Abstract
The greater cane rat (Thryonomys swinderianus) demonstrated numerous dermal architectural peculiarities hitherto unreported. This investigation assessed and evaluated certain histologic features of skin samples from the fore and hind limbs, the neck, head, proximal to the ear and oro-nasal regions for follicular evaluations and micro anatomic assessments in the adult species. Twenty wild taken animals of equal gender distribution were used for histologic assessments of structural elements and histo-morphometric evaluations. Hair follicular density, size, distribution and orientations as well as sexual dimorphisms observed in the body regions studied with Motic Image Plus software analysis were also reported. Statistical analysis revealed sexual dimorphism in this feature as females demonstrated significantly higher (P < .05) follicular density and epidermal thickness at about twice the recorded values for males at similar sites evaluated, but half (P < .05) of follicular diameter of values of males. Mean follicular density for oro-nasal area, head, neck, fore and hind limbs were 50 ± 3.55 and 70 ± 3.34n/µm2, 16.24 ± 3.02 and 12 ± 4.00, 8.00 ± 2.68 and 83.66 ± 4.08, 8.02 ± 4.00 and 3.23 ± 3.85, 4.32 ± 3.02 and 2.05 ± 2.04 for females and males respectively. Follicular area decreased proportionally with density increase but it was inversely proportional with epidermal thickness in all evaluated regions. This investigation suggests that the peculiarities observed in dermal structures adapt this species to environmental forces, defense and self-preservation including thermoregulation, foraging and predator evasion, whereas histo-morphometric evaluation result suggests that thermoregulation and other skin sensory modalities may differ between genders in the greater cane rat.
Keywords: Epidermal architecture, Follicular evaluations, Histology, Hystricomorpha, Morphology, Thryonomys
1. Introduction
The family Hystricomorpha comprises the Hystricidae (family of the porcupines) and includes the Thryonomys (Grass cutters); also known as the greater cane rat, they are found exclusively in Africa and described as the biggest of all African rodents [1], [2] and are represented by a single species, Thryonomys. The species inhabits dense, reedy grassland with damp or wet places [3], [4]. Distribution of cane rats is determined basically by the availability of adequate or preferred grass species for food [2]. The greater cane rat is a semi-aquatic inhabitant whereas the lesser breed is found in the upland areas.
Most of its species, subspecies and breeds described may be allied to either: T. swinderianus or the T. gregorianus (the smaller grasscutter) [5]. The thickest body measurement ranged from 40 to 60 cm in addition to 20–25 cm tail [6]. Body weight fluctuates between 2 and 4 kg and furs could be mixtures of brown, reddish and grey hairs that change with habitat. Nowadays, particularly in Benin, there is a selected breed of these rats adapted to narrow captivity [6].
Some authors have reported that the skin and hairs (fur) of the head region as well as the limbs and tails in this species are easily torn out due to thinness of the skin [7], [8], a character makes the animal intractable both in wild and captivity (See Fig. 1).
Fig. 1.

Picture of adult greater cane rat (Thryonomys swinderianus) in captivity.
Recent researches suggest that a reaction-diffusion process may be responsible for the development of cutaneous appendages such as hair, feathers or scales due to the activation of Sonic hedgehog, a paracrine factor that acts locally without much diffusion [9]. Inhibitors believed to be involved are paracrine factors; BMP4 or BMP2 [10], [11]. BMPs prevent dermal fibroblasts from aggregating, while Sonic hedgehog may support the formation and retention of the dermal papilla [9].
The importance of dermatologic oriented studies in this species cannot be overemphasized since indeed skin morphology may be a better indicator of habitat and lifestyle than phylogeny [12]. When commercial farming and laboratory investigations entails maintaining a reasonable population of cane rats for scientific research and economic purposes is a priority; then skin related investigations become a consideration. However, due to paucity of literary evidence on cane rat dermatology, any derived information from such study should contribute to an establishment of new reference and basic data.
Existing works on the cane rat include studies on the skull and mandible [13], the brain [14], the gastrointestinal tract [15] and on reproductive performance [16]. This, to the best of the author’s knowledge is the first regional dermal morphologic assessment in the greater cane rat.
