Abstract
Coccidiosis is one of the most economically important diseases of poultry. This study determined the preponderance of chicken Eimeria in southern Nigeria and assessed the parasite’s resistance to three anticoccidial drugs: Amprolium hydrochloride; Amprolium hydrochloride + Sulfaquinoxaline-Sodium; and Toltrazuril. Multiplex PCR amplification of the SCAR region was used to confirm Eimeria preponderance. Resistance was assessed following the inoculation of 2.32 × 105 infective oocysts into broilers. Data on weight gain, feed intake, feed conversion and fecal oocyst shed were recorded. At 7 days post inoculation 9 birds per treatment were sacrificed and assessed for macroscopic lesions in four intestinal regions. Percent optimum anticoccidial activity (POAA), Anticoccidial index (ACI) and Anticoccidial sensitivity test (AST) were used to access resistance. The preponderance of Eimeria spp. were E. tenella (77%), E. necatrix (55%), E. acervulina (44%) and E. mitis (11%), with multi-species infection occurring in 55% of samples assessed. Fecal oocyst shedding was low (P < 0.05) in the medicated groups. Lesions in the cecal region were present in all infected groups regardless of treatment and accounted for 27.8% of lesion scores by severity and 37.5% of lesion scores by frequency. Overall, lesion scores were less (P < 0.05) in birds of the medicated groups compared with the infected-unmedicated group. The high preponderance of E. tenella in the field, and the occurrence of cecal lesions – caused mainly by E. tenella- despite drug administration, indicate resistance in populations of this species in our isolate. Based-on the POAA, ACI and AST values, the Eimeria isolate showed reduced sensitivity to toltrazuril.
Keywords: Amprolium, Cecal Coccidiosis, Drug resistance, Preponderance, Sulphaquinoxaline, Toltrazuril
1. Introduction
Poultry coccidiosis caused by the Ampicomplexan parasite Eimeria, is one of the world’s most economically important diseases of poultry birds. Eimeria primarily targets the tissues of the intestinal epithelium which consequently results in a decline in growth and feed utilization in poultry[1], [2]. The disease is therefore associated with high production losses, high morbidity and mortality rates of above 50% [3], [4]. In broilers, mild or subclinical infections are also important as even minor intestinal lesions can significantly impede feed efficiency and profitability [5].
The control of poultry coccidiosis can often be achieved by a combination of several strategies including; the use of anticoccidial drugs, delivery of live Eimeria vaccines, good husbandry practice and optimum biosecurity standards [6], [7]. Inclusion of anticoccidial drugs in the diet of poultry birds is unarguably the most widespread means of controlling poultry coccidiosis [8], [9]. Usage rates upward of 70% have previously been reported in poultry farms across the USA and Europe [10], and up to 100% in Nigeria [11], [12]. This is particularly so because modern poultry systems are characterized by high stocking density that encourages parasite accumulation and transmission [13] and anticoccidial drugs are a convenient, and affordable method for keeping parasite challenge at a minimum [14]. In Nigeria, anticoccidial drug use is very popular, particularly because the majority (over 60%) of our poultry farms are characterized by poor hygiene conditions and low biosecurity levels [15]. These drugs seem to be indispensable for the sustainability of poultry production systems.
Unfortunately, as with the problems encountered with the widespread use of antimicrobials in veterinary and public health, the extensive use of chemotherapeutic drugs unavoidably leads to the development of drug resistance among Eimeria species [16]. Heavy drug dependence could have profound impact on the biology of Eimeria species in Nigeria with regards to the development of drug resistance, which can limit the effectiveness of such products [17].
Following personal conversations with some local veterinarians in Rivers state, it was established that the anticoccidials Toltrazuril, Amprolium hydrochloride and Amprolium hydrochloride + Sulphaquinoxaline Sodium, are the drugs of choice for the control of chicken coccidiosis in the state. The presence of resistant Eimeria spp. in Rivers state are a threat to local poultry production and could compromise local control efforts. This study was therefore embarked upon to assess drug resistance of Eimeria spp. to these three drugs using preponderance studies and drug sensitivity trials.
2. Materials and methods
2.1. Ethical approval
The sampling protocol and the use of animals for experimental studies applied here were approved by the University of Port Harcourt Research Ethics Review Committee with ethics approval number UPH/CEREMAD/REC/04.
2.2. Field study
2.2.1. Sampling location
Port Harcourt is the capital of Rivers State and the third largest city in southern Nigeria (Fig. 1). It is a diverse city located in the Niger Delta region (within latitude 4049′27″N 702′1″E) and is economically significant as the centre of Nigeria’s oil industry. Port Harcourt metropolis is home to two local government areas (LGA): Port Harcourt LGA and Obio-akpor LGA. Nine major Live Bird Markets (LBMs) in Port Harcourt city were selected purposively based on the fact that being a densely populated city, live poultry trade traffic would very likely flow into the city from different parts of the state including the rural, urban as well as suburban areas.
Fig. 1.
Map of Nigeria: The study area is in Rivers state (highlighted in green) in the southern region of Nigeria. The sampling locations cover Port Harcourt and Obio/Akpor local government areas (The Cartography Unit, University of Port Harcourt).
2.2.2. Sample collection and processing
From September to November 2017, field studies were conducted and fresh fecal droppings were randomly collected from across cages where live chickens were housed. Samples from each market were pooled into a bulk sample. Each bulk sample was homogenized (in a blender or by stirring with a rod) and filtered using a 106 µm mesh sieve. Eimeria oocysts were harvested using the saturated NaCl floatation method (10 min at 1000×g). The harvested oocysts were re-suspended in distilled water and washed by centrifugation three times to remove the flotation solution (500×g for 5 min). The sediment containing the oocysts was transferred into beakers, suspended in 2.5% (w/v) K2Cr2O7 solution and allowed to sporulate at room temperature for seven days with regular stirring. After sporulation, oocysts from each sample were cleaned with sodium hypochlorite (4% active chlorine) and washed with distilled water three times as described before [18].
2.3. Molecular characterization of chicken Eimeria
2.3.1. Genomic DNA extraction
Total genomic DNA extraction from oocysts of Eimeria species was done as follows: 20 mL of each oocyst suspension, were centrifuged at 750g for 10 min to pellet the oocysts. Each pellet was re-suspended in the minimum volume residual supernatant and transferred to a 2 mL screw top plastic tube containing glass beads (0.1–0.5 mm; ZR BashingBeadTM, SA) and covered with sterile phosphate buffered saline (PBS; pH 8.0). The pelleted oocysts were then disrupted using a Mini Beadbeater-8, (Biospec Products Bartlesville, USA) for three minutes. Total genomic DNA (gDNA) was isolated from the smashed oocysts homogenate using a Quick-DNATM extraction kit (ZYMO RESEARCH) following the manufacturers protocol. Briefly, 1200 µL of Genomic Lysis Buffer were added to the filtrate and 800µL of the filtrate plus Lysis Buffer suspension transferred to a Zymo-SpinTM IIC column in a collection tube and centrifuged at 10,000×g for 60 s. Then 500 µL of genomic DNA Wash Buffer were added to the previous step and centrifuged at 10,000×g for 60 s. The suspension was then transferred to a 1.5 mL centrifuge tube and 100µL of DNA Elution Buffer added directly to the column matrix and centrifuged at 10,000×g for 30 s to elute the DNA. Finally, the eluted DNA was transferred into a prepared Zymo-SpinTM IV-HRC Spin Filter placed in a 1.5 mL micro-centrifuge tube and centrifuged at 8000×g for 60 s. The amount of DNA extracted from each sample (Table 1) was quantified in Nano grams/µL using a NanoDrop nucleic acid quantification UV spectrophotometer (Thermo fisher Scientific).
