Abstract
Background and aims
Nephrolithiasis is known to be associated with several systemic diseases including chronic kidney disease and renal failure, which can also occur as a complication of chronic liver disease (CLD). This study aimed to assess the prevalence of nephrolithiasis in patients with CLD.
Methods
A short survey was completed by 198 patients with CLD and 322 controls matched by age, sex, and state of residence. A primary diagnosis of liver disease was confirmed with health record review.
Results
The median age of the liver disease group was 63 years and 128 (65%) were male; the median age of the control group was 63 and 199 (63%) were male. Body mass index was higher in the liver disease group (27.8 vs 26.7, P < .01). The most common liver disease diagnosis was hepatitis C (60 [30%]) followed by alcoholic cirrhosis (42 [21.2%]). The self-reported prevalence of nephrolithiasis in the liver disease group was 26%, compared to 14% in the control group (P < .01). This association remained significant after adjusting for age, sex, body mass index, and family history of kidney stones or liver disease.
Conclusions
In this case–control, survey-based study, the prevalence of nephrolithiasis was 2 times higher in patients with CLD.
Abbreviations: BMI, Body Mass Index; CLD, Chronic Liver Disease; NASH, Nonalcoholic Steatohepatitis; NL, Nephrolithiasis; OR, Odds Ratio
Keywords: urolithiasis, hepatic failure, steatohepatitis
Nephrolithiasis (NL) is a common and painful malady, occurring in 7–8% of adults in the United States.1 Authors of a recent review suggested that urinary stone disease encompasses more than just the stone event, as it is associated with significant long-term comorbidity, and that it deserves concerted preventive efforts.2 Likewise, Chronic Liver Disease (CLD) represents a growing national health concern that can lead to the development of cirrhosis, liver cancer, or other complications including acute or chronic renal insufficiency.3 Of note, the prevalence of NL in CLD may be higher than in the general population; however, the evidence supporting that notion is sparse. As such, providing any empirical evidence that indeed NL is more common among those with CLD would advance support for implementing awareness and prevention efforts among patients with CLD.
Motivated by this, we performed a cross sectional case control study to compare the prevalence of NL between patients with CLD and control individuals with no history of CLD. Moreover, we administered questionnaires and performed a medical chart review to obtain data on important covariates (i.e., risk factors for NL as well as liver disease diagnoses) to enhance our comparisons between the case and control groups.
Methods
Study Population
At our institution, we maintain as part of routine clinical practice a registry of CLD patients. As such, we leveraged this clinical resource to recruit adult patients with liver disease who were seen at Mayo Clinic Florida between January 1, 2013 and December 31, 2014, and to confirm primary and secondary liver disease diagnosis. We contacted 384 patients to solicit their participation and were able to cross-reference disease diagnosis with our database. For the cases, inclusion criterion centered on a diagnosis of liver disease. For a comparison group, we identified control patients without a history of liver disease from the Mayo Clinic Biobank,4 Jacksonville, Florida, supported by the Mayo Clinic Center for Individualized Medicine, and frequency matched them to the CLD cases on age, sex, and state of residence. All participants were adults who could complete a survey on their own and all provided written informed consent to be included in this IRB approved study.
All participants completed a short mail-based survey with questions related to personal or family history of kidney stones or liver disease. Our study coordinator collaborated with the physician staff to confirm a primary diagnosis of liver disease via chart review.
Statistical Analyses
We summarize continuous data using median and range while for our categorical data we display counts and percentages. To compare continuous variables between our cases and controls the statistical team employed Wilcoxon rank sum tests. In contrast, for comparisons between cases and controls for categorical variables our team used Fisher Exact test due to the small sample size. For our primary analysis evaluating the association between CLD and NL, we employed univariable and multivariable logistic regression models with the dependent variable as history of NL (yes/no). All statistical tests were two-sided, and the threshold of significance was set at P = .05. All analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, North Carolina). All authors had access to the study data and have reviewed and approved the final manuscript.
Results
In Table 1, we provide the characteristics of the 198 CLD patients (cases) and 322 matched controls who were recruited and completed a short survey. Median age in the liver disease group was 63 years, and 64.6% were male. By comparison, the control group had a median age of 63 years, with 62.6% being male. Body Mass Index (BMI) was slightly higher in the liver disease group (27.8 vs 26.7, P < .01). In Table 2 we display the primary liver disease diagnosis of the patients with CLD. The most common liver disease diagnosis was hepatitis C (30%) followed by alcoholic cirrhosis (21.2%) and Nonalcoholic Steatohepatitis (NASH: 14.1%).
