Graphical abstract
Keywords: Gastrointestinal nematodes, Haemonchus contortus, Ovicidal and larvicidal activities, Phytochemical, Tannin
Abstract
Grape pomace obtained as a byproduct of industrial processing of grapes retains nutrients and substances with anthelmintic potential such as saponins, tannins, and flavonoids. Therefore, this study evaluated the in vitro ovicidal and larvicidal activity of hydroalcoholic grape pomace extract against gastrointestinal nematodes of sheep. The anthelmintic evaluation was performed by in vitro assays with eggs and larvae of nematodes obtained from naturally infected donor sheep. The grape pomace extract showed high in vitro ovicidal and larvicidal activity with LD50 values of 0.30 mg/mL for egg hatching inhibition, 1.01 mg/mL for larval development inhibition and 100% efficacy in larval migration inhibition assays at all concentrations evaluated. The effect of tannins was evaluated by the addition of 50 mg/mL polyvinyl polypyrrolidone to grape pomace extract at the concentration of 12.5 mg/mL. The in vitro ovicidal activity of grape pomace was reduced by only 15% after polyvinyl polypyrrolidone addition, indicating that other phytochemicals also contribute to the ovicidal activity displayed by the extract. Our results demonstrate that grape pomace exhibits in vitro anthelmintic activity, suggesting that, beyond its nutritional potential, this pomace can also be an ally for gastrointestinal nematode control in sheep.
1. Introduction
Infection by gastrointestinal nematodes (GINs) is one of the principal problems causing losses in sheep production due to the resulting lower productivity, the costs of anthelmintic treatments and the mortality of highly infected sheep [1], especially in developing countries such as Brazil. For decades, GINs in sheep have been controlled by the administration of synthetic anthelmintics to the animals; however, the incorrect use of these drugs and absence of epidemiological control has selected resistant nematodes [2]. This emergence of anthelmintic resistance has brought about the need to develop new alternatives in the control of sheep parasitosis.
Medicinal plants have emerged as an alternative to the use of synthetic anthelmintics because they contain phytochemicals with anthelmintic properties such as saponins, flavonoids, and tannins [3], [4]. These phytochemicals are present in considerable quantities in the residues obtained from the industrial processing of the fruit for the production of juices and wines [5], [6]. Among the many fruit crops, grapes are one of the most productive worldwide, annually generating millions of tons of waste predominantly through the wine industry. After the fermentation stage of wine production, some of the original phenolic content remains in the pomace [5], [7].
Studies by Yu and Ahmedna [8] and Nistor et al. [9] found that grape pomace is safe and can be added to ruminant feed at rates of up to 100 g/kg/day as a good nutritional source. Although grape pomace contains phytochemicals with anthelmintic properties, including gallic acid [10], gallocatechin, epigallocatechin [11], [12] and resveratrol [13], there are no scientific reports about its anthelmintic activity. Given the need to develop new alternatives for the control of GINs, this study evaluated the ovicidal and larvicidal activities of grape pomace hydroalcoholic extract in in vitro assays with eggs and larvae of helminths obtained from naturally infected sheep.
2. Materials and methods
2.1. Ethical approval
Animal use was approved by the Animal Use and Ethics Committee of the Faculty of Engineering, UNESP/Ilha Solteira, according to international standards of animal use in research (clearance certificate number: 12/2013/CEUA).
2.2. Preparation of the grape pomace extract and fractions
Grape pomace of the variety Máximo IAC 138-22 was donated by a winemaker from the Jales region (São Paulo, Brazil) in July 2017. The grape pomace was dried in a circulating air oven (50 °C). The grape pomace extract (GPE) was prepared from 10 g of powdered grape pomace in 100 mL of ethanol (70%) using the exhaustive maceration method for 7 days. The mixture was filtered and the resulting liquid rotaevaporated, furnishing the crude GPE. Crude GPE (1.5 g) was dissolved in 20 mL of ethanol (70%) and fractionated by successive extractions with hexane, dichloromethane, and ethyl acetate. The partition process was performed by diluting 1.5 g of the crude GPE with 20 mL of ethanol (70%) and successively extracting with hexane, dichloromethane, and ethyl acetate. The extraction process was carried out with 50 mL of each solvent and repeated three times for each extract. All fractions and GPE were frozen until use.