Table 1.
Nucleic acid content in each sample following DNA extraction.
| Sample ID | ng/µL |
|---|---|
| Oil Mill | 28.94 |
| Choba | 24.95 |
| Slaughter | 39.15 |
| Mile 1 | 27.85 |
| Rumuokuta | 28.53 |
| Fruit garden | 66.81 |
| Creek-road | 263.33 |
| Rumuokoro | 23.83 |
| Rumuomasi | 36.58 |
Nucleic acid was quantified using the Nano drop nucleic acid spectrophotometer as explained in the text. Nucleic acid concentration is quantified here in ng/µL.
2.3.2. Multiplex PCR amplification
The Eimeria species was verified by a multiplex PCR assay as earlier described by Fernandez et al., [19]. Six pairs of species-specific primers were used (Table 2). The PCR amplification was based upon a 25 µL volume consisting of 1 µL genomic DNA template, 0.5 µL of each primer, 12.5 µL Taq DNA polymerase (Sigma, USA) and made up to 25 µL with nuclease free water. The standardized cycling conditions consisted of initial denaturation: 96 °C for 5 min followed by 30 cycles of 95 °C denaturation for 1 min, 59 °C annealing for 2 min; 72 °C extension for 1 min, and a final extension: 72 °C for 7 min.
Table 2.
The primers used in multiplex PCR amplification.
| S/N | Species | Primer sequences 5′–3′ | Expected amplicon size (bp) | |
|---|---|---|---|---|
| 1 | E. acervulina | F | AGTCAGCCACACAATAATGGCAAACATG | 811 |
| R | AGTCAGCCACAGCGAAAGACGTATGTG | |||
| 2 | E. tenella | F | CCGCCCAAACCAGGTGTCACG | 539 |
| R | CCGCCCAAACATGCAAGATGGC | |||
| 3 | E. mitis | F | AGTCAGCCACCAGTAGAGCCAATATTT | 460 |
| R | AGTCAGCCACAAACAAATTCAAACTCTAC | |||
| 4 | E. maxima | F | GGGTAACGCCAACTGCCGGGTATG | 272 |
| R | AGCAAACCGTAAAGGCCGAAGTCCTAGA | |||
| 5 | E. necatrix | F | TTCATTTCGCTTAACAATATTTGGCCTCA | 200 |
| R | ACAACGCCTCATAACCCCAAGAAATTTTG | |||
| 6 | E. brunetti | F | TGGTCGCAGAACCTACAGGGCTGT | 626 |
| R | TGGTCGCAGACGTATATTAGGGGTCTG |
(Adapted from Fernandez et al. [19]).
F: forward primer; R: reverse primer; bp: base pairs.
2.3.3. Agarose gel electrophoresis
Agarose gel was prepared as follows: 1.5% (w/v) Agarose was prepared with 100 mL of distilled water and TBE buffer. The mixture was microwaved for 3 min. The Agarose solution was allowed to cool and 1µL of ethidium bromide added per 100 mL of agarose solution. The solution was loaded onto a casting tray with well position casting combs and refrigerated for 20 min to solidify.
The solidified Agarose gel was placed into the gel electrophoresis unit. The unit was filled with TBE until the gel was completely submerged. DNA samples extracted previously were well loaded with loading dye. A molecular ladder of 1000 base pairs was loaded into lane 4 and the other samples loaded into additional wells. Gel was run at 120 V for 25 min. DNA fragments (Bands) were visualized under UV light and images captured using a digital camera.
2.4. Drug sensitivity of chicken Eimeria species in Rivers state.
2.4.1. Isolate selection, propagation and enumeration
Eimeria oocysts isolated from Slaughter market were selected from amongst the others for propagation and drug sensitivity trials. Based on the results of our field survey, Slaughter market is the second largest live bird market in Port Harcourt in terms of the number of live poultry traders present and the sources of live birds. It was therefore expected that Eimeria oocysts from this market would be more representative of oocysts from across the state.
The isolate selected was inoculated into five 2-3-week - old susceptible chickens to provide sufficient oocysts for drug sensitivity trials. Fecal droppings were collected between days 5 and 7 post inoculation and processed for total Eimeria oocysts using standard procedures [20]. The concentration of oocysts and total number of oocysts collected were estimated using a Neubauer counting chamber as described previously [21]. On day eight post inoculation, birds were killed by decapitation. The intestines were removed and oocysts harvested as previously described [21]. The recovered oocysts were washed three times with distilled water, suspended in K2Cr2O7 (2.5% w/v) and allowed to sporulate at room temperature with regular stirring. The total number of sporulated oocysts were enumerated using a Neubauer counting chamber as described by Holdsworth et al. [21].
2.4.2. Anticoccidial drugs
Three kinds of anticoccidial drugs were studied. Toltrazuril, Amprolium and Sulphaquinoxaline Sodium. Toltrazuril is a broad spectrum anticoccidial used in planned treatment programmes of poultry by inclusion in their drinking water [14]. Amprolium affects co-factor uptake and synthesis and is particularly effective against E. acervulina, E. necatrix and E. tenella [22]. Sulphaqunoxaline like other sulphonamides is a broad spectrum anticoccidial drug with a broad spectrum activity and the longest history of use in the preventative and curative management of Coccidiosis in poultry [22].
These drugs were administered according to the recommended dosage and usage as follows: 2.5% Toltrazuril (Bio-Pharmachemie, Vietnam) (Tolt): 1 mL per L of water for 2 days; 250 mg Amprolium hydrochloride (DSL Pharma, Nigeria) (Amp): 0.5 g per 2.5 L of water for 7 days; and 165 mg Amprolium hydrochloride + 185 mg Sulfaquinoxaline sodium (Bremer Pharma, GMBH Germany) (Amp + Sul): 1 g per L of water for 5 days. The anticoccidial drugs above were used in the state according to the local veterinarians.
