Abstract
Background:
Sedentary behavior is pervasive among the general population, but little is known about the epidemiology of this behavior in multiple sclerosis (MS).
Objective:
We compared self-reported sitting time (ST), as a measure of sedentary behavior, between persons with MS and healthy controls, and examined ST across demographic and clinical characteristics of those with MS.
Methods:
1081 persons with MS and 150 healthy controls self-reported ST based on the International Physical Activity Questionnaire (IPAQ), and completed the Godin Leisure-Time Exercise Questionnaire (GLTEQ) and a demographic/clinical scale. Data were analyzed using analysis of variance, bivariate correlations, and stepwise regression analysis.
Results:
There was not a significant difference in ST between persons with MS and controls (F=0.01, p=0.95), and persons with MS reported 450.9±220.6 minutes of ST per day. ST was weakly associated with GLTEQ scores in MS (r=−.21, p<.001), but not controls. ST significantly differed as functions of marital status, physical activity level, employment status, education, and degree of ambulatory impairment among those with MS.
Conclusions:
ST does not differ between persons with MS and healthy controls, but those with MS report a large amount of this sedentary behavior that is potentially an independent correlate of health and disease outcomes.
Keywords: Multiple sclerosis, sedentary behavior, sitting, health behavior, physical inactivity
INTRODUCTION
Sedentary behavior (i.e., behavior involving sitting or lying that does not increase energy expenditure during the waking hours) is pervasive among adults in Western countries1 and world-wide2. Adults in countries around the world accumulate between 3 and 8 hours of sitting time (ST) per day2. ST has been detrimentally associated with disease risk factors such as blood glucose and obesity3, as well as increased risks of morbidity and mortality2,4 independent of physical activity. Accordingly, researchers have begun focusing on sedentary behavior, particularly reducing or breaking-up daily ST, as an important target of behavioral interventions for reducing health risks5,6.
To date, the majority of research on sedentary behavior has focused on the general population without a chronic disease condition, and there is minimal research on sedentary behavior in adults with progressive diseases that result in mobility disability7 such as multiple sclerosis (MS). MS is a common immune-mediated disease of the central nervous system (CNS) that causes axonal demyelination, transection, and loss as well as neurodegeneration over time. Such damage of the CNS tissue results in the clinical expressions of MS that progressively result in the accumulation of ambulatory impairment8. The clinical expression of MS and associated ambulatory impairment might increase the rate of sedentary behavior in persons with MS. One small study has quantified sedentary time as an estimated 8 hours per day in persons with MS9. This study indicates that sedentary time differs based on disability status, but did not examine other variables for a good description of demographic and clinical variables that might explain differences in ST. There further was not comparison with controls.
The present study involved a secondary, exploratory analysis of existing data and compared self-reported ST between persons with MS and controls without MS or any other disease conditions. We secondly examined self-reported ST among demographic and clinical characteristics of persons with MS. This paper provides additional information regarding the possible public health crisis involving ST in persons with MS, and potentially identifies a new avenue for behavioral interventions for improving health and possibly disease status in this population, if there is a high rate of sedentary behavior.
METHOD
Participants
The current study involved a secondary analysis of data amalgamated from 13 previous investigations10–22 of physical activity and its associations with quality of life, social cognitive and symptomatic outcomes; all data from those investigations had been previously de-identified. Studies were included in the data amalgamation that included an assessment of clinical characteristics (i.e. disability status and disease duration), sociodemographic variables (i.e. sex, age, BMI, education, etc.), and ST (questions 7 of the International Physical Activity Questionnaire (IPAQ)). There were 13 studies that contained those data and the de-identified data were compiled across those studies into a single data set for analyses. MS participant recruitment occurred throughout the United States, but primarily within the Midwest and the state of Illinois. Potential participants were contacted through print and email flyers and advertisement on the National Multiple Sclerosis Society website. Healthy controls were recruited through the University community via public email postings. Persons with MS were recruited based on the following common inclusion criteria: (1) diagnosis of MS; (2) relapsefree in the previous 30 days; and (3) ambulatory with or without assistance. The final combined samples of convenience included 1081 persons with MS and 150 healthy controls.
Self-report ST and physical activity measures
ST was measured using item seven from the abbreviated version of the IPAQ23, and scores from this item have been validated with accelerometry24. This item reads, “During the last 7 days, how much time did you spend sitting on a weekday?” The item further has instructions regarding the location and opportunity for ST such as at work or home and while doing course work or during leisure time. The item includes examples of sitting activities such as sitting at a desk, visiting friends, reading, or watching television. Participants indicated their ST by writing in the number of hours in a single blank below the instructions.
Physical activity was measured with the Godin Leisure-Time Exercise Questionnaire (GLTEQ)25. The GLTEQ measures the frequency of strenuous, moderate, and mild leisure time physical activity performed for periods of 15 minutes or more over a usual week. The overall GLTEQ score was calculated by multiplying the frequencies of strenuous, moderate, and mild by 9, 5, and 3 METs, respectively, and yielded a continuous measure of leisure physical activity in arbitrary units26.
