Abstract
Objective:
Serrated lesions such as sessile serrated adenomas or polyps (SSA/Ps) are important colorectal cancer precursors, but etiological factors for these lesions are largely unknown. We aimed to determine the effects of calcium and vitamin D supplementation on the incidence of serrated polyps (SPs) in general and hyperplastic polyps and SSA/Ps specifically.
Design:
Participants with one or more adenoma at baseline were randomized to receive 1200 mg/day of elemental calcium, 1,000 IU/day of vitamin D3, both, or neither agent. Treatment continued for 3 or 5 years, when risk of polyps was determined from surveillance colonoscopy (treatment phase). Outcomes after treatment ceased were also assessed (observational phase). Adjusted risk ratios (aRRs) of SPs were determined via multivariable generalized linear models.
Results:
SPs were diagnosed in 565 of 2058 (27.5%) participants during the treatment phase, and 329/1108 (29.7%) during the observational phase. In total, 211 SSA/Ps were identified during follow up. In the treatment phase, there was no effect of either calcium or vitamin D on incidence of SSA/Ps. However, during the later observational phase, we observed elevated risks of SSA/Ps associated with calcium alone and calcium + vitamin D treatment [aRR (95% CI): 2.66 (1.44–4.89) and 3.82 (1.26–11.57 respectively].
Conclusion:
In a large multicenter chemoprevention study, we found evidence that calcium and vitamin D supplementation increased the risk of SSA/Ps. This appeared to be a late effect: 6–10 years after supplementation began. These possible risks must be weighed against the benefits of calcium and vitamin D supplementation.
Keywords: colonoscopy, epidemiology, carcinogenesis, mineral supplementation
INTRODUCTION:
Some serrated polyps (SPs) are now established precursor lesions for colorectal cancer (CRC), and are estimated to give rise to 20–30% of sporadic cases.[1, 2] The term “serrated” is derived from the infolding of crypt epithelium seen on histological cross-section, which is a shared feature of these polyps. SPs comprise hyperplastic polyps (HP), in addition to two more advanced precursor lesions, the traditional serrated adenoma (TSA) and the sessile serrated adenoma or polyp (SSA/P).[3] Though HPs are the most common type of SP overall, SSA/Ps are the most common premalignant SP, and are thought to give rise to CRC via the “serrated pathway” involving CpG island methylation (CIMP) and BRAF mutation and often accompanied by microsatellite instability (MSI).[2] These lesions are distinct from conventional adenomas and differ in their biology, endoscopic appearance, typical location, histopathology, and natural history.[4]
Prevention of CRC incidence and mortality are important public health goals given the fact that CRC is a common cancer worldwide, and is responsible for significant cancer mortality.[5] In addition to screening, chemoprevention with various agents has been frequently studied as a means of reducing the incidence of CRC and its precursors, though results have been mixed.[6] Given the biologic differences between SPs and conventional adenomas, it is plausible that they respond differently to chemopreventive agents.[7, 8] Thus, it is important to understand the effects of such agents on both types of premalignant lesions.
By analyzing findings from a large chemoprevention trial with randomized calcium and vitamin D treatment, we aimed to determine whether these agents reduced the risk of SPs, particularly SSA/Ps, both during treatment and observational follow-up after treatment ended. Based on evidence for antineoplastic effects,[9–11] we hypothesized that calcium and vitamin D supplementation would have either a null or protective effect on SP occurrence.
METHODS:
Study design and participants
We analyzed the risk of serrated colorectal polyps among participants in the Vitamin D/Calcium Polyp Prevention Study. This was a randomized, multicenter, double blind, placebo-controlled trial that took place at 11 geographically diverse centers across the US. Participants were enrolled in the trial between July 2004 and July 2008, and outcomes were collected up to June 2016. Detailed methods regarding study design and procedures have been previously published, including the study protocol.[12] Briefly, patients between ages 45 and 75 who had a clearing colonoscopy with at least 1 adenomatous polyp detected and removed within the preceding 4 months, and who were scheduled for surveillance colonoscopy at either 3 or 5 years were invited to participate. Exclusion criteria included patients with known familial CRC syndromes (e.g. polyposis or Lynch syndrome), inflammatory bowel disease, other serious health conditions, contraindication or requirement for either agent, or abnormal blood creatinine, 25-hydroxyvitamin D or calcium levels.
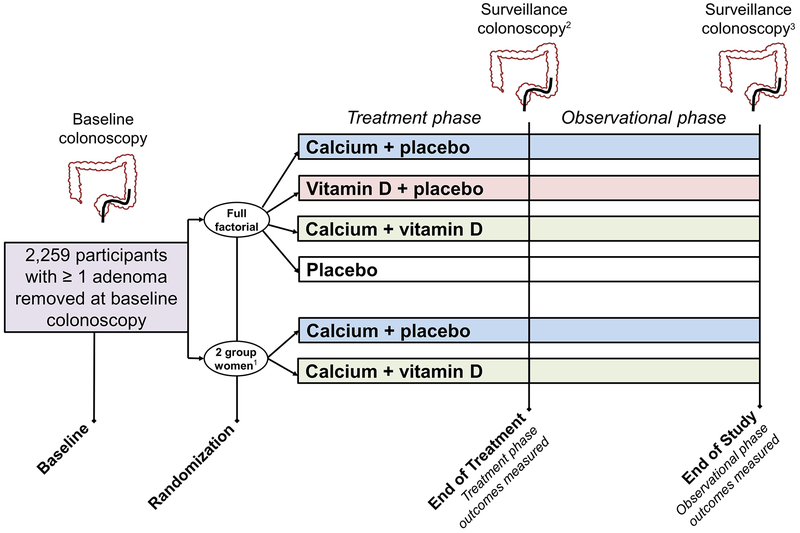
After enrollment, all patients underwent a blinded 2–3 month run-in period to confirm minimum pill taking adherence of 80%. Following the run-in, eligible participants were randomized to one of four treatment groups using a partial 2 × 2 factorial design: A) calcium carbonate (1200mg elemental calcium/day), B) vitamin D3 (1000 IU/day), C) both agents, or D) placebo. Women who did not want to forego supplemental calcium could elect to be randomized to one of the two groups with calcium supplementation and thus were only randomized to vitamin D vs. placebo. Treatment was to be continued until the first surveillance colonoscopy anticipated at 3 or 5 years (treatment period). Data collection continued after this colonoscopy for participants who consented to additional follow-up (observational period) (Figure 1), however, subjects who did not have an End of Treatment colonoscopy were not included in observational analyses.
FIGURE 1:
Study design and outcome measurement
Footnotes:
1Women could elect to be randomized to either calcium-containing group outside of factorial randomization (2 group randomization).
2Interim colonoscopies taking place before 3 years were included in the treatment interval analysis. In the absence of an anticipated year 3 or 5 colonoscopy, any colonoscopy taking place more than 1 year after the baseline examination was accepted.
3End of Study colonoscopy is defined as the first surveillance colonoscopy that took place at least 3 years after the End of Treatment colonoscopy. For subjects who did not have a surveillance colonoscopy at least 3 years following End of Treatment, non-surveillance exams and interim colonoscopies taking place before 3 years were included in the observational phase analysis.
The End of Study (i.e. end of observational phase) colonoscopy was the first surveillance colonoscopy at least 3 years after the End of Treatment colonoscopy. Data from all procedures between the End of Treatment colonoscopy up to and including the End of Study colonoscopy were considered as outcome data for the observational phase. If the surveillance colonoscopy was incomplete (e.g. surgery was needed to excise a lesion seen at the End of Study colonoscopy) or there was a very short follow-up interval to the next exam (< 6 months), the results of this later procedure are included as observational data. For subjects who did not have a surveillance colonoscopy at least 3 years after the End of Treatment colonoscopy but had other procedures, we included outcome data from all those procedures. This includes any colonoscopies prior to the 3 year mark and non-surveillance colonoscopies after 3 years. The majority of participants had outcomes determined from a single colonoscopy in both phases: In the treatment phase, only 84/2058 (4%) had > 1 exam. In the observational phase, only 82/1108 (7.4%) had >1 exam. Participants were unblinded to their treatment assignment on 11/1/2013. Among observational phase participants, 405/1108 (37%) were still blinded at the time of their End of Study colonoscopy.
The study was approved by the institutional review boards at the Dartmouth central coordinating site and all participating clinical sites, and all participants provided informed consent prior to enrollment. The study was registered at ClinicalTrials.gov (number NCT00153816). All authors had access to study data and reviewed and approved the final manuscript.
Measurement of outcomes and covariates
At enrollment, data were collected by trained research assistants on demographics, medical history, family history medications and supplement use, health habits, and diet (Block Brief 2000 questionnaire). Height and weight were determined by self-report or measurement, and used to calculate body mass index (BMI). During the trial, patients were contacted by a study coordinator every 6 months to verify adherence, health status and adverse events, concomitant medications and supplement use, and performance of colonoscopy or other colorectal imaging. Blood levels of 25-hydroxyvitamin D, calcium, and creatinine were measured during the trial with standard assays as previously described.[12]
Categorization of baseline specimens was based on abstraction of pathology records at the clinical centers. All lesions found during post-randomization colonoscopies were reviewed centrally by an expert in GI pathology, specifically regarding serrated neoplasia (DCS). The study pathologist was blinded to treatment assignment. For this study, the outcome of interest included all SPs, subtyped as HPs [including microvesicular (MVHP), goblet-cell rich (GCHP), and mucin-poor (MPHP)], SSA/Ps (with or without cytological dysplasia), and TSAs, classified according to WHO criteria.[3] In certain cases due to specimen fragmentation, orientation, or small size, it was not possible to make a clear determination of SP subtype, in which case lesions were labeled as “serrated polyp not subclassified”. Polyp location was categorized as right-sided if proximal to the splenic flexure, and lesions more distal were categorized as left-sided.
