Abstract
Warm water aquaculture is widely practiced in Kenya and is dominated by the culture of Nile tilapia (Oreochromis niloticus) (75% of total production) followed by African catfish (Clarias gariepinus) at 18%. Aquaculture started in Kenya in 1920’s and has been on upward trend until 2014 when it peaked at 24,096 MT. However, production reduced drastically in the past 3 years, with 14,952 metric tonnes (MT) reported in 2016. Most farmers practice earthen pond based semi-intensive culture system. Commercial intensive culture of Nile tilapia (O. niloticus) in cages in Lake Victoria has grown significantly in the last five years with a production of 12 million kg of fish every cycle (about 8 months). Recirculation aquaculture system (RAS) is also gaining popularity mainly in intensive hatcheries. The freshwater cages have been marred by increasing frequencies of fish kills with obvious financial and environmental implications. Although limited information exists on fish disease outbreaks across the country, certain well known diseases in farmed fish have been reported. These include; fungal, mainly saprolegniasis, bacterial, mainly hemorrhagic disease and pop-eye diseases. Parasites have also been documented in farmed O. niloticus and C. gariepinus. Although prophylactic treatments are used in some hatcheries in order to prevent infections, limited biosecurity measures are in place to prevent diseases in farmed fish. This is because of inadequate knowledge of the economics of fish diseases, poor infrastructure and inadequate human resource specialized in fish diseases. This review describes the aquaculture production and health mangement practices of farmed fish in Kenya in order to document actions required for effective monitoring and regulation of future fish health problems across the country.
Keywords: Aquaculture, Culture systems, Fish health, Hemorrhagic disease, Nile tilapia, Saprolegniasis
1. Introduction
Aquaculture in Kenya, which stands at 14,952 MT [1], comprises of freshwater and mariculture. Mariculture involves the farming of finfish (Milk fish) (Chanos chanos) and Grey mullets (Mugil cephalus); Shellfish (Mud crabs) (Scylla serrata), Oysters (Saccosteria cucullata), shrimp (Penaeus monodon) and Seaweeds (mainly Kappaphycus alvarezii) [2], [3]. Mariculture is underdeveloped mainly due to accessibility problems, conflicts over land ownership, and lack of clear policies [4]. Production statistics of marine aquaculture for Kenya have not been captured in the FAO/national fisheries database since it has not been commercialized despite its great potential [4]. Current mariculture production data indicates that there are over 100 MT of seaweeds, milkfish, shrimps, and mud crabs produced in small scale [2], [4] This is lower than the production of freshwater aquaculture which is currently at 14,852 MT [1], [4].
Fresh water aquaculture involves cold and warm water culture. Cold water culture involves Rainbow trout (Oncorhynchus mykiss) in the Mount Kenya region while warm water fishes comprises of Nile tilapia (Oreochromis niloticus) constituting 75%, African catfish (Clarias gariepinus), and other species comprising 25% [5], [6]. There have been efforts to culture some indigenous fish, like the African carp (Labeo victorianus), Ngege (O. esculentus and Victoria tilapia (O. variabilis) [7], [8], [9]. However, culture of these indigenous species have remained on experimental basis and are not widely adopted by farmers due to low survival and poor yields [2], [7].
The average per capita annual fish consumption in 2010 was 5 kg person−1 year−1 which is below the FAO recommended average of 20 kg person−1 year−1 [10] and the contribution of fish to overall animal protein intake in Kenya is still very low (5.7%) [11]. Freshwater fish consumption in 2014 was estimated at 195,206 tonnes. However, taking into account post-harvest food losses and negative trade balance, the total fish consumption may be lower [12]. To meet the gap between fish production locally and the increasing demand for food fish, Kenya imports about 5900 MT annually from other countries such as China, India, Pakistan, Japan, Korea and Uganda [13]. The bulk of imports in 2013 were frozen tilapia (14%) originating from China. Other imported fish include; frozen mackerel, tuna and herring. Total fish imports reached 5853 MT in 2014, whilst those of Nile tilapia increased from 14% (2013) to 30.8% (2014) [13], [14].
As freshwater aquaculture increases, so does the movement of live fish across borders leading to higher risk of introduction of fish with unknown health histories. There are well documented indications across Africa and internationally that increase in incidences of diseases in aquaculture can cause huge economic losses. For example, in Asian countries, massive expansion and intensification of aquaculture have been reported to be followed by fish health/disease issues leading to significant costs due to losses [12]. This calls for serious management of diseases outbreaks and application of strict biosecurity measures to prevent diseases. Kenya has policies and measures in place to address aquatic animal health issues but lacks expertise in fish health disease, diagnostic laboratories and quarantine facilities, for effective surveillance and diagnosis of diseases [15], [16]. The expansive growth of cage farming in Lake Victoria warrants monitoring of fish health [16]. This paper reviews aquaculture production systems and fish health management practices in Kenya in order to establish and document actions needed to monitor, mitigate and regulate effectively for future fish health problems across the country.
2. Fresh water aquaculture production in Kenya
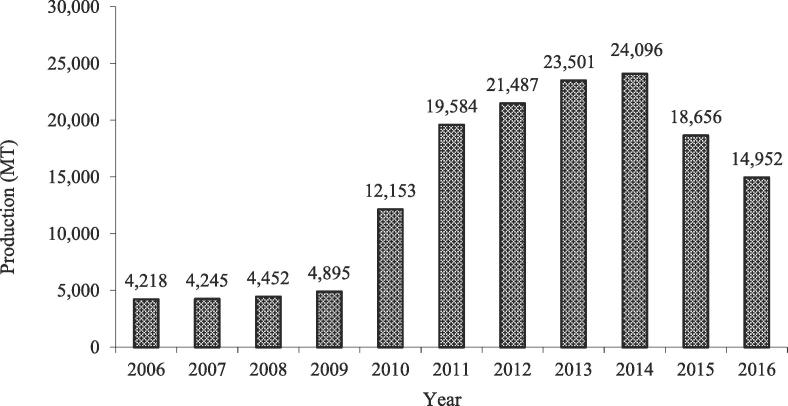
Freshwater aquaculture in Kenya started in 1920’s and became popular in 1960’s. However, it stagnated until 2003 when the production rose from 1000 MT to 4000 MT following numerous efforts to boost production through the “Eat More Fish Campaigns” championed by the government [11]. Between the years 2006 and 2009, aquaculture production remained below 4895 MT until 2010 when 12,153 MT was realized (Fig. 1) [1], [11]. The government nationwide Economic Stimulus Project - Fish Farming Enterprise Productivity Program (ESP-FFEPP), which for the first time, received substantial funding triggered a rapid growth in the sector [10], [17], and supported fish farmers by subsidizing fingerlings, feed and pond construction.
