Abstract
Concomitant cannabis and nicotine use is more prevalent than cannabis use alone; however, to date, most of the literature has focused on associations of isolated cannabis and nicotine use limiting the generalizability of existing research. To determine differential associations of concomitant use of cannabis and nicotine, isolated cannabis use and isolated nicotine use on brain network connectivity, we examined systems-level neural functioning via independent components analysis (ICA) on resting state networks (RSNs) in cannabis users (CAN, n = 53), nicotine users (NIC, n = 28), concomitant nicotine and cannabis users (NIC+CAN, n = 26), and non-users (CTRL, n = 30). Our results indicated that the CTRL group and NIC+CAN users had the greatest functional connectivity relative to CAN users and NIC users in 12 RSNs: anterior default mode network (DMN), posterior DMN, left frontal parietal network, lingual gyrus, salience network, right frontal parietal network, higher visual network, insular cortex, cuneus/precuneus, posterior cingulate gyrus/middle temporal gyrus, dorsal attention network, and basal ganglia network. Post-hoc tests showed no significant differences between (1) CTRL and NIC+CAN and (2) NIC and CAN users. These findings of differential associations of isolated vs. combined nicotine and cannabis use demonstrate an interaction between cannabis and nicotine use on RSNs. These unique and combined mechanisms through which cannabis and nicotine influence cortical network functional connectivity are important to consider when evaluating the neurobiological pathways associated with cannabis and nicotine use.
Keywords: addiction, cannabis, nicotine, resting state fMRI, functional connectivity, Independent Component Analysis
Introduction
Concomitant nicotine use is highly prevalent in cannabis users with rates as high as 39% (Substance Abuse and Mental Health Services Administration 2014). Studies have demonstrated modulating effects of cannabinoid receptor antagonists (e.g., rimonabant) that lead to reduction of reward-related effects of nicotine (Cohen et al.; Le Foll 2004; Forget et al. 2005; Shoaib 2008) and increase the odds of smoking cessation in humans (Rigotti et al. 2009; Cahill and Ussher 2011) whereas agonists have the opposite effect (Gamaleddin et al. 2012). Nicotine also potentiates the acute pharmacological and biochemical effects of delta9-tetrahydrocannabinol (THC), the primary psychoactive ingredient in cannabis (Valjent et al. 2002; Amos et al. 2004; Viveros et al. 2006).
While numerous studies suggest an adverse additive effect of combined cannabis and nicotine use on physiology (i.e., respiratory function) (Agrawal and Lynskey 2009), their combined effects on the brain and related behavior are not well understood. In terms of behavior, evidence from the literature is conflicting. Agrawal and colleagues (2012) reported higher rates of substance-related problems and psychopathology in comorbid nicotine and cannabis users (Agrawal et al. 2012). On the other hand, Bonn-Miller et al (2010) showed that nicotine-only individuals had greater symptoms of depression and anxiety relative to those with combined use groups (Bonn-Miller et al. 2010). Cognitively, some suggest that the degree of combined use has differential effects such that those who primarily use cannabis but also sporadically use nicotine show wide-ranging impairment on cognitive function including learning and memory than those who use nicotine more regularly (Schuster et al. 2015). The authors posit that greater combined use may attenuate cognitive deficits and therefore reinforce concurrent use. Others have failed to observe differences in cognition, although differences in correlations between cognitive performance and brain structure have been reported (Filbey et al. 2015).
How these combined effects may manifest in the brain remains understudied. Individually, each substance has been associated with alterations in brain structure (cannabis: Cousijn et al. 2012; Gilman et al. 2014; Filbey et al. 2014; nicotine: Brody et al. 2004; Yu et al. 2011; Liao et al. 2012) and function (cannabis: Filbey and Yezhuvath 2013; Cousijn et al. 2014; nicotine: Claus et al. 2013). A widely utilized approach of looking at the brain’s resting state networks (RSNs) provides baseline information on the brain’s functional network architecture based on the temporal correlations of spatially distributed brain regions in the absence of a task (Biswal et al. 1997). Existing studies in primarily cannabis and nicotine users indicate opposing effects of each substance (Subramaniam et al. 2016), including in RSNs (Vergara et al. 2018). For example, increased connectivity has been reported in cannabis users (Pujol et al. 2014; Filbey et al. 2014), whereas reduced connectivity has been observed in nicotine users (Weiland et al. 2015). In adolescents, nicotine users exhibited decreased activity in the nucleus accumbens during a monetary reward task compared to poly-substance. alcohol, and control groups, but no difference compared to the cannabis group (Karoly et al. 2015). Currently, we are only aware of one study that examined combined vs. unique associations of cannabis and nicotine on resting state networks (RSNs). Using a seed-based analysis that focused on posterior cingulate gyrus (PCG) connections in the brain’s default mode network (DMN), Wetherill and colleagues (2015) found decreased connectivity in users of only cannabis, only nicotine, and concurrent users compared to non-using controls (Wetherill et al. 2015).
To date, however, associations of concurrent use on alterations in other resting state networks (RSNs) besides the DMN have not yet been examined. Emergent studies on RSNs and substance abuse show altered functional connectivity in substance abusing populations in other RSNs such as the executive control network (ECN) (Sutherland et al. 2012). Thus, we tested the hypothesis that concomitant and isolated cannabis and nicotine use is associated with differential activation in several RSNs, including DMN and ECN.