Statistical analysis
The primary outcomes for this analysis were one or more serrated polyps (any type) overall, as well as SSA/Ps and HPs specifically. Participants were included in the treatment phase analysis if they had a surveillance colonoscopy performed at least 1 year following randomization. Participants were included in the observational phase if they had a treatment phase colonoscopy, consented to additional follow up, and had a subsequent surveillance exam before all follow up ceased (June 2016). For the primary analysis, contingency tables and Chi-squared tests were used to compare SP occurrence between randomized treatment groups. Multivariable generalized linear models for binary data were used to estimate adjusted risk ratios (aRR) and 95% confidence intervals (CI) for different SP types both during the treatment phase, and during the observational phase after treatment was complete (Figure 1). Model covariates included age, sex, race, BMI, smoking status, clinical center, 2 group vs. full-factorial randomization, number of baseline SPs (0, 1, 2+), and anticipated treatment phase surveillance interval (3 or 5 year; treatment phase only). We also performed a sensitivity analysis of observational phase outcomes excluding participants with SSA/Ps either at baseline or during the treatment phase.
Treatment phase subgroup analyses were conducted based on stratification by baseline 25-hydroxyvitamin D levels, baseline dietary calcium intake, sex, BMI, baseline vitamin D and calcium supplement use, smoking history, alcohol use, baseline nonsteroidal anti-inflammatory drug (NSAID) or aspirin use, and anticipated surveillance interval. Analyses of SP occurrence during the observational period also included subgroup analyses by end-of-treatment metrics (e.g. end of treatment serum 25-hydroxyvitamin D levels). To further explore the relationship between calcium and vitamin D and SPs using study data, we examined the association between dietary calcium intake (among those not given calcium) and 25-hydroxyvitamin D levels (among those not given vitamin D) on SP occurrence. We did this both for treatment phase (analyzing baseline levels) and observational phase (analyzing end of treatment serum levels) SPs. Interactions were assessed using Wald tests or one-degree-of-freedom tests for trends within strata. All analyses were by intention to treat. Two-sided p values of < 0.05 were considered statistically significant. Statistical analyses were conducted with the use of SAS software, version 9.4 (SAS Institute, Cary, NC) or STATA software, version 12 (StataCorp, College Station, TX).
RESULTS:
Participants and baseline characteristics
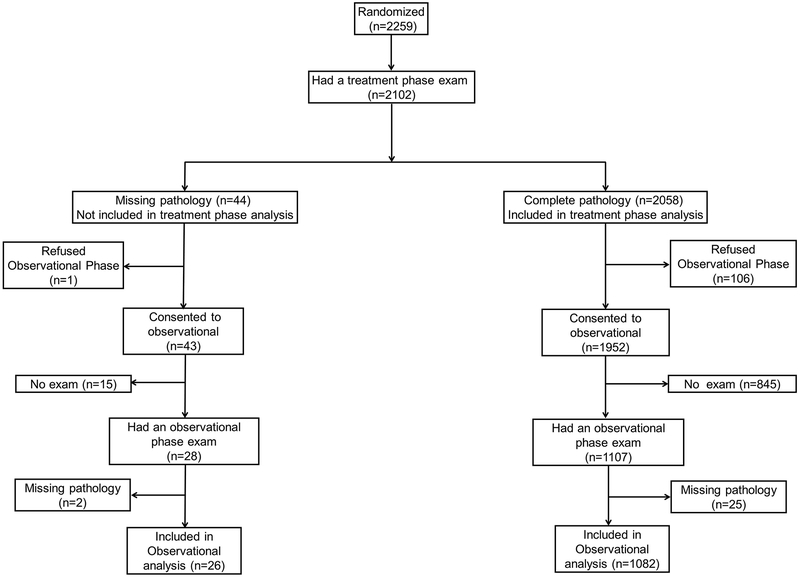
A total of 2,813 participants were enrolled and 2,259 underwent randomization to one of the six treatment groups. 419 were randomized to calcium alone, 420 to vitamin D alone, 421 to calcium + vitamin D, and 415 to placebo. A total of 584 women elected to be randomized to one of 2 arms with calcium supplementation, including 295 to calcium alone and 289 to calcium + vitamin D. A total of 2,058 participants had a colonoscopy at least 1 year after randomization and had sufficient pathology data to determine polyp outcomes during treatment phase. 1108 participants had at least 1 colonoscopy with sufficient pathology data following end of treatment and were included in the observational phase analysis (Figure 2). Randomized participants were mostly male (63%) and the average age was 58.1 (SD 6.8). The majority of participants (88%) were Caucasian. Participant characteristics at enrollment were similar between treatment groups (Table 1). Baseline characteristics of the subgroup entering the observational phase were also similar between groups (Supplementary Table 1).
FIGURE 2:
Flow chart of treatment and observational phase composition
TABLE 1:
Baseline characteristics of randomized participants
| Treatment Assignment | |||||||
|---|---|---|---|---|---|---|---|
| Full factorial randomization | 2 group randomization1 | ||||||
| Placebo | Calcium + placebo | Vitamin D + placebo | Calcium + vitamin D | Calcium + Placebo | Calcium + vitamin D | ||
| N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | N (%) or mean ± SD | ||
| Total N | 415 | 419 | 420 | 421 | 295 | 289 | |
| Sex Female | 60 (14.5) | 63 (15.0) | 62 (14.8) | 67 (15.9) | 295 (100) | 289 (100) | |
| Male | 355 (85.5) | 356 (85.0) | 358 (85.2) | 354 (84.1) | 0 | 0 | |
| Age | 58.2 ± 7.0 | 58.7 ± 7.0 | 58.3 ± 7.0 | 58.7 ± 6.9 | 56.7 ± 6.0 | 57.0 ± 6.6 | |
| Race Caucasian | 357 (88.8) | 354 (87.6) | 364 (90.1) | 350 (86.6) | 238 (86.6) | 237 (87.5) | |
| African American | 27 (6.7) | 33 (8.2) | 25 (6.2) | 46 (11.4) | 28 (10.2) | 25 (9.2) | |
| Asian/Pacific Islander | 10 (2.5) | 10 (2.5) | 12 (3.0) | 6 (1.5) | 8 (2.9) | 7 (2.6) | |
| Other | 8 (2.0) | 7 (1.7) | 3 (0.7) | 2 (0.5) | 1 (0.4) | 2 (0.7) | |
| BMI (kg/m2) | 29.0 ± 4.9 | 29.5 ± 5.1 | 29.1 ± 4.6 | 29.0 ± 5.1 | 28.8 ± 6.0 | 28.3 ± 5.4 | |
| <25 | 80 (19.3) | 74 (17.7) | 76 (18.1) | 97 (23.0) | 91 (31.0) | 99 (34.4) | |
| 25–29.9 | 187 (45.2) | 180 (43.0) | 181 (43.1) | 180 (42.8) | 91 (31.0) | 97 (33.7) | |
| ≥ 30 | 147 (35.5) | 165 (39.4) | 163 (38.8) | 144 (34.2) | 112 (38.1) | 92 (31.9) | |
| Smoking status | |||||||
| Never | 187 (45.1) | 212 (50.6) | 217 (51.7) | 204 (48.5) | 205 (69.5) | 169 (58.5) | |
| Former | 193 (46.5) | 174 (41.5) | 164 (39.1) | 169 (40.1) | 62 (21.0) | 88 (30.5) | |
| Current | 35 (8.4) | 33 (7.9) | 39 (9.3) | 48 (11.4) | 28 (9.5) | 32 (11.1) | |
| Alcohol intake (drinks/day) | 0.89 ± 1.08 | 0.82 ± 1.01 | 0.91 ± 1.11 | 0.89 ± 1.08 | 0.40 ± 0.72 | 0.48 ± 0.69 | |
| Dietary fat intake (g/day) | 71.4 ± 34.3 | 71.3 ± 31.6 | 69.0 ± 28.2 | 66.4 ± 25.7 | 53.9 ± 24.2 | 58.2 ± 26.7 | |
| Dietary fiber intake (g/day) | 15.5 ± 7.3 | 16.1 ± 7.1 | 15.0 ± 6.7 | 14.9 ± 7.2 | 13.9 ± 6.3 | 14.6 ± 6.4 | |
| Red meat intake (servings/day) | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.7 | 0.7 ± 0.6 | 0.5 ± 0.5 | 0.5 ± 0.5 | |
| Processed meat intake (servings/day) | 0.4 ± 0.5 | 0.4 ± 0.5 | 0.4 ± 0.4 | 0.4 ± 0.4 | 0.2 ± 0.3 | 0.3 ± 0.3 | |
| Dietary calcium intake (mg/day) | 672 ± 313 | 718 ± 326 | 643 ± 286 | 652 ± 303 | 604 ± 295 | 656 ± 313 | |
| Baseline supplemental calcium2 (mg/day) | |||||||
| 0 | 201 (51.9) | 199 (50.6) | 207 (52.3) | 212 (53.0) | 64 (24.9) | 56 (22.0) | |
| 1–499 | 173 (44.7) | 172 (43.8) | 172 (43.4) | 169 (42.3) | 94 (36.6) | 86 (33.7) | |
| ≥500 | 13 (3.4) | 22 (5.6) | 17 (4.3) | 19 (4.8) | 99 (38.5) | 113 (44.3) | |
| Dietary vitamin D intake (IU/day) | 138 ± 97 | 146 ± 96 | 131 ± 102 | 130 ± 92 | 124 ± 96 | 134 ± 98 | |
| Supplemental vitamin D2 (IU/day) | |||||||
| 0 | 204 (52.2) | 203 (52.2) | 209 (52.6) | 210 (52.9) | 84 (34.2) | 71 (30.5) | |
| 1–499 | 175 (44.8) | 170 (43.7) | 162 (40.8) | 175 (44.1) | 120 (48.8) | 110 (47.2) | |
| ≥500 | 12 (3.1) | 16 (4.1) | 26 (6.6) | 12 (3.0) | 42 (17.1) | 52 (22.3) | |
| Serum 25-OH- Vitamin D (ng/ml) | 24.24 ± 7.84 | 24.58 ± 8.42 | 24.88 ± 8.09 | 24.45 ± 8.06 | 25.03 ± 8.90 | 24.29 ± 7.71 | |
| Aspirin use | |||||||
| <4 days/week | 255 (61.5) | 249 (59.4) | 255 (60.7) | 270 (64.1) | 227 (77.0) | 206 (71.3) | |
| ≥4 days/week | 160 (38.6) | 170 (40.6) | 165 (39.3) | 151 (35.9) | 68 (23.1) | 83 (28.7) | |
| NSAID use (non-aspirin) | |||||||
| <4 days/week | 372 (89.6) | 367 (87.6) | 379 (90.2) | 388 (92.2) | 261 (88.5) | 253 (87.5) | |
| ≥4 days/week | 43 (10.4) | 52 (12.4) | 41 (9.8) | 33 (7.8) | 34 (11.5) | 36 (12.5) | |
Numbers for some characteristics may not sum to total N due to missing data; Abbreviations: SD, standard deviation; BMI, body mass index
Participants not randomized to calcium but were given calcium
Supplemental values in elemental mg/day and include separate supplements and multivitamins. Participants were asked to cease these supplements at enrollment as a condition of study entry.
