaction (F(2, 62)= 1.342, p = .259) or main effect of group (F(1, 62) = .420, p = .659), but we noted a main effect of emotion (F(1, 62) = 3.169 p = .046) in which disengagement was more delayed during viewing of angry faces compared to viewing of sad (p = .01) and happy (p = .048) faces. This suggests that angry faces held the initial attention of participants longer than did sad or happy faces. Planned follow-up ANOVA revealed no group differences for disengagement for any emotion condition.
Figure 3.
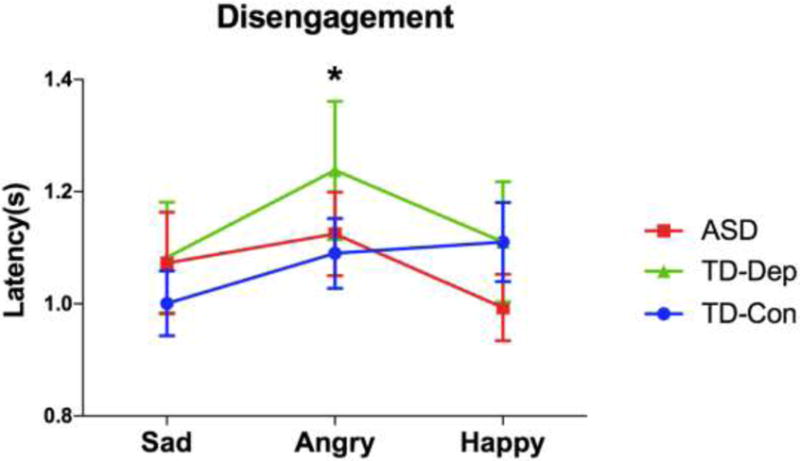
Latency to disengage from affective stimuli for participants with ASD, TD-Dep and TD-Con. Across cohorts, participants were slower to disengage from angry faces, compared to sad and happy. Error bars indicate standard error of the mean. ASD, autism spectrum disorder; TD-Con, typically developing controls; TD-Dep, typically developing with depression
Preference
A 3 (emotion: sad, angry, happy) × 3 (group: ASD, TD-Dep, TD-Con) RM-ANOVA was conducted to determine whether participants differed in overall look time to emotional stimuli (note that “disengagement” discussed above was the viewing duration only during the first “visit” to that particular emotional face). Group means are displayed in Figure 4. There was a significant emotion × group interaction (F(2, 62)= 7.300, p < .001). There was also a significant main effect of emotion (F(1, 62) = 3.698, p = .028), but no significant main effect of group (F(1, 62) = 2.443, p = .095). Follow-up ANOVA revealed that this interaction was driven by mean differences in the TD-Con group. This group displayed increased preference for happy faces compared to ASD and TD-Dep groups (F(1, 62) = 16.103, p < .001), similar to the pattern of findings noted for prioritization (latency to first look).
Figure 4.
