Abstract
Objective:
Adult body mass index (BMI) is strongly associated with venous thromboembolism (VTE), however whether earlier-life adiposity or other measures of adult adiposity are associated with VTE risk remains largely unknown.
Materials and Methods:
We evaluated associations of childhood somatotype, BMI in early adulthood, adult adiposity, and change in weight since early adulthood with incident VTE risk over ≥20 years of follow-up among 205,935 participants from Nurses’ Health Studies (NHS/NHS II) and Health Professionals Follow-Up Study (HPFS), ages 29–76 at baseline. We estimated multivariable-adjusted hazard ratios for VTE using Cox proportional hazards models.
Results and Conclusions:
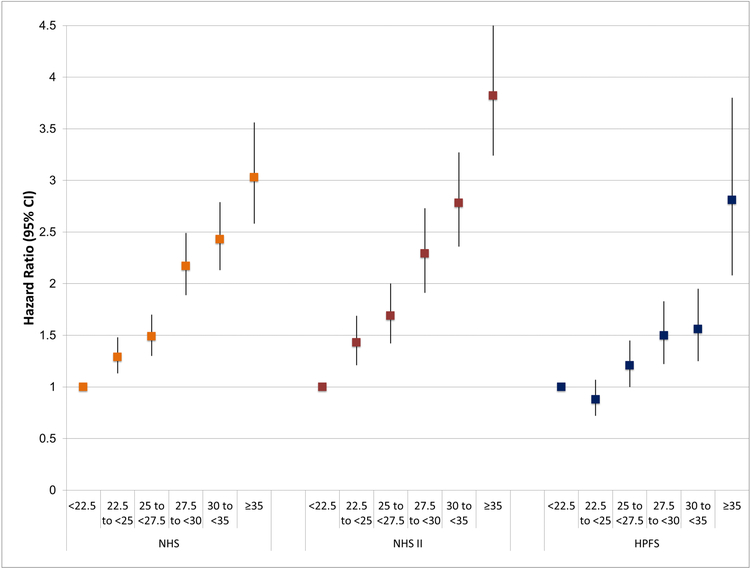
Somatotype in childhood and young adulthood BMI were not significantly associated with VTE risk, after accounting for adult BMI. Adult BMI was strongly associated with VTE in all three cohorts (e.g., multivariable-adjusted HRs comparing ≥35 kg/m2 vs. <22.5 kg/m2: NHS:3.03[95% CI: 2.58, 3.56], NHS II:3.82[95% CI: 3.24, 4.51], HPFS:2.81 [95% CI: 2.08, 3.80]; all p-trends<0.01). Adult waist circumference was associated with greater VTE risk, even after adjusting for adult BMI (all p-trends<0.01). Increasing weight gain from young adulthood was significantly associated with VTE after adjusting for current BMI among women (HR comparing gain ≥ 20kg vs. no change: NHS:1.36[95% CI: 1.13, 1.65], NHS II:1.48[95% CI: 1.17, 1.87]) and not men (HPFS:1.20[95% CI: 0.97, 1.50]). These results indicate that BMI and adiposity are likely more important acutely than cumulatively over time in the etiology and prevention of VTE. Clinically, encouraging weight loss in individuals who are overweight or obese could help reduce VTE risk.
Keywords: Venous Thromboembolism, Adiposity, Somatotype, Body Mass Index, Cohort Study
INTRODUCTION
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). It is the third most common cardiovascular disease in the United States and has an annual incidence rate (1 to 2 per 1000 person-years) similar to that of stroke.1 Prior observational studies have consistently found higher body mass index (BMI) to be a risk factor for VTE.2–6 In addition, a recent Mendelian randomization study of >7,000 VTE cases and >52,000 controls from the INVENT consortium provided evidence for a causal relationship between high BMI and risk of VTE.7
Fewer studies have focused on other measures of adult adiposity, childhood and early adulthood adiposity, or on weight gain throughout adulthood in relation to VTE risk. Thus, we evaluated associations of childhood and young adulthood adiposity (childhood somatotype, BMI in early adulthood), measures of adult adiposity, and change in weight since early adulthood with incident VTE risk among over 200,000 participants from the Nurses’ Health Study (NHS), the Nurses’ Health Study II (NHS II), and the Health Professionals Follow-Up Study (HPFS).
METHODS
Study Population
The NHS began in 1976, when 121,700 female registered nurses, aged 30–55 years, completed a mailed questionnaire about their health and lifestyle. The NHS II began in 1989 when 116,429 female registered nurses, aged 25–42 years, completed and returned a similar questionnaire. The HPFS included 51,529 U.S. male health professionals who were aged 40–75 years at enrollment in 1986. All three cohorts use identical methods for data collection and follow-up, including biennial mailed questionnaires to update health and lifestyle information. The follow-up rate remains approximately 85–90% across all three cohorts. Study protocols were approved by the institutional review boards at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
For the current analysis, we began follow-up in 1986 (NHS), 1993 (NHS II), and 1988 (HPFS) when relevant data on childhood and adult adiposity were first collected. We excluded participants who reported a diagnosis of VTE or died prior to the analytic baseline. We also excluded participants missing data required to calculate BMI and those with BMI <15 kg/m2 or >60 kg/m2. Thus, the present analysis included 88,597 women from NHS, 80,132 women from NHS II, and 37,206 men from HPFS.
