Abstract
Background
Kabul (Afghanistan) is a major focus of cutaneous leishmaniasis (CL) caused by Leishmania tropica. Microscopy remains the reference test for diagnosis despite its low performance. We evaluated whether Loopamp™ Leishmania Detection Kit (Loopamp) and CL Detect™ Rapid Test (CL Detect), detecting Leishmania DNA and antigen, respectively could improve CL diagnosis.
Methods
A diagnostic accuracy study with prospective inclusion was conducted in a leishmaniasis reference clinic in Kabul. Slit skin samples from CL suspects were analysed by microscopy. Samples taken with a dental broach were tested with CL Detect, Loopamp, and PCR. All samples were transferred to the Academic Medical Center (AMC, the Netherlands) for PCR and Loopamp analyses. The diagnostic performance of the tests was evaluated against a reference combining microscopy and PCR.
Findings
274 CL suspects were included in the study. In Kabul, CL Detect had a 65·4% sensitivity [95% Confidence Interval (CI): 59.2–71.2%] and a 100% specificity [95% CI: 80.5–100%], while these were 87.6% [95%CI: 82.9–91.3%] and 70.6% [95% CI: 44.0–89.7%] for Loopamp. At AMC the Loopamp's sensitivity (92.2% [95% CI: 88.2–95.2%]) and specificity (94.1% [95% CI: 71.3–99.8%]) were higher. An algorithm where CL Detect negative suspects would be tested by Loopamp yielded a 93.4% sensitivity [95% CI: 89.6–96.1%] and a 94.1% specificity [95% CI: 71.3–99.8%] when Loopamp's performance at AMC was used.
Interpretation
The high specificity of CL Detect and the performance of Loopamp allow their use in a diagnostic algorithm that would minimize the number of CL patients referred for confirmation.
Fund
Federal Ministry of Education and Research, Germany.
Research in context.
Evidence before this study
We searched PubMed from 1 January 2016 up to July 2018 to understand the current limitations in the diagnosis of cutaneous leishmaniasis (CL) and identify any update, technical or strategic, to address them and to gather information for the design of this study and discussion of the results obtained. Early diagnosis of CL is of paramount importance to limit the disease impact (severity of lesions and stigma), to treat and manage patients adequately and to protect the community from new infections (when transmission is anthroponotic). However, diagnosing CL cases remains a challenge in 2018. Observing Leishmania amastigotes under a microscope in Giemsa's stained skin scrapings or fine needle aspirates remains the reference test despite its low and variable sensitivity. Molecular diagnostic tests, which are more sensitive, are rarely used in endemic countries due to their complexity.
Afghanistan is one of the high burden countries for CL, according to WHO, and Kabul has traditionally been the largest focus of anthroponotic CL in the world. Yet laboratory confirmation of reported cases is as low as 5%, despite the potential toxicity of the pentavalent antimonials used to treat this disease.
To address unmet diagnostic needs in CL diagnosis we evaluated two newly available test: (i) a simple leishmanial DNA detection test that brings the sensitivity of molecular diagnosis to the point-of-care, and that showed good diagnostic performance in the diagnosis of visceral leishmaniasis in Sudan (Loopamp™ Leishmania Detection Kit), and (ii) a rapid diagnostic test for leishmanial antigen detection that showed high sensitivity and specificity in the diagnosis of early CL lesions in Tunisia (CL Detect™ Rapid Test for Cutaneous Leishmaniasis).
Added value of this study
We provide estimates of the diagnostic performance of two new tests for CL in the context of a leishmaniasis clinic in a high burden country, Afghanistan. To our knowledge this is the first evaluation of these tests in parallel and of any of them in Afghanistan.
We also presented the option of combining the two tests in a diagnostic algorithm that enables diagnosis at peripheral level and reduces the number of patients that needs to be referred to a specialized clinic for confirmation.
Implications of all the available evidence
The implementation of the proposed diagnostic algorithm should overcome the limitations of the current diagnostic process for CL that relies in low sensitive parasitological tests or technically complex molecular methods. The combined use of Loopamp and CL detect represents an important advance in access to early diagnosis of dermal leishmaniasis; contributing to improvement in management and control of the disease.
Alt-text: Unlabelled Box
1. Introduction
Cutaneous leishmaniasis (CL) is caused by several species of protozoa of the Leishmania genus, which are transmitted by the bite of infected female sand flies. It is often referred to as a group of diseases because of its wide range of clinical manifestations, which spans a range from small cutaneous nodules to severe mucosal tissue destruction. Although not lethal, CL is responsible for chronic and disfiguring skin lesions and is an important cause of morbidity and social stigma. A total of 197,311 new cases were reported world-wide to the World Health Organization (WHO) in 2015, 70% of them from the Eastern Mediterranean Region [1,2].
Afghanistan is one of the high burden countries for CL, with 36% of its population at risk of acquiring the disease and a reported yearly incidence of 17.9 new cases/10,000 inhabitants in endemic regions. In 2015, Afghanistan reported 29,392 new cases to the WHO, though a 5–10 fold underreporting is estimated. Out of the reported cases, the laboratory confirmation proportion is as low as 5%. Leishmania major and L. tropica are the causative agents of CL in the country, which are transmitted in a zoonotic and an anthroponotic cycle, respectively [1,[3], [4], [5]].
Early and accurate diagnosis is necessary because the CL parasite burden in wounds decreases over time, despite growth of the lesions. The broad variety of clinical manifestations as well as its extensive differential diagnosis complicates the clinical diagnosis of CL, which makes confirmatory testing necessary. Treatment of CL is usually based on intralesional or systemic antimonials, which are potentially toxic for patients. In order to avoid over-treatment, parasitological confirmation is thus important. Also, in an anthroponotic cycle such as for L. tropica, early CL diagnosis and treatment reduces the risk of transmission to other people.
