Abstract
Ochratoxin A (OTA) formation prevention is not usually available so counteracting strategies are urgent. This study investigated the toxic effects of OTA on Nile tilapia with trials to neutralize these effects by using some feed additives. Supplements used as one percent (Garlen Extra4, Nigella sativa, Garlen Extra4 plus Nigella sativa and a commercial mycotoxins binder, Fero Bind Pro) were added to 500 ppb/kg OTA-contaminated diet. Fish were fed at 3% body weight per day for 10 weeks. The clinical signs recorded in OTA – intoxicated positive control group were sluggish swimming and off food before death with reduction in survivability (53%) and growth performance. Several post-mortem lesions were in liver, kidneys and spleen. Serum levels of ALT, AST, creatinine and urea were significantly increased with reduction in total protein, albumin and globulin in ochratoxicated fish group compared to the negative control group. Concerning the pathological changes that have been noticed in ochratoxin treated fish were almost completely alleviated in examined tissues of fish that were fed on diet with Garlen Extra4 plus Nigella sativa or Nigella sativa and partially in fish that were fed on diet with Garlen Extra4 or Fero Bind Pro, respectively. Best detoxifying results were obtained by using 30 g/kg Nigella sativa plus 0.1 g/kg Garlen Extra4 followed by Nigella sativa, Garlen Extra4 then Fero Bind Pro as a commercial Mycotoxins binder. It could be concluded that inclusions of 30 g/kg Nigella sativa plus 0.1 g/kg Garlen Extra4 in Oreochromis niloticus (O. niloticus) diets could partially reduce OTA toxic effects.
Keywords: Detoxifying agents, Feed additives, Garlen, Mycotoxins binder, Nigella sativa
1. Introduction
Mycotoxins have negative impacts on fish production, growth as well as immune system [1]. Ochratoxins are small mycotoxins groups and Ochratoxin A (C20H18ClNO6) is a secondary metabolite produced by Aspergillus ochraceus (A. ochraceus) and Penicillium viridicatum. Ochratoxin A (OTA) is a potential fungal toxin for fish and it is associated with feed contamination as corn, oilseeds and cereal grains that affecting animals' performance through damaging kidney function [2]. OTA mode of action can be nephrotoxic, immunosuppressive [3], teratogenic and carcinogenic to animals. OTA has public health importances as association with human kidney disease and Balkan Endemic Nephropathy (BEN) probable causative agent [4]. Many chemical, physical and biological mycotoxin nuterlizing techniques had been developed [1]. In fish diseases controling strategies, medicinal plants interest is considered cheap, practical and effective mean that has burgeoned by increasing the efficiency of new plant-derived drugs besides the growing natural products interest generally. Garlen Extra4 is a garlic extract (allicin). Garlic (Allium sativum) is one of the medicinal plants that has antibacterial, antifungal, antiviral in addition to antiprotozoal properties as well as beneficial effects on the immunity [5]. Garlic bulb extract inhibited completely A. ochraceus growth [6]. Nigella sativa, has immune-stimulant, antioxidant, anti-tumour, anti-inflammatory and antibacterial activities [7]. Fero Bind Pro is a commercial mycotoxins binder feed supplement that had many strategies for a good mycotoxin defense as antioxidant and immuno-stimulant properties of vitamin E [8]. Moreover, Fero Bind Pro had OTA degrading ability besides to fish immune enhancing by yeast cell that is a one of Fero Bind Pro ingredients [9], [10] as well as Fero Bind Pro had a clay absorbing activity [11]. Effects of dietary OTA have not been investigated intensively in Nile tilapia, therefore the present work was conducted to investigate the adverse effects of OTA on growth performance, immuno-physiological parameters and histopathological alterations in O. niloticus and to evaluate the best detoxification effects of Garlen Extra4 (Garlic), Nigella stiva and Fero Bind Pro.
2. Material and methods
2.1. Fish
A total of 90 apparently healthy O. niloticus with average body weight of 55 ± 5 ɡ/fish were kept in aquaria measuring (90 × 45 × 45 cm) at Animal Health Research Institute, Kafr El-Sheikh branch. Aquaria were supplied with chlorine-free tap water [5] and were aerated continuously at 27 ± 2 °C. Half of the water was changed daily. Fish were acclimated and were fed on the basal diet only for 14 days.
2.2. Experimental design and feeding diets
Fish were divided randomly into six groups (15 fish each) and were fed the prepared pelleted experimental diets for two months as the experimental design (Table 1). Diets were formulated according to [12] (Table 2) and were analysed chemically for different nutrients and OTA according to [13], [14] respectively. Digestible energy was calculated (kcal/kg) according to [12]. OTA was produced by growing Aspergillus ochraceus. The selected isolates were previously found to be producers of OTA. Spore suspensions of each isolate were prepared from colonies of toxigenic A. ochraceus, which were previously grown on Czapeck yeast agar (CYA) for 7 days at 25 °C then used for ochratoxin production [15]. Toxigenic A. ochraceus strain culture was kindly obtained from Prof. Dr. Ramadan Salem at Mycology Dept., Ainmal Health Research institute, Dokki, Giza, Egypt on sterilized mycotoxin-free crushed yellow corn. The moldy crushed yellow corn was steamed to kill the fungus, dried, milled and analyzed for OTA determination using thin-layer chromatography (TLC) [14]. The diet was provided daily at 3% of fish body weight [16]. Feed was offered six days/ week twice daily. Feed amount was adjusted bi-weekly based on the actual body weight changes of fish. Fish body weight, body weight gain, feed conversion ratio, Relative Growth Rate (RGR) and survival rate were calculated at the end of the experiment [17].
Table 1.
Outline of the experimental design.
| Group | Diet |
|---|---|
| 1 | Basal diet + 0% (OTA)* (negative control) |
| 2 | Basal diet + 500 ppb OTA (positive control) |
| 3 | Basal diet + 500 ppb OTA + 0.1 g/kg Garlen Extra4** |
| 4 | Basal diet + 500 ppb OTA + 30 g/kg Nigella sativa*** |
| 5 | Basal diet + 500 ppb OTA + 0.1 g/kg Garlen Extra4 + 30 g/kg Nigella sativa |
| 6 | Basal diet + 500 ppb OTA + 0.5 g/kg Fero Bind Pro****(Mycotoxins binder) |
Ochratoxin A.
Garlen Extra4: Commercial feed additive, contain Garlic extract (25%), blend of volatile oil (10%).A product from Media Vet. Co. for Imp. And Exp. Kafr El-Sheikh. Egypt. Manufactured by: Hefei Royal Eagle Imp. & Exp. Co. Ltd. China.
Nigella sativa: Fresh prepared seeds powder. The proximate analysis of Nigella sativa dry biomass as feed base provided: 92.93% dry matter, 7.07% moisture, 19.04% crude protein, 21.4% crude fat, 5.3% Ash, 47.19% total carbohydrate, 0.27% Ca, 0.51% total phosphorus, 4316.11 Metabolizable Energy (Metabolizable Energy was calculated according to [55].
Fero Bind Pro: Commercial feed supplement acting as mycotoxins binder consisted of yeast (cell wall extract) vitamin E & Se, enzymes, butylated hydroxyl toluene (BHT), bentonite and clay minerals, Betaine and herbal extract (Quillaya saponaria).
Table 2.
Ingredients Proximate analysis of the experimental different dietary treatments.
| Ingredients | Treatments | Basal diet | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Ochratoxin A | – | + | + | + | + | + | |
| Garlen Extra4 | – | – | 0.010 | – | 0.010 | – | |
| Nigella sativa | – | – | – | 3.0 | 3.0 | – | |
| Fero Bind Pro | – | – | – | – | – | 0.050 | |
| Yellow corn (7.5%) | 34 | 34 | 36.29 | 34.9 | 34.89 | 33.95 | |
| Soybean meal (48%) | 39 | 39 | 39 | 38.7 | 38.7 | 39 | |
| Fish Meal (Menhaden, 64%) | 10 | 10 | 10 | 10 | 10 | 10 | |
| Wheat bran (16.4%) | 5 | 5 | 2.1 | 2 | 2 | 5 | |
| Corn glutine (60%) | 5 | 5 | 5.5 | 5 | 5 | 5 | |
| Soybean oil | 5 | 5 | 5.100 | 4.4 | 4.4 | 5 | |
| Vitamins & mineral mixture* | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | |
| Dicalcium phosphate | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | |
| Total (kg) | 100 | 100 | 100 | 100 | 100 | 100 | |
| Values between parentheses are determined crude protein content (N×6.25) | |||||||
| Proximate analysis | |||||||
| Dry matter (DM) | 90.12 | 90.12 | 90.12 | 90.17 | 90.17 | 90.13 | |
| Moisture | 9.88 | 9.88 | 9.88 | 9.83 | 9.83 | 9.87 | |
| Crude protein (CP) | 31.54 | 31.54 | 31.54 | 31.54 | 31.54 | 31.54 | |
| Ether extract (EE) | 7.755 | 7.755 | 7.83 | 7.715 | 7.71 | 7.753 | |
| Crude fiber (CF) | 2.75 | 2.75 | 2.52 | 2.73 | 2.73 | 2.75 | |
| Nitrogen free extract (NFE**) | 41.275 | 41.275 | 41.54 | 41.385 | 41.39 | 41.247 | |
| Ash | 6.80 | 6.80 | 6.69 | 6.80 | 6.80 | 6.84 | |
| Methionine | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | 0.60 | |
| Lysine | 1.84 | 1.84 | 1.84 | 1.84 | 1.84 | 1.84 | |
| Calcium | 1.01 | 1.01 | 1.01 | 1.02 | 1.02 | 1.02 | |
| Total phosphorus | 1.03 | 1.03 | 1.01 | 1.01 | 1.01 | 1.03 | |
| Digestable Energy (DE kcal /kg) | 3090.76 | 3090.76 | 3090.33 | 3094.74 | 3094.52 | 3089.66 | |
The used vitamins & mineral mixture* (Multivita Co.) composed of vitamin A 12000000 IU, vitamin D3 2200000 IU, vitamin E 10000 mg, vitamin K3 2000 mg, vitamin B1 1000 mg, vitamin B2 5000 mg, vitamin B6 1500 mg, vitamin B12 10 mg, Niacin 30000 mg, Biotin 50 mg, Folic acid 1000 mg, Pantothenic acid 10000 mg, Iron 30000 mg, Manganese 60000 mg, Copper 4000 mg, Zinc 50000 mg, Iodine 1000 mg, Cobalt 100 mg, Selenium 100 mg, calcium carbonate (CaCO3) carrier to 3000 g.**NFE = Nitrogen free extract and calculated by difference {100 − (moisture% + CP% + EE% + CF% + Ash%). Carbohydrates = NFE + CF.