Exposure measurement
Body fatness at age 10 was assessed by recall of somatotypes of 9-level pictograms developed by Sunkard et al.8 representing sizes ranging from extreme thinness (category 1) to obesity (category 9). The use of recalled somatotypes has been validated in both older 9 and younger women10 by comparison with childhood records. Somatotypes at age 10 correlated moderately with recorded BMI (r= 0.65).9 The validity did not differ by adult BMI at the time of the report.9 Overweight was defined as somatotype ≥5.11
Adult height was obtained from the 1976 (NHS), 1989 (NHS II), and 1986 (HPFS) questionnaires and presumed to be constant over the follow-up period. We obtained self-reported body weight at age 18 in 1980 for NHS participants and in 1986 for NHS II participants and body weight at age 21 years in 1986 for HPFS participants. In a validation study of 118 NHS II participants, recalled weight and height age 18 were compared with college or nursing school entrance records and the correlation between reported weight and measured weight was 0.84.12 Therefore, the validity of recalled weight is high in these cohorts. Current weight was obtained from the baseline questionnaire and then updated every 2 years thereafter. BMI was calculated as weight in kilograms/(height in meters)2. BMI was divided into 6 categories (<22.5, 22.5 to <25, 25 to <27.5, 27.5 to <30, 30 to <35, ≥35 kg/m2). In a validation study of 184 NHS participants, the correlation between self-reported and measured weight was 0.96.13
In 1986 for NHS, 1993 for NHS II, and 1988 for HPFS, a tape measure was enclosed with the questionnaire, which directed participants to measure their waist at the umbilicus. Participants were instructed to take measurements while standing and avoid measuring over bulky clothing. Waist circumference was updated using the same procedure in 1996 (NHS and HPFS), 2000 (NHS), 2005 (NHS II), and 2008 (HPFS). In all three cohorts, circumference measures were recorded to the nearest one-quarter inch. The self-reported anthropometric assessments have been previously validated with technicians’ measurements with a correlation of 0.95 for men (HPFS) and 0.89 for women (NHS).14
Weight change since the age of 18 years (NHS/NHS II) or 21 years (HPFS) was calculated by subtracting current weight (updated biennially) from the reported weight at age 18 or 21. Weight change was categorized into 6 groups: loss of ≥4kg, no change (± 4kg), gain of 4 to <10kg, gain of 10 to <15kg, gain of 15 to <20kg, and gain of ≥20kg.
Outcome ascertainment
All VTE cases were identified by the report of the study participant on the biennial questionnaire. Disease diagnoses were identified on the questionnaire using a blank line requesting participants to write-in any physician-diagnosed major disease diagnosed since the last questionnaire, or by specifically asking about DVT and PE. In NHS, physician-diagnosed DVT was identified when participants wrote this diagnosis on the biennial questionnaires from 1976 until present, on a blank line reserved for “other conditions”. Although DVT was not confirmed by medical record review, in a validation study of 101 self-reported cases of DVT, it was found that 94% of cases were confirmed in medical records, 2% were probable, and only 4% were not confirmed.15 For PE, NHS biennial questionnaires have included a specific question asking participants whether they had a physician-diagnosed PE during the preceding two years.
On NHS II questionnaires, a single question specifically asked participants whether they had a physician-diagnosed VTE (“DVT/PE”) in the past two years. On HPFS questionnaire, participants were asked about physician-diagnosed PE and DVT in the past two years on two separate questions. Given the severity of the diagnosis, followed by extensive treatment, therapeutic monitoring, and clinical follow-up required of patients with VTE, cases are likely reported with high accuracy in this population of nurses and health professionals with high health literacy and we likely are not missing cases that participants neglected to report.