Table 1.
Patient Characteristics.
| Characteristic | Cases (N = 198)a | Controls (N = 322)a | P value |
|---|---|---|---|
| Age | (n = 198) 62.5 (23.3–74.7) |
(n = 318) 63.0 (20.0–91.0) |
.22 |
| Sex | .71 | ||
| N | (n = 198) | (n = 318) | |
| Female | 70 (35.4) | 119 (37.4) | |
| Male | 128 (64.6) | 199 (62.6) | |
| Race | (n = 196) | (n = 316) | .97 |
| White | 185 (94.4) | 294 (93.0) | |
| Black/African American | 5 (2.6) | 9 (2.8) | |
| Asian | 2 (1.0) | 4 (1.3) | |
| Other | 4 (2.0) | 9 (2.8) | |
| BMI | 27.8 (18.0–48.4) (n = 198) |
26.7 (16.6–57.4) (n = 319) |
<.01 |
| BMI < 25 | 54 (27.3) | 112 (35.1) | |
| BMI 25–30 | 70 (35.4) | 126 (39.5) | |
| BMI > 30 | 74 (37.4) | 81 (25.4) |
Abbreviation: BMI, Body Mass Index.
Continuous variables are reported as median (range); categorical as No. (%).
Table 2.
Primary Liver Disease Diagnosis in 198 Cases.
| Primary diagnosis | Cases, No. (%) |
|---|---|
| Alcoholic cirrhosis | 42 (21.2) |
| Alpha-1 antitrypsin deficiency | 3 (1.5) |
| Autoimmune hepatitis | 8 (4.0) |
| Caroli disease | 2 (1.0) |
| Cholangiocarcinoma/HCC | 3 (1.5) |
| Chronic hepatitis B | 3 (1.5) |
| Chronic hepatitis C | 60 (30.3) |
| Cirrhosis: cryptogenic (idiopathic) | 15 (7.6) |
| Cirrhosis: fatty liver (NASH) | 28 (14.1) |
| Cryptogenic | 2 (1.0) |
| Graft failure | 5 (2.5) |
| Hemochromatosis | 1 (0.5) |
| Inborn metabolic disease | 3 (1.5) |
| PBC | 9 (4.5) |
| PSC | 13 (6.6) |
| Polycystic liver disease | 1 (0.5) |
Abbreviations: HCC, Hepatocellular Carcinoma; NASH, Nonalcoholic Steatohepatitis; PBC, Primary Biliary Cholangitis; PSC, Primary Sclerosing Cholangitis.
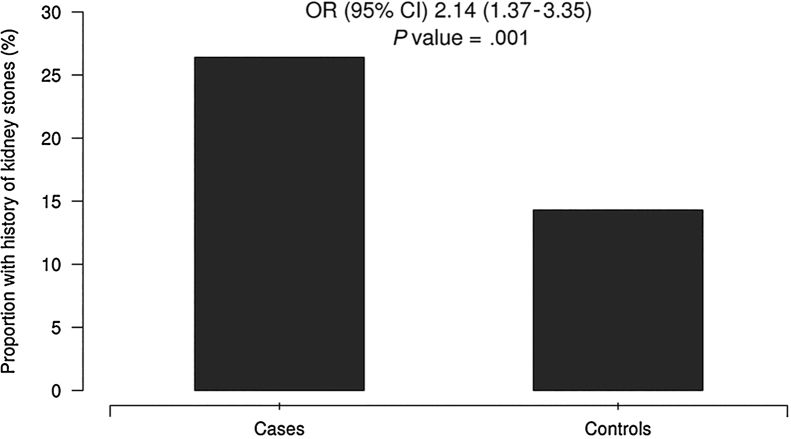
We provide a comparison between the cases and controls for the information obtained from the surveys in Table 3. Among the CLD cases, 52 (26.4%) had been told they had kidney stones, compared to only 46 (14.3%) in the control group (P < .01) (Figure 1). In Table 4, we report odds ratios from our logistic regression analysis estimating the magnitude of the association between CLD and history of NL. Moreover, we provide ORs after age adjustment and then again after adjustment for age, sex, BMI, and family history of kidney stones or liver disease. From our logistic regression analysis, patients with CLD are two times more likely to have a self-reported history of kidney stones compared to those without history of liver disease (OR = 2.14; 95% CI 1.37, 3.35; P < .01). Of keen interest, this association remains apparent after adjustment for age, sex, BMI, and family history of kidney stones or liver disease (OR = 2.14; 95% CI 1.32, 3.47; P < .01). Likewise this association remains regardless of whether there was just one or more than one kidney stone event (OR = 2.15; 95% CI 1.04, 4.43; P = .04 vs OR = 2.21, 95% CI 1.24, 3.96; P < .01). In addition, the association was not attenuated by removing those with NASH diagnosis from the analysis (OR = 2.04, 95% CI 1.28, 3.25; P < .01).