Laboratory diagnosis is generally based on microscopic examination of Giemsa's stained smears from skin scrapings or fine needle aspirates. Sensitivity of microscopy, however, is in general low, variable, and examiner dependent [2]. Molecular diagnostic tests, notably polymerase chain reaction (PCR), are highly sensitive tests for Leishmania detection, which is especially relevant in chronic cutaneous lesions with lower parasite loads [6,7]. However, PCR tests require well-equipped laboratory facilities, trained laboratory personnel and sufficient financial resources, which are often not available in CL endemic foci. Thus there is a need to move towards more user-friendly and field-amenable diagnostic options. A DNA detection test that may meet these requirements is the loop-mediated isothermal amplification (LAMP) assay: it requires a constant temperature (60–65 °C) for target DNA amplification rather than thermocycling, is highly specific, and the results can be visualized using simple detection methods, which make LAMP tests an attractive option for POC diagnosis [8]. One of such tests that can be used for the diagnosis of CL is the Loopamp™ Leishmania Detection Kit (Eiken Chemical Co., Tokyo, Japan) [9]. Another promising option for the POC diagnosis of CL is the CL Detect™ Rapid Test (InBios International Inc., Seattle, USA), an immunochromatographic rapid diagnostic test (RDT) for the detection of Leishmania in CL skin lesions using polyclonal antibodies against amastigote peroxidoxin.
The objective of this study was to evaluate the sensitivity and specificity of these two new tests for the diagnosis of CL cases in Kabul, Afghanistan, comparing their diagnostic performance to that of microscopy and PCR. In addition we also evaluated the accuracy of a diagnostic algorithm in which CL Detect and Loopamp are used sequentially to improve and simplify CL diagnosis in Afghanistan.
2. Methods
2.1. Study site and participants
The study was conducted at the leishmaniasis clinic of the National Malaria and Leishmaniasis Control Programme (NMLCP) in Kabul, Afghanistan. Study participants were recruited from patients with skin lesions compatible with CL presenting themselves at the NMLCP clinic or being referred from other health facilities in Kabul province from the 16th April to the 22nd June 2016. The CL suspects were prospectively and consecutively enrolled in the study if they fulfilled all of the following inclusion criteria: (i) aged >2 years, (ii) clinical samples can be obtained (see details below), and (iii) provision of informed consent. Patients already receiving treatment for CL were not eligible for the study.
2.2. Sample collection
Before sample collection the lesion and surrounding skin was washed with water and soap. In ulcerated lesions the sore was debrided using forceps and rubbing with a gauze pad soaked in sterile saline solution. When judged necessary a 5% emulsion of lidocaine/prilocaine ointment or a 5% lidocaine hydrochloride injectable anesthetic could be applied before sample collection.
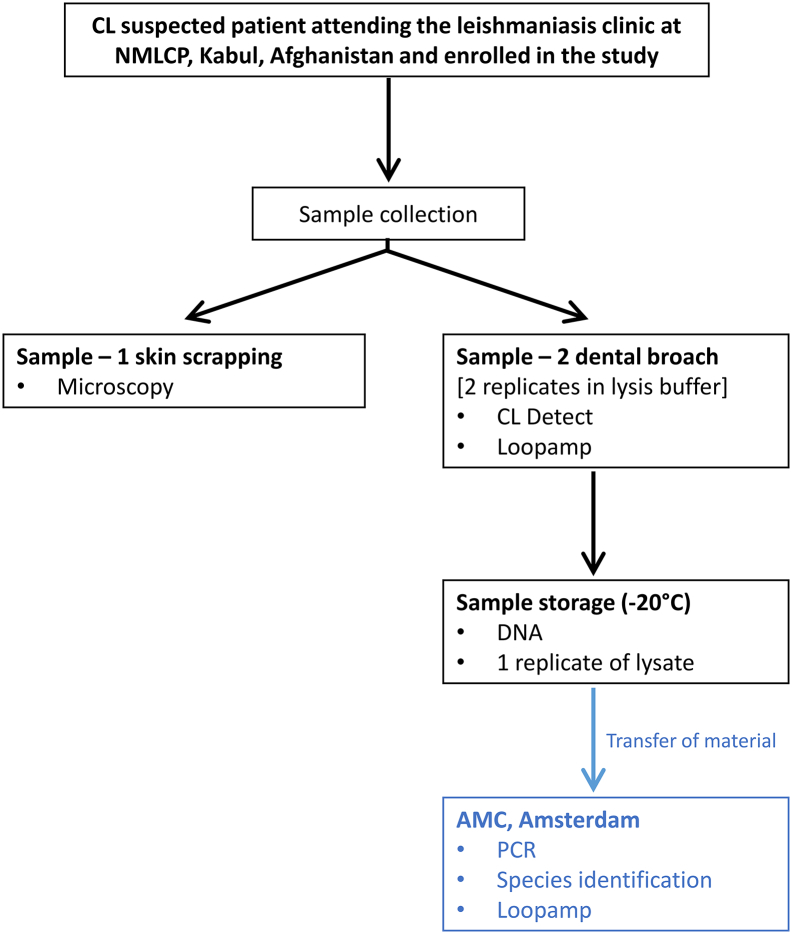
As detailed in Fig. 1, two samples were obtained from each study participant. The first sample (Sample-1) was taken following the routine practice to diagnose CL cases at the NMLCP. For this, the selected site of the lesion was scraped, using a disposable scalpel blade. The material obtained was smeared onto a microscope slide that was further prepared for microscopic examination (explained below).
Fig. 1.
Work flow of clinical samples and tests conducted at the National Malaria and Leishmaniasis Control Programme (NMLCP) in Kabul, Afghanistan and at the Academic Medical Center (AMC), Amsterdam, The Netherlands. The test conducted were: CL Detect™ Rapid Test (CL Detect), Loopamp™ Leishmania Detection Kit (Loopamp) and polymerase chain reaction (PCR).
The second sample (Sample-2) was obtained using the dental broach provided in the CL Detect™ Rapid Test for Cutaneous Leishmaniasis kit. Briefly: the dental broach was inserted near the border of the lesion to a depth of approximately ½ the length of the dental broach's barb from the edge of the ulcer towards the inflamed area. Once inserted it was twice gently rotated and removed with a quick sharp pull, twisting slightly. The dental broach was then placed with the barbed-end down in an Eppendorf tube containing 3 drops (~150 μl) of the lysis buffer provided in the kit. The material collected in the lysis buffer was used for testing as described below and one aliquot was preserved at −20 °C for DNA extraction and further molecular testing (i.e. LAMP, PCR, and species identification).
When a patient presented multiple lesions Sample-1 and -2 were collected from close positions in the most active lesion.
2.3. DNA extraction
Fifty microliters of the lysate were processed for DNA extraction using the QIAamp DNA Mini Kit (QIAGEN) following the instructions provided in the kit. The DNA was eluted in 100 μl PCR grade water and processed immediately or stored at −20 °C until further analysis. Aliquots of lysate and DNA (50 μl each) were shipped to the Academic Medical Center (AMC) in Amsterdam, the Netherlands for PCR, Leishmania species identification and repeated LAMP testing.
