Abstract
Background/objectives
Individuals with Gilbert's syndrome (GS) harbor mutations in the UGT1A1 gene and are known to have elevated levels of bilirubin, which enhances the risk for gall stone formation. The aim of this study is to screen Indian patients with GS for the incidence of gall stone disease.
Methods
Individuals with persistently elevated serum bilirubin levels were genotyped for two polymorphisms (rs8175347; rs4148323) in UGT1A1 gene to confirm GS in them. Flanking regions of the above polymorphisms were amplified followed by direct sequencing. Ultrasonography was done to detect gallstone disease. Clinical data, including assessment of liver function, circulating levels of total and direct bilirubin, as well as routine hematological parameters were obtained as per standard procedures (Autoanalyzer).
Results
Of the total 1621 individuals subjected to genotyping, 1191 (1149 males of 29.6 ± 11.3 years with mean BMI of 22.1 ± 3.7 kg/m2 and 42 females of 30.8 ± 14.8 years with mean BMI of 20.9 ± 3.7 kg/m2) were confirmed to have GS. Gall bladder abnormalities including cholelithiasis (n = 106/1191; 8.9%), polyps (n = 18/1191; 1.5%) and gallbladder wall thickening (n = 17/1191; 1.4%) were noted. Incidence of gall stone disease was observed in 103 males (out of 1149) and 3 females (out of 42) indicating the risk of the disease to be 9.0% and 7.1% respectively in males and females with GS.
Conclusion
Early recognition of GS by genetic analysis is required before these patients with intermittent episodes of jaundice run the risk of unnecessary operations on their bile ducts from the mistaken assumption ascribing the jaundice to a stone which has been left behind.
Abbreviations: ALT, alanine transaminase; AST, aspartate amino transferase; BMI, body mass index; DNA, deoxyribose nucleic acid; ERCP, Endoscopic Retrograde Cholangio Pancreatography; EUS, endoscopic ultrasongram; GD, gall stone disease; GS, Gilbert's syndrome; GWAS, genome wide association disease; MRCP, Magnetic Resonance Cholangio Pancreatography; PCR, polymerase chain reaction; SNPs, single nucleotide polymorphisms; UGT1A1, UDP glucuronosyltransferase family 1 member A1
Keywords: Gilbert's syndrome, gallstone disease, genetic polymorphisms, unconjugated bilirubin, UGT1A1gene
Gallstone disease (GD) is a common gastrointestinal ailment causing substantial burden to the health care system with 10–20% of the patients developing symptoms.1 While genetic predisposition and environmental factors have a profound influence on disease causation,2 demographic and environmental risk factors3 (including age, sex and body mass index,) are relevant in this regard. The prevalence of Gilbert's syndrome is 6% in South Indian ethnicity4 and it affects 7.4% of adult population in North India,5 where in cholesterol stones, composed principally of cholesterol monohydrate crystals are encountered as opposed to pigment stones (composed of calcium bilirubinate) that are encountered in South India.5, 6
Pigment gallstones are formed due to excess secretion of bilirubin,7 arising from hemolysis, ineffective erythropoiesis, or pathologic enterohepatic cycling of unconjugated bilirubin, into the bile. An augmented enterohepatic cycling of bilirubin emanating from increased absorption of unconjugated bilirubin has been proposed as the cause of pigment gallstones.8 Pigment gallstones are classified descriptively as “black” or “brown”, and are mostly composed of calcium hydrogen bilirubinate, which is polymerized and oxidized in “black” stones but remains unpolymerized in “brown” stones. Bilirubin monoconjugates that are increased in several conditions, such as in Gilbert's syndrome and chronic hemolysis, may play a pivotal role in pigment stone formation where the unconjugated monohydrogenated bilirubin co-precipitates with calcium. Generally, pigment stones are formed by the precipitation of bilirubin in bile, with black stones associated with chronic hemolytic states, and Gilbert's syndrome.9
Gilbert's syndrome (GS) is a genetic disorder that results from increased serum unconjugated bilirubin levels in the absence of liver disease or overt hemolysis.10 Raised bilirubin alone can be a manifestation of more serious disease.11 Various tests have been proposed in an attempt to provide a firm diagnosis of Gilbert's syndrome. Two polymorphisms in UGT1A1 namely G71R in exon 1(rs4148323) and A(TA)7TAA in the TATA box (rs8175347) of the promoter region are the most prevalent and are responsible for GS.12, 13A simple dinucleotide repeat polymorphism(TA)5–8, in the TATAbox of the UGT1A1 gene is associated with reduced expression of the hepatic enzyme and results in the chronic unconjugated hyperbilirubinemia that occurs in Gilbert syndrome.14, 15
A polymorphism (rs8175347) in UGT1A1 gene in homozygous state is associated with GS and unconjugated hyperbilirubinemia.16 It is revealed that the length of TA repeats in the promoter region is an important modulator of enzyme activity; the greater the number of repeats higher the serum bilirubin levels and cholelithiasis risk.16, 17 Furthermore, genome-wide association studies (GWAS) of three large Caucasian cohorts have identified single nucleotide polymorphisms (SNPs) in UGT1A1 gene that are responsible for 18% of clinical variability of serum bilirubin.18 Cholelithiasis risk is partially mediated by genes impacting bilirubin metabolism,19, 20 and earlier studies from our lab have identified the association of a variant in UGT1A1 gene with pigment gallstone disease in South Indian population.21 Previous studies reported coexistence of gallstones with Gilbert's Syndrome.22, 23, 24, 25 In the present study we report the coexistence of gallstone disease in Indian patients affected with Gilbert syndrome.