2.3. Measurement of total phenolics and tannins
2.3.1. Total phenolic content
Grape pomace (200 mg) contained in a test tube was mixed with 10 mL of ethanol (70%) and subjected to ultrasonic treatment for 20 min at room temperature and then centrifuged for 10 min (2054g) at 4 °C. Aliquots of the supernatant (0.02, 0.05 and 0.1 mL) were added to test tubes containing distilled water (0.48, 0.45 and 0.4 mL respectively), 0.25 mL of Folin-Ciocalteu reagent and 1.25 mL of sodium carbonate solution (20%). The tubes were shaken in a vortex and absorbance was recorded at 725 nm after 40 min. The total phenol content was measured as tannic acid equivalent from the calibration curve (concentration range: 2–10 µg/mL). The total phenolic content was expressed as dry matter (x%) [14].
2.3.2. Non-tannin phenol and tannin contents
In a test tube, 100 mg of PVPP, 1.0 mL of distilled water, and 1.0 mL of the supernatant obtained in Section 2.3.1 were mixed and agitated in a vortex, kept at 4 °C for 15 min, then centrifuged for 10 min (2054g) and the supernatant collected. The Folin-Ciocalteu procedure was carried out on this supernatant. The content of non-tannin phenols was measured as tannic acid equivalent per gram of dry matter (y%). The difference between the total phenolic content (x%) and the content of non-tannin phenols (y%) indicated the tannin content [14].
2.4. Recovery and preparation of eggs
The eggs were obtained from faeces of sheep naturally infected with gastrointestinal nematodes (95% H. contortus and 5% Trichostrongylus sp.). The eggs were recovered using a sequence of sieves [15], and the eggs retained in the 25-μm sieve were washed with distilled water, transferred to Falcon tubes and centrifuged for 5 min at 2054g. The supernatant was removed and mixed with saturated NaCl to re-suspend the eggs. After centrifugation, the supernatant was transferred to 25-µm sieves and the retained eggs were washed and transferred to a Falcon tube, in which the final concentration of eggs was adjusted to 100 eggs/100 µL.
2.5. Egg hatching assay (EHA)
To each well of a 24-well microplate, 100 eggs/100 µL were added and the total volume was adjusted to 200 µL with distilled water. All concentrations of GPE and fractions (0.78, 1.56, 3.12, 6.25, 12.5, 25.0 mg/mL), water (negative control 1), DMSO at 0.5% (negative control 2), and albendazole at 25 µg/mL (positive control) were tested in five replicates. After incubation of the microplate for 48 h at 28 °C, the number of eggs and L1 per well were counted under an inverted microscope. To evaluate the effect of tannins, another series of incubations were made. The procedure was similar to that described above using the negative controls, GPE in the concentration with the greatest effect (12.5 mg/mL) with PVPP (50 mg/mL) and the GPE (12.5 mg/mL) without PVPP. Five replicates were made for each treatment [16].
2.6. Larval development assay (LDA)
A 24-well microplate containing 100 eggs/100 μL in each well was incubated for 48 h at 28 °C and after hatching 90 μL of nutrient medium (consisting of 1 g of yeast in 90 mL of normal saline and 10 mL of Earle’s balanced salt), 10 μL of amphotericin (25 μg/mL) and 200 μL of GPE were added to each well [17]. All concentrations of GPE (0.19, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25.0 mg/mL), water (negative control 1), DMSO at 0.5% (negative control 2), and ivermectin at 10 µg/mL (positive control) were tested in five replicates. The plates were incubated for 5 days, and the numbers of L1 and L3 were counted under an inverted microscope.
2.7. Larval migration assays (LMA)
For these assays, the L3 were obtained by culture of faeces from a naturally infected donor sheep and then collected by sedimentation using Baermann devices [17], [18]. One thousand live L3 (500 µL) were added to centrifuge tubes containing 1000 µL of GPE solution (0.19, 0.39, 0.78, 1.56, 3.12, 6.25, 12.5, 25 mg/mL) or commercial anthelmintic control (levamisole hydrochloride at 1.25 mg/mL). PBS (the negative control, pH 7.2) was added to the tubes to obtain a final volume of 2000 μL. After incubation for 3 h at 28 °C the tubes were capped with a 25-µm sieve and turned upside down over a Petri dish containing distilled water. The number of L3 that migrated through the mesh within 3 h was counted under an inverted microscope. The percentage inhibition of larval migration was determined relative to the number of larvae tested.