Fig. 1.
Aquaculture production in Kenya (metric tonnes, MT) trends between 2006 and 2015. Source: [1], [11], [18].
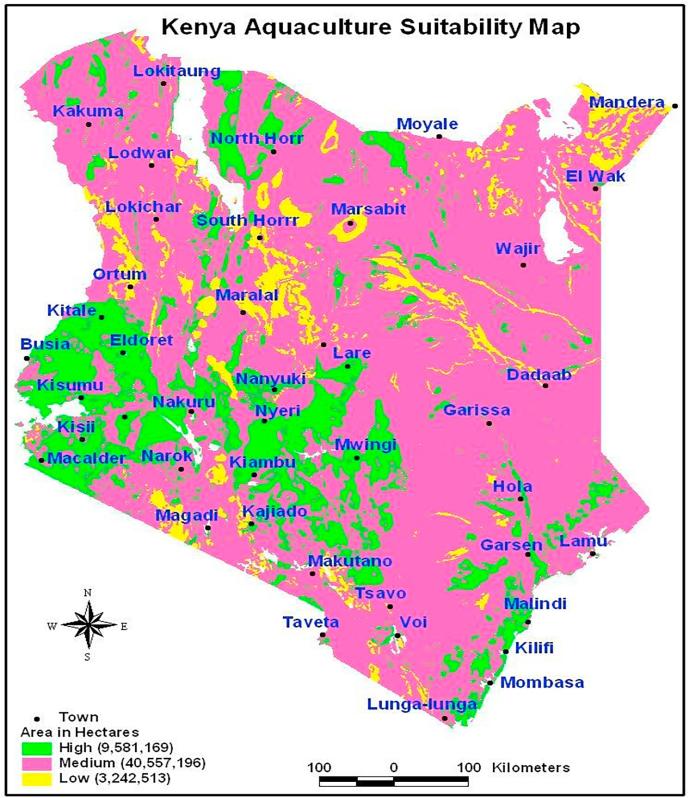
During the ESP-FFEPP, earthen ponds were constructed in most parts of the country after mapping areas which were suitable for aquaculture (Fig. 2). Areas with high suitability were recorded at 9, 581,169 ha, areas with medium suitability at 40,557,196 ha, whilst the areas of low suitability (mainly the arid and semi-arid lands (ASAL) regions of the country) at 3, 242,515 ha (Fig. 2) [19]. The ESP-FFEPP was implemented within the 2009 and 2010 financial year, leading to an increase in fish pond area from 220 ha in 2008 to 468 ha in 2009 and a total gross land for aquaculture from 728 ha (2008) to 825 ha (2009) [20].
Fig. 2.
Map of Kenya indicating areas suitable for freshwater aquaculture: green, highly suitable, pink, medium suitable and yellow, low suitable aquaculture areas based on water availability, climatic conditions, soil type, topography, land use, access to inputs and markets. Source: https://www.ajol.info/index.php/ajfand/article/view/149194[19].
Despite the gains in growth following the ESP-FFEPP, aquaculture production in Kenya reduced from 24,096 MT in 2014 to 18,656 MT in 2015 and further to 14, 952 MT in 2016 (Fig. 1) [20]. Similarly, the number of operational fish ponds reduced from 69, 194 (2013) to 60,277 (2015) shrinking the operational area from 2105 to 1873 ha in 2013 and in 2015 respectively [18], [19]. Reduction in fish production was as a result of poor water retention capacity of ponds in some counties especially the Coastal and the Eastern region; poor extension services, inadequate capacity support, poor husbandry practices, low quality and quantity of fish farm inputs, poor marketing infrastructure, dependency syndrome on government/donor support and lack of value addition. The establishment of county governments and subsequent removal of aquaculture from the functions of the national government to county governments also led to a reduction in aquaculture activities in several counties in Kenya which lacked support programs for fish farming [18].
The distribution of aquaculture activities by region indicates a high concentration of activities in a number of counties and low concentration in others (Table 1). Highest pond numbers and aquaculture related activities are found in Kakamega, Bungoma, Busia, Kisii, Meru, Nyeri, Kisumu, Muranga, Embu counties, among others, while relatively lower activity are noted in Kitui, Lamu and Elgeyo Marakwet [11], [18].
Table 1.
Number of ponds per county in Kenya and respective pond area in 2015.
| S/N | County | Ponds area (ha) 2015 | No of ponds in 2015 |
|---|---|---|---|
| 1 | Kakamega | 259.2 | 8640 |
| 2 | Bungoma | 119.16 | 3972 |
| 3 | Kisii | 93.78 | 3126 |
| 4 | Meru | 88.5 | 2950 |
| 5 | Nyeri | 71.43 | 2381 |
| 6 | Kisumu | 66.66 | 2222 |
| 7 | Muranga | 66.6 | 2220 |
| 8 | Embu | 62.37 | 2079 |
| 9 | Migori | 61.86 | 2062 |
| 10 | Trans Nzoia | 61.29 | 2043 |
| 11 | Machakos | 53.34 | 1778 |
| 12 | Siaya | 52.35 | 1745 |
| 13 | Busia | 48.51 | 1617 |
| 14 | Tharaka Nithi | 48 | 1600 |
| 15 | Kiambu | 45.96 | 1532 |
| 16 | Homa Bay | 42.69 | 1423 |
| 17 | Makueni | 41.34 | 1378 |
| 18 | Kirinyaga | 38.91 | 1297 |
| 19 | Bomet | 38.4 | 1280 |
| 20 | Nyamira | 36.6 | 1220 |
| 21 | Nakuru | 36.48 | 1216 |
| 22 | Laikipia | 36.45 | 1215 |
| 23 | Uasin Gishu | 32.97 | 1099 |
| 24 | Vihiga | 31.11 | 1037 |
| 25 | Nyandarua | 28.62 | 954 |
| 26 | Nandi | 28.29 | 943 |
| 27 | Kericho | 28.05 | 935 |
| 28 | Nairobi | 26.1 | 870 |
| 29 | Kwale | 26.04 | 868 |
| 30 | Taita Taveta | 25.29 | 843 |
| 31 | Baringo | 21.96 | 732 |
| 32 | Kilifi | 18.57 | 619 |
| 33 | Narok | 17.01 | 567 |
| 34 | Tana River | 15.6 | 520 |
| 35 | Kajiado | 11.88 | 396 |
| 36 | Kitui | 10.26 | 342 |
| 37 | Lamu | 9.21 | 307 |
| 38 | Elgeyo Marakwet | 7.47 | 249 |
| TOTAL | 1808 | 60,277 |
3. Freshwater fish species reared in Kenya
Aquaculture species in Kenya includes Nile tilapia (O. niloticus) and African catfish (C. gariepinus). Tilapia represents 75% of the total fish produced from aquaculture, followed by African catfish (18%), common carp (6%) and trout (<1%) [14]. Tilapia farming is mainly carried out in monoculture systems. A survey conducted in Western Kenya targeting 1000 farmers indicated that a high proportion of farmers (74%) cultured Nile tilapia and African catfish in monoculture systems, while 26% of farmers carried out polyculture of the two species [21]. This was attributed to inadequate knowledge of polyculture by farmers [11], [14]. In addition to the production of food fish, ornamental fishes are also produced at small scale for local and internal markets [5].