Methods
Study Population
A total of 1621 individuals (1506 men and 115 women), with high bilirubin levels, who visited the hepatology clinics between September 2009 and April 2017, were enrolled into the study. Anthropometric data were obtained from these individuals and blood samples (2 ml) were collected from the patients for biochemical and genetic analyses. The study was approved in institutional ethics committee.
Genotyping
Gilbert's syndrome in the patients was confirmed by genetic screening of variants in UGT1A1 gene. Genomic DNA was isolated from peripheral blood leukocytes of whole blood by commercial kit (Bioserve Biotechnologies, India) following manufacturer's instructions. DNA concentration and purity was measured with NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) and agarose gel electrophoresis respectively. Variants namely UGT1A1 (TA)n (rs8175347) and G7R(G>A) (rs4148323) in UGT1A1 gene were genotyped in all patients by polymerase chain reaction (PCR) followed by direct DNA sequencing. Briefly, 100 ng of DNA along with 10 pmol/μl primers (Forward primer F-5′ TCCCTGCTACCTTTGTGGAC3′ and Reverse primer R-5′ CTGGGTAGCCTCAAATTCCA3′), dNTPs (10 mM each) and Taq polymerase (5 U/μl) to generate a 700-bp fragment. The PCR reactions conditions were initial denaturation at 95 °C for 5 min; followed by 35 cycles at 95 °C for 60 s, 60 °C for 60 s, and 72 °C for 60 s and by a final extension step at 72 °C for 7-min. The amplicons were purified using AMPure magnetic beads (Agencourt AMPure XP-Beckman Coulter) and dye Termination cycling Sequencing was done by sequencing ready reaction Kit (Beckman) and was directly sequenced by Beckman coulter (Genomelab GeXp). Sequence analysis was performed using software (Beckman Coulter CEQ™ 8000, Fullerton, CA 92834-3100).
Biochemical Analyses
Total and direct bilirubin levels, alanine transaminase (ALT), Aspartate amino transferase (AST) and other liver function parameters were estimated in serum using commercially available kits (Randox laboratories, Crumlin, Antrim, UK). Hemoglobin level, reticulocyte count and other hemogram parameters were estimated by auto analyzer (Beckman coulter LX750, Miami, FL).
Results
Clinical Characteristics
Of the 1621 individuals referred for genetic testing for Gilbert's syndrome, 1157 were of South Indian (SI) and 464 were of East Indian (EI) ethnicity with a BMI (mean ± SD) of 22.04 ± 3.8 and 22.01 ± 3.2 respectively. The ultrasound investigations were conducted for 1240 individuals in whom Gilberts syndrome could be confirmed in 1191 individuals including 1149 males (96.5%mean age 29.6 ± 11.3 years; mean BMI 22.1 ± 3.7 kg/m2) and 42 females (3.5%mean age 30.8 ± 14.8 years; mean BMI 20.9 ± 3.7 kg/m2). Coexistence of GS with gallbladder abnormality was noted in greater number of males (137/141; 97.2%) than in females. Demographic and clinical characteristics between Gilbert's syndrome with and without gallstone disease (GS-GD) for 1191 patients are as presented in Table 1. Even though no significant difference was observed, total bilirubin levels were high and indirect bilirubin was higher than the direct bilirubin in both the groups. Related parameters indicative of liver function as well as hematological parameters did not reveal any significant changes as shown in Table 1. It was also interesting to note that there was no significant difference in the BMI between the groups.