At baseline, most participants had 1 or 2 low risk tubular adenomas, but 385 (18%) had advanced adenomas and 179 (8%) had 3 or more conventional adenomas. There were 544 (24%) participants who had additional baseline SPs including 521 (23%) with HPs, 28 (1%) with SSA/Ps, and 1 with a TSA (Table 2). There were 30 baseline SSA/Ps among 28participants; 12 of these had cytological dysplasia. Six participants had synchronous advanced adenomas and SSA/Ps at baseline, and these were evenly distributed across treatment groups (Supplementary Table 2).
TABLE 2:
Total serrated polyps diagnosed at baseline, and during treatment and observational phases
| Diagnosis1 | Baseline2 | Treatment phase | Observational phase | |||
|---|---|---|---|---|---|---|
| Polyps | Subjects3 | Polyps | Subjects3 | Polyps | Subjects3 | |
| Sessile serrated adenoma/polyp | 18 | 17 | 128 | 96 | 79 | 62 |
| SSA/P with cytological dysplasia | 12 | 11 | 4 | 4 | 3 | 3 |
| Traditional serrated adenoma | 1 | 1 | 8 | 8 | 8 | 6 |
| Serrated polyp not subclassified | 4 | 4 | 16 | 14 | 22 | 18 |
| Hyperplastic polyp | 839 | 521 | 955 | 494 | 498 | 271 |
| Total | 874 | 544 | 1111 | 565 | 607 | 329 |
Diagnosis at baseline is determined by the local pathologist at each clinical center. Post randomization polyps were all reviewed by the study pathologist.
Includes all randomized subjects and lesions found within 1 year of randomization
Participants with more than one type of serrated polyp may be represented multiple times. Total is the number of subjects with any serrated polyp, counting each subject only once if they had multiple types of serrated polyps
Occurrence of serrated polyps
During the treatment phase, a total of 1,111 SPs were found, including 955 HPs and 132 SSA/Ps (4 with cytological dysplasia) (Table 2). On the participant level, 565 (27%) of 2058 participants had at least 1 SP, including 494 (24%) with HPs and 100 (5%) with SSA/Ps. Of the 565 participants with ≥ 1 SP in the treatment phase, 213 had an adenoma and a SP at baseline, 327 had only adenoma(s), and 1 had only an SSA/P at baseline (24 had missing serrated status at baseline). During the observational phase, a total of 607 SPs were identified among participants with data from colonoscopies after the end of study treatment. This included 498 HPs and 79 SSA/Ps. On the participant level, 329 of 1108 (30%) had SPs during this period, including 271 (24%) with HPs and 62 (6%) with SSA/Ps. Of the 329 participants ≥ 1 SP in the observational phase, 123 had SPs at baseline, 143 had SPs during treatment phase, and 73 had SPs at both baseline and treatment phase. Of the 62 observational phase participants with SSA/Ps, 11 had prior SSA/Ps during the treatment phase. In both treatment and observational phases, the majority of SSA/Ps (70%) were located in the right colon (Supplementary Table 3).
Effects of calcium and vitamin D supplementation
The effects of randomized calcium and vitamin D supplementation on risk of SPs during the treatment and observational phases are shown in Table 3. During the treatment phase, we found no statistically significant effects of either calcium, vitamin D, or the combination on occurrence of SPs overall or HP or SSA/P subtypes. During the observational phase, there were no observed effects of calcium and/or Vitamin D on the risk of SPs overall or on HPs. There was also no statistically significant effect of vitamin D supplementation on risk of SSA/Ps (aRR = 1.30, 95% CI 0.81–2.09). However, among subjects randomized to calcium, we observed an increased risk of SSA/Ps (aRR 2.65, 95% CI 1.43 – 4.91). We also observed an increased risk of SSA/Ps among those randomized to the combination of vitamin D and calcium (aRR 3.81, 95% CI 1.25 – 11.64). We performed a sensitivity analysis excluding observational phase participants who had prior SSA/Ps during the treatment phase. In this analysis, the risks of incident SSA/P in the observational phase were similar to results of the full cohort shown in Table 3 [Vitamin D aRR: 1.27 (95% CI 0.75, 2.13); Calcium aRR: 2.99 (95% CI 1.54, 5.78) and Vitamin D + calcium: aRR: 4.56 (95% CI 1.55, 13.42)].
TABLE 3:
Calcium and vitamin D treatment and the risk of serrated polyps during treatment and observational phase
| Outcome | Treatment phase: n=20581 | Observational: n=11082 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N with event/N followed (%) | Crude RR (95% CI) | p | Adjusted3 aRR (95% CI) | p | N with event/Nfollowed (%) | Crude RR (95% CI) | p | Adjusted3 aRR (95% CI) | p | |
| Any serrated polyp | ||||||||||
| No Vitamin D | 278/1031 (27.0) | Reference | Reference | 159/550 (28.9) | Reference | Reference | ||||
| Vitamin D | 287/1027 (28.0) | 1.04 (0.90–1.19) | 0.62 | 1.01 (0.87–1.17) | 0.90 | 170/558 (30.5) | 1.05 (0.88–1.26) | 0.57 | 1.04 (0.86–1.25) | 0.71 |
| No Calcium | 203/763 (26.6) | Reference | Reference | 114/418 (27.3) | Reference | Reference | ||||
| Calcium | 231/764 (30.2) | 1.14 (0.97–1.33) | 0.12 | 1.15 (0.98–1.36) | 0.10 | 136/428 (31.8) | 1.17 (0.95–1.44) | 0.15 | 1.21 (0.97–1.50) | 0.09 |
| Placebo | 99/378 (26.2) | Reference | Reference | 56/205 (27.3) | Reference | Reference | ||||
| Vitamin D + Calcium | 117/384 (30.5) | 1.16 (0.93–1.46) | 0.19 | 1.15 (0.92–1.46) | 0.23 | 72/218 (33.0) | 1.21 (0.90–1.62) | 0.20 | 1.24 (0.92–1.67) | 0.16 |
| Any HP | ||||||||||
| No Vitamin D | 249/1029 (24.2) | Reference | Reference | 132/548 (24.1) | Reference | Reference | ||||
| Vitamin D | 245/1027 (23.9) | 0.99 (0.85–1.15) | 0.86 | 0.96 (0.82–1.13) | 0.65 | 139/555 (25.1) | 1.04 (0.85–1.28) | 0.71 | 1.03 (0.83–1.27) | 0.80 |
| No Calcium | 176/763 (23.1) | Reference | Reference | 101/418 (24.2) | Reference | Reference | ||||
| Calcium | 204/763 (26.7) | 1.16 (0.97–1.38) | 0.10 | 1.18 (0.99–1.42) | 0.07 | 103/423 (24.4) | 1.01 (0.79–1.28) | 0.95 | 1.05 (0.82–1.35) | 0.71 |
| Placebo | 87/378 (23.0) | Reference | Reference | 50/205 (24.4) | Reference | Reference | ||||
| Vitamin D + Calcium | 102/384 (26.6) | 1.15 (0.90–1.48) | 0.26 | 1.16 (0.90–1.49) | 0.26 | 53/215 (24.7) | 1.01 (0.72–1.42) | 0.95 | 1.03 (0.73–1.45) | 0.87 |
| Any SSA/P | ||||||||||
| No Vitamin D | 42/1008 (4.2) | Reference | Reference | 26/536 (4.9) | Reference | Reference | ||||
| Vitamin D | 58/1006 (5.8) | 1.38 (0.94–2.04) | 0.10 | 1.27 (0.86–1.88) | 0.22 | 36/550 (6.6) | 1.35 (0.83–2.20) | 0.23 | 1.31 (0.82–2.10) | 0.26 |
| No Calcium | 36/745 (4.8) | Reference | Reference | 13/409 (3.2) | Reference | Reference | ||||
| Calcium | 39/740 (5.3) | 1.09 (0.70–1.70) | 0.70 | 1.03 (0.66–1.60) | 0.91 | 36/417 (8.6) | 2.72 (1.47–5.03) | 0.001 | 2.66 (1.44–4.89) | 0.002 |
| Placebo | 14/369 (3.8) | Reference | Reference | 5/201 (2.5) | Reference | Reference | ||||
| Vitamin D + Calcium | 21/372 (5.7) | 1.49 (0.77–2.88) | 0.24 | 1.26 (0.67–2.39) | 0.47 | 22/216 (10.2) | 4.09(1.60–10.50) | 0.003 | 3.82 (1.26–11.57) | 0.02 |
Analyses of no vitamin D vs. vitamin D include all randomized participants; analyses of no calcium vs. calcium and of neither agent vs. both agents are restricted to full factorial participants.
