Abstract
Context
Postprandial hyperinsulinemia might be an important cardiometabolic risk determinant in black compared with white women. However, the contributions of insulin clearance and β-cell function to racial differences in postprandial insulin response are unknown.
Objective
To compare, by race and menopause, early insulin response to oral and intravenous glucose and to measure postprandial intact glucagon-like peptide 1 (GLP-1) concentrations, insulin clearance, and β-cell function.
Design and Participants
119 federally employed women without diabetes [87 premenopausal (52 black, 35 white) and 32 postmenopausal (19 black, 13 white)] underwent an oral glucose tolerance test, insulin-modified frequently sampled intravenous glucose test (IM-FSIGT), and mixed meal tolerance test (MMTT).
Outcome Measures
Early insulin response was measured as follows: (i) insulinogenic index (oral glucose tolerance test); (ii) acute insulin response to glucose (IM-FSIGT); and (iii) ratio of incremental insulin/glucose area under the curve in the first 30 minutes of the MMTT. Insulin clearance was assessed during the IM-FSIGT and MMTT. During the MMTT, intact GLP-1 was measured and β-cell function assessed using the insulin secretion rate and β-cell responsivity indexes.
Results
Black pre-menopausal and postmenopausal women had a greater insulin response and lower insulin clearance and greater dynamic β-cell responsivity (P ≤ 0.05 for all). No differences were found in the total insulin secretion rates or intact GLP-1 concentrations.
Conclusions
Greater postprandial hyperinsulinemia in black pre-menopausal and postmenopausal women was associated with lower hepatic insulin clearance and heightened β-cell capacity to rapid changes in glucose, but not to higher insulin secretion. The relationship of increased β-cell secretory capacity, reduced insulin clearance, and ambient hyperinsulinemia to the development of cardiometabolic disease requires further investigation.
A greater postprandial insulin response, relative to glucose, in black compared with white women was associated with lower insulin clearance but not increased insulin secretion.
In the United States, black women have a twofold increased risk of type 2 diabetes and a 1.3-fold increased risk of heart disease compared with white women (1). Multiple socioenvironmental factors might contribute to this greater cardiometabolic disease risk. However, emerging evidence has suggested that hyperinsulinemia (high insulin relative to ambient glucose concentrations) might be an important mediating risk determinant (2). Hyperinsulinemia is a compensatory response to increasing insulin resistance and results from increased insulin secretion (enhanced β-cell responsiveness) or reduced insulin clearance, or both. Whether hyperinsulinemia directly mediates the greater cardiometabolic disease risk in black women is uncertain but is of high clinical significance because hyperinsulinemia, relative to insulin resistance, is a prominent feature in black individuals of African ancestry (3). For example, using intravenous (nonphysiologic) or oral glucose challenges, black individuals (African Americans and black Africans) had greater insulin concentrations compared with individuals of European or Asian ancestry (3–11). However, the underlying etiology for the greater insulin response in black compared with white adults under physiologic conditions is unclear, because only a few studies have examined the insulin response after a meal, and these studies were performed in pubertal youth and not in an adult population with a greater risk of cardiovascular disease (12–14). Clarifying racial differences in the insulin response to physiologic and nonphysiologic conditions could help to elucidate the mechanisms contributing to greater cardiometabolic disease risk in black populations.
Reduced hepatic insulin clearance is an important contributor to hyperinsulinemia, because the liver and regulation of insulin-degrading enzyme (IDE) accounts for most of the insulin elimination from the body (15, 16). The greater insulin response in black compared with white individuals has mostly been attributed to reduced insulin clearance rather than an increase in insulin secretion (9, 10, 17, 18). However, reduced insulin clearance has largely been observed in the context of overall lower insulin resistance in black individuals, and the study conclusions could have been confounded by a greater prevalence of abnormal glucose tolerance in the black compared with the white groups (8, 10, 18). The metabolic phenotype in women of African ancestry is also characterized by less visceral and hepatic fat content compared with white women, despite a similar body mass index (19). A lower hepatic fat content has been more commonly associated with an improved metabolic profile (and greater hepatic insulin clearance). However, whether this relationship of hepatic fat with insulin clearance differs by race is unknown.
An increased β-cell secretory capacity has also been posited as a major determinant of hyperinsulinemia in blacks, and increased insulin secretion has been observed using the hyperglycemic clamp (the reference standard measure of insulin secretory capacity) (5, 20). Further evidence supporting greater β-cell responsivity in blacks has come from studies measuring gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1), gastrointestinal incretin hormones that mediate 50% to 80% of postprandial insulin secretion (21). The greater insulin concentrations in blacks compared with whites were associated with a 30% greater increase in GLP-1 concentrations in black adults (22, 23). However, a consistent relationship between incretin levels and hyperinsulinemia is lacking, with little to no increase in GLP-1 or GIP concentrations found in youth after an oral or meal glucose challenge (12, 24, 25). Because age and menopause are associated with increasing insulin resistance and alterations in incretin response, these contradictory findings raise the question of whether age- and/or menopause-related increases in insulin resistance could be confounding the assessment of racial differences in incretin and insulin response (26, 27).
To fully appreciate the potential racial differences in hyperinsulinemia and its relationship to cardiometabolic disease risk, it is also important to measure the insulin response under physiologic conditions [i.e., after a meal when the enteroinsular axis has been optimally activated and physiologic insulin-mediated suppression of free fatty acid (FFA) concentrations, a marker of adipose-tissue insulin resistance], can be assessed. Previously, in a small group of black compared with white premenopausal women, we observed a multiphasic pattern of glucose concentrations and a trend toward higher insulin and total FFA concentrations after a mixed-meal tolerance test (MMTT) (14). In the present study, we sought to elucidate the spectrum of insulin response to both oral and intravenous glucose stimuli and used a MMTT to maximally stimulate the incretin response. Our objectives were to compare the insulin response to three separate glucose challenges [oral glucose tolerance test (OGTT), insulin-modified intravenous glucose test (IM-FSIGT), and MMTT] in black women of African ancestry (African American and African immigrant) with white women and evaluate incretin concentrations, insulin clearance, and β-cell function [insulin secretion rate (ISR) and β-cell responsivity] postprandially.
Research Design and Methods
Study cohort
We recruited women to the Federal Women’s Study (ClinicalTrials.gov identifier, NCT01809288), a trial designed to examine the racial/ethnic variations in the risk of diabetes and heart disease in African, African-American, and white women who were federally employed and lived in the Washington DC metropolitan area (28). Recruitment was achieved by newspaper advertisement, flyers, and referral by previous participants. The participant flow diagram is shown in Supplemental Fig. 1: 147 were screened and 119 women were enrolled. Black women of African ancestry were defined as African American (parents and participant identified as African American born in the United States) or African (parents and participant born in Africa). White women were those who had identified themselves and both parents as white. The participants self-identified as healthy, did not report a history of diabetes, and were not taking medications that influenced glucose or lipid metabolism. They had also undergone a history, a physical examination, urinalysis, electrocardiography, and routine laboratory tests to exclude chronic illnesses and anemia. Menopause was defined as having irregular periods and/or an FSH >21 U/L. The National Institute of Diabetes, Digestive and Kidney Diseases institutional review board approved the present study. All enrollees gave written informed consent before participation and were seen at the National Institutes of Health Clinical Center (Bethesda, MD).