Table 1.
Demographic and Clinical Characteristics.
| GS with GD | GS | Ref. Range | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age | 34.3 ± 13.6 | 29.1 ± 11.2 | |
| Gender n(M/F) | 103/3 | 1012/38 | |
| BMI | 20.75 ± 2.95 | 22.04 ± 3.72 | |
| Total Bilirubin (mmol/L) | 3.67 ± 1.92 | 3.8 ± 1.8 | 0.3–1.2 |
| Direct Bilirubin (mmol/L) | 0.70 ± 0.63 | 0.9 ± 0.4 | Up to 0.2 |
| Indirect Bilirubin (mmol/L) | 2.97 ± 1.68 | 2.9 ± 1.7 | |
| ALT (U/L) | 33.97 ± 18.08 | 31.6 ± 19.3 | 0–40 |
| AST (U/L) | 30.17 ± 10.49 | 29.5 ± 16.4 | Up to 40 |
| ALP (U/L) | 82.91 ± 27.97 | 84.2 ± 28.7 | 30–120 |
| Total protein (mmol/L) | 7.35 ± 0.58 | 7.6 ± 0.4* | 6–8 |
| Albumin (mmol/L) | 4.29 ± 0.67 | 4.6 ± 0.3* | 3.5–5.2 |
| Globulin (mmol/L) | 3.01 ± 0.42 | 3.0 ± 0.3 | 2.3–3.5 |
| Total WBC (cell/mm3) | 7807.61 ± 2831.04 | 7679.4 ± 2467.1 | 4000–10000 |
| RBC (millions/mm3) | 4.50 ± 0.93 | 4.9 ± 1.0* | 3.8–4.8 |
| Hemoglobin (mmol/L) | 13.51 ± 2.46 | 14.6 ± 2.6* | 12.0–15.0 |
| HCT (%) | 39.24 ± 6.65 | 41.7 ± 8.5* | 36–46 |
| Platelets (×106/mm3) | 1.93 ± 0.76 | 2.4 ± 0.7* | 1.5–4.1 |
GS, Gilbert's syndrome; GD, gallstone disease; Ref, reference; BMI, body mass index; ALT, alanine transaminase; AST, aspartate amino transferase; ALP, alkaline phosphatase; WBC, white blood cells; RBC, red blood cells; HCT, hematocrit.
P > 0.05.
UGT1A1 Gene Polymorphism and Gilbert's Syndrome
In a total of 1240 individuals suspected for Gilbert's syndrome, 58 subjects were wild, 258 were heterozygous and 924 were homozygous for the rs8175347 dinucleotide repeat polymorphism (TA)5–8. For the rs4148323 single nucleotide polymorphism 1102 subjects were wild, 129 were heterozygous and 9 were homozygous. Of these, 129 were compound heterozygous for both the variants. Gilbert's syndrome was confirmed in 1191 of the 1240 subjects (96%) based on the genotype.
Gilbert's Syndrome and Gall Stone Disease
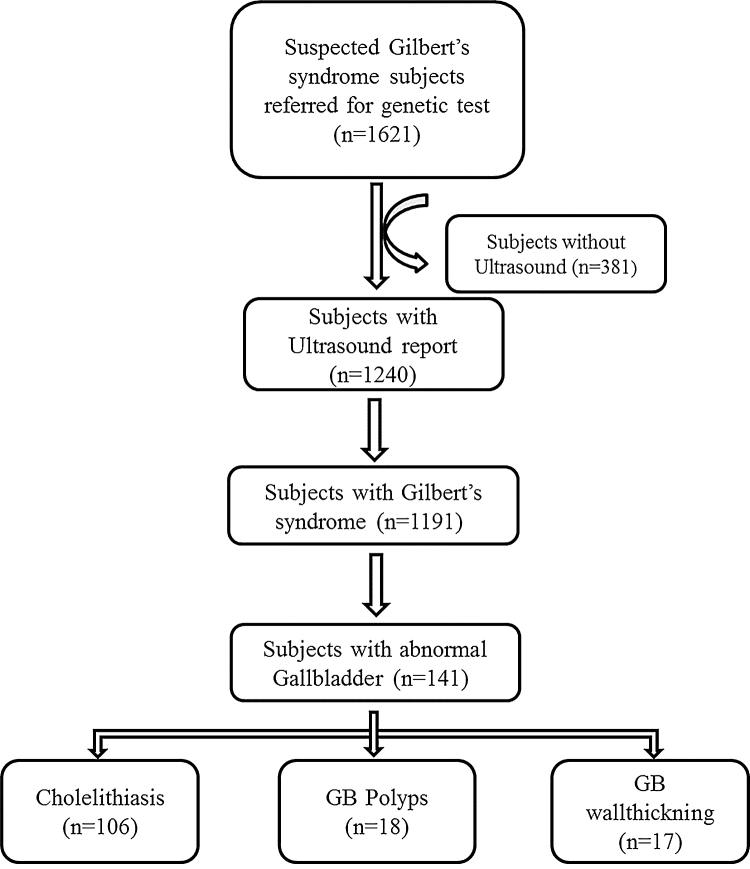
A total of 1191 subjects were positive for Gilberts syndrome, of these 11.8% (n = 141) had gall bladder abnormalities including cholelithiasis (n = 106/1191; 8.9%), polyps (n = 18/1191; 1.5%) and gallbladder wall thickening (n = 17/1191; 1.4%) respectively (Figure 1). In the present analysis, it was seen that 9.0% of males (n = 103/1149) and 7.1% of females (n = 3/42) were at risk for gallstone disease.