2.3. Clinical symptoms and postmortem examination of fish
OTA clinical signs and postmortem lesions in Nile tilapia were detected according to [18] and [19] respectively.
2.4. Blood collection
By the end of experiment (2nd month), 2 mL of blood/fish via the caudal vessels were collected from seven fish from each experimental group [5]. Part of blood was collected by syringe containing EDTA anticoagulant for differential leukocytic count [20]. Another part of blood samples was centrifugated for serum collection for biochemical determination of serum total protein, serum albumin, serum globulin, albumin/ globulin ratio, activities of ALT (GPT) and AST (GOT), urea and creatinine [5].
2.5. Assessment of Ochratoxin A level in musculatures
As musculatures are the edible part of fish, OTA detection in fish musculatures samples using TLC was conducted as mentioned before [14].
2.6. Histopathological examination
At the end of the experiment, specimens from liver, kidneys, muscles and heart were fixed immediately in 10% formalin and were processed by using routine paraffin embedding method. Sections of 4 μm thickness were stained by hematoxylin and eosin [21] then examined microscopically.
2.7. Biosafety measures
All biosafety measures were applied throughout the whole research and after finishing the experiment. All equipment and surfaces were disinfected. Fish and blood samples were decontaminated before discarding [22].
2.8. Statistical analysis
The data were analyzed statistically by one way ANOVA test [23].
3. Results
3.1. Clinical signs and postmortem lesions
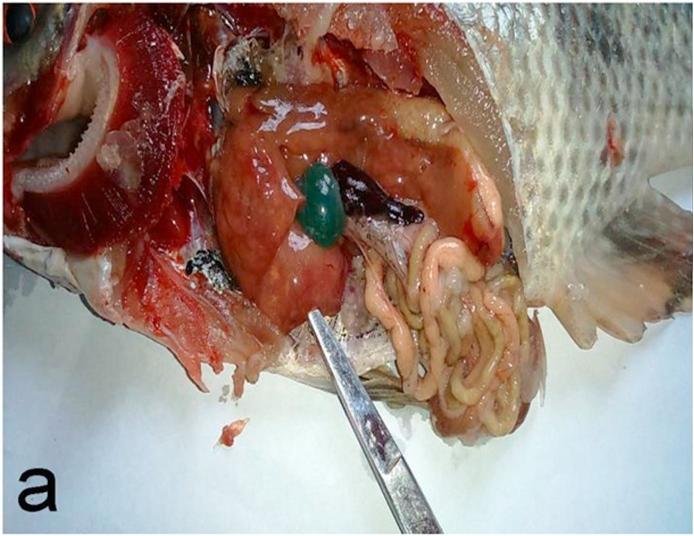
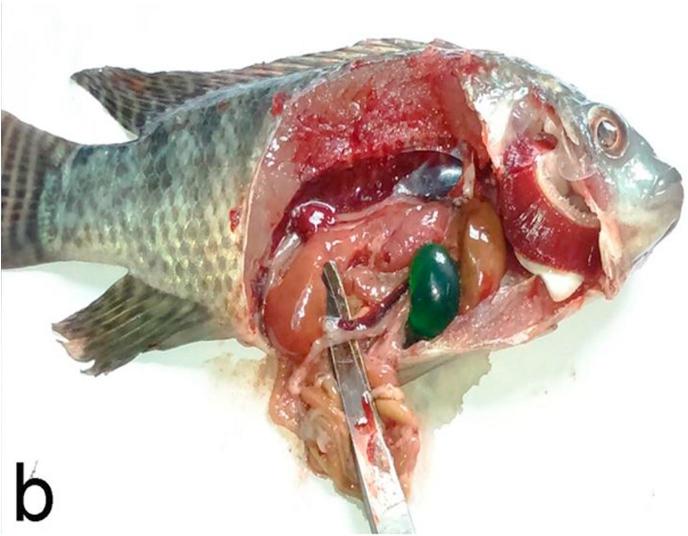
There was no noticeable change in color of skin or fins, but there were decreasing in feed intake, sluggish swimming and off food before death in OTA intoxicated positive control group in comparison with negative control and other experimental groups. Post-mortem examination revealed congested gills, enlarged pale liver with necrotic foci, enlarged gall bladder and congested enlarged spleen and kidneys (Fig. 1a, Fig. 1b). Mortalities began two weeks after starting the experiment and continued until the fourth week then stopped.
Fig. 1a.
Experimentally OTA intoxicated O. niloticus showing enlarged pale liver with necrotic foci, enlarged gall bladder, congested enlarged spleen and congested gills.
Fig. 1b.
Experimentally OTA intoxicated O. niloticus showing enlarged pale liver, enlarged gall bladder, congested enlarged spleen, congested gills and kidneys.
3.2. Growth performance
Ochratoxin intoxicated fish positive control (2nd group) had lower survival rate than the negative control group. Moreover, the 5th group had the same survival rate as the negative control group. The best body weight gain, final body weight, relative growth rate as well as feed conversion ratio were observed in negative control followed by 5th and 4th groups, respectively, while the lowest results were observed in the 2nd group. The 3rd and 6th groups had nearly the same body weight gain but the 6th group was better than the 3rd group in the relative growth rate and feed conversion rate (Table 3).
Table 3.
Effect of different treatments on growth performance and survivability of Oreochromis niloticus at the end of the experiment.
| Group | Survival rate | Growth parameters |
||||
|---|---|---|---|---|---|---|
| Initial body weight | Final body weight | Body weight gain | F.C.R* | RGR** | ||
| 1 | 100% | 60.69 ± 0.09a | 98.55 ± 0.1a | 37.86 ± 0.04a | 1.7 ± 0.07c | 47.55 |
| 2 | 53% | 60 ± 3.05a | 70.46 ± 0.1f | 10.46 ± 0.1e | 3 ± 0.1a | 16.04 |
| 3 | 93% | 56.75 ± 0.15a | 76.79 ± 0.03d | 20.04 ± 0.14d | 2.8 ± 0.05ab | 30.01 |
| 4 | 53% | 55.67 ± 0.21a | 77.16 ± 0.04c | 22.49 ± 0.03c | 2.5 ± 0.02b | 33.86 |
| 5 | 100% | 53.67 ± 1.4a | 77.86 ± 0.01b | 24.51 ± 0.07b | 2.4 ± 0.02b | 37.26 |
| 6 | 93% | 55 ± 1.15a | 75.23 ± 0.04e | 20.23 ± 0.12d | 2.4 ± 0.2b | 31.07 |
Data represented as Mean ± SE, FCR*: Feed Conversion Ratio. RGR**: Relative Growth Rate. Means within the same column of different letters are significantly different at (P < .05).
3.3. Immuno-physiological parameters
Effects of OTA on the differential leukocytes were illustrated on (Table 4). There was only significant difference in neutrophils count among all treatments. On the other hand, there was no significant difference in other leukocytes types numbers among different treatments.
Table 4.