Study protocol
After the screening visit, the women returned to the National Institutes of Health Clinical Center after a 10- to 12-hour overnight fast for each of the three visits (OGTT, IM-FSIGT, and MMTT), 7 to 14 days apart, and completed within a 4- to 6-week period. The OGTT, IM-FSIGT, and MMTT were completed consecutively, except for in 10 women (five black and five white) who had undergone the MMTT before the IM-FSIGT because of scheduling difficulties. During the standard 75-g OGTT, the plasma glucose, insulin, and C-peptide concentrations were measured at −15, 0, 30, 60, 90, and 120 minutes after glucose ingestion. Glucose tolerance status was defined according to the American Diabetes Association guidelines. Prediabetes was defined as fasting glucose ≥100 but <126 mg/dL and/or 2-hour glucose level ≥140 but <200 mg/dL (29). During the IM-FSIGT visit, one intravenous catheter was placed in each arm near or in the antecubital veins. Baseline blood samples were obtained, and dextrose (0.3 g/kg) was administered intravenously. Insulin was given as a bolus at 20 minutes (0.03 U/kg/min). As previously described, plasma glucose and insulin concentrations were obtained at 32 time points between baseline and 180 minutes (7). For the MMTT, the test meal consisted of a bagel with cream cheese, a cheese omelet, and orange juice to meet 33% of the estimated daily energy needs (52% carbohydrate, 15% protein, 33% fat), which was calculated using the Mifflin St. Jeor equation plus an activity factor of 1.5 (30). All women completed the breakfast meal within 20 minutes (mean, 13.8 ± 4.9 minutes). Blood samples were taken for glucose, insulin, and C-peptide concentrations at 0, 10, 20, 30, 40, 50, 60, 90, and 120 minutes. In a subgroup of 64 premenopausal women (40 black and 24 white) and 18 postmenopausal women (9 black and 9 white), additional samples were collected for intact GLP-1, GIP, and glucagon analysis.
Calculations and variables
OGTT
The early insulin response was calculated using the insulinogenic index (31).
IM-FSIGT
The acute insulin response to glucose (AIRg) was defined as the insulin area under the curve (AUC) greater than basal at 0 to 10 minutes (32). The insulin sensitivity index (SI) was calculated via the minimal model (MinMOD Millenium, version 6.02) (32), and the disposition index was calculated as the product of the AIRg and insulin SI. Hepatic insulin clearance was estimated as the basal and total fractional hepatic insulin extraction using a model for clearance from the liver and the periphery (33), combined with the classic C-peptide deconvolution method to estimate the ISR (34).
MMTT
The early insulin response was calculated as the ratio of insulin/glucose concentrations for the incremental area under the respective curves for the first 30 minutes during the MMTT . Insulin clearance was calculated using two methods: (i) the molar ratio of C-peptide/insulin concentrations for the incremental area under the respective curves during the first 30 minutes of the test (35); and (ii) the fractional hepatic insulin extraction from the three-compartment model for insulin kinetics [liver, plasma, and extravascular; model VII in the study by Piccinini et al. (36)]. The total ISR (ISRtotal) during the MMTT was computed using the standard two-compartment model for C-peptide kinetics (34). The β-cell responsivity indexes were estimated from plasma glucose and C-peptide concentrations measured during the MMTT using the oral C-peptide minimal model (36). In brief, this C-peptide secretion model provides two β-cell responsivity indexes of pancreatic secretion: (i) Фd, the dynamic component that likely represents secretion of promptly releasable insulin and is proportional to the glucose rate of increase through the constant; and (ii) Фs, the static component that is proportional to delayed glucose concentration and likely represents the new insulin entering into the releasable pool (36). The ISRtotal was partitioned into its three components: basal, dynamic, and static, as previously described (36). All modeling calculations were performed using MATLAB R2017b (Natick, MA).
Because of technical difficulties with sample collection, results were unavailable for the following: insulinogenic index (n = 2), SI (n = 8), IM-FSIGT insulin clearance (n = 22), MMTT fractional hepatic extraction (n = 10), and β-cell responsivity indexes (n = 16).
Biochemical analyses
Glucose, insulin, and C-peptide concentrations were measured in serum using the Roche Cobas 6000 analyzer (Roche Diagnostics, Indianapolis, IN). Samples for incretin, glucagon, and total FFA analysis were collected in EDTA tubes with protease inhibitor, immediately centrifuged, and the plasma was stored at −80°C until analysis. Total GIP and intact GLP-1 concentrations were measured with Mesoscale ELISA kits [Gaithersburg, MD; GIP detection range, 73.4 to 2500 pg/mL; intact GLP-1-(7-36) detection range, 0.9 to 1000 pg/mL]. Glucagon was measured using a Mercodia kit (Winston-Salem, NC; glucagon detection range, 1.5 to 120 pmol/L). The total FFA concentrations were measured using an in vitro enzymatic colorimetric assay (Wako Diagnostics, Mountain View, CA).
Imaging studies
Whole body composition measurements were performed using dual-energy X-ray absorptiometry (Hologic Discovery, Bedford, MA). Hepatic fat content was measured using proton magnetic resonance spectroscopy at 3T (MAGNETOM Verio; Siemens, Tarrytown, NY) (37). Additionally, the visceral fat measurement was obtained using T1-weighted images at the level of L2-L3 (38). Because of difficulties with data acquisition, we were unable to obtain visceral fat in 14 black and 4 white women and hepatic fat in 17 black and 8 white women.
Statistical analysis
The data are presented as the mean ± SD, except as indicated otherwise. An early response was defined as the incremental AUC during the first 30 minutes of the MMTT (iAUC30), calculated using a cubic spline to determine the integral (STATA, version 15.1; StataCorp, College Station, TX). The Student t test and Kruskal-Wallis test were used to compare the continuous parametric and nonparametric variables, respectively. The χ2 test was used to compare categorical variables. Spearman correlations were performed for continuous variables. Mixed effects models were used to analyze the glucose, hormone, and FFA response during the MMTT (within-subject factor of time; between-subject factor of race; time × race interaction). Statistical analyses were performed with STATA, version 15.1 (StataCorp), and P values < 0.05 were considered statistically significant.
Results
Of the 119 women enrolled, 87 were premenopausal (13 African immigrant, 39 African American, and 35 white) and 32 were postmenopausal (two African immigrant, 17 African American, and 13 white; Supplemental Fig. 1). Except for body mass index, African-immigrant and African-American women had similar demographic and metabolic characteristics (P > 0.05 for all; Supplemental Table 1) and therefore were combined into one group for the subsequent analyses.
