Abstract
ABSTRACT: We report the case of a 37-year old primigesta, primipara (IGIP) patient with a singleton, naturally obtained pregnancy, diagnosed with type I diabetes mellitus from the age of three, carrier of an insulin pump for 11 years. The patient was diagnosed in adolescence with with a tumor of the ischio-rectal fossa with multiple attempts of excision which failed due to the particular situation of the tumor. Ultrasound examination diagnosed in the first trimester of pregnancy a voluminous right ovarian cystic tumor. The patient presented pregnancy-induced hypertension starting with 28 gestational weeks. Maternal-fetal and obstetric management assumed sequential ultrasound examination, ovarian tumor and maternal blood pressure drug control, and also the surgical management of the ischio-rectal tumor. Cesarean section was performed at 38 gestational weeks, outcoming with a live fetus, normal weight, good neonatal progression and favorable postoperative progression of the mother. In this case report, we emphasize the fact that in pregestational diabetes mellitus and pregnancy-induced hypertension, constant glycemic control, performed by the insulin pump, prior and during gestation, and the maternal blood pressure control are essential for maternal-fetal outcome.
Keywords: glycemic control, insulin pump, blood pressure control, ischio-rectal tumor, surgery
Introduction
Due to the spectacular progress of maternal-fetal medicine at present, perinatal morbidity and mortality in pregnancy complicated with pregestational or gestational diabetes are comparable to those of healthy pregnant women [1].
It is also recognized the association between maternal diabetes and the increased incidence of fetal abnormalities, but, by the remarkable development of prenatal ultrasound diagnostics on one hand, and recent acquisitions of ultrasound equipment and obstetric ultrasound software on the other hand, congenital malformations or abnormal development of the fetus can be detected effectively from the first trimester of pregnancy.
Pregnancy associated with diabetes is associated with a higher rate of hypertensive complications than normal pregnancy and a slightly increased risk of preeclampsia (15-20% vs. 5-7%) [2, 3, 4, 5].
Pregestational diabetes mellitus refers to type I diabetes, which is valid for most cases, and the incidence of type I diabetes during pregnancy varies between 0.2 and 0.5% [4,6, 7, 8].
Also, most studies converge to the idea that in pregnant women with type I diabetes, pregnancy is associated with an increased risk of preeclampsia, intrauterine growth restriction (IUGR), neonatal morbidity and perinatal mortality [4,9, 10, 11, 12, 13,14].
Pregnancy complicated with type I diabetes mellitus presents the risk of significant deviation of fetal growth [1].
Fetal macrosomia is diagnosed in approximately 25% of newborns from mothers with diabetes, being directly correlated with maternal hyperglycemia that induces hyperglycemia and fetal hyperinsulinism [1,4,15, 16, 17].
On the other side, diabetes mellitus may associate IUGR with both vascular complications of the underlying disease and maternal metabolic disorders [1,4,18, 19, 20].
Case presentation
The paper presents the case of a 37-year old IGIP patient with a singleton, naturally obtained pregnancy (1).
Figure 1.
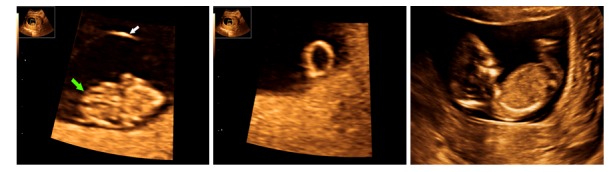
Transvaginal scan at 8 weeks (+2 days) demonstrating the embryo (green arrow), amniotic sac (white arrow) and the extraembryonic coelom. B. Transvaginal scan at 8 weeks (+2 days) demonstrating the Yolk sac. C. Transabdominal, sagittal scan at 12 weeks (+2 days) showing normal aspects
The study was performed according to the tenets of the Declaration of Helsinki and was approved by the University Ethics Committee. The patient was fully informed about the possible consequences of the present study, and she filled in an informed consent form to participate.
The patient was diagnosed with type I diabetes mellitus from the age of three and she is the carrier of an insulin pump for 11 years.
Also, the patient was diagnosed in adolescence with a tumor of the ischio-rectal fossa with multiple attempts of excision, both extra-abdominal route and two attempts to resolve through abdominal laparotomy, both of which failed due to the particular situation of the tumor, practically unapproachable to the resection.
Sequential ultrasound (US) examination diagnosed in the first trimester of pregnancy a voluminous right ovarian cystic tumor (9/13cm), drug controlled (Progesterone 200-400mg/day, Dydrogesterone 20-40mg/day) during gestation (2) (1).
Figure 2.
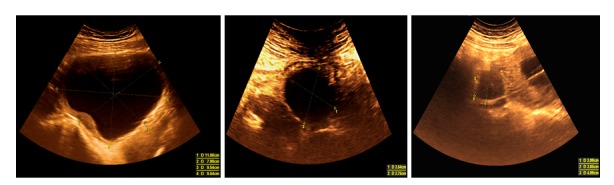
Systematic evaluation of the right ovarian cyst in the first and second trimester of pregnancy demonstrating its favorable evolution
Table 1.