Materials and Methods
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Participants
We analyzed resting state fMRI data and anatomical MPRAGE data acquired from 137 participants categorized into four distinct substance use groups: nicotine users (NIC, n = 28, mean age = 32.7 ± 10.0, 16 males), cannabis users (CAN, n = 53, mean age = 24.4 ± 7.9, 38 males), nicotine and cannabis users (NIC+CAN, n = 26, mean age = 26.5 ± 8.0, 19 males), and non-users (CTRL, n = 30, mean age = 28.8 ± 8.9, 14 males). All of the participants were recruited for larger studies aimed at examining the neurocognitive mechanisms related to symptoms of cannabis and nicotine use disorders (Filbey et al., 2014; Filbey, McQueeny, Kadamangudi, Bice, & Ketcherside, 2015; Filbey, Schacht, Myers, Chavez, & Hutchison, 2009). All of the participants were recruited through flyers and media advertisement in the Albuquerque, New Mexico and Dallas, Texas metro areas. Written informed consent was obtained from all participants in accordance with the Institutional Review Board (IRB) of University of New Mexico and University of Texas at Dallas. The inclusion criteria for all of the participants were: (1) English as the primary language, (2) no current or history of psychosis, traumatic brain injury or neurological disorder. Cannabis users (CAN) were included if they currently use cannabis regularly (at least four times per week) over the last six months (confirmed via self-report and positive THC-COOH urinalysis). Nicotine users (NIC) were included if they reported nicotine use (verified by CO breath monitor) of 10 or more times daily and had less than three months of abstinence in the past year. Participants who met criteria for both regular cannabis and nicotine use as described were included in the cannabis and nicotine group (NIC+CAN). Non-using controls were included if they indicated no regular use of cannabis or nicotine in the last year and no current use of either substance. Participants were excluded if they met current or lifetime abuse or dependence criteria for cocaine, hallucinogens, opiates, sedatives, or stimulants according to the Structured Clinical Interview for DSM-IV (SCID; First et al. 2002). Participants were not excluded for current or lifetime abuse or dependence for alcohol. Four NIC, nine CAN, and four NIC+CAN participants reported past cocaine use. One NIC, 13 CAN, four NIC+CAN, and one CTRL participant reported past hallucinogen use. Two NIC and six CAN participants reported past sedatives use. Three NIC, three CAN, and two NIC+CAN participants reported past stimulants use. Six CAN participants reported past opiates use. Table 1 summarizes the demographic information, behavioral measures, and total number of participants per cohort. To account for potential acute effects, participants were instructed to abstain from cannabis for 72 hours and from nicotine for 12 hours prior to the fMRI scan. Abstinence was verified using self-report and CO breath level of 0.0.
Table 1.
Participant characteristics for cannabis only (CAN), nicotine only (NIC), combined cannabis and nicotine groups (NIC+CAN), and non-using control group (CTRL).
| CAN Mean (SD) |
NIC Mean (SD) |
NIC+CAN Mean (SD) |
CTRL Mean (SD) |
F/t, p | |
|---|---|---|---|---|---|
| N | 53 | 28 | 26 | 30 | - |
| Demographic variables | |||||
| Age | 24.4 (7.9) | 32.7 (10.0) | 26.5 (8.0) | 28.8 (8.9) | F(3, 131) = 5.9, p = 0.001* |
| Males (N, %) | 38 (71.7) | 16 (57) | 19 (73.1) | 14 (46.7) | χ2(3) = 8.1; p = 0.043* |
| Years of Education | 13.3 (2.7) | 14.3 (2.4) | 13.3 (2.1) | 16.6 (1.7) | F(3, 131)= 12.7, p < 0.001* |
| IQ | 105.5 (13.0) | 111.2 (11.7) | 107.3 (12.2) | 111.6 (9.9) | F(3, 131) = 2.2, p = 0.09 |
| Substance use variables | |||||
| # cannabis use/last 90 days | 80.5 (15.2) | 1.43 (6.60) | 83.8 (12.0) | 0.0 | F(3, 131) = 576.2, p < 0.001* |
| Duration of regular cannabis use (years) | 6.5 (6.4) | n/a | 8.0 (7.6) | n/a | t(78) = −0.78, p = 0.44 |
| # cigarette smoking days/last 90 days | 7.3 (15.8) | 90 (0.0) | 89.5 (1.9) | 0.0 | F(3, 131) = 824.1, p < 0.001* |
| Pack yearsa | n/a | 8.6 (11.0) | 8.9 (14.8) | n/a | t(53) = −0.05, p = 0.96 |
| # drinking days/last 90 days | 23.1 (26.6) | 16.8 (22.9) | 21.0 (21.6) | 9.1 (19.5) | F(3, 131) = 2.31, p = 0.08 |
| Average drink per drinking day | 4.0 (2.9) | 3.3 (2.7) | 5.1 (3.2) | 1.5 (1.2) | F(3, 131) = 8.8, p < 0.001* |
| DSM-IV current alcohol abuse (N) | 9 | 2 | 5 | 1 | χ2(3) = 7.5; p = 0.28 |
| DSM-IV current alcohol dependence (N) | 6 | 1 | 2 | 0 | χ2(3) = 8.1; p = 0.043* |
| DSM-IV lifetime alcohol abuse (N) | 32 | 15 | 10 | 6 | χ2(3) = 20.4; p < 0.001* |
| DSM-IV lifetime alcohol dependence (N) | 24 | 10 | 5 | 3 | χ2 (3) = 8.0; p = 0.047* |
# cigarette packs per day/# years regular cigarette use
Behavioral measures
Sample Characteristics
Age, sex, and number of years of formal education were obtained using a standard demographics questionnaire. A Time Line Follow-Back (TLFB) (Sobell and Sobell 1992) approach was used to quantify alcohol, nicotine, and cannabis use patterns for 90 days prior to study participation. The DSM-IV cannabis use disorder (CUD) SCID (First et al. 2002) symptom count (current) was used as a measure of cannabis dependence in the CAN and NIC+CAN users and the Fagerstrom Test for Nicotine Dependence (FTND; Fagerstrom and Schneider 1989) assessed the severity of nicotine dependence in the NIC and NIC+CAN users.