Participant characteristics
The participant characteristics stratified by race and menopausal status are listed in Table 1. Overall, black women had lower triglyceride concentrations and lower visceral fat and tended to have lower hepatic fat. To determine the effect of menopause on metabolic characteristics, we compared the black pre- and postmenopausal women and white pre-menopausal and postmenopausal women (Table 1). Black premenopausal women had a lower fasting glucose, 2-hour glucose, triglyceride concentrations, and a lower prevalence of prediabetes compared with black postmenopausal women (P ≤ 0.01 for all). A similar metabolic pattern was observed between white pre-menopausal and postmenopausal women (Table 1). Therefore, subsequent analyses were stratified by menopausal status.
Table 1.
Participant Characteristics Stratified by Race and Menopausal Status
| Characteristic | Entire Cohort (n = 119) |
Premenopausal Women (n = 87) |
Postmenopausal Women (n = 32) |
P Value (Pre- vs Postmenopausal) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Black (n = 71) | White (n = 48) | ||||||||||
| Black (n = 71) | White (n = 48) | P Value | Black (n = 52) | White (n = 35) | P Value | Black (n = 19) | White (n = 13) | P Value | |||
| Age, y | 42.3 ± 9 | 43.3 ± 11 | 0.63 | 39 ± 7 | 38 ± 7 | 0.70 | 53 ± 4 | 57 ± 4 | < 0.01a | < 0.01a | < 0.01a |
| BMI, kg/m2 | 30.6 ± 5.6 | 29.3 ± 4.9 | 0.20 | 30.7 ± 6.0 | 29.9 ± 5.0 | 0.48 | 30.1 ± 4.6 | 27.7 ± 4.4 | 0.15 | 0.66 | 0.17 |
| Obesity, n (%) | 35 (49) | 23 (48) | 0.88 | 28 (54) | 19 (54) | 0.97 | 7 (37) | 4 (31) | 0.72 | 0.21 | 0.15 |
| Prediabetes, n (%) | 23 (33) | 13 (27) | 0.54 | 10 (19) | 6 (17) | 0.81 | 13 (68) | 7 (54) | 0.40 | < 0.01a | 0.01a |
| Total fat mass, % | 33.5 ± 13.2 | 32.3 ± 9.6 | 0.62 | 37.2 ± 6.9 | 39.1 ± 7.0 | 0.21 | 38.6 ± 5.4 | 37.2 ± 6.1 | 0.50 | 0.44 | 0.39 |
| Visceral fat L2-3, cm2 | 67 (42–111) | 95 (67–138) | 0.01a | 58 (34–104) | 97 (66–132) | 0.02a | 89 (58–128) | 92 (68–149) | 0.54 | 0.04a | 0.81 |
| Subcutaneous fat L2-3, cm2 | 265 (197–407) | 291 (193–344) | 0.96 | 265 (177–407) | 298 (191–358) | 0.69 | 277 (218–375) | 240 (194–337) | 0.48 | 0.64 | 0.48 |
| Hepatic fat, %) | 0.7 (0.4–1.5) | 1.0 (0.6–2.2) | 0.09 | 0.6 (0.5–1.3) | 1.0 (0.7–2.5) | 0.04a | 1.2 (0.4–3.7) | 0.9 (0.5–1.7) | 0.90 | 0.45 | 0.58 |
| Fasting lipid profile, mg/dL | |||||||||||
| Total cholesterol | 168 ± 33 | 171 ± 28 | 0.63 | 162 ± 30 | 163 ± 23 | 0.94 | 183 ± 36 | 192 ± 32 | 0.47 | 0.02a | 0.01a |
| LDL cholesterol | 95 ± 29 | 99 ± 26 | 0.51 | 91 ± 29 | 93 ± 23 | 0.75 | 107 ± 29 | 114 ± 29 | 0.46 | 0.04a | 0.01a |
| HDL cholesterol | 60 ± 17 | 56 ± 14 | 0.21 | 60 ± 17 | 55 ± 14 | 0.18 | 62 ± 18 | 60 ± 16 | 0.78 | 0.69 | 0.30 |
| Triglycerides | 57 (41–69) | 70 (58–98) | < 0.01a | 53 (41–67) | 68 (56–99) | < 0.01a | 61 (52–78) | 75 (67–94) | 0.06 | 0.04a | 0.27 |
| OGTT | |||||||||||
| Fasting glucose, mg/dL | 92 ± 7 | 92 ± 7 | 0.67 | 90 ± 7 | 91 ± 7 | 0.55 | 95 ± 7 | 95 ± 7 | 0.89 | 0.01a | 0.12 |
| 2-h Glucose, mg/dL | 125 ± 24 | 127 ± 28 | 0.65 | 121 ± 24 | 124 ± 27 | 0.60 | 135 ± 21 | 136 ± 32 | 0.99 | 0.03a | 0.22 |
| IM-FSIGT | |||||||||||
| SI (mU/L)−1min−1 × 10−4 | 2.3 (1.7–3.2) | 3.0 (2.1–5.2) | < 0.01a | 2.3 (1.6–3.2) | 2.8 (1.8–4.4) | 0.06 | 2.4 (1.7–3.0) | 4.5 (2.4–6.0) | 0.04a | 0.95 | 0.29 |
| Disposition index | 1676 (1118–2776) | 1331 (972–1758) | 0.02a | 1867 (1255–2780) | 1481 (972–1817) | 0.02a | 1240 (930–1967) | 1234 (970–1557) | 0.47 | 0.08 | 0.28 |
Data presented as mean ± SD or median (25th to 75th percentile).
Abbreviations: HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Statistically significant.
Among the premenopausal women, the prevalence of obesity and prediabetes, glucose concentrations, and fasting cholesterol profile were similar in black and white women (Table 1). Black premenopausal women had lower fasting triglyceride concentrations and lower visceral and hepatic fat content compared with the white women (P < 0.05 for all). In the postmenopausal women, blacks were 4.8 ± 1.5 years younger and tended to have lower fasting triglycerides but otherwise had similar rates of obesity and prediabetes, a similar body composition, and similar glucose concentrations.
SI was lower in both premenopausal (P = 0.06) and postmenopausal (P = 0.04) black compared to white women (Table 1). The disposition index was greater in black premenopausal women only (P < 0.02; Table 1).