Statistical analysis
Participants contributed person-time from baseline (NHS: 1986, NHS II: 1993, HPFS: 1988) until June 1, 2014 (NHS), June 1, 2013 (NHS II), December 1, 2012 (HPFS), or until they were censored due to VTE, death, or loss to follow-up. We performed age- and multivariable-adjusted Cox proportional hazards analysis to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of VTE associated with measures of childhood, early adulthood, and adult adiposity. Multivariable models were adjusted for potential confounding factors found on unadjusted analysis to be associated with VTE in our cohorts: age (continuous), physical activity (measured as average metabolic equivalents/week across 6 common activities), smoking status (never, past, current), history of hypertension, hypercholesterolemia, coronary heart disease (defined as myocardial infarction or angina), cancer, rheumatological disease, aspirin use, postmenopausal hormonal therapy use (NHS and NHS II only), and oral contraceptive use (NHS II only).16 Data on covariates were first obtained from the 1986 (NHS), 1993 (NHS II), and 1988 (HPFS) questionnaires and updated every two years. Very few participants were missing data for the covariates. However, when data were missing, we carried forward responses from the previous questionnaire cycle. If data remained missing after two cycles, missing indicator variables were created for that variable and included in models as covariates. We performed a test for trend across categories of interest by treating categories as an ordinal variable in the proportional hazards models and assigning the median value for that category. We also performed sensitivity analyses in which we excluded participants with cancer as cancer may have an impact on body weight and waist circumference as well as the diagnosis of VTE, due to increasing use of computerized tomography scans for cancer staging and surveillance. All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Over ≥20 years of follow-up, there were 2,721 incident VTE cases in NHS, 1,975 cases in NHS II, and 1,415 cases in HPFS. Table 1 shows age-adjusted baseline characteristics according to categories of BMI in each of the cohorts. The mean age at baseline was 53 years in NHS, 40 years in NHS II, and 56 years in HPFS. Individuals with higher BMI at baseline were less physically active and had a higher prevalence of hypertension, high cholesterol, and type 2 diabetes. Additionally, across all three cohorts, participants with a higher BMI at baseline also tended to report a higher somatotype at age 10, higher BMI in young adulthood, and have a larger waist circumference at baseline.
Table 1.
Age-adjusted baseline characteristics according to body mass index (kg/m2) at baseline in the Nurses’ Health Study (NHS), Nurses’ Health Study II (NHS II), and Health Professionals Follow-Up Study (HPFS).
| NHSa | NHS IIb | HPFSc | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <25 n=50,835 | 25 to <30 n=25,117 | >30 n=12,645 | <25 n=47,210 | 25 to <30 n=18,560 | >30 n=14,362 | <25 n=17,331 | 25 to <30 n=16,816 | >30 n=3,059 | |
| Age, yd | 52.4 (7.3) | 53.7 (7.1) | 53.2 (7.0) | 39.4 (4.4) | 40.3 (4.3) | 40.4 (4.2) | 56.3 (10.1) | 56.6 (9.6) | 55.9 (9.0) |
| Somatotype at age 10 | 2.3 (1.4) | 2.7 (1.5) | 3.2 (1.5) | 2.5 (1.3) | 3.0 (1.4) | 3.6 (1.4) | 2.5 (1.5) | 2.9 (1.6) | 3.5 (1.8) |
| Young adult BMIe, kg/m2 | 20.4 (2.3) | 21.8 (2.7) | 24.0 (3.8) | 20.0 (2.2) | 21.9 (2.9) | 24.8 (4.5) | 20.8 (4.8) | 22.6 (5.3) | 25.1 (6.1) |
| Adult waist circumference, cm | 73.6 (6.4) | 84.8 (7.3) | 97.3 (10.3) | 72.5 (6.8) | 84.5 (8.1) | 99.9 (118) | 89.5 (6.0) | 98.2 (6.7) | 111.3 (8.8) |
| Physical activity, MET-h/weekf | 15.9 (22.8) | 12.6 (18.8) | 0 7 n S | 23.8 (31.2) | 20.2 (27.4) | 15.6 (22.0) | 28.0 (31.6) | 24.8 (29.2) | 19.3 (25.2) |
| Smoking, % | |||||||||
| Never | 42 | 45 | 48 | 64 | 64 | 65 | 50 | 44 | 42 |
| Past | 33 | 35 | 37 | 23 | 22 | 22 | 41 | 47 | 49 |
| Current | 25 | 20 | 15 | 13 | 14 | 13 | 9 | 9 | 9 |
| Hypertension, % | 17 | 30 | 47 | 4 | 7 | 19 | 19 | 28 | 44 |
| High cholesterol, % | 11 | 15 | 17 | 12 | 18 | 25 | 21 | 23 | 24 |
| Coronary heart diseaseg, % | 1 | 1 | 2 | 1 | 1 | 1 | 8 | 9 | 11 |
| Type 2 diabetes, % | 2 | 4 | 10 | 1 | 1 | 3 | 3 | 3 | 7 |
| Cancer, % | 6 | 6 | 6 | 2 | 2 | 1 | 5 | 5 | 5 |
| Aspirin use, % | 55 | 55 | 54 | 12 | 12 | 14 | 38 | 43 | 43 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study; y, years; BMI, body mass index; kg, kilograms; m, meters; MET, metabolic equivalent of task; h, hours.
Values are means(SD) or percentages and are standardized to the age distribution of the study population.
Baseline=1986
Baseline=1993
Baseline=1988
Value is not age adjusted
BMI at age 18 (NHS, NHS II), BMI at age 21 (HPFS).
Hours of metabolic equivalent tasks.
Coronary heart disease includes any history of myocardial infarction, coronary artery stenosis, or angina.