Disability status
The Patient Determined Disease Steps (PDDS)27,28 scale is a single-item, self-reported measure of disability status. The PDDS ranges from 0 (Normal) to 8 (Bedridden), and has been recommended as a surrogate for the physician-rated Expanded Disability Status Scale (EDSS)28 when neurological examination is not suitable (e.g., survey-based research). Scores from the PDDS have been strongly and linearly associated with EDSS scores (r=.783)28. PDDS scores were trichotomized into groups of ambulatory disability. Scores of 0-2 were categorized as “no ambulatory impairment,” scores of 3-4 were categorized as “mild ambulatory impairment” and scores above 5 were categorized as “moderate-severe ambulatory impairment.” Similar classifications have been reported previously29,30.
Sociodemographic and clinical variables
Sociodemographic and clinical characteristics were measured with a standard scale. Sex, age, body mass index (BMI), marital status, number of children, employment status, race, education, and income were included as sociodemographic variables. The following sociodemographic variables were collapsed into groups for ease of analysis: age (18-39 vs. 40-59 vs. >60), BMI (normal vs. overweight vs. obese), marital status (married vs. unmarried), children (no children vs. children), race (Caucasian vs. non-Caucasian), education (college graduate vs. non-college graduate), and income (< $40,000/year vs. >$40,000/year). Clinical course of MS was determined by standard definitions31 and disease duration was identified as the time since the date of confirmed MS diagnosis. The following clinical variables were collapsed into groups as a justifiable means to segment the data for clinically relevant groups: disease type (Relapsing Remitting MS vs. Progressive MS), PDDS (no impairment, mild ambulatory impairment and moderate-severe ambulatory impairment), and disease duration (<10 years, 10-20 years, and >20 years).
Procedure
All studies were approved by the same University Institutional Review Board, and all data were de-identified before amalgamation. All participants provided written informed consent. Participants were either sent a battery of questionnaires through the United States Postal Service (USPS) with a stamped, pre-addressed return envelope or completed questionnaires during a baseline testing session in the laboratory. If the questionnaires were received via USPS, the researchers verified the questionnaires were complete upon return. Any participant with missing information was collected over the phone. If the questionnaires were completed during a baseline testing session, the questionnaires were checked for completion prior to the participant completing the session and any missing information was obtained. All participants received compensation for successful completion of the questionnaires.
Data analysis
All data analyses were conducted on de-identified data in IBM SPSS Statistics Version 21 for Windows (SPSS, Inc., Chicago, IL). Descriptive statistics are provided in text and tables as mean (M) with standard deviation (SD) unless otherwise noted (e.g., medians or percentages). We initially compared the MS and control groups for differences in demographic and physical activity variables using independent samples t-test and/or chi-square tests. The primary analytic model for examining sitting behavior involved a between-subjects analysis of variance (ANOVA) on self-reported ST per day from question seven of the IPAQ. The between-subjects factors were based on group (i.e., MS vs. healthy control), or sociodemographic (i.e., sex, age, BMI, marital status, number of children, employment, race, education, and income) and clinical (i.e., MS type, disease duration, and ambulatory status based on PDDS score) factors in those with MS separately. Bonferroni follow-up analyses were performed to identify significant differences in ST for significant between-subjects effects involving 3 or more groups in the ANOVAs. Effect size estimates based on Cohen’s d (i.e., difference between mean scores for two groups divided by the pooled standard deviation) are provided and delineated as small, medium, and large based on the criteria of 0.2, 0.5, and 0.8, respectively32. We further examined the association between ST and physical activity (i.e., GLTEQ scores) using a Pearson product-moment correlation in persons with MS and controls separately. We performed multiple linear regression analyses with stepwise entry to examine the independent contributions of variables associated ST in only the MS sample.
RESULTS
Sociodemographic and clinical characteristics of samples
Table 1 provides the sociodemographic characteristics and identified differences between the samples of participants with MS and healthy controls, and the clinical characteristics of the participants with MS. The mean PDDS score for the MS participants was 2.0 (range=0–8), indicating that the sample was characterized by moderate disability (i.e. no restrictions in walking but significant limitations to daily activity due to MS). The disability ranged between normal and bilateral assistance (e.g. rollator or frame). The mean duration of MS was 9.7 years with 90% (n=973) of the sample reporting a diagnosis of relapsing remitting multiple sclerosis (RRMS). There were no differences between MS and controls for marital status, number of children, and income, but there were differences in sex, age, BMI, employment, race, and education (p<0.05).
Table 1.