2. Materials and methods
2.1. Ethical approval
Ethical approval was obtained from the Faculty of Veterinary Medicine, University of Ibadan, Nigeria. UI/ACUREC/App/2017/0028.
2.2. Animals
The animals used for the present study belong to the Genus Thryonomys (larger grasscutter- T. swinderianus). A total of twenty wild caught adult animals in equal gender composition were used for this investigation. Aging was through body length (head-tail) estimation [17].
2.3. Sampling and histological protocol
Adult animals used were sedated using Ketamine HCl at 7 mg/kg body weight. Skin tissue samples of between 1 and 2 cm2 sizes were taken from 14 selected body sites on each animal. These skin samples were taken with scalpel blades immediately after sedation and fixed in buffered 10% formol saline for 24 h before transferring to 10% formalin solution. Tissues were then dehydrated in graded increment of concentration in alcohol (50%, 70% and 90%) before clearing with Xylene followed by embedding and sectioning with the Microtome. Routine tissue processing and histologic staining (after mounting with diphenyl xanthine (DPX) of tissues with Haematoxylin and Eosin stains [18] were carried out using the Leica® automatic tissue processor in the Department of Pathology, University of Ibadan, Nigeria
2.4. Histologic assessments
A digital microscope with (Motic®) Camera 2.0 attached was used for both slide image processing and histologic assessment of micrographs after a uniform scale bar of 1:100 µm was set as shown on the left top corner of micrographs. Pictures were taken at X 100 objective lens and transferred to a programmable compatible computer, where measurements and evaluations were done with the provided conversions.
2.5. Histo-morphometric evaluations
Size, arrangement and distribution of the hair follicle and sweat glands were studied in a 2 cm2 field area of light microscope slide using Motic Image Plus software 2.0 ©China, [19] in the irregular measure module of this software for follicle area and perimeter at X10 objective lens magnifications
2.6. Statistical analysis
The data obtained were recorded as means and standard deviation (Mean ± SD) and subjected to statistical analysis using Paleontological statistics (PAST) 3.01 [20]. Statistical differences between the mean values of epidermal, reticular and papillary dermal thickness and those of hair follicles at the regions evaluated were determined using Student ‘t’ test. Paired samples test was used to determine the differences among the means values of epidermis, dermal layers and hair follicles. The statistical significance was assigned at the P < .05.