Subjects who had an exam at least one year after randomization and had sufficient pathology to determine at least one of the serrated outcomes
Subjects followed after treatment period who had sufficient pathology to determine at least one of the serrated outcomes
Adjusted for age, sex, clinical center, race (white, black, other/missing), BMI, smoking status (never, former, current), anticipated surveillance interval (3 or 5 years; treatment phase only), randomization arm of randomization (2 group or full factorial), number of baseline serrated polyps (0, 1, 2+). Where necessary due to sparse data, clinical center was grouped by prevalence of serrated lesions at baseline into low (Georgia, Iowa, Minnesota, and California), medium (New Hampshire, Colorado, South Carolina, and Puerto Rico), high (Texas, Ohio, and North Carolina).
We also examined the effect of calcium and vitamin D on HP subtypes (MVHP, GCHP, and MPHP) (Supplementary Table 4). We found no statistically significant effects of either agent on specific HP subtypes in the treatment or observational phases.
Subgroup analyses
Results were stratified by baseline characteristics to determine presence or absence of effect modification during both treatment and observational phases (Tables 4 and 5). During the treatment phase, the effects of vitamin D and calcium did not differ substantially in subgroups based on baseline vitamin D levels, dietary calcium intake, sex, race, BMI, alcohol use, or use of NSAIDs or aspirin. However, current smoking apparently modified the effect of calcium supplementation on SPs such that current smokers experienced higher calcium aRRs than former or never smokers. Among non-smokers, the calcium aRR for any serrated polyp was 1.04 (95% CI 0.87–1.24), while among current smokers it was 2.16, 95% CI 1.32 – 3.51, p interaction = 0.02). There were similar interactive patterns for HPs and SSA/Ps, though with the smaller number of endpoints, these interactions were compatible with chance.
TABLE 4:
Subgroup analyses of vitamin D and calcium supplementation and risk of serrated polyps during treatment phase (N=2058)
| Any Serrated Polyp | Hyperplastic Polyp | Sessile Serrated Adenoma/Polyp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUBGROUP | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) |
| Baseline serum vitamin D | ||||||||||||
| ≤ 23.16 ng/ml | 145/523: 130/494 | 0.94 (0.76–1.15) | 91/372: 118/380 | 1.18 (0.92–1.50) | 132/521: 111/494 |
0.88 (0.70–1.11) | 81/372: 105/379 | 1.22 (0.94–1.57) | 17/511: 28/484 | 1.61 (0.87–2.98) | 15/365: 16/366 | 0.92 (0.45–1.86) |
| > 23.16 ng/ml | 133/508: 157/533 | 1.09 (0.88–1.33) | 112/391: 113/384 | 1.02 (0.81–1.29) | 117/508: 134/533 | 1.04 (0.83–1.30) | 95/391: 99/384 |
1.07 (0.83–1.38) | 25/497: 30/522 | 1.09 (0.66–1.81) | 21/380: 23/374 | 1.07 (0.61–1.89) |
| p | 0.30 | 0.42 | 0.29 | 0.49 | 0.35 | 0.77 | ||||||
| Baseline dietary calcium | ||||||||||||
| ≤ 597 mg/day | 133/466: 130/484 | 0.92 (0.74–1.14) | 93/350: 101/326 | 1.14 (0.88–1.47) | 124/464: 106/484 | 0.79 (0.63–1.01) | 81/350: 90/325 | 1.18 (0.90–1.55) | 17/454: 29/472 | 1.67 (0.92–3.06) | 93/350: 101/326 | 1.07 (0.53–2.15) |
| > 597 mg/day | 131/500: 133/463 | 1.05 (0.85–1.30) | 101/355: 108/383 | 1.01 (0.80–1.28) | 111/500: 117/463 | 1.09 (0.86–1.38) | 87/355: 93/383 | 1.02 (0.78–1.32) | 25/489: 26/457 | 0.99 (0.57–1.74) | 101/355: 108/383 | 1.08 (0.56–2.05) |
| p | 0.39 | 0.52 | 0.07 | 0.46 | 0.21 | 0.79 | ||||||
| Baseline vitamin D/calcium supplementation3 | ||||||||||||
| <400 IU or mg/day | 147/523: 141/514 | 0.97 (0.80–1.19) | 192/721: 211/719 | 1.09 (0.92–1.29) | 132/522: 120/514 | 0.91 (0.73–1.14) | 167/721: 185/718 | 1.11 (0.92–1.34) | 20/508: 30/500 | 1.37 (0.78–2.39) | 32/704: 38/697 | 1.11 (0.70–1.76) |
| ≥400 IU or mg/day | 130/502: 144/511 | 1.04 (0.84–1.29) | 11/40: 19/41 | 1.60 (0.84–3.06) | 116/501: 123/571 | 0.99 (0.78–1.24) | 9/40: 18/41 | 1.88 (0.92–3.86) | 22/494: 28/504 | 1.17 (0.67–2.04) | 4/39: 1/36 | 0.09 (0.01–1.54) |
| p | 0.65 | 0.30 | 0.60 | 0.21 | 0.63 | 0.25 | ||||||
| Sex | ||||||||||||
| Male | 182/649: 178/656 | 0.96 (0.80–1.15) | 171/656: 189/649 | 1.11 (0.92–1.33) | 163/649: 154/656 | 0.92 (0.76–1.12) | 150/656: 167/649 | 1.12 (0.92–1.37) | 28/635: 36/640 | 1.21 (0.74–1.98) | 28/642: 36/633 | 1.20 (0.74–1.95) |
| Female | 96/382: 109/371 | 1.10 (0.86–1.40) | 32/107: 42/115 | 1.19 (0.81–1.76) | 86/380: 91/371 | 1.02 (0.78–1.33) | 26/107: 37/114 | 1.28 (0.82–1.98) | 14/373: 22/366 | 1.39 (0.71–2.74) | 8/103: 3/107 | 0.42 (0.10–1.83) |
| p | 0.33 | 0.73 | 0.55 | 0.56 | 0.60 | 0.10 | ||||||
| Race | ||||||||||||
| White | 226/873: 255/874 | 1.08 (0.92–1.26) | 181/669: 199/646 | 1.12 (0.94–1.34) | 197/871: 214/874 | 1.03 (0.87–1.23) | 154/669: 172/645 | 1.15 (0.95–1.40) | 40/855: 55/857 | 1.29 (0.87–1.92) | 36/653: 37/628 | 1.00 (0.64–1.56) |
| Black | 26/79: 17/81 | 0.63 (0.36–1.10) | 10/42: 19/69 | 1.09 (0.54–2.21) | 26/79: 17/81 | 0.63 (0.36–1.10) | 10/42: 19/69 | 1.09 (0.54–2.21) | 1/75: 2/79 | 1.93 (0.26–14.47) | 0/41: 2/65 | NE |
| Other/Multiple | 13/36: 9/32 | 0.76 (0.34–1.70) | 10/26: 6/24 | 0.62 (0.21–1.83) | 13/36: 8/32 | 0.65 (0.28–1.51) | 10/26: 6/24 | 0.62 (0.21–1.83) | 0/35: 1/31 | NE | 0/25: 0/23 | NE |
| p | 0.38 | 0.69 | 0.39 | 0.66 | 0.48 | NE | ||||||
| Body mass index | ||||||||||||
| <25 kg/m2 | 53/285: 67/253 | 1.08 (0.78–1.49) | 35/147: 48/157 | 1.35 (0.91–2.00) | 43/224: 56/253 | 1.06 (0.73–1.53) | 28/147: 41/157 | 1.54 (0.98–2.41) | 13/221: 15/248 | 1.06 (0.52–2.15) | 10/141: 8/154 | 0.64 (0.27–1.50) |
| 25–29.9 kg/m2 | 111/423: 110/414 | 1.03 (0.82–1.31) | 83/337: 94/329 | 1.16 (0.89–1.51) | 101/423: 90/414 | 0.96 (0.74–1.24) | 73/337: 80/329 | 1.13 (0.85–1.50) | 19/416: 20/409 | 1.04 (0.55–1.97) | 9/335: 22/319 | 2.46 (1.13–5.34) |
| ≥ 30 kg/m2 | 114/382: 110/360 | 0.98 (0.78–1.23) | 85/278: 89/278 | 1.01 (0.78–1.32) | 105/381: 99/360 | 0.94 (0.74–1.20) | 75/278: 83/277 | 1.07 (0.81–1.41) | 10/370: 23/349 | 2.25 (1.08–4.69) | 17/268: 9/267 | 0.43 (0.19–0.98) |
| p | 0.71 | 0.16 | 0.65 | 0.21 | 0.12 | 0.16 | ||||||
| Smoking status | ||||||||||||