2.4.3. Experimental design
The study performed involved 135 one-day-old MARSHAL broiler chicks purchased from a local breeding center (Obasanjo farms, Otta Nigeria). Chicks were housed under coccidian free condition at a simulated animal biosafety level 2 containment area in the Department of Animal and Environmental Biology facility, University of Port Harcourt. Birds were offered tap water and a basal diet ad libitum throughout the trial. The temperature within each cage was maintained at 30 to 35 °C by electric heating using 100 W bulbs. At 12 days of age the chicks were randomly assigned to five groups of 27 chicks. Each group comprised three replicates of nine birds. The various coccidiostats mentioned above were administered to the medicated groups (Amp, Amp + Sul and Tolt) from day 12 and continued up to day 19 under the recommended usage stated above. At 14 days of age, all birds except those in the non-infected-unmedicated groups (NegCntr) were orally infected with 2.32 × 105 sporulated oocysts of the Eimeria spp. isolate. All birds were weighed individually on the day they were inoculated, and 7 days post inoculation. Data regarding weight gain, feed intake, feed conversion and oocysts shed per gram of faeces were recorded. On the seventh day post inoculation, three chickens were randomly selected from each group, euthanized, necropsied and lesion scores recorded for the four regions of the intestine as described by Johnson and Reid, [23].
2.4.4. Evaluation of resistance
Drug resistance of Eimeria was evaluated using three indexes:
-
1.
The Anticoccidial Index (ACI). ACI = (rate of relative body weight gain + survival rate) – (lesion score + oocyst value). An ACI value of ≥ 160 indicated sensitivity; a value < 160 indicated resistance [24]. Oocyst value was calculated as follows: (OPG output of each group/OPG output of PosCntr group) × 100.
-
2.
The Anticoccidial Sensitivity Test (AST) [25]. AST = (average lesion score in infected-unmedicated group – average lesion score in medicated group)/average lesion score in infected-unmedicated group × 100%. AST ≥ 50% was judged to be sensitive and <50% was resistance; and
-
3.
Percent Optimum Anticoccidial Activity (POAA). POAA = (GSR in medicated group – GSR in infected-unmedicated group)/(GSR in uninfected-unmedicated group – GSR in infected-unmedicated group) × 100%. GSR (Growth and Survival Ratio) was defined as final body weight divided by initial body weight. POAA > 50% was judged to be sensitive and ≤50% was resistance [5].
The overall assessment of drug resistance of our LBM Eimeria isolate was adapted from Lan et al., [26]. Briefly, if 3 of 3 indexes showed resistance, our isolate was considered as severely drug resistant (+++). Two of 3 meant moderate drug resistance (++), 1 of 3 meant slight drug resistance (+) and none meant no drug resistance.
2.5. Statistical analyses
Data were analyzed by the software R 3.1.0 using a Windows XP system. The body weights, feed intake, lesion scores and oocysts shed per gram of feces were analyzed by one-way ANOVA and a post hoc analysis using Tukey’s multiple comparison test to identify statistically significant variations. The difference was considered significant if P < 0.05. Box plots and bar charts were generated using R 3.1.0.
3. Results
3.1. Preponderance of chicken Eimeria spp. in Rivers state
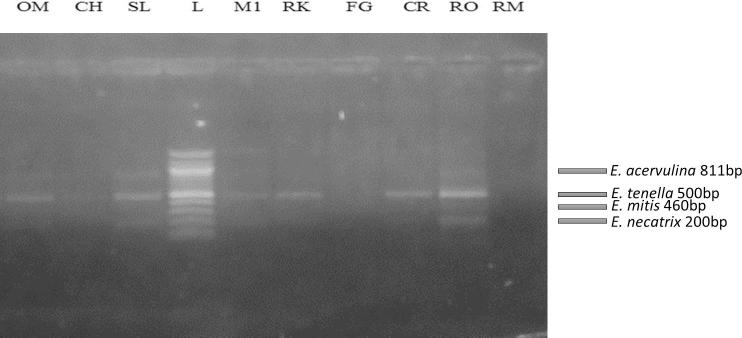
The results of the molecular identification showed that E. tenella, E. necatrix, E. acervulina and E. mitis are the predominant Eimeria species in Rivers state. E. tenella was present at 7 of 9 locations (77%), E. necatrix at 5 of 9 (55%), E. acervulina at 4 of 9 (44%) and E mitis at 1 of 9 (11%). The primers used allowed for the discrimination of all four Eimeria species present in the samples. The different sizes of DNA fragments amplified were displayed on Agarose gel as follows: E. acervulina (811 bp), E. tenella (539 bp) E. mitis (310 bp) and E. necatrix (200 bp) (Fig. 2). Multi - Eimeria species infections were found in 5 of 9 (55%) locations with Eimeria tenella being the dominant species.
Fig. 2.
Agarose gel electrophoresis showing the amplified SCAR gene of the Eimeria spp. Lane OM: E. acervulina (811 bp), E. tenella (500 bp); Lane CH: E. acervulina (811 bp), E tenella (500 bp); Lane SL: E. acervulina (811 bp), E. tenella (500 bp), E. necatrix (200 bp); Lane M1: E. tenella (500 bp), E. necatrix (200 bp); Lane RK: E. tenella (500 bp), E. necatrix (200 bp); Lane FG: No band; Lane CR: E. tenella (500 bp), E. necatrix (200 bp); Lane RO: E acervulina (811 bp), E tenella (500 bp), E. mitis (460), E. necatrix (200 bp); Lane RM: No band; Lane L: 1000 bp molecular ladder. Abbreviations: OM: Oil Mill; CH: Choba; SL: Slaughter; M1, Mile 1: RK, Rumuokuta; FG, Fruit garden; CR, Creek road; RO, Rumuokoro; RM, Rumuomasi; bp, base pairs.
3.2. Drug sensitivity of chicken Eimeria spp. isolate in Rivers state.
3.2.1. Performance results: Body weight gain (BWG), feed intake and feed conversion ratio (FCR)
In all treatments, body weight gain (BWG) of the medicated groups and the uninfected-unmedicated groups (NegCntr) were significantly higher than the infected-unmedicated group (PosCntr) (Table 3). In Amprolium hydrochloride and Amprolium hydrochloride + Sulfaquinoxaline sodium treated groups, BWG, was better than PosCntr group. Toltrazuril did not result in a significant increase in BWG. Feed consumption was highest in the NegCntr group and lowest in the PosCntr group (Table 4). The highest weight gain and the lowest feed conversion ratio was recorded in the NegCntr group (Table 5).
Table 3.
Average body weight gain (g) of experimental birds between 14 days old and 21 days old.
| Treatment | Replicate 1 | Replicate 2 | Replicate 3 |
|---|---|---|---|
| Amp | 161.08 ± 57.98* | 163.06 ± 21.78* | 157.23 ± 36.36* |
| Amp + Sul | 176.22 ± 42.31* | 165.27 ± 18.78* | 173.34 ± 50.24* |
| Tolt | 149.34 ± 35.11 ns | 167.94 ± 55.75 ns | 142.51 ± 50.51 ns |
| NegCntr | 185.56 ± 9.75* | 182.43 ± 10.43* | 183.00 ± 17.85* |
| PosCntr | 112.27 ± 39.95 | 140.41 ± 21.35 | 131.02 ± 45.10 |
Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control. Data represent mean ± SD (∗: P < 0.05 vs. PosCntr; ns: no significant differences).