2.8. Scanning electron microscopy (SEM) processing of L3 stage
The larvae (L3 stage) for SEM analysis were recovered from the negative control and GPE 0.78 mg/mL treatment in the larval migration inhibition assay. The L3 were individually preserved in 2% glutaraldehyde solution in phosphate buffer (0.1 M, pH = 7.4) and refrigerated at 4 °C until analysis. The larvae were processed for SEM analysis at the Scanning Electron Microscopy Laboratory at Faculdade de Engenharia de Ilha Solteira UNESP. The fixed larvae were washed in solution buffer and dehydrated in graded acetone series (15%, 30%, 50%, 70%, 95%, and 100%). The larvae were then critical point dried and sputter coated with gold. Immediately following gold coating, the larvae were observed with a scanning electron microscope (Carl-Zeiss, EVO/LS15, Scanning Electron Microscope, Germany).
2.9. Statistical analysis
The mean percentage of egg hatching inhibition, larval development inhibition and larval migration inhibition assays at different concentrations of extract versus the control was compared by one-way ANOVA (95%) and the Dunnett test (5%). The final concentrations of the GPE and fractions in each well of the microplate or tubes were used for LD50 determination. The value of LD50 for the extract is the dose required to kill half the members of a tested population after a specified test duration. The LD50 values were calculated by means of nonlinear regression analysis with the aid of GraphPad Prism version 5.0.
3. Results
3.1. Yield of extracts from the plant material
After the extraction with ethanol (70%), 10 g of dried grape pomace yielded 1.97 g of GPE. The partition of 1.5 g of GPE to solvents of increasing polarity furnished 0.12 g, 0.32 g, 0.45 g, and 0.61 g respectively, of the fractions in hexane (FH), dichloromethane (FD), ethyl acetate (FAC) and residual aqueous solution (FRA).
3.2. Total phenolic and total tannin content
The total phenolic content in GPE was 3.95 mg/g (tannic acid equivalent) in 100 mg of dried pomace, which corresponded to 4.2% total phenolic content in the dry matter. The total tannin content of GPE was 1.92 mg/g (tannic acid equivalent) in 100 mg of dried pomace, which corresponded to 2.03% total tannin content in the dry matter.
3.3. Egg hatch assay
The percentage of inhibition of egg hatch by the GPE and fractions is presented in Table 1. The GPE significantly (P < .05) inhibited egg hatching in a dose-dependent manner with an LD50 value of 1.01 mg/mL. The fractions presented satisfactory inhibition only at the highest concentration, with LD50 values of 25.6, 9.10, 5.05 and 3.25 mg/mL, respectively, for FH, FD, FAC and FRA. The use of PVPP decreased GPE egg hatch inhibition by 15%. Due to its effect on eggs, GPE was the only extract evaluated in the other in vitro tests.
Table 1.
Percentages of inhibition of egg hatching (mean ± SD) of sheep gastrointestinal nematodes (95% Haemonchus contortus) by the crude grape pomace extract (GPE) and fractions (FH, FD, FAC and FRA) and LD50 of the crude grape pomace extract (GPE) and fractions (FH, FD, FAC and FRA).