Table 3.
Kidney Stone and Liver Disease Survey.
| Survey question | Cases (N = 198)a | Controls (N = 322)a | P value |
|---|---|---|---|
| 1. Has a doctor ever told you that you have kidney stones? | <.01 | ||
| Missing | 1 | 1 | |
| Yes | 52 (26.4) | 46 (14.3) | |
| No | 145 (73.6) | 275 (85.7) | |
| 2a. If so, how many kidney stones have you had? | 2 (1–40) (n = 45) |
2 (1–75) (n = 39) |
.48 |
| Age when first kidney stone was diagnosed? | 45 (16–70) (n = 50) |
43 (16–72) (n = 44) |
.83 |
| Age when last kidney stone was passed? | 57 (16–73) (n = 45) |
53 (15–78) (n = 41) |
.48 |
| 4. Have any of your blood relatives had kidney stones? | .11 | ||
| Missing | 9 | 23 | |
| Yes | 56 (29.6) | 69 (23.1) | |
| No | 133 (70.4) | 230 (76.9) | |
| 5. Has a doctor ever told you that you have liver disease? | |||
| Missing | 1 | 3 | |
| Yes | 197 (100.0) | 0 (0.0) | |
| No | 0 (0.0) | 319 (100.0) | |
| 7. Have any of your blood relatives had liver disease? | <.01 | ||
| Missing | 5 | 12 | |
| Yes | 47 (24.4) | 43 (13.9) | |
| No | 146 (75.6) | 267 (86.1) |
Continuous variables are reported as median (range); categorical as No. (%).
Figure 1.
History of nephrolithiasis. Proportion of subjects with kidney stones, cases and controls.
Table 4.
History of Kidney Stones in Patients With CLD Vs Controls.
| Has a doctor ever told you that you have kidney stones? | OR (95% CI)a | P Value |
|---|---|---|
| Unadjusted | 2.14 (1.37–3.35) | <.01 |
| Adjusting for age, sex, BMI | 2.15 (1.37–3.39) | <.01 |
| Adjusting for age, sex, BMI, blood relatives had kidney stones | 2.14 (1.33–3.44) | <.01 |
| Adjusting for age, sex, BMI, blood relatives had kidney stones, blood relatives had liver disease | 2.14 (1.32–3.47) | <.01 |
Abbreviations: BMI, Body Mass Index; CLD, Chronic Liver Disease; OR, Odds Ratio.
Reported as median (range).
Discussion
In this case–control, survey-based study, we assessed history of NL in patients with CLD compared with controls. We observed that the prevalence of NL in the CLD group was nearly double that of the control group. Thus, our data suggest that there is an association between NL and CLD. If this is confirmed in future investigations with more robust study designs (i.e., prospective cohorts) it would support the notion of a link between NL and CLD and therefore a need for increased awareness of this association among practitioners caring for these patients.
NL is known to be associated with several systemic diseases including chronic kidney disease and renal failure,5 which can also occur as a complication of CLD. The linkage between CLD and kidney disease is well known. A plausible explanation for the present findings may be that NL and CLD share common pathogenesis (e.g., obesity)6, 7 or exacerbating factors (e.g., dehydration).8 Further elucidation of these factors was beyond the scope of this study and is the subject of further study. Regardless of the cause, a 2 times higher risk of NL in patients with CLD is important to report. The comorbidities associated with NL added to those of CLD could create potentially critical complications, and efforts to raise awareness and implement prevention strategies for this group are warranted.
Whether cause or consequence, associations between NL and a host of other diseases have been described including genetic, endocrine, inflammatory, and vascular diseases, as well as obesity.2, 7 Previous studies of liver disease and NL are sparse. Two imaging-based studies9, 10 found an increased frequency of NL in patients with NASH. Comparing patients with and without NASH based on computed tomography findings in those with NL, Nam9 described a 19% increased frequency of NL in patients with NASH. In a similar study utilizing ultrasound diagnosis of NASH and NL, Einollahi et al.10 reported that 17% of those with NASH had NL compared to 8% without liver disease. The differences in frequency in these studies may relate to the lower sensitivity and specificity of ultrasound to detect NL compared to computed tomography. In our CLD cohort, 14.1% had a primary diagnosis of NASH. Our findings of increased risk of NL in patients with CLD were not diminished by the removal of the NASH group from our analysis, thereby suggesting that the risk is not limited to just those with NASH. After adjusting for BMI, age, sex, and family history, we still found a 2 times higher risk of NL in our CLD cohort. Whether this risk is explained by other shared risk factors remains unclear.