Results of differential leukocyte count among different groups at the end of the experiment.
| Group | N | Neutrophils | Lymphocytes | Monocytes | Eosinophils | Basophils |
|---|---|---|---|---|---|---|
| 1 | 7 | 31 ± 2.8a | 58 ± 10.3 a | 4 ± 0.57 a | 4 ± 1.15 a | 3 ± 0.57 a |
| 2 | 7 | 17 ± 0.57c | 72 ± 4.04 a | 5 ± 0.57 a | 3 ± 0.57 a | 3 ± 1.15 a |
| 3 | 7 | 22 ± 0.57 bc | 68.3 ± 1.6 a | 5 ± 0.57 a | 3 ± 0.57 a | 2 ± 0.57 a |
| 4 | 7 | 27 ± 1.7 ab | 63 ± 2.8 a | 4.6 ± 0.33 a | 2.6 ± 0.33 a | 1 ± 0 a |
| 5 | 7 | 30 ± 2.3 a | 61 ± 2.3 a | 4 ± 1.15 a | 4 ± 0.57 a | 2 ± 0.57 a |
| 6 | 7 | 24 ± 4.04 abc | 63 ± 2.3 a | 6 ± 1.15 a | 3 ± 0.57 a | 4 ± 1.7 a |
Data represented as Mean ± SE. Means within the same column of different letters are significantly different at (P < .05).
The immune suppressive effect of OTA was illustrated (Table 5). There were significant reductions in serum total protein, albumin, globulin in the positive control group when compared to the negative control group. The 5th group had significant improvement in serum total protein and globulin levels when compared to other treatments (3rd, 4th and 6 th groups).
Table 5.
Results of serum total protein, albumin, globulin and albumin/globulin ratio among different groups at the end of the experiment.
| Group | N | Total protein (g/dL) | Albumin (g/dL) | Globulin (g/dL) | A/G ratio |
|---|---|---|---|---|---|
| 1 | 7 | 5.60 ± 0.29a | 2.11 ± 0.63 a | 3.500 ± 0.2 ab | 0.58 ± 0.15 |
| 2 | 7 | 3.38 ± 0.03b | 1.21 ± 0.06b | 2.17 ± 0.01b | 0.55 ± 0.03 |
| 3 | 7 | 3.730 ± 0.6bc | 1.23 ± 0.07b | 2.500 ± 0.17 ab | 0.49 ± 0.03 |
| 4 | 7 | 4.4 ± 0.4abc | 1.35 ± 0.03 ab | 3.023 ± 0.07 ab | 0.46 ± 0.003 |
| 5 | 7 | 5.130 ± 0.5ab | 1.36 ± 0.03 ab | 3.77 ± 0.076 a | 0.38 ± 0.008 |
| 6 | 7 | 3.500 ± 0.c | 1.26 ± 0.06 ab | 2.24 ± 0.18b | 0.62 ± 0.05 |
Data represented as Mean ± SE. Means within the same column of different letters are significantly different at (P < .05).
Hepato renal toxicity of OTA was illustrated in (Table 6). There was a significant elevation in ALT and AST enzymes, urea and creatinine levels in positive control group when compared to the negative control one. Moreover, these parameters were significantly reduced by using different feed supplements.
Table 6.
Effect of different treatments on serum AST, ALT, urea and creatinine of Oreochromis niloticus at the end of the experiment.
| Group | Parameters |
|||
|---|---|---|---|---|
| AST (uL/mL) | ALT (uL/mL) | Urea (mg/dL) | Creatinine (mg/dL) | |
| 1 | 23 ± 2.3b | 4 ± 1b | 6.3 ± 2.3b | 0.35 ± 0.07c |