4. Aquaculture production systems
In Kenya, current aquaculture culture systems are made up of extensive and semi-intensive systems (Table 2). Truly intensive systems exist in a relatively small number. Reports indicate that fish farmers operating at a subsistence level are turning into commercial intensive fish farming with some earning as much as US$ 11,000.00 ha−1 year−1 in gross income [5]. More than 90% of farmers practice semi-intensive fish farming while the intensive system is practiced by only 3% due to high cost of electricity and non-availability of cheaper quality feeds [11]. In the semi-intensive systems, ponds are fertilized with either cattle, sheep, poultry or rabbit manure and supplementary feed inform of cereal bran (wheat, rice, maize) and low protein formulated feeds are given to supplement natural foods [22]. Aquaculture farm systems in Kenya are in most cases integrated with either crop or livestock production (Vegetables, bananas, goats, cattle and chicken) [23]. Crop farming is generally done at subsistence level while livestock rearing is often done for commercial purposes especially for milk and meat production [19].
Table 2.
The Kenya national distribution of fish culture systems and respective cover area (m2).
| Region | Semi intensive systems (Ponds) |
Extensive systems (Dams) |
Intensive systems (Tanks) |
|||
|---|---|---|---|---|---|---|
| Area | Number | Area | Number | Area | Number | |
| Central | 1609 | 506,605 | 167 | 1,933,809 | 83 | 18,744 |
| Coast | 434 | 58,698 | – | – | 9 | 180 |
| Eastern | 752 | 423,628 | 20 | 113,018 | 3 | 118 |
| Nyanza | 2070 | 453,423 | 15 | 41,220 | 1 | 27 |
| Rift Valley | 1,531 | 761,856 | 129 | 3,385,298 | 65 | 4015 |
| Western | 2720 | 549,486 | – | – | – | – |
Source: [2].
4.1. Extensive fish farming
This system is mainly conducted in dams and water reservoirs. The farmed fishes depend on primary productivity of the culture water and no artificial feed is given. The species mainly cultured in this system are O. niloticus and C. gariepinus which are stocked to prevent breeding of mosquitoes in dams put in place for watering livestock. The dams are mainly found in Central and Rift valley regions (Table 2). Production from this system ranges between 500 and 1,500 kg ha−1 year−1, contributing 10% of farmed fishes in Kenya [24].
4.2. Semi-intensive systems
Semi-intensive farming is the main system adopted in Kenya. These systems are mainly used to produce O. niloticus and C. gariepinus either in monoculture or polyculture. They consist of earthen ponds, liner ponds and concrete ponds. Ponds are fertilized using organic manures (cow dung, sheep, poultry or rabbit manure) [22]. Feeding is done using supplementary feeds formulated on farm or purchased from cottage fish feed production industries. In some cases, cereal brans are used as feeds to increase pond productivity. Production from this system ranges between 1000 and 2500 kg ha−1 year−1 [24]. Most farmers prefer this system since it is less expensive in terms of feed inputs.
4.3. Intensive systems
4.3.1. Raceways
This system is mainly used for production of rainbow trout (Oncorhynchus mykiss). There are 6 commercial trout farms in Kenya concentrated in Mount Kenya region. According to the Kenya’s State Department of Fisheries, production of trout from the raceways in 2014 was 241 MT valued at U$ 1,430,000 [13]. The contribution of rainbow trout is therefore higher in monetary value than by weight since a kg costs between U$ 3–12 [5]. Production in these systems ranges between 10,000 and 80,000 kg ha−1 year−1 [11]. The system requires high quality feed which are expensive and can only be afforded by a few farmers.
4.3.2. Recirculating aquaculture systems (RAS)
Recirculating aquaculture systems in Kenya are mainly tank-based systems used for culturing O. niloticus and C. gariepinus. Fish are reared in tanks indoors or under green houses. There exist 8 farms operating recirculating systems in form of hatcheries and grow-out farms in Kenya. Fish are grown at high density ranging between 5 and 20 fish m−3 under controlled conditions. Production from RAS is at 200 tonnes ha−1 year−1 [18]. The adoption of the system is low due to high cost of initial capital investment in tanks, greenhouses and high cost of electricity required in running the system. Investment in recirculation aquaculture systems (RAS) for Nile tilapia production and intensive catfish production is carried out in peri-urban areas near towns like Nairobi, Kiambu, Nyeri, Meru, Kisumu, Machakos, Kilifi, Homa Bay, Kakamega and Busia [18].