3. Results
3.1. Histologic assessments of hair follicle
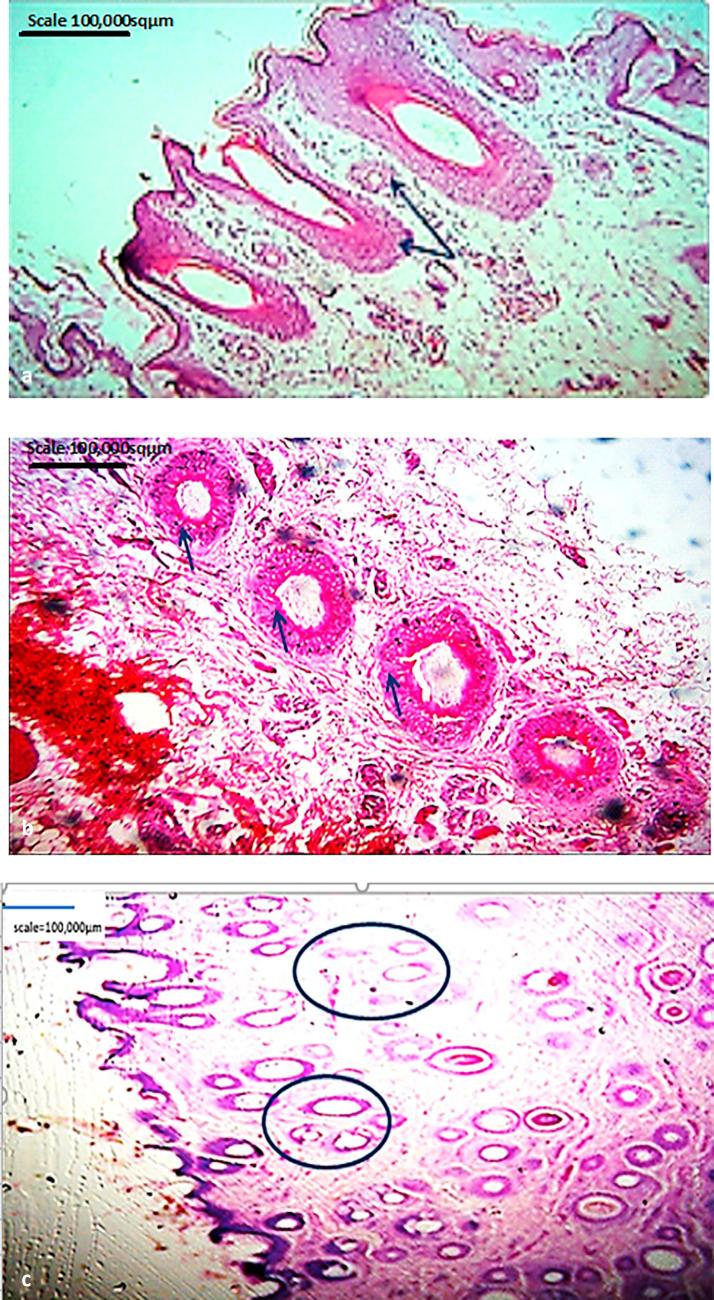
Hair follicle groupings resembled the types earlier reported in literature. Groupings varied and ranged from as few as two in a group to twelve in another. Some appeared in rows on dorsum and ventrum of cane rat body skin; some alternate in relative size, the small ones alternating with the larger ones (Fig. 2a) in the neck area compared to other body regions.
Fig. 2.
(a) Micrograph of skin around neck region showing alternating hair follicle size arrangement peculiar to cane rat (b) Micrograph of skin from lateral part of the head proximal to the ear showing hair follicles diagonal orientation arrangement peculiar to cane rat (c) Micrograph of skin around oro-nasal area in head region showing hair follicle groupings of various sizes similar to that of pigs and dogs. Stain: H&E. Magnification: ×100.
Arrangement of follicles was also peculiar as some showed linear disposition while others seemed to show diagonal orientation from the epidermal surface down the dermis at some angle especially at the sides of the head medial to the ear lobes in the skin from the lateral part of head proximal to the ear (Fig. 2b)
The skin surface was smooth where hairs were absent, projections of the epidermis but on hairy skin, the surface was broken by hair follicles and sweat pores. Skin samples taken from around oro-nasal area in the head region (Figs. 2c and 3a) showed hair follicle groupings of relative size variations peculiar only to other species (dog and pig) in similar regions of the body. The forelimb skin also demonstrated marked corrugations of epidermal layer and indistinct strata (Fig. 3b). The hind limb (Fig. 3c) revealed a different pattern where both undulating and projecting epidermises were present and the dermis was well supported with dense irregular connective tissue.
Fig. 3.
(a–d) Micrograph of skin from around oral area showing hair follicular grouping peculiar to cane rat (b) Micrograph of skin from giant cane rat forelimb showing marked corrugation of epidermis and indistinct epidermal strata (c) Micrograph of skin from giant cane rat hind limb showing undulating and projecting epidermis. (d) Micrograph of skin from dorsal part of neck of the cane rat showing degree of outward projection that was not dependent on presence, absence or extent of dermal papillae (green arrow = with dermal papilla, blue arrow = without dermal papilla) Stain: H&E. Magnification: ×100.
