Abstract
Hepatic Encephalopathy (HE) is a neuropsychiatric syndrome that occurs in up to 30% of patients with cirrhosis. HE may be a consequence of pure liver failure, as in patients with fulminant hepatitis, or of the combination of liver failure and portal-systemic shunting, as in patients with liver cirrhosis. Several clinical and pathophysiologic observations suggest the importance of portal-systemic shunts in the development of HE. Episodes of HE are usually related to precipitating events, such as infections or gastrointestinal bleeding; a minority of cirrhotic patients experienced a chronic HE, refractory to standard medical treatment. This latter type of HE should be related to spontaneous or radiological (such as Transjugular Intrahepatic Portosystemic Shunt (TIPS)) portal systemic shunts, that could be restricted or occluded in patients with chronic HE. Both TIPS reduction and shunt occlusion are radiological procedures, safe and effective to ameliorate neurological symptoms in patients with refractory HE.
Abbreviations: HE, Hepatic Encephalopalthy; TIPS, Transjugular Intrahepatic Porto-systemic Shunt; SPSSs, Spontaneous Portal-systemic Shunts
Keywords: transjugular intrahepatic porto-systemic shunt, hepatic encephalopathy, spontaneous portal-systemic shunts
Hepatic Encephalopathy (HE) is a major complication of cirrhosis and refers to potentially reversible neuropsychiatric abnormalities related to an accumulation of toxins due to hepatocellular dysfunction and portosystemic shunting.1, 2, 3 The prevalence of overt HE at the time of diagnosis of cirrhosis is 10–14% in general, 16–21% in those with decompensated cirrhosis and 10–50% in patients with Transjugular Intrahepatic Portosystemic Shunt (TIPS).4 According to the recent guidelines,4 HE could be subdivided into: episodic HE, fully reversible; recurrent HE characterized by bouts of HE that occur with a time interval of 6 months or less and persistent HE that denotes a pattern of behavioral alterations that are always present and interspersed with relapses of overt HE. While in some patients HE is related to a precipitating event as infection or gastrointestinal bleeding, other patients have chronic HE, refractory to the conventional medical therapy with lactulose, nonabsorbable antibiotics and an appropriate protein-restricted diet characterized by persistent alterations in the mental status, often without evident precipitating events.4 Paradoxically, some of these patients may present clinically with a relatively mild hepatocellular disease, without ascites or esophageal varices, and this contrasts with the severity of the neurological impairment. The treatment results are usually unsatisfactory, and these patients are hospitalized several times with consequent deterioration of their quality of life. In these patients the chronicity of HE may be sustained by presence of unrecognized large Spontaneous Portal-systemic Shunts (SPSSs).3, 4, 5 Another condition that often increased the risk of recurrent or persistent HE is the placement of TIPS, a radiological procedure widely used in the management of complications of portal hypertension.6, 7
Portosystemic Shunts in Cirrhosis—Classification, Types, Clinical Implications, Natural History
SPSSs are, as the name implies, potential communications between the portal venous system and the systemic venous circulation than can open and grow when portal pressure increases. These SPSS act as “release valves” to reduce the portal pressure, but also act as bypasses to normal liver flow. At one point, the shunt becomes large enough that it starts contributing to the progression of the liver disease.8 The main types of SPSSs are: paraumbilical vein, splenorenal, splenoiliac with internal hemorrhoids, esophageal varices and gastrocaval, indirect gastrocaval, gastrorenal associated with gastric varices.8 SPSSs can be classified into left-sided and right-sided (central) shunts; the most frequent right-side shunt is represented by recanalization of paraumbilical vein, while the most common left-sided shunts are gastrorenal shunt, observed in 85% of patients with gastric varices, gastrocaval shunt and splenorenal shunt. The clinical manifestations of these latter shunts comprise gastric variceal bleeding, HE and portal vein thrombosis if the shunt is very large. Moreover, SPSSs could be classified in large or small size according to its maximum diameter, with a cut-off of 8 mm; this value was chosen since it was the smallest size of a symptomatic shunt embolized reported in the literature.9, 10
SPSSs have been described in both patients with cirrhosis and in patients without significant alterations of liver function.11 The presence of the shunt alters hepatic hemodynamics and increases the bioavailability of intestinally derived agents, such as ammonium, increasing the risk of HE. Several clinical and pathophysiologic observations suggest the importance of portal-systemic shunts in the development of HE and previous reports suggested that 46–70% of cirrhotic patients with refractory HE show SPSSs upon radiological screening.3, 5, 6 Our group demonstrated the presence of SPSSs in 71% of cirrhotic patients with chronic HE, refractory to the standard medical treatment.3
A large shunt therefore may be the cause of HE even in the absence of significant liver damage; this type of encephalopathy is currently classified as type B (from the word “bypass”) HE.4 In patients with Non-cirrhotic Portal Hypertension (NCPH) with minimal alterations of liver function, the presence of large portal-systemic shunts may be associated with neurologic abnormalities, abnormal ammonia levels, and magnetic resonance spectroscopy pattern similar to that observed in cirrhotic patients with HE.12, 13, 14
The term “Portosystemic Shunt Syndrome” (PSS) includes the signs and symptoms observed in cirrhotic patients with SPSSs.15, 16 In the Saad classification, three stages were described. In the early stage (A), the patient is asymptomatic with well-preserved hepatic function and large SPS. In the late stage (B), the patient is symptomatic with recurrent/persistent HE and fairly-preserved hepatic function. In the terminal/end stage (C), HE related to both shunt and liver failure, thrombosis of the portal vein (due to a larger fraction of shunted blood), ascites and jaundice are present. Thrombocytopenia (seen in >90%) is also frequently observed in patients with PSS.