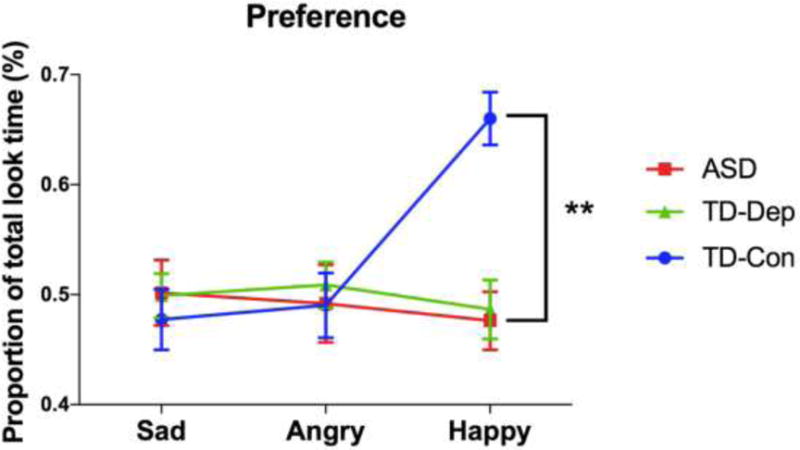
Preference for affective stimuli for participants with ASD, TD-Dep, and TDCon. Participants in the TD-Con cohort spent the most time looking at happy faces, compared to participants with ASD and TD-Dep. Error bars indicate standard error of the mean. **, p < .01; ASD autism spectrum disorder, TD-Con typically developing controls, TD-Dep typically developing with depression
Aim 2: Repetitive thinking in ASD
To test the hypothesis that autism-specific repetitive thinking, as indexed by intensity of circumscribed interests and insistence on sameness, is associated with depression-specific repetitive thinking (rumination) and depression symptomology, we performed a series of Pearson bivariate correlations between psychometric self-report instruments, focusing only on participants in the ASD cohort. Insistence on sameness (RBSR-IS) was significantly correlated with depression-specific repetitive thinking (RRS; r = .584, p = .009) and depressive symptomology (BDI-II; r = .640, p = .003). Similarly, CI severity also showed significant correlations with the RRS (r = .709, p = .001) and BDI-II (r = .763, p < .001). These results suggest that individuals with ASD who have more severe patterns of ASD-specific repetitive thinking also experience a greater intensity of ruminative thought and depressive symptoms.
Aim 3: Influence of repetitive thinking and depressive symptoms on affective bias in ASD
We next examined the effect of repetitive thinking and depressive symptoms on patterns of visual attention in ASD by grouping participants according to “high” and “low” scores within self-report measures of depression, rumination, and repetitive behavior. Participants with ASD were grouped independently by median split on BDI-II total scores, RRS total scores, RBSR-IS severity scores, and CI severity scores. Separate 3 (emotion: angry, sad, happy) × 2 (group: high, low) RM-ANOVAs were conducted for each eye-tracking variable (prioritization, disengagement, preference) across each psychometric measure. These analyses have been deemed exploratory because of sample size and therefore are interpreted in the context of effect sizes (Cohen’s d) rather than significance values from F statistics. For ease of interpretation in these analyses, prioritization was converted to a difference score that indexed the latency to fixate on an emotional image, relative to the latency to fixate on the neutral image (i.e. prioritization = latency to emotional image – latency to neutral image).
Across measures, individuals with higher repetitive/depressive symptom scores were faster to fixate on angry faces than those with lower scores (BDI-II: F(1,17) = 2.529, p = .129, d = .75; RRS: (F(1,17) = 3.160, p = .093, d = .86; RBSR-IS: (F(1,17) = 7.517, p = .014, d = 1.38; CI: F(1,17) = 3.647, p = .073, d = .94). A similar pattern was noted for sad faces, with a notable exception: Participants with higher insistence on sameness scores were slower to fixate on sad faces (RBSR-IS: F(1,17) = 2.624, p = .124, d = .81) and faster to fixate on happy faces (F(1,17) = 2.485, p = .133, d = .98) compared to peers with low insistence on sameness scores. Finally, participants with more severe circumscribed interests spent more time looking at sad faces than low-severity peers (CI: F(1,17) = 3.800, p = .068, d = .96).
Discussion
The purpose of this paper was to examine affective bias in adults with ASD (a) in comparison to typically developing adults with and without depression, and (b) in relation to repetitive thinking and depressive symptoms within ASD. We hypothesized that individuals with ASD would demonstrate patterns of attention more similar to individuals in the TD-Dep cohort, reflected as increased attention to sad images and decreased attention to happy images, compared to never-depressed TD controls. Results revealed attentional similarities between ASD and TD-Dep cohorts, as well as patterns unique to ASD. Across both of these clinical cohorts, participants showed biased attention toward angry stimuli, reflected as a prioritization of angry images and an increased latency to disengage with this information. ASD and TD-Dep cohorts did not display an attentional bias toward happy stimuli, such that neither group prioritized fixating on happy faces and showed decreased overall preference for happy images compared to the TD-Con cohort. Finally, participants with ASD were unique in prioritization of (e.g., significantly faster attending to) sad images.