Sociodemographic and clinical characteristics with MS and controls. Note: MS: Multiple Sclerosis; RRMS: Relapsing-Remitting Multiple Sclerosis; PDDS: Patient Determined Disease Steps scale; GLTEQ: Godin Leisure-Time Exercise Questionnaire. Note: Values shown are mean ± standard deviation, unless otherwise noted.
| Group | |||
|---|---|---|---|
| Variable | Control (n=150) |
MS (n=1081) |
p-value |
| Sex (% female) | 91% | 84% | 0.01 |
| Age (years) | 43.2 ± 9.9 | 47.0 ± 10.3 | <.001 |
| BMI (cm/kg2) | 26.2 ± 5.8 | 27.7 ± 6.7 | .007 |
| Marital Status (% married) | 63% | 68% | 0.19 |
| Children (% with children) | 72% | 71% | 0.76 |
| Employment (% employed) | 95% | 61% | <.001 |
| Race (% Caucasian) | 82% | 93% | <.001 |
| Education (% college graduate) | 81% | 58% | <.001 |
| Income (% over $40,000) | 81% | 68% | 0.37 |
| MS Type (% RRMS) | - | 90% | - |
| Disease duration (% less than 10 years) | - | 61% | - |
| PDDS score | |||
| - No Ambulatory Impairment (% PDDS 0-2) | 47% | ||
| - Mild Ambulatory Impairment (% PDDS 3-4) | 26% | ||
| - Moderate-Severe Ambulatory Impairment (% PDDS ≥5) | 10% | ||
| Time sitting (minutes) | 449.6 ± 169.6 | 450.9 ± 220.6 | 0.95 |
| GLTEQ | 41.6 ± 26.7 | 23.9 ± 23.1 | <0.001 |
ST and physical activity in MS versus controls
The ST for persons with MS versus controls are presented in Figure 1. The ANOVA did not identify a significant difference in minutes of ST between MS and controls (F=0.01, p=0.95). The mean scores for ST (M±SD) were 449.6±169.6 and 450.9±220.6 minutes per day for control and MS participants, respectively. This was unchanged after controlling for sociodemographic variables that differed between groups in ANCOVA (F=0.49, p=0.49). The ANOVA identified significant differences between groups in physical activity based on GLTEQ scores (F=81.258, p<0.001). The mean scores were 23.9±23.1 and 41.6±26.7 for MS and controls, respectively. The difference of 17.7 was moderate in magnitude based on Cohen’s d of 0.7. ST was significantly and weakly associated with GLTEQ scores in those with MS (r=−0.21, p<0.001), but not in the control sample (r=0.01, p=0.90).
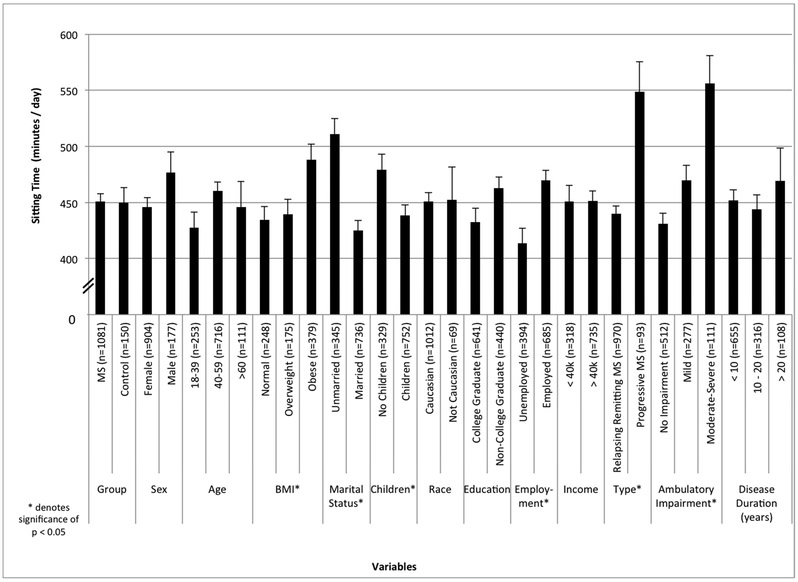
Figure 1.
Minutes of sitting time across sociodemographic and clinical characteristics among persons with MS. Note: MS: Multiple Sclerosis; PDDS: Patient Determined Disease Steps scale; BMI: Body Mass Index.