In multivariable-adjusted models, we found somatotype at age 10 to be significantly associated with risk of VTE in all three cohorts. In NHS, women who reported a somatotype of 5 or more at age 10 had a 29% increased risk (95% CI: 1.16, 1.48) of VTE compared to women who reported a somatotype of 1. We found similar associations in NHS II (HR=1.47, 95% CI: 1.26, 1.71) and in HPFS (HR=1.21, 95% CI: 1.02, 1.44) (Table 2). However, associations were no longer significant after adjusting for adult BMI (all p-trends>0.05).
Table 2.
Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of venous thromboembolism according to somatotype at age 10 in Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1993–2015), and Health Professionals Follow-Up Study (1988–2012).
| Cases | Person-years | Multivariable-adjusted modela | Multivariable-adjusted model + BMIb | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Somatotype at Age 10 | ||||
| NHS | ||||
| 1 | 720 | 545,611 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 609 | 460,807 | 1.07 (0.96, 1.19) | 1.02 (0.92, 1.14) |
| 3 | 406 | 307,607 | 1.07 (0.94, 1.20) | 0.96 (0.85, 1.09) |
| 4 | 315 | 221,993 | 1.15 (1.00, 1.31) | 0.99 (0.86, 1.13) |
| >5 | 306 | 196,353 | 1.29 (1.13, 1.48) | 1.09 (0.95, 1.25) |
| p-trend | <0.01 | 0.53 | ||
| NHS II | ||||
| 1 | 328 | 352,569 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 499 | 579,711 | 0.97 (0.84, 1.11) | 0.89 (0.78, 1.03) |
| 3 | 453 | 430,130 | 1.15 (1.00, 1.33) | 0.93 (0.80, 1.07) |
| 4 | 323 | 298,815 | 1.13 (0.97, 1.32) | 0.83 (0.70, 0.97) |
| >5 | 334 | 227,285 | 1.47 (1.26, 1.71) | 1.02 (0.87, 1.20) |
| p-trend | <0.01 | 0.93 | ||
| HPFS | ||||
| 1 | 315 | 138,610 | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 415 | 188,890 | 1.03 (0.88, 1.91) | 0.99 (0.85, 1.15) |
| 3 | 231 | 105,378 | 1.06 (0.89, 1.26) | 0.99 (0.83, 1.18) |
| 4 | 133 | 65,112 | 1.01 (0.82, 1.24) | 0.92 (0.74, 1.13) |
| >5 | 241 | 101,813 | 1.21 (1.02, 1.44) | 1.07 (0.90, 1.28) |
| p-trend | 0.05 | 0.68 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study; BMI, body mass index; HR, hazard ratio; CI, confidence interval.
Adjusted for age, physical activity, smoking, prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, prevalent rheumatological disease, aspirin use, hormonal therapy use (NHS and NHS II only), oral contraceptive use (NHS II only)
Further adjustment for adult BMI
Similarly, higher young adult BMI was significantly associated with a greater risk of VTE; however, after adjusting for adult BMI the associations were no longer significant (Table 3). For example, in NHS, the multivariable-adjusted hazard ratio comparing participants with a young adult BMI ≥27.5 kg/m2 to those with a young adult BMI of 15 to <19 kg/m2 was 1.52 (95% CI: 1.23, 1.88, p-trend<0.01). However, after adjusting for current BMI, this was attenuated to a HR of 0.99 (95% CI: 0.79, 1.23, p-trend=0.60). Similar patterns were also observed in NHS II and HPFS (Table 3).
Table 3.
Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of venous thromboembolism according to young adult body mass index in Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1993–2015), and Health Professionals Follow-Up Study (1988–2012).
| Cases | Person-years | Multivariable-adjusted modela | Multivariable-adjusted model + BMIb | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Young Adult BMI (kg/m2) | ||||
| NHSc | ||||
| 15 to<19 | 409 | 339,746 | 1.00 (Reference) | 1.00 (Reference) |
| 19 to <20.4 | 568 | 443,983 | 1.10 (0.97, 1.25) | 1.03 (0.91, 1.18) |
| 20.4 to <21.9 | 557 | 441,065 | 1.07 (0.94, 1.22) | 0.93 (0.82, 1.06) |
| 21.9 to <24.9 | 596 | 440,738 | 1.12 (0.98, 1.27) | 0.88 (0.77, 1.00) |
| 24.9 to <27.5 | 209 | 115,273 | 1.48 (1.25, 1.75) | 1.05 (0.88, 1.25) |
| >27.5 | 111 | 57,928 | 1.51 (1.22, 1.87) | 0.99 (0.79, 1.23) |
| p-trend | <0.01 | 0.60 | ||
| NHS IIc | ||||
| 15 to<19 | 323 | 414,749 | 1.00 (Reference) | 1.00 (Reference) |
| 19 to <20.4 | 428 | 495,499 | 1.12 (0.97, 1.30) | 0.97 (0.84, 1.12) |
| 20.4 to <21.9 | 406 | 412,886 | 1.26 (1.09, 1.45) | 0.94 (0.81, 1.09) |
| 21.9 to <24.9 | 468 | 397,726 | 1.42 (1.23, 1.64) | 0.89 (0.76, 1.04) |
| 24.9 to <27.5 | 188 | 115,685 | 1.78 (1.48, 2.14) | 0.96 (0.79, 1.17) |
| >27.5 | 158 | 81,505 | 2.01 (1.65, 2.44) | 0.97 (0.79, 1.21) |
| p-trend | <0.01 | 0.77 | ||
| HPFSd | ||||
| 15 to<19 | 87 | 31,917 | 1.00 (Reference) | 1.00 (Reference) |
| 19 to <20.4 | 132 | 64,906 | 0.78 (0.59, 1.02) | 0.76 (0.58, 1.00) |
| 20.4 to <21.9 | 270 | 119,635 | 0.88 (0.69, 1.12) | 0.84 (0.65, 1.07) |
| 21.9 to <24.9 | 521 | 259,944 | 0.85 (0.68, 1.08) | 0.76 (0.60, 0.96) |
| 24.9 to <27.5 | 252 | 102,279 | 1.02 (0.79, 1.31) | 0.79 (0.61, 1.03) |
| >27.5 | 103 | 30,876 | 1.36 (1.01, 1.82) | 0.94 (0.69, 1.28) |
| p-trend | <0.01 | 0.94 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study; BMI, body mass index; HR, hazard ratio; CI, confidence interval; kg, kilogram; m, meter.