| 2 | 58.5 ± 20.4a | 10 ± 2 a | 11.3 ± 1.8a | 0.88 ± 0.07a |
| 3 | 49.5 ± 1.4 ab | 8 ± 4ab | 6.8 ± 1.4b | 0.47 ± 0.09bc |
| 4 | 46 ± 2.3 ab | 8 ± 1 ab | 6.85 ± 1.05b | 0.44 ± 0.07bc |
| 5 | 31 ± 0.57ab | 7.9 ± 0.12 ab | 6.55 ± 0.75b | 0.43 ± 0.08bc |
| 6 | 57 ± 5.7a | 8 ± 3 ab | 8.95 ± 0.45ab | 0.58 ± 0.14b |
Data represented as Mean ± SE. Means within the same column of different letters are significantly different at (P < .05).
3.4. Ochratoxin A level in fish musculatures
Assessment of Ochratoxin A level in musculatures of all experimental groups for OTA residues indicated that Ochratoxin was not detected in fish musculature of any fish group.
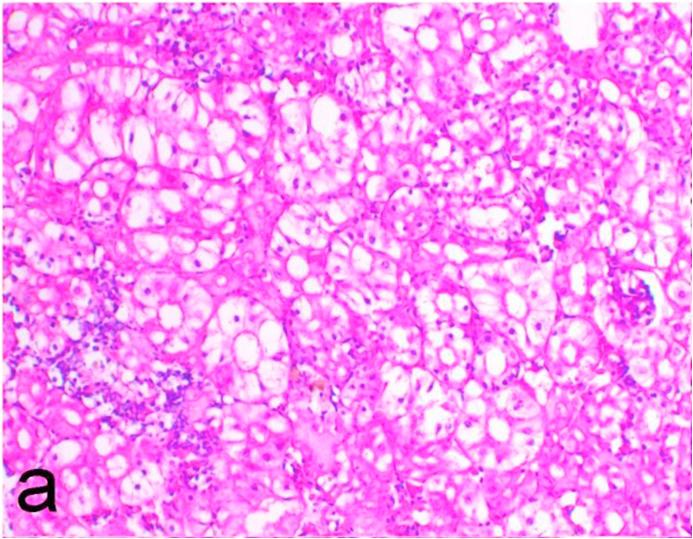
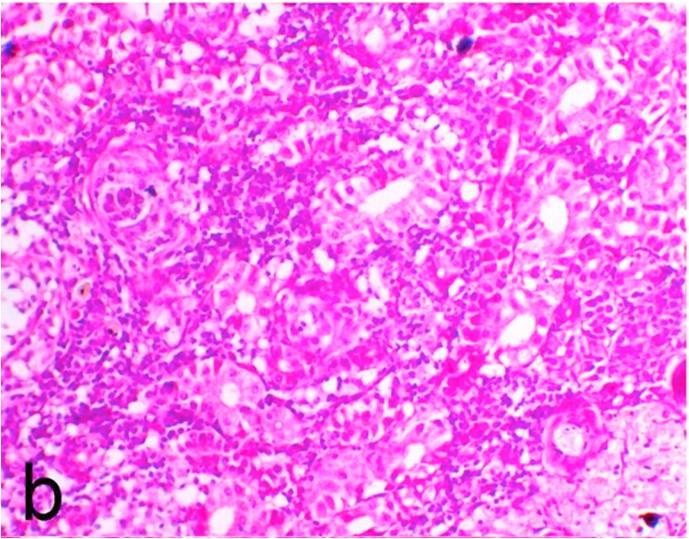
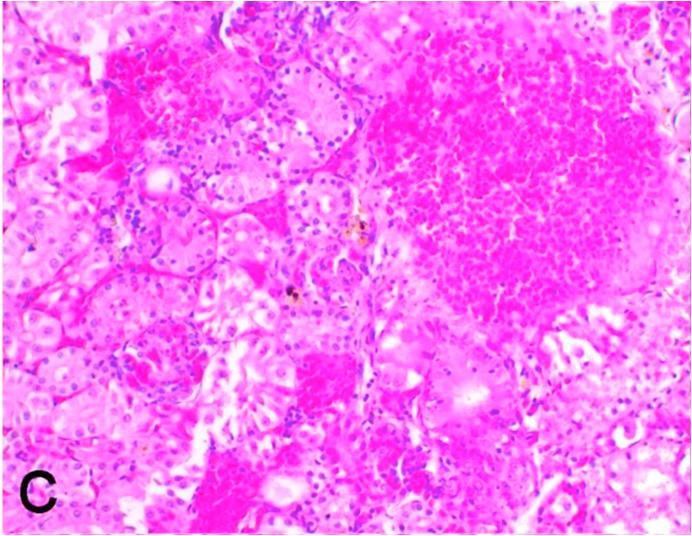
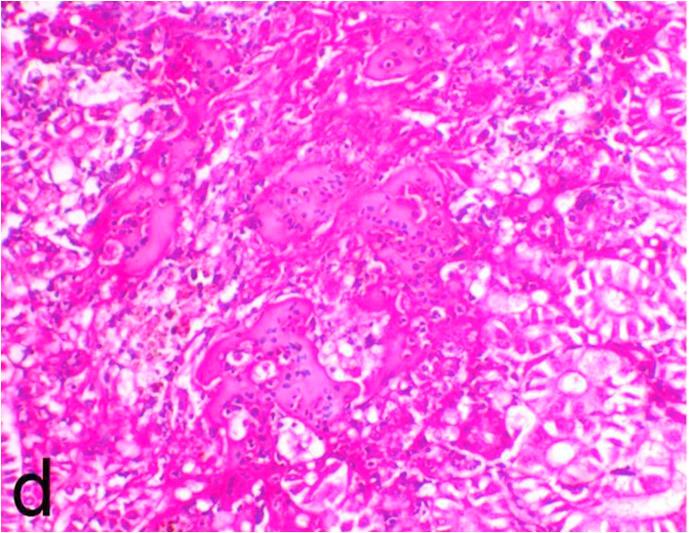
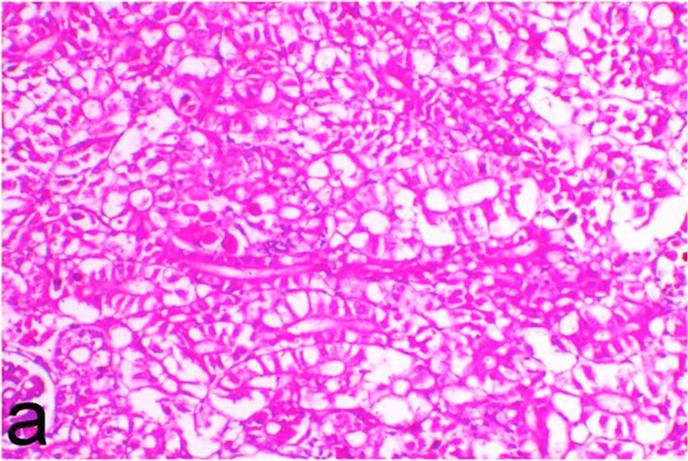
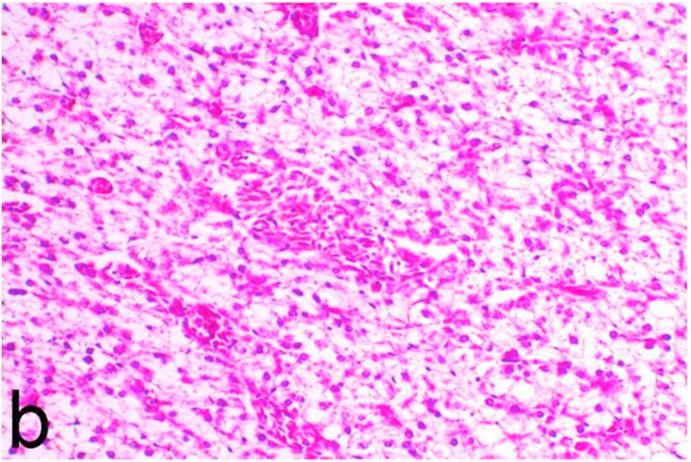
3.5. Pathological examination
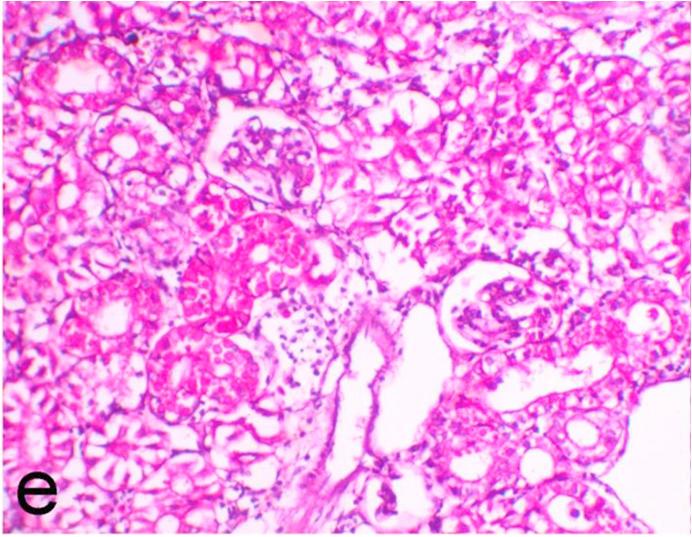
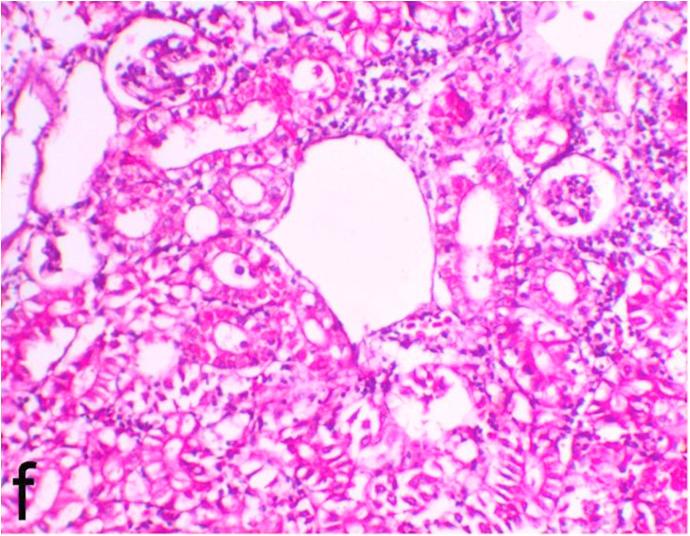
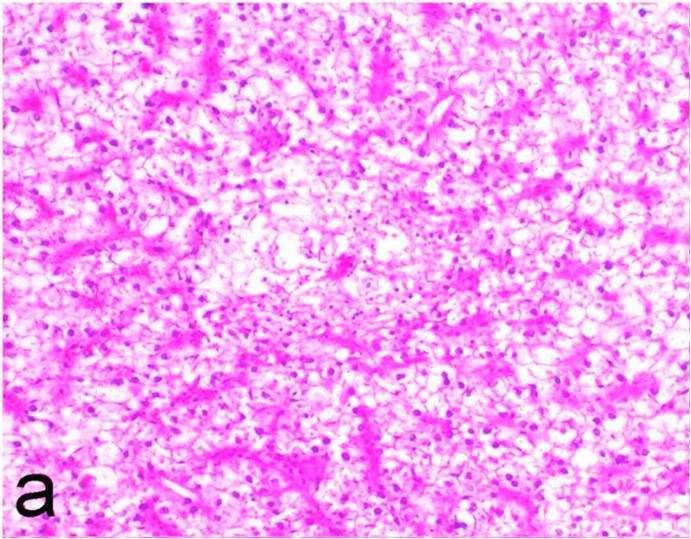
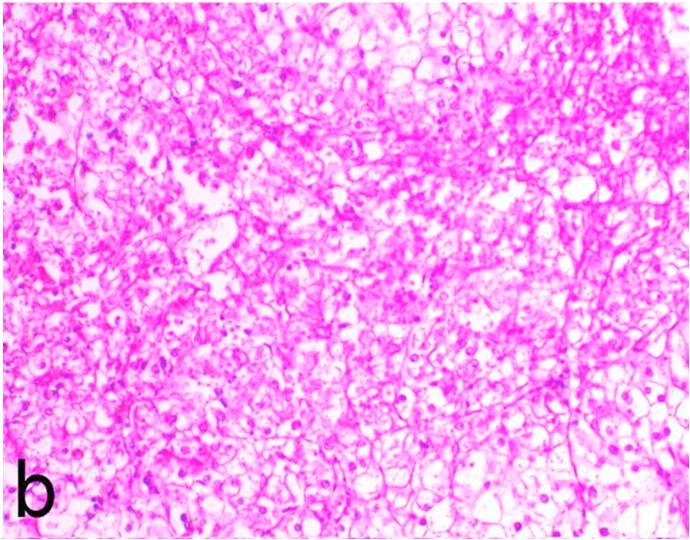
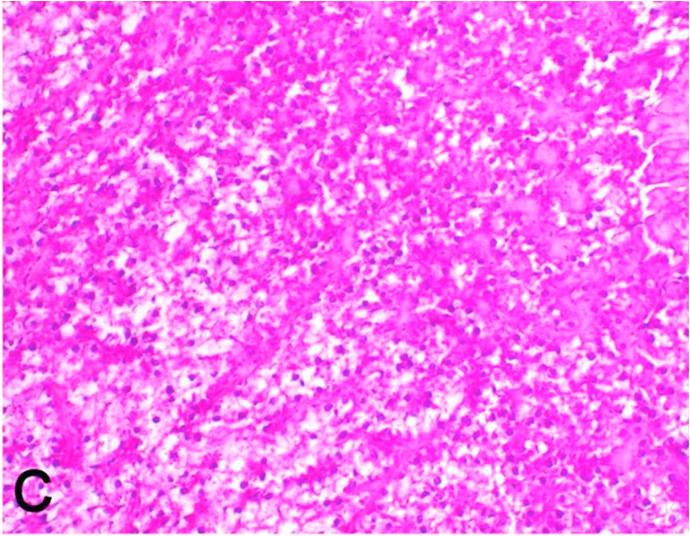
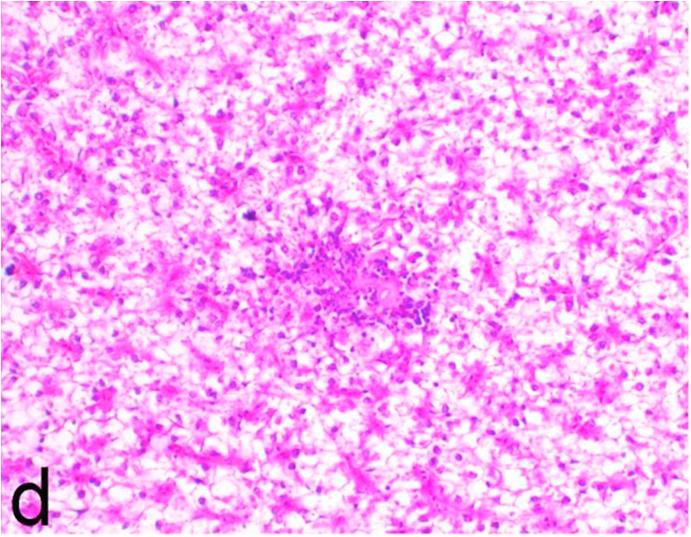
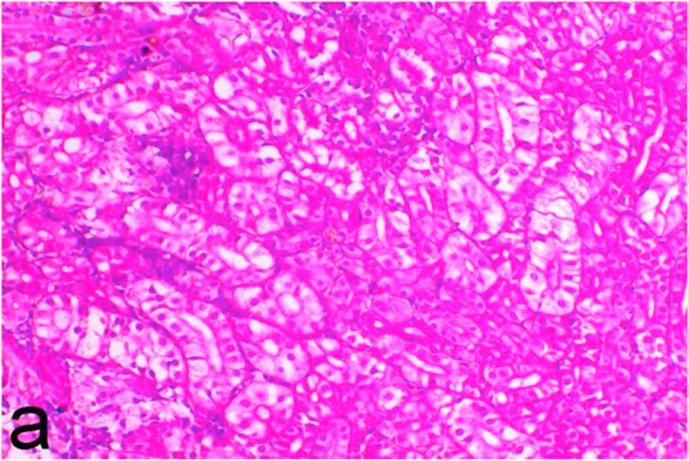
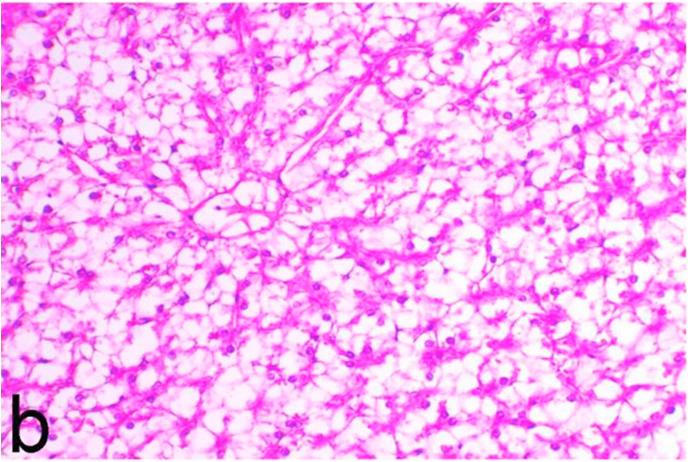
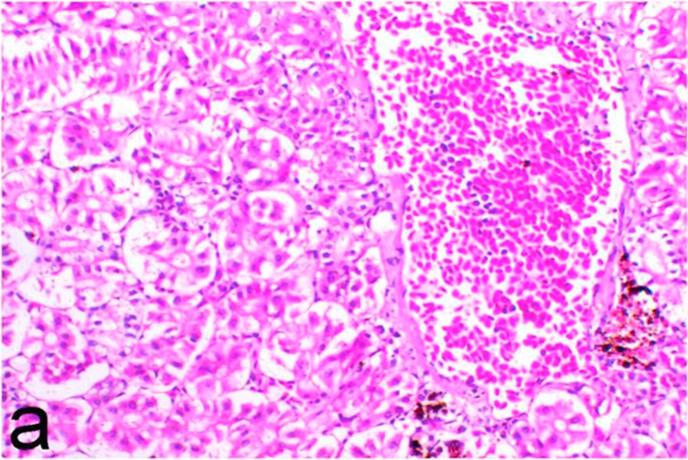
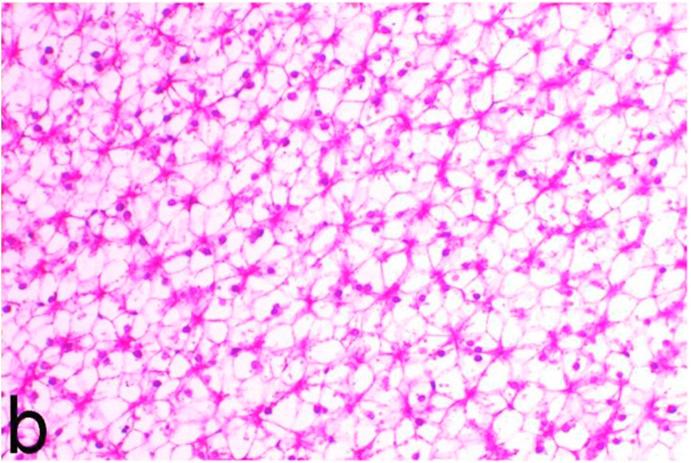
Histopathological findings of kidneys, liver, muscle and heart of negative control fish showed normal morphological appearances (Fig. 2a, Fig. 2b) whereas ochratoxin-treated group revealed marked enlargement and congestion of kidneys and livers (Fig. 1a, Fig. 1b) macroscopically. Microscopically, the kidney showed, marked vacuolar degeneration of the renal tubular epithelium (Fig. 3a) as well as focal interstitial mononuclear cell infiltrations predominantly macrophages and lymphocytes (Fig. 3b) which sometimes admixed with few heterophils. Wide dilatation of renal blood vessels (Fig. 3c) was also observed together with focal to massive tubular necrosis (Fig. 3d) and cystic tubular dilatation (Fig. 3e). Marked activation of melanomacrophage cells especially around blood vessels as well as an increase of glomerular space and shrinkage of the glomerular tufts (Fig. 3f) were also observed. The liver showed wide dilatation of hepatic sinusoids and blood vessels (Fig. 4a), hydropic degeneration of the hepatocytes, meanwhile multi focal hepatocellular necrosis of the hepatocytes represented by increased eosinophilia of the cytoplasm (Fig. 4b) with pyknosis of the nuclei (Fig. 4c) which in some areas infiltrated with mononuclear cells (Fig. 4d) and heterophils. Perivascular mononuclear cells sometimes admixed with heterophil infiltrations as well as focal activation of melano macrophage cells were also observed. Heart revealed focal pericarditis and myocarditis in the form of mononuclear cell and heterophil infiltrations, degeneration and necrosis of the myocardial muscles and activation of melanomacrophages.