A recent multicenter retrospective study shown that, among cirrhotic patients, the prevalence of SPSS increases as liver function deteriorates (more frequent in cirrhotic patients with MELD score above 10), probably as a consequence of worsening portal hypertension, but without achieving an effective protection against its complications. Patients with good liver function and SPSSs develop more portal hypertension-related complications (GI bleeding and ascites) and have a lower transplant free survival. In patients with preserved liver function, the search for SPSSs allows to identify patients with a higher risk of worse outcomes, so these patients would probably benefit from a closer surveillance and more intensive therapy.10
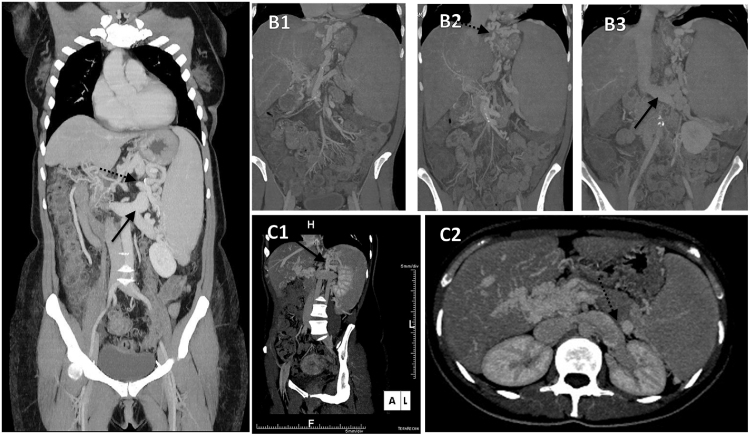
Some cirrhotic patients with HE who appear normal on the first view show extrapyramidal and cerebellar symptoms at precise neurological examination.17 Some develop a progressive hypokinetic-rigid syndrome which has lately been referred to as “cirrhosis-related Parkinsonism”, but features such as progressive ataxia, dystonia, choreoathetosis or spastic paraparesis may also be present and may be accompanied by progressive cognitive dysfunction. This chronic progressive form of HE is usually refractory to standard medical treatment and is frequently associated to the presence of SPSSs. Philips et al. demonstrated that in five of seven patients with hepatic Parkinsonism, the embolization of the shunt significantly improved neurological symptoms (Figure 1).18
Figure 1.
(A) Direct spleno-renal shunt: Maximum Intensity Projection (MPR) coronal reconstruction; the shunt consist in a tortuous varicosities between the splenic vein (dotted arrow) and its confluence within an ectatic left renal vein (full arrow) in the absence of varicosities along the gastric wall. (B1–B2–B3) Splenogastrorenal or indirect splenorenal shunt: consecutive MPR coronal reconstruction depicting a splenogastrorenal shunt. The anastomosis connects, toward a short gastric vein, the gastric vein wall (gastric and perigastric varices) (dotted arrow, panel B2), to the left renal vein (full arrow, panel B3) via the left inferior phrenic vein with connection with the splenic vein. (C1–C2) Gastrorenal shunt: MPR coronal oblique reconstruction depicting how gastric varices are connected to the left renal vein (full arrow, panel C1). Axial images at the splenic hilum clearly demonstrate a cleavage plane among the splenic vein (dotted arrow, panel C2) and the ectatic left renal vein.