Adjusted for age, physical activity, smoking, prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, prevalent rheumatological disease, aspirin use, hormonal therapy use (NHS and NHS II only), oral contraceptive use (NHS II only)
Further adjustment for adult BMI
Young adult BMI is BMI at age 18 years
Young adult BMI is BMI at age 21 years
In Figure 1, we present multivariable adjusted HRs (95% CI) for risk of VTE across categories of adult BMI. Across all three cohorts, we found a strong, positive association between higher adult BMI and risk of VTE (all p-trends <0.01).
Figure 1.
Multivariable adjusted hazard ratios and 95% confidence intervals of venous thromboembolism according to categories of adult body mass index (kg/m2) in Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1993–2015), and Health Professionals Follow-Up Study (1988–2012).
Increasing waist circumference was also associated with a greater risk of VTE in all three cohorts independent of adult BMI (Table 4). For example, in NHS the multivariable-adjusted HR comparing women with the greatest waist circumference (≥96 cm) to those with the lowest (<72 cm) was 2.08 (95% CI: 1.57, 2.76), p-trend<0.01. Similar associations were also found for NHS II (HR=2.26, 95% CI: 1.58, 3.23, p-trend<0.01) and HPFS participants (HR=2.00, 95% CI: 1.53, 2.62 comparing men with waist circumference ≥107 cm vs. <89 cm, p-trend<0.01).
Table 4.
Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of venous thromboembolism according to waist circumference in Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1993–2015), and Health Professionals Follow-Up Study (1988–2012).
| Cases | Person-years | Multivariable-adjusted modela | Multivariable-adjusted model + BMIb | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Waist Circumference (cm) | ||||
| NHS | ||||
| <72 | 117 | 200,749 | 1.00 (Reference) | 1.00 (Reference) |
| 72 to <80 | 227 | 242,662 | 1.46 (1.16, 1.83) | 1.43 (1.14, 1.80) |
| 80 to <88 | 265 | 221,825 | 1.64 (1.31, 2.06) | 1.53 (1.21, 1.94) |
| 88 to <96 | 247 | 133,250 | 2.41 (1.91, 3.05) | 2.08 (1.61, 2.70) |
| >96 | 318 | 142,933 | 2.77 (2.20, 3.49) | 2.08 (1.57, 2.76) |
| p-trend | <0.01 | <0.01 | ||
| NHS II | ||||
| <72 | 112 | 231,394 | 1.00 (Reference) | 1.00 (Reference) |
| 72 to <80 | 148 | 219,412 | 1.45 (1.13, 1.86) | 1.42 (1.11, 1.83) |
| 80 to <88 | 155 | 166,870 | 1.93 (1.50, 2.48) | 1.80 (1.37, 2.36) |
| 88 to <96 | 111 | 94,756 | 2.45 (1.86, 3.21) | 2.07 (1.46, 2.88) |
| >96 | 194 | 119,464 | 3.11 (2.41. 4.02) | 2.26 (1.58, 3.23) |
| p-trend | <0.01 | <0.01 | ||
| HPFS | ||||
| <89 | 138 | 111,149 | 1.00 (Reference) | 1.00 (Reference) |
| 89 to <95 | 202 | 116,279 | 1.23 (0.98, 1.53) | 1.18 (0.94, 1.47) |
| 95 to <101 | 287 | 112,328 | 1.68 (1.36, 2.07) | 1.54 (1.23, 1.93) |
| 101 to <107 | 188 | 78,474 | 1.48 (1.18, 1.86) | 1.31 (1.02, 1.69) |
| >107 | 253 | 62,980 | 2.30 (1.84, 2.87) | 2.00 (1.53, 2.62) |
| p-trend | <0.01 | <0.01 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study; BMI, body mass index; HR, hazard ratio; CI, confidence interval; cm, centimeter.