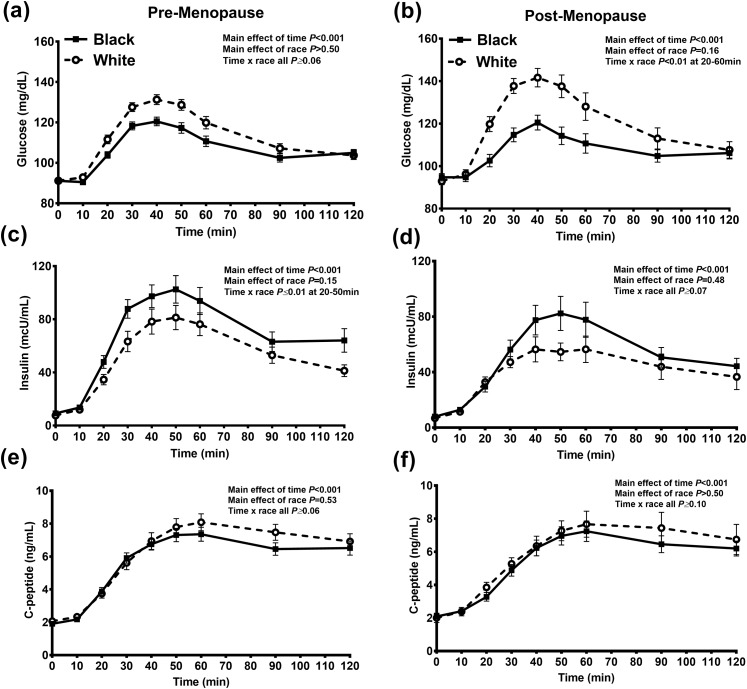
During the MMTT, black women compared with white women had greater insulin and lower glucose concentrations (at 20 to 40 minutes), regardless of menopausal status [Fig. 1(a)–(d)]. No differences were found in the C-peptide concentrations [Fig. 1(e) and 1(f)]. Intact GLP-1 and GIP concentrations were similar in black and white pre-menopausal and postmenopausal women (Table 2; Supplemental Fig. 2). Glucagon concentrations were similar in premenopausal women (P = 0.93); however, postmenopausal white women had lower glucagon concentrations during the first 30 minutes of the MMTT (P = 0.03; Table 2). The postprandial FFA concentrations were greater in the black pre- and postmenopausal women; however, this was only statistically significant at 30 minutes after the meal (Supplemental Fig. 3).
Figure 1.
The change in (a, b) glucose, (c, d) insulin, and (e, f) C-peptide concentrations during the MMTT in black (solid squares and lines) and white (white circles and dotted lines) pre-menopausal and postmenopausal women. Data presented as mean ± SEM.
Table 2.
Early Incretin and Glucagon Response in Pre-menopausal and Postmenopausal Women Stratified by Race During the MMTT
| Variable | Premenopausal (n = 64) |
Postmenopausal (n = 18) |
||||
|---|---|---|---|---|---|---|
| Black (n = 40) | White (n = 24) | P Value | Black (n = 9) | White (n = 9) | P Value | |
| Intact GLP-1 iAUC30, pg/mL/min | 101 (62–167) | 92 (32–144) | 0.41 | 53 (32–84) | 77 (33–115) | 0.57 |
| Total GIP iAUC30, pg/mL/min | 2600 (1355–3636) | 1687 (1238–3044) | 0.34 | 3184 (1862–3654) | 1872 (1338–2941) | 0.38 |
| Glucagon iAUC30, pmol/L/min | 47 (26–74) | 45 (16–75) | 0.93 | 41 (26–86) | 17 (6–23) | 0.03 |
Early response was defined as the iAUC30 of the meal test.
Data presented as mean ± SD or median (25th to 75th percentile).
Insulin response
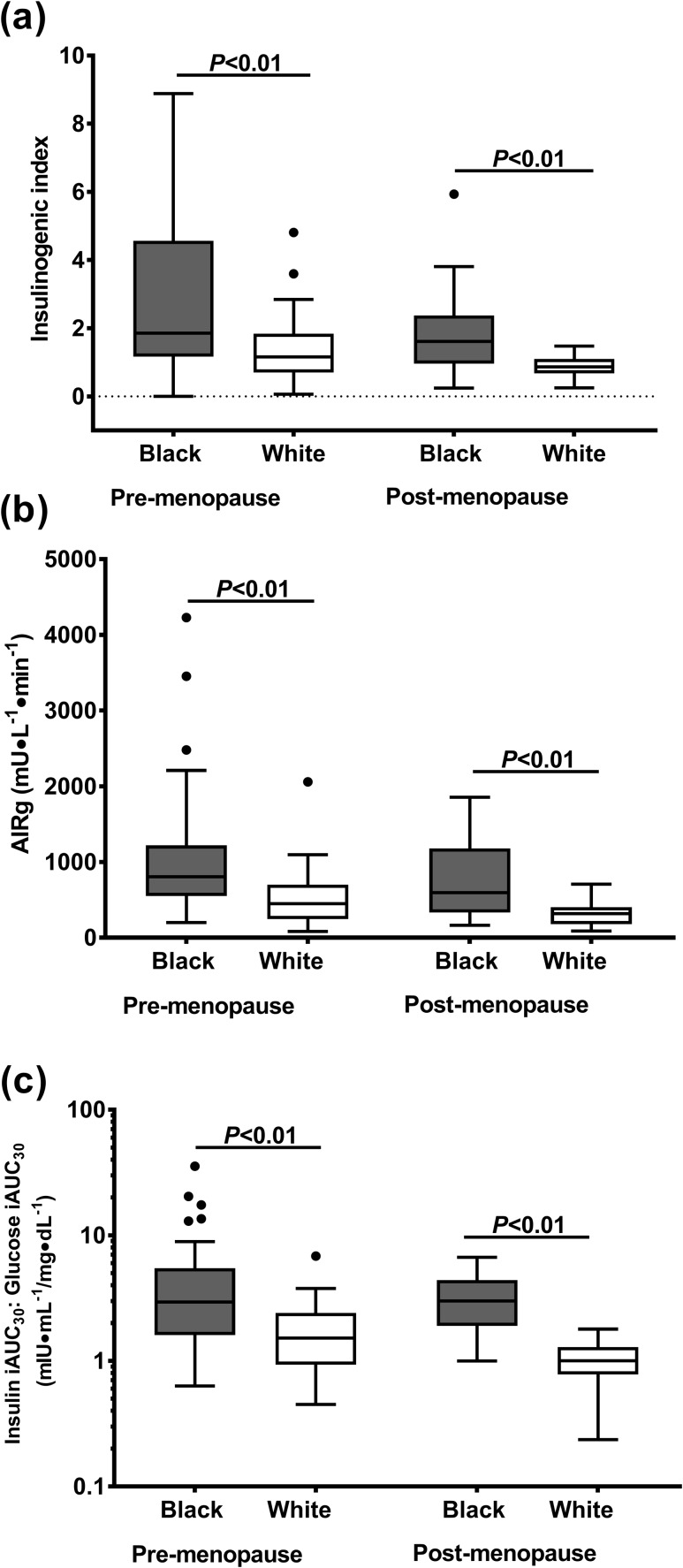
Black women had a greater insulin response, relative to glucose, during all three tests (Fig. 2). The insulinogenic index (OGTT) and AIRg (IM-FSIGT) was 40% to 50% and 80% greater in black pre-menopausal and postmenopausal women, respectively [P ≤ 0.01; Fig. 2(a) and (2b)]. Similarly, during the MMTT, black compared with white women had 2.5-fold greater early postprandial insulin to glucose response [Fig. 2(c)].
Figure 2.
Early insulin response to three different glucose stimuli. Early response was defined as (a) insulinogenic index during OGTT, (b) AIRg during IM-FSIGT, and (c) insulin iAUC30/glucose iAUC30 during MMTT. Data are presented as Tukey box and whiskers plots. Black circles represent any value that lie outside the range of the whiskers.