Figure 1.
Samples consort diagram.
Discussion
Gilbert's syndrome is associated with mildly elevated levels of unconjugated bilirubin in absence of liver disease and is characterized by episodes of mild, intermittent jaundice. It is well known that a polymorphism in the promoter region of UGT1A1 gene is responsible for the elevated unconjugated hyperbilirubinaemia leading to GS,26 which is usually diagnosed incidentally during a lab investigation or when a light yellowish tinge of conjunctivae is noticed; both the features (elevated bilirubin levels and yellow eyes) tend to be repetitive causing undue anxiety in the patients and their attendants. Individuals with Gilbert's syndrome also develop gallstones necessitating cholecystectomy. It is not uncommon in clinical practice that the intermittent episodes of jaundice are ascribed to gall stone(s) left behind, leading to the risk of unwarranted operations on their bile ducts. Thus, it is prudent to determine the coexistence of Gilbert's syndrome in individuals with gallstone disease. In absence of detailed knowledge in the Indian population in this regard, we conducted the present study to determine how frequently these conditions coexist.
The present study involved individuals with increased levels of circulating bilirubin and comparisons were made between patients confirmed for Gilbert's syndrome with or without concomitant gall stone disease in them. Bilirubin biosynthesis starts from hemoglobin metabolism, where heme is metabolized into biliverdin and then is converted to bilirubin which is termed as unconjugated or indirect bilirubin. The unconjugated bilirubin bound with albumin is transported via the bloodstream to the liver where it undergoes glucuronidation into conjugated bilirubin by the UDP-glucuronosyltransferase 1(UGT family enzyme); only UGT1A1 is involved in bilirubin conjugation.27 In our results we found lower conjugated bilirubin and higher unconjugated bilirubin levels but it's not statistically significant and there was significantly lower albumin and total proteins in GS subjects between with GD and without GD; albumin and UGT family of enzymes play an important role in conversion of unconjugated bilirubin into conjugated bilirubin. An increased unconjugated bilirubin has been proposed as the cause of pigment gallstones.8 We also found significantly lower hemoglobin, RBC and hematocrit which were haemolytic cause for the hyperbilirubinaemia.
Results obtained in the present study indicated certain interesting findings about the incidence of gallstone disease in males and females. It is well known that middle aged, obese females also tend to develop gallstone disease. The obtained results (Table 1) indicated higher prevalence of Gilbert's syndrome and gallstone disease in males, as opposed to females. While the increased tendency of females to develop gallstone disease is well recognized in Northern India.28 It is known that female gender,29 obesity30 are risk factors for gallstone disease but majority of the subjects were males in the present study. GS is predominantly a disease of males22, 31 and in this study all subjects had normal BMI; which shows that GS independently of these parameters is associated with gallstone disease. Gilbert's syndrome subjects have been found to have gallstones ∼5% in previous reports25 and in the present study we found that 8.9% of Gilbert's syndrome (UGT1A1(TA)n promoter polymorphism) patients had gallstones.