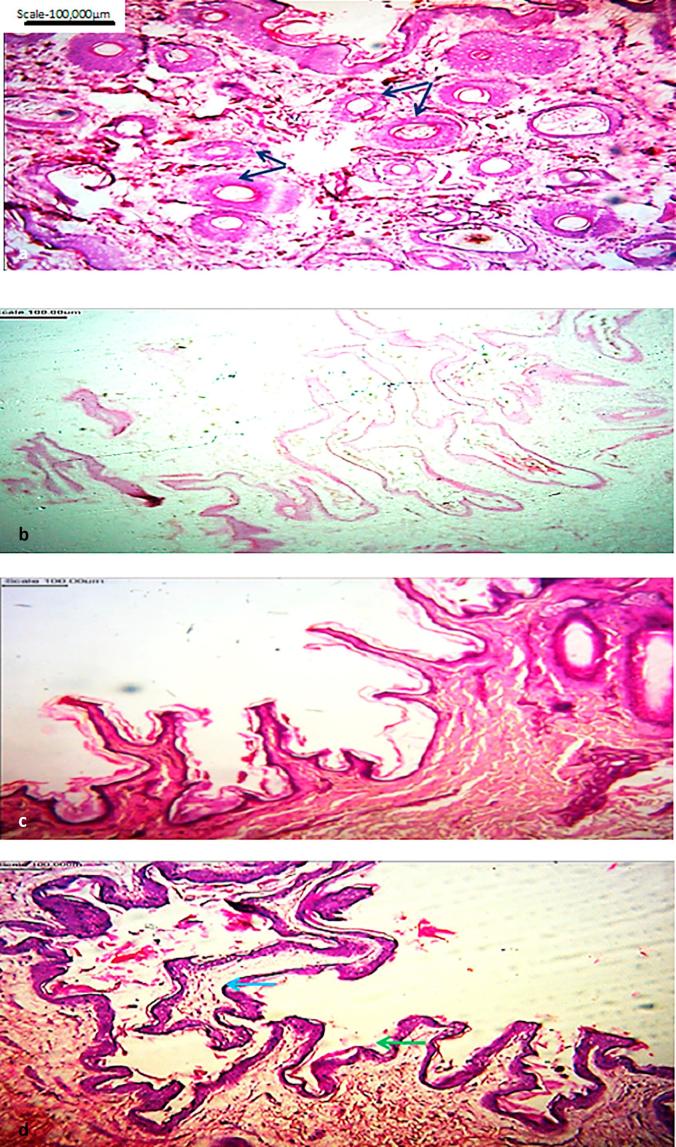
The dorsum skin surface (Fig. 3d) was smooth with only projections of the epidermis pronounced but hairs are conspicuously absent. In some areas, the stratum basale is widened showing rete ridges and rete pegs in the dermis of the skin (Figs. 3d and 4a).
Fig. 4.
(a) Micrograph of skin from the eye area of cane rat head showing absence of epidermal pegs: (b) Micrograph of skin from eye area showing hair follicles/sweat glands/adipocytes of giant cane rat. H&E. Magnification: ×100.
Summary statistics in Table 1 on follicular density across sexes revealed a least significant difference (P < .2) with Shapiro Wilks value 0.8312 but the one-way ANOVA residual vs normal order statistical median was significant P < .05, whereas follicular area showed significant (P < .001) dimorphism with higher values in the head, neck, fore limb and hind limb of males but not in the oro-nasal area, Welsh F test or unequal variances F = 56.24, df = 2.37. Similar result was observed in epidermal thickness (P < .001) but no significant differences were recorded in the epidermal thickness in neck and hindlimb region values (Welsh F = 123, df = 2.36).
Table 1.
Showing results of sites, follicular assessments and epidermal dimensions in both females and males cane rats.
| Site | Females |
Males |
||||
|---|---|---|---|---|---|---|
| Mean follicular density ± SD (n/µm2) | Mean follicular area ± SD (sqµm) | Mean epidermal thickness ± SD (µm) | Mean follicular density ± SD (n/µm2) | Mean follicular area ± SD (sqµm) | Mean epidermal thickness ± SD (µm) | |
| Oro-nasal | 55 | 132.48 ± 23.04 | 10.01 ± 0.61 | 70 | 424.5 ± 112.83 | 15.74 ± 5.77 |
| Head proximal to ear) | 16 | 4041.34 ± 44.23 | 61.22 ± 4.12 | 12 | 5917.96 ± 145.78 | 88.4 ± 20.11 |
| Neck | 8 | 7053.33 ± 63.22 | 12.44 ± 3.19 | 8 | 7844.75 ± 5379.09 | 15.37 ± 1.91 |
| Fore limb | 8 | 564.33 ± 112.31 | 111.22 ± 71.11 | 3 | 740.7 ± 328.05 | 117.46 ± 45.85 |
| Hind limb | 4 | 2001.99 ± 166.55 | 30.11 ± 3.22 | 2 | 2219.26 ± 1701.62 | 27.8 ± 5.41 |