ST per sociodemographic characteristics with MS
Figure 1 displays ST per sociodemographic characteristics in persons with MS. The ANOVA identified statistically significant differences in ST when considering BMI (F=4.793, p=0.009), marital status (F=20.272, p<0.001), children (F=7.00, p=0.008), and employment status (F=6.99, p=0.01). When considering BMI, a Bonferroni follow up analysis indicated significant differences between normal and obese participants (p=0.011) and overweight and obese participants (p=0.039). Obese participants spent 49 and 53 more minutes per day sitting than normal (d=0.3) and overweight (d=0.2) participants, respectively. The analysis of marital status revealed a difference of 64.2 minutes of sitting per day (d=0.3) with unmarried persons sitting more than married persons with MS. When comparing children, persons without offspring sat for 38.5 more minutes of sitting per day (d=0.2) between those persons with children. The analysis of employment status indicated a difference of 36.8 minutes per week of sitting that was small in magnitude (d=0.2) between those who were employed, who sat more, versus unemployed. Sex, age, race, education, and income were not significantly associated with time sitting in the ANOVA (p>0.05). We further confirmed the lack of associations between ST and age (r=0.040, p=0.188), and income (r=−0.037, p=0.230) using bivariate correlations with those variables on non-categorical units (e.g. age in actual years rather than groups). The bivariate correlation revealed a weak, yet significant association between ST and education level in persons with MS (r=0.066, p=0.030).
ST per clinical characteristics in persons with MS
Figure 1 further includes ST per clinical characteristics in persons with MS. Within the MS sample, the ANOVA revealed statistically significant differences in ST when considering MS Type (F=21.089, p<0.001) and PDDS score (F=15.853, p<0.001). When considering MS Type, the average ST was 440.0±213.8 minutes per day and 548.7±257.7 minutes per day in RRMS and Progressive MS, respectively. The analysis of MS type revealed a difference of 108.6 minutes of sitting per day (d=0.5) with persons with Progressive MS sitting more than those with RRMS. This difference was moderate according to its Cohen’s d value. When considering ambulatory impairment, a Bonferroni follow up analysis indicated significant differences among all comparisons between the three groups (p<0.05). The average ST was 431.2±203.3 minutes per day for those without ambulatory impairment, 469.8±218.3 per day for those with mild ambulatory impairment and 556.2±260.4 minutes per day in those with moderate-severe ambulatory impairment. Persons with mild disability spent 38.6 more minutes sitting per day (d=0.2) than persons without ambulatory disability, but 86.4 minutes less sitting per day than persons with moderate-severe disability (d=0.4). These differences were considered small according to their Cohen’s d values. Persons with moderate-severe disability spent 125.0 more minutes sitting per day than persons without ambulatory impairment and this difference was considered moderate based on a Cohen’s d of 0.6. There was no difference by disease duration (F=2.064, p=0.127). We confirmed the lack of associations between ST with disease duration (r=0.045, p=0.140) using a bivariate correlation.
Stepwise multiple linear regression
The regression analysis with stepwise entry was conducted to determine the sociodemographic and clinical variable(s) that independently predicted ST in the MS sample. We only included variables that explained variation in sitting behavior from the previous analyses (i.e. BMI, marital status, children, employment, education, MS Type, PDDS, and GLTEQ). The results of the regression analysis are presented in Table 2. Marital status entered in Step 1 (R2=0.034, p<0.001), followed by GLTEQ score in Step 2 (ΔR2=0.033, p<0.001). Employment status entered the model in Step 3 (ΔR2=0.017, p<0.001), followed by PDDS in Step 4 (ΔR2=0.017, p<0.001). Education entered the model in Step 5 (ΔR2=0.006 for step 5, p=0.023). BMI, Children and MS Type did not enter into the regression model. The variables of GLTEQ, marital status, employment status, PDDS score, and education level explained 9% of variance (ΔR2=0.09) in sitting behavior.
Table 2.
Summary of hierarchical regression analysis for variables predicting sitting time in persons with MS. Note: MS: Multiple Sclerosis; B: unstandardized beta-coefficient; SE B: standard error of the unstandardized beta-coefficient; β: standardized beta-coefficient; GLTEQ: Godin Leisure-Time Exercise Questionnaire; PDDS: Patient Determined Disease Steps. Note: Marital status coding: 0=unmarried; 1=married; GLTEQ range: 0-198 units/day; Employment coding: 0=unemployed; 1=employed; PDDS coding: 0=no ambulatory impairment; 1=mild ambulatory impairment, 2=moderate ambulatory impairment; Education coding: 1=less than 7th grade, 2=9th grade, 3=partial high school, 4=high school graduate, 5=1-3 years of college, 6=college/university graduate, 7=master’s degree, 8=PhD or equivalent. R2 = 0.034 for step 1, p<0.001; ΔR2=0.033 for step 2, p<0.001; ΔR2=0.017 for step 3, p<0.001; ΔR2=0.017 for step 4, p<0.001; ΔR2=0.006 for step 5, p=.023.