Table 4.
Total feed consumed within the infection period.
| Treatment | Feed consumed (g) |
|---|---|
| Amp | 4090.59 ± 86.20 |
| Amp + Sul | 4227.52 ± 135.48 |
| Tolt | 4036.94 ± 76.39 |
| NegCntr | 4389.27 ± 75.06 |
| PosCntr | 3561.93 ± 136.38 |
Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control. Data represent mean ± SD.
Table 5.
Feed Conversion ratio of birds between 14 days old and 21 days old.
| Treatment | Replicate 1 | Replicate 2 | Replicate 3 |
|---|---|---|---|
| Amp | 2.76 | 2.78 | 2.95 |
| Amp + Sul | 2.75 | 2.75 | 2.71 |
| Tolt | 3.01 | 2.72 | 3.08 |
| NegCntr | 2.66 | 2.69 | 2.61 |
| PosCntr | 3.40 | 2.80 | 3.14 |
Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control.
3.2.2. Lesion scores
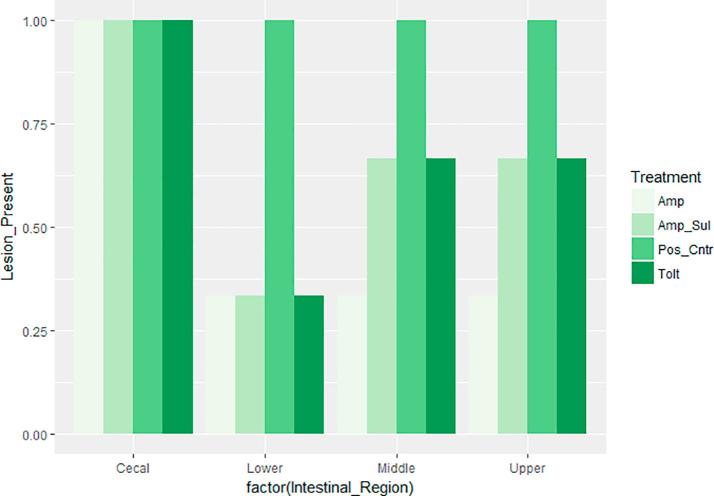
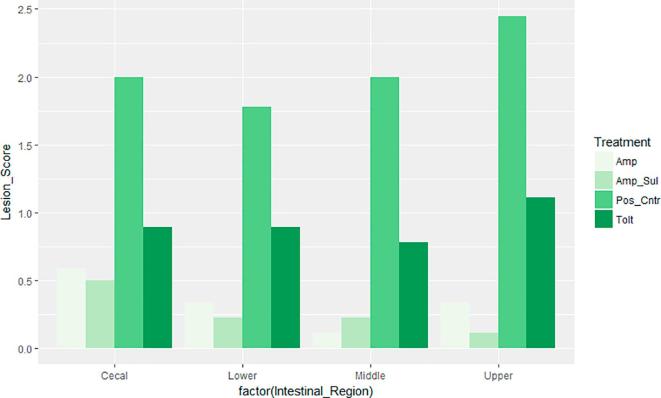
Intestinal macroscopic lesions were completely absent in birds of the NegCntr group, and present in the PosCntr group. Fig. 3, Fig. 4 show the occurrence and specific mean lesion scores for the four regions of the intestine assessed respectively. Lesions in the cecal region were present in all medicated groups as well as the PosCntr group. However, lesion scores of birds in the PosCntr group were markedly higher. The three anticoccidial drugs were effective in reducing the occurrence of lesions to varying degrees in the lower, middle and upper intestinal regions but not in the cecal region. A combined assessment of the average intestinal lesion scores of birds in our drug sensitivity trial showed that, Amp + Sul group had the least lesion score i.e. most effective, and Tolt group had the highest scores, i.e. least effective. Overall, intestinal lesion scores were significantly less (P < 0.05) in birds treated with the anticoccidial drugs (Table 6.) compared with birds in PosCntr group.
Fig. 3.
Occurrence of lesions in the four regions of the intestine. Keys: Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; PosCntr: infected-unmedicated positive control.
Fig. 4.
Mean lesion scores in the four regions of the intestine. Keys: Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; PosCntr: infected-unmedicated positive control.
Table 6.
Overall lesion score of birds infected with a mixed Eimeria spp. isolate assessed by taking an average of the individual scores for the four intestinal regions.
| Treatment | Lesion score |
|---|---|
| Amp | 0.333 ± 0.36* |
| Amp + Sul | 0.305 ± 0.21* |
| Tolt | 0.944 ± 1.28* |
| NegCntr | 0 |
| PosCntr | 2.083 ± 0.25 |
Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control. Data represent mean ± SD (∗: P < 0.05 vs. PosCntr).