We additionally sought to understand how markers of repetitive thinking traditionally conceived of as “autism-specific” were related to “depression-specific” repetitive thinking (e.g., rumination), and further, to what degree these forms of repetitive thinking appear to influence patterns of affective bias. We hypothesized that more severe behaviors associated with repetitive thought in ASD (insistence on sameness, circumscribed interests) would be associated with higher rumination and depression scores, and that individuals with ASD who exhibited high scores on various repetitive thinking measures would also demonstrate increased attention to negative emotional images. Results revealed strong positive correlations between both insistence on sameness and circumscribed interest severity and depressive symptoms and depressive rumination. Exploratory analyses suggested both general and specific influences of repetitive cognition on patterns of emotional attention in ASD: Faster initial attention to angry stimuli was observed in participants who endorsed greater repetitive cognition and depression severity. These results suggest that, similar to general depression findings, negative biases span attentional, cognitive, and affective domains when present in ASD. Because our autism-specific (IS and CI) and depression-specific (BDI-II and RRS) measures were highly correlated, it is unclear whether autism-specific or depression-specific mechanisms were driving the observed bias to faster fixations on negative affective stimuli (or indeed to what degree the emotion bias contributed to the thinking patterns and mood).
We did, however, observe some specific effects of autism-related cognition on distinct patterns of emotional attention. Along with faster look times to angry faces, increased overall attention to sad faces was noted in participants with more severe circumscribed interests, indicating greater general bias toward negative stimuli in these individuals. In contrast, participants with more severe insistence on sameness showed diverse patterns of attention, looking more quickly to angry and happy faces but more slowly to sad faces. Of note, we had collected post-task ratings of valence and arousal of the emotional images, so we conducted post hoc analyses to assess whether the high-IS ASD group had an anomalous response to sad images in comparison to other conditions or cohorts. Controlling for multiple comparisons, there were no significant differences in ratings of valence and arousal across any cohorts or IS-groups for any emotion conditions. We thus infer that slower orienting responses to sad images in the high-IS ASD group do not appear to be an artifact of difficulty decoding this particular emotion. However, it was noteworthy that high-IS ASD participants had the highest mean ratings of arousal to sad faces and low-IS ASD participants the lowest (Sad Arousal: TD-con M=1.75, SD=0.91; TD-dep M=1.84, SD=0.87; ASD Low-IS M=1.23, SD=0.56; ASD High-IS M=2.00, SD=0.91). It is possible that the ASD High-IS group’s slower initial response to sad images may be associated with affective blunting to highly affectively arousing stimuli. The current findings of a more comprehensive negativity bias associated with CI and the absence of a dysphoric bias (or possible avoidance) associated with IS suggest that different mechanisms may underlie these cognitive patterns and differentially contribute to risk for developing depression. However, more precise measurement tools are needed to determine the degree to which repetitive cognition is associated with these and other repetitive behaviors.
Repetitive cognition likely involves coordination between multiple cortical networks, including those devoted to introspection and memory, salience detection, and executive function (Kelley et al., 2002; Murray, Schaer, & Debbané, 2012), although the specific mechanisms contributing to its pathological forms (e.g., rumination, insistence on sameness) have yet to be determined. In depression, negative patterns of repetitive thought are hypothesized to develop via dysfunctional modulation of large-scale neural networks, including the default mode network (DMN) and salience network (Burrows, Usher, Schwartz, Mundy, & Henderson, 2016). This hypothesis is supported by studies indicating that individuals with depression show variation in functional connectivity between key nodes of these networks: the insula and the anterior cingulate cortex (ACC; Berman et al., 2014; Connolly et al., 2013), which is related to rumination severity (Kaiser et al., 2015). There is also evidence for dysfunctional connectivity within the DMN and salience network in ASD (e.g., Hahamy, Behrmann, & Malach, 2015; Kennedy & Courchesne, 2008; Nomi & Uddin, 2015; Uddin et al., 2013), although variation in the connectivity between these two networks has yet to be explicitly tested. Interestingly, a recent study demonstrated that activity in key nodes of the DMN (ACC) and salience network (insula) was able to distinguish individuals with ASD from peers during autism-specific repetitive cognition (engagement with items of circumscribed interest; Cascio et al., 2014). These studies provide preliminary but converging evidence that functional alterations in the DMN and salience network may contribute to an endophenotype that is common to both autism and depression and warrant future cross-diagnostic research.