4.3.3. Cages
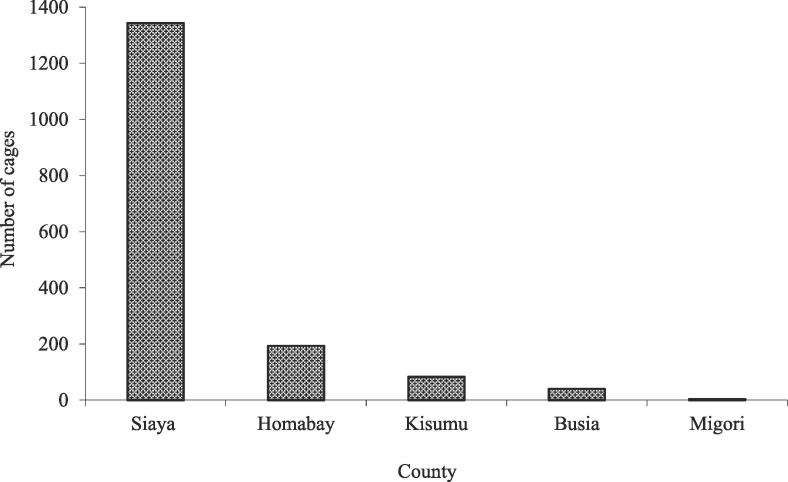
Cage farming is growing fast in Lake Victoria with the highest number of the cages located in Siaya County (Fig. 3) [25]. Intensive cage culture started in 2013 after cage trials were conducted successfully at Dunga beach in Kisumu County by Kenya Marine and Fisheries Research Institute (KMFRI) and Dunga Beach Cooperative Society under the Association for Strengthening Agriculture Research in East and Central Africa (ASARECA) project [25], [26]. Currently, cage farming is practiced in five riparian counties (Migori, Siaya, Homabay, Busia and Kisumu counties) (Fig. 3). Stocking density in the cages ranges between 60 and 250 fish m−3 with cage sizes ranging from 8 to 125 m3. The number of cages increased from 1663 in 2016 to 3398 cages in 2017 [26]. Nile tilapia is the only fish cultured in cages producing 12 million kg of fish every cycle (about 8 months in a year) [25], [26].
Fig. 3.
Distribution of fish cages in five riparian counties of Lake Victoria in 2016. Adapted from [25].
The largest cage farming enterprise in Lake Victoria is Winnie’s farm in Anyanga beach which started with 60 cages in 2013 and currently owns more than 550 cages together with other groups consisting of 100 farmers [12]. Currently, the enterprises operating cages are about 43 with over 4000 cages stocked with >3 million individual tilapia fingerlings [26]. Cage farming has a huge potential to increase aquaculture production and support economic growth around the Lake Victoria region [25].
4.4. Ponds
Most of smallholder farmers have a minimum of 1 pond to a maximum of 60 fish ponds. The level of operations of farmers are rated as small scale, medium or large scale [20]. Large scale operators represent a pond surface area of 4000–80,000 m2 and more than 13 ponds while medium scale operators represent 601–3999 m2 and 5–12 ponds. Small scale farmers have less than 5 ponds and in most cases use their own individual labour to produce fish mainly for household consumption and excess fish are sold to neighbors [20], [24]. A stocking rate of 3 fish m−2 is commonly used in ponds in Kenya to achieve yields of 1 kg m−2. At this stocking rate daily weight gain ranges from 1.5 to 2.0 g in well managed systems. Rare cases in Kenya have stocking densities of 6 juveniles m−2 in ponds giving a production of 3 kg m−2 [11].
Most fish farmers practicing pond culture add manure or inorganic fertilizer to ponds to increase the supply of natural food organisms to fish so as to reduce production costs arising from feeds [5]. The manures in use are; cow dung, sheep, poultry and rabbit manure. These manures increase the risk of introduction of pathogens into the system [27], [28]. Culture periods of 6 months or more are needed to produce fish that weigh between 250 and 300 g from the ponds. The size of fish attained at the end of the growth period depends on the climatic conditions of the area especially temperature with areas having an average temperature lower than 25 °C having smaller fish at harvest. Type of feed used and management practices like water quality management, feeding regimes and stocking density also affect the growth of fish.
5. Husbandry practices
5.1. Source of water
Water used for aquaculture activities in Kenya is mainly sourced from streams, springs, rivers and boreholes which constitutes 72% of water sources for aquaculture activities [21]. Only 28% of farmers obtain water from boreholes, shallow wells and municipal tap water [20]. The water is either pumped to the culture units or directed to flow by gravity. Most farmers do not treat the water before use. Water is allowed to settle in a reservoir before being channeled to production units. This practice is not recommended since it provides opportunity for potential introduction of pathogens to the culture facility from the water source.
5.2. Feeds inputs
Various feeds are used by fish farmers in Kenya, ranging from mash (for fingerlings) to farm made pellets, pressed pellets (made locally by a number of companies) and extruded floating feeds (Table 3). Extruded floating feeds are mainly imported from other countries including, the Netherlands, Norway, Denmark, Israel, Mauritius, Uganda and Ghana [2], [22]. Companies producing extruded pellets in Kenya are Sigma Ltd, Unga Feeds Ltd, Jewlet Enterprises, Lenalia Feeds Ltd and Food Tech Africa (Table 3) [2]. Due to unavailability of cheaper feeds in the country, some farmers have been using pig pellets and poultry feed (grower and layer mash) to feed fish [21], [29]. Some of these livestock feeds are supplemented with antibiotics, probiotics and growth promoters which farmers could be introducing to fish unknowingly. For example pig pellets contain enzymes like phytases, β-glucanases, xylanases, α-galactosidases, proteases, amylase, lipases, mannanases, cellulases, hemicellulases and pecti-nases while poultry feed are supplemented with salinomycin, sodium and virginiamycin so as to promote growth and reduce mortality [30]. The use of pig or poultry feed for fish is not recommended since fish and other livestock have different dietary requirements for efficient growth [31]. This implies that fish get nutrients in proportions, which are limited leading to wastage of feed, poor growth and occurrence of deformities and nutritional diseases.
Table 3.