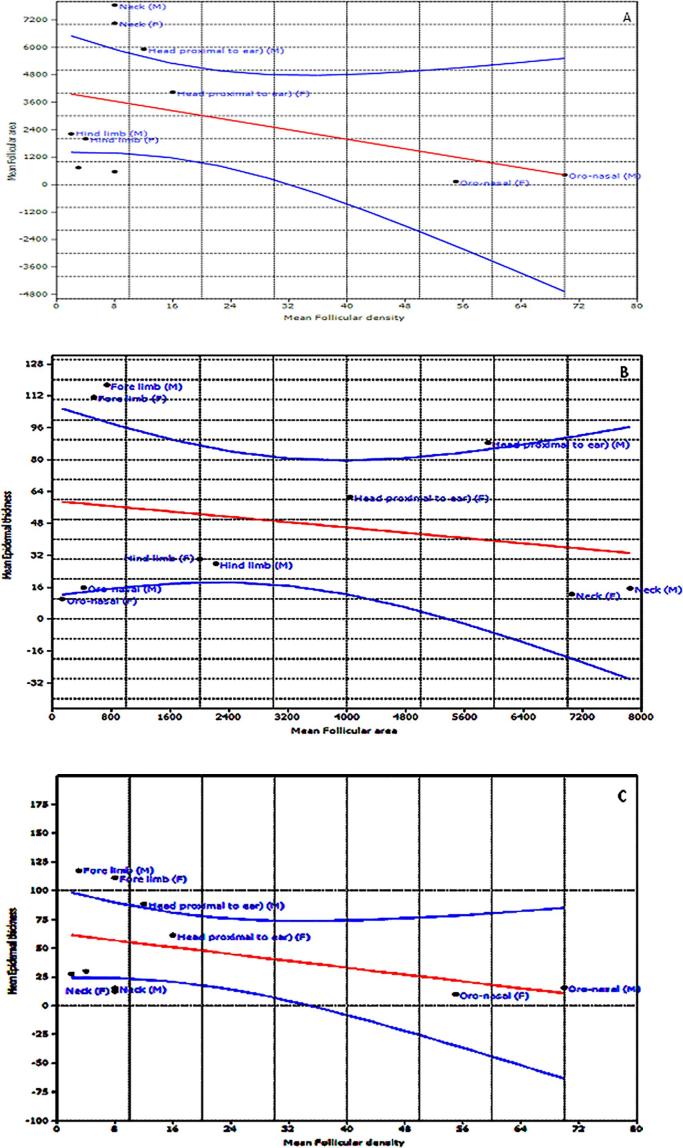
The hair follicular assessments and evaluation are shown in Table 1. Hair follicle density and thickness (cross sectional area) values were higher in the head, proximal to the ear and neck region than similar parameters from the hind limb and oro-nasal area where they were found fewer irrespective of sex as shown in the regression of follicular density but with an increase in follicular size (Fig. 5, Fig. 6A). Slope parameters were negative indicating that follicular area decreased as their density increased, intercept (positive) and significantly different from zero but not significantly (P < .2) different between sexes. Follicular density was however higher in the hind limb compared to the fore limb, females had a higher follicle density than males in the body regions evaluated.
Fig. 5.
Sex-based analysis of variation (ANOVA) showing residual vs group means/centroid size using the parameters distribution at the sites assessed.
Fig. 6.
A–C (A) A gender based ordinary least square regression of mean follicular density on mean follicular cross-sectional area at the sites evaluated at 95% confidence interval, Slope = −51.992, intercept = 4061.1, r2 = 0.17877 (B) A gender based ordinary least square regression of mean epidermal thickness on mean follicular dimensional area at 95% confidence interval at the sites evaluated, slope = -0.00334, intercept = 59.306. r2 = 0.05304. (C) A gender based ordinary least square regression of mean epidermal thickness on mean follicular density at 95% confidence interval at the sites evaluated, slope = −4.836, intercept = 27.588. r2 = 0.173.
The regressions of mean epidermal thickness on mean follicular area demonstrated a similar result at 95% confidence interval with negative slope parameters (Fig. 6B). Also indicated that epidermal thickness decreased in the regions studied as follicular area dimension increases. This observation was more pronounced in the forelimb and head regions proximal to the ear when compared to the hind limb, oro-nasal and neck regions.