3.2.3. Fecal oocyst shedding
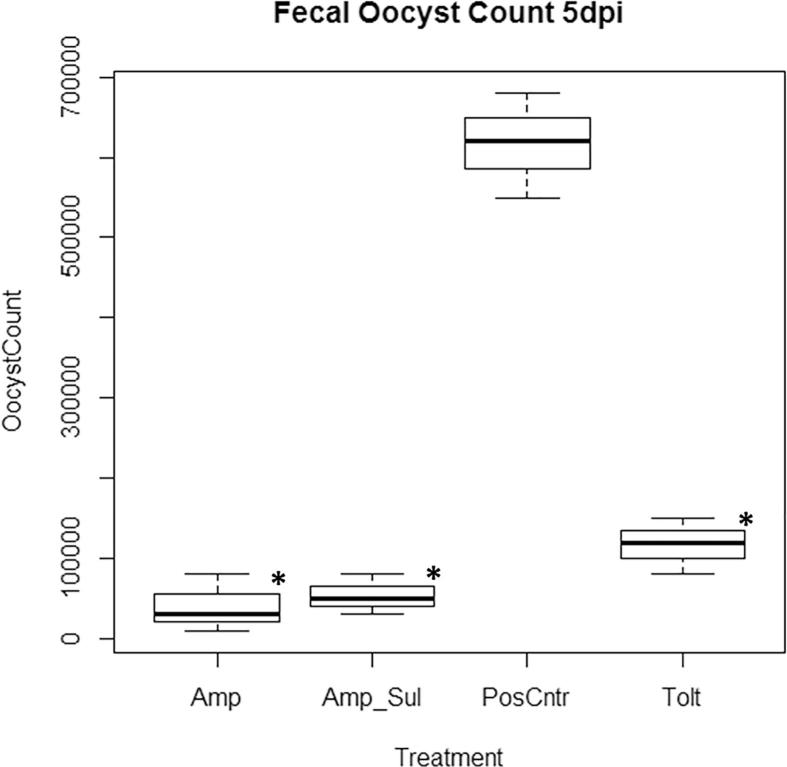
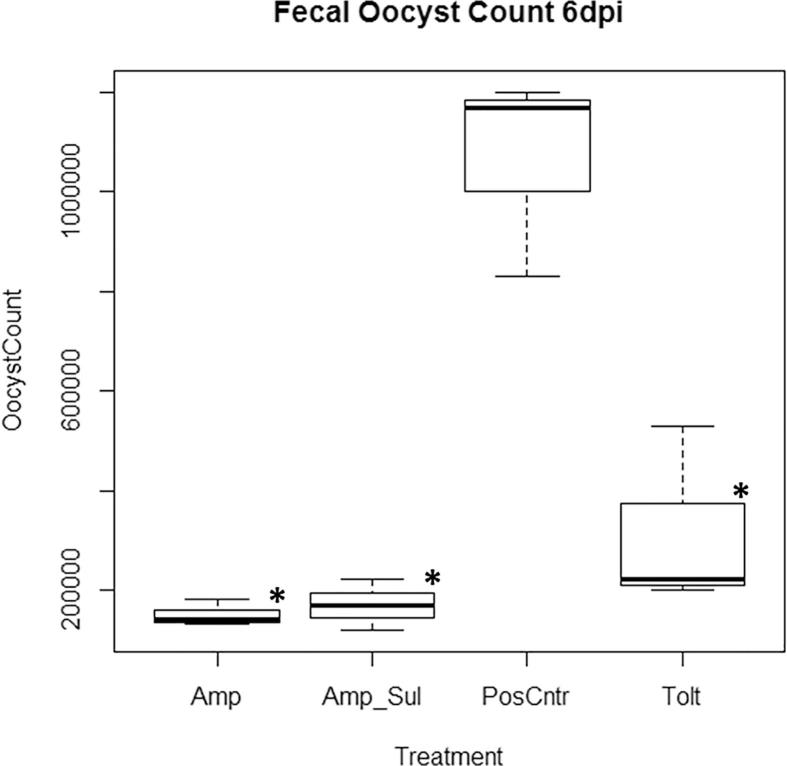
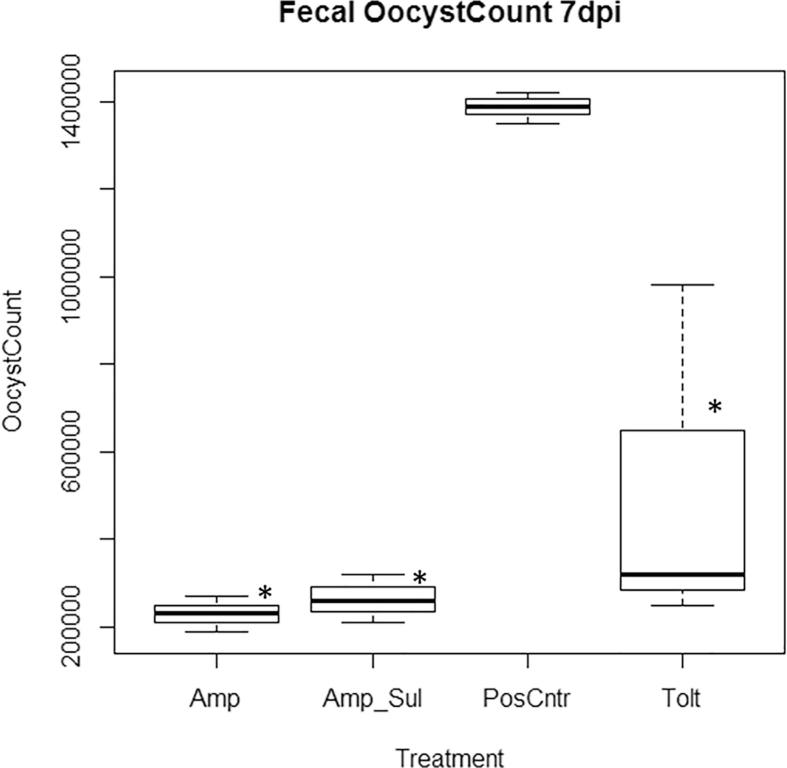
Oocysts shed in the feces of birds from 5 days post infection (dpi), 6 dpi and 7 dpi in all treatment groups is shown in Fig. 5, Fig. 6, Fig. 7 respectively. Fecal oocysts shedding increased with the duration of infection with the least oocysts count on day 5 and the highest oocyst count on day 7. Among the medicated groups, Amp group recorded the least number of fecal oocysts excreted with 1.46 × 105 ± 0.24 oocysts shed per gram of feces. Tolt group had the highest with 3.17 × 105 ± 1.98 oocysts shed per gram of feces. Overall, fecal oocyst counts in all three medicated groups were significantly less (P < 0.05) than that in the PosCntr group (Table 7).
Fig. 5.
Oocysts shed per gram of feces 5 days post inoculation. Keys: Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; PosCntr: infected-unmedicated positive control. ∗: P < 0.05 vs. PosCntr.
Fig. 6.
Oocysts shed per gram of feces 6 days post inoculation. Keys: Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; PosCntr: infected-unmedicated positive control. ∗: P < 0.05 vs. PosCntr.
Fig. 7.
Oocysts shed per gram of feces 7 days post inoculation. Keys: Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; PosCntr: infected-unmedicated positive control. ∗: P < 0.05 vs. PosCntr.
Table 7.
Overall oocyst shed per gram (OPG) of feces assessed by taking an average of the oocyst shed from day 5 to day 7 post inoculation.
| Treatment | OPG feces (105) |
|---|---|
| Amp | 1.40 ± 0.24* |
| Amp + Sul | 1.62 ± 0.39* |
| Tolt | 3.17 ± 1.98* |
| NegCntr | – |
| PosCntr | 10.23 ± 0.92 |
Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control. Data represent mean ± SD (∗: P < 0.05 vs. PosCntr).
3.2.4. Anticoccidial index (ACI), anticoccidial sensitivity test (AST) and percent optimum anticoccidial activity (POAA).
The results of ACI, AST and POAA are summarized in Table 8, Table 9. No bird mortality was recorded throughout the trial. The ACI values of Amp and Amp + Sul groups (>160) indicated that these drugs were able to treat the chickens infected with our isolate while maintaining bird productivity. Resistance to toltrazuril was expressed as the value of 150.99. Both POAA and AST values (>50%) showed that our isolate is sensitive to all three coccidiostats tested, however, sensitivity to toltrazuril was much less. In the present study, an overall drug sensitivity assessment based on a combined analysis of the three indexes revealed slight resistance of Eimeria isolate to toltrazuril (Table 10).
Table 8.