MRI acquisition
MRI scans of the NIC, CAN, NIC+CAN users, and ten participants in the CTRL group were performed at the Mind Research Network in Albuquerque, NM on a Siemens 3 Tesla Trio scanner using the standard 12-channel phased array head coil. Whole brain high-resolution T1-weighted anatomical images were collected using a multi-echo Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence with the following parameters: TR/TE/TI = 2530/1.64, 3.5, 5.36, 7.22, 9.08/1200 ms, flip angle = 7°, field of view (FOV) = 256×256×192 mm3, voxel size = 1×1×1 mm3, and NEX = 1. The sequence parameters for the resting state fMRI were: FOV = 240×240, matrix = 64×64, slice thickness = 4.55 mm, no gap between slices, voxel size = 3.75×3.75×4.55 mm2, 32 axial slices, TR/TE = 2000/29 ms, flip angle = 60°, 158 image volumes, and scan duration = 5 minutes. MRI scans of the remaining 20 CTRL participants were collected at the Advanced Imaging Research Center at the University of Texas, Southwestern Medical Center using a 3T Philips whole-body scanner with Quasar gradient subsystem (40 mT/m amplitude, a slew rate of 220 mT/m/ms. The following parameters were used to collect high-resolution T1-weighted anatomical images using a MPRAGE sequence: TR/TE/T1 = 2100/3.70/1100 ms; flip angle = 12°, FOV = 256×256×160 mm3, and voxel size = 1×1×1 mm3. The sequence parameters for the resting state fMRI were: FOV = 220×136×220 mm3, matrix = 64×64, slice thickness = 3.88 mm, voxel size=1×1×1 mm3, 39 axial slices, TR/TE = 2000/29 ms, flip angle = 75°, 150 image volumes, and scan duration = 5.2 minutes.
Data pre-processing
Each of the participants’ resting state BOLD fMRI data and anatomical MPRAGE data were processed using the processing scheme based on SPM (Wellcome Department of Imaging Neuroscience, London, UK), AFNI (National Institute of Mental Health Scientific and Statistical Computing Core, Bethesda, MD), and FSL (FMRIB Software Library v5.0. Analysis Group, FMRIB, Oxford, UK). In the first step, the first 5 time points were removed from each BOLD fMRI data to account for T1-relaxation effects. BOLD fMRI data were then motion corrected by aligning each of the time points with the mean of the data using SPM’s realign function. Following motion correction, the data were co-registered with each of the participants’ anatomical MPRAGE scan. After co-registration, the anatomical MPRAGE images were segmented in to grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) images and probability maps were created using SPM’s new segment tool. The BOLD fMRI data were transformed into MNI standard space using the deformation field derived during the anatomical segmentation. In order to remove the effects of physiological noises and motion related artifacts from the BOLD fMRI data, a GLM based regression model was implemented. CSF and WM masks were created using the probability images derived during segmentation step were thresholded at p > 0.95. These masks were used to extract the CSF and WM time-series from the BOLD fMRI data in MNI space. A principal component analysis was performed on the time series data and first principal component (PC) from CSF and WM time series was extracted. Our GLM regression model included 6 motion parameters derived during motion correction step, 6 autoregressive version of the motion parameters and 12 quadratics of these motion parameters in order to reduce the effect of motion on the BOLD fMRI data. In total, 26 regressors were used in the GLM model (1 pc for CSF, 1 pc for WM, 6 motion parameters and 6 one-time point delayed motion parameters, 12 squared motion parameters). Following regression, the BOLD fMRI data were band-pass filtered between the frequency bands 0.01–0.1 Hz and spatially smoothed with a 6 mm FWHM Gaussian filter using the 3dBandPass command in AFNI.
Motion analysis
Because participant head motion during resting state can affect group level differences, we applied multiple motion criteria. For each participant, we calculated motion parameters during the motion correction step. In addition, we also calculated frame-wise displacement for each participant based on the model defined by Jenkinson and colleagues (Jenkinson et al. 2002). A participant was only included in the analysis if the maximum motion in any direction was less than 1 voxel (<3.75 mm) and if the mean frame-wise displacement was less than 0.5mm. To determine if the groups showed different motion profile, group level two-sample t-tests were performed to derive group level differences in motion between each group.