A final regression of mean epidermal thickness on mean follicular density showed a similar disposition of slope variables (Fig. 6C). Also demonstrated an inverse variation of epidermal thickness with increment in follicular density values, this status was shown by all regions evaluated except at the oro-nasal area.
Sexual dimorphism in hair follicle parameters results revealed significant (P < .05) differences between the sexes. A two-sample t-test on follicular density and epidermal thickness: in oro-nasal region showed a difference between means = 49.62 (P < .02), head, proximal to ears = 60.81 (P < .04), at fore limbs = 108.84 (P < .001) and hind limbs = 25.95 (P < .003). Whereas follicular area and epidermal thickness were not significantly different between sexes at oro-nasal region. Differences were reported in follicular area and epidermal thickness in head, proximal to ear; 4904.8 (P < .03), at the neck area = 7435.1 (P < .002), at forelimbs = 538.18 (P < .002) and at the hind limbs = 2081.7 (P < .002).
4. Discussion
This study describes regional variations in epidermal morphology, hair follicular dimensions in the greater cane rat as a pilot study and is to the best of the authors knowledge a first report in this species. Hair follicle density and thickness (cross sectional area) values were higher in the head, proximal to the ear and neck region than similar parameters from the hind limb and oro-nasal area where they were found fewer irrespective of sex but with concomitant increase in size. Similarly, it was observed that follicular area decreased proportionally with density increase but inversely proportional with epidermal thickness in all evaluated regions. Density in the hind limbs was significantly higher than in the fore limb.
Alternating large and small hair follicle arrangement observed in the neck area and peculiar to greater cane rat suggest a denser aggregate of hair follicles and sensory receptors compared to other body regions probably for nursing, homeothermic and defense purposes as suggested by Joseph et al. [21]. Considerable amounts of adipose tissue were also seen at this site. Such arrangements were not noticed in an on-going research on the porcupine (Hystrix hystrix cristata) a member of the same family. Similarly, the diagonal orientation arrangement of follicles in the skin from the lateral part of head proximal to the ear is also not common to the mammalian skin but peculiar to this species and postulated to be of value in sound depth and direction assessment in foraging in the wild [9], [22]. Skin samples taken from around oro-nasal area in the head region showed hair follicle groupings of various sizes similar to those of pigs and dogs [23] but are distinctly absent in other hystricomorphs; the naked mole rat (Heterocephalus glaber) and the common mole rat (Cryptomys hottentotus) [21].
Mammalian cutaneous appendages such as hair, feathers, or scales grow with spaces between them in histo-typic aggregations, and these spaces (for instance, on the scalp) are similar from region to region [24] and are variations of a basic principle [22] whereas morphogenetic determinants (mRNA) mediate species-specific protein synthesis necessary for skin structural modifications.
The unique findings in this species are consistent with the findings of Fuch, [25] that molecular interactions involved in mesenchymal-epithelial cues required for follicle morphogenesis, hair cycling, and/or follicle differentiation may interfere with wnt pathways in regulating hair follicle patterning and morphogenesis among species [25] to produce the hair follicle number, size, distribution, patterning and arrangement unique to the current species. Follicular diameter, number and density vary with body regions and are consistent with observations of Mark-James and Miller [26].
The forelimb skin showed marked corrugations of epidermal layer and indistinct strata, it was very thin with irregular dermal junction and no discernible reticular and papillary layers. Similar findings have been reported in the common mole rat (Chryptomys hottentotus) [21]. An arrangement congenial with liberal manipulation of the limb is for self-care purposes including survival in aquatic environments. A reverse of the foregoing skin morphologic description was observed in the hind limb where both undulating and projecting epidermises were present and the dermis was well supported with dense irregular connective tissue and typical of rodents [27]. The basal cells of the stratum germinativum found typical of rodents may be considered the stem cells of epidermis [23] as they are undifferentiated and possess the capability of pluripotency [9], [23].
The dorsum skin surface was smooth where hairs were absent though projections of the epidermis are conspicuous and independent of absence dermal papilla. This arrangement is thought to serve as predilection site for skin parasites as mites on hairy skin where the surface was broken by hair follicles and sweat pores. These mammalian type characteristics reflect the environment type where this species thrives [4] and possible optimal metabolic requirements [28].