2.4. Index tests
2.4.1. CL Detect™ rapid test for cutaneous leishmaniasis (CL detect)
The CL Detect was performed at the NMLCP following the manufacturer's instructions. Briefly: the dental broach used to collect the sample was inserted barbed-end down for 25 min in an Eppendorf tube containing 3 drops of lysis buffer. Twenty microliters of the lysate were then transferred to a new Eppendorf tube containing 3 drops of Chase Buffer Type A provided in the kit. The CL Detect strip was then dipped in this solution for 20 min before recording the results. A positive result was recorded when a control line and test line appeared in the test area, and a negative result when only the control line appeared. Whenever an invalid result was obtained (no control line visible), the test was repeated immediately. Staff reading the results was blinded from clinical information and from microscopy and Loopamp results. The remaining lysate was kept at −20 °C in the freezers of the Health Protection and Research Organization (HPRO) laboratories until further use.
2.4.2. Loopamp™ leishmania detection kit (Loopamp)
Thirty microliters of DNA solution, both test samples and controls were used in the LAMP reaction, which was prepared following the manufacturer's instructions. In Kabul, test samples were run for 40 min at 65 °C followed by an inactivation step for 2 min at 95 °C in a MiniOpticon Real Time PCR System (Bio-Rad). DNA extraction and Loopamp testing in Kabul was done at the HPRO laboratory, which collaborates with the NMLCP. At AMC reactions were run in a Loopamp LF-160 incubator (Eiken Chemical Co., Tokyo, Japan) with the inactivation step at 80 °C for 2 min. Results were visualized under illumination with UV light, read by personnel blinded from clinical information and from the results of the microscopy and CL Detect. Loopamp reactions included positive and negative controls. A Loopamp was considered positive if green fluorescence was observed.
2.5. Reference tests (used in a composite reference standard)
2.5.1. Microscopy
Giemsa stained smears were examined by experienced microscopists from the NMLCP using a light microscope. Leishmania amastigotes were confirmed under 1000× magnification. The results were recorded and photographically documented, by staff blinded to clinical information and other test results.
2.5.2. Real-time PCR and Leishmania species identification
At AMC, DNA detection by PCR was performed using 1.25 μl DNA and following the method targeting the 18SrRNA gene as described elsewhere [10]. Positive samples were further analysed with a second PCR method, consisting of the amplification of the Leishmania mini-exon and the digestion with Eae I enzyme [11]. Staff reading the PCR results was blinded from clinical information and earlier test results. Restriction fragment length polymorphism (RFLP) patterns were compared to those from L. major (MHOM/IR/1972/NADIM5 and MHOM/SU/73/5ASKH) and L. tropica (MHOM/SU/74/SAF-K27 and MHOM/IL/75/LV140) strains to allow Leishmania species identification.
2.6. Statistics and analyses
2.6.1. Sample size
The sample size required to evaluate the new tests, based on an expected sensitivity (and specificity) of 80% and a desired error margin of ±10% and an alpha level of 5%, was estimated to be 62 cases and 62 non-cases. With an expected prevalence of CL among suspects of 70%, a minimum of 89 CL subjects should be screened in order to identify 62 CL cases; a minimum of 207 subjects should be screened in order to identify 62 non-cases. To take into account prevalence variability (e.g. 80%), we aimed to recruit 300 CL suspects.
2.6.2. Definition of a CL case
A CL case was defined based on a composite reference standard, thus any CL suspect testing positive by microscopy and/or PCR was considered a CL case. And CL suspects returning a negative result in both microscopy and PCR were considered as non-cases. This combined approach has been used in other studies, and is justified by inter-site and inter-sampling variability in parasite load [[12], [13], [14]].
2.6.3. Diagnostic accuracy assessment
We used the groups CL case and non-case to estimate the sensitivity and specificity of Loopamp and CL Detect. We also estimated the sensitivity of both tests depending on the duration and type of lesion. In addition we evaluated the sensitivity and specificity of the tests against skin scraping microscopy alone, as this is the test used to routinely diagnose CL at NMLCP's leishmaniasis clinic in Kabul. We also assessed the agreement between CL Detect and microscopy performed in Kabul and between Loopamp and PCR performed at the AMC using Cohen's kappa statistics (κ). Finally we estimated the accuracy of a diagnostic algorithm where CL Detect and Loopamp would be used sequentially to improve and simplify diagnosis of CL cases in the Afghan context. The sensitivity and specificity estimates and their 95% exact binomial confidence intervals as well as the Cohen's kappa statistics were calculated using the package epiR in R 3.5.0 [15]. Subgroups analyses were performed using Stata software [16].
2.7. Ethical considerations
The study was carried out in conformity with the Helsinki Declaration, and ethical approval was obtained from the Institutional Review Board of the Afghanistan National Public Health Institute, Ministry of Public Health, Islamic Republic of Afghanistan (Reference No: 361549). Participants were informed about the objectives and procedures of the study, and benefits and risks were also explained. Written informed consent was obtained from all participants, or their parents or guardians in the case of minors (<18 years old) in the presence of independent witnesses before enrolment. Confidentiality was assured by assigning a study code to each participant. The study was registered at ClinicalTrials.gov, identifier: NCT03435419.
2.8. Role of the funding source
The funders did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
3. Results
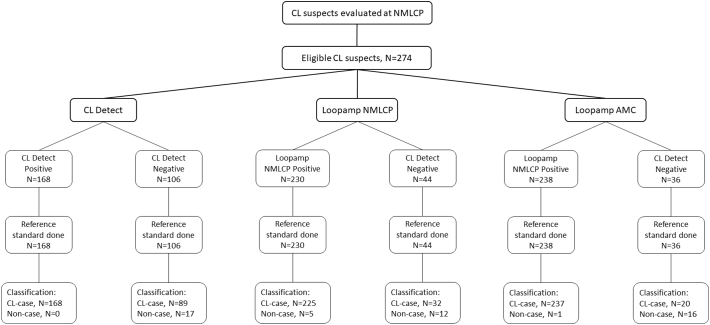
A total of 274 patients suspected of having CL were included in the study. Two hundred and four of them were positive by microscopy while 252 were positive by PCR. When results from both tests were combined, 257 patients were considered as true cases for the analysis, and 17 as non-cases (Table 1). A STARD workflow figure showing sample flow and tests results is shown as supplementary information (Fig. S1).