| Step | Variable | B | SE B | β |
|---|---|---|---|---|
| Step 1 | Marital Status | −85.28 | 16.45 | −0.19 |
| Step 2 | Marital Status | −85.77 | 16.18 | −0.19 |
| GLTEQ | −1.81 | 0.35 | −0.18 | |
| Step 3 | Marital Status | −87.42 | 16.05 | −0.19 |
| GLTEQ | −1.92 | 0.35 | −0.19 | |
| Employment | 57.73 | 15.64 | 0.13 | |
| Step 4 | Marital Status | −85.16 | 15.92 | −0.19 |
| GLTEQ | −1.74 | 0.35 | −0.17 | |
| Employment | 78.71 | 16.46 | 0.18 | |
| PDDS | 47.41 | 12.53 | 0.14 | |
| Step 5 | Marital Status | −85.02 | 15.88 | −0.19 |
| GLTEQ | −1.79 | 0.35 | −0.18 | |
| Employment | 73.64 | 16.57 | 0.17 | |
| PDDS | 48.88 | 12.51 | 0.15 | |
| Education | −34.78 | 15.22 | −0.08 |
DISCUSSION
Sedentary behavior is prevalent and problematic in developed society1 and world-wide2. We know very little about sedentary behavior in persons with chronic, disabling diseases, particularly MS. To that end, the current study compared the self-reported ST of persons with MS versus healthy controls. The primary analysis did not indicate a significant difference in ST between persons with MS and controls, even after controlling for sociodemographic variables. Both groups spent approximately 450 minutes per day, or 7.5 hours per day, sitting. Our results for persons with MS and healthy persons are generally consistent with the estimates of 8 hours and 7.7 hours spent sitting per day in previous studies9,33. This indicates that persons with MS, overall, are as sedentary as persons without MS or any other chronic condition, and developing interventions for reducing the volume of sedentary behavior is equally important in MS as the general population.
Our findings are comparable with three studies of persons with mobility disability, including Parkinson’s disease34, Stroke35, and MS36, where similar amounts of sedentary time were reported between the disabled group and the control group. One notable difference between our study and previous research34-36 is that we did not examine the way activity was accumulated throughout the day and if this was different in those with disability compared with the control group. The use of the IPAQ limited our ability to examine such differences in the way sedentary behavior is accumulated in MS, but this is an important line of future research for designing behavioral interventions. For instance, persons with Parkinson’s accumulate sedentary time in longer bouts as compared to controls34. Persons with stroke have half as many daily sit to stand transitions compared with controls35, and those with MS have greater amounts of static activity than controls36. Research with healthy non-disabled populations supports the health benefits of breaking-up long bouts of sedentary time5,6,37,38(changing the pattern of activity). Accordingly, there is a strong rationale for examining the volume and pattern of sedentary behavior in persons with MS as this might inform the design of future behavioral interventions.
Interestingly, the present study indicated significant differences in physical activity levels between persons with MS and controls. The sample of persons with MS engaged in approximately 17.7 less units of physical activity than healthy controls, but physical activity was weakly associated with self-reported ST in those with MS. The difference in physical activity mirrors previous research in persons with MS39–42 and furthers the notion that persons with MS are inadequately participating in physical activity. Overall, our findings demonstrate that persons with MS are less physically active, but equally sedentary based on ST, compared with controls. Our results further support the notion that physical activity and ST are relatively independent constructs1 in MS, and sedentary behavior should not be considered the absence of moderate to vigorous physical activity43. Sitting behavior might be considered an important target of interventions in MS.
The secondary analysis of the present study identified clinical and sociodemographic variables that were associated with ST in the sample of persons with MS. The results indicated that BMI, marital status, children, employment status, GLTEQ score, MS Type, and PDDS score were statistically associated with ST, whereas sex, age, race, education, income, and disease duration were not associated with ST. Our results regarding subgroups of MS are statistically significant, but do not indicate clinically meaningful differences between subgroups; there is no established value for judging clinically meaningful differences based on IPAQ ST scores. The highest amounts of ST were evident in participants with progressive MS and moderate-severe ambulatory impairment who were obese, unmarried, without children, and employed. The stepwise linear regression analysis additionally indicated GLTEQ score, marital status, employment status, education level, and disability status via PDDS score individually explained variance in ST, although collectively only accounting for 9 percent of total variance. Those with severe disability, in particular, might have a greater burden of disease symptoms (e.g., walking dysfunction and fatigue) and this may result in a great amount of ST per day.
Of note, the ST for persons with moderate-severe ambulatory impairment (9.3 hours sitting per day), persons with mild ambulatory impairment (7.8 hours sitting per day) and persons without ambulatory impairments (7.2 hours sitting per day) was slightly lower, but of the same ratio documented in a previous study with a small sample, but with an objective outcome9. That study quantified sedentary time as the percentage of the day spent inactive using a Step Activity Monitor worn during the waking hours of the day in 21 persons with MS. The data indicated that, on average, 80 percent of the waking hours of the day were spent inactive, and, if we adopt a minimal wear-time of 10-hours per day, this translates into an estimated 8 hours of sedentary time per day in persons with MS. Moreover, there was a difference in sedentary time between disability levels such that those with mobility impairment spent 85% of waking hours inactivate compared with 76% of those without mobility disability. Importantly, there are notable limitations of this previous study including the small sample size, minimal comparison of sedentary time across clinical and demographic characteristics, and a non-MS control sample. Collectively, our secondary analysis identifies the subgroups of the MS population that might be important recipients for inclusion in behavioral interventions for decreasing ST42.