| Never/Former | 242/942: 246/922 | 1.00 (0.85–1.17) | 183/696: 194/690 | 1.04 (0.87–1.24) | 215/940: 210/922 | 0.96 (0.81–1.14) | 158/696: 171/689 | 1.07 (0.88–1.30) | 36/922: 49/907 | 1.34 (0.88–2.06) | 34/682: 33/671 | 0.90 (0.56–1.45) |
| Current | 36/89: 41/105 | 1.01 (0.68–1.50) | 20/67: 37/74 | 2.16 (1.32–3.51) | 34/89: 35/105 | 0.89 (0.59–1.35) | 18/67: 33/74 | 1.93 (1.16–3.20) | 6/86: 9/99 | 1.24 (0.44–3.48) | 2/63: 6/69 | 4.60 (1.11–19.05) |
| p | 0.93 | 0.02 | 0.68 | 0.06 | 0.85 | 0.15 | ||||||
| Alcohol use | ||||||||||||
| 0 drinks/day | 87/327: 72/300 | 0.85 (0.65–1.13) | 51/205: 59/205 | 1.17 (0.85–1.63) | 74/325: 62/300 | 0.89 (0.65–1.21) | 43/205: 50/204 | 1.26 (0.87–1.82) | 17/318: 14/291 | 0.98 (0.49–2.00) | 12/199: 11/194 | 0.95 (0.42–2.19) |
| 0.1–1 drinks/day | 97/393: 111/380 | 1.08 (0.85–1.37) | 78/270: 80/284 | 0.95 (0.72–1.25) | 85/393: 89/380 | 1.00 (0.77–1.31) | 66/270: 66/284 | 0.91 (0.67–1.23) | 16/388: 24/377 | 1.32 (0.70–2.46) | 11/265: 16/282 | 1.24 (0.57–2.67) |
| > 1 drink/day | 80/246: 80/267 |
0.91 (0.69–1.20) | 65/230: 70/220 | 1.12 (0.82–1.51) | 76/246: 72/267 | 0.85 (0.63–1.14) | 59/230: 67/220 | 1.24 (0.90–1.71) | 9/237: 17/261 | 1.68 (0.77–3.68) | 11/223: 11/212 | 0.94 (0.42–2.11) |
| p | 0.79 | 0.58 | 0.61 | 0.46 | 0.34 | 0.90 | ||||||
| Baseline NSAID/ASA use | ||||||||||||
| < 4 days/week | 145/575: 160/587 | 1.08 (0.88–1.31) | 95/402: 124/419 | 1.21 (0.95–1.54) | 134/574: 135/587 | 0.96 (0.77–1.19) | 84/402: 111/418 | 1.25 (0.97–1.62) | 24/562: 34/575 | 1.33 (0.80–2.23) | 19/391: 20/406 | 0.92 (0.49–1.70) |
| ≥ 4 days/week | 133/456: 127/440 | 0.93 (0.75–1.15) | 108/361: 107/345 | 1.03 (0.82–1.30) | 115/455: 110/440 | 0.94 (0.75–1.19) | 92/361: 93/345 | 1.05 (0.81–1.36) | 18/446: 24/431 | 1.30 (0.70–2.43) | 17/354: 19/334 | 1.11 (0.58–2.13) |
| p | 0.34 | 0.33 | 0.88 | 0.32 | 0.94 | 0.71 | ||||||
| Surveillance interval | ||||||||||||
| 3 year | 154/526: 147/531 | 0.92 (0.76–1.12) | 106/390: 119/402 | 1.09 (0.87–1.37) | 139/525: 130/531 | 0.91 (0.74–1.13) | 94/390: 106/401 | 1.11 (0.86–1.43) | 23/517: 23/519 | 0.89 (0.51–1.56) | 19/381: 16/391 | 0.86 (0.45–1.66) |
| 5 year | 124/505: 140/496 | 1.13 (0.91–1.39) | 97/373: 112/362 | 1.17 (0.92–1.48) | 110/504: 115/496 | 1.03 (0.81–1.31) | 82/373: 98/362 | 1.22 (0.93–1.58) | 19/491: 35/487 | 1.82 (1.05–3.15) | 19/364: 23/349 | 1.17 (0.65–2.11) |
| p | 0.20 | 0.64 | 0.48 | 0.60 | 0.09 | 0.44 | ||||||
All models adjusted for age, sex, center, treatment arm (where appropriate), and number of baseline serrated polyps (0, 1, 2+). Where necessary due to sparse data, clinical center was grouped by prevalence of serrated lesions at baseline into low (Georgia, Iowa, Minnesota, and California), medium (New Hampshire, Colorado, South Carolina, and Puerto Rico), high (Texas, Ohio, and North Carolina). Analyses of no vitamin D vs. vitamin D include all randomized participants; analyses of no calcium vs. calcium are restricted to full factorial participants. p values are for interaction. NSAID: nonsteroidal anti-inflammatory drug; ASA: aspirin; NE: no estimate.
# events/N in the group receiving Vitamin D: # events/N in the group not receiving Vitamin D
# events/N in the group receiving Calcium: # events/N in the group not receiving Calcium
Effects of Vitamin D supplementation are presented for baseline vitamin D use and effects of calcium supplementation are presented for baseline calcium use
TABLE 5:
Subgroup analyses of vitamin D and calcium supplementation and risk of serrated polyps during observational phase (N=1108)
| Any Serrated Polyp | Hyperplastic Polyp | Sessile Serrated Adenoma/Polyp | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SUBGROUP | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) | Events/N1 | Vitamin D aRR (95% CI) | Events/N2 | Calcium aRR (95% CI) |
| Baseline serum vitamin D | ||||||||||||
| ≤ 23.16 ng/ml | 78/273: 81/264 | 1.12 (0.86–1.46) | 55/202: 69/207 | 1.18 (0.86–1.60) | 66/273: 66/263 | 1.09 (0.80–1.47) | 48/202: 55/206 | 1.06 (0.75–1.49) | 11/267: 17/262 | 1.60 (0.75–3.42) | 4/199: 18/203 | 4.37 (1.20–15.86) |
| > 23.16 ng/ml | 81/277: 89/294 | 1.01 (0.77–1.32) | 59/216: 67/221 | 1.07 (0.79–1.46) | 66/275: 73/292 | 1.02 (0.74–1.39) | 53/216: 48/217 | 0.86 (0.60–1.23) | 15/269: 19/288 | 1.31 (0.67–2.57) | 9/210: 18/214 | 1.80 (0.81–3.97) |
| p | 0.52 | 0.77 | 0.72 | 0.40 | 0.66 | 0.23 | ||||||
| Serum vitamin D at EOT | ||||||||||||
| ≤ 23.16 ng/ml | 109/386: 54/193 | 1.02 (0.77–1.36) | 55/217: 69/223 | 1.13 (0.83–1.54) | 92/385: 46/193 | 1.02 (0.74–1.40) | 49/217: 56/222 | 1.03 (0.73–1.45) | 14/375: 11/191 | 1.62 (0.76–3.45) | 5/212: 14/217 | 2.51 (0.93–6.72) |
| > 23.16 ng/ml | 48/158: 115/360 | 1.04 (0.78–1.40) | 59/196: 66/201 | 1.11 (0.82–1.49) | 38/157: 93/357 | 1.06 (0.75–1.50) | 52/196: 46/197 | 0.88 (0.62–1.26) | 11/155: 24/354 | 1.08 (0.53–2.21) | 8/192: 21/196 | 2.45 (1.08–5.53) |
| p | 0.94 | 0.87 | 0.81 | 0.47 | 0.47 | 0.96 | ||||||
| Baseline dietary calcium | ||||||||||||
| ≤ 597 mg/day | 79/249: 92/274 | 1.07 (0.82–1.38) | 58/201: 67/190 | 1.19 (0.87–1.62) | 69/245: 80/273 | 1.06 (0.80–1.41) | 53/201: 57/188 | 1.11 (0.79–1.54) | 11/241: 15/269 | 1.43 (0.66–3.12) | 4/197: 15/186 | 3.19 (1.00–10.18) |
| > 597 mg/day | 75/274: 69:243 | 1.02 (0.77–1.35) | 51/190: 64/207 | 1.16 (0.84–1.60) | 58/273: 53/241 | 1.00 (0.71–1.41) | 43/190: 43/204 | 0.95 (0.64–1.39) | 15/265: 17/241 | 1.27 (0.66–2.44) | 9/186: 18/200 | 1.77 (0.77–4.08) |
| p | 0.88 | 0.95 | 0.85 | 0.52 | 0.94 | 0.42 | ||||||
| Dietary calcium at EOT | ||||||||||||
| ≤ 378.4 mg/day | 82/262: 87/270 | 1.03 (0.79–1.34) | 41/166: 61/164 | 1.45 (1.03–2.04) | 68/262: 73/268 | 1.05 (0.78–1.41) | 38/166: 47/162 | 1.20 (0.82–1.77) | 11/254: 17/266 | 1.67 (0.78–3.61) | 2/161: 13/158 | 5.90 (1.34–25.98) |
| > 378.4 mg/day | 77/287: 83/286 | 1.02 (0.78–1.33) | 73/251: 75/262 | 1.03 (0.78–1.36) | 64/285: 66/285 | 0.95 (0.70–1.30) | 63/251: 56/259 | 0.88 (0.64–1.21) | 15/281: 19/282 | 1.19 (0.64–2.23) | 11/247: 23/257 | 1.99 (0.95–4.16) |
| p | 0.77 | 0.10 | 0.93 | 0.20 | 0.55 | 0.21 | ||||||
| Baseline vitamin D/calcium supplementation3 | ||||||||||||