Table 1.
Results of microscopy and PCR on clinical samples (slit skin and dental broach respectively) from 274 cutaneous leishmaniasis (CL) suspects attending the NMLCP in Kabul, Afghanistan.
| Microscopy |
||||
|---|---|---|---|---|
| Positive | Negative | |||
| PCR | Positive | 199 | 53 | Cases: 257 |
| Negative | 5 | 17 | Non-cases: 17 | |
PCR-positive samples were subjected to RFLP analysis, which showed that all these CL cases were due to L. tropica.Table 2 shows the demographic and clinical data from the 274 patients with suspected CL in the study. Most of the CL cases (78.2%) were between 5 and 40 years old and 52.5% were females. CL lesions were mostly single (70.8%) and of the nodular type (73.1%), and duration varied between 1 and 9 months, with a large majority (96.9%) existing for 4 months or less.
Table 2.
Clinical and demographic data of 274 patients with suspected cutaneous leishmaniasis (CL) attending the NMLCP clinic in Kabul. Cases were patients testing positive on microscopy or PCR or both.
| Cases (N = 257) | Non-cases (N = 17) | ||
|---|---|---|---|
| Gender | Female | 135 (52·5%) | 8 (47·1%) |
| Male | 122 (47·5%) | 9 (52·9%) | |
| X2 = 0·19; p = 0·6619 | |||
| Age (years) | 5–14 | 99 (38.5%) | 7 (41·2%) |
| 15–39 | 102 (39·7%) | 6 (35·3%) | |
| 40–59 | 41 (15·9%) | 3 (17·6%) | |
| ≥60 | 15 (5·8%) | 1 (5·9%) | |
| Median | 20 years | 24 years | |
| IQR | 11–35 years | 13–35 years | |
| Z = 0·18; p = 0·8570 | |||
| Number of lesions | Single | 182 (70·8%) | 15 (88·2%) |
| Multiple | 75 (29·2%) | 2 (11·8%) | |
| X2 = 2·39; p = 0·1217 | |||
| Number of patients with a lesion at a specific locationa | Facial/head/neck | 45 (17·5%) | 3 (17·6%) |
| X2 = 0·00; p = 0·9885 | |||
| Limbs/trunk | 82 (31·9%) | 5 (29·4%) | |
| X2 = 0·04; p = 0·8305 | |||
| Hands | 151 (58·7%) | 10 (58·8%) | |
| X2 = 0; p = 0·9955 | |||
| Feet | 11 (4·3%) | 1 (5·9%) | |
| X2 = 0·10; p = 0·7546 | |||
| Lesion typeb | Nodule | 188 (73·1%) | 6 (35·3%) |
| Ulcer | 65 (25·3%) | 11 (64·7%) | |
| Plaque | 3 (1·2%) | 0 (0%) | |
| Nodule + Ulcer | 1 (0·4%) | 0 (0%) | |
| X2 = 12·42; p = 0·0061¥ | |||
| Lesion duration (months)b | <2 | 24 (9·3%) | 1 (5·9%) |
| 2 | 105 (40·8%) | 4 (23·5%) | |
| 3 | 69 (26·8%) | 6 (35·3%) | |
| 4 | 51 (19·8%) | 6 (35·3%) | |
| 5 | 4 (1·5%) | 0 (0%) | |
| ≥6 | 2 (0·8%) | 0 (0%) | |
| Unknown | 2 (0·8%) | 0 (0%) | |
| Median | 2 months | 3 months | |
| IQR | 2–3 months | 2–4 months | |
| Z = −1·577; p = 0·1149 |
IQR: Interquartile range.
Multiple locations are possible.
Main lesion (lesion sampled).
Statistically significant.
No adverse reactions were observed after collecting samples by either dental broach or skin scraping.
CL Detect returned a positive result in 168 of the 257 CL cases and it was negative in all non-cases. Loopamp in 230 of 257 cases and in 5 of 17 non-cases. When compared to the reference standard, the CL Detect showed a very high specificity (100%), however its sensitivity was low (65.4%). Loopamp showed lower performance in Kabul (87.6% sensitivity and 70.6% specificity) than in the AMC (92.2% sensitivity and 94.1% specificity) (Table 3).
Table 3.
Sensitivity and specificity of CL Detect and Loopamp in 274 patients with suspected CL attending the NMLCP clinic in Kabul, Afghanistan. Reference for the analysis: combined skin-scraping microscopy and dental broach PCR.
| Index tests | Cases, n = 257 |
Non-cases, n = 17 |
Diagnostic performance |
|||
|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Sensitivity [95% CI] | Specificity [95% CI] | |
| Tests in Kabul | ||||||
| CL Detect | 168 | 89 | 0 | 17 | 65·4% [59·2–71·2] | 100% [80·5–100] |
| Loopamp | 225 | 32 | 5 | 12 | 87·6% [82·9–91·3] | 70·6% [44·0–89·7] |
| Test at AMC | ||||||
| Loopamp-AMC | 237 | 20 | 1 | 16 | 92·2% [88·2–95·2] | 94·1% [71·3–99·8] |
CL Detect: CL Detect™ Rapid Diagnostic Test; Loopamp: Loopamp™ Leishmania Detection Kit, CI: Confidence Interval.
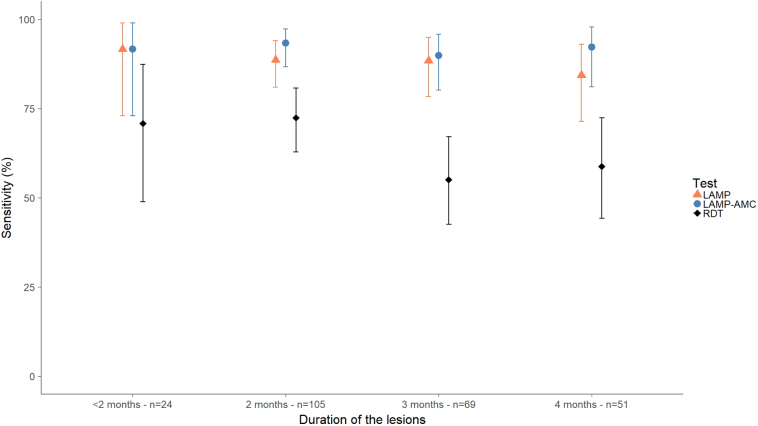
The variation of the sensitivity with respect to the duration of the lesions was assessed in 249 confirmed CL cases. Eight out of the 257 confirmed cases (3%) were not included in this analysis as a limited number had lesions older than 4 months (n = 6) or with an unrecorded duration (n = 2) (Table 2). The sensitivity of Loopamp performed in Kabul and at the AMC was high (around 90%) and did not vary with the duration of the lesions. However, the sensitivity of CL Detect decreased in lesions with durations of 3 months or more, but this is not statistically significant (Fig. 2). Similarly the sensitivity of CL Detect and Loopamp was calculated for 253 CL cases with either ulcerated or nodular lesions. Four CL cases with other lesion types (plaque or nodule-ulcer lesions) were not considered in this analysis (Table 2). CL Detect showed lower sensitivity in ulcers (55.4% [95% CI: 42.5–67.7]) compared to nodular lesions (68.6% [95% CI: 61.5–75.2]). This was not observed for Loopamp, which presented similar sensitivity independently of the type of lesion and the laboratory where the test was conducted (Table 4).