The strengths of the current study include its large sample size and inclusion of both sociodemographic and clinical variables; however, this study is not without limitations. Further, this study involved a secondary analysis of data that were collected for other primary purposes, making the study, but not data collection, retrospective in nature. This limits a focal examination of all possible determinants of ST, but does allow for a descriptive epidemiology. The current sample of persons with MS was primarily Caucasian (93%) and female (84%) with an income above $40,000 per year (68%). The disease characteristics of this sample included mostly RRMS (90%) and a relatively low disability status based on the PDDS mean of 2.0. The current results cannot necessarily be applied to other sub-populations of persons with MS. Considering the retrospective nature of this study, there were sociodemographic differences in sex, age, BMI, employment, race, and education between persons with MS and controls, making the comparison between the two groups not ideal. Future research should consider utilizing a matched sample. Further, the control data may vary in ways unforeseen that might have influenced ST. Finally, ST was measured with selfreport and not an objective assessment, yet our estimate of ST was similar to a previous study that utilized an accelerometer to measure sedentary behavior in a small sample of persons with MS9. Due to the self-reported nature of our data, aspects of sitting time, such as sedentary breaks, could not be identified. Future research should aim to examine the volume and structure of sedentary time using objective measures, like accelerometry.
We believe that future researchers should identify the consequences and determinants of sedentary behavior in MS. For example, outcomes such as cardiovascular health4,44, fatigue45, and health-related quality of life46, have been associated with sedentary behavior in healthy persons, but have yet to be examined as consequences in persons with MS. The combinatory effects of chronic disease, like MS, and sedentary behavior should be examined considering the potential for greater health problems than either alone. The identification of possible determinants of sedentary behavior will help identify modifiable targets for future interventions designed to reduce sedentary behavior. Outcomes from such studies could inform future public policy, health initiatives, and clinical guidelines47 in order to reduce ST in the MS population, thus decreasing this population’s risk of developing comorbidities2,48.
Overall, the current study indicates that self-reported ST does not differ between persons with MS and healthy controls. Nevertheless, the amount of ST in persons with MS is high (~7.5 hours/day) and ST has been associated with morbidity and mortality, independent of physical activity level, in healthy populations4. This suggests a future line of research directed toward decreasing ST among persons with MS through interventions and examining the secondary effects on morbidity and disease-specific manifestations2.
Acknowledgements:
We are grateful of the volunteers who participated in this research.
Source(s) of support: National Multiple Sclerosis Society and National Institutes of Health.
Footnotes
Ethics approval: The University of Illinois at Urbana-Champaign’s Institutional Review Board approved this study. All participants gave written informed consent before data collection.
Presentation: Accepted for a poster presentation at the 2014 Consortium of MS Centers ACTRIMS Annual Meeting.
Competing interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Sedentary Behaviour Research Network. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours.” Appl Physiol Nutr Metab. 2012;37(3):540–542. doi :10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 2.Bauman A, Ainsworth BE, Sallis JF, et al. The descriptive epidemiology of sitting: A 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011;41(2):228–235. doi:10.1016/j.amepre.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Healy GN, Matthews CE, Dunstan DW, Winkler EAH, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003–06. Eur Heart J. 2011;32(5):590–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel AV, Bernstein L, Deka A, et al. Leisure Time Spent Sitting in Relation to Total Mortality in a Prospective Cohort of US Adults. Am J Epidemiol. 2010;172(4):419–429. doi:10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duvivier BMFM Schaper NC, Bremers MA, et al. Minimal Intensity Physical Activity (Standing and Walking) of Longer Duration Improves Insulin Action and Plasma Lipids More than Shorter Periods of Moderate to Vigorous Exercise (Cycling) in Sedentary Subjects When Energy Expenditure Is Comparable. Plos One. 2013;8(2):1–8. doi:10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr. 2013;98(2):358–366. doi:10.3945/ajcn.112.051763. [DOI] [PubMed] [Google Scholar]