Resting state functional connectivity (RSFC) analyses
We evaluated resting state functional connectivity using a group independent component analysis (ICA)-based dual regression approach. In the first step, group ICA was performed using a temporal concatenation approach available in FSL MELODIC. For this step, we combined all of the participants across the four groups. In order to combine the processed fMRI data across the participants, we restricted our analysis to first 140 timepoints across all the participants. We performed two distinct ICAs. For the first analysis, we selected the dimensionality to be 20 components and for the second analysis we extracted 40 components. For both of the analyses, each IC was quantitatively compared with the IC maps from 1000 Functional Connectome Project to identify resting state networks (Taylor et al. 2012). Specifically, we calculated DICE coefficients between each pair of IC maps derived from the current analysis and the IC maps from the 1000 Functional Connectome Project using 3dMATCH program in AFNI (Taylor and Saad 2013). The highest DICE coefficient values along with visual inspection identified the RSNs. Based on this comparison, we observed increased parcellation of RSNs in multiple ICs in ICA with 40 components compared to ICA with 20 components. Thus, we selected the ICA with 20 components for further analysis. Each of the 20 ICs were back-projected onto each individual participant’s brain using GLM to derive participant-level component time series. Participant-level component time series were used in the second regression model to derive participant-level IC maps. For each of the participant-level IC maps, z-stat images were calculated.
Between-group analyses
A one way 4-factor ANOVA was performed to determine differences between the groups. Additionally, post/hoc two-sample t-test comparisons were performed comparing each of the four groups in the ICs displaying significant group level ANOVA differences. In sum, a total of six two-sample tests were performed for each of the ICs, comparing NIC users with NIC+CAN users, CAN users with NIC+CAN users, CTRL group with NIC users, and CTRL group with CAN users. We found typical dissimilarities in sex and education characteristics (Gfroerer et al. 1997) between the substance using and non-using groups; thus, we covaried for these variables, in addition to age in each of the group level comparisons. Additionally, we used recent alcohol use (average drinks per drinking day in the preceding 90 days) and lifetime alcohol abuse and dependence as covariates to control for effects of alcohol use on RSNs. In the NIC+CAN vs. CAN post-hoc comparison, cannabis and nicotine use in the preceding 90 days was also added as a covariate to control from the effects of recent use. Similar to other multi-site studies (Biswal et al. 2010; Turner et al. 2013), we also added MRI site as a covariate. Group level statistics were performed using permutation tests available in FSL randomize and group level differences were derived using p < 0.05 with FWE correction.
Correlational analyses between RSFC and behavioral measures
For each IC exhibiting significant group level ANOVA differences between all the groups, we extracted brain regions pertaining to these differences. An ROI mask was created using this group level difference and average connectivity strength was extracted for each participant using the z-stat maps created during the dual regression steps. Each average connectivity score was then correlated with measures of substance use severity (CAN: DSM-IV CUD symptom count; NIC: FTND) and p-values were corrected for multiple comparisons using p < 0.05 with FDR correction for 72 tests (12 ICs x 6 contrasts).
Results
Motion effects
All of the participants passed the motion criterion with maximum motion in any direction < 1 voxel size (3.75 mm) and mean frame-wise displacement < 0.5 mm. In addition, none of the groups revealed significant group level differences in motion (NIC vs. CAN, FDjenkinson p = 0.78, NIC vs. NIC+CAN, FDjenkinson p = 0.40, CAN vs.. NIC+CAN, FDjenkinson p = 0.53, CTRL vs. NIC, FDjenkinson p = 0.70, CTRL VS. CAN, FDjenkinson p = 0.82, and CTRL vs. NIC+CAN, FDjenkinson p = 0.86). Based on these results, motion was not considered to have a significant effect on the group level results.
Group level RSNs
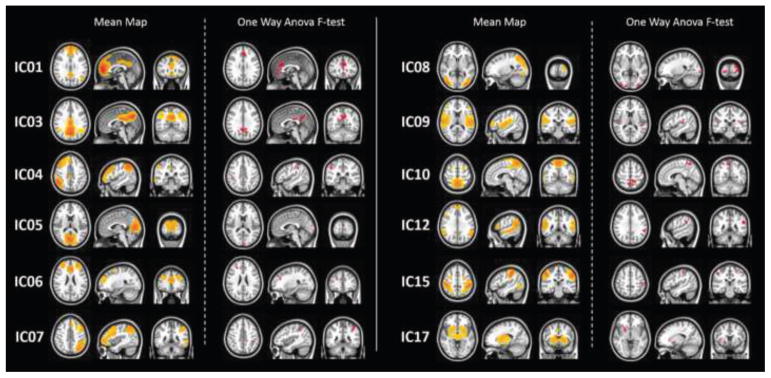
A total of 15 ICs were identified as representations of known resting state networks based on the comparison with FCP-1000 (Biswal et al. 2010). Based on the ANOVA results, we identified 12 components with significant effect of group. Figure 1 illustrates the group level IC maps from the interaction effects of NIC and CAN vs. CTRL and NIC+CAN for each of the 12 RSN. The RSNs were: (A) anterior default mode network (DMN; IC01), (B) posterior DMN (IC03), (C) left frontal parietal network (IC04), (D) lingual gyrus (IC05), (E) salience network (IC06), (F) right frontal parietal network (IC07), (G) higher visual network (HVN; IC08), (H) insular cortex (IC09), (I) cuneus/precuneus (IC10), (J) bilateral inferior temporal gyrus, bilateral superior temporal gyrus and superior frontal gyrus (IC12), (K) dorsal attention network (DAN; IC15), and (L) basal ganglia network (IC17). For each of the 12 ICs, we performed six post-hoc two-sample t test comparisons to determine the direction of the effects: CTRL vs. NIC, CTRL vs. CAN, CTRL vs. NIC+CAN, NIC vs. CAN, NIC vs. NIC+CAN, and CAN vs. NIC+CAN.