In some parts of the skin, the stratum basale was widened at spaced intervals, forming the rete ridges upwards and rete pegs which extended down into the dermis. Feldhamer et al. [29] stated that the frequency of mitotic figures in the Malpighian layer corresponds to the intensity of desquamation in the first and second layers of the spinosum often called stratum Malpighi. A relationship between membrane morphology and the palisading of the adjacent basal layer exists; showing marked palisading at locations where the subjacent basement membrane is usually thin, but staining deeply where palisading is non-existent [24], [30]. The basement membrane is variable in width, fibrillated or stratified, reticulated or fragmented, indistinct or even absent. The integrity of the dermo-epidermal junction depends on the adherence between the basal cell layer of the epidermis, and the dermis at the actual interface, and also upon the bonds uniting the individual cells of the basal layer, the basal malpighii cells or keratinocytes and the basal melanocytes [22].
The present species offers new opportunities into novel dermatological evaluations of the adult skin due to histologic peculiarities observed unfamiliar to other rodents. Higher hair follicle density and thickness (cross sectional area) in the head, proximal to the ear and neck regions portrays a gender dimorphic pattern and regional furry outlook coupled with high scruff skin mobility [30] especially in males which permit an easy twist of neck for possible quick bite delivery [13], [31]. This feature may also be associated with escape mechanism; an adaptation in deceptive grip hold on the cane rat likely to be lost with the characteristic body mass jerk.
5. Conclusions
The sexually dimorphic regional skin architectural features observed in greater cane rats in the current investigation may be adaptations for nursing purposes. Follicular area decreases proportionally with density increase but it is inversely proportional with epidermal thickness in all evaluated regions. Further dermatological studies on immunohistochemistry may be valuable in description and characterization of the cane rat skin, and give further insight into clinical application in skin integrity and peculiarities in wound-immune response/closure.
Competing interests
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Asibey E.O.A. The grass cutter (Thyonomys swinderianus), TEMMINCK in Ghana. Symp, Zool. Soc., London. 1974;34:161–170. S Biol Cons 6, 1974, 32– 39. [Google Scholar]
- 2.National Research Council (NRC) National Academy Press; Washington DC: 1991. Micro livestock; Little Known Small Animals with a Promising Economic Future; p. 449. [Google Scholar]
- 3.Ajayi S.S. Wildlife as a source of protein in Nigeria, Some Priorities of Development. Nig Field. 1971;36:115–127. [Google Scholar]
- 4.Abioye F.O., Udah A.C., Opara M.N., Onyema A.C., Aju P.C. Adaptability Study of Grasscutter (Thryonomys swinderianus) in natural and Domestic Environments. Ann Conf Assoc Nig. 2008:155–159. [Google Scholar]
- 5.Alogninouwa T., Agba K.C., Agossou E., Kpodekon M. Anatomical Histological and functional specificities of the digestive tract in the male grascutter (Thryonomys swinderianus, Temminck) Anat Histol Embryol. 1996;25:15–21. doi: 10.1111/j.1439-0264.1996.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 6.Senou M., Yawadan L.T., Schrage R.C. Proc 1st conf. Grasscutter prod.; Cotonou, Benin: 1992. Contribution to genetic improvement of grasscutter (Thryonomys swinderianus) pp. 175–185. [Google Scholar]
- 7.Happolds D.C.D., Happolds E. Clarendon Press; Oxford: 1987. Mammals of Nigeria; pp. 122–144. [Google Scholar]
- 8.Kingdon J. Academic Press; London and New York: Nat. world: 1997. The Kingdon Field Guide to African Mammals; p. 45. [Google Scholar]
- 9.Gilbert E.F. 8th edn. Sinauer; Sunderland, MA: 2008. Developmental Biology; pp. 266–287. [Google Scholar]