| Company Name | Type | Location | Type of Feed |
|---|---|---|---|
| Commercial manufacturers | |||
| Sigma Feeds Ltd | Local | Rongai, Kajiado County | Floating pellets |
| Lenalia Fish feeds | Local | Limuru, Kiambu County | Floating and sinking pellets |
| Maisha Bora Fish Feeds Ltd | Local | Kikuyu, Kiambu County | Sinking pellets |
| Kwality Fish Feeds Limited | Local | Ruiru, Kiambu County | Sinking pellets |
| Sare Millers Ltd | Local | Kisumu County | Floating and sinking pellets |
| Jewlet Fish Farm Enterprises | Local | Kendubay, Homabay County | Floating and sinking pellets |
| Unga Feeds Ltd-Nairobi | Local | Industrial Area Nairobi | Floating pellets |
| Ugachick Fish Feeds | Imported | Uganda | Floating pellets |
| Raanan Fish Feeds | Imported | Nairobi County | Floating pellets |
| Nile Aqua | Imported | Uganda | Floating pellets |
| Skretting Fish Feeds | Imported | Nairobi County | Floating pellets |
| Aller Aqua fish Feeds | Imported | Nairobi County | Floating pellets |
| LFL Riche Terre | Imported | Nairobi County | Floating pellets |
| Food Tech Africa | Local | Nairobi County | Floating pellets |
| Cottage feed industries | |||
| Othaya Fish Feeders S.H.G | Local | Othaya, Nyeri County | Sinking pellets |
| Chumara Fish Feeds | Local | Chuka, Meru County | Sinking pellets |
| Mabro Fish Farm Enterprises | Local | Usigu, Siaya County | Sinking pellets |
| Bidii Fish Farmers S.H.G | Local | Luanda- Emuhaya | Floating and Sinking pellets |
| Osifeeds Ltd. | Local | Kajiado County | Sinking pellets |
| Zibag Fish producers & Processors | Local | Nyandarua County | Sinking pellets |
| Hesao Integrated Fish Farming Organization | Local | Nyalenda B, Kisumu County | Sinking pellets |
| Dominion Fish Feed limited | Local | Siaya County | Sinking pellets |
| Nyawara Animal Feed Plant | Local | Gem, Siaya County | Sinking pellets |
| Kenya Marine and Fisheries Research Institute | Local | Sangoro, Kisumu County | Sinking pellets |
5.2.1. Type of feeds used
Commercial fish feeds in Kenya, usually contain 24–30% and 30–40% crude protein for O. niloticus and C. gariepinus respectively [32]. These feeds are too expensive for some farmers such that, most farmers use locally formulated mixed feeds [33]. The feed are made by mixing dried freshwater shrimp (Caridina niloticus), commonly known as Ochonga with rice bran or maize bran with Omena (Rastrineobola argentea) meal [20], [30]. This practice does not lead to formulation of balanced diets required by the fish leading to poor growth and nutritional deficiencies [31]. Other feed materials and ingredients available locally and commonly used by fish farmers in Kenya are; terrestrial plants (grasses, leaves (e.g. cassava) and seeds of leguminous shrubs and trees vegetables); aquatic plants (water hyacinth, water lettuce, duckweed); small terrestrial animals (earthworms, termites); Aquatic animals (trash fish, by catch fish); rice (broken, bran, hulls); wheat (middling, germ, bran); maize (gluten feed, germ, gluten meal); seed cakes (mustard, coconut, groundnut, cotton, sunflower, soybean); brewers waste; slaughterhouse wastes: offal, and blood [22], [32], [34].
6. Fry and fingerling supply
Fish seed are sourced from hatcheries which are either owned by the government or private farmers. Between the years 2010 to 2016, the government owned National Aquaculture Research and Development Training Centre, Sagana supplied 30.3% of fry and fingerlings while private hatcheries contributed 69.7% [18], [35]. The total demand for both African catfish and tilapia fingerlings across Kenya was estimated at 100 million yr−1 in 2010 [17]. The common methods used in Kenya for fingerling production are; open ponds, tanks and hapas in ponds. Fry are collected from the spawning units at 0.03–0.05 g and stocked into nursery units for rearing to the fingerling stage (5 g) before they are stocked into grow out facilities [35]. Currently, there are a total of 127 authenticated hatcheries in Kenya with a capacity to produce 96 million fingerlings annually [35]. On average, the hatcheries record a survival of 70% of the hatched crop which are sold to farmers at fry or fingerling stage [17]. The hatcheries are located in different parts of the county to allow for ease of access by farmers [18], [35]. The fingerlings for Nile tilapia produced include all male tilapia produced through sex reversal, naturally male tilapia produced by use of super YY males and mixed sex tilapia fingerlings [35].
7. Record keeping
This is one of the important aspects in fish farming used to determine profitability of the business. Majority of farmers (77%) have reported to keep records on their fish farming activities [21]. The records kept are for the species of fish reared, the number of fish stocked per pond, feeding records and water quality records (temperature, dissolved oxygen and pH). The records are kept in form of notebooks and files depending on the farmers’ production scale. Fish health records are not kept by farmers since they don’t record diseased fish and cannot establish the cause of fish mortalities. The number of dead fish is recorded without any diagnosis to determine the cause of mortality [15], [21].
8. Fish disease occurrence in Kenya
Very limited information exists on disease outbreaks in fish farms in Kenya. Most fish health studies have focused on parasites in two most cultured species, O. niloticus and C. gariepinus [15]. These studies focused on the parasite descriptions, biology and pathology [36]. The lack of information on fish diseases could be linked to lack of diagnostic infrastructure, lack of human resource with expertise in fish health, high cost of diagnosis, lack of well-equipped veterinary laboratories for identification of pathogens, absence of outbreak reports due to poor record keeping by farmers and socio-economic status of the farmers [15]. However, some farmers have experienced mortality of fish in their farms losing between 40 and 100% of the stock in both cages and ponds [25], [26]. While this is usually associated with water quality problems, it is possible that it could be health related since no diagnosis is done at the farm level to rule out diseases. Most small scale and medium scale farmers do not bother to establish the cause of mortalities, and when they do, they consult officers from the universities or fisheries officers who also have little or no knowledge on fish health [15]. A study conducted in 2014 in some fish hatcheries investigating bacterial and fungal infections in farmed fish established that the hatcheries lost most of their stocks to diseases [37]. The small scale hatcheries were reported to experience more mortality due to inadequate biosecurity measures and poor management practices to prevent infections. Most reported diseases in fish farms are; fungal mainly saprolegniasis, bacterial mainly hemorrhagic and pop eye diseases [15], [38].
Some O. niloticus hatcheries have been affected by Streptococcus iniae which makes the affected fish to have a C- shape especially the newly stocked fish larvae [37], [38], [39]. Grow out O. niloticus have also been affected by fish louse (Argulus spp.) while C. gariepinus have been affected by freshwater white spot disease (Ichthyophthirius multilifis) [37]. Disease occurrences in farms have been attributed to poor husbandry practices including use of on-farm formulated feed with high bacterial load and use of water directly from the source without prior treatment [37], [38]. Water directly sourced from the river or streams can introduce high levels of bacterial loads which affect younger fish more than adults indicating poor hatchery practices within Kenya aquaculture systems. The bacterial infections affecting pond cultured fish in Kenya are caused by Aeromonas hydrophila, Pseudomonas fluorescens and P. aeruginosa, Edwardsiella tarda, Flavobacterium columnare, Mycobacterium fortuitum and Streptococcus iniae [15], [40]. In cages, symptoms like fin rot, cloudy eyes and skin lesions, have been reported indicating possibility of bacterial and fungal infections [25].