Adjusted for age, physical activity, smoking, prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, prevalent rheumatological disease, aspirin use, hormonal therapy use (NHS and NHS II only), oral contraceptive use (NHS II only)
Further adjustment for adult BMI
Weight gain since age 18, which is the difference between current weight and weight at age 18, was significantly associated with risk of VTE in women after adjustment for current BMI (Table 5). Compared with women in NHS who had little change in weight (± 4kg), women who gained ≥20 kg since age 18 had 35% increased risk of VTE (95% CI: 1.13, 1.65, p-trend<0.01). Among women in NHS II, the risk of VTE in women who gained ≥20 kg since age 18 was 1.48 (95% CI: 1.17, 1.87, p-trend=0.01) times the risk of women with little change (± 4kg) in weight. In men, we observed a borderline significant risk of VTE for men who gained ≥20 kg compared to men with little change in weight since age 21 (HR=1.20, 95% CI: 0.97, 1.50, p-trend=0.38).
Table 5.
Multivariable adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) of venous thromboembolism according to change in weight (kg) since age 18 in Nurses’ Health Study (1986–2014), Nurses’ Health Study II (1993–2015), and age 21 in Health Professionals Follow-Up Study (1988–2012).
| Cases | Person-years | Multivariable-adjusted modela | Multivariable-adjusted model + BMIb | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Weight Change | ||||
| NHS | ||||
| Loss of >4 kg | 115 | 107,142 | 1.13 (0.91, 1.41) | 1.15 (0.92, 1.43) |
| No change (± 4kg) | 258 | 298,452 | 1.00 (Reference) | 1.00 (Reference) |
| Gain 4 to <10 kg | 398 | 413,586 | 1.13 (0.97, 1.33) | 1.04 (0.88, 1.22) |
| Gain 10 to 15 kg | 359 | 307,500 | 1.33 (1.13, 1.57) | 1.12 (0.93, 1.33) |
| Gain 15 to 20 kg | 368 | 240,713 | 1.70 (1.45, 2.00) | 1.30 (1.07, 1.57) |
| Gain >20kg | 952 | 471,341 | 2.18 (1.89, 2.52) | 1.36 (1.13, 1.65) |
| p-trend | <0.01 | <0.01 | ||
| NHS II | ||||
| Loss of >4 kg | 56 | 70,651 | 1.49 (1.10, 2.03) | 1.37 (1.01, 1.87) |
| No change (± 4kg) | 148 | 295,185 | 1.00 (Reference) | 1.00 (Reference) |
| Gain 4 to <10 kg | 284 | 440,454 | 1.25 (1.03, 1.53) | 1.14 (0.93, 1.40) |
| Gain 10 to 15 kg | 242 | 300,435 | 1.54 (1.26, 1.90) | 1.22 (0.97, 1.52) |
| Gain 15 to 20 kg | 242 | 229,682 | 1.96 (1.59, 2.41) | 1.36 (1.07, 1.72) |
| Gain >20kg | 999 | 581,644 | 2.92 (2.44, 3.49) | 1.48 (1.17, 1.87) |
| p-trend | <0.01 | 0.01 | ||
| HPFS | ||||
| Loss of >4 kg | 154 | 64,581 | 1.16 (0.94, 1.43) | 1.12 (0.91, 1.38) |
| No change (± 4kg) | 235 | 133,222 | 1.00 (Reference) | 1.00 (Reference) |
| Gain 4 to <10 kg | 322 | 178,957 | 1.04 (0.87, 1.23) | 0.98 (0.82, 1.17) |
| Gain 10 to 15 kg | 244 | 107,560 | 1.21 (1.01, 1.45) | 1.06 (0.88, 1.29) |
| Gain 15 to 20 kg | 163 | 66,702 | 1.26 (1.02, 1.54) | 1.03 (0.83, 1.28) |
| Gain > 20kg | 297 | 81,451 | 1.72 (1.43, 2.05) | 1.20 (0.97, 1.50) |
| p-trend | <0.01 | 0.38 |
Abbreviations: NHS, Nurses’ Health Study; NHS II, Nurses’ Health Study II; HPFS, Health Professionals Follow-Up Study; BMI, body mass index; HR, hazard ratio; CI, confidence interval; kg, kilogram.