Comparison of current findings to the depression literature
The affective bias task used in this study was originally developed to assess patterns of emotional bias in adults with major depressive disorder (MDD; Duque & Vázquez, 2015). In a sample of 16 adults with MDD and 34 never-depressed controls, Duque and colleagues reported significant biases toward negative information in the MDD cohort, such that these individuals made significantly longer initial fixations to sad faces and spent a greater amount of total time viewing these faces compared to healthy controls, the latter of which was positively correlated with BDI-II scores. The Duque et al. MDD cohort also demonstrated marginally less overall attention to happy faces, compared to controls. In the current study, we did not find similar evidence of a negativity bias in our TD-Dep cohort. While BDI-II scores were comparable between study samples, two notable differences may have accounted for the current lack of replication. First, these study samples were from different western cultures (Spain versus United States), which has been shown in some contexts to influence differences in emotional attention that may not be detected in emotion labeling or valence ratings (Ko, Lee, Yoon, Kwon, & Mather, 2011). Perhaps more importantly, our TD-dep sample was comprised of significantly fewer females than Duque et al. (54%, compared to 87%) in order to compare this subsample to the male-dominant ASD population. Exploration of rumination and attention bias by sex within ASD is an important future direction of this work.
Limitations
Several limitations should be considered when interpreting and generalizing the results presented here. First, statistical power was limited due to a small sample size across the three groups. The value of this small sample, however, lies in the novel direct comparison of individuals with autism to those with depression, with both clinical cohorts characterized according to gold standard diagnostic measures (ADOS-2, SCID-5). Second, this study limited inclusion to individuals with verbal fluency and a minimum of 5th grade-level reading comprehension, which is not representative of the full spectrum of individuals with ASD. These inclusion criteria were deemed necessary in order to use self-report measures of internal cognitive styles and psychiatric symptomology. Finally, it is well known known that visual attention can be guided by reward, cognition, and stimulus salience (Chelazzi, Perlato, Santandrea, & Della Libera, 2013; Connor, Egeth, & Yantis, 2004), and so caution must be exhibited when drawing conclusions about the mechanistic processes underlying the observed gaze patterns. Stimuli were presented in grayscale and cropped to include only facial features to equate low-level salience properties across images and counterbalanced to account for presentation location biases; therefore, it is unlikely that emotion condition effects were driven by such processes. Although our study and Duque et al. (2015) demonstrated relationships between autism and depression symptomology and gaze patterns, respectively, determination of the physiological or psychological processes underlying these patterns will require multi-method studies (e.g., joint eye-tracking and functional imaging).