Some of these subjects (with undiagnosed Gilbert's syndrome) undergo unnecessary investigations like MRCP (Magnetic Resonance Cholangio Pancreatography), ERCP (Endoscopic Retrograde Cholangio Pancreatography) or EUS (Endoscopic Ultrasongram) when “jaundice” is found to be associated with symptomatic or asymptomatic gallstones. In few subjects, elevated levels of bilirubin may appear during follow-up after laparoscopic cholecystectomy has been done, resulting again in needless investigations for a suspected retained common bile duct stone. Similar situation can arise in patients who have recovered after complete resolution from an episode of acute hepatitis when bilirubin is found to be “still elevated” during follow-up. Hence it is prudent to be aware of Gilbert's syndrome in any one with unconjugated hyperbilirubinemia with normal hemoglobin levels associated with gallstone disease.
In conclusion, individuals with increased levels of circulating unconjugated bilirubin in gallstone disease are to be investigated for polymorphisms associated with Gilbert's syndrome.
Conflicts of Interest
The authors have none to declare.
Acknowledgements
The authors acknowledge the individuals for consenting to participate in the study. We acknowledge the help rendered by Mrs. Ashwini Kaitha in genotyping the samples. Our sincere thanks are due to Professor C. Subramanyam and Dr. M. Sasikala for critical review and suggestions on the manuscript.
References
- 1.Friedman G.D., Raviola C.A., Fireman B. Prognosis of gallstones with mild or no symptoms: 25 years of follow-up in a health maintenance organization. J Clin Epidemiol. 1989;42:127–136. doi: 10.1016/0895-4356(89)90086-3. [DOI] [PubMed] [Google Scholar]
- 2.Lammert F., Sauerbruch T. Mechanisms of disease: the genetic epidemiology of gallbladder stones. Nat Clin Pract Gastroenterol Hepatol. 2005;2:423–433. doi: 10.1038/ncpgasthep0257. [DOI] [PubMed] [Google Scholar]
- 3.Miquel J.F., Covarrubias C., Villaroel L. Genetic epidemiology of cholesterol cholelithiasis among Chilean Hispanics, Amerindians, and Maoris. Gastroenterology. 1998;115:937–946. doi: 10.1016/s0016-5085(98)70266-5. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni R.G., Lakshmidevi K.B., Ronghe V., Dinesh U.S. Gilbert's syndrome in healthy blood donors what next? Asian J Transfus Sci. 2016;10(January):63. doi: 10.4103/0973-6247.165835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon R.K. Prevalence and type of biliary stones in India. World J Gasteroenterol. 2000;6:4–5. [Google Scholar]
- 6.Jayanthi V., Palanivelu C., Prasanthi R., Methew S., Srinivasan V. Composition of gall stones in Coimbatore district of Tamil Nadu state. Indian J Gastroenterol. 1998;17:134–135. [PubMed] [Google Scholar]
- 7.Buch S., Schafmayer C., Volzke H. Loci from a genome-wide analysis of bilirubin levels are associated with gallstone risk and composition. Gastroenterology. 2010;139:1942–1951. doi: 10.1053/j.gastro.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Vitek L., Carey M.C. Enterohepatic cycling of bilirubin as a cause of “black” pigment gallstones in adult life. Eur J Clin Invest. 2003;33:799–810. doi: 10.1046/j.1365-2362.2003.01214.x. [DOI] [PubMed] [Google Scholar]
- 9.Lambou-Gianoukos S., Heller S.J. Lithogenesis and bile metabolism. Surg Clin North Am. 2008;88:1175–1194. doi: 10.1016/j.suc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert A., Lereboullet P. La cholémie simple familiale. Semaine Med. 1901;21:241–243. [Google Scholar]
- 11.Levine R.A., Klatskin G. Unconjugated hyperbilirubinemia in the absence of overt hemolysis: importance of acquired disease as an etiologic factor in 366 adolescent and adult subjects. Am J Med. 1964;36:541–552. doi: 10.1016/0002-9343(64)90102-0. [DOI] [PubMed] [Google Scholar]
- 12.Borlak J., Thum T., Landt O., Erb K., Hermann R. Molecular diagnosis of a familial nonhemolytic hyperbilirubinemia (Gilbert's syndrome) in healthy subjects. Hepatology. 2000;32:792–795. doi: 10.1053/jhep.2000.18193. [DOI] [PubMed] [Google Scholar]