Adjusted for age, physical activity, smoking, prevalent diabetes, prevalent hypertension, prevalent hypercholesterolemia, prevalent MI/angina, prevalent cancer, prevalent rheumatological disease, aspirin use, hormonal therapy use (NHS and NHS II only), oral contraceptive use (NHS II only)
Further adjustment for adult BMI
In sensitivity analyses, we excluded participants who developed cancer over follow-up and found similar results. We continued to find a strong, positive association between higher adult BMI and risk of VTE (all p-trends <0.01) among this subgroup of participants without cancer (results not shown). Additionally, increasing waist circumference continued to be associated with a greater risk of VTE in all three cohorts independent of adult BMI (results not shown). For example, in NHS the multivariable-adjusted HR comparing women with the greatest waist circumference (≥96 cm) to those with the lowest (<72 cm) was 2.17 (95% CI: 1.59, 2.98), p-trend<0.01. Similar associations were also found for NHS II (HR=2.41, 95% CI: 1.66, 3.50, p-trend<0.01) and HPFS participants (HR=1.94, 95% CI: 1.44, 2.62 comparing men with waist circumference ≥107 cm vs. <89 cm, p-trend<0.01).
DISCUSSION
To our knowledge, this is the first study to investigate the association between measures of adiposity and VTE throughout the life course, from childhood to later-life. We hypothesized that since adult BMI is strongly related to risk of VTE, earlier-life adiposity may predict VTE risk in later life. In multivariable-adjusted models, we did find that childhood body fatness at age 10 as well as increasing young adult BMI were associated with an increased risk of VTE, however these associations were no longer significant after adjustment for adult BMI. These findings suggest that BMI is likely more important acutely, rather than cumulatively over time, in the etiology of VTE.
We confirmed a strong, positive association between adult BMI and risk of VTE in three large prospective cohorts of female and male health professionals. BMI is a well-established risk factor for VTE, with many prior studies demonstrating an increased risk of VTE with increasing levels of adult BMI.5,6,17–19 Among these, prior research in the NHS, albeit with less follow-up, reported a strong, positive relationship between adult BMI and PE.5 Moreover, two recent independent Mendelian randomization studies demonstrated consistent significant associations between genetically predicted BMI and VTE, suggesting the relationship is causal. 7,20 In our current analysis, we expanded on these findings including longer (22–28 years) follow-up, two additional cohorts (including one of men), and both DVT and PE. Overall, we found associations similar to those previously reported for higher categories of BMI, demonstrating that higher BMI in adulthood is a strong and significant risk factor for VTE in both men and women across middle and older age. To our knowledge, the current analysis is the largest and most comprehensive prospective study evaluating the relationship between obesity and VTE. To expand our understanding of the BMI-VTE association, we also explored other measures of adult adiposity: waist circumference and weight change since early adulthood. We found that larger waist circumference was associated with a greater risk of VTE in all three cohorts and that this association was independent of adult BMI. While prior studies have found a significant relationship between increased waist circumference and risk of VTE18,21, these studies did not simultaneously adjust for adult BMI and thus it could not be determined if the association between central adiposity and VTE was independent of overall adiposity. In models that evaluated weight change since age 18 (NHS/NHS II) or age 21 (HPFS), we found a greater VTE risk for those adults who gained the most weight since young adulthood, indicating that limiting weight gain in adults should be strongly encouraged to help reduce VTE risk. Few prior studies have investigated the association of weight change in relation to VTE risk, and those published have had much smaller sample sizes and significantly shorter follow-up compared to our analysis.22
Our study had numerous strengths including the prospective design with long follow-up and a high follow-up rate. In addition, we were able to adjust for many potential confounders and evaluate whether the associations with early life body size were independent of adult BMI. Potential limitations also need to be considered. First, reliance on participant’s memory to recall their earlier life body size may lead to measurement error. However, our previous work has indicated good validity of recalled childhood and adolescence body size, even among older adults. Also, because the recall of early life body size occurred years before VTE diagnosis, misclassification is likely to be random and lead to underestimation of the association. Second, our study population is primarily white and results may not be generalizable to other ethnic and racial groups. Although adiposity and VTE risk should be examined in minority populations, at the same time, the homogeneity and the uniform health access of this population reduces confounding by many factors and provides strong interval validity. Lastly, there is also potential for misclassification of VTE since this study identified VTE events via participant self-report. However, in validation studies, 94% of self-reported cases were confirmed in medical records.15 In addition, we would expect bias towards the null if participants who experienced a VTE were classified as not have had a VTE, or vice versa. Thus, our findings are unlikely to overestimate the associations between body size and VTE.
CONCLUSIONS
In conclusion, adult BMI, waist circumference, and weight change are independently, positively associated with incident VTE risk. Early life body size is not significantly associated with VTE risk after accounting for adult BMI, indicating that BMI and adiposity are likely more important acutely, than cumulatively over time, in the etiology of VTE. Clinically, encouraging weight maintenance or loss in adults could help reduce VTE risk.
Highlights:
We confirmed a strong, positive association between adult BMI and risk of VTE.
Childhood and young adult adiposity were not significantly associated with VTE.
Increasing weight gain from young adulthood was significantly associated with VTE.
Adiposity is more important acutely in the etiology and prevention of VTE.
ACKNOWLEDGEMENTS
We thank the participants and staff of the Nurses’ Health Study, Nurses’ Health Study II, and Health Professionals Follow-Up Study for their valuable contributions.