| Concentration (mg/mL) | GPE | FH | FD | FAC | FRA |
|---|---|---|---|---|---|
| 12.5 | 100 ± 0 aA | 33.7 ± 1.5 aD | 80.5 ± 1.3 aC | 98.2 ± 1.2 aB | 95.5 ± 3.8 aB |
| 6.25 | 94.0 ± 2.94 bA | 28.0 ± 1.4 bC | 9.0 ± 1.4 bD | 70.5 ± 1.0 bB | 74.2 ± 3.9 bB |
| 3.12 | 85.2 ± 3.8 cA | 6.0 ± 1.1cD | 4.2 ± 0.5 cE | 40.5 ± 0.6 cC | 47.2 ± 0.5 cB |
| 1.56 | 61.2 ± 2.2 dA | 2.8 ± 0.8 dC | 3.0 ± 0.8 cC | 21.7 ± 2.3 dB | 20.0 ± 0.8 dB |
| 0.78 | 37.75 ± 2.63 eA | 3.0 ± 0.7 eC | 3.2 ± 1.2 cB | 15.5 ± 1.1 eB | 18.2 ± 2.2 dB |
| 0.39 | 28.75 ± 2.22 fA | 3.3 ± 1.1 eD | 2.8 ± 1.8cD | 7.1 ± 0.9 fC | 13.2 ± 1.5 eB |
| Negative control | 3.8 ± 1.5 g | 3.8 ± 1.5e | 3.8 ± 1.5c | 3.8 ± 1.5 g | 3.8 ± 1.5f |
| Positive control | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a | 100 ± 0 a |
| LD50 | 1.01 | 25.6 | 9.10 | 5.05 | 3.25 |
FH – fraction in hexane; FD – fraction in dichloromethane; FAC – fraction in ethyl acetate; FRA – fraction residual aqueous. Small letters compare means between columns and capital letters compare means between rows (P < .05).
3.4. Larval development assay
The results obtained showed that GPE had a larvicidal effect. The GPE blocked the development of L1 to L3 even at the lowest evaluated concentration (LD50 = 0.30 mg/ mL) and increased efficacy in a dose-dependent manner (P < .05). Inhibition of larval development reached 100% in the positive control and approximately 4% in the negative control (Table 2).
Table 2.
Percentages of inhibition of larval development and inhibition of larval migration (mean ± SD) of sheep gastrointestinal nematodes (95% Haemonchus contortus) by the grape pomace extract (GPE) and LD50 of the grape pomace extract (GPE).
| Concentration mg/mL | LDA | LMA |
|---|---|---|
| 12.5 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 6.25 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 3.12 | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| 1.56 | 92.00 ± 5.48b | 100.00 ± 0.00 a |
| 0.78 | 82.00 ± 7.70c | 100.00 ± 0.00 a |
| 0.39 | 62.25 ± 1. 70 d | 100.00 ± 0.00 a |
| 0.195 | 45.12 ± 1.52 e | 100.00 ± 0.00 a |
| 0.097 | 29.00 ± 1.87f | 100.00 ± 0.00 a |
| Negative control | 4.60 ± 1.10 g | 0.00 ± 0.00b |
| Positive control | 100.00 ± 0.00 a | 100.00 ± 0.00 a |
| LD50 | 0.30 | <0.097 |
Different small letters in the same column indicate significant differences (P < .05).
3.5. Larval migration inhibition assay and scanning electron microscopy (SEM)
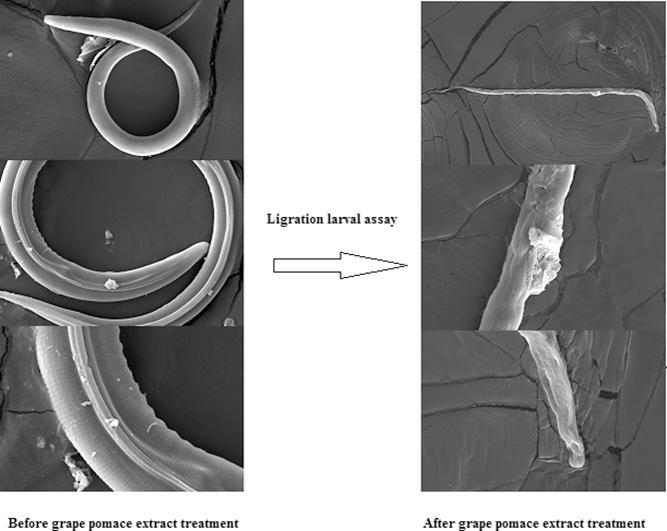
After a 3-hour exposure of the L3 to GPE, 100% inhibition of larval migration was observed at all concentrations evaluated (Table 2). SEM micrographs of L3 recovered from the negative control and GPE 0.78 mg/mL treatment are presented in Fig. 1, Fig. 2, respectively.