9. Fish health management practices
Some fish farms in Kenya especially hatcheries, use preventive measures to reduce chances of disease occurrence [16]. Unlike in grow-out systems, disinfection of farm equipment and culture facilities are routinely included in fish health management schemes in hatcheries. The choice of management practices and application of prophylactics are based on the farmers’ knowledge and experience [41]. Commonly used drugs and chemicals in aquaculture systems in Kenya are; potassium permanganate and sodium chloride to eliminate bacterial and fungal infections [42]. Treatments in the hatchery are done at the egg incubation stage or at the fry stages to increase survival of the hatched fry [41], [42]. The only antibiotic which is used in Kenya by a private hatchery is oxytetracycline [35] to prevent bacterial infections in African catfish broodstock. Use of oxytetracycline in food fish have raised concerns on antibiotic resistance in fish [43], [44] which has become globally relevant issue [45], [46].
9.1. Challenges in fish health management
Quarantine facilities are non-existent in Kenya and limited biosecurity measures have been put in place to monitor new introductions and occurrence of diseases in fish [20]. This is due to non-reported fish diseases and inadequate human resource specialized in fish diseases, making the establishment of such facilities unappealing. The quarantine facilities would be important with the increase in importation of broodstock especially the non-indigenous species of Nile tilapia which may lead to introduction of diseases and parasites [37]. Inadequate measures to prevent escapes of cultured fish to the wild also poses a great danger to the wild stocks [47]. These inadequate biosecurity measures may result in rapid spread of disease pathogens within the country [47]. In fact, the recent sampling and detection of Tilapia lake virus (TiLV) in Tanzanian and Ugandan parts of Lake Victoria in both fish in cages and open waters [48] put farmed fish in cages in lake Victoria at high risk of diseases. Although the TiLV was detected, there have not been any external clinical signs, clinical disease or mortality resulting from the virus [48]. However, the TiLV has led to serious losses of Nile tilapia in most countries including Israel, Ecuador and Egypt [49]. With the intensification of cage culture of tilapia in lake Victoria, more biosecurity measures need to be in place to avoid possible infections since cages are open systems that can allows exchange of pathogens between cultured and wild fish in the lake [26].
Kenya has no specialized fish diagnostic laboratories recognized by the World Animal Health Organization (known by its French name “Office International des Epizooties” (OIE). In the event of disease outbreaks, diagnoses are performed at local universities and public research institutes that conduct research on fisheries and aquaculture. Kenya has several universities, including Moi, Kenyatta, Egerton and Maseno, as well as the Kenya Marine and Fisheries Research Institute conducting research in fisheries and aquaculture. A recommendation was made by [14] that since specialists in fish disease are not common in Kenya, farmers need to use preventative measures like maintaining a suitable environment for fish, stocking healthy fish, using quality feeds and limiting stress to prevent diseases in intensive farming systems.
10. Conclusions and recommendations
The high potential of aquaculture in Kenya if exploited will lead to increased production of fish. It is evident that there is no health management strategy for farmed fish in Kenya due to inadequate capacity both in human resource, infrastructure and lack of funding for fish health management. Capacity building of the various stakeholders needs to be enhanced to acquire basic skills required in the identification of sick fish. A fish pathology and diagnostic laboratory with appropriately trained and experienced staff should be established to help in diagnosis of diseased fish. Simple realistic and low cost biosecurity measures should be adopted by farmers in order to prevent occurrence of diseases in farmed fish. From earlier reports, no baseline information exists on fish health management in Kenya. A survey emphasizing the health of farmed fish and health management practices need to be carried out to provide basic information for planning necessary interventions for fish health management in the country. More research with focus on fish health need to be emphasized and setting up of a specialized government fish health inspectorate is necessary for fish disease surveillance. An understanding of the existing health management practices, dynamics, infrastructure and regulatory practices in other African countries like Egypt should be encouraged for better fish health management in Kenya.
Acknowledgments
Acknowledgments
This study was conducted as part of baseline information for the Imaqulate project “Evaluating Costs and Benefits of Prophylactic Health Products and Novel Alternatives on Smallholder Aquaculture Farmers in Asia and Africa (IMAQulate), Project Ref: BB/N005082/1”. The project is being implemented under funding from Biotechnology and Biological Sciences Research Council/ Department for International Development (BBSRC/DfID), UK and the Department of Biotechnology, India.
Competing interests
There is no conflict of interest to declare.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
Contributor Information
Mary A. Opiyo, Email: marybede@gmail.com.
William Leschen, Email: william.leschen@stir.ac.uk.
Harrison Charo-Karisa, Email: h.karisa@cgiar.org.
References
- 1.KNBS, Kenya National Bureau of Statistics. Economic Survey 2017. Nairobi: Kenya National Bureau of Statistics (KNBS); 2017. p. 333. http://www.devolutionplanning.go.ke/images/hb/Economic Survey 2017.pdf.
- 2.Munguti J.M., Kim J., Ogello E.O. An overview of kenyan aquaculture: current status, challenges and opportunities for future development. Fish Aquat Sci. 2014;17:1–11. [Google Scholar]
- 3.Mirera D.O. Experimental Polyculture of Milkfish (Chanos chanos) and Mullet (Mugil cephalus) Using Earthen Ponds in Kenya. West Indian Ocean J Mar Sci. 2011;10:59–71. https://www.ajol.info/index.php/wiojms/article/view/74184/64836 [Google Scholar]
- 4.KMFRI. Kenya’s, Aquaculture Brief 2017: Status, Trends, Challenges and Future Outlook Mombasa: Kenya Marine and Fisheries Research Institute (KMFRI) 2017 p. 12. www.kmfri.co.ke/images/pdf/Kenya_Aquaculture_Brief_2017.pdf.