Implications for future research
Immediate next steps for this study include replication in a larger sample to better assess potential sex effects and to set the stage for future longitudinal study of ASD characteristics that may be risk factors for the development of co-occurring depression. More work is needed to determine mechanistic similarities between these two populations. The affective bias task presented here would be easily applicable to functional imaging or electrophysiological studies, which may shed light on neural mechanisms underlying repetitive cognition. Specifically, these studies should capitalize upon existing evidence in depression of dysfunctional connectivity between networks supporting salience detection and executive function (Berman et al., 2014; Burrows et al., 2016; Connolly et al., 2013), which may contribute to patterns of cognitive rigidity. Finally, we suggest future work to probe etiological similarities between the two groups. For example, behavioral genetic studies of depression have found that individuals with specific polymorphisms (two copies of the short allele) of the promotor region of the serotonin transporter gene (5HTTLPR, SLC64A) show increased rumination in response to current life stress (Clasen, Wells, Knopik, McGeary, & Beevers, 2011; Canli et al., 2006) and in adults who have been exposed to early life stress (Antypa & Van der Does, 2010), implicating this genetic variant as a moderator in depression susceptibility. Etiological studies of ASD have yielded mixed findings in variants of this gene conferring risk for ASD although converging evidence does indicate overtransmission of the 5HTTLPR short allele in this population (Devlin et al., 2005; Huang & Santangelo, 2008; McCauley et al., 2004; Sutcliffe et al., 2005). There is also limited evidence to suggest heterogeneity in SLC64A is related to rigid behavior (McCauley et al., 2004; Sutcliffe et al., 2005). Stratification of individuals with ASD based on allelic profile may reveal differential susceptibility for developing depression and ultimately provide opportunities for earlier and targeted intervention strategies. This work may also capitalize upon the substantial contribution of heritability to both ASD and depression (McGuffin, Katz, Watkins, & Rutherford, 1996; Sandin et al., 2014) by designing family-based studies to investigate repetitive cognition as an endophenotype for depression.
Conclusions
Repetitive cognition plays a critical role in both the onset and maintenance of depression and in the expression of autism. The increased prevalence of depression in the autism population suggests that while these symptoms differ practically, they may share common neural mechanisms. In the current study, we used an affective bias task to reveal similarities between autism and depression samples in visual attention to social-emotional stimuli. We further demonstrated that depression-specific and autism-specific repetitive thinking appear to be associated within ASD, and to correlate with negative attention biases. The latter finding was more clearly evident for those with more intense circumscribed interests. These commonalities suggest susceptibilities in ASD that may contribute to the development of depression.
Acknowledgments
Funding: This study was funded by grants from the National Institutes of Health (NIMH K01-MH103500, R01-MH113576, T32-MH18921; EKS NICHD U54HD08321).
Footnotes
Compliance with Ethical Standards
Ethical approval: This research was performed in accordance with the Declaration of Helsinki. All procedures described herein received ethical review and approval from the Institutional Review Board of Vanderbilt Medical Center (Behavioral Sciences Committee).
Informed consent: Informed consent was obtained from all individual participants included in the study. All participants were 18 years of age or older and served as their own legal representatives; we assessed capacity to provide informed consent for all participants, and obtained informed consent in writing from all participants, with consent forms approved by the board named above.
Contributor Information
Kathryn E. Unruh, Vanderbilt Brain Institute, Vanderbilt University, 1215 21st Ave S, Medical Center East, Room 10245, Nashville, TN 37215
James W. Bodfish, Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, Department of Hearing & Speech Sciences, Vanderbilt University, 1215 21st Ave S, Medical Center East, Room 10245, Nashville, TN 37215
Katherine O. Gotham, Department of Psychiatry and Behavioral Sciences, Vanderbilt University Medical Center, 1500 21st Ave S, Room 2272, Village at Vanderbilt, Nashville, TN 37212.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Vol. 5. Washington, D.C.: 2013. [Google Scholar]
- Antypa N, Van der Does AJW. Serotonin transporter gene, childhood emotional abuse and cognitive vulnerability to depression. Genes, Brain and Behavior. 2010;9(6):615–620. doi: 10.1111/j.1601-183X.2010.00593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauminger & Kasari, 2000; Howlin, Goode, Hutton, & Rutter, 2004; Lasgaard, Nielsen, Eriksen, & Goossens, 2010; Lord et al. 2000; White & Roberson-Nay, 2009; Bauminger N, Kasari C. Loneliness and friendship in high-functioning children with autism. Child Development. 2000;71(2):447–456. doi: 10.1111/1467-8624.00156. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK, et al. Beck depression inventory. 1996 Retrieved from http://www.nctsnet.org/content/beck-depression-inventory-second-edition.