Insulin clearance, secretion, and β-cell responsivity
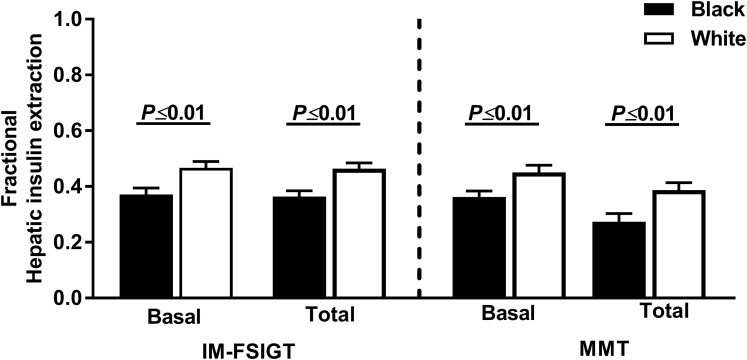
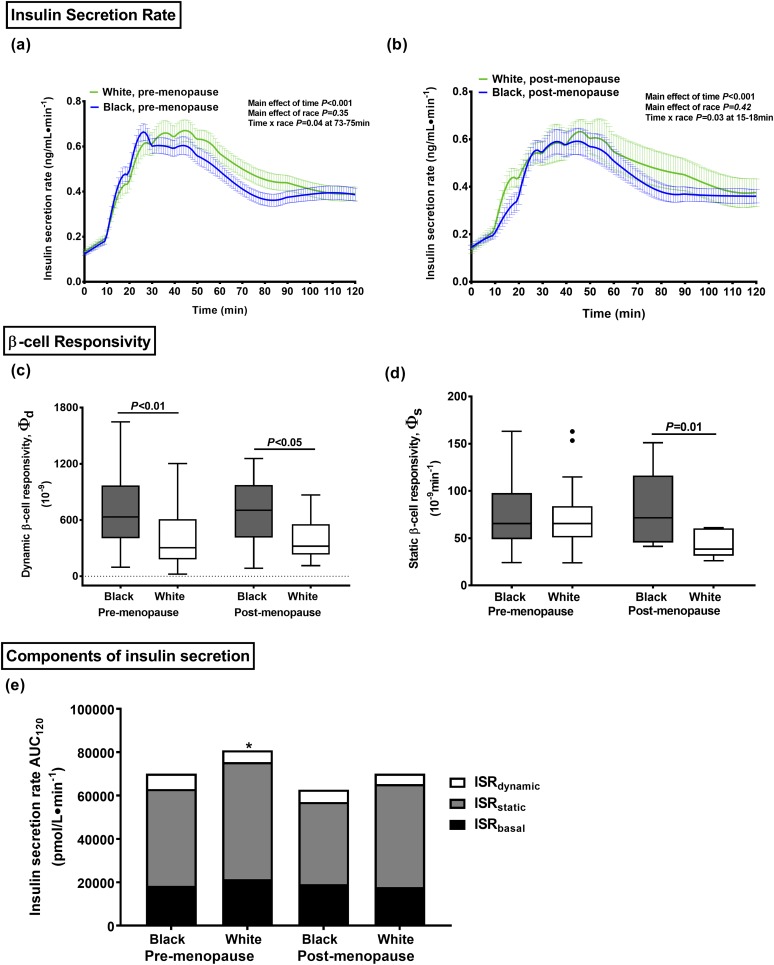
Using modeling parameters, the basal and total fractional hepatic insulin extraction was ~11% and 9% lower in black women during the IM-FSIGT and the MMTT, respectively (P ≤ 0.01; Fig. 3). During the MMTT, the insulin clearance ratio (C-peptide iAUC30/insulin iAUC30) was also 20% lower in the black compared with white, pre-menopausal and postmenopausal women and correlated positively with both basal fractional hepatic insulin extraction (IM-FSIGT: r = 0.35, P < 0.001; MMTT: r = 0.75, P < 0.001; Supplemental Fig. 4). The absolute ISR was not different when stratified by race or menopausal status [Fig. 4(a) and 4(b)]. Dynamic β-cell responsivity (Фd) and dynamic ISR was greater in black women [P < 0.01; Fig. 4(c) and 4(e)]. No differences were found in static β-cell responsivity (Фs), static ISR, or basal ISR [Fig. 4(d) and 4(e)].
Figure 3.
Basal and total fractional hepatic insulin extraction during IM-FSIGT and MMTT in black (black bars) and white (white bars) women. Data presented as mean ± SEM.
Figure 4.
Model estimates of insulin secretion and β-cell function during the MMTT. ISR in black (blue lines) and white (green lines) (a) premenopausal and (b) postmenopausal women. β-cell responsivity in black (gray boxes) and white (white boxes) women by menopausal status: (c) dynamic and (d) static. (e) The total insulin secretion AUC rate during the MMTT partitioned into its components: ISRbasal (black box), ISRstatic (gray box) and ISRdynamic (white box). Data presented as (a, b) line graphs, (c, d) Tukey Box and whisker plots, and (e) bar graphs. Black circles represent any value that lie outside the range of the whiskers. *P < 0.01 premenopausal white vs black women.
Correlations
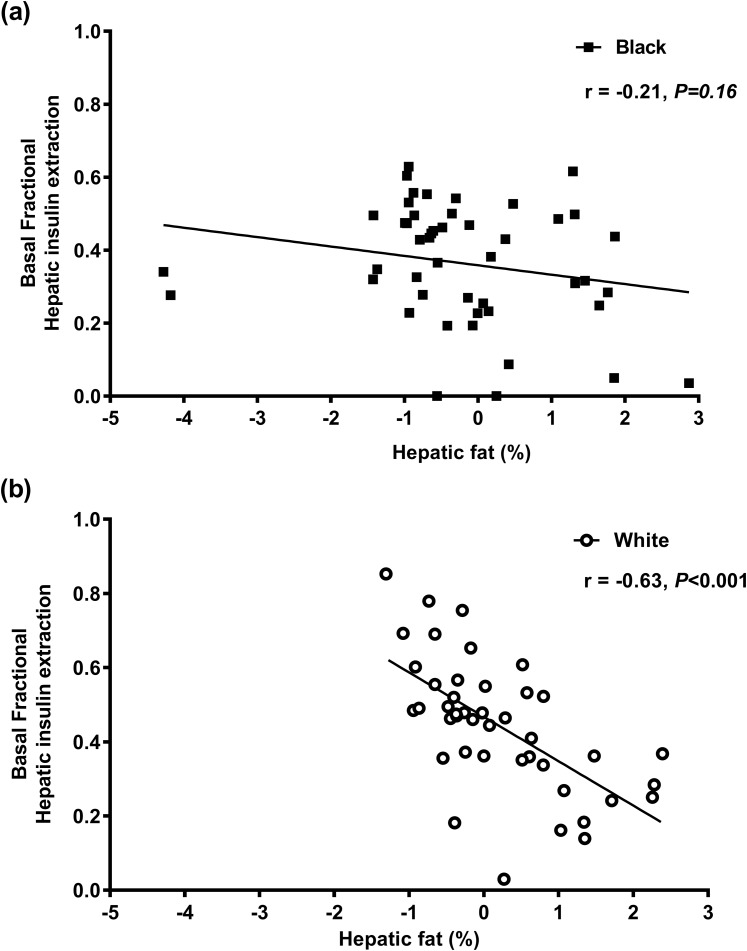
Hepatic fat correlated with the MMTT early insulin response regardless of race or menopausal status (r = 0.33 and P < 0.001 for all). The relationship of MMTT basal hepatic insulin extraction with hepatic fat differed by race. The strong negative correlation observed in whites was absent in black women (Fig. 5). The early GIP and intact GLP-1 response correlated with insulin secretion (r = 0.42, P < 0.001 and r = 0.30, P < 0.001, respectively) but not with Фd or Фs (r ≤ 0.10 and P ≥ 0.17 for all). The FFA level at 30 minutes did not correlate with the insulin response (r = 0.16, P = 0.11).