Fig. 2.
Variation of the sensitivity of CL Detect and Loopamp according to the duration of the lesions in 249 confirmed CL cases attending the NMLCP's clinic in Kabul, Afghanistan. Reference for the analysis: combined skin-scraping microscopy and dental broach PCR.
Table 4.
Sensitivity of CL Detect and Loopamp according to the type of lesion in 253 CL cases attending the NMLCP's clinic in Kabul, Afghanistan. Reference for the analysis: combined skin-scraping microscopy and dental broach PCR. Only nodules and ulcerated lesions were included, due to the low number of other lesion types (shown in Table 2).
| Nodule |
Ulcer |
|||
|---|---|---|---|---|
|
N = 188 |
N = 65 |
|||
| Positive | Sensitivity (95% CI) | Positive | Sensitivity (95% CI) | |
| Tests in Kabul | ||||
| CL Detect | 129 | 68·6% (61·5–75·2) | 36 | 55·4% (42·5–67·7) |
| Loopamp | 165 | 87·8% (82·2–92·1) | 57 | 87·7% (77·2–94·5) |
| Test at AMC | ||||
| Loopamp-AMC | 176 | 93·6% (89·1–96·7) | 58 | 89·2% (79·1–95·6) |
CL-Detect: CL Detect™ Rapid Diagnostic Test; Loopamp: Loopamp™ Leishmania Detection Kit; PCR: Polymerase Chain Reaction targeting the mini-exon. CI: Confidence Interval.
When compared to skin-scraping microscopy alone, CL Detect detected 81.9% of the microscopy positive samples [95% CI: 75.9–86.9] and tested negative in 98.6% of the microscopy negative [95% CI: 92.3–100]. On the other hand Loopamp, performed either at NMLCP or AMC, detected >89% of the microscopy positive samples, but tested negative only in a small proportion of the microscopy negative (<40%) (Table S1). We observed a good agreement between CL Detect and microscopy (κ = 0.69 [95% CI: 0.57–0.80]) and the two molecular tests (Loopamp and PCR) conducted at AMC (κ = 0.69 [95%CI: 0.58–0.81]); a McNemar's test showed no significant differences in the performance of Loopamp between the centres (X2 = 1.52; p = 0.2170).
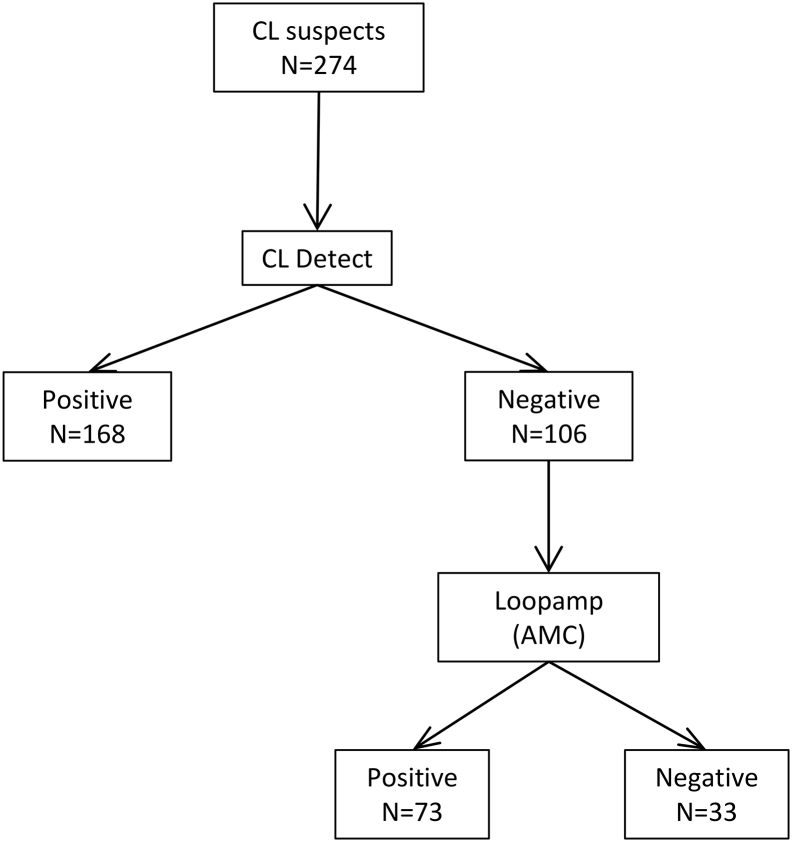
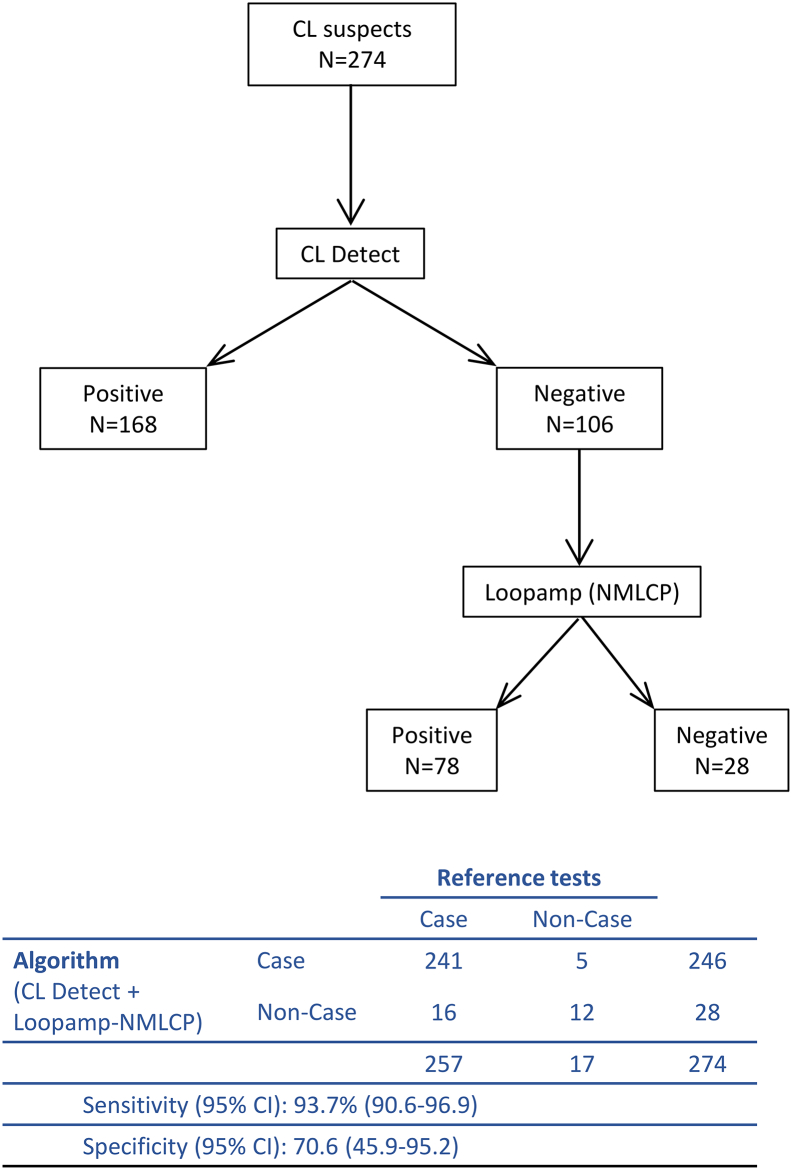
Based on the performance of CL Detect and Loopamp observed in this study we evaluated the sensitivity and specificity of an algorithm where CL suspects would be tested first by CL Detect, and CL Detect-negative individuals would then be tested by Loopamp (Fig. 3). Applying this algorithm to our sample of 274 CL suspects in Kabul would give a sensitivity of 93.4% [95% CI: 89.6–96.1] and a specificity of 94.1% [95% CI: 71.3–99.8] using the performance of Loopamp at AMC (Table 5). Using the Loopamp performance observed in Kabul, the algorithm will maintain a high sensitivity (93.8% [95% CI: 90.1–96.4]) but the specificity would decrease to 70.6% [95% CI: 44.0–89.7] (Fig. S2).
Fig. 3.
Results of the proposed algorithm where CL Detect and Loopamp would be used sequentially in the 274 cutaneous leishmaniasis (CL) suspects attending the NMLCP clinic in Kabul, Afghanistan. The Loopamp results obtained at the AMC were used and CL cases were defined using the results of skin-scraping microscopy and dental broach PCR as a reference.