Portosystemic Shunts Occlusion—Methods, Techniques, Indications, Contraindications, Outcomes, Adverse Effects, Literature Review of Large Significant Studies
The treatment of PSS has become important in daily clinical practice. Interventional radiology treatments using a catheter have become a leading treatment for portal hypertension because of the extensive range of indications it provides using many kinds of procedures.18 With Balloon-occluded Retrograde Transvenous Obliteration (BRTO), a balloon catheter is placed retrogradely in the gastrorenal shunt or in the inferior phrenic vein flowing into the inferior vena cava so that a sclerosant can be injected into the gastric varices for thrombus formation while the blood flow is cut off. The diameter of the gastrorenal shunt is measured at the base of the shunt at the communication point with the left renal vein, which where the occlusion balloon will be placed. The balloon diameter is chosen to match the diameter of the shunt. BRTO has been shown to be very effective in treating gastric varices and refractory HE associated with porto-systemic shunts with very low recurrence and rebleeding rates (0–9%).19, 20
The main indications for BRTO are: acute or previous bleeding from gastric varices sustained by a gastrorenal shunt; recurrent HE due to the presence of a SPSS or bleeding attributable to portal hypertension such as duodenal varices and mesenteric varices. Regarding contraindications, BRTO should be avoided when the contrast agent flows easily from the shunt into the portal vein under balloon-occluded retrograde venography, because there is a risk of portal thrombosis due to sclerosing agent migrated into the portal vein.18 Ethanolamine oleate has been used most commonly in Asia as a sclerosant.21 However, reported complications include renal dysfunction, pulmonary edema, cardiogenic edema, and anaphylaxis.20, 22 To avoid these complications, sclerosants such as Sodium Tetradecyl Sulfate (STS) have been utilized instead of ethanolamine.23 The most common shunt to be occluded during a BRTO procedure is a gastrorenal shunt, which provides venous outflow in 90% of gastric varices cases with the remaining 10% draining through a gastrocaval shunt.24
In some circumstances, sclerosant injected do not flow into the gastric varices but rather drains into the inferior vena cava or the azygos venous system through the collateral veins. Therefore, the pre-operative evaluation by CT scan and the occlusion of the collateral veins is necessary for the sclerosant to retain in the gastric varices.18
Recently, newly developed techniques such as Plug Assisted Retrograde Transvenous Obliteration (PARTO) or Coil Assisted Retrograde Transvenous Obliteration (CARTO) have been developed in order to shorten the procedure time of conventional BRTO. In PARTO, a vascular plug is used as a substitute for the balloon and this has been found to be technically and clinically equivalent. PARTO decreases procedure time and risk of potential complications as an indwelling balloon catheter is not required.25 However, there are some disadvantages, which lead to incomplete occlusion of the shunt with high recurrence rate or pulmonary embolization due to the embolus passing through the collateral vessels.
Gwon et al. conducted a prospective multicenter study to evaluate technical and clinical outcomes of PARTO for the treatment of gastric varices and HE in 73 patients and they demonstrated that PARTO can be rapidly performed with high technical success and durable clinical efficacy in patients with SPSSs. In CARTO, large-sized coils are used instead of the plug.26
The main complications, even if rare, associated to shunt's embolizations are: hematuria, pulmonary edema and shock due to the use of ethanolamine oleate, allergic reactions to the sclerosant, portal thrombosis caused by inflow of the sclerosant from the left gastric vein into the portal vein or from the splenorenal shunt into the splenic vein.27
Different studies demonstrated that shunt-related HE due to the increased shunt blood flow can be dramatically improved by closing the shunts.18, 28, 29, 30 In many cases, however, many other shunts exist, and mild HE may remain when only the gastrorenal shunt is closed. In these cases, it is necessary to consider closing the other shunts.
The largest European multicenter study28 showed that embolization of SPSSs in 37 patients with chronic HE significantly improved neurological symptoms and reduced the incidence of HE after the procedure. Considering secondary parameters of success, defined as either improved autonomy, or decreased number of hospitalizations or severity of the worst HE episode after embolization, an improvement was observed in three-quarters of the patients. Laleman et al. also found that MELD > 11 was a significant risk factor for the development of recurrence of HE in the long-term.28 In a separate study, Mukund et al. evaluated 20 patients who underwent BRTO specifically for recurrent HE29; the clinical response was 80% at 24 months. Clinical success was defined as improvement in HE (preferably by objective psychometric/cognitive criteria), reduction in serum ammonia levels, and reduction of medications used to manage HE. Recently, an Indian group demonstrated in 21 cirrhotic patients that embolization of SPSSs significantly ameliorated HE symptoms, serum ammonia levels and Child–Pugh score.18 In this study they found that patients with Child–Pugh score > 11 had very high mortality. Furthermore, Patil et al.,30 performed a systematic review and meta-analysis of six studies involving occlusion/embolization of SPSS for medically refractory HE in cirrhotics with Child–Pugh A disease and MELD score < 15. Lienorenal shunts were predominant, and 90% of the procedures performed were technically successful and did not result in any procedure-related complications. Improvement in HE was seen in a pooled percentage of 76.2%. De novo variceal disease was seen in 6%, and new onset or worsening ascites was seen in 14%. The authors concluded that PSS occlusion or embolization was safe with minimal complications in patients with adequate functional liver reserve. The main studies reporting the effect of spontaneous shunt occlusion in patients with chronic/recurrent refractory HE are reported in Table 1.
Table 1.