Fig. 1.
SEM micrographs of Haemonchus contortus L3s recuperated from the migration larval assays (MLA) after 3 h of incubation at room temperature with negative control (5% DMSO).
Fig. 2.
SEM micrographs of Haemonchus contortus L3s recuperated from the migration larval assays (MLA) after 3 h of incubation at room temperature with GPE at concentration of 0.78 mg/mL.
4. Discussion
The use of extracts and oils from plants as well as fruit pomace has been extensively studied as an alternative to anthelmintics in the treatment of gastrointestinal parasites in sheep [18], [19], [20], [21], [22], as they may be sources of phytoconstituents, which display various biological properties [22]. However, excessive consumption can pose risks to the health of animals. It is in this context that plant products already used to feed animals, such as pomaces generated by fruit processing, are suitable for anthelmintic evaluation, since several pomaces have pre-set dosage limits based on experiments with different levels of inclusion in animal feed [9], [23], [24], [25], [26]. The grape pomace used in animal feed contains a significant quantity of lipids, proteins, non-digestible fibre and minerals. Oilseeds in grape pomace consist of 13–19% essential fatty acids, and about 11% protein and 60–70% non-digestible carbohydrates, in addition to containing non-phenolic antioxidants in the form of tocopherols and betacarotenoids [8]. Moreover, grape pomace is rich in phytoconstituents such as flavonoids, saponins and tannins, which have many biological properties, including anthelmintic activity [6], [12], [27], [28]. Several studies have demonstrated the activity of plants containing tannins in the control of parasitosis in sheep [29], [30], [31]. Tannins are known to form strong complexes with proteins and other macromolecules causing anti-nutritional effect in animals. However, these effects are more related to the nature of the chemical structure than to the amount present in the plant [32], [33], [34]. Plants as the Dalea purpa, that produces a moderate concentration of tannins (6–9% DM), have more beneficial effect [32] than the Onobrychis viciifolia with lower concentration in tannin (2.5–3.4% DM) [35].
Recent papers have demonstrated that moderate amounts of tannins (depending on the type of tannins) can bring benefits such as the protection of alimentary protein against excessive ruminal degradation, reduction of ammonia loss and increased absorption of dietary amino acids in the small intestine which improve digestion and animal performance [33], [36], [37], [38]. The pomace used in our study contained 4.19% of total phenols and 2.04% of total tannin in the dry matter.
The results of the EHA, LDA and LMA assays showed that GPE actively affects the eggs and larvae of GINs. GPE presented high dose-dependent ovicidal activity, which was only reduced by 15% by the addition of PVPP when compared with the value obtained at the same extract concentration in the absence of PVPP. This suggests that the activity presented by GPE is related to its tannin content; however, tannins are not solely responsible for the ovicidal activity observed.
The FH, FD, FAC and FRA obtained from fractionation of the crude GPE also showed ovicidal activity, but at a lower level than the GPE.
In LDA, the effect was dose-dependent and the larvae were more sensitive to GPE than the eggs, with lower LD50 values. GPE blocked 100% passage from stage L1 to L3 at higher concentrations, and the larvae recovered from LDA at the lowest concentrations were all dead. An SEM micrograph obtained from L3 recovered from the negative control of LMA showed no alterations in the larva’s cylindrical shape or in its integument (Fig. 1). However, the L3 recovered from the treatment with GPE at the concentration of 0.78 mg/mL had lost its cylindrical shape and the integument was seriously damaged (Fig. 2). In GPE the active compounds may interact with cell membranes, resulting in destabilization and subsequent increased cell permeability that facilitates the action on intracellular proteins in the parasite [39]. However, other different mechanisms may contributing to the observed effect.
The present study contributes valuable information regarding the use of industrial bagasse rich in phytochemicals in veterinary parasitology. Phytochemicals such as polyphenols and flavonoids present in GPE are known for their antioxidant and anti-inflammatory properties and may promote improvement in the animal's immune system when added to the diet, helping to increase resistance to infection [8], [40]. GPE may be a new alternative for the control of gastrointestinal nematodes in sheep, providing a sustainable method for the organic production of these animals. In the case of GPE, there is an advantage over the use of essential oils or medicinal plants, since it has already been evaluated for use in sheep feed and can be used safely at doses up to 100 g/kg/day without toxic effects, as reported by Nistor et al. [9] and Baumgärtel et al. [41].