- 7.Manns PJ, Dunstan DW, Owen N, Healy GN. Addressing the nonexercise part of the activity continuum: A more realistic and achievable approach to activity programming for adults with mobility disability? Phys Ther. 2012;92(4):614–625. doi:10.2522/ptj.20110284. [DOI] [PubMed] [Google Scholar]
- 8.Maghzi A-H, Borazanci A, McGee J, Steven Alexander J, Gonzalez-Toledo E, Minagar A. 1 - Multiple Sclerosis: Pathophysiology, Clinical Features, Diagnosis, and Management In: Minagar A, ed. Neuroinflammation. London: Elsevier; 2011:1–23. http://www.sciencedirect.com/science/article/pii/B9780123849137000010. Accessed November 11, 2013. [Google Scholar]
- 9.Cavanaugh JT, Gappmaier VO, Dibble LE, Gappmaier E. Ambulatory activity in individuals with multiple sclerosis. J Neurol Phys Ther Jnpt. 2011;35(1):26–33. doi :10.1097/NPT.0b013e3182097190. [DOI] [PubMed] [Google Scholar]
- 10.Motl RW, Snook EM, McAuley E, Scott JA, Douglass ML. Correlates of Physical Activity Among Individuals With Multiple Sclerosis. Ann Behav Med. 2006;32(2): 154–161. doi:10.1207/s15324796abm3202_13. [DOI] [PubMed] [Google Scholar]
- 11.Suh Y, Weikert M, Dlugonski D, Sandroff B, Motl RW. Social cognitive correlates of physical activity: findings from a cross-sectional study of adults with relapsing-remitting multiple sclerosis. J Phys Act Health. 2011;8(5):626–635. [DOI] [PubMed] [Google Scholar]
- 12.Sandroff BM, Motl RW, Suh Y. Accelerometer output and its association with energy expenditure in persons with multiple sclerosis. J Rehabil Res Dev. 2012;49(3):467–475. [DOI] [PubMed] [Google Scholar]
- 13.Motl RW, Dlugonski D, Wojcicki TR, McAuley E, Mohr DC. Internet intervention for increasing physical activity in persons with multiple sclerosis. Mult Scler. 2011;17(1): 116–128. [DOI] [PubMed] [Google Scholar]
- 14.Dlugonski D, Motl RW, Mohr DC, Sandroff BM. Internet-delivered behavioral intervention to increase physical activity in persons with multiple sclerosis: Sustainability and secondary outcomes. Psychol Health Med. 2012;17(6):636–651. [DOI] [PubMed] [Google Scholar]
- 15.Pilutti L, Dlugonski D, Sandroff B, Klaren R, Motl R. Randomized controlled trial of a behavioral intervention targeting symptoms and physical activity in multiple sclerosis. Mult Scler J. 2013. [DOI] [PubMed] [Google Scholar]
- 16.Motl RW, Snook EM, Schapiro RT. Symptoms and physical activity behavior in individuals with multiple sclerosis. Res Nurs Health. 2008;31(5):466–475. [DOI] [PubMed] [Google Scholar]
- 17.Motl RW, McAuley E, Wynn D, Suh Y, Weikert M, Dlugonski D. Symptoms and physical activity among adults with relapsing-remitting multiple sclerosis. J Nerv Ment Dis. 2010;198(3):213. [DOI] [PubMed] [Google Scholar]
- 18.Sandroff BM, Motl RW, Kam JP, Pula JH. Accelerometer measured physical activity and the integrity of the anterior visual pathway in multiple sclerosis. Mult Scler Relat Disord. 2014;3(1). doi:10.1016/j.msard.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 19.Suh Y, Joshi I, Olsen C, Motl RW. Social Cognitive Predictors of Physical Activity in Relapsing-Remitting Multiple Sclerosis. Int J Behav Med.1–8. doi:10.1007/s12529-013-9382-2. [DOI] [PubMed] [Google Scholar]
- 20.Ranadive SM, Yan H, Weikert M, et al. Vascular dysfunction and physical activity in multiple sclerosis. Med Sci Sports Exerc. 2012;44(2):238–243. doi:10.1249/MSS.0b013e31822d7997. [DOI] [PubMed] [Google Scholar]
- 21.Sandroff BM, Motl RW. Fitness and cognitive processing speed in persons with multiple sclerosis: A cross-sectional investigation. J Clin Exp Neuropsychol. 2012;34(10): 1041–1052. doi:10.1080/13803395.2012.715144. [DOI] [PubMed] [Google Scholar]
- 22.Dlugonski D, Motl RW. Marital status and motherhood: implications for physical activity. Women Health. 2013;53(2):203–215. doi:10.1080/03630242.2013.767304. [DOI] [PubMed] [Google Scholar]
- 23.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;195(9131/03): 1381–1395. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg DE, Bull FC, Marshall AL, Sallis JF, Bauman AE. Assessment of sedentary behavior with the International Physical Activity Questionnaire. J Phys Act Health. 2008;5:S30–S44. [DOI] [PubMed] [Google Scholar]
- 25.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Appl Sport Sci. 1985; 10(3): 141–146. [PubMed] [Google Scholar]