- 13.Burchell B., Hume R. Molecular genetic basis of Gilbert's syndrome. J Gastroenterol Hepatol. 1999;14:960–966. doi: 10.1046/j.1440-1746.1999.01984.x. [DOI] [PubMed] [Google Scholar]
- 14.Bosma P., Chowdhury J., Bakker C. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 15.Monaghan G., Ryan M., Seddon R., Hume R., Burchell B. Genetic variation in bilirubin UDP-glucuronosyltransferase gene promoter and Gilbert's syndrome. Lancet. 1996;347:578–581. doi: 10.1016/s0140-6736(96)91273-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsezou A., Thetis M., Giannatou E. Gilbert syndrome as a predisposing factor for cholelithiasis risk in the Greek adult population. Gen Test. 2009;13:143–146. doi: 10.1089/gtmb.2008.0095. [DOI] [PubMed] [Google Scholar]
- 17.Bosma P.J., Chowdhury J.R., Bakker C. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert's syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 18.Benjamin E.J., Dupuis J., Larson M.G. Genome-wide association with select biomarker traits in the Framingham heart study. BMC Med Genet. 2007;8(suppl 1):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel A., Kneip S., Barut A. Genetic link of hepatocellular carcinoma with polymorphisms of the UDP-glucuronosyltransferase UGT1A7 gene. Gastroenterology. 2001;121:1136–1144. doi: 10.1053/gast.2001.28655. [DOI] [PubMed] [Google Scholar]
- 20.Vasavda N., Menzel S., Kondaveeti S. The linear effects of alpha-thalassaemia, the UGT1A1 and HMOX1 polymorphisms on cholelithiasis in sickle cell disease. Br J Haematol. 2007;138:263–270. doi: 10.1111/j.1365-2141.2007.06643.x. [DOI] [PubMed] [Google Scholar]
- 21.Ravikanth V.V., Rao G.V., Govardhan B. Polymorphisms in UGT1A1 gene predispose south Indians to pigmentous gallstones. J Clin Exp Hepatol. 2016;6:216–223. doi: 10.1016/j.jceh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peel A.L., Ritchie H.D. Gilbert's syndrome in patients with gallbladder stones. Ann R Coll Surg Engl. 1974;55:184. [PMC free article] [PubMed] [Google Scholar]
- 23.Milton J.N., Sebastiani P., Solovieff N. A genome-wide association study of total bilirubin and cholelithiasis risk in sickle cell anemia. PLoS ONE. 2012;27:e34741. doi: 10.1371/journal.pone.0034741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foulk W.T., Butt H.R., Owen C.A., Jr., Whitcomb F.F., Jr., Mason H.L. Constitutional hepatic dysfunction (Gilberts disease): its natural history and related syndromes. Medicine. 1959;38:25–46. [PubMed] [Google Scholar]
- 25.Powell L.W., Hemingway E., Billing B.H. Idiopathic unconjugated hyperbilirubinemia (Gilbert's syndrome). A study of 42 families. N Engl J Med. 1967;277:1108–1112. doi: 10.1056/NEJM196711232772102. [DOI] [PubMed] [Google Scholar]
- 26.Krawczyk M., Wang D.Q., Portincasa P. Dissecting the genetic heterogeneity of gallbladder stone formation. Semin Liver Dis. 2011;31:157–172. doi: 10.1055/s-0031-1276645. [DOI] [PubMed] [Google Scholar]
- 27.Perera M.A., Innocenti F., Ratain M.J. Pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 polymorphisms: are we there yet? Pharmacotherapy. 2008;28:755–768. doi: 10.1592/phco.28.6.755. [DOI] [PubMed] [Google Scholar]
- 28.Unisa S., Jagannath P., Dhir V., Khandelwal C., Sarangi L., Roy T.K. Population-based study to estimate prevalence and determine risk factors of gallbladder diseases in the rural Gangetic basin of North India. HPB. 2011;13:117–125. doi: 10.1111/j.1477-2574.2010.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novacek G. Gender and gallstone disease. WMW Wiener Medizinische Wochenschrift. 2006;156:527–533. doi: 10.1007/s10354-006-0346-x. [DOI] [PubMed] [Google Scholar]
- 30.Stampfer M.J., Maclure K.M., Colditz G.A., Manson J.E., Willett W.C. Risk of symptomatic gallstones in women with severe obesity. Am J Clin Nutr. 1992;55:652–658. doi: 10.1093/ajcn/55.3.652. [DOI] [PubMed] [Google Scholar]
- 31.Kornberg A. Latent liver disease in persons recovered from catarrhal jaundice and in otherwise normal medical students as revealed by the bilirubin excretion test. J Clin Invest. 1942;21:299. doi: 10.1172/JCI101304. [DOI] [PMC free article] [PubMed] [Google Scholar]