Fig. 2a.
Negative control group, kidney showing normal histological structure. H&E. ×200.
Fig. 2b.
Negative control group, liver showing normal lobular architecture with normal vacuolation of fat and glycogen. H&E. ×200.
Fig. 3a.
Ochratoxin positive control group, kidney showing marked vacuolar degeneration of the renal tubular epithelium. H&E. ×200.
Fig. 3b.
Ochratoxin positive control group, kidney showing focal interstitial mononuclear cell infiltrations predominantly macrophages and lymphocytes. H&E. ×200.
Fig. 3c.
Ochratoxin positive control group, kidney showing wide dilatation of renal blood vessels. H&E. ×200.
Fig. 3d.
Ochratoxin positive control group, kidney showing massive tubular necrosis. H&E. ×200.
Fig. 3e.
Ochratoxin positive control group, kidney showing focal vacuolation and necrosis of the renal tubules and cystic tubular dilatation. H&E. ×200.
Fig. 3f.
Ochratoxin positive control group, kidney showing cystic tubular dilatation, focal mononuclear cells infiltrations, increase of glomerular space and shrinkage of the glomerular tufts. H&E. ×200.
Fig. 4a.
Ochratoxin positive control group, liver showing dilatation of hepatic blood vessels and vacuolation of the hepatocytes. H&E. ×200.
Fig. 4b.
Ochratoxin positive control group, liver showing hydropic degeneration of the hepatocytes, rarefaction, increased eosinophilia of the cytoplasm and pyknosis of the nuclei. H&E. ×200.
Fig.4c.
Ochratoxin positive control group, liver showing necrosis of the hepatocytes. H&E. ×200.
Fig. 4d.
Ochratoxin positive control group, liver showing area of necrosis of the hepatocytes infiltrated with mononuclear cells. H&E. ×200.
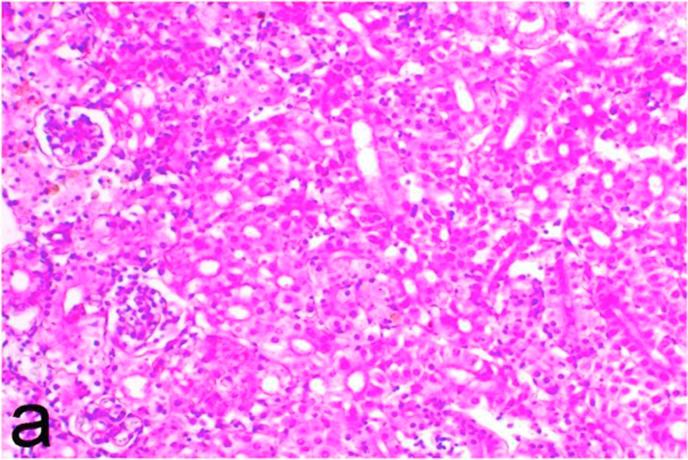
Garlen Extra4 treated group, kidney revealed mild to moderate pathological changes including dilatation of renal blood vessels, vacuolation and necrosis of the renal tubular epithelium (Fig. 5a) as well as infiltration of mononuclear cells and activation of melano macrophages. Marked tubular basophilia as a feature of tubular regeneration was observed. Hydropic degeneration (Fig. 5b) and individual cell necrosis of hepatocytes were the only pathological changes observed in the liver. The heart only showed few foci of mononuclear cells infiltration.