Main Studies Reporting the Effect of Spontaneous Shunt Occlusion in Patients With Chronic/Recurrent Refractory HE.
| Studies | No. of pts. with refractory HE/treated with shunt occlusion | Procedure | Technical success rate (%) | HE improvement rate (%) | Adverse events after shunt occlusion | Causes of death |
|---|---|---|---|---|---|---|
| Sakurabayashi, 19979 | 7/7 | CARTO BRTO |
100 | 57 | Fever, transient pleural effusion, ascites, and mild esophageal varices | – |
| Chikamori, 200051 | 5/5 | BRTO | 100 | 100 | Fever 1 Bleeding 1 |
Progression of HCC 1 |
| Zidi, 200752 | 7/7 | CARTO BRTO |
100 | 15 | – | End stage liver disease 3 |
| Mukund, 201229 | 7/7 | BRTO | 86 | 86 | Abnormal liver function test results, acute kidney injury with leukocytosis 2 | – |
| Laleman, 201328 | 37/37 | CARTO PARTO |
100 | 78 | Varices 19 (de novo 2) Ascites 15 (de novo 6) Bleeding 1 |
No deaths |
| Naeshiro, 201453 | 14/14 | BRTO | 100 | 93 | Varices 4 | Other causes not related to liver failure 5 |
| An, 201440 | 17/17 | CARTO PARTO |
100 | 59 | Ascites 3 Varices 8 Bleeding 0 |
Progression of HCC 2 Sepsis 1 Hepatorenal Syndrome 3 |
| Gwon, 201519 | 16/73 | PARTO | 100 | 100 | Ascites 5 Varices 4 Bleeding 0 |
Deterioration of liver function 4 Progression of HCC 1 |
| Philips, 201718 | 21/21 | CARTO PARTO BRTO |
95 | 75 | Ascites 8 (de novo 3) Varices 15 (de novo 1) Bleeding 1 |
Hemoperitoneum and multiple organ failure 1 |
In conclusion, patients who seems to benefit from shunt's occlusion are patients with symptomatic SPSSs larger than 8 mm and with a relatively mild liver disease (Child Pugh score < 11; MELD score < 11); in the others shunt occlusion should be evaluate with caution and liver transplantation could be considered.
Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt (TIPS)—Risk Factors, Implications, Natural History of Portal Hypertension After TIPS
TIPS has been used since more than 25 years to treat some of the complications of portal hypertension, especially variceal bleeding and ascites refractory to conventional therapy.7 TIPS establishes a communication between the portal and hepatic veins, inducing the blood to shift from the splanchnic circulation into the systemic vascular bed with the aim of decompressing the portal venous system, and avoids the major complications of portal hypertension. To date, with the use of the new Polytetrafluoroethylene (PTFE)-covered stents that have reduced significantly the incidence of shunt insufficiency,31 the main problem in the long-term management of patients submitted to TIPS is the development of overt HE.32 Two main causes are involved in post-TIPS HE: portal blood diversion from the liver due to portal-systemic shunting, variable and dependent on the stent diameter and the portosystemic gradient,33 and the decrease in liver metabolic capacity which, however, may be further reduced by the procedure. The incidence and severity of HE are higher during the first month after a TIPS procedure and decrease progressively because the diameter of the shunt tends to decrease spontaneously.32 Different trials investigated the main risk factors of post-TIPS HE which are: history of previous HE, older age, high creatinine levels, low serum sodium and low albumin values,34 low Portacaval Pressure Gradient (PPG) and Child–Pugh score.35, 36 Two recent studies demonstrated that also minimal HE37 and sarcopenia pre-TIPS38 have a strong impact on overt HE development after TIPS placement.
The overall incidence of post-TIPS HE ranges between 10% and 50%4 and can be derived by several Randomized Controlled Trials (RCTs) in which TIPS was compared with standard non-derivative therapy for the prevention of variceal rebleeding or the treatment of refractory ascites.32
In the RCTs for the prevention of variceal rebleeding, the incidence of post-TIPS HE was significantly higher than that reported in patients submitted to non-derivative treatment. This observation was not confirmed in the trials comparing TIPS with large volume paracentesis for intractable ascites in which the rate of HE was similar in the 2 groups of patients, probably because of the very high incidence of HE, independent of TIPS, in patients with very advanced liver disease and refractory ascites. However, even in this kind of patients, chronic HE was more frequent in those submitted to TIPS than in those treated with repeated paracentesis.32
Techniques of TIPS Reduction—Clinical Implications and Literature Review
Chronic recurrent HE refractory to standard treatment is the most important problem faced when a patients has to be treated with TIPS that occurs in 5–10% of patients. In some cases, the occurrence of this complication may deeply reduce the patient's quality of life and can be treated by reducing the diameter or by occluding the stent.39 In fact, HE ameliorated in most patients submitted to stent revision, however, the procedure is not without dangers and may not solve the problem in all patients, and the complications of portal hypertension, such as varices or refractory ascites may recur. Therefore, the decision of revising the shunt should be taken with caution, on a strict definition of refractory HE and only in patients free of portal hypertension before the revision. Although the procedure adopted for the reduction of the diameter permits the portal pressure modulation, it is very difficult to establish which portosystemic pressure gradient values should be reached to avoid further episodes of HE as well as events eventually occurring after the recurrence of portal hypertension.40
In the treatment of patients with chronic HE, various percutaneous techniques have been described that alter the hemodynamics through the shunt by occluding it with coils or balloons or by reducing its diameter by inserting constrained stents or stent-grafts.41 These methods based on partial or complete occlusion of the shunt have been adopted to control the portosystemic shunt overflow that can occur after a TIPS placement.42 Although initial attempts at percutaneous shunt occlusion with coils or detachable balloons were successful,43 these techniques were associated with a high morbidity and mortality44 related to the recurrence of variceal bleeding or to balloon migration into the right side of the hearth or balloon rupture. Now a days the Amplatzer Vascular Plug (AVP; AGA Medical, Golden Valley, MN, USA) is favored in occluding large-diameter high-flow vessels because of the lack of migration and ease of placement.45 The complete stent's occlusion, however, is associated with a high risk of variceal rebleeding consequent to an irreversible increase in portal pressure.