| < 400 IU or mgs/day | 76/268: 87/278 | 1.11 (0.85–1.46) | 107/393: 130/404 | 1.14 (0.92–1.41) | 64/267: 75/278 | 1.14 (0.85–1.55) | 95/393: 98/399 | 0.97 (0.76–1.25) | 11/260: 13/274 | 1.16 (0.52–2.59) | 12/384: 35/393 | 2.70 (1.38–5.27) |
| ≥ 400 IU or mgs/day | 83/280: 81/278 | 0.93 (0.71–1.22) | 7/25: 5/22 | 1.03 (0.30–3.47) | 68/279: 62/275 | 0.89 (0.65–1.21) | 6/25: 4/22 | 1.08 (0.27–4.26) | 15/274: 23/274 | 1.46 (0.77–2.78) | 1/25: 1/22 | 0.81 (0.16–4.21) |
| p | 0.34 | 0.89 | 0.24 | 0.92 | 0.64 | 0.58 | ||||||
| Vitamin D/calcium supplementation at EOT3 | ||||||||||||
| < 400 IU or mgs/day | 143/474: 149/476 | 1.04 (0.85–1.26) | 110/397: 131/414 | 1.12 (0.90–1.39) | 117/472: 125/474 | 1.05 (0.84–1.32) | 97/397: 98/409 | 0.96 (0.75–1.23) | 24/462: 28/468 | 1.21 (0.71–2.06) | 13/388: 34/403 | 2.40 (1.26–4.56) |
| ≥ 400 IU or mgs/day | 15/74: 21/80 | 1.34 (0.73–2.45) | 4/20: 5/12 | 2.00 (0.61–6.59) | 14/74: 14/79 | 1.00 (0.50–2.00) | 4/20: 5/12 | 2.00 (0.61–6.59) | 2/72: 8/80 | 4.00 (0.80–20.05) | 0/20: 2/12 | NE |
| p | 0.47 | 0.25 | 0.74 | 0.17 | 0.16 | NE | ||||||
| Sex | ||||||||||||
| Male | 105/362: 109/367 | 1.01 (0.81–1.28) | 105/363: 109/366 | 1.00 (0.80–1.26) | 88/360: 86/365 | 0.95 (0.73–1.25) | 93/363: 81/362 | 0.85 (0.65–1.10) | 16/351: 27/362 | 1.61 (0.88–2.93) | 13/356: 30/357 | 2.24 (1.16–4.32) |
| Female | 54/188: 61/191 | 1.13 (0.82–1.56) | 9/55: 27/62 | 2.62 (1.39–4.95) | 44/188: 53/190 | 1.21 (0.84–1.74) | 8/55: 22/61 | 2.50 (1.22–5.13) | 10/185: 9/188 | 1.00 (0.40–2.45) | 0/53: 6/60 | NE |
| p | 0.67 | 0.004 | 0.36 | 0.004 | 0.40 | NE | ||||||
| Race | ||||||||||||
| White | 137/468: 155/493 | 1.05 (0.86–1.27) | 102/377: 124/375 | 1.21 (0.97–1.51) | 112/466: 124/490 | 1.02 (0.81–1.28) | 90/377: 91/370 | 1.02 (0.79–1.32) | 24/456: 36/486 | 1.46 (0.88–2.43) | 13/370: 35/365 | 2.60 (1.36–4.98) |
| Black | 13/46: 8/39 | 0.86 (0.37–2.01) | 7/22: 8/38 | 0.52 (0.21–1.28) | 11/46: 8/39 | 0.97 (0.39–2.37) | 6/22: 8/38 | 0.59 (0.23–1.52) | 2/45: 0/39 | NE | 0/21: 1/38 | NE |
| Other/Multiple | 3/17: 5/10 | 4.01 (0.58–27.49) | 3/7: 2/10 | 0.13 (0.01–2.47) | 3/17: 510 | 4.01 (0.58–27.49) | 3/7: 2/10 | 0.13 (0.01–2.47) | 0/17: 0/9 | NE | 0/6: 0/10 | NE |
| p | 0.40 | 0.28 | 0.43 | 0.58 | NE | NE | ||||||
| Body mass index | ||||||||||||
| <25 kg/m2 | 36/110: 41/132 | 0.95 (0.64–1.42) | 23/74: 26/84 | 1.10 (0.65–1.86) | 31/110: 33/131 | 0.96 (0.61–1.50) | 21/74: 17/83 | 0.73 (0.40–1.33) | 7/108: 7/130 | 0.89 (0.36–2.19) | 3/72: 10/84 | 2.81 (0.99–8.03) |
| 25–29.9 kg/m2 | 57/235: 65/233 | 1.08 (0.79–1.49) | 46/189: 53/192 | 1.10 (0.78–1.57) | 46/234: 50/231 | 1.02 (0.71–1.47) | 39/189: 39/189 | 0.96 (0.65–1.44) | 11/229: 20/232 | 1.81 (0.90–3.64) | 6/186: 18/188 | 2.78 (1.09–7.12) |
| ≥ 30 kg/m2 | 66/205: 64/193 | 1.03 (0.77–1.39) | 45/155: 57/152 | 1.39 (0.99–1.95) | 55/204: 56/193 | 1.10 (0.79–1.53) | 41/155: 47/151 | 1.17 (0.81–1.69) | 8/199: 9/188 | 1.20 (0.46–3.15) | 4/151: 8/145 | 2.04 (0.61–6.80) |
| p | 0.76 | 0.23 | 0.37 | 0.16 | 0.99 | 0.70 | ||||||
| Smoking status | ||||||||||||
| Never/Former | 143/502: 146/497 | 1.01 (0.83–1.24) | 104/385: 118/382 | 1.14 (0.91–1.43) | 118/500: 117/494 | 0.99 (0.79–1.24) | 92/385: 87/377 | 0.94 (0.73–1.23) | 24/491: 30/489 | 1.30 (0.77–2.20) | 12/377: 32/373 | 2.50 (1.28–4.89) |
| Current | 16/48: 24/61 | 1.47 (0.82–2.64) | 10/33: 18/46 | 1.73 (0.78–3.81) | 14/48: 22/61 | 1.42 (0.78–2.59) | 9/33: 16/46 | 1.24 (0.59–2.61) | 2/45: 6/61 | 2.36 (0.51–10.98) | 1/32: 4/44 | 4.33 (0.92–20.42) |
| p | 0.43 | 0.55 | 0.47 | 0.40 | 0.44 | 0.84 | ||||||
| Alcohol use | ||||||||||||
| 0 drinks/day | 46/167: 43/160 | 1.03 (0.71–1.48) | 25/112: 31/104 | 1.52 (0.94–2.47) | 37/166: 37/159 | 1.10 (0.73–1.66) | 22/112: 24/102 | 1.36 (0.80–2.33) | 8/164: 3/158 | 0.43 (0.14–1.32) | 2/109: 5/102 | 7.66 (0.47–123.68) |
| 0.1–1 drinks/day | 70/222: 65/199 | 1.00 (0.75–1.34) | 50/154: 59/166 | 1.17 (0.85–1.62) | 56/221: 53/198 | 0.99 (0.71–1.39) | 44/154: 43/164 | 0.94 (0.65–1.36) | 15/216: 15/196 | 1.21 (0.59–2.48) | 5/151: 20/160 | 3.47 (1.27–9.49) |
| > 1 drink/day | 38/131: 53/158 | 1.17 (0.80–1.69) | 34/125: 41/127 | 1.04 (0.69–1.56) | 34/131: 43/157 | 1.02 (0.67–1.54) | 30/125: 33/126 | 0.98 (0.62–1.54) | 3/126: 14/156 | 3.81 (1.09–13.25) | 6/123: 8/124 | 1.30 (0.45–3.74) |
| p | 0.60 | 0.50 | 0.94 | 0.63 | 0.02 | 0.22 | ||||||
| Baseline NSAID/ASA use | ||||||||||||
| < 4 days/week | 88/312: 94/297 | 1.09 (0.85–1.40) | 56/211: 69/227 | 1.15 (0.85–1.56) | 74/312: 77/295 | 1.05 (0.79–1.40) | 51/211: 52/225 | 0.93 (0.66–1.31) | 12/306: 18/294 | 1.60 (0.79–3.27) | 5/206: 17/225 | 3.04 (0.98–9.45) |
| ≥ 4 days/week | 71/238: 76/261 | 1.03 (0.77–1.36) | 58/207: 67/201 | 1.21 (0.89–1.63) | 58/236: 62/260 | 1.00 (0.72–1.38) | 50/207: 51/198 | 1.06 (0.75–1.50) | 14/230: 18/256 | 1.24 (0.63–2.43) | 8/203: 19/192 | 2.36 (1.07–5.24) |
| p | 0.61 | 0.75 | 0.72 | 0.58 | 0.62 | 0.66 | ||||||
| NSAID/ASA use at EOT | ||||||||||||
| < 4 days/week | 76/264: 94/287 | 1.10 (0.85–1.43) | 53/193: 66/192 | 1.23 (0.90–1.67) | 62/264: 76/285 | 1.09 (0.81–1.47) | 47/193: 49/190 | 1.04 (0.73–1.49) | 11/259: 18/281 | 1.60 (0.77–3.30) | 5/186: 16/190 | 2.74 (0.92–8.16) |
| ≥ 4 days/week | 83/284: 76/269 | 1.01 (0.77–1.33) | 61/224: 70/234 | 1.12 (0.82–1.51) | 70/282: 63/268 | 0.99 (0.73–1.34) | 54/224: 54/231 | 0.95 (0.67–1.33) | 15/275: 18/267 | 1.27 (0.64–2.50) | 8/222: 20/225 | 2.47 (1.10–5.56) |
| p | 0.50 | 0.75 | 0.5 | 0.78 | 0.67 | 0.77 | ||||||
All models adjusted for age, sex, center, treatment arm (where appropriate), number of baseline serrated polyps (0, 1, 2+), surveillance interval (where appropriate). Where necessary due to sparse data, clinical center was grouped by prevalence of serrated lesions at baseline into low (Georgia, Iowa, Minnesota, and California), medium (New Hampshire, Colorado, South Carolina, and Puerto Rico), high (Texas, Ohio, and North Carolina). p values are for interaction. Analyses of no vitamin D vs. vitamin D include all randomized participants; analyses of no calcium vs. calcium are restricted to full factorial participants.