Table 5.
Sensitivity and specificity of the proposed algorithm (CL Detect and Loopamp used sequentially) to diagnose cutaneous leishmaniasis (CL) cases in Kabul, Afghanistan. The Loopamp results obtained at the AMC were used and CL cases were defined using combined skin-scraping microscopy and dental broach PCR as a reference.
| Reference tests |
||||
|---|---|---|---|---|
| Case | Non-case | |||
| Algorithm (CL Detect + Loopamp-AMC) | Case | 240 | 1 | 241 |
| Non-Case | 17 | 16 | 33 | |
| 257 | 17 | 274 | ||
4. Discussion
The two POC test evaluated in this study showed complementary performances: CL Detect came out as a rapid diagnostic test for leishmanial antigen detection with high specificity that enables diagnosis at the community level, while Loopamp acted as a well-performing, simple and robust test that brings the accuracy of molecular diagnosis to the point-of-care. The sequential use of these two POC tests in a diagnostic algorithm allows CL diagnosis with a high sensitivity and specificity. Moreover, in this algorithm most of the patients would be diagnosed with the CL Detect at the peripheral level in poorly equipped health centres and less CL suspects would need to be referred for testing with Loopamp. This proposed algorithm will reduce the number of true cases left untreated compared to the use of microscopy. This strategy remains to be validated in the field.
We have evaluated the CL Detect™ Rapid Test (InBios International Inc., Seattle, USA) in a L. tropica endemic focus in Afghanistan [4,17]. This RDT was first evaluated in Tunisia in a L. major endemic focus where it yielded >90% sensitivity and specificity [18]. A study in Sri Lanka, where CL is caused by L. donovani, returned a very low sensitivity (36%) but 100% specificity [19]. Another study conducted in Morocco also returned high specificity (94%), however the sensitivity was different in cases from L. tropica endemic regions (73%) and L. major endemic regions (59%) [20]. Differences in sensitivity across regions might be explained by either differences in the levels of the target peroxidoxin at the species levels or in its sequence or conformation. The reference standard used may also have an impact on the estimated sensitivity and can affect comparison of different studies. In the studies described above microscopy was the reference test in Tunisia and PCR in Sri Lanka, the study in Morocco used the same composite reference standard we used in our work, a combination of microscopy and PCR.
Although in our study CL Detect detects less cases than microscopy, its very high specificity (98.6–100%) enables its use in settings with limited infrastructure where microscopy is not available.