Figure 1. Group level IC maps and corresponding f-test comparisons exhibiting interaction effects between nicotine and cannabis.
ICA analyses identified the following RSNs: anterior default mode network (DMN) (IC01), posterior DMN (IC03), left frontal parietal network (IC04), lingual gyrus (IC05), salience network (IC06), right frontal parietal network (IC07), higher visual network (IC08), insular cortex (IC09), cuneus/precuneus (IC10), bilateral inferior temporal gyrus, bilateral superior temporal gyrus and superior frontal gyrus (IC12), dorsal attention network (IC15), and basal ganglia network (IC17).
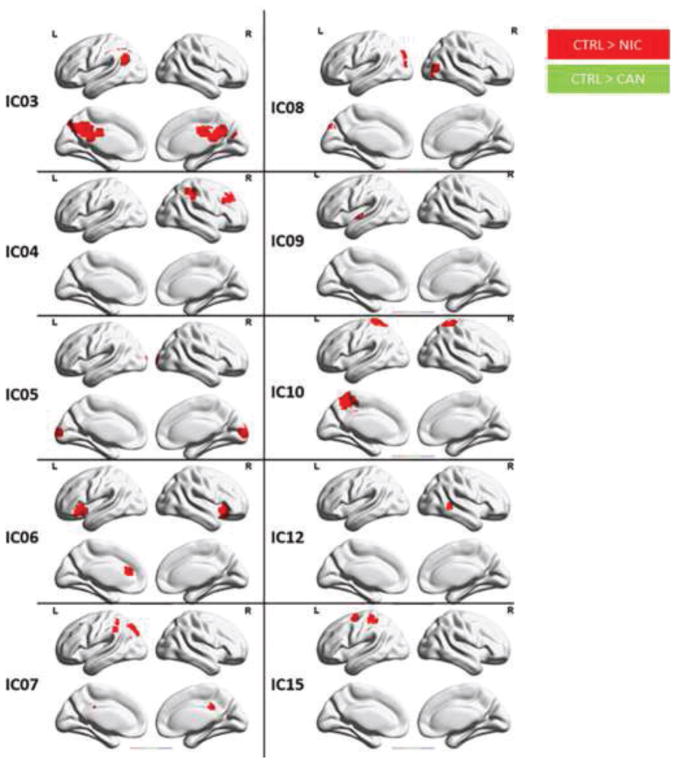
Figure 2 displays group level differences between the CTRL group and NIC and CAN, users. The CTRL group exhibited significantly greater connectivity than NIC users across all identified ICs, except the anterior DMN (IC01) and the basal ganglia network (IC17) (all p < 0.05, with FWE using FSL randomise). Additionally, the CTRL group exhibited significantly greater connectivity compared to CAN users in the salience network (IC06) and posterior cingulate gyrus (IC11) (p < 0.05, with FWE correction using FSL randomise).
Figure 2. Differences between the control group and single substance using groups via t-tests.
The control (CTRL) group exhibited significantly greater connectivity in the salience network (IC06) compared to nicotine (NIC) and cannabis (CAN) users. The CTRL group also exhibited greater connectivity in posterior DMN (IC03), left frontal parietal network (IC04), lingual gyrus (IC05), right frontal parietal network (IC07), higher visual network (IC08), insular cortex (IC09), cuneus/precuneus (IC10), bilateral inferior temporal gyrus, bilateral superior temporal gyrus and superior frontal gyrus (IC12) and dorsal attention network (IC15) compared to NIC users (p < 0.05, FWE corrected using FSL randomise).