5. Conclusions
In conclusion, our results demonstrate for the first time the anthelmintic activity of GPE, which successfully inhibited the hatching, development and mobility of free-living stages of H. contortus. The results obtained in this study demonstrate that GPE is a potential source of bioactive compounds with significant ovicidal and larvicidal properties. In vitro efficiencies were comparable to a commercial anthelmintic; however, further in vivo tests at different concentrations of GPE are needed to better understand the potential of GPE in sheep parasite control.
Competing interests
The authors declare that they have no competing interests
Acknowledgments
The authors thank CAPES (Coordenacão de Aperfeicoamento de Pessoal de Nível Superior) and FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo, 2014/25294-0) for scholarships.
Footnotes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- 1.Aguerre S., Jacquiet P., Brodier H., Bournazel J.P., Grisez C., Prevót F. Resistance to gastrointestinal nematodes in dairy sheep: genetic variability and relevance of artificial infection of nucleus rams to select for resistant ewes on farms. Vet Parasitol. 2018;256:16–23. doi: 10.1016/j.vetpar.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Jabbar A., Iqbal Z., Kerboeuf D., Muhammad G., Khan M.N., Afaq M. Anthelmintic resistance: the state of play revisited. Life Sci. 2006;79:2413–2431. doi: 10.1016/j.lfs.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Oliveira A.F., Costa Junior L.M., Lima A.S., Silva C.R., Ribeiro M.N., Mesquita J.W. Anthelmintic activity of plant extracts from Brazilian savanna. Vet Parasitol. 2017;236:121–127. doi: 10.1016/j.vetpar.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Mengistu G., Hoste H., Karonen M., Salminen J.P., Hendriks W.H., Pellikaan W.F. The in vitro anthelmintic properties of browse plant species against Haemonchus contortus is determined by the polyphenol content and composition. Vet Parasitol. 2017;237:110–116. doi: 10.1016/j.vetpar.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 5.Vergara-Salinas J.R., Vergara M., Altamirano C., Gonzalez A., Pérez-Correa J.R. Characterization of pressurized hot water extracts of grape pomace: chemical and biological antioxidant activity. Food Chem. 2015;171:62–69. doi: 10.1016/j.foodchem.2014.08.094. [DOI] [PubMed] [Google Scholar]
- 6.Pintać D., Majkić T., Torović L., Orčić D., Beara I., Simin N. Solvent selection for efficient extraction of bioactive compounds from grape pomace. Ind Crops Prod. 2018;111:379–390. [Google Scholar]
- 7.Ky I., Teissedre P.L. Characterization of Mediterranean grape pomace seed and skin extracts: polyphenolic content and antioxidant activity. Molecules. 2015;20:2190–2207. doi: 10.3390/molecules20022190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J., Ahmedna M. Functional components of grape pomace: their composition, biological properties and potential applications. Int J Food Sci Technol. 2013;48:221–237. [Google Scholar]
- 9.Nistor E., Dobrei A., Dobrei A., Bampidis V., Ciolac V. Grape pomace in sheep and dairy cows feeding. J Hortic Forest Biotechnol. 2014;18:146–150. [Google Scholar]
- 10.Patel M., Ganeshpurkar A., Bansal D., Dubey N. Experimental evaluation of anthelmintic effect of gallic acid. Pharmacogn Commun. 2015;5:145–147. [Google Scholar]
- 11.Molan A.L., Meagher L.P., Spencer P.A., Sivakumaran S. Effect of flavan-3-ols on in vitro egg hatching, larval development and viability of infective larvae of Trichostrongylus colubriformis. Int J Parasitol. 2003;33:1691–1698. doi: 10.1016/s0020-7519(03)00207-8. [DOI] [PubMed] [Google Scholar]