Results for Anticoccidial Sensitivity Test and Anticoccidial Index of each drug.
| Treatment | Survival rate% | BWG rate % |
Oocyst value | AST | ACI |
|---|---|---|---|---|---|
| Amp | 100 | 87.36 | 13.68 | 84.01 | 173.35 |
| Amp + Sul | 100 | 93.44 | 15.85 | 85.36 | 177.29 |
| Tolt | 100 | 82.88 | 30.95 | 54.68 | 150.99 |
| NegCntr | 100 | 100 | 0 | – | 200 |
| PosCntr | 100 | 69.64 | 100 | – | 67.56 |
AST: Anticoccidial sensitivity test = (average lesion score in infected-unmedicated group – average lesion score in medicated group)/average lesion score in infected-unmedicated group × 100%. AST ≥ 50% was judged to be sensitive and < 50% was resistance; ACI: Anticoccidial index = (rate of relative body weight gain + survival rate) – (lesion score + oocyst value). An ACI value of ≥160 indicated sensitivity; a value < 160 indicated resistance, Oocyst value = (OPG output of each group/OPG output of PosCntr group) × 100; Tolt: toltrazuril; Amp: amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control.
Table 9.
Result for Percentage Optimum Anticoccidial Activity (POAA).
| Treatment | Initial Body Weight (g) | Final Body Weight (g) | GSR | POAA % |
|---|---|---|---|---|
| Amp | 213.74 ± 2.44 | 374.20 ± 0.66 | 1.75 | 86.58 |
| Amp + Sul | 227.25 ± 1.59 | 398.86 ± 4.10 | 1.76 | 88.96 |
| Tolt | 217.55 ± 14.52 | 369.77 ± 21.97 | 1.70 | 59.49 |
| NegCntr | 236.69 ± 4.53 | 420.35 ± 3.40 | 1.78 | 100.00 |
| PosCntr | 217.64 ± 7.15 | 345.54 ± 7.85 | 1.59 | 0.00 |
POAA: Percent Optimum Anticoccidial Activity = (GSR in medicated group – GSR in infected-unmedicated group)/(GSR in uninfected-unmedicated group – GSR in infected-unmedicated group) × 100%, GSR (growth and survival ratio) = final body weight divided by initial body weight. POAA > 50% was judged to be sensitive and ≤50% was resistance; Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; NegCntr: uninfected-unmedicated negative control; PosCntr: infected-unmedicated positive control.
Table 10.
Overall assessment of the sensitivity of Eimeria LBM isolate.
| Treatment | ACI | AST | POAA | Resistance |
|---|---|---|---|---|
| Amp | – | – | – | None |
| Amp + Sul | – | – | – | None |
| Tolt | + | – | – | Slight |
(+): resistance expressed within experimental period; (–): no resistance expressed within experimental period; Tolt, toltrazuril; Amp, amprolium hydrochloride; Amp + Sul: Amprolium hydrochloride + sulfaquinoxaline sodium; ACI: Anticoccidial Index; AST: Anticoccidial Sensitivity Test; POAA: Percentage Anticoccidial Activity.
4. Discussion
Eimeria spp. isolates were identified by the multiplex PCR amplification of the SCAR rDNA region using species-specific primers [19]. The preponderance of chicken Eimeria species recorded in Rivers state were E. tenella: 77%, E. necatrix: 55%, E. acervulina: 44% and E. mitis 11%. Our results are in agreement with a related study involving the molecular characterization of chicken Eimeria in northern Nigeria where preponderance rates of 75% for E. tenella, 25% E. necatrix, 33% E acervulina and 50% E. mitis were reported [27]. Our study further revealed that E. burnetti and E. maxima were absent from our samples. This result is also in agreement with the study by Jatau et al., [27] where these species were also absent. The absence of E. maxima and E burnetti in these two regions is an indication that these species may not play significant roles in the epidemiology of chicken coccidiosis in Nigeria.
Analysis of our results showed that E. tenella was present at every positive sample location and had the highest prevalence (77.8%). The same observation was reported by Jatau et al. [27] in northern Nigeria. High preponderance rates such as these are often an indication of drug resistance in this species [10]. E. tenella therefore remains highly invasive amongst others and is possibly the most economically important Eimeria species causing chicken coccidiosis in southern Nigeria. Similar results have been obtained in Ethiopia [28], India [29] and China [9], [26], [30] where E. tenella was reported as the most prevalent.
The co-occurrence of multiple Eimeria species in a given location was also evident in this study. Our finding of the high prevalence of mixed Eimeria species infections (55%) is in agreement with related results of 68% and 67% found in northern Nigeria by Jatau et al., [31] and Jatau et al., [27] respectively.
E. tenella and E. necatrix are considered as highly pathogenic species of Eimeria [32], [33]. While the presence of Eimeria oocysts in chicken feces is not a definitive diagnosis of chicken coccidiosis [28], the circulation of such pathogenic species can contribute to high morbidity and mortality in young chickens particularly when conditions of poor nutrition, poor sanitation and hygiene and co-infection with other pathogens persist [34]. Pathogenic species such as these could compromise poultry productivity in the majority of Nigerian chicken farms, particularly among the medium to small scale farms that are often characterized by poor sanitation and minimal-to-no biosecurity [15]. Based on our result, the species with the least preponderance is E. mitis. E. mitis is not often associated with severe disease in chickens, however, it can result in the reduction of feed efficiency in chickens [32].
Given the high prevalence of poultry coccidiosis reported in Nigeria, Nigerian poultry farms rely heavily on the use of anticoccidials for the control of coccidiosis [11]. It has been established that such practice as the extensive use of anticoccidial drugs could greatly influence the development of anticoccidial resistance in poultry Eimeria [35], [36].
In this study, Eimeria oocysts were isolated from chicken fecal samples collected at a live bird market in Port Harcourt. By targeting all Eimeria species from a live bid market instead of a single poultry farm, our parasite detection rates should be higher [37] and our anticoccidial profile should be more representative of what is happening in the average chicken population in the field [35]. This is particularly so because live birds for sale at the market are often sourced from multiple farms [38], making such places important pathogen accumulation and distribution points [39].
The Eimeria oocysts were isolated, propagated, characterized and assessed for sensitivity to three anticoccidial drugs – Amprolium hydrochloride, Amprolium hydrochloride + Sulfaquinoxaline sodium and Toltrazuril. The sensitivity of these drugs was accessed on a prophylactic basis as experimental birds were inoculated with the Eimeria spp. isolate two days after drug administration commenced. The isolates were subsequently confirmed to contain E. tenella, E. necatrix, and E. acervulina, as described above. The common assay of including the respective anticoccidial drugs into the diet of infected birds was employed.