From the data available, partial occlusion of a TIPS appears to be more attractive than complete occlusion because partial occlusion allows reversal of flow-related complications and control of portal hypertension. To overcome the problems associated with shunt occlusion, several techniques have been developed that diminish flow by creating turbulence within the shunt lumen. Initially bare metal stents were constrained to make an hourglass shape either with a silk suture or within a parallel balloon-expandable stent.46, 47 However, bare metal stents had poor accuracy in regulating flow across the shunt.
This ushered the development of constrained covered stents which provided a measurable, effective and immediate method of shunt reduction. Polytetrafluoroethylene (PTFE)-covered stents have been found to be superior in maintaining the correct reduced shunt diameter without early or late shunt occlusion.48, 49 Fanelli et al.,50 used in 12 patients with refractory HE a balloon-expandable stent with a suture in the middle and dynamic dilation of the shunt according to the needs of the patient. Technically successful shunt reduction with an immediate increase in portosystemic gradient was achieved in all patients. Symptoms of HE disappeared within 24 h after the procedure. During the follow-up, no recurrence of HE was found.
However, endovascular shunt reduction is not always successful in controlling HE32; in non-responding patients, probably the presence of large collaterals or the deterioration of the liver function is more important than the portal blood diversion and in these patients liver transplantation remains the ultimate treatment.
The main studies reporting the effect of shunt reduction in patients with chronic/recurrent refractory post-TIPS HE are reported in Table 2.
Table 2.
Main Studies Reporting the Effect of Shunt Reduction in Patients with Chronic/Recurrent Refractory Post-TIPS HE.
| Studies | No. with refractory HE/treated with TIPS | Child–Pugh class | No. of pts. improved | Adverse events after TIPS reduction | PPG pre (mmHg) | PPG post (mmHg) |
|---|---|---|---|---|---|---|
| Bureau, 201755 | 1/29 | C:1 | 1 | – | – | – |
| De Santis, 201656 | 2/38 | B:1 C:1 |
2 | Ascites 1 Bleeding 1 |
6.5 ± 2.6 | 12.7 ± 3.8 |
| Nardelli, 201637 | 3/82 | B:1 C:2 |
3 | – | 5.6 ± 3.2 | 12.1 ± 2.7 |
| Cookson, 201157 | 8/NR | B:3 C:5 |
5 | Bleeding 3 Deaths 2 |
4.9 ± 3.6 | 10.5 ± 3.9 |
| Fanelli, 200950 | 12/189 | A:1 B:5 C:6 |
12 | Ascites 1 OLT 1 Deaths 4 |
6.6 ± 2.69 |
15.1 ± 3.4 |
| Riggio, 200834 | 6/78 | 6 | Ascites 1 Death for bleeding 1 |
5.5 ± 2.1 |
14.7 ± 1.9 |
|
| Chung, 200840 | 4/113 | C:4 | 4 | – | – | |
| Maleux, 200748 | 16/266 | A:2 B:13 C:1 |
10 | Ascites 1 Bleeding 1 OLT 1 |
5.25 ± 2.4 |
11 ± 3.1 |
| Kochar, 200639 | 38/733 | 21 | Bleeding 3 Ascites 3 Deaths 3 |
– | – | |
| Kerlan, 199558 | 5/NR | 4 | Bleeding 1 | – | – |
Taking into consideration the risk of complications related to the recurrence of portal hypertension, TIPS reduction should be evaluated only in patients with at least 3 episodes of non-precipitant-induced severe encephalopathy requiring hospitalization in the last 3 months despite continuous treatment with non-absorbable disaccharides or when there was a persistent HE, defined as the presence of a continuously detectable altered mental state with further episodic deterioration despite protein restriction to 1 g/kg of body weight and treatment with non-absorbable disaccharides.32
Conclusion
Shunt-related HE is a difficult clinical problem that is usually managed conservatively. When HE is refractory to these conservative treatments, more invasive techniques are often required. In these kind of patients, endovascular management of HE by occluding the portosystemic shunt has been demonstrated largely safe with an objective improvement in neurological symptoms.
Conflicts of Interest
The authors have none to declare.