There are notable strengths and potential limitations relating to this study. In an effort to reduce selection bias and enhance generalizability to patients with significant liver disease, the cases were recruited from a clinical database of consecutive patients with a primary diagnosis of CLD being evaluated at a single large hepatology practice. Liver disease diagnosis and other data such as BMI and demographics were confirmed by medical chart review. However, information relating to other potential risk factors was not collected for this study, and we cannot rule out on the basis of the current analysis the possibility that 1 or more risk factors may contribute to or explain this association. Further study will be required to elucidate such factors. We acknowledge the inherent limitations relating to use of survey data which include recall bias and tendencies toward socially acceptable responses.11 Related to the issue of recall bias, we would offer that it is more likely that the “exposure” of history of NL would be under reported (i.e., due to subclinical disease) than over reported. Moreover, we would also offer that this would occur equally in both cases (patients with liver disease) and controls (those without liver disease). Thus, the resulting effect of this non-differential misclassification would likely be to bias our reported odds ratio toward the null value of zero.
Conclusion
This case–control, survey-based study increases the understanding of the previously reported risk of NL in patients with CLD. Patients with CLD demonstrated a significantly higher prevalence of NL, with nearly double the risk. This association remained statistically significant after adjusting for age, sex, BMI, and family history of kidney stones or liver disease. Identification of other factors that may relate to this risk and investigation of responsible mechanisms requires further study. Key points of interest will be stratifying risks and individualization of directed therapies. Meanwhile, these findings underscore the need to increase awareness of the risk of NL in patients with CLD and to apply appropriate screening, management, and preventive strategies aimed at reducing stone formation and its attendant morbidity and complications.
Authors’ Contribution
IP, WP, AP, WH: study concept, drafting of manuscript, critical revision; DH, ND: statistical analysis.
Conflicts of Interest
The authors have none to declare.
References
- 1.Scales C.D., Jr., Smith A.C., Hanley J.M. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scales C.D., Jr., Tasian G.E., Schwaderer A.L. Urinary stone disease: advancing knowledge, patient care, and population health. Clin J Am Soc Nephrol. 2016;11:1305–1312. doi: 10.2215/CJN.13251215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . 2016. Chronic Liver Disease and Cirrhosis. [01.02.17]. Available from: https://www.cdc.gov/nchs/fastats/liver-disease.htm. [Google Scholar]
- 4.Olson J.E., Ryu E., Johnson K.J. The Mayo Clinic Biobank: a building block for individualized medicine. Mayo Clin Proc. 2013;88:952–962. doi: 10.1016/j.mayocp.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shoag J., Halpern J., Goldfarb D.S. Risk of chronic and end stage kidney disease in patients with nephrolithiasis. J Urol. 2014;192:1440–1445. doi: 10.1016/j.juro.2014.05.117. [DOI] [PubMed] [Google Scholar]
- 6.James O.F., Day C.P. Non-alcoholic steatohepatitis (NASH): a disease of emerging identity and importance. J Hepatol. 1998;29:495–501. doi: 10.1016/s0168-8278(98)80073-1. [DOI] [PubMed] [Google Scholar]
- 7.Taylor E.N., Stampfer M.J., Curhan G.C. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293:455–462. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 8.Cheungpasitporn W., Rossetti S., Friend K. Treatment effect, adherence, and safety of high fluid intake for the prevention of incident and recurrent kidney stones: a systematic review and meta-analysis. J Nephrol. 2016;29 doi: 10.1007/s40620-015-0210-4. 211-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nam I.C. Association of non-alcoholic fatty liver disease with renal stone disease detected on computed tomography. Eur J Radiol Open. 2016;3:195–199. doi: 10.1016/j.ejro.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einollahi B., Naghii M.R., Sepandi M. Association of nonalcoholic fatty liver disease (NAFLD) with urolithiasis. Endocr Regul. 2013;47:27–32. doi: 10.4149/endo_2013_01_27. [DOI] [PubMed] [Google Scholar]
- 11.Haley W.E., Gilbert O.N., Riley R.F. The association between Self-Reported Medication Adherence scores and systolic blood pressure control: a SPRINT baseline data study. J Am Soc Hypertens. 2016;10:857–864. doi: 10.1016/j.jash.2016.08.009. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]