Figure 5.
The relationship of basal fractional hepatic insulin extraction during the MMTT and hepatic fat in (a) black and (b) white women. Data presented as individual data points for black (black squares) and white (white circles) women with the corresponding regression line.
Discussion
Greater hyperinsulinemia in black compared with white pre-menopausal and postmenopausal women
The present study has provided insight into racial differences in insulin response in a large group of women stratified by race and menopausal status. We have demonstrated that blacks have a greater insulin response to oral (liquid glucose drink and solid mixed meal) and intravenous glucose stimuli compared with white women and confirmed that racial differences in insulin response are detectable in both pre-menopausal and postmenopausal women. Although greater hyperinsulinemia had been previously observed during intravenous glucose tests, the postprandial insulin response was less clear (5, 12, 14, 22–24, 27). Our study has extended these observations by confirming that hyperinsulinemia is also prominent under physiologic conditions in response to a mixed meal, regardless of menopausal status. Additionally, by including two groups of women of African descent, we found that greater insulin response is likely related to intrinsic biological factors that occur across the African diaspora. Clinically, our findings imply that interventions to target greater hyperinsulinemia in blacks in the United States could be applicable to other populations of African descent.
Postprandial hyperinsulinemia is related to racial differences in insulin clearance
To elucidate the physiologic mechanisms leading to greater hyperinsulinemia in black women, we estimated the fractional hepatic insulin extraction, ISR, and β-cell responsivity (a measure of secretory capacity) after the solid breakfast meal. Black women had lower basal and total hepatic insulin extraction estimated by two independent measures during the IM-FSIGT and MMTT. These findings confirmed the lower hepatic insulin extraction during the IM-FSIGT in a large group of black pre-menopausal and postmenopausal women and are in agreement with previous studies (5, 17, 39). Moreover, by also demonstrating lower basal and total hepatic insulin extraction in black women during the MMTT, we found that a lower insulin clearance was also an important physiologic feature after a solid meal (Fig. 3). As expected, the lower insulin clearance in black women was in the context of a lower SI. However, the absolute racial difference in insulin clearance was only 10%, which would not fully explain the variance in hyperinsulinemia observed in pre-menopausal and postmenopausal women.
Because greater insulin secretion has also been proposed as a key contributor to the racial differences in hyperinsulinemia (5, 20, 25, 27), we also modeled the ISR and partitioned pancreatic secretion into its three components: basal, dynamic, and static. The total ISR and the pattern of insulin secretion were similar when stratified by race and menopause status (Fig. 4). However, the glucose concentrations were lower in the black women during the first 40 minutes of the MMTT, implying that they had greater β-cell responsivity. The dynamic insulin secretion was also higher in black women but no difference was found in basal or static insulin secretion. The higher dynamic responsivity (Фd), defined as the β-cell’s secretory capacity proportional to the rate of increase of glucose concentrations, posits that black women have a larger quantity of insulin in the rapidly releasable pool compared with white women. These findings are in keeping with the greater AIRg after an IM-FSIGT in black women compared with white women, a well-established phenotypic characteristic that has been replicated in the present study. The lack of difference by race in static responsivity implies that the capacity to deliver new insulin granules to the plasma membrane in response to maintained increases in glucose is similar. This component is the dominant source of the cumulative release of insulin during the MMTT. These findings have corroborated the greater β-cell function observed in black compared with white pubertal youth using an alternative C-peptide and glucose mathematical model during an OGTT (25) but argue against greater total insulin secretion as a major determinant of the greater postprandial hyperinsulinemia in black women.
Postprandial hyperinsulinemia is not related to racial differences in incretin response
In contrast to our hypothesis, the greater insulin response and greater dynamic β-cell responsivity in black women were not related to greater postprandial incretin concentrations. Previous studies that found a greater insulin and intact GLP-1 response were limited by small sample sizes and had been performed in response to an OGTT in which incretin secretion might not have been maximally stimulated (22, 23). Rather, our results are in agreement with the findings from youth, in which a greater early insulin response in black compared with white youth was associated with lower or no differences in incretin responses after either an oral glucose load or a mixed macronutrient meal (12, 24, 25).
Relationship of postprandial insulin response to hepatic fat and FFA
Because the liver accounts for ~80% of insulin clearance (15), increased hepatic and visceral adiposity are typically close correlates of hyperinsulinemia and reduced insulin clearance (40, 41). This lower insulin clearance in blacks has been paradoxically associated with lower visceral and hepatic fat accumulation (19), a finding replicated in our cohort. The relationship of hepatic insulin clearance with hepatic fat differed by race and was most prominent in premenopausal women. The expected inverse correlation of hepatic fat with insulin extraction was observed in white women but not in the black women (Fig. 4). Therefore, black women had lower hepatic insulin extraction across a wide range of hepatic fat content compared with white women. Other intrinsic factors, such as racial differences in IDE activity, may play a role in the differential regulation of hepatic insulin clearance (17). IDE is an evolutionarily conserved zinc metalloprotease whose activity is regulated by the liver, suppressed in obesity, and associated with reduced insulin clearance (16, 42, 43). Polymorphisms in the gene encoding IDE have been variably linked to an increased risk of type 2 diabetes, although these studies have been in largely Asian and white populations (43–47). The effects of obesity and liver fat accumulation on IDE activity and whether these factors mediate racial variations among individuals of African descent remain to be elucidated.
Greater postprandial hyperinsulinemia was also observed in the context of lower overall insulin sensitivity (SI) and could indicate greater tissue-specific insulin resistance. The higher FFA concentrations during the MMTT in black women (Supplemental Fig. 3), likely reflected resistance to insulin-mediated suppression of adipose tissue lipolysis, because the FFA rate of appearance from the diet is negligible during the first 2 hours of the MMTT (14). These observations validate previous findings, in a smaller biracial group of 15 black and 13 white women, in which the modeling of FFA kinetics demonstrated reduced suppression of lipolysis in black women (14) and underscore the importance of assessing hormonal markers in the physiologic state, rather than during intravenous glucose tests (7, 14).