ASA: aspirin; EOT: end of treatment; NE: no estimate; NSAID: nonsteroidal anti-inflammatory drug;
# events/N in the group receiving Vitamin D: # events/N in the group not receiving Vitamin D
# events/N in the group receiving Calcium: # events/N in the group not receiving Calcium
Effects of Vitamin D supplementation are presented for baseline vitamin D use and effects of calcium supplementation are presented for baseline calcium use
In the observational phase, similarly, the effect of vitamin D and calcium were consistent within most subgroups. There was evidence that among women, calcium was associated with a higher risk of SPs overall (aRR 2.62, 95% CI 1.39–4.95, p for trend 0.004) and HPs specifically (aRR 2.50, 95% CI 1.22–5.13, p for trend 0.004). Although current smokers had higher calcium RR’s than non-smokers during the observational phase, the differences were not as marked as during the treatment phase, and the interactions were not statistically significant (Table 5).
Associations with baseline vitamin D levels and dietary calcium
Analysis of the association of serum vitamin D levels and dietary calcium intake among participants not given the respective supplements (independent of treatment effects) is shown in Supplementary Tables 5 and 6. We did not find evidence that either variable was strongly associated with the risk of SPs in either the treatment or observational phases.
DISCUSSION:
In this randomized trial of calcium and vitamin D3 supplementation for the prevention of colorectal adenomas, we surprisingly found that calcium and the combination of calcium and vitamin D3 increased the risk of SSA/Ps in the observational period of the trial, 3–5 years after supplementation ended. In addition, we found evidence that the effect of calcium was modified by both sex and smoking status; women and current smokers had higher risks of SPs when exposed to supplemental calcium. We did not observe an association between Vitamin D alone and the risk of SSA/Ps.
Serrated neoplasia gives rise to a significant proportion of sporadic CRC cases. Optimal prevention of CRC therefore involves targeting SPs in addition to conventional adenomas, so it is important to understand the effects of potential chemopreventive agents on both polyp types. Prior studies[13–15] and a meta-analysis[16] have found that calcium supplementation reduces the risk of adenomatous polyps, though the primary outcome of our original trial did not, at least among individuals who were overweight or obese.[12] A previous trial by our group analyzed the effect of calcium treatment alone on polyp recurrence, and found a beneficial effect of calcium on adenoma recurrence (RR 0.85, 95% CI 0.74 – 0.98)[14] but a null effect of calcium on right sided serrated polyps (RR 0.93, 95% CI 0.63, 1.38).[8] However, these analyses were limited to early effects, at either 1 or 4 years after treatment initiation and thus are consistent with the results for SPs presented here.
Interestingly, other authors have reported inverse associations between dietary calcium intake and HPs, the most prevalent SP subtype. In a case-control study of 560 patients with HPs, Davenport et al. reported that the highest quartile of calcium intake was associated with lower risk of HPs (> 1217 mg/day vs. ≤ 595.8 mg/day: OR 0.66, 95% CI 0.44 – 0.99, p for trend 0.03).[17] Similarly, another smaller case-control study of 81 patients with HPs by Martinez al. reported an inverse association between dietary calcium intake and HPs (≥ 1094 mg/day vs. ≤ 558 mg/day: OR 0.41, 95% CI 0.19 – 0.90, p for trend 0.05).[18] In contrast, our study found a null association between treatment with calcium supplements and HPs. We did evaluate the association with dietary calcium as well (as in the previous observational studies), and also did not find any statistically significant associations with risk of HPs in either the treatment or observational phase (Supplementary Table 2). Taken together, our results would suggest that only calcium supplementation, not dietary calcium intake, is associated with SPs overall and SSA/Ps specifically. However, it should be noted that our study design evaluated risk of incident SSA/Ps in contrast to observational studies, which generally evaluate associations between dietary factors and prevalent polyps.
The literature is sparse with respect to other analyses of the effect of calcium on more advanced serrated neoplasia such as SSA/Ps. One case-control study with 214 patients with SSA/Ps reported a non-significant inverse association between calcium intake and risk of SSA/Ps (OR for highest vs. lowest quartile of calcium intake: 0.54 (0.28, 1.06).[17] However, this study measured dietary calcium not supplemental use, and is subject to confounding and healthy user effects, and thus cannot be directly compared to results presented here.
If calcium increases the risk of premalignant serrated polyps, one would expect that calcium would also be linked to serrated pathway cancers, which are typically characterized by CIMP and MSI.[2] There are limited data on the association between calcium supplementation and colorectal cancer with features of serrated neoplasia. One study reported a small increase in risk of CIMP positive tumors associated with calcium supplementation, but this was not statistically significant (OR 1.22, 95% CI 0.95 – 1.58, p = 0.13).[19] Other studies have investigated the association between dietary calcium intake and MSI high colorectal cancer and have not found a clear association.[20, 21] However, this does not necessarily exclude an effect of calcium on the later phases of serrated carcinogenesis as SSA/Ps can give rise to microsatellite stable cancers as well.[2]
The possible mechanisms by which calcium (alone or in addition to vitamin D) could influence serrated neoplasia are uncertain. Our findings of the relatively long latency period would suggest that calcium (with or without vitamin D) likely influences early events in the serrated neoplasia pathway rather than late effects such as progression to dysplasia or cancer. Calcium is involved in myriad cellular functions, but most of its proposed mechanisms and actions are anti-neoplastic.[22] Serrated pathway lesions have been associated with Annexin A10. Annexins are a family of calcium-regulated phospholipid binding proteins that have varied cellular functions. The specific function of Annexin A10 is uncertain, but it has been shown to be highly expressed in both SSA/Ps and serrated adenocarcinomas.[23–26] Therefore, it is possible that calcium supplementation could influence Annexin A10 expression and thereby alter progression through the serrated pathway. Calcium is involved with Mucin 5AC production,[27] another marker of the serrated pathway.[28, 29]
We also found evidence that smoking modified the risk of SSA/Ps associated with calcium. This is interesting, given consistent evidence that tobacco use increases the risk of SPs and specifically SSA/Ps from other studies.[11, 30] Another CRC chemoprevention trial found evidence that smokers were at higher risk of recurrent conventional adenomas when given a combination of aspirin, calcium, and calcitriol. These results suggest that smokers may be particularly sensitive to calcium-mediated effects that promote colorectal neoplasia.
If calcium and its combination with vitamin D are truly associated with increased risk of premalignant SPs, this has important public health implications. Calcium and vitamin D supplements are taken by roughly 40% of the US population, which equates to over 100 million persons.[31] Women represent the majority of those taking calcium supplements in the general population.[32] It is interesting therefore that SSA/Ps are at least as common if not more common in women than men,[33, 34] in contrast to conventional adenomas, which are more common in men.
The strengths of this study include its randomized design and large sample size, in addition to use of a run-in period to optimize adherence to study medications, and quantification of other sources of calcium and vitamin D intake. Study groups were similar at baseline with respect to dietary practices. In addition, polyps occurring during the trial were centrally read in a blinded fashion by an expert in serrated neoplasia, which minimizes any bias associated with pathologist under-interpretation and inter-pathologist variation for SSA/P readings.