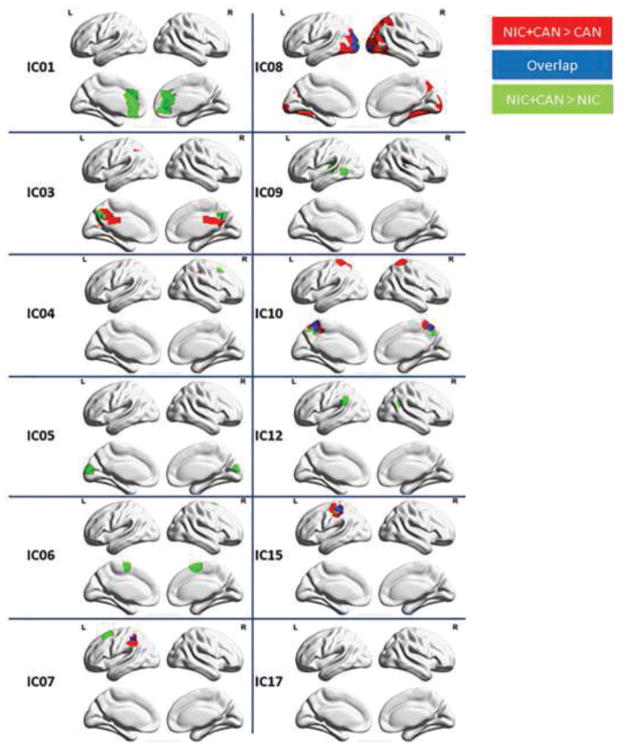
Figure 3 illustrates group level differences in RSFC between the NIC users, CAN users, and NIC+CAN users. NIC+CAN users exhibited significantly greater RSFC in the posterior DMN (IC03), left frontal parietal network (IC04), right frontal parietal network (IC07), higher visual network (IC08), cuneus/precuneus (IC10), and dorsal attention network (IC15) compared to both CAN and NIC users. NIC+CAN also exhibited significantly greater RSFC in the anterior DMN (IC01), lingual gyrus (IC05), salience network (IC06), and insular cortex (IC09) compared to NIC users. There was no significant difference between the NIC and CAN users.
Figure 3. Differences in resting state functional connectivity between substance using groups via t-tests.
Concurrent nicotine and cannabis users (NIC+CAN) exhibited significantly greater connectivity in posterior DMN (IC03), left frontal parietal network (IC04), right frontal parietal network (IC07), higher visual network (IC08), cuneus/precuneus (IC10) and dorsal attention network (IC15) compared to NIC and CAN users. NIC+CAN users also exhibited greater connectivity in the anterior DMN (IC01), lingual gyrus (IC05), salience network (IC06), and insular cortex (IC09) compared to NIC users (p < 0.05, FWE corrected using FSL randomise).
Correlations between RSFC and substance use severity
Correlation analyses were performed based on the ANOVA group level RSFC differences. No significant correlations were observed between the ICs and substance use severity measures in any of the groups at p < 0.05 with FDR correction. However, using a less stringent threshold of FDR-correction for each correlation analysis (i.e., 12 ICs) rather than the total of 72 ICs from all correlation analyses conducted for the study, we observed a significantly positive correlation between the left frontal parietal network (IC04) and current CUD symptom count in CAN users (r = 0.48, p = 0.0014).
Discussion
To address the important issue of differential associations of isolated vs. combined nicotine and cannabis use with brain function, we compared the brain’s resting state functional connectivity in cannabis users (CAN), nicotine users (NIC), concomitant cannabis and nicotine users (NIC+CAN), and a non-using control group (CTRL). We found that RSFC was greatest in the NIC+CAN users among the substance use groups. We observed an interaction between CAN and NIC such that RSN connectivity was greater in the concurrent users relative to the single substance users. Specifically, RSFC was greater in the CTRL group compared to NIC users in all of the identified ICs, except the anterior DMN and basal ganglia network, and greater than CAN users in the salience network and posterior cingulate gyrus. Interestingly, there were no significant differences in connectivity between the CTRL group and NIC+CAN users. Additionally, NIC+CAN users exhibited greater RSFC compared to NIC users in all identified ICs, except the left frontal parietal network, posterior cingulate gyrus, dorsal attention network, and basal ganglia network, and compared to CAN users in the posterior DMN, right frontal parietal network, higher visual network, cuneus/precuneus, and dorsal attention network. There were no significant differences in RSFC between NIC and CAN users. These findings suggest an interaction between cannabis and nicotine use such that isolated users differ from concomitant users, but not to each other.
This interaction may be due to reported opposing effects of cannabis and nicotine on the brain. For instance, in previous studies reporting enhanced RSFC in cannabis users have suggested potential compensatory mechanisms following THC-induced alterations in cerebral blood flow or down regulation of CB1 receptors (Gilman et al. 2014; Weiland et al. 2015). Support for compensation is based on increased RSFC in regions associated with cognitive load effect such as those in frontal, medial, and cerebellar areas (Chang 2006). Conversely, nicotine’s cognitive enhancing properties via nicotinic acetylcholine receptor activity may obviate neuroadaptive compensatory mechanisms (Poorthuis et al. 2009). Studies have also shown that one potential mechanism underlying nicotine’s cognitive enhancing effects is its suppression on RSFC such as in the DMN (Hahn et al. 2007; Tanabe et al. 2011). The DMN is comprised of brain regions important for processing internal information. Thus, de-activation of DMN may lead to more effective processing of external stimuli while engaged in tasks that could lead to augmented cognitive functioning.