FUNDING: This work was supported by the National Institutes of Health (Grants UM1 CA186107, R01 HL034594, UM1 CA176726, UM1 CA167552, R01 HL35464, T32 HL098048, and R01 HL116854).
Abbreviations used:
- BMI
(body mass index)
- VTE
(venous thromboembolism)
- DVT
(deep vein thrombosis)
- PE
(pulmonary embolism)
- NHS
(Nurses’ Health Study)
- NHS II
(Nurses’ Health Study II)
- HPFS
(Health Professionals Follow-Up Study)
- HR
(hazard ratio)
- CI
(confidence interval)
- kg
(kilogram)
- m
(meter)
- cm
(centimeter)
Footnotes
DECLARATION OF INTEREST: Dr. Kabrhel reports grants from Janssen, grants from Diagnostica Stago, grants from Siemens Healthcare Diagnostics, outside the submitted work. All other authors declare no conflict of interest.
MEETING PRESENTATION: Results from this analysis were presented at the American Heart Association Epi Lifestyle Scientific Session (March 2018) in New Orleans, Louisiana.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost 2005;3:1611–1617. [DOI] [PubMed] [Google Scholar]
- 2.Ageno W, Becattini C, Brighton T, Selby R, Kamphuisen PW. Cardiovascular risk factors and venous thromboembolism: a meta-analysis. Circulation 2008;117:93–102. [DOI] [PubMed] [Google Scholar]
- 3.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol 2005;162:975–982. [DOI] [PubMed] [Google Scholar]
- 4.Goldhaber SZ, Grodstein F, Stampfer MJ, et al. A prospective study of risk factors for pulmonary embolism in women. JAMA 1997;277:642–645. [PubMed] [Google Scholar]
- 5.Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity (Silver Spring) 2009;17:20402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stein PD, Beemath A, Olson RE. Obesity as a risk factor in venous thromboembolism. Am J Med 2005;118:978–980. [DOI] [PubMed] [Google Scholar]
- 7.Lindström S, Germain M, Crous-Bou M, et al. Assessing the causal relationship between obesity and venous thromboembolism through a Mendelian Randomization study. Hum Genet 2017;136:897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stunkard AJ, Sorensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–120. [PubMed] [Google Scholar]
- 9.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol 1993;138:56–64. [DOI] [PubMed] [Google Scholar]
- 10.Field AE, Franko DL, Striegel-Moore RH, Schreiber GB, Crawford PB, Daniels SR. Race differences in accuracy of self-reported childhood body size among white and black women. Obes Res 2004;12:1136–1144. [DOI] [PubMed] [Google Scholar]
- 11.Schernhammer ES, Tworoger SS, Eliassen AH, et al. Body shape throughout life and correlations with IGFs and GH. Endocr Relat Cancer 2007;14:721–732. [DOI] [PubMed] [Google Scholar]
- 12.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord 1995;19:570–572. [PubMed] [Google Scholar]
- 13.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol 1983;117:651–658. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of selfreported waist and hip circumferences in men and women. Epidemiology 1990;1:466–473. [DOI] [PubMed] [Google Scholar]
- 15.Pun VC, Hart JE, Kabrhel C, Camargo CA Jr., Baccarelli AA, Laden F. Prospective Study of Ambient Particulate Matter Exposure and Risk of Pulmonary Embolism in the Nurses’ Health Study Cohort. Environ Health Perspect 2015;123:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varraso R, Kabrhel C, Goldhaber SZ, Rimm EB, Camargo CA Jr. Prospective study of diet and venous thromboembolism in US women and men. Am J Epidemiol 2012;175:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Severinsen MT, Kristensen SR, Johnsen SP, Dethlefsen C, Tjonneland A, Overvad K. Anthropometry, body fat, and venous thromboembolism: a Danish follow-up study. Circulation 2009;120:1850–1857. [DOI] [PubMed] [Google Scholar]
- 18.Horvei LD, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Obesity measures and risk of venous thromboembolism and myocardial infarction. Eur J Epidemiol 2014;29:821–830. [DOI] [PubMed] [Google Scholar]
- 19.Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klarin D, Emdin CA, Natarajan P, Conrad MF, INVENT Consortium, Kathiresan S. Genetic Analysis of Venous Thromboembolism in UK Biobank Identifies the ZFPM2 Locus and Implicates Obesity as a Causal Risk Factor. Circ Cardiovasc Genet 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borch KH, Braekkan SK, Mathiesen EB, et al. Anthropometric measures of obesity and risk of venous thromboembolism: the Tromso study. Arterioscler Thromb Vasc Biol 2010;30:121–127. [DOI] [PubMed] [Google Scholar]
- 22.Horvei LD, Braekkan SK, Hansen JB. Weight Change and Risk of Venous Thromboembolism: The Tromso Study. PloS One 2016;11:e0168878. [DOI] [PMC free article] [PubMed] [Google Scholar]