The extent to which an anticoccidial drug can result in a marked reduction or in some cases, the complete elimination of intestinal lesions is often explored as an indication of drug sensitivity and/or parasite resistance [5], [9]. Also, presence and characteristic nature of lesions in specific areas of the intestine can be used to identify the species expressing resistant traits in a mixed inoculum of Eimeria [23]. Analysis of our results showed that all three medicated groups resulted in marked reduction in the occurrence of lesions in the upper, middle and lower intestinal regions (Fig. 3). The putative Eimeria species in our isolate that cause lesions in these parts of the intestine are E. acervulina and E. necatrix [40]. This indicates that populations of E. acervulina and E. necatrix in our isolate are sensitive to the three anticoccidial drugs tested. On the contrary, cecal lesions due primarily to infection by E. tenella [40] were present in all birds inoculated with Eimeria spp. isolate despite medication. Cecal lesions accounted for 27.8% of lesion scores by severity and 37.5% of lesion scores by frequency. These results indicated reduced sensitivity of all three anticoccidial drugs to populations of E. tenella in our study. Overall, birds in the medicated groups had significantly less severe lesion scores, which is an indication of a healthier gut and a greater chance of recovery from disease [41].
Fecal oocyst shedding was apparent in all groups except the uninfected-unmedicated group. Our results are in agreement with similar studies conducted in China [26], Egypt [42] and Iran [5] where birds continued to shed large amounts of oocysts regardless of anticoccidial medication. Oocysts shed per gram of feces by birds in all three medicated groups was however, significantly less than those in the infected-unmedicated groups. Our results therefore showed that the transmission of coccidiosis was sustained in the presence of drug pressure though the transmission potential (amount of oocysts shed) is reduced. This is very important in the epidemiology of the disease and should be factored in when considering disease control options.
Birds in all three medicated groups had higher body weight gains than those in the infected-unmedicated group. However the difference was statistically significant in Amprolium hydrochloride and Amprolium hydrochloride + Sulfaquinoxaline treated groups only. Our results further showed that the body weight gains and feed efficiency of birds in the uninfected-unmedicated groups were significantly better than those in the medicated groups. These results are in agreement with similar studies [9], [26] and therefore indicates that regardless of the prophylactic treatment of birds with anticoccidial drugs, coccidiosis can still reduce production efficiency in infected birds [5]. This highlights the economic importance of chicken coccidiosis.
Three anticoccidial efficacy indexes were adopted. An overall assessment of the drug sensitivity of our field isolate using the ACI, AST and the POAA showed sensitivity to Amprolium hydrochloride and Amprolium hydrochloride + Sulfaquinoxaline sodium and slight resistance to Toltrazuril. Our results are markedly different from a related study in China where severe resistance to Amprolium hydrochloride, Toltrazuril and Sulfaquinoxaline sodium was reported [26]. The differences reported in poultry Eimeria drug sensitivity results perhaps express the unique ecological and/ or epidemiological factors that modulate the development of drug resistance between regions.
5. Conclusions
The study aimed to evaluate drug resistance of Eimeria spp. in southern Nigeria using preponderance and drug sensitivity assays. Four species, E. tenella, E. necatrix, E. acervulina and E. mitis were identified with E. tenella being the most dominant species present in 7 of 7 Eimeria positive locations. The higher preponderance of E. tenella was an indication of drug resistance in this species and the drug sensitivity results corroborated this finding as populations of E. tenella causing cecal lesions resulted in varying degrees of pathology in birds despite treatment with anticoccidial drugs. Overall, slight resistance to Toltrazuril was observed within the experimental period.
Acknowledgement
We are grateful to Dr. Ononyelu Ojimelukwe who provided the funds, encouragement and support needed to complete this work. We thank Dr. Isa Jatau for his guidance through the parasite isolation and propagation phase of this work. Many thanks to the Department of Animal and Environmental Biology for supporting this research. Lastly, many thanks to all poultry traders who participated in the study.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Levine N.D. University Park Press; Baltimore: 1982. Taxonomy and life cycles of coccidia. The Biology of the Coccidia. [Google Scholar]
- 2.Reid W.M., Hines T.K., Johnson J., Stino K.R. Coccidiosis: effects of high environmental temperatures on anticoccidial protection. Poult Sci. 1976;55:1436–1441. doi: 10.3382/ps.0551436. [DOI] [PubMed] [Google Scholar]
- 3.Karaer Z., Guven E., Akcay A., Kar S., Nalbantoglu S., Cakmak A. Prevalence of subclinical coccidiosis in broiler farms in Turkey. Trop Anim Health Prod. 2012;44:589–594. doi: 10.1007/s11250-011-9940-z. [DOI] [PubMed] [Google Scholar]
- 4.Györke A., Pop L., Cozma V. Prevalence and distribution of Eimeria species in broiler chicken farms of different capacities. Parasite. 2013;20:50. doi: 10.1051/parasite/2013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arabkhazaeli F., Modrisanei M., Nabian S., Mansoori B., Madani A. Evaluating the resistance of Eimeria spp. field isolates to anticoccidial drugs using three different indices. Iran J Parasitol. 2013;8:234–241. [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman H.D. Practical use of vaccines for the control of coccidiosis in the chicken. Worlds Poult Sci J. 2000;56:7–20. [Google Scholar]
- 7.Shirley M.W., Smith A.L., Tomley F.M. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv Parasitol. 2005;60:285–330. doi: 10.1016/S0065-308X(05)60005-X. [DOI] [PubMed] [Google Scholar]
- 8.Peek H.W., Landman W.J.M. Coccidiosis in poultry: Anticoccidial products, vaccines and other prevention strategies. Vet Q. 2011;31:143–161. doi: 10.1080/01652176.2011.605247. [DOI] [PubMed] [Google Scholar]
- 9.Tan L., Li Y., Yang X., Ke Q., Lei W., Mughal M.N. Genetic diversity and drug sensitivity studies on Eimeria tenella field isolates from Hubei Province of China. Parasit Vectors. 2017;10:137. doi: 10.1186/s13071-017-2067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman H.D., Barta J.R., Blake D., Gruber A., Jenkins M., Smith N.C. A selective review of advances in coccidiosis research. Adv Parasitol. 2013;83:93–171. doi: 10.1016/B978-0-12-407705-8.00002-1. [DOI] [PubMed] [Google Scholar]
- 11.Etuk E.B., Okoli I.C., Uko M.U. Prevalence and management issues associated with poultry coccidiosis in abak agricultural zone of Akwa Ibom State, Nigeria. Int J Poult Sci. 2004;3:135–139. [Google Scholar]