Since LAMP was first developed different diagnostic tests using this methodology have been advanced [8,21,22]. The usefulness of LAMP in the diagnosis of CL has been proven but its use is still limited to in-house methods [[23], [24], [25]]. In our study, we used the Loopamp™ Leishmania Detection Kit; to the best of our knowledge this is the first commercially available LAMP kit for the diagnosis of leishmaniasis, already showing a very good diagnostic performance for the diagnosis of visceral leishmaniasis [9,22]. The advantages of molecular tests over microscopy in the diagnosis of CL have been described elsewhere [7,[26], [27], [28], [29]], and in this study we have shown that these advantages are also present when using LAMP, a test which is more amenable than PCR for use in resource limited settings. These advantages relate mainly to a higher sensitivity, which is sustained in long duration lesions in contrast to direct detection methods such as microscopy. We have confirmed this in our study where LAMP did not show a decrease in sensitivity with lesion duration, while the sensitivity of CL Detect decreased in lesions older than 2 months (Fig. 2). The performance of CL Detect also decreased in ulcerated lesions, which might reflect an advanced stage of CL, and thus a lower parasite load. It is also worth noting that lesion type distribution across cases and non-cases was found to be statistically significant, with a higher proportion of nodules/ulcers in true cases. We observed a slightly lower specificity of LAMP at the NMLCP compared to the AMC; a reason for this may be that the laboratory personnel at the AMC is highly experienced in the use of LAMP, while this was the first time this procedure was performed at the NMLCP. Nevertheless it worth noting that the different performance of Loopamp in the two sites was not found to be statistically significant. We would also like to highlight that in order to enhance the use of molecular diagnostics in less equipped settings it would be important to develop and use simple methods for DNA preparation. While in our study we used silica based columns (QIAamp DNA Mini Kit), there are other simpler options that may be suitable for DNA preparation from CL lesions, such as the PURE method (Eiken Chemical Co, Japan), and that should be further explored [25].
A limitation in this study has been the inability of applying all tests on the same sample: we used skin scraps for microscopy, whereas CL Detect, Loopamp and PCR were done in samples obtained with a dental broach. We wanted to evaluate the new tests against the routine diagnostic at NMLCP which is skin-scraping microscopy. At the same time CL Detect requires a dental broach for sampling and we decided to use the sample collected by this method also for DNA extraction in order to limit nuisance to the patients. Sampling of CL lesions using a dental broach is not a procedure exclusive to the CL Detect™ Rapid Test; other authors have prepared slide smears for microscopy from this sample [14,30,31], and we have shown that this simple method can be used for molecular diagnosis. Nevertheless it remains to be explored if other sampling methods can be applied, especially those suitable for lesions that are not susceptible to be sampled with a dental broach, so CL Detect could be used in all type of lesions and its overall performance can be assessed.
Our study shows the performance of two novel approaches to the diagnosis of CL, a disease for which there are still many unmet needs. We believe that integrating these two test in a diagnostic algorithm will bring benefits to the affected communities by enabling early diagnosis with a rapid test, reducing the number of patients to be referred, and accurate confirmation in specialized centres with a molecular test.
The following are the supplementary data related to this article.
Fig. S1.
STARD workflow showing sample flow and tests' results.
Fig. S2.
Results of the proposed algorithm where CL Detect and Loopamp would be used sequentially in the 274 cutaneous leishmaniasis (CL) suspects attending the attending the NMLCP clinic in Kabul, Afghanistan. The Loopamp results obtained in Kabul were used and CL cases were defined using the results of skin-scraping microscopy and dental broach PCR as a reference.
Sensitivity and specificity of CL Detect, Loopamp and PCR in 274 CL suspects attending the NMLCP, Kabul. Reference test for the analysis: skin-scraping microscopy performed at NMLCP.
Acknowledgments
Acknowledgements
We are especially grateful to all the study participants and the local team in Kabul. We are also grateful to InBios International Inc., USA for the donation of the CL Detect™ Rapid Diagnostic Test; the company had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr. Maarten Schim van der Loeff for the critical review of the manuscript.
This work was largely supported by funds from the Federal Ministry of Education and Research, Germany (KfW grant reference number 202060457, Development of Products for the Prevention, Diagnosis and Treatment of Neglected and Poverty Related Diseases; https://www.bmbf.de/en). UK aid from the UK Government, the Government of Switzerland and the Government of Netherlands also contributed to FIND's participation in this work. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of interests
AP and IC are employees of the Foundation for Innovative New Diagnostics (FIND). FIND contributed to the development of the Loopamp Leishmania Detection Kit. However, FIND does not have any financial interest in the product. The other authors declare that they have no conflict of interest.
Authors contribution
Martijn M. T. Vink: Data curation, formal analysis, investigation, project administration, supervision, writing review & editing.
Sami M. Nahzat: Investigation, resources, writing review & editing.
Habiburrahman Rahimi: Investigation, supervision, writing review & editing.
Cyril Buhler: Formal analysis, methodology, validation, writing review & editing.
Bashir A. Ahmadi: Investigation, resources, writing review & editing.
Mohammad Nader: Investigation, resources, writing review & editing.
Fazal R. Zazai: Investigation, resources, writing review & editing.
Abdul S. Yousufzai: Investigation, project administration, resources, writing review & editing.
Merlin van Loenen: Investigation, resources, writing review & editing.
Henk D. F. H. Schallig: formal analysis, Investigation, resources, validation, writing review & editing.
Albert Picado: Conceptualization, formal analysis, funding acquisition, methodology, project administration, resources, software, visualization, writing original draft, writing review & editing.
Israel Cruz: Conceptualization, data curation, formal analysis, funding acquisition, methodology, project administration, resources, supervision, visualization, writing original draft, writing review & editing.