Fig. 5a.
Garlen Extra4 treated group (Group 3), kidney showing vacuolation of the renal tubular epithelium as well as mononuclear cell infiltrations. H&E. ×200.
Fig. 5b.
Garlen Extra4 treated group (Group 3), liver showing hydropic degeneration of the hepatocytes. H&E. ×200.
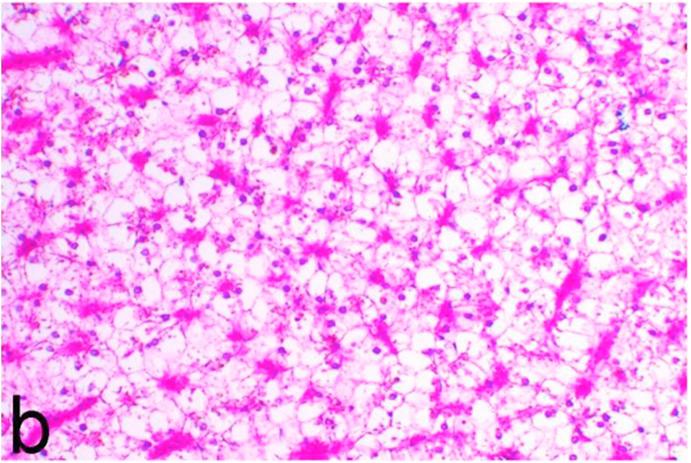
Kidneys of Nigella sativa treated group showed marked vacuolation of the renal tubular epithelium and dilatation of renal blood vessels (Fig. 6a) along with focal increase in the glomerular space, mild activation of melanomacrophages and very mild interstitial mononuclear cell infiltration. Moreover, necrosis of the renal tubules was not detected by any degree in all examined kidneys of such group. Liver showed vacuolation of hepatocytes and mild to moderate congestion (Fig. 6b).
Fig. 6a.
Group 4 (Nigella sativa treated group), kidney showing marked vacuolation of renal tubular epithelium and dilatation of renal blood vessels. H&E. ×200.
Fig. 6b.
Group 4 (Nigella sativa treated group), liver showing congestion and vacuolation of the hepatocytes. H&E. ×200.
In Garlen Extra4 and Nigella sativa treated group, kidney revealed minor pathological changes including dilatation of renal blood vessels, vacuolation and necrosis of the renal tubular epithelium (Fig. 7a) as well as intertubular infiltration of mononuclear cells and activation of melano macrophages. Mild focal tubular basophilia as a feature of tubular regeneration was observed. Liver showed hydropic degeneration of hepatocytes, focal necrosis, mild congestion (Fig. 7b) and foci of mononuclear cells and heterophils especially perivascular.
Fig. 7a.
Group 5 (Garlen Extra4 and Nigella sativa treated group), kidney showing mild vacuolation of the renal tubular epithelium. H&E. ×200.
Fig. 7b.
Group 5 (Garlen Extra4 and Nigella sativa treated group), liver showing hydropic degeneration and congestion. H&E. ×200.
Fero Bind Pro treated group revealed moderate to marked changes including dilatation of renal blood vessels, tubular vacuolation and necrosis (Fig. 8a), as well as activation of melano macrophages. Moreover, mild infiltration of mononuclear cells and increase glomerular space was also observed in few sections. Liver showed marked congestion and vacuolation (Fig. 8b). Muscles showed no significant pathological changes in all experimental groups.
Fig. 8a.
Group 6 (Fero Bind Pro treated group), kidney showing marked tubular vacuolation and focal necrosis of renal tubular epithelium. H&E. ×200.
Fig. 8b.
Group 6 (Fero Bind Pro treated group), liver showing marked dilatation of hepatic blood vessels and vacuolation of the hepatocytes. H&E. ×200.
4. Discussion
Despite paucity of studies on the effects of ochratoxins in aquaculture species, there is increasing evidence that ochratoxins cause several growth problems and pathological changes in different fish species so it is urgent to try finding suitable ways to control the problem.
At the molecular level, OTA interferes with synthesis of DNA, RNA and protein by inhibition of phenylalanine – tRNA synthetase enzyme in addition, OTA decreases electrolyte absorption and increase water consumption by inducing damage of the renal proximal tubules epithelium [24], [25]. OTA renal toxicity mode of action is by inhibition of various kidney enzyme activities [26]. Many studies conducted on different animals including fish had demonstrated that the kidney is the primary target for OTA [27], [28].
OTA induces renal and hepato-toxicity as well as a tubular-interstitial nephropathy in both humans and animals [29]. There are three mechanisms of OTA toxicity that have been proposed including inhibition of protein synthesis, induction of lipid peroxidation and inhibition of mitochondrial ATP production [30], [31].
In the present study, the post-mortem lesions induced by OTA in Nile tilapia were similar to those observed in rainbow trout [32]. The observed post-mortem lesions coincided with the histopathological findings which revealed degenerative and necrotic changes in the kidney and liver similar to that reported by [33], [34] in rainbow trout intra peritoneally injected with OTA and channel catfish, respectively.
It was previously reported that the characteristic features of ochratoxin toxicity in rats include necrosis of single epithelial cells, tubular dilation and formation of cysts similar to what observed in the present study. In another study on mice fed on diets containing 40 µg/g ochratoxins caused nephropathy, characterized by cystic dilatation [35]. In general, several proposed causes of cystic tubular dilatation were reported including primarily from direct toxic injury to the tubule epithelium interfering with absorption and secretion. It may also occur secondary to renal ischemia or from prolonged diuresis related to drug administration. Secondary mechanisms of tubular dilatation may result from lower urinary tract obstruction, deposition of tubular crystals, interstitial inflammation and/or fibrosis, and chronic progressive nephropathy [36], [37].
In the present study, there was relatively low survival rate (53%) of the positive control group in comparison to 100% in the negative control group. The obtained results are in agreement with a previous study [38] which found that the survival rate of mono-sex Nile tilapia fingerlings was significantly decreased by feeding 5 mg of OTA Ochratoxin A contaminated diet in comparison with control.
Significant decrease in growth performance including; weight gain, final weight and feed conversion ratio (FCR) in OTA intoxicated O. niloticus group compared to negative control group was similar to that observed by Manning et al. [34] in channel catfish at 8 weeks where weight gain was significantly reduced in catfish fed diets containing 1.0 mg OTA/kg or above as well as FCR was significantly reduced for catfish fed diets containing 4.0 or 8.0 mg OTA/kg of diet. In addition, Abo Hagger et al. [38] reported similar findings in O. niloticus fingerlings. Growth rate reduction by OTA may be due to renal toxicity, loss of appetite and protein synthesis disturbance.
Haematological and biochemical observations are considered fish health valuable monitoring tools. Regarding to neutropenia of OTA positive control group comparing to the negative control group as a negative effect of OTA on the differential leukocytic count might be related to OTA immunosuppressive effects. Similarly, Mansour et al. [39] observed decreasing in WBCs count in O. niloticus intoxicated by OTA.
Plasma proteins were significantly decreased in ochratoxin intoxicated African catfish (Clarias gariepinus) and in Nile tilapia [40], [41] respectively. Similar results were observed in the present study as well as total protein and globulin levels were significantly decreased in OTA positive control group than the negative control group. Similar results were also obtained by Mansour et al. [39] in O. niloticus after stomach intubations of OTA as 80 μg/kg fish as low dose and 160 μg/kg fish as high dose by Abo Hagger et al. [38] through feeding 5 mg OTA diet to O. niloticus fingerlings. The serum total protein reduction may be due to increasing of the protein breakdown as corticosteroid hormones stimulation which enhances the proteins breakdown to provide amino acids as well as enhancing gluconeogenesis for providing glucose to compensate increase in energy demands under stressful condition [41]. Moreover, OTA found to be nephrotoxic, hepatotoxic and increasing gastrointestinal tract permeability so the hypoproteinemia might be related to renal loss of protein, hepatic insufficiency and gastrointestinal loss [39].
Significant increase of ALT and AST enzymes observed in the present study may be attributed to OTA hepatotoxic effects that lead to an increase in enzymes liberation in blood [42]. Moreover, this may be related to the fact that liver is considered OTA detoxification site [43]. Similar findings of increasing of AST and ALT were reported in O. niloticus before [39].
The present biochemical results of creatinine and urea in ochratoxicated group were similarly to results previously described in OTA intoxicated O. niloticus [38], [39] as well as urea was significantly increased in aflatoxin intoxicated O. niloticus [44]. Creatinine and urea levels increasing may be related to reduction of kidney function [45] and OTA genotoxicity since the main target organ is kidney where DNA single-strand breaks induced and DNA adducts in kidney [46].