Overall, the cumulative effect of ambient hyperinsulinemia on long-term cardiometabolic risk remains to be elucidated. However, the greater postprandial insulin response in black women was not associated with greater glucose, triglyceride, or cholesterol concentrations or a greater prevalence of obesity or prediabetes in the present study and a previous cross-sectional study found no relationship of impaired postprandial endothelial function in insulin-resistant women (black or white) with an exaggerated insulin response (48). Nevertheless, the collective effect of ambient hyperinsulinemia, in the context of reduced insulin clearance and increased β-cell capacity to respond to rapid increases in glucose, is unknown. Recent murine studies have found that hyperinsulinemia alone, without insulin resistance, increases heart failure risk by inducing cardiac hypertrophy (49, 50).
Strengths and weaknesses
To the best of our knowledge, the present study is the first to systematically compare by race the contribution of insulin secretion and clearance to postprandial hyperinsulinemia in women who were similar in glucose tolerance status and stratified by menopause. Detailed modeling analyses of fractional hepatic extraction were used in two separate tests, and the magnitude of lower insulin clearance in black women was similar. The study limitations included the cross-sectional design and relatively smaller sample of postmenopausal women. Additionally, we did not measure gastric emptying, an important factor for the rate of glucose absorption and postprandial insulin response; however, the meal completion time was similar when stratified by race and menopausal status.
Conclusions
Compared with white women, black pre-menopausal and postmenopausal women had a greater insulin response to oral and intravenous glucose loads. Lower insulin clearance and a heightened β-cell capacity to secrete insulin in response to rapid changes in glucose concentrations were associated with this greater postprandial hyperinsulinemia, and the etiological mechanisms and cardiovascular implications of this response require further investigation.
Supplementary Material
Acknowledgments
We thank the volunteers whose participation made this study possible. We thank Dr. Vipul Periwal and Dr. Andrea Kelly for their contributions in modeling insulin secretion and statistical analyses, respectively. We thank Drs. Francesca Piccinini, Chiara Dalla Man, Michele Schiavoni, and Vincent Sparacino for their helpful advice and sharing computer codes.
Financial Support : S.T.C., A.E.S., A.M.G., M.W., and A.S.S. are supported by the Intramural Program of the National Institute of Diabetes, Digestive and Kidney Diseases, National Institutes of Health. A.B.C. is supported by the National Institutes of Health Clinical Center, and A.E.S. is also supported by the National Institute on Minority Health Disparities, National Institutes of Health.
Clinical Trial Information: ClinicalTrial.gov no. NCT01809288 (registered 12 March 2013).
Author Contributions: S.T.C. designed the study, collected the data, conducted the analysis, and wrote the manuscript. A.E.S. designed the study, collected data, and revised and edited the manuscript. A.B.C., A.S.S., M.G.-D.L.C., P.C.A., L.S.M., C.W.D., A.M.G., and M.W. contributed to the study design and data collection and analysis and revised and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- AIRg
acute insulin response to glucose
- AUC
area under the curve
- FFA
free fatty acid
- iAUC30
incremental area under the curve during the first 30 minutes of the mixed meal tolerance test
- GLP-1
glucagon-like peptide 1
- IDE
insulin-degrading enzyme
- IM-FSIGT
insulin-modified frequently sampled intravenous glucose test
- ISR
insulin secretion rate
- ISRtotal
total insulin secretion rate
- MMTT
mixed meal tolerance test
- OGTT
oral glucose tolerance test
- SI
insulin sensitivity index
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Riehle C, Abel ED. Insulin signaling and heart failure. Circ Res. 2016;118(7):1151–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response: a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rasouli N, Spencer HJ, Rashidi AA, Elbein SC. Impact of family history of diabetes and ethnicity on beta-cell function in obese, glucose-tolerant individuals. J Clin Endocrinol Metab. 2007;92(12):4656–4663. [DOI] [PubMed] [Google Scholar]
- 5. Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87(5):2218–2224. [DOI] [PubMed] [Google Scholar]
- 6. Haffner SM, D’Agostino R, Saad MF, Rewers M, Mykkänen L, Selby J, Howard G, Savage PJ, Hamman RF, Wagenknecht LE, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742–748. [DOI] [PubMed] [Google Scholar]
- 7. Chow CC, Periwal V, Csako G, Ricks M, Courville AB, Miller BV III, Vega GL, Sumner AE. Higher acute insulin response to glucose may determine greater free fatty acid clearance in African-American women. J Clin Endocrinol Metab. 2011;96(8):2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osei K, Schuster DP. Metabolic characteristics of African descendants: a comparative study of African-Americans and Ghanaian immigrants using minimal model analysis. Diabetologia. 1995;38(9):1103–1109. [DOI] [PubMed] [Google Scholar]
- 9. Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabet Med. 1994;11(8):755–762. [DOI] [PubMed] [Google Scholar]
- 10. Osei K, Schuster DP, Owusu SK, Amoah AG. Race and ethnicity determine serum insulin and C-peptide concentrations and hepatic insulin extraction and insulin clearance: comparative studies of three populations of West African ancestry and white Americans. Metabolism. 1997;46(1):53–58. [DOI] [PubMed] [Google Scholar]
- 11. Nyenwe E, Owei I, Wan J, Dagogo-Jack S. Parental history of type 2 diabetes abrogates ethnic disparities in key glucoregulatory indices. J Clin Endocrinol Metab. 2018;103(2):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins PB, Férnández JR, Garvey WT, Granger WM, Gower BA. Entero-insular axis and postprandial insulin differences in African American and European American children. Am J Clin Nutr. 2008;88(5):1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Punyadeera C, Crowther NJ, van der Merwe MT, Toman M, Immelman AR, Schlaphoff GP, Gray IP. Metabolic response to a mixed meal in obese and lean women from two South African populations. Obes Res. 2002;10(12):1207–1216. [DOI] [PubMed] [Google Scholar]
- 14. Li Y, Chow CC, Courville AB, Sumner AE, Periwal V. Modeling glucose and free fatty acid kinetics in glucose and meal tolerance test. Theor Biol Med Model. 2016;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983;244(6):E517–E527. [DOI] [PubMed] [Google Scholar]
- 16. Wei X, Ke B, Zhao Z, Ye X, Gao Z, Ye J. Regulation of insulin degrading enzyme activity by obesity-associated factors and pioglitazone in liver of diet-induced obese mice. PLoS One. 2014;9(4):e95399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Piccinini F, Polidori DC, Gower BA, Bergman RN. Hepatic but not extrahepatic insulin clearance is lower in African American than in European American women. Diabetes. 2017;66(10):2564–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang X, Srinivasan SR, Radhakrishnamurthy B, Dalferes ER, Berenson GS. Racial (black-white) differences in insulin secretion and clearance in adolescents: the Bogalusa heart study. Pediatrics. 1996;97(3):357–360. [PubMed] [Google Scholar]