- 5.Mbugua M.H. State Department of Fisheries; Nairobi: 2008. Aquaculture in Kenya. Status, Challenges and Opportunities; p. 10. [Google Scholar]
- 6.Ngugi C., Manyala J. Review of aquaculture extension service in Kenya. Rome: Food and Agriculture Organization of the United Nations (FAO) Rome: Fisher Dept. 2004 p. 20. www.fao.org/docrep/007/y5641e/y5641e09.htm. [Google Scholar]
- 7.Orina P.S., Charo-Karisa H., Munguti J.M., Boera P., Abwao J., Kyule D. A comparative study of Labeo victorianus (Bouelenger, 1901) and Oreochromis niloticus (Linnaeus, 1758) grown in polyculture systems. Lakes Reservoirs: Res Manage. 2018;23:56–62. https://onlinelibrary.wiley.com/doi/abs/10.1111/lre.12202. [Google Scholar]
- 8.Charo-Karisa H., Maithya J. Stirling: Sustainable Aquaculture Research Networks in Sub-Saharan Africa (SARNISSA); 2010. Contribution of fish farmers to conservation of endangered Lake Victoria Basin fish species - the case of Oreochromis variabilis and O. esculentus; pp. 1–14. http://www.sarnissa.org/SARNISSA+Project+Publications. [Google Scholar]
- 9.Maithya J., Mbithi N., Wanjala P. Growth performance of Oreochromis variabilis larvae: a case study of effect of live and formulated diets on growth and survival rates. Int J Fish Aquac. 2017;9:14–23. http://academicjournals.org/journal/IJFA/article-abstract/DE35A7F63124. [Google Scholar]
- 10.Rothuis A., van Duijn A.P., van Rijsingen J., van der Pijl W., Rurangwa E. LEI report 2011–067/IMARES report C131/11. Wageningen University; Wageningen: 2011. Business opportunities for aquaculture in Kenya; With special reference to food security; p. 28. http://library.wur.nl/WebQuery/wurpubs/fulltext/273483. [Google Scholar]
- 11.Food and Agriculture Organization of the United Nations (FAO). Fishery and Aquaculture Country Profiles. Kenya, 2016. Country Profile Fact Sheets. Rome: 2016. p. 42. http://www.fao.org/fishery/facp/KEN/en.
- 12.FARM AFRICA Report on Market Study of the Aquaculture Market in Kenya. Kenya Market-Led Aquaculture Programme (KMAP) Nairobi: FARM AFRICA. 2016:76. [Google Scholar]
- 13.State Department of Fisheries (SDF). Fisheries Annual Statistical Bulletin 2014. Nairobi, State Department of Fisheries: 2014. p. 59.
- 14.FARM AFRICA . FARM AFRICA; Nairobi: 2016. Kenya Market-Led Aquaculture Programme Strategic Environmental Assessment and Environmental Management Plan; p. 112. [Google Scholar]
- 15.Akoll P., Mwanja W. Fish health status, research and management in East Africa: past and present. Afr J Aquat Sci. 2012;37:117–129. [Google Scholar]
- 16.AU-IBAR. Mapping study of aquatic animal diseases in Africa – Eastern Africa. Nairobi: Interafrican Bureau for Animal Resources (AU-IBAR) 2016 59.
- 17.Musa S.A., Aura C.M., Owiti G.E., Nyonje B.E., Orina P.A., Charo-Karisa H.A. Fish farming enterprise productivity program (FFEPP) as an impetus to Oreochromis niloticus (L.) farming in Western Kenya: Lessons to learn. Afr J Agric Res. 2012;(7):1324–1330. [Google Scholar]
- 18.State Department of Fisheries (SDF) State Department of Fisheries; Nairobi: 2016. Kenya Fish Farming Enterprise Productivity Capacity Assessment and Gap Analysis Report 2016. [Google Scholar]
- 19.Ogello E.O., Munguti J. Aquaculture: a promising solution for food insecurity, poverty and malnutrition in Kenya. Afr J Food, Agric Nutr Dev. 2016;16:11331–11350. [Google Scholar]
- 20.Obwanga B., Lewo M.R., Bolman B.C., van der Heijden P.G.M. Report 2017–092 3R Kenya. Wageningen University & Research; Wageningen: 2017. From aid to responsible trade: driving competitive aquaculture sector development in Kenya; Quick scan of robustness, reliability and resilience of the aquaculture sector. 10.18174/421667. [Google Scholar]
- 21.Jacobi N. University of Akureyri, Iceland; Akureyri: 2013. Examining the potential of fish farming to improve the livelihoods of farmers in the Lake Victoria region, Kenya: Assessing Impacts of Governmental Support; p. 98. Masters Thesis. [Google Scholar]
- 22.Munguti J.M., Musa S., Orina P.S., Kyule D.N., Opiyo M.A., Charo-Karisa H. An overview of current status of Kenyan fish feed industry and feed management practices, challenges and opportunities. Int J Fish Aquat Sci. 2014;1:128–137. http://www.fisheriesjournal.com/vol1issue6/Pdf/145.1.pdf [Google Scholar]
- 23.Ogello E.O., Mlingi F., Nyonje B., Charo-Karisa H., Munguti J. Can integrated livestock-fish culture be a solution to East Africa’s food insecurity? A review. Afr J Food, Agric Nutr Dev. 2013;13:8058–8076. http://www.bioline.org.br/pdf?nd13066 [Google Scholar]
- 24.Ngugi C., Bowman J.R., Omolo B. Oregon University; 2007. A new guide to fish farming in Kenya. Oregon: Aquaculture Collaborative Research Support Program (ACRSP) Aquaculture CRSP Management Office; p. 100. [Google Scholar]
- 25.Aura C.M., Musa S., Yongo E., Okechi J.K., Njiru J.M., Ogari Z. Integration of mapping and socio-economic status of cage culture: towards balancing lake-use and culture fisheries in Lake Victoria, Kenya. Aquac Res. 2018;49:532–545. [Google Scholar]
- 26.Njiru J.M., Aura C.M., Okechi J.K. Cage fish culture in Lake Victoria: a boon or a disaster in waiting? Fish Manag Ecol. 2018:1–9. [Google Scholar]
- 27.Mente E., Karalazos V., Karapanagiotidis I.T., Pita C. Nutrition in organic aquaculture: an inquiry and a discourse. Aquac Nutr. 2011;17:e798–e817. [Google Scholar]
- 28.Little D.C., Edwards P. Integrated livestock-fish farming systems. Rome: Food and Agriculture Organization of the. United Nations. 2003:22. [Google Scholar]
- 29.Liti D., Cherop L., Munguti J., Chhorn L. Growth and economic performance of Nile tilapia (Oreochromis niloticus, L.) fed on two formulated diets and two locally available feeds in fertilized ponds. Aquac Res. 2005;36:746–752. [Google Scholar]
- 30.Odede R.O. The University of Nairobi; Nairobi: 2015. Use of Non-Antibiotic Growth Promoters in Chicken Broiler Production in Kenya; p. 98. [Google Scholar]
- 31.National Research Council (NRC) Nutrient Requirements of Fish. Feb 1 National Academies Press 105 1993.p. https://books.google.co.ke/books?hl=en&lr=&id=M2aBtOUhRagC&oi=fnd&pg=PA1&ots=ZVikISPMzn&sig=F3tHfbsEsXmgVkjosuzoJf8hm8&rediresc=y#v=onepage&q&f=false.