Although the exact mechanism for the crosstalk between cannabis and nicotine is complex and has yet to be determined, the notion of ‘gateway drug’ effects suggests that despite accounts of progression of drug use from nicotine to cannabis, animal studies demonstrate potentiating effects of cannabis exposure on the rewarding effects of nicotine. For instance, 94% of THC-exposed rats were more likely to self-administer nicotine compared to 65% of non-exposed rats (Panlilio et al. 2007). This response following exposure to THC persisted with increasing response requirement, indicating increased reward-salience for nicotine that was not seen for heroin or cocaine self-administration in THC-exposed rats. These findings indicate a specific interaction between endocannabinoid and nicotinic pathways that may lead to greater combined use of both, and, perhaps, greater combined effects as demonstrated in the current study. Interestingly, PET studies of the dopamine system, considered the final common pathway for the reward and reinforcement properties of drugs of abuse and implicated in both cannabis and nicotine (i.e., decreased dopamine function), do not strongly support a combined effect of concomitant cannabis and nicotine use on dopamine transporter availability (Leroy et al. 2012). It is possible that our findings of enhanced RSFC in NIC+CAN reflect the previously suggested compensatory mechanisms noted as a result of THC (Filbey et al. 2014) as well as recent findings suggesting attenuating effects of nicotine on neurocognitive decrements in concurrent cannabis and nicotine users (Filbey et al. 2015).
Given the existing literature showing associations between substance use and functional connectivity, the absence of significant associations between substance use severity and functional connectivity in our sample was surprising. For example, Filbey et. al. (2014) reported that greater duration of cannabis use was associated with greater RSFC in OFC networks (Filbey et al. 2014) and Wetherill et. al. (2015) found a positive correlation between connectivity between the posterior cingulate cortex and anterior insula and duration of cannabis use (Wetherill et al. 2015). Notably, at a more lenient FDR-corrected threshold, we a significantly positive association between CUD symptom count and left frontal parietal network emerged in CAN. Taken together, these findings suggest a weak relationship between substance use severity and RSNs.
Limitations and conclusions
Although we made an attempt to statistically correct for the differences between groups on demographic variables of no interest as well as scanner site, it is important to replicate these findings in more closely matched groups to minimize potential confounding effects of these variables. Additionally, it is important to note that while a resting scan duration of 5 minutes that has been previously reported to be sufficient to provide robust correlation strengths (Van Dijk et al. 2010) reliability of findings increase with longer scan duration (Bim et al. 2013). Thus, a longer resting state duration may bolster these effects. To conclude, our findings add to the growing literature on drug abuse by demonstrating that the concurrent use of cannabis and nicotine is associated with enhanced RSFC that is similar to that of non-using controls but greater than that of cannabis only or nicotine only users. This effect is observable despite a potentially opposing attenuating effect of nicotine on RSFC, therefore, suggesting a greater role of cannabis on systems-level neural mechanisms that should be considered in intervention programs.
Acknowledgments
This study was funded by the National Institutes of Health (grants K01 DA021632, R01DA030344-01A1, and R01 DA038895).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Agrawal A, Budney AJ, Lynskey MT. The co-occurring use and misuse of cannabis and tobacco: a review: Cannabis and tobacco review. Addiction. 2012;107:1221–1233. doi: 10.1111/J.1360-0443.2012.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A, Lynskey MT. Tobacco and cannabis co-occurrence: Does route of administration matter? Drug Alcohol Depend. 2009;99:240–247. doi: 10.1016/j.drugalcdep.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos A, Wiltshire S, Bostock Y, et al. ‘You can’t go without a fag… you need it for your hash’—a qualitative exploration of smoking, cannabis and young people. Addiction. 2004;99:77–81. doi: 10.1111/j.1360-0443.2004.00531.x. [DOI] [PubMed] [Google Scholar]
- Bim RM, Molloy EK, Patriat R, et al. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Kylen JV, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10:165–170. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<165::aid-nbm454>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, et al. Toward discovery science of human brain function. Proc Natl Acad Sci. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonn-Miller MO, Zvolensky MJ, Johnson KA. Uni-morbid and co-occurring marijuana and tobacco wue: Examination of concurrent associations with negative mood states. J Addict Dis. 2010;29:68–77. doi: 10.1080/10550880903435996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55:77–84. doi: 10.1016/S0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD005353.pub4. Art. No.: CD005353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129:1096–1112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Claus ED, Blaine SK, Filbey FM, et al. Association between nicotine dependence severity, BOLD response to smoking cues, and functional connectivity. Neuropsychopharmacology. 2013;38:2363–2372. doi: 10.1038/npp.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Voltz C, et al. SR141716, a central cannabinoid (CB1) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav Pharmacol. 13:451–463. doi: 10.1097/00008877-200209000-00018. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Vingerhoets WAM, Koenders L, et al. Relationship between working-memory network function and substance use: a 3-year longitudinal fMRI study in heavy cannabis users and controls: Working-memory and substance use. Addict Biol. 2014;19:282–293. doi: 10.1111/adb.l2111. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, et al. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. NeuroImage. 2012;59:3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- Fagerstrom K-O, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom Tolerance Questionnaire. J Behav Med. 1989;12:159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Asian S, Calhoun VD, et al. Long-term effects of marijuana use on the brain. Proc Natl Acad Sci. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, McQueeny T, Kadamangudi S, et al. Combined effects of marijuana and nicotine on memory performance and hippocampal volume. Behav Brain Res. 2015;293:46–53. doi: 10.1016/j.bbr.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse. 2013;39:382–391. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009;106(31):13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Forget B, Hamon M, Thiébot M-H. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology (Berl) 2005;181:722–734. doi: 10.1007/s00213-005-0015-6. [DOI] [PubMed] [Google Scholar]