Negative results even in OTA positive control group obtained by examination of musculatures of all groups for OTA residues are supported by the finding of Fuchs et al. [42]. Where radiography studies to whole-body in rainbow trout proved that the highest OTA or its metabolites concentrations were presented in kidney, urine then bile, but not in the blood or muscles tissue. This may be explained on the basis that residues of OTA or its metabolites depend on many factors as including; time of feeding, length, the dose, use of naturally contaminated grain or crystalline OTA, the route of toxicity, serum binding degree and half-life of OTA [47]. Oral gastric tube or intravenous toxico-kinetics of 50 μg/kg b.w. OTA have been studied in carp (Cyprimus carpio) and serum half-life was 8.3 h and 0.68 h after intravenous and oral dosing, respectively. It was suggested that low bioavailability was related to hydrolysis or conjugation [48] and may be due to another reason as in the present study O. niloticus was orally intoxicated with OTA. Moreover ochratoxin A is fat soluble and not readily excreted, it accumulates in the fat of affected animals and tilapia musculatures have low lipid content. The obtained results were supported by the observed histopathological pictures.
The adverse effects of OTA were reduced in the group supplemented with Garlen Extra4 where a significant increase in growth and decrease in feed conversion rates. Similar results were previously documented [5]. Moreover, increased growth and total protein with decreasing in AST and ALT activities in comparison to OTA positive control were detected previously [49] after adding 3% garlic (Allium sativum) to rainbow trout fish diet comparing to negative control group. In addition to enhanced total protein and albumin or enhancement of total protein and globulin were also observed by [50] in Labeo rohita fingerlings after supplementation of 0.1%, 0.5% and 1.0% garlic (Allium sativum) to diets and [5] in O. niloticus feeding garlic supplemented diet respectively.
Nigella sativa treated group have improved OTA drastic effects. This may be due to Nigella sativa active compounds as thymoquinon, nigellaone and thymohydroquinon which inhibit bacteria and improve body function and so the performance. Also, Nigella sativa contains fat soluble unidentified factors as well as essential fatty and amino acids which play an important role in growth. Moreover many macro and micro elements are responsible for all vital functions regulation in the body and improve immunity. The observed results are similar to those obtained by Abo Hagger et al. [38] in O. niloticus.
The best of the present results was detoxifying results that were achieved by the synergism between Garlen Extra4 and Nigella sativa (group 5) and to the best of our knowledge this was not recorded before as a method to improve the OTA adverse effects in O. niloticus. These results supported by the present haematological, biochemical and histpathological findings.
Fero Bind Pro as a commercial mycotoxins binder succeeded in decreasing OTA drastic effects on O. niloticus treated group. This may be due to its components as yeast cell walls where in addition to its nutritional value and adsorbent activity for mycotoxins [24]. Yeast cell wall extract has good adsorbent activity for mycotoxins because of the presence of some specific molecules, as manno- proteins in addition to beta glucans [51]. Theses components when added to feeds bind to mycotoxins in the gastrointestinal tract so prevent OTA absorption in the gastrointestinal tract [52]. Moreover, yeast increase total protein, albumin, globulin and enhance immune response. Similar results obtained by Reyes-Becerril et al. [10] in juvenile leopard grouper. In addition, vitamin E contained in Fero Bind Pro had antioxidant activity and was associated with aflatoxin metabolism reduction [8]. Moreover, clays in general, were early used for their adsorptive properties to reduce the toxic effects of aflatoxins and ochratoxin [53] in O. niloticus. As well as Quillaja (Quillaja saponaria) if used in small amount of extracts improves weight gain and food utilization in tropical fish carp and tilapia [54].
The best detoxification supplementation was Garlen Extra4 plus Nigella sativa followed by Nigella sativa then Garlen Extra4 and the last was the commercial mycotoxins binder (Fero Bind Pro). These results were supported by the histopathological examination as varying degrees of pathological changes were observed in the challenged experimental groups. Very mild to mild, mild, mild to moderate, moderate to marked pathological changes including congestion, vacuolation and necrosis of the renal tubular epithelium as well as infiltration of mononuclear cells and activation of melano macrophages were observed in the kidney in Garlen Extra4 and Nigella sativa followed by Nigella sativa then Garlen Extra4 and the last was the commercial mycotoxins binder (Fero Bind Pro), respectively in comparison to ochratoxin treated group.
5. Conclusions
Inhibition or reduction of ochratoxin production in fish feed is not always possible, so it is crucial to try to eliminate or reduce ochratoxin drastic effects on fish. Addition of 0.1 g/kg Garlen Extra4 plus 30 g/kg Nigella sativa as detoxifying feed additives to fish (O. niloticus) diet has a positive impact on fish health through improving survivability and growth performance. It markedly reduces the toxic effects of ochratoxin on kidneys and liver as well as improves fish immunity. The detoxifying effects of different percentages of the feed additives added separately or in combination with each other need further studies.
6. Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7. Competing interests
There is no competing interest to declare.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Agouz H.M., Anwer W. Effect of Biogen and Myco-Ad on the growth performance of Common Carp (Cyprinus carpio) fed a mycotoxin contaminated aquafeed. J Fish Aquat Sci. 2011;6:334–345. [Google Scholar]
- 2.Cast A.D. Power and the ability to define situation. Soc Psychol Q. 2003:185–201. [Google Scholar]
- 3.Clark H.A., Snedeker S.M. Ochratoxin A: its cancer risk and potential for exposure. J Toxicol Environ Health B Crit Rev. 2006;9:265–296. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 4.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: an overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 5.Diab Amany M., Saker O.A., Eldakroury M.F., Elseify M.M. Effects of garlic (Allium sativum) and curcumin (Turmeric, Curcuma longa Linn) on Nile tilapia immunity. Vet Med J Giza. 2014;60:C1–C19. [Google Scholar]
- 6.Reddy K.R.N., Reddy C.S. Muralid haran K. Exploration of ochratoxin A contamination and its management in rice. Am J Plant Physiol. 2007;2:206–213. [Google Scholar]
- 7.Mona S.Z., Olfat M.F., Suzan O., Medhat Kh., Mostafa F., Isiz A. Diminution of aflatoxicosis in Tilapia zilli fish by dietary supplementation with fix in toxin and Nigella sativa oil. J Nat Sci. 2010;8:43–49. [Google Scholar]
- 8.Ricardo Y.S., Álvaro J.A.B., Josée P.C. Growth and hematology of juvenile pacu, Piaractus mesopotamicus. (Holmberg 1887) fed with increasing levels of vitamin E (DL-α-tocopheryl acetate). Zbornik Matice srpskezaprirodnenauke. Proc Natl Acad Sci USA. 2005;109:73–79. [Google Scholar]
- 9.Péteri Z., Téren J., Vágvölgyi C., Varga J. Ochratoxin degradation and adsorption caused by astaxanthin-producing yeasts. Food microbiol. 2007;24:205–210. doi: 10.1016/j.fm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Reyes-Becerril M., Tovar-Ramírez D., Ascencio-Valle F., Civera-Cerecedo R., Gracia-López V., Barbosa-Solomieu V. Effects of dietary live yeast Debaryomyces hansenii on the immune and antioxidant system in juvenile leopard grouper (Mycteroperca rosacea) exposed to stress. Aquaculture. 2008;280:39–44. [Google Scholar]
- 11.Phllips T.D., Lemke S.L., Grant P.G. Characterization of clay-based entero-sorbents for the prevention of aflatoxicosis. Adv Exp Med Biol. 2002;504:157–171. doi: 10.1007/978-1-4615-0629-4_16. [DOI] [PubMed] [Google Scholar]
- 12.NRC (Nutrition requirements of fish). National Research Council. Washington, D. C: National Academy Press; 1993.
- 13.AOAC. Official methods of analysis of the Association of Official Analytical Chemists. 18th Ed. Aithersburg, MD., USA; 2005.
- 14.AOAC. Official methods of analysis of the Association of Official Analytical Chemists. 13th Ed. Aithersburg, MD., USA; 1980.
- 15.Trenk H.L., Butz M.E., Chu F.S. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl Microbiol. 1971;21:1032–1035. doi: 10.1128/am.21.6.1032-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eurell T.E., Lewis S.D.H., Grumbles L.C. Comparison of selected diagnostic tests for detection of motile Aeromonas septicaemia in fish. Am J Vet Res. 1978;39:1384–1386. [PubMed] [Google Scholar]
- 17.Abeer El-Keredy M.S., Abeer M.E., Diab Amany M., Ali Gehan I.E., Walied S.K. Effect of dietary Vitamin C and Β-Glucan to alleviate the toxic effect of Copper Sulphate in tilapia fish. Alex J Vet Sci. 2017;55:36–49. [Google Scholar]
- 18.Austin B, Austin DA. Bacterial fish pathogens: diseases in farmed and wild fish. 4th ed. Ellis Harwood limited England; 2007.
- 19.Plumb JA, Bowser PR. Microbial fish diseases laboratory manual. Alabama agricultural experiment station. Alabama: Auburn Univ.; 1983.