- 19. Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology. 2009;49(3):791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31(7):1445–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008;60(4):470–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, Lustig RH, Cashion AK, Burghen GA. Racial differences in glucagon-like peptide-1 (GLP-1) concentrations and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obes Relat Metab Disord. 2003;27:1359–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Velasquez-Mieyer PA, Umpierrez GE, Lustig RH, Cashion AK, Cowan PA, Christensen M, Spencer KA, Burghen GA. Race affects insulin and GLP-1 secretion and response to a long-acting somatostatin analogue in obese adults. Int J Obes Relat Metabol Disord. 2004;28:330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velásquez-Mieyer PA, Cowan PA, Pérez-Faustinelli S, Nieto-Martínez R, Villegas-Barreto C, Tolley EA, Lustig RH, Alpert BS. Racial disparity in glucagon-like peptide 1 and inflammation markers among severely obese adolescents. Diabetes Care. 2008;31(4):770–775. [DOI] [PubMed] [Google Scholar]
- 25. Michaliszyn SF, Lee S, Bacha F, Tfayli H, Farchoukh L, Mari A, Ferrannini E, Arslanian S. Differences in β-cell function and insulin secretion in black vs. white obese adolescents: do incretin hormones play a role? Pediatr Diabetes. 2017;18(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahrén B, Larsson H, Holst JJ. Reduced gastric inhibitory polypeptide but normal glucagon-like peptide 1 response to oral glucose in postmenopausal women with impaired glucose tolerance. Eur J Endocrinol. 1997;137(2):127–131. [DOI] [PubMed] [Google Scholar]
- 27. Chandler-Laney PC, Phadke RP, Granger WM, Fernández JR, Muñoz JA, Man CD, Cobelli C, Ovalle F, Gower BA. Age-related changes in insulin sensitivity and β-cell function among European-American and African-American women. Obesity (Silver Spring). 2011;19(3):528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chung ST, Ha J, Onuzuruike AU, Kasturi K, Galvan-De La Cruz M, Bingham BA, Baker RL, Utumatwishima JN, Mabundo LS, Ricks M, Sherman AS, Sumner AE. Time to glucose peak during an oral glucose tolerance test identifies prediabetes risk. Clin Endocrinol (Oxf). 2017;87(5):484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. American Diabetes Association 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. [DOI] [PubMed] [Google Scholar]
- 30. Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–247. [DOI] [PubMed] [Google Scholar]
- 31. Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. J Clin Endocrinol Metab. 2005;90(1):493–500. [DOI] [PubMed] [Google Scholar]
- 32. Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther. 2003;5(6):1003–1015. [DOI] [PubMed] [Google Scholar]
- 33. Polidori DC, Bergman RN, Chung ST, Sumner AE. Hepatic and extrahepatic insulin clearance are differentially regulated: results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 2016;65(6):1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels: comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes. 1992;41(3):368–377. [DOI] [PubMed] [Google Scholar]
- 35. Meier JJ, Holst JJ, Schmidt WE, Nauck MA. Reduction of hepatic insulin clearance after oral glucose ingestion is not mediated by glucagon-like peptide 1 or gastric inhibitory polypeptide in humans. Am J Physiol Endocrinol Metab. 2007;293(3):E849–E856. [DOI] [PubMed] [Google Scholar]
- 36. Piccinini F, Dalla Man C, Vella A, Cobelli C. A model for the estimation of hepatic insulin extraction after a meal. IEEE Trans Biomed Eng. 2016;63(9):1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ouwerkerk R, Pettigrew RI, Gharib AM. Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology. 2012;265(2):565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muniyappa R, Noureldin R, Ouwerkerk R, Liu EY, Madan R, Abel BS, Mullins K, Walter MF, Skarulis MC, Gharib AM. Myocardial fat accumulation is independent of measures of insulin sensitivity. J Clin Endocrinol Metab. 2015;100(8):3060–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J. Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51(10):3014–3019. [DOI] [PubMed] [Google Scholar]
- 40. Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135(1):122–130. [DOI] [PubMed] [Google Scholar]
- 41. Rungan S, Finucane K, Gentles T, Gibbs HC, Hu R, Ruygrok PN. Heart transplantation in pediatric and congenital heart disease: a single-center experience. World J Pediatr Congenit Heart Surg. 2014;5(2):200–205. [DOI] [PubMed] [Google Scholar]
- 42. Brandimarti P, Costa-Júnior JM, Ferreira SM, Protzek AO, Santos GJ, Carneiro EM, Boschero AC, Rezende LF. Cafeteria diet inhibits insulin clearance by reduced insulin-degrading enzyme expression and mRNA splicing. J Endocrinol. 2013;219(2):173–182. [DOI] [PubMed] [Google Scholar]
- 43. Rudovich N, Pivovarova O, Fisher E, Fischer-Rosinsky A, Spranger J, Möhlig M, Schulze MB, Boeing H, Pfeiffer AF. Polymorphisms within insulin-degrading enzyme (IDE) gene determine insulin metabolism and risk of type 2 diabetes. J Mol Med (Berl). 2009;87(11):1145–1151. [DOI] [PubMed] [Google Scholar]
- 44. Karamohamed S, Demissie S, Volcjak J, Liu C, Heard-Costa N, Liu J, Shoemaker CM, Panhuysen CI, Meigs JB, Wilson P, Atwood LD, Cupples LA, Herbert A, Study NFH; NHLBI Framingham Heart Study . Polymorphisms in the insulin-degrading enzyme gene are associated with type 2 diabetes in men from the NHLBI Framingham Heart Study. Diabetes. 2003;52(6):1562–1567. [DOI] [PubMed] [Google Scholar]
- 45. Kwak SH, Cho YM, Moon MK, Kim JH, Park BL, Cheong HS, Shin HD, Jang HC, Kim SY, Lee HK, Park KS. Association of polymorphisms in the insulin-degrading enzyme gene with type 2 diabetes in the Korean population. Diabetes Res Clin Pract. 2008;79(2):284–290. [DOI] [PubMed] [Google Scholar]
- 46. Furukawa Y, Shimada T, Furuta H, Matsuno S, Kusuyama A, Doi A, Nishi M, Sasaki H, Sanke T, Nanjo K. Polymorphisms in the IDE-KIF11-HHEX gene locus are reproducibly associated with type 2 diabetes in a Japanese population. J Clin Endocrinol Metab. 2008;93(1):310–314. [DOI] [PubMed] [Google Scholar]
- 47. Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445(7130):881–885. [DOI] [PubMed] [Google Scholar]
- 48. Muniyappa R, Sachdev V, Sidenko S, Ricks M, Castillo DC, Courville AB, Sumner AE. Postprandial endothelial function does not differ in women by race: an insulin resistance paradox? Am J Physiol Endocrinol Metab. 2012;302(2):E218–E225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pires KM, Buffolo M, Schaaf C, David Symons J, Cox J, Abel ED, Selzman CH, Boudina S. Activation of IGF-1 receptors and Akt signaling by systemic hyperinsulinemia contributes to cardiac hypertrophy but does not regulate cardiac autophagy in obese diabetic mice. J Mol Cell Cardiol. 2017;113:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, Wei W, Abel ED, Xiang YK. Inhibiting insulin-mediated β2-adrenergic receptor activation prevents diabetes-associated cardiac dysfunction. Circulation. 2017;135(1):73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.