- 12.Williams A.R., Ropiak H.M., Fryganas C., Desrues O., Mueller-Harvey I., Thamsborg S.M. Assessment of the anthelmintic activity of medicinal plant extracts and purified condensed tannins against free-living and parasitic stages of Oesophagostomum dentatum. Parasit Vectors. 2014;7:518. doi: 10.1186/s13071-014-0518-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giri B.R., Bharti R.R., Roy B. In vivo anthelmintic activity of Carex baccans and its active principle resveratrol against Hymenolepis diminuta. Parasitol Res. 2015;114:785–788. doi: 10.1007/s00436-014-4293-y. [DOI] [PubMed] [Google Scholar]
- 14.Makkar H.P.S. 1st ed. Springer Science & Business Media; 2003. Quantification of tannins in tree and shrub foliage – a laboratory manual. [Google Scholar]
- 15.Coles G.C., Bauer C., Borgsteede F.H., Geerts S., Klei T.R., Taylor M.A. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 16.Alonso-Díaz M.A., Torres-Acosta J.F., Sandoval-Castro C.A., Aguilar-Caballero A.J., Hoste H. In vitro larval migration and kinetics of exsheathment of Haemonchus contortus larvae exposed to four tropical tanniniferous plant extracts. Vet Parasitol. 2008;153:313–319. doi: 10.1016/j.vetpar.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 17.Féboli A., Laurentiz A.C., Soares S.C., Augusto J.G., Anjos L.A., Magalhães L.G. Ovicidal and larvicidal activity of extracts of Opuntia ficus-indica against gastrointestinal nematodes of naturally infected sheep. Vet Parasitol. 2016;226:65–68. doi: 10.1016/j.vetpar.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 18.Wagland B.M., Jones W.O., Hribar L., Bendixsen T., Emery D.L. A new simplified assay for larval migration inhibition. Int J Parasitol. 1992;22:1183–1185. doi: 10.1016/0020-7519(92)90040-r. [DOI] [PubMed] [Google Scholar]
- 19.Rajeswari V.D. Anthelmintic activity of plants: a review. Res J Phytochem. 2014;8:57–63. [Google Scholar]
- 20.Akkari H., Ezzine O., Dhahri S., B’chir F., Rekik M., Hajaji S. Chemical composition, insecticidal and in vitro anthelmintic activities of Ruta chalepensis (Rutaceae) essential oil. Ind Crops Prod. 2015;74:745–751. [Google Scholar]
- 21.Gaínza Y.A., Domingues L.F., Perez O.P., Rabelo M.D., López E.R., Chagas A.C.S. Anthelmintic activity in vitro of Citrus sinensis and Melaleuca quinquenervia essential oil from Cuba on Haemonchus contortus. Ind Crops Prod. 2015;76:647–652. [Google Scholar]
- 22.Djilas S., Jasna C.B., Ćetković G. By-products of fruits processing as a source of phytochemicals. Chem Ind Chem Eng Q. 2009;15:191–202. [Google Scholar]
- 23.Barroso D.D., Araujo G.G.L., Silva D.S., Medina F.T. Resíduo desidratado de vitivinícolas associado a diferentes fontes energéticas na alimentação de ovinos: consumo e digestibilidade aparente. Ciênc Agrotec. 2006;30:767–773. [Google Scholar]
- 24.Nogueira F.A., Fonseca L.D., da Silva R.B., de Paiva Ferreira A.V., Nery P.S., Geraseev L.C. In vitro and in vivo efficacy of aqueous extract of Caryocar brasiliense Camb to control gastrointestinal nematodes in sheep. Parasitol Res. 2012;111:325–330. doi: 10.1007/s00436-012-2843-8. [DOI] [PubMed] [Google Scholar]
- 25.Domingues L.F., Giglioti R., Feitosa K.A., Fantatto R.R., Rabelo M.D., de Sena Oliveira M.C. In vitro and in vivo evaluation of the activity of pineapple (Ananas comosus) on Haemonchus contortus in Santa Inês sheep. Vet Parasitol. 2013;197:263–270. doi: 10.1016/j.vetpar.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Pieszka M., Gogol P., Pietras M., Pieszka M. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann Anim Sci. 2015;15:475–491. [Google Scholar]