- Gamaleddin I, Wertheim C, Zhu AZX, et al. Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking: Cannabinoid receptors and nicotine. Addict Biol. 2012;17:47–61. doi: 10.1111/j.1369-1600.2011.00314.x. [DOI] [PubMed] [Google Scholar]
- Gfroerer JC, Greenblatt JC, Wright DA. Substance use in the US college-age population: differences according to educational status and living arrangement. Am J Public Health. 1997;87:62–65. doi: 10.2105/ajph.87.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Kuster JK, Lee S, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34:5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Yang Y, et al. Nicotine Enhances Visuospatial Attention by Deactivating Areas of the Resting Brain Default Network. J Neurosci. 2007;27:3477–3489. doi: 10.1523/JNEUROSCI.5129-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neurolmage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Bryan AD, Weiland BJ, et al. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015;16:5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B. Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther. 2004;312:875–883. doi: 10.1124/jpet.104.077974. [DOI] [PubMed] [Google Scholar]
- Leroy C, Karila L, Martinot J-L, et al. Striatal and extrastriatal dopamine transporter in cannabis and tobacco addiction: a high-resolution PET study: DAT availability in addictions. Addict Biol. 2012;17:981–990. doi: 10.1111/j.1369-1600.2011.00356.x. [DOI] [PubMed] [Google Scholar]
- Liao Y, Tang J, Liu T, et al. Differences between smokers and non-smokers in regional gray matter volumes: a voxel-based morphometry study: Gray matter changes in smokers. Addict Biol. 2012;17:977–980. doi: 10.1111/j.1369-1600.2010.00250.x. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–657. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Goriounova NA, Couey JJ, Mansvelder HD. Nicotinic actions on neuronal networks for cognition: General principles and long-term consequences. Biochem Pharmacol. 2009;78:668–676. doi: 10.1016/j.bcp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, et al. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigotti NA, Gonzales D, Dale LC, et al. A randomized controlled trial of adding the nicotine patch to rimonabant for smoking cessation: efficacy, safety and weight gain. Addiction. 2009;104:266–276. doi: 10.1111/j.1360-0443.2008.02454.x. [DOI] [PubMed] [Google Scholar]
- Schuster RM, Crane NA, Mermelstein R, Gonzalez R. Tobacco may mask poorer episodic memory among young adult cannabis users. Neuropsychology. 2015;29:759–766. doi: 10.1037/neu0000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoaib M. The cannabinoid antagonist AM251 attenuates nicotine self-administration and nicotine-seeking behaviour in rats. Neuropharmacology. 2008;54:438–444. doi: 10.1016/j.neuropharm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back. In: Litten RZ, Allen JP, et al., editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Subramaniam P, McGlade E, Yurgelun-Todd D. Comorbid Cannabis and Tobacco Use in Adolescents and Adults. Curr Addict Rep. 2016;3:182–188. doi: 10.1007/s40429-016-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neurolmage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Nyberg E, Martin LF, et al. Nicotine effects on default mode network during resting state. Psychopharmacology (Berl) 2011;216:287–295. doi: 10.1007/s00213-011-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Gohel S, Di X, et al. Functional covariance networks: Obtaining resting-state networks from intersubject variability. Brain Connect. 2012;2:203–217. doi: 10.1089/brain.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Saad ZS. FATCAT: (An Efficient) Functional And Tractographic Connectivity Analysis Toolbox. Brain Connect. 2013;3:523–535. doi: 10.1089/brain.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JA, Damaraju E, van Erp TGM, et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Front Neurosci. 2013;7 doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Mitchell JM, Besson M-J, et al. Behavioural and biochemical evidence for interactions between Δ9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135:564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, et al. Intrinsic Functional Connectivity As a Tool For Human Connectomics: Theory, Properties, and Optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara VM, Weiland BJ, Hutchison KE, Calhoun VD. The Impact of Combinations of Alcohol, Nicotine, and Cannabis on Dynamic Brain Connectivity. Neuropsychopharmacology. 2018;43:877–890. doi: 10.1038/npp.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros M, Marco E, File S. Nicotine and cannabinoids: Parallels, contrasts and interactions. Neurosci Biobehav Rev. 2006;30:1161–1181. doi: 10.1016/j.neubiorev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Weiland BJ, Sabbineni A, Calhoun VD, et al. Reduced executive and default network functional connectivity in cigarette smokers. Hum Brain Mapp. 2015;36:872–882. doi: 10.1002/hbm.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fang Z, Jagannathan K, et al. Cannabis, cigarettes, and their co-occurring use: Disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–123. doi: 10.1016/j.drugalcdep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhao L, Lu L. Regional Grey and White Matter Changes in Heavy Male Smokers. PLoS ONE. 2011;6:e27440. doi: 10.1371/journal.pone.0027440. [DOI] [PMC free article] [PubMed] [Google Scholar]