- Berman MG, Misic B, Buschkuehl M, Kross E, Deldin PJ, Peltier S, et al. Does resting-state connectivity reflect depressive rumination? A tale of two analyses. Neuroimage. 2014;103:267–279. doi: 10.1016/j.neuroimage.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Pickles A, Murphy M, Rutter M. Autism, affective and other psychiatric disorders: patterns of familial aggregation. Psychological Medicine. 1998;28(2):385–395. doi: 10.1017/s0033291797006004. [DOI] [PubMed] [Google Scholar]
- Buck TR, Viskochil J, Farley M, Coon H, McMahon WM, Morgan J, Bilder DA. Psychiatric comorbidity and medication use in adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2014;44(12):3063–3071. doi: 10.1007/s10803-014-2170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows CA, Usher LV, Schwartz CB, Mundy PC, Henderson HA. Supporting the Spectrum Hypothesis: Self-Reported Temperament in Children and Adolescents with High Functioning Autism. Journal of Autism and Developmental Disorders. 2016;46(4):1184–1195. doi: 10.1007/s10803-015-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hughes ME, Waite LJ, Hawkley LC, Thisted RA. Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychology and Aging. 2006;21(1):140. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Canli T, Qiu M, Omura K, Congdon E, Haas BW, Amin Z, Lesch KP. Neural correlates of epigenesis. Proceedings of the National Academy of Sciences. 2006;103(43):16033–16038. doi: 10.1073/pnas.0601674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron C, Rutter M. Comorbidity in child psychopathology: Concepts, issues and research strategies. Journal of Child Psychology and Psychiatry. 1991;32(7):1063–1080. doi: 10.1111/j.1469-7610.1991.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Cascio CJ, Foss‐Feig JH, Heacock J, Schauder KB, Loring WA, Rogers BP, Cao A. Affective neural response to restricted interests in autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2014;55(2):162–171. doi: 10.1111/jcpp.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederlund M, Hagberg B, Gillberg C. Asperger syndrome in adolescent and young adult males. Interview, self-and parent assessment of social, emotional, and cognitive problems. Research in Developmental Disabilities. 2010;31(2):287–298. doi: 10.1016/j.ridd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Chelazzi L, Perlato A, Santandrea E, Della Libera C. Rewards teach visual selective attention. Vision Research. 2013;85:58–72. doi: 10.1016/j.visres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes, Brain and Behavior. 2011;10(7):740–746. doi: 10.1111/j.1601-183X.2011.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry. 2013;74(12):898–907. doi: 10.1016/j.biopsych.2013.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S. Visual attention: bottom-up versus top-down. Current Biology. 2004;14(19):R850–R852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Crane L, Goddard L, Pring L. Autobiographical memory in adults with autism spectrum disorder: The role of depressed mood, rumination, working memory and theory of mind. Autism. 2013;17(2):205–219. doi: 10.1177/1362361311418690. [DOI] [PubMed] [Google Scholar]
- de Wit S, Watson P, Harsay HA, Cohen MX, van de Vijver I, Ridderinkhof KR. Corticostriatal connectivity underlies individual differences in the balance between habitual and goal-directed action control. Journal of Neuroscience. 2012;32(35):12066–12075. doi: 10.1523/JNEUROSCI.1088-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong R. Autism and familial major mood disorder: are they related? The Journal of Neuropsychiatry and Clinical Neurosciences. 2004;16(2):199–213. doi: 10.1176/jnp.16.2.199. [DOI] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Spence MA. Autism and the serotonin transporter: the long and short of it. Molecular Psychiatry. 2005;10(12):1110. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behaviour Research and Therapy. 2007;45(11):2664–2678. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory–II. Psychological Assessment. 1998;10(2):83. [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Annals of the New York Academy of Sciences. 1999;877(1):614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Duque A, Vázquez C. Double attention bias for positive and negative emotional faces in clinical depression: Evidence from an eye-tracking study. Journal of Behavior Therapy and Experimental Psychiatry. 2015;46:107–114. doi: 10.1016/j.jbtep.2014.09.005. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Benjamin LS, Williams JB. Structured clinical interview for DSM-IV axis II personality disorders: SCID-II. American Psychiatric Pub; 1997. [Google Scholar]