- 20.Lucky Z. Methods for the Diagnosis of Fish Diseases. Ameruno Publishing Co. 1977 [Google Scholar]
- 21.Bancroft JD, Christopher Layout, Suvarna SK. Bancroft's theory and practice of histological Techiques. 7th ed. Churchil Livingston Elsevier; 2013
- 22.WHO (World Health Organization). Laboratory biosafety manual. 3rd ed. 2004. WHO/CDS/CSR/LYO/2004.11
- 23.SPSS (Statistical package for the social sciences) Revisions 6, spss Inc, Chicago; 1997.
- 24.Patrícia C., Anna L., Dionýz M. Prevention of Ochratoxin A contamination of food and Ochratoxin A detoxification by microorganisms. A review. Czech J Food Sci. 2010;28:465–474. [Google Scholar]
- 25.Yang D.J., Chang W.K., Byoung K.A., Jong S.A., Radka B. Effects of Ochratoxin A and preventive action of a mycotoxin-deactivation Product in broiler chickens. Vet Med Zoot T. 2013;61:22. [Google Scholar]
- 26.Braunberg R.C., Barton C.N., Gantt O.O., Friedman L. Interaction of citrinin and ochratoxin A. Nat Toxins. 1994;2:124–131. doi: 10.1002/nt.2620020307. [DOI] [PubMed] [Google Scholar]
- 27.Bayman P., Baker J.L. Ochratoxins: a global perspective. Mycopathologia. 2006;162:215–223. doi: 10.1007/s11046-006-0055-4. [DOI] [PubMed] [Google Scholar]
- 28.Liang R., Shen X.L., Zhang B., Li Y., Xu W., Zhao Y. Apoptosis signal-regulating kinase 1 promotes Ochratoxin A-induced renal cytotoxicity. Sci Rep. 2015;5:8078. doi: 10.1038/srep08078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Froquet R., Le Drean G., Parent-Massin D. Effect of Ochratoxin A on human haemato- poietic progenitors proliferation and differentiation: an in vitro study. Hum Exp Toxicol. 2003;22:393–400. doi: 10.1191/0960327103ht375oa. [DOI] [PubMed] [Google Scholar]
- 30.Marquardt R.R., Frohlich A.A. A review of recent advances in understanding ochratoxicosis. Anim Sci J. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- 31.Schilter B., Marin-Kuan M., Delatour T., Nestler S., Mantle P., Cavin C. Ochratoxin A: potential epigenetic mechanisms of toxicity and carcinogenicity. Food Addit Contam. 2005;22:88–93. doi: 10.1080/02652030500309319. [DOI] [PubMed] [Google Scholar]
- 32.Hendricks J.D. Carcinogenecity of aflotoxins in nonmammalian organisms. In: Eaton D.L., Groopman J.D., editors. Toxicology of Aflotoxins: Human Health, veterinary, and Agricultural Significance. Academic Press; San Diego: 1994. pp. 36–103. [Google Scholar]
- 33.Doster R.C., Sinnhuber R.O., Wales J.H. Acute intraperitoneal toxicity of ochratoxins A and B in rainbow trout (Salmo gairdneri) Food Cosmet Toxicol. 1972;10:85–92. doi: 10.1016/0015-6264(74)90063-7. [DOI] [PubMed] [Google Scholar]
- 34.Manning B.B., Ulloa R.M., Li M.H., Robinson E.H., Rottinghaus G.E. Ochratoxin A fed to channel catfish (Ictalurus punctatus) causes reduced growth and lesions of hepatopancreatic tissue. Aquaculture. 2003;219:739–750. [Google Scholar]
- 35.Wanda M, Haschek KAV, Val RB. Selected mycotoxins affecting animal and human health in Handbook of Toxicologic Pathology. 2nd ed. 2002, p. 645–699.
- 36.Greaves P. 4th Ed. Elsevier; Amsterdam: 2012. Histopathology of Preclinical Toxicity Studies. [Google Scholar]
- 37.Lameire N. The pathophysiology of acute renal failure. Crit Care Clin. 2005;21:197–210. doi: 10.1016/j.ccc.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Abo Hagger A.A., El-Melegy KhM., Abdel-Ghany Z.M., Abou-Seif R.A. Dietary Nigella sativa and yeast cell wall for reducing the toxicity of ochratoxin A in cultured Nile tilapia in Egypt. Mansoura Journal of Animal and Poultry Production. J Animal and Poultry Prod Mansoura Univ. 2011;2:327–336. [Google Scholar]
- 39.Mansour T.A., Safinaz G.M., Soliman M.K., Eglal A.O., Srour T.M., Mona S.Z. Ameliorate the drastic effect of Ochratoxin A by using yeast and whey in cultured Oreochromis niloticus. Egypt Life Sci J. 2011;8:68–81. (ISSN: 1097 – 8135) [Google Scholar]
- 40.Mousa M.A., Khattab Y.A. The counteracting effect of vitamin C (L- ascorbic acid) on the physiological perturbations induced by ochratoxin intoxication in the African catfish (Clarias gariepinus) J Egypt Acad Environ Develop. 2003;4:117–128. http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.541.1395 [Google Scholar]
- 41.Adel ME. Shalaby. The opposing effect of ascorbic acid (vitamin C) on ochratoxin toxicity in Nile tilapia (Oreochromis niloticus). In: Proceedings of the 6th International Symposium on Tilapia in Aquaculture; 2004. http://citeseerx.ist.psu.edu/viewdoc/summary? doi = 10.1.1.541.1395.
- 42.Fuchs R., Appelgren L.E., Hult K. Distribution of 14 C-ochratoxin A in the rainbow trout (Salmo-gairdneri) Acta Pharmacol Toxicol. 1986;59:220–227. doi: 10.1111/j.1600-0773.1986.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 43.Stormer F.C., Pederson J.I. Formation of (4R)- and (4S)-hydroxy ochratoxin A from ochratoxin A by rat liver microsomes. Appl Environ Microbiol. 1980;39:971–975. doi: 10.1128/aem.39.5.971-975.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.EL-Boshy ME, EL-Ashram AMM, Nadia AA. Effect of dietary Beta-1, 3 glucans on immunomodulation on diseased Oreochromis niloticus experimentally infected with aflatoxin B1. In: 8th International symposium on tilapia in aquaculture 2008; 2:1109–1127. https://www.researchgate.net/publication/258205846.
- 45.Zotti F.D., Visonà E., Massignani D., Abaterusso C., Lupo A., Gambaro G. General practitioners’ serum creatinine recording styles. J Nephrol. 2008;21:106–109. [PubMed] [Google Scholar]
- 46.Hossein zadeh H., Parvardeh S.M.N., Asl H.R., Sadeghnia T.Z. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 47.Kuiper-Goodman T., Scott P.M. Risk Assessment of the Mycotoxin Ochratoxin A. Biomed Environ Sci. 1989;2:179–248. [PubMed] [Google Scholar]
- 48.Hagelberg S., Hul T.K., Fuchs R. Toxico kinetics of ochratoxin A in several species and its plasma binding properties. J Appl Toxicol. 1989;9:91–96. doi: 10.1002/jat.2550090204. [DOI] [PubMed] [Google Scholar]
- 49.Nya E.J., Austin B. Use of garlic, Allium sativum, to control Aeromonas hydrophila infection in rainbow trout, Oncorhynchus mykiss (Walbaum) J Fish Dis. 2009;32:963–970. doi: 10.1111/j.1365-2761.2009.01100.x. [DOI] [PubMed] [Google Scholar]
- 50.Sahu S., Das B.K., Mishra B.K., Pradhan J. Sarang N. Effect of Allium sativum on the immunity and survival of Labeo rohita infected with Aeromonas hydrophila. j. Appl Ichthyol. 2007;23:80–86. [Google Scholar]
- 51.Ringot D., Lerzy B., Chaplain K., Bonhoure J., Auclair E., Larondelle Y. In vitro biosorption of ochratoxin A on the yeast industry by-products: comparison of isotherm models. Bioresource Technol. 2007;98:1812–1821. doi: 10.1016/j.biortech.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 52.Kabak B., Dobson A.D.W., Var I. Strategies to prevent mycotoxin contamination of food and animal feed. Crit Rev Food Sci Nutr. 2006;46:593–619. doi: 10.1080/10408390500436185. [DOI] [PubMed] [Google Scholar]
- 53.Abdelaziz M.A., Anwer W., Abeer H.A. Field study on the mycotoxin binding effects of clay in Oreochromis niloticus feeds and their impacts on the performance as well as the health status throughout the culture season. IBC (Interdisciplinary Bio Central) 2010;2:1–5. [Google Scholar]
- 54.Francis G., Makkar H.P.S., Becker K. Dietary supplementation with Quillaja saponin mixture improves growth performance and metabolic efficiency in common carp (Cyprinus carpio L.) Aquaculture. 2002;203:311–320. [Google Scholar]
- 55.Shiau S.Y., Hang S.I. Optimal dietary protein level for hybrid tilapia (Oreochromis niloticus × O. aureus) reared in seawater. Aquaculture. 1990;81:119–127. [Google Scholar]