References
- 1.WHO Global leishmaniasis update, 2006–2015: A turning point in leishmaniasis surveillance. Relev Epidemiol Hebd. 2017;92:557–565. [PubMed] [Google Scholar]
- 2.WHO. Control of the leishmaniases. World Health Organ Tech Rep Ser 2010; : xii–xiii, 1–186, (back cover). [PubMed]
- 3.Leishmaniasis in high-burden countries: an epidemiological update based on data reported in 2014Relev Epidemiol Hebd. 2016;91:287–296. WHO. [PubMed] [Google Scholar]
- 4.WHO . WHO; 2018. Control of cutaneous leishmaniasis in Afghanistan: Achievements and challenges. [Google Scholar]
- 5.Reithinger R., Mohsen M., Aadil K., Sidiqi M., Erasmus P., Coleman P.G. Anthroponotic cutaneous leishmaniasis, Kabul, Afghanistan. Emerg Infect Dis. 2003;9:727–729. doi: 10.3201/eid0906.030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weigle K.A., Labrada L.A., Lozano C., Santrich C., Barker D.C. PCR-based diagnosis of acute and chronic cutaneous leishmaniasis caused by Leishmania (Viannia) J Clin Microbiol. 2002;40:601–606. doi: 10.1128/JCM.40.2.601-606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eroglu F., Uzun S., Koltas I.S. Comparison of clinical samples and methods in chronic cutaneous leishmaniasis. Am J Trop Med Hyg. 2014;91:895–900. doi: 10.4269/ajtmh.13-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Notomi T., Mori Y., Tomita N., Kanda H. Loop-mediated isothermal amplification (LAMP): Principle, features, and future prospects. J Microbiol. 2015;53:1–5. doi: 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- 9.Adams ER., Schoone G., Versteeg I, Gomez MA, Diro E., Mori Y., Perlee D., Downing T., Saravia N., Assaye A., Hailu A., Albertini A., Ndung'u JM., Schallig H. Development and Evaluation of a Novel Loop-Mediated Isothermal Amplification Assay for Diagnosis of Cutaneous and Visceral Leishmaniasis. J Clin Microbiol. 2018 Jun 25;56(7) doi: 10.1128/JCM.00386-18. pii: e00386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adams E.R., Gomez M.A., Scheske L. Sensitive diagnosis of cutaneous leishmaniasis by lesion swab sampling coupled to qPCR. Parasitology. 2014;141:1891–1897. doi: 10.1017/S0031182014001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marfurt J., Niederwieser I., Makia N.D., Beck H.-P., Felger I. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115–124. doi: 10.1016/s0732-8893(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 12.Suárez M., Valencia B.M., Jara M. Quantification of Leishmania (Viannia) kinetoplast DNA in ulcers of cutaneous leishmaniasis reveals inter-site and inter-sampling variability in parasite load. PLoS Negl Trop Dis. 2015;9 doi: 10.1371/journal.pntd.0003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharquie K.E., Hassen A.S., Hassan S.A., Ia Al-Hamami. Evaluation of diagnosis of cutaneous leishmaniasis by direct smear, culture and histopathology. Saudi Med J. 2002;23:925–928. [PubMed] [Google Scholar]
- 14.Ul Bari A., Azam S., Mahmood T. Comparison of various cytodiagnostic tests in the rapid diagnosis of cutaneous leishmaniasis. J Pakistan Assoc Dermatol. 2010;20:63–69. [Google Scholar]
- 15.Stevenson M. 2017. epiR: Tools for the analysis of epidemiological data. [Google Scholar]
- 16.StataCorp Stata Statistical Software. 2017. https://www.stata.com/
- 17.Faulde M., Schrader J., Heyl G., Amirih M. Differences in transmission seasons as an epidemiological tool for characterization of anthroponotic and zoonotic cutaneous leishmaniasis in northern Afghanistan. Acta Trop. 2008;105:131–138. doi: 10.1016/j.actatropica.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Ben Salah A., Zaatour A., Gharbi A., Bettaieb J., Ghawar W., Khedher A. ASTMH; New Orleans: 2014. Clinical evaluation of CL detect TM rapid test for cutaneous leishmaniasis: Performance characteristics when compared to smear microscopy at multiple test sites. [Google Scholar]
- 19.De Silva G., Somaratne V., Senaratne S. Efficacy of a new rapid diagnostic test kit to diagnose Sri Lankan cutaneous leishmaniasis caused by Leishmania donovani. PLoS One. 2017;12:1–14. doi: 10.1371/journal.pone.0187024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennis I., Verdonck K., El Khalfaoui N. Accuracy of a rapid diagnostic test based on antigen detection for the diagnosis of cutaneous leishmaniasis in patients with suggestive skin lesions in Morocco. Am J Trop Med Hyg. 2018;99:716–722. doi: 10.4269/ajtmh.18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besuschio S.A., Llano Murcia M., Benatar A.F. Analytical sensitivity and specificity of a loop-mediated isothermal amplification (LAMP) kit prototype for detection of Trypanosoma cruzi DNA in human blood samples. PLoS Negl Trop Dis. 2017;11 doi: 10.1371/journal.pntd.0005779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhtar M., Ali S.S., Boshara S.A. Sensitive and less invasive confirmatory diagnosis of visceral leishmaniasis in Sudan using loop-mediated isothermal amplification (LAMP) PLoS Negl Trop Dis. 2018;12:1–14. doi: 10.1371/journal.pntd.0006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nzelu C.O., Cáceres A.G., Guerrero-Quincho S. A rapid molecular diagnosis of cutaneous leishmaniasis by colorimetric malachite green-loop-mediated isothermal amplification (LAMP) combined with an FTA card as a direct sampling tool. Acta Trop. 2016;153:116–119. doi: 10.1016/j.actatropica.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Kothalawala H.S., Karunaweera N.D. Loop-mediated isothermal amplification assay as a sensitive diagnostic tool for Leishmania donovani infections in Sri Lanka. Ceylon Med J. 2016;61:68. doi: 10.4038/cmj.v61i2.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imai K., Tarumoto N., Amo K. Non-invasive diagnosis of cutaneous leishmaniasis by the direct boil loop-mediated isothermal amplification method and MinION™ nanopore sequencing. Parasitol Int. 2018;67:34–37. doi: 10.1016/j.parint.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Reithinger R., Dujardin J.-C. Molecular diagnosis of leishmaniasis: Current status and future applications. J Clin Microbiol. 2007;45:21–25. doi: 10.1128/JCM.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chargui N., Bastien P., Kallel K. Usefulness of PCR in the diagnosis of cutaneous leishmaniasis in Tunisia. Trans R Soc Trop Med Hyg. 2005;99:762–768. doi: 10.1016/j.trstmh.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Bensoussan E., Nasereddin A., Jonas F., Schnur L.F., Jaffe C.L. Comparison of PCR assays for diagnosis of cutaneous leishmaniasis. J Clin Microbiol. 2006;44:1435–1439. doi: 10.1128/JCM.44.4.1435-1439.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Vries H.J.C., Reedijk S.H., Schallig H.D.F.H. Cutaneous leishmaniasis: Recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16:99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiths W., Dutz W. Repeated tissue sampling with a dental broach. A trial in cutaneous leishmaniasis. Br J Dermatol. 1975;93:43–45. doi: 10.1111/j.1365-2133.1975.tb06474.x. [DOI] [PubMed] [Google Scholar]
- 31.Al-Heany A.R., Sharquie K.E., Al-Najar S.A., Prof A., Noaimi A.A. Cutaneous leishmaniasis: Comparative techniques for diagnosis. IOSR J Dent Med Sci. 2014;13:33–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sensitivity and specificity of CL Detect, Loopamp and PCR in 274 CL suspects attending the NMLCP, Kabul. Reference test for the analysis: skin-scraping microscopy performed at NMLCP.