- Gotham K, Bishop SL, Brunwasser S, Lord C. Rumination and perceived impairment associated with depressive symptoms in a verbal adolescent–adult ASD sample. Autism Research. 2014;7(3):381–391. doi: 10.1002/aur.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Unruh K, Lord C. Depression and its measurement in verbal adolescents and adults with autism spectrum disorder. Autism. 2015;19(4):491–504. doi: 10.1177/1362361314536625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Cane DB. Construct accessibility and clinical depression: A longitudinal investigation. Journal of Abnormal Psychology. 1987;96(3):199–204. doi: 10.1037//0021-843x.96.3.199. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information-processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004;113(3):386. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Hahamy A, Behrmann M, Malach R. The idiosyncratic brain: distortion of spontaneous connectivity patterns in autism spectrum disorder. Nature Neuroscience. 2015;18(2):302–309. doi: 10.1038/nn.3919. [DOI] [PubMed] [Google Scholar]
- Howlin P, Goode S, Hutton J, Rutter M. Adult outcome for children with autism. Journal of Child Psychology and Psychiatry. 2004;45(2):212–229. doi: 10.1111/j.1469-7610.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- Huang CH, Santangelo SL. Autism and serotonin transporter gene polymorphisms: A systematic review and meta-analysis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147(6):903–913. doi: 10.1002/ajmg.b.30720. [DOI] [PubMed] [Google Scholar]
- Joormann J, Dkane M, Gotlib IH. Adaptive and maladaptive components of rumination? Diagnostic specificity and relation to depressive biases. Behavior Therapy. 2006;37(3):269–280. doi: 10.1016/j.beth.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Whitfield-Gabrieli S, Dillon DG, Goer F, Beltzer M, Minkel J, Pizzagalli DA. Dynamic resting-state functional connectivity in major depression. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.352. Retrieved from http://www.nature.com.proxy.library.vanderbilt.edu/npp/journal/vaop/ncurrent/full/npp2015352a.html. [DOI] [PMC free article] [PubMed]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Courchesne E. Functional abnormalities of the default network during self-and other-reflection in autism. Social Cognitive and Affective Neuroscience. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerns CM, Roux AM, Connell JE, Shattuck PT. Adapting Cognitive Behavioral Techniques to Address Anxiety and Depression in Cognitively Able Emerging Adults on the Autism Spectrum. Cognitive and Behavioral Practice. 2016;23(3):329–340. [Google Scholar]
- Ko SG, Lee TH, Yoon HY, Kwon JH, Mather M. How does context affect assessments of facial emotion? The role of culture and age. Psychology and Aging. 2011;26(1):48. doi: 10.1037/a0020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasgaard M, Nielsen A, Eriksen ME, Goossens L. Loneliness and social support in adolescent boys with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2010;40(2):218–226. doi: 10.1007/s10803-009-0851-z. [DOI] [PubMed] [Google Scholar]
- Lam KS, Aman MG. The Repetitive Behavior Scale-Revised: Independent validation in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(5):855–866. doi: 10.1007/s10803-006-0213-z. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism diagnostic observation schedule: ADOS-2. Western Psychological Services; Los Angeles, CA: 2012. [Google Scholar]
- Lugnegard T, Hallerbäck MU, Gillberg C. Psychiatric comorbidity in young adults with a clinical diagnosis of Asperger syndrome. Research in Developmental Disabilities. 2011;32(5):1910–1917. doi: 10.1016/j.ridd.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Öhman A. The Karolinska directed emotional faces (KDEF) CD ROM from Department of Clinical Neuroscience, Psychology Section, Karolinska Institutet. 1998:91–630. [Google Scholar]
- Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with Asperger syndrome/high-functioning autism: A community-and clinic-based study. Journal of Autism and Developmental Disorders. 2010;40(9):1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- Mazefsky CA, Oswald DP, Day TN, Eack SM, Minshew NJ, Lainhart JE. ASD, a psychiatric disorder, or both? Psychiatric diagnoses in adolescents with high-functioning ASD. Journal of Clinical Child & Adolescent Psychology. 2012;41(4):516–523. doi: 10.1080/15374416.2012.686102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Olson LM, Dowd M, Amin T, Steele A, Blakely RD, Sutcliffe JS. Linkage and association analysis at the serotonin transporter (SLC6A4) locus in a rigid-compulsive subset of autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127(1):104–112. doi: 10.1002/ajmg.b.20151. [DOI] [PubMed] [Google Scholar]
- McGuffin P, Katz R, Watkins S, Rutherford J. A Hospital-Based Twin Register of the Heritability of DSM-IV Unipolar Depression. Archives of General Psychiatry. 1996;53(2):129–136. doi: 10.1001/archpsyc.1996.01830020047006. [DOI] [PubMed] [Google Scholar]
- Murray RJ, Schaer M, Debbané M. Degrees of separation: A quantitative neuroimaging meta-analysis investigating self-specificity and shared neural activation between self-and other-reflection. Neuroscience & Biobehavioral Reviews. 2012;36(3):1043–1059. doi: 10.1016/j.neubiorev.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2001;25(4):781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504. [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. Journal of Personality and Social Psychology. 1991;61(1):115. doi: 10.1037//0022-3514.61.1.115. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspectives on Psychological Science. 2008;3(5):400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage: Clinical. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piven J, Palmer P. Psychiatric disorder and the broad autism phenotype: evidence from a family study of multiple-incidence autism families. American Journal of Psychiatry. 1999;156(4):557–563. doi: 10.1176/ajp.156.4.557. [DOI] [PubMed] [Google Scholar]
- Qualter P, Brown SL, Munn P, Rotenberg KJ. Childhood loneliness as a predictor of adolescent depressive symptoms: an 8-year longitudinal study. European Child & Adolescent Psychiatry. 2010;19(6):493–501. doi: 10.1007/s00787-009-0059-y. [DOI] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Larsson H, Hultman CM, Reichenberg A. The Familial Risk of Autism. JAMA. 2014;311(17):1770–1777. doi: 10.1001/jama.2014.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Nils F, Sanchez X, Philippot P. Assessing the effectiveness of a large database of emotion-eliciting films: A new tool for emotion researchers. Cognition and Emotion. 2010;24(7):1153–1172. [Google Scholar]
- Segal ZV, Gemar M, Truchon C, Guirguis M, Horowitz LM. A priming methodology for studying self-representation in major depressive disorder. Journal of Abnormal Psychology. 1995;104(1):205. doi: 10.1037//0021-843x.104.1.205. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. Repetitive Behavior Profiles in Asperger Syndrome and High-Functioning Autism. Journal of Autism & Developmental Disorders. 2005;35(2):145–158. doi: 10.1007/s10803-004-1992-8. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. The American Journal of Human Genetics. 2005;77(2):265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: A psychometric analysis. Cognitive Therapy and Research. 2003;27(3):247–259. [Google Scholar]
- Turner-Brown LM, Lam KSL, Holtzclaw TN, Dichter GS, Bodfish JW. Phenomenology and measurement of circumscribed interests in autism spectrum disorders. Autism. 2011;15(4):437–456. doi: 10.1177/1362361310386507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Lynch CJ, Khouzam A, Phillips J, Feinstein C, Menon V. Salience network–based classification and prediction of symptom severity in children with autism. JAMA Psychiatry. 2013;70(8):869–879. doi: 10.1001/jamapsychiatry.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SW, Roberson-Nay R. Anxiety, social deficits, and loneliness in youth with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(7):1006–1013. doi: 10.1007/s10803-009-0713-8. [DOI] [PubMed] [Google Scholar]