Some limitations bear mentioning as well. Our findings are derived from a secondary analysis of a trial designed and sized to evaluate the occurrence of conventional adenomas. SSA/Ps are significantly less common than conventional adenomas, so we had limited statistical power in many of our analyses. To better understand the intervention effects, we performed multiple subgroup analyses, and it is possible that some statistically significant findings from these analyses were due to chance. Therefore, subgroup analyses in particular should be considered hypothesis generating, not conclusive. SSA/Ps are also prone to underdetection,[35] which could bias our results toward the null and affect the precision of our estimates. Additionally, there is evidence that the detection of SSA/Ps is improving over time, likely due to better recognition by endoscopists,[35] which likely occurred in our trial as well. However, we would not expect detection to differ based on treatment group, which was randomly assigned. Additionally, it is well known that pathology classification of SSA/Ps is variable. This may explain the higher proportion of SSA/Ps identified during the study compared to baseline examinations, for which pathology was not centrally reviewed. Baseline examinations also had a higher proportion of dysplastic SSA/Ps than later colonoscopies, which could be attributed to differences in pathology reads. A majority of participants were unblinded as to their treatment group at the time their observational phase colonoscopy was performed. While this is unlikely to influence the accuracy of these colonoscopies, it is possible that unblinding could have impacted behaviors that influence polyp development. Lastly, to be included in the trial, all participants had to have a conventional adenoma, so this study does not provide data on participants who may develop SPs in the absence of conventional adenomas. However, we have no reason to expect that effects of vitamin D and calcium would differ based on this distinction.
In conclusion, in a randomized trial of calcium and vitamin D supplementation for prevention of colorectal polyps, we found evidence that calcium supplementation and the combination of calcium and vitamin D may increase the risk of SPs and specifically SSA/Ps during later follow up. This is in contrast to conventional adenomas, where there was no apparent effect of these interventions in this study, and other studies that support a protective effect of calcium.[14, 36] Further studies are recommended to confirm these results, which may have important implications for CRC screening and prevention.
Supplementary Material
SUMMARY BOX.
1. What is already known about this subject
Serrated polyps, particularly sessile serrated adenomas/polyps (SSA/Ps) are important colorectal cancer precursor lesions that contribute to the problem of interval cancer.
Established risk factors for SSA/Ps include tobacco and alcohol use, white race, and obesity.
There are limited data on association between diet and micronutrient intake and premalignant serrated polyps.
2. What are the new findings?
Using data from a recently completed randomized chemoprevention trial of vitamin D and calcium in patients with colorectal adenomas, we found that calcium and the combination of calcium and vitamin D increased the risk of SSA/Ps 6–10 years after supplementation began.
Women and current smokers had higher risks of serrated polyps when exposed to supplemental calcium.
3. How might it impact on clinical practice in the foreseeable future?
Patients with a history of premalignant serrated polyps, especially women and smokers, may wish to avoid vitamin D and calcium supplementation.
Acknowledgments
Grant Support: This study was funded a grant from the National Institutes of Health, National Cancer Institute (CA098286, PI: Baron). Dr. Crockett’s effort was supported in part by a grant from the NIH (KL2TR001109).
Clinical Trial Registration: ClinicalTrials.gov NCT00153816
REFERENCES
- 1.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology 2010;138(6):2088–100. [DOI] [PubMed] [Google Scholar]
- 2.Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011;42(1):1–10. [DOI] [PubMed] [Google Scholar]
- 3.Snover DC, Ahnen D, Burt R, Odze RD Serrated Polyps of the Colon and Rectum and Serrated Polyposis In. WHO Classification of Tumours of the Digestive System, IARC; 4th ed. Lyon, France; 2010. [Google Scholar]
- 4.Crockett SD, Snover DC, Ahnen DJ, et al. Sessile serrated adenomas: an evidence-based guide to management. Clin Gastroenterol Hepatol 2015;13(1):11–26 e1. [DOI] [PubMed] [Google Scholar]
- 5.Colorectal Cancer Facts & Figures 2017. 2017.
- 6.Crockett SD, Baron JA. A Chemopreventive Cocktail on the Rocks. Gastroenterology 2016;150(1):26–9. [DOI] [PubMed] [Google Scholar]
- 7.Haque TR, Bradshaw PT, Crockett SD. Risk factors for serrated polyps of the colorectum. Dig Dis Sci 2014;59(12):2874–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wallace K, Grau MV, Ahnen D, et al. The association of lifestyle and dietary factors with the risk for serrated polyps of the colorectum. Cancer Epidemiol Biomarkers Prev 2009;18(8):2310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14(5):342–57. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Giovannucci E. Calcium, vitamin D and colorectal cancer chemoprevention. Best Pract Res Clin Gastroenterol 2011;25(4–5):485–94. [DOI] [PubMed] [Google Scholar]
- 11.Bailie L, Loughrey MB, Coleman HG. Lifestyle Risk Factors for Serrated Colorectal Polyps: A Systematic Review and Meta-analysis. Gastroenterology 2017;152(1):92–104. [DOI] [PubMed] [Google Scholar]
- 12.Baron JA, Barry EL, Mott LA, et al. A Trial of Calcium and Vitamin D for the Prevention of Colorectal Adenomas. N Engl J Med 2015;373(16):1519–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonithon-Kopp C, Kronborg O, Giacosa A, et al. Calcium and fibre supplementation in prevention of colorectal adenoma recurrence: a randomised intervention trial. European Cancer Prevention Organisation Study Group. Lancet 2000;356(9238):1300–6. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med 1999;340(2):101–7. [DOI] [PubMed] [Google Scholar]
- 15.Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res 1993;290(1):87–95. [DOI] [PubMed] [Google Scholar]
- 16.Keum N, Lee DH, Greenwood DC, et al. Calcium intake and colorectal adenoma risk: dose-response meta-analysis of prospective observational studies. Int J Cancer 2015;136(7):1680–7. [DOI] [PubMed] [Google Scholar]
- 17.Davenport JR, Su T, Zhao Z, et al. Modifiable lifestyle factors associated with risk of sessile serrated polyps, conventional adenomas and hyperplastic polyps. Gut 2016; 10.1136/gutjnl-2016-312893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez ME, McPherson RS, Levin B, et al. A case-control study of dietary intake and other lifestyle risk factors for hyperplastic polyps. Gastroenterology 1997;113(2):423–9. [DOI] [PubMed] [Google Scholar]
- 19.Weisenberger DJ, Levine AJ, Long TI, et al. Association of the colorectal CpG island methylator phenotype with molecular features, risk factors, and family history. Cancer Epidemiol Biomarkers Prev 2015;24(3):512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satia JA, Keku T, Galanko JA, et al. Diet, lifestyle, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev 2005;14(2):429–36. [DOI] [PubMed] [Google Scholar]
- 21.Slattery ML, Anderson K, Curtin K, et al. Dietary intake and microsatellite instability in colon tumors. Int J Cancer 2001;93(4):601–7. [DOI] [PubMed] [Google Scholar]
- 22.Newmark HL, Lipkin M. Calcium, vitamin D, and colon cancer. Cancer Res 1992;52(7 Suppl):2067s–2070s. [PubMed] [Google Scholar]
- 23.Sajanti SA, Vayrynen JP, Sirnio P, et al. Annexin A10 is a marker for the serrated pathway of colorectal carcinoma. Virchows Arch 2014; 10.1007/s00428-014-1683-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim JH, Rhee YY, Kim KJ, et al. Annexin A10 expression correlates with serrated pathway features in colorectal carcinoma with microsatellite instability. APMIS 2014;122(12):1187–95. [DOI] [PubMed] [Google Scholar]
- 25.Tsai JH, Lin YL, Cheng YC, et al. Aberrant expression of annexin A10 is closely related to gastric phenotype in serrated pathway to colorectal carcinoma. Mod Pathol 2014; 10.1038/modpathol.2014.96. [DOI] [PubMed] [Google Scholar]
- 26.Macaron C, Lopez R, Pai RK, et al. Expression of Annexin A10 in Serrated Polyps Predicts the Development of Metachronous Serrated Polyps. Clin Transl Gastroenterol 2016;7(12):e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitrovic S, Nogueira C, Cantero-Recasens G, et al. TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. Elife 2013;2:e00658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khaidakov M, Lai KK, Roudachevski D, et al. Gastric Proteins MUC5AC and TFF1 as Potential Diagnostic Markers of Colonic Sessile Serrated Adenomas/Polyps. Am J Clin Pathol 2016;146(5):530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh MD, Clendenning M, Williamson E, et al. Expression of MUC2, MUC5AC, MUC5B, and MUC6 mucins in colorectal cancers and their association with the CpG island methylator phenotype. Mod Pathol 2013;26(12):1642–56. [DOI] [PubMed] [Google Scholar]
- 30.Figueiredo JC, Crockett SD, Snover DC, et al. Smoking-associated risks of conventional adenomas and serrated polyps in the colorectum. Cancer Causes Control 2014; 10.1007/s10552-014-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr 2010;140(4):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief 2011, http://www.ncbi.nlm.nih.gov/pubmed/21592424 (61):1–8. [PubMed] [Google Scholar]
- 33.Burnett-Hartman AN, Passarelli MN, Adams SV, et al. Differences in epidemiologic risk factors for colorectal adenomas and serrated polyps by lesion severity and anatomical site. Am J Epidemiol 2013;177(7):625–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol 2010;63(8):681–6. [DOI] [PubMed] [Google Scholar]
- 35.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol 2010;105(12):2656–64. [DOI] [PubMed] [Google Scholar]
- 36.Shaukat A, Scouras N, Schunemann HJ. Role of supplemental calcium in the recurrence of colorectal adenomas: a metaanalysis of randomized controlled trials. Am J Gastroenterol 2005;100(2):390–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.