- 10.Jung H.S., Francis-West P.H., Widelitz R.B., Jiang T.X., Ting-Berreth S., Tickle C. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- 11.Noramly S., Morgan B.A. BMPs mediate lateral inhibition at successive stages in feather tract development. Dev. 1998;125:3775–3787. doi: 10.1242/dev.125.19.3775. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand M. 3rd ed. John Wiley & Sons; New York: 1988. Analysis of Vertebrate Structure. [Google Scholar]
- 13.Samuel O.M., Parés-Casanova P.M., Nwaogu I.C., Olopade J.O. Geometric morphometrics of the skull of two African rodents, Thryonomys swinderianus and Cricetomys gambianus. Ann Exp Biol. 2015;3:21–30. [Google Scholar]
- 14.Slominski A.T., Zmijewski M.A., Skobowiat C., Zbytek B., Slominski R.M., Steketee J.D. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012;212(vii):1–115. doi: 10.1007/978-3-642-19683-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Opara M.N., Ike K.A., Okoli I.C. Haematology and plasma biochemistry of the wild adult African Grasscutter (Thryonomys. swinderianus Temmninck) J Am Sci. 2006;2:17–22. [Google Scholar]
- 16.Nguema-Ntsame M., Edderai D. Act. du Sém. Int. sur l’élévage intensif de gibier a’ but alim; en Afr Libreville: 2000. Resultats zootechniques de la station d’aulacodiculture d’Owendo; pp. 75–77. [Google Scholar]
- 17.Dendrinos P., Karamanlidis A.A., Kotomatas S., Paravas V., Adamantopoulou S. Report of a new mediterranean monk seal (Monachus monachus) breeding colony in the aegean sea. Gre. Aq. Mam. 2008;34:355–361. [Google Scholar]
- 18.Elizabeth J.K. 4th ed. Churchill Livingstone; Philadelphia Pa: 2000. Wheater’s Functional Histology: A Text and Color Atlas; p. 424. [Google Scholar]
- 19.Motic Images software ® China, 2007.
- 20.Hammer Ø., Harper D.A.T., Ryan P.D. (PAST) Paleontological Statistics Software Package for Education and Data Analysis. Palaeont Elect. 2013;1:9. [Google Scholar]
- 21.Joseph T., Daly M., Buffenstein R. Skin morphology and its role in thermoregulation in Delmolerats, Heterocephalus glaber and Cryptomys hottentotus. J. Anat. 1998:495-2. doi: 10.1046/j.1469-7580.1998.19340495.x. Printed in the United Kingdom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nohno T., Kawakami Y., Wada N., Ishikawa T., Ohuchi H., Noji S. Differential expression of the two closely related LIM-class homeobox genes LH-2A and LH-2B during limb development. Biochem Biophys Res Commun. 1997;238:506–511. doi: 10.1006/bbrc.1997.7320. [DOI] [PubMed] [Google Scholar]
- 23.Dellmann H., Brown E.M. Lea and Fibiger; Philadelphia: 1976. Textbook of Veterinary Histology; pp. 456–457. [Google Scholar]
- 24.Yahav S., Buffenstein R. Huddling behaviour facilitates homeothermy in the naked mole-rat Heterocephalus glaber. Phys Zool. 1999;1164:871–884. [Google Scholar]
- 25.Fuch E. Beauty is skin deep: the fascinating biology of the epidermis and its appendages. Harvey Lect. 1998;94:47–77. [PubMed] [Google Scholar]
- 26.Mark-James G., Miller J. 4th Edn. Elsevier Inc.; 2006. Looking bill and Mark’s Principles of Dermatology; p. 6. [Google Scholar]
- 27.Kusi C., Tuah A.K., Annor S.Y., Djang-Fordjour K.T. Determination of dietary crude protein level required for optimum growth of the grasscutter in captivity. Livest Res Rural Dev. 2012;24:10. http://www.lrrd.org/lrrd24/10/kusi24176.htm [Google Scholar]
- 28.Widelitz R.B., Early Chuong CM. Events in skin appendage formation: induction of epithelial placodes and condensation of dermal mesenchyme. J Invest Derm Sympos Proc. 1998;4:302–306. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- 29.Feldhamer G., Thompson B., Chapman J. The John Hopkins University Press; Baltimore and London: 2003. Wild mammals of North America; p. 117. [Google Scholar]
- 30.Fitzinger F. Cane Rats. In: Nowak R., editor. Walker's Mammals of the World. The Johns Hopkins University Press; 1995. pp. 1650–1651. [Google Scholar]
- 31.Weiss L., Greep R.O. 4th ed. Mc Graw-Hill Incorp.; US: 1977. Histology; p. 133. [Google Scholar]