- 26.Gosney JL, Scott JA, Snook EM, Motl RW. Physical activity and multiple sclerosis - Validity of self-report and objective measures. Fam Community Health. 2007;30(2). [DOI] [PubMed] [Google Scholar]
- 27.Rizzo MA, Hadjimichael OC, Preiningerova J, Vollmer TL. Prevalence and treatment of spasticity reported by multiple sclerosis patients. Mult Scler. 2004;10(5):589–595. doi:10.1191/1352458504ms1085oa. [DOI] [PubMed] [Google Scholar]
- 28.Learmonth YC, Motl RW, Sandroff BM, Pula JH, Cadavid D. Validation of Patient Determined Disease Steps (PDDS) Scale Scores in Persons with Multiple Sclerosis. Bmc Neurol. 2013. doi:10.1186/1471-2377-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marrie RA, Horwitz R, Cutter G, Tyry T, Campagnolo D, Vollmer T. Comorbidity delays diagnosis and increases disability at diagnosis in MS. Neurology. 2009;72(2): 117–124. doi:10.1212/01.wnl.0000333252.78173.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sosnoff JJ, Boes MK, Sandroff BM, Socie MJ, Pula JH, Motl RW. Walking and thinking in persons with multiple sclerosis who vary in disability. Arch Phys Med Rehabil. 2011;92(12):2028–2033. doi:10.1016/j.apmr.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46(4):907–911. doi:10.1212/WNL.46.4.907. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 33.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167(7):875–881. doi:10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chastin SFM, Baker K, Jones D, Burn D, Granat MH, Rochester L. The Pattern of Habitual Sedentary Behavior Is Different in Advanced Parkinson’s Disease. Mov Disord. 2010;25(13):2114–2120. doi:10.1002/mds.23146. [DOI] [PubMed] [Google Scholar]
- 35.Alzahrani MA, Ada L, Dean CM. Duration of physical activity is normal but frequency is reduced after stroke: an observational study. J Physiother. 2011;57(1):47–51. [DOI] [PubMed] [Google Scholar]
- 36.Rietberg MB, Van Wegen EEH, Kollen BJ, Kwakkel G. Do Patients With Multiple Sclerosis Show Different Daily Physical Activity Patterns From Healthy Individuals? Neurorehabil Neural Repair. 2014. doi:10.1177/1545968313520412. [DOI] [PubMed] [Google Scholar]
- 37.Dunstan DW, Kingwell BA, Larsen R, et al. Breaking Up Prolonged Sitting Reduces Postprandial Glucose and Insulin Responses. Diabetes Care. 2012;35(5):976–983. doi:10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Healy GN, Dunstan DW, Salmon J, et al. Breaks in Sedentary Time Beneficial associations with metabolic risk. Dia Care. 2008;31(4):661–666. doi:10.2337/dc07-2046. [DOI] [PubMed] [Google Scholar]
- 39.Motl RW. Physical activity and its measurement and determinants in multiple sclerosis. Minerva Med. 2008;99(2):157–165. [PubMed] [Google Scholar]
- 40.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11(4):459–463. [DOI] [PubMed] [Google Scholar]
- 41.Sandroff BM, Dlugonski D, Weikert M, Suh Y, Balantrapu S, Motl RW. Physical activity and multiple sclerosis: new insights regarding inactivity. Acta Neurol Scand. 2012;126(4):256–262. doi:10.im/j.1600-0404.20n.01634.x. [DOI] [PubMed] [Google Scholar]
- 42.Klaren RE, Motl RW, Dlugonski D, Sandroff BM, Pilutti LA. Objectively quantified physical activity in persons with multiple sclerosis. Arch Phys Med Rehabil. 2013;94(12):2342–2348. [DOI] [PubMed] [Google Scholar]
- 43.Rhodes RE, Mark RS, Temmel CP. Adult Sedentary Behavior: A Systematic Review. Am J Prev Med. 2012;42(3):e3–e28. doi:10.1016/j.amepre.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Chomistek AK, Manson JE, Stefanick ML, et al. Relationship of Sedentary Behavior and Physical Activity to Incident Cardiovascular Disease: Results From the Women’s Health Initiative. J Am Coll Cardiol Jacc. 2013;61(23):2346–2354. doi:10.1016/j.jacc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellingson LD, Kuffel AE, Vack NJ, Cook DB. Active and sedentary behaviors influence feelings of energy and fatigue in women. 2013. [DOI] [PubMed] [Google Scholar]
- 46.Balboa-Castillo T, León-Muñoz LM, Graciani A, Rodríguez-Artalejo F, Guallar-Castillón P. Longitudinal association of physical activity and sedentary behavior during leisure time with health-related quality of life in community-dwelling older adults. Health Qual Life Outcomes. 2011;9(1):47–56. doi:10.1186/1477-7525-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen N Sedentary behavior: Understanding and influencing adults’ prolonged sitting time. Prev Med. 2012;55(6):535–539. doi:10.1016/j.ypmed.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Chau JY, Grunseit AC, Chey T, et al. Daily Sitting Time and All-Cause Mortality: A Meta-Analysis. Plos One. 2013;8(11). doi:10.1371/journal.pone.0080000. [DOI] [PMC free article] [PubMed] [Google Scholar]