References
- 1.Cordoba J., Minguez B. Hepatic encephalopathy. Semin Liver Dis. 2008;28:70–80. doi: 10.1055/s-2008-1040322. [DOI] [PubMed] [Google Scholar]
- 2.Shawcross D.L., Olde Damink S.W., Butterworth R.F. Ammonia and hepatic encephalopathy: the more things change, the more they remain the same. Metab Brain Dis. 2005;20:169–179. doi: 10.1007/s11011-005-7205-0. [DOI] [PubMed] [Google Scholar]
- 3.Riggio O., Efrati C., Catalano C. High prevalence of spontaneous portal-systemic shunts in persistent hepatic encephalopathy: a case–control study. Hepatology. 2005;42(5):1158–1165. doi: 10.1002/hep.20905. [DOI] [PubMed] [Google Scholar]
- 4.Vilstrup H., Amodio P., Bajaj J. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 5.Ohnishi K., Sato S., Saito M. Clinical and hemodynamic features in cirrhotic patients having a large spontaneous splenorenal and/or gastrorenal shunt. Am J Gastroenterol. 1986;81:450–455. [PubMed] [Google Scholar]
- 6.Lam K.C., Juttner H.U., Reynold T.B. Spontaneous portosystemic shunt: relationship to spontaneous encephalopathy and gastrointestinal hemorrhage. Dig Dis Sci. 1981;26:346–352. doi: 10.1007/BF01308377. [DOI] [PubMed] [Google Scholar]
- 7.Rössle M. TIPS: 25 years later. J Hepatol. 2013;59(5):1081–1093. doi: 10.1016/j.jhep.2013.06.014. Review. [DOI] [PubMed] [Google Scholar]
- 8.Saad W.E. Vascular anatomy and the morphologic and hemodynamic classifications of gastric varices and spontaneous portosystemic shunts relevant to the BRTO procedure. Tech Vasc Interv Radiol. 2013;16(2):60–100. doi: 10.1053/j.tvir.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Sakurabayashi S., Sezai S., Yamamoto Y. Embolization of portal-systemic shunts in cirrhotic patients with chronic recurrent hepatic encephalopathy. Cardiovasc Intervent Radiol. 1997;20:120–124. doi: 10.1007/s002709900118. [DOI] [PubMed] [Google Scholar]
- 10.Simón-Talero M., Roccarina D., Martínez J. Association between portosystemic shunts and increased complications and mortality in patients with cirrhosis. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.01.028. . [DOI] [PubMed] [Google Scholar]
- 11.Park J.H., Cha S.H., Han J.K. Intrahepatic portosystemic venous shunt. Am J Roentgenol. 1990;155:527–528. doi: 10.2214/ajr.155.3.2117349. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe A. Portal-systemic encephalopathy in non-cirrhotic patients: classification of clinical types, diagnosis and treatment. J Gastroenterol Hepatol. 2000;15:969–979. doi: 10.1046/j.1440-1746.2000.02283.x. [DOI] [PubMed] [Google Scholar]
- 13.Minguez B., Garcia Pagan J.C., Bosch J. Noncirrhotic portal vein thrombosis exhibits neuropsychological and MR changes consistent with minimal hepatic encephalopathy. Hepatology. 2006;43:707–714. doi: 10.1002/hep.21126. [DOI] [PubMed] [Google Scholar]
- 14.Nicoletti V., Gioia S., Lucatelli P. Hepatic encephalopathy in patients with non-cirrhotic portal hypertension: Description, prevalence and risk factors. Dig Liver Dis. 2016;48(9):1072–1077. doi: 10.1016/j.dld.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Kumamoto M., Toyonaga A., Inoue H. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25(6):1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]
- 16.Saad W.E. Portosystemic shunt syndrome and endovascular management of hepatic encephalopathy. Semin Intervent Radiol. 2014;31(3):262–265. doi: 10.1055/s-0034-1382795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tryc A.B., Goldbecker A., Berding G. Cirrhosis-related Parkinsonism: prevalence, mechanisms and response to treatments. J Hepatol. 2013;58(4):698–705. doi: 10.1016/j.jhep.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 18.Philips C.A., Kumar L., Augustine P. Shunt occlusion for portosystemic shunt syndrome related refractory hepatic encephalopathy—A single-center experience in 21 patients from Kerala. Indian J Gastroenterol. 2017;36(5):411–419. doi: 10.1007/s12664-017-0787-8. [DOI] [PubMed] [Google Scholar]
- 19.Kanagawa H., Mima S., Kouyama H. Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol. 1996;11:51–58. doi: 10.1111/j.1440-1746.1996.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 20.Hirota S., Matsumoto S., Tomita M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999;211:349–356. doi: 10.1148/radiology.211.2.r99ma25349. [DOI] [PubMed] [Google Scholar]
- 21.Saad W.E., Darcy M.D. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011;28:339–349. doi: 10.1055/s-0031-1284461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chikamori F., Kuniyoshi N., Shibuya S. Eight years of experience with transjugular retrograde obliteration for gastric varices with gastrorenal shunts. Surgery. 2001;129:414–420. doi: 10.1067/msy.2001.112000. [DOI] [PubMed] [Google Scholar]