- 12.Kabir J., Umoh V.J., Audu-okoh E., Umoh J.U., Kwaga J.K.P. Veterinary drug use in poultry farms and determination of antimicrobial drug residues in commercial eggs and slaughtered chicken in Kaduna State, Nigeria. Food Control. 2004;15:99–105. [Google Scholar]
- 13.Chapman H.D., Jeffers T.K. Vaccination of chickens against coccidiosis ameliorates drug resistance in commercial poultry production. Int J Parasitol Drugs Drug Resist. 2014;4:214–217. doi: 10.1016/j.ijpddr.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chapman H.D. Biochemical, genetic and applied aspects of drug resistance in Eimeria parasites of the fowl. Avian Pathol. 1997;26:221–244. doi: 10.1080/03079459708419208. [DOI] [PubMed] [Google Scholar]
- 15.The F.A.O. Structure and importance of the commercial and village based poultry industry in. Nigeria. 2008:44–49. [Google Scholar]
- 16.Blake D.P., Tomley F.M. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Permin A., Hansen J.W. FAO Animal Health Manual; Rome: 1998. The Epidemiology, Diagnosis and Control of Poultry Parasites. [Google Scholar]
- 18.Eckert J., Braun R., Shirley M.W., Coudert P. Office for Official Publications of the European Communities; Brussels: 1995. Biotechnology: Guidelines on techniques in coccidiosis research. [Google Scholar]
- 19.Fernandez S., Pagotto A.H., Furtado M.M., Katsuyama Â.M., Madeira A.M.B.N., Gruber A. A multiplex PCR assay for the simultaneous detection and discrimination of the seven Eimeria species that infect domestic fowl. Parasitology. 2003;127:317–325. doi: 10.1017/s0031182003003883. [DOI] [PubMed] [Google Scholar]
- 20.Ryley J.F., Meade R., Hazelhurst J., Robinson T.E. Methods in coccidiosis research: Separation of oocysts from faeces. Parasitology. 1976;73:311–326. doi: 10.1017/s0031182000046990. [DOI] [PubMed] [Google Scholar]
- 21.Holdsworth P.A., Conway D.P., McKenzie M.E., Dayton A.D., Chapman H.D., Mathis G.F. World Association for the Advancement of Veterinary Parasitology (WAAVP) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys. Vet Parasitol. 2004;121:189–212. doi: 10.1016/j.vetpar.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Kant V., Singh P., Verma P.K., Bais I., Parmar M.S., Gopal A. Anticoccidial Drugs Used in the Poultry: An Overview. Sci Int. 2013;1:261–265. [Google Scholar]
- 23.Johnson J., Reid W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp Parasitol. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- 24.Fei C., Fan C., Zhao Q., Lin Y., Wang X., Zheng W. Anticoccidial effects of a novel triazine nitromezuril in broiler chickens. Vet Parasitol. 2013;198:39–44. doi: 10.1016/j.vetpar.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 25.McDougald L.R., Da Silva J.M., Solis J., Braga M. A survey of sensitivity to anticoccidial drugs in 60 isolates of coccidia from broiler chickens in Brazil and Argentina. Avian Dis. 2015;31:287–292. [PubMed] [Google Scholar]
- 26.Lan L., Sun B., Zuo B., Chen X., Du A. Prevalence and drug resistance of avian Eimeria species in broiler chicken farms of Zhejiang province, China. Poult Sci. 2017;96:1–6. doi: 10.3382/ps/pew499. [DOI] [PubMed] [Google Scholar]
- 27.Jatau I.D., Lawal I.A., Kwaga J.K.P., Tomley F.M., Blake D.P. Nok AJ. Three operational taxonomic units of Eimeria are common in Nigerian chickens and may undermine effective molecular diagnosis of coccidiosis. BMC Vet Res. 2016;12. doi: 10.1186/s12917-016-0713-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luu L., Bettridge J., Christley R.M., Melese K., Blake D., Dessie T. Prevalence and molecular characterisation of Eimeria species in Ethiopian village chickens. BMC Vet Res. 2013;9. doi: 10.1186/1746-6148-9-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chengat Prakashbabu B., Thenmozhi V., Limon G., Kundu K., Kumar S., Garg R. Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol. 2017;233:62–72. doi: 10.1016/j.vetpar.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clark E.L., Macdonald S.E., Thenmozhi V., Kundu K., Garg R., Kumar S. Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int J Parasitol. 2016;46:537–544. doi: 10.1016/j.ijpara.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jatau I.D., Sulaiman N.H., Musa I.W., Lawal A.I., Okubanjo O.O., Isah I. Prevalence of coccidia infection and preponderance Eimeria species in free range indigenous and intensively managed exotic chickens during hot-wet season, in Zaria, Nigeria. Asian J Poult Sci. 2012;6:79–88. [Google Scholar]
- 32.Conway D.P., Sasai K., Gaafar S.M., Smothers C.D. Effects of different levels of oocyst inocula of Eimeria acervulina, E. tenella, and E. maxima on plasma constituents, packed cell volume, lesion scores, and performance in chickens. Avian Dis. 2013;37:118–123. [PubMed] [Google Scholar]
- 33.Blake D.P., Clark E.L., Macdonald S.E., Thenmozhi V., Kundu K., Garg R. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci. 2015;112:E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams R.B., Marshall R.N., Pagès M., Dardi M., Cacho E. del. Pathogenesis of Eimeria praecox in chickens: Virulence of field strains compared with laboratory strains of E. praecox and Eimeria acervulina. Avian Pathol. 2009;38:359–366. doi: 10.1080/03079450903186028. [DOI] [PubMed] [Google Scholar]
- 35.Peek H.W., Landman W.J.M. Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1999 and 2001. Avian Pathol. 1996;2003(32):391–401. doi: 10.1080/0307945031000121149. [DOI] [PubMed] [Google Scholar]
- 36.Hong Y.H., Kim E.S., Lillehoj H.S., Lillehoj E.P., Song K.D. Association of resistance to avian coccidiosis with single nucleotide polymorphisms in the zyxin gene. Poult Sci. 2009;88:511–518. doi: 10.3382/ps.2008-00344. [DOI] [PubMed] [Google Scholar]
- 37.Turner J.C.M., Feeroz M.M., Hasan M.K., Akhtar S., Walker D., Seiler P. Insight into live bird markets of Bangladesh: An overview of the dynamics of transmission of H5N1 and H9N2 avian influenza viruses. Emerg Microbes Infect. 2017 doi: 10.1038/emi.2016.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagani P., Abimiku J.E., Emeka-Okolie W. Food and Agricultural Organization of the United Nations; Rome: 2008. Assessment of the Nigerian poultry market chain to improve biosecurity. [Google Scholar]
- 39.Elelu N. Epidemiological risk factors of knowledge and preventive practice regarding avian influenza among poultry farmers and live bird traders in Ikorodu, Lagos State, Nigeria. Int J Vet Sci Med. 2017 doi: 10.1016/j.ijvsm.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor M.A., Coop R.L., Wall R.L. John Wiley & Sons; Oxford: 2016. Veterinary parasitology. [electronic book]. Fourth Edi. [Google Scholar]
- 41.Ritzi M.M., Abdelrahman W., Mohnl M., Dalloul R.A. Effects of probiotics and application methods on performance and response of broiler chickens to an Eimeria challenge. Poult Sci. 2014;93:2772–2778. doi: 10.3382/ps.2014-04207. [DOI] [PubMed] [Google Scholar]
- 42.Abdelrahman W., Mohnl M., Teichmann K., Doupovec B., Schatzmayr G., Lumpkins B. Comparative evaluation of probiotic and salinomycin effects on performance and coccidiosis control in broiler chickens. Poult Sci. 2014;93:3002–3008. doi: 10.3382/ps.2014-04212. [DOI] [PubMed] [Google Scholar]