- 32.Liti D.M., Mugo R.M., Munguti J.M., Waidbacher H. Growth and economic performance of Nile tilapia (Oreochromis niloticus, L.) fed on three brans (maize, wheat and rice) in fertilized ponds. Aquac Nutr. 2006;12:239–245. [Google Scholar]
- 33.Charo-Karisa H., Opiyo M.A., Munguti J., Marijani E., Nzayisenga L. Cost-benefit analysis and growth effects of pelleted and unpelleted farm made feeds on African catfish (Clarias gariepinus, Burchell 1822) in earthen ponds. Afr J Food, Agric Nutr Dev. 2013;13:8019–8030. https://www.ajol.info/index.php/ajfand/article/view/94597 [Google Scholar]
- 34.Munguti J., Charo-Karisa H., Opiyo M., Ogello E., Marijani E., Nzayisenga L. Nutritive value and availability of commonly used feed ingredients for farmed Nile tilapia (Oreochromis niloticus, L.) and African catfish (Clarias gariepinus, Burchell) in Kenya, Rwanda and Tanzania. Afr J Food, Agric Nutr Dev. 2012;12:6135–6155. [Google Scholar]
- 35.Nyonje B.M., Opiyo M.A., Orina P.S., Abwao J., Wainaina M., Charo-Karisa H. Current status of freshwater fish hatcheries, broodstock management and fingerling production in the Kenya aquaculture sector. Livest Res Rural Dev. 2018;30 [Google Scholar]
- 36.Ochieng V., Matolla G., Khyria S. A Study of Clinostomum affecting Oreochromis niloticus in small water bodies in Eldoret-Kenya. Int J Sci Eng Res. 2012;3:1–6. https://www.ijser.org/viewPaperDetail.aspx?M0R0035 [Google Scholar]
- 37.Njagi I. Overcoming Challenges to Export in Kenyan. Aquaculture. 2016 [Google Scholar]
- 38.Walakira J., Akoll P., Engole M., Sserwadda M., Nkambo M., Namulawa V. Common fish diseases and parasites affecting wild and farmed Tilapia and catfish in Central and Western Uganda. Uganda J Agric Sci. 2014;15:113–125. https://www.ajol.info/index.php/ujas/article/view/126198 [Google Scholar]
- 39.Florio D., Gustinelli A., Caffara M., Turci F., Quaglio F., Konecny R. Veterinary and public health aspects in tilapia (Oreochromis niloticus) aquaculture in Kenya. Uganda and Ethiopia. Ittiopatologia. 2009;6:51–93. https://www.researchgate.net/publication/262731822_Veterinary_and_public_health_ [Google Scholar]
- 40.Parasites Paperna I. infections and diseases of fishes in Africa: an update (No. 31). Food and Agriculture Organization of the United Nations (FAO) Rome: FAO Fisher Dept. 1996:26–28. [Google Scholar]
- 41.Magondu E.W., Rasowo J., Oyoo-Okoth E., Charo-Karisa H. Evaluation of sodium chloride (NaCl) for potential prophylactic treatment and its short-term toxicity to African catfish Clarias gariepinus (Burchell, 1822) yolk-sac and swim-up fry. Aquaculture. 2011;319:307–310. [Google Scholar]
- 42.Rasowo J., Okoth O.E., Ngugi C.C. Effects of formaldehyde, sodium chloride, potassium permanganate and hydrogen peroxide on hatch rate of African catfish Clarias gariepinus eggs. Aquaculture. 2007;269:271–277. [Google Scholar]
- 43.Done H.Y., Venkatesan A.K., Halden R.U. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J. 2015;17:513–524. doi: 10.1208/s12248-015-9722-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subasinghe R.P., Barg U., Tacon A. Use Chem. Aquac. Asia, Loilo, Philippines: SEAFDEC Aquaculture Department; 1996. Chemicals in Asian aquaculture: need, usage, issues and challenges; pp. 1–6. [Google Scholar]
- 45.Cabello F.C., Godfrey H.P., Buschmann A.H., Dölz H.J. Aquaculture as yet another environmental gateway to the development and globalization of antimicrobial resistance. Lancet Infect Dis. 2016;16:e127–e133. doi: 10.1016/S1473-3099(16)00100-6. [DOI] [PubMed] [Google Scholar]
- 46.Henriksson P.J.G., Rico A., Troell M., Klinger D.H., Buschmann A.H., Saksida S. Unpacking factors influencing antimicrobial use in global aquaculture and their implication for management: a review from a systems perspective. Sustain Sci. 2018;13:1105–1120. doi: 10.1007/s11625-017-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hickley P., Muchiri M., Britton R., Boar R. Economic gain versus ecological damage from the introduction of non-native freshwater fish: case studies from Kenya. Open Fish Sci J. 2008;1:36–46. [Google Scholar]
- 48.Mugimba K.K., Chengula A.A., Wamala S., Mwega E.D., Kasanga C.J., Byarugaba D.K. Detection of tilapia lake virus (TiLV) infection by PCR in farmed and wild Nile tilapia (Oreochromis niloticus) from Lake Victoria. J Fish Dis. 2018:1–9. doi: 10.1111/jfd.12790. [DOI] [PubMed] [Google Scholar]
- 49.Bacharach E., Mishra N., Briese T., Zody M.C., Kembou Tsofack J.E., Zamostiano R. Characterization of a Novel Orthomyxo-like Virus Causing Mass Die-Offs of Tilapia. MBio. 2016;7:e00431–e516. doi: 10.1128/mBio.00431-16. 10.1128/mBio. 00431-1610.1128/mBio. 00431-16. [DOI] [PMC free article] [PubMed] [Google Scholar]