- 23.Sabri S.S., Swee W., Turba U.C. Bleeding gastric varices obliteration with balloon-occluded retrograde transvenous obliteration using sodium tetradecyl sulfate foam. J Vascular Inter Radiol. 2011;22:309–316. doi: 10.1016/j.jvir.2010.11.022. quiz 16. [DOI] [PubMed] [Google Scholar]
- 24.Al-Osaimi A.M., Sabri S.S., Caldwell S.H. Balloon-occluded retrograde transvenous obliteration (BRTO): preprocedural evaluation and imaging. Semin Intervent Radiol. 2011;28(3):288–295. doi: 10.1055/s-0031-1284455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gwon D., Kim Y., Ko G., Kim J., Ko H., Kim J. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol. 2015;26:1589–1595. doi: 10.1016/j.jvir.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Lee E.W., Saab S., Gomes A.S. Coil-assisted retrograde transvenous obliteration (CARTO) for the treatment of portal hypertensive variceal bleeding: preliminary results. Clin Transl Gastroenterol. 2014;5:e61. doi: 10.1038/ctg.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota S., Kobayashi K., Kako Y. Balloon-occluded retrograde transvenous obliteration of varices: focusing on the portalhemodynamics and the recent techniques. Hepatol Int. 2018;12(suppl 1):102–111. doi: 10.1007/s12072-017-9813-2. [DOI] [PubMed] [Google Scholar]
- 28.Laleman W., Simon-Talero M., Maleux G. EASL-CLIF-Consortium. Embolization of large spontaneous portosystemic shunts for refractory hepatic encephalopathy: a multicenter survey on safety and efficacy. Hepatology. 2013;57(6):2448–2457. doi: 10.1002/hep.26314. [DOI] [PubMed] [Google Scholar]
- 29.Mukund A., Rajesh S., Arora A. Efficacy of balloon-occluded retrograde transvenous obliteration of large spontaneous lienorenal shunt in patients with severe recurrent hepatic encephalopathy with foam sclerotherapy: initial experience. J Vasc Interv Radiol. 2012;23:1200–1206. doi: 10.1016/j.jvir.2012.05.046. [DOI] [PubMed] [Google Scholar]
- 30.Patil R., Rassameehiran S., Patel R. Embolization for closure of spontaneous porto-systemic shunts in patient with cirrhosis and refractory hepatic encephalopathy: a systematic review and meta-analysis. Gastroenterology. 2017;152:S1140. [Google Scholar]
- 31.Bureau C., Garcia-Pagan J.C., Otal P. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469–475. doi: 10.1053/j.gastro.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Riggio O., Nardelli S., Moscucci F. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16(1):133–146. doi: 10.1016/j.cld.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez W., Piera C., Bandi J.C. Quantification of the extent of portal systemic shunting before and after TIPS: relationship with portal systemic encephalopathy. Hepatology. 1995;22:296A. [Google Scholar]
- 34.Riggio O., Angeloni S., Salvatori F.M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738–2746. doi: 10.1111/j.1572-0241.2008.02102.x. [DOI] [PubMed] [Google Scholar]
- 35.Casado M., Bosch J., Garcia-Pagan J.C. Clinical events after TIPS: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296–1303. doi: 10.1016/s0016-5085(98)70436-6. [DOI] [PubMed] [Google Scholar]
- 36.Bai M., Qi X., Yang Z. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: a systematic review. J Gastroenterol. Hepatol. 2011;26:943–951. doi: 10.1111/j.1440-1746.2011.06663.x. [DOI] [PubMed] [Google Scholar]
- 37.Nardelli S., Gioia S., Pasquale C. Cognitive impairment predicts the occurrence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2016;111(4):523–528. doi: 10.1038/ajg.2016.29. [DOI] [PubMed] [Google Scholar]
- 38.Nardelli S., Lattanzi B., Torrisi S. Sarcopenia is risk factor for development of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt placement. Clin Gastroenterol Hepatol. 2017;15(6):934–936. doi: 10.1016/j.cgh.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 39.Kochar N., Tripathi D., Ireland H. Transjugular intrahepatic portosystemic stent shunt (TIPSS) modification in the management of post-TIPSS refractory hepatic encephalopathy. Gut. 2006;55:617–623. doi: 10.1136/gut.2005.089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chung H.H., Razavi M.K., Sze D.Y. Portosystemic pressure gradient during transjugular intrahepatic portosystemic shunt with Viatorr stent graft: what is the critical low threshold to avoid medically uncontrolled low pressure gradient related complications? J Gastroenterol Hepatol. 2008;23:95–101. doi: 10.1111/j.1440-1746.2006.04697.x. [DOI] [PubMed] [Google Scholar]