- 27.Villalba J.J., Provenza F.D., Hall J.O., Lisonbee L.D. Selection of tannins by sheep in response to gastrointestinal nematode infection. J Anim Sci. 2010;88:2189–2198. doi: 10.2527/jas.2009-2272. [DOI] [PubMed] [Google Scholar]
- 28.D’Addabbo T., Carbonara T., Leonetti P., Radicci V., Tava A., Avato P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem Rev. 2011;10:503–519. [Google Scholar]
- 29.Hoste H., Jackson F., Athanasiadou S., Thamsborg S.M., Hoskin S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006;22:253–261. doi: 10.1016/j.pt.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Quijada J., Fryganas C., Ropiak H.M., Ramsay A., Mueller-Harvey I., Hoste H. Anthelmintic Activities against Haemonchus contortus or Trichostrongylus colubriformis from small ruminants are influenced by structural features of condensed tannins. J Agric Food Chem. 2015;63:6346–6354. doi: 10.1021/acs.jafc.5b00831. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira L.E., Castro P.M., Chagas A.C., França S.C. Beleboni RO. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp Parasitol. 2013;134:327–332. doi: 10.1016/j.exppara.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Jin L., Wang Y., Iwaasa A.D., Xu Z.J., Schellenberg M.P., Zhang Y.G. Effect of condensed tannins on ruminal degradability of purple prairie clover (Dalea purpurea Vent.) harvested at two growth stages. Anim Feed Sci Technol. 2012;176:17–25. [Google Scholar]
- 33.Jayanegara A., Goel G., Makkar H.P.S., Becker K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim Feed Sci Technol. 2015;209:60–68. [Google Scholar]
- 34.Häring D.A., Scharenberg A., Heckendorn F., Dohme F., Lüscher A., Maurer V. Tanniferous forage plants: agronomic performance, palatability and efficacy against parasitic nematodes in sheep. Renew Agric Food Syst. 2008;23:19–29. [Google Scholar]
- 35.Theodoridou K., Aufrère J., Andueza D., Pourrat J., Le Morvan A., Stringano E. Effects of condensed tannins in fresh sainfoin (Onobrychis viciifolia) on in vivo and in situ digestion in sheep. Anim Feed Sci Technol. 2010;160:23–38. doi: 10.1017/S1751731111001510. [DOI] [PubMed] [Google Scholar]
- 36.Waghorn G. Benefical and detrimental effects of dietary condensed tannins for sustainable sheep and goat production - Progress and challenges. Anim Feed Sci Technol. 2008;147:116–139. [Google Scholar]
- 37.Ghaffari M.H., Tahmasbi A.M., Khorvash M., Naserian A.A., Ghaffari A.H., Valizadeh H. Effects of pistachio by-products in replacement of alfalfa hay on populations of rumen bacteria involved in biohydrogenation and fermentative parameters in the rumen of sheep. J Anim Physiol Anim Nutr. 2014;98:578–586. doi: 10.1111/jpn.12120. [DOI] [PubMed] [Google Scholar]
- 38.Valenti B., Natalello A., Vasta V., Campidonico L., Roscini V., Mattioli S. Effect of different dietary tannin extracts on lamb growth performances and meat oxidative stability: comparison between mimosa, chestnut and tara. Animal. 2018 doi: 10.1017/S1751731118001556. [DOI] [PubMed] [Google Scholar]
- 39.Santos A.C.V., Santos F.O., Lima H.G., Silva G.D.D., Uzêda R.S., Dias Ê.R. In vitro ovicidal and larvicidal activities of some saponins and flavonoids against parasitic nematodes of goats. Parasitology. 2018 doi: 10.1017/S0031182018000689. [DOI] [PubMed] [Google Scholar]
- 40.Kerboeuf D., Riou M., Guégnard F. Flavonoids and related compounds in parasitic disease control. Mini Rev Med Chem. 2008;8:116–128. doi: 10.2174/138955708783498168. [DOI] [PubMed] [Google Scholar]
- 41.Baumgärtel T., Kluth H., Epperlein K., Rodehutscord M. A note on digestibility and energy value for sheep of different grape pomace. Small Rumin Res. 2007;67:302–306. [Google Scholar]