- 41.Madoff D.C., Wallace M.J., Ahrar K. TIPSrelated hepatic encephalopathy: management options with novel endovascular techniques. Radiographics. 2004;24(1):21–36. doi: 10.1148/rg.241035028. discussion 36–7. [DOI] [PubMed] [Google Scholar]
- 42.Pereira K., Carrion A.F., Martin P. Current diagnosis and management of post-transjugular intrahepatic portosystemic shunt refractory hepatic encephalopathy. Liver Int. 2015;35:2487–2494. doi: 10.1111/liv.12956. [DOI] [PubMed] [Google Scholar]
- 43.Haskal Z.J., Cope C., Soulen M.C. Intentional reversible thrombosis of transjugular intrahepatic portosystemic shunts. Radiology. 1995;195:485–488. doi: 10.1148/radiology.195.2.7724771. [DOI] [PubMed] [Google Scholar]
- 44.Paz-Fumagalli R., Crain M.R., Mewissen M.W., Varma R.R. Fatal hemodynamic consequences of therapeutic closure of a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1994;5:831–834. doi: 10.1016/s1051-0443(94)71616-x. [DOI] [PubMed] [Google Scholar]
- 45.Pattynama P.M.T., Wils A., van der Linden E., van Dijk L.C. Embolization with the amplatzer vascular plug in TIPS patients. Cardiovasc Intervent Radiol. 2007;30:1218–1221. doi: 10.1007/s00270-007-9089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haskal Z.J., Middlebrook M.R. Creation of a stenotic stent to reduce flow through a transjugular intrahepatic portosystemic shunt. J Vasc Interv Radiol. 1994;5:827–829. doi: 10.1016/s1051-0443(94)71614-6. discussion 9–30. [DOI] [PubMed] [Google Scholar]
- 47.Forauer A.R., McLean G.K. Transjugular intrahepatic portosystemic shunt constraining stent for the treatment of refractory postprocedural encephalopathy: a simple design utilizing a Palmaz stent and Wallstent. J Vasc Interv Radiol. 1998;9:443–446. doi: 10.1016/s1051-0443(98)70296-9. [DOI] [PubMed] [Google Scholar]
- 48.Maleux G., Verslype C., Heye S. Endovascular shunt reduction in the management of transjugular portosystemic shunt-induced hepatic encephalopathy: preliminary experience with reduction stents and stent-grafts. AJR Am J Roentgenol. 2007;188:659–664. doi: 10.2214/AJR.05.1250. [DOI] [PubMed] [Google Scholar]
- 49.Clarke G., Patel R., Tsao S. Treatment of refractory post-transjugular portosystemic stent-shunt encephalopathy: a novel case of stent luminal reduction. Eur J Gastro Hepatol. 2004;16:1387–1390. doi: 10.1097/00042737-200412000-00025. [DOI] [PubMed] [Google Scholar]
- 50.Fanelli F., Salvatori F.M., Rabuffi P. Management of refractory hepatic encephalopathy after insertion of TIPS: long-term results of shunt reduction with hourglassshaped balloon-expandable stent-graft. Am J Roentgenol. 2009;193:1696–1702. doi: 10.2214/AJR.09.2968. [DOI] [PubMed] [Google Scholar]
- 51.Chikamori F., Kuniyoshi N., Shibuya S., Takase Y. Transjugular retrograde obliteration for chronic portosystemic encephalopathy. Abdom Imaging. 2000;25:567–571. doi: 10.1007/s002610000046. [DOI] [PubMed] [Google Scholar]
- 52.Zidi S.H., Zanditenas D., Gelu-Simeon M. Treatment of chronic portosystemic encephalopathy in cirrhotic patients by embolization of portosystemic shunts. Liver Int. 2007;27:1389–1393. doi: 10.1111/j.1478-3231.2007.01602.x. [DOI] [PubMed] [Google Scholar]
- 53.Naeshiro N., Kakizawa H., Aikata H. Percutaneous transvenous embolization for portosystemic shunts associated with encephalopathy: long-term outcomes in 14 patients. Hepatol Res. 2014;44:740–749. doi: 10.1111/hepr.12181. [DOI] [PubMed] [Google Scholar]
- 55.Bureau C., Thabut D., Oberti F. Transjugular intrahepatic portosystemic shunts with covered stents increase transplant-free survival of patients with cirrhosis and recurrent ascites. Gastroenterology. 2017;152(1):157–163. doi: 10.1053/j.gastro.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 56.De Santis A., Nardelli S., Bassanelli C. The modification of splenic stiffness on acoustic radiation force impulse parallels the variation of portal pressure induced by transjugular intrahepatic portosystemic shunt. J Gastroenterol Hepatol. 2017 doi: 10.1111/jgh.13907. [DOI] [PubMed] [Google Scholar]
- 57.Cookson D.T., Zaman Z., Gordon-Smith J. Management of transjugular intrahepatic portosystemic shunt (TIPS)-associated refractory hepatic encephalopathy by shunt reduction using the parallel technique: outcomes of a retrospective case series. Cardiovasc Intervent Radiol. 2011;34:92–99. doi: 10.1007/s00270-010-0016-7. [DOI] [PubMed] [Google Scholar]
- 58.Kerlan R.K., Jr., LaBerge J.M., Baker E.L. Successful reversal of hepatic encephalopathy with intentional occlusion of transjugular intrahepatic portosystemic shunts. J Vasc Interv Radiol. 1995;6:917–921. doi: 10.1016/s1051-0443(95)71212-x. [DOI] [PubMed] [Google Scholar]