Maternal - fetal and obstetric management of the case
Maternal-fetal management |
Obstetric ultrasound |
8gw |
- normal living embryo |
11-13 (+6)gw |
- First-trimester anomaly screening (+ maternal serum biochemistry) |
||
- NT - normal - NB - normal - DV - normal - TF - normal - First trimester cardiac scan - normal | |||
16-18gw |
- normal |
||
18-23gw |
Second-trimester anomaly scan - normal |
||
28-32gw |
- normal |
||
34gw |
- normal |
||
37gw |
- normal |
||
Glycemic control |
Insulin pump |
||
Ovarian cystic tumor control |
- Progesterone 200-400 mg/ day - Dydrogesterone 20-40 mg/ day |
||
Maternal blood pressure control |
- Metildopa 500 mg/ day |
||
Surgical management of the ischio-rectal tumor |
- Incision, evacuation and vaginal drainage |
||
Obstetric management and neonatal outcome |
- low-segment transverse cesarean section at 38gw - male live fetus, weighing 3270g - Apgar score - 9 |
||
gw-gestational weeks; NT-Nuchal translucency thickness; NB-Nasal bone; DV-Ductus venosus flow; TF-Tricuspid flow | |||
The US examination at 11-13 (+6 days) gestational weeks (gw), 18-23gw and other routine sonographic examinations revealed favorable fetal aspects to a good prognosis (3,4) (Table 1).
Figure 3.
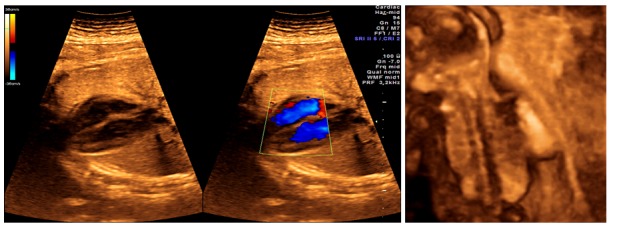
A. Second-trimester anomaly scan. Dual image (2D and color Doppler). The heart is seen in a lateral four-chamber view, demonstrating normal ventricular septum and normal cardiac anatomy. B. 3D rendering demonstrating normal fetal spine
Figure 4.
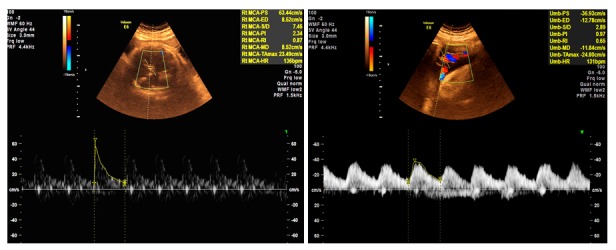
A. Middle cerebral artery (MCA) Doppler in the third trimester demonstrating normal flow. B. Umbilical artery (UA) Doppler in the third trimester interrogated close to the placental end of the cord, demonstrating normal flow
The patient presented pregnancy-induced hypertension (PIH) starting with 28gw. PIH control up to the time of obstetric intervention was obtained by administering Metildopa 500mg/day.
The recurrence of the ischio-rectal tumor at 34-35gw, due to its high volume (600ml), distension and compression (5), required incision, evacuation and vaginal drainage.
Figure 5.
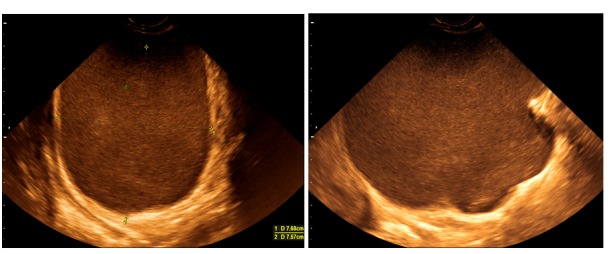
Transvaginal scan demonstrating the increasing in size bulky ischio-rectal tumor at 32 and 34gw
Obstetrical management assumed the low-segment transverse cesarean section at 38gw, extracting a male live fetus, weighing 3270g, Apgar score-9, which had a good neonatal progression. The postoperative progression of the patient was favorable (1).
The patient and the newborn have been discharged home, seven days after the obstetric intervention.
Discussions
Regarding to hypertensive disorders associated with pregestational diabetes mellitus (PIH and preeclampsia), in a meta-analysis of Bar et al., these range between 9-66% [4]. Most studies highlight the fact that preeclampsia rates increase proportional to the severity of diabetes, with particular reference to disease duration, pre-existing hypertension, microalbuminuria before pregnancy, glycemic control prior to 20 gestational weeks, nulliparity, proteinuria and nephropathy [4,6,21, 22, 23,24].
Regarding the presented case, constant glycemic control, performed by the insulin pump, at least in the last 11 years prior to gestation, is essential for maternal-fetal prognosis.
Macrosomia is defined by a birth weight of 4000g and above or a birth weight above the 90th centile fetal growth curve for the gestational age and sex [1].
Abnormal maternal glucose homeostasis and subsequent variations in fetal glucose values are the cause of fetal macrosomia in diabetic pregnancy [1,25].
Fetal macrosomia in these cases is estimated at 35-40% [1,26].
Not only maternal hyperglycemia is responsible for fetal macrosomia of diabetic pregnancy, but also other components of metabolic syndrome contribute to fetal over-growth, such as increased levels of lipids or amino acids [27].
Normal fetal weight, consistent with gestational age and fetal sex in the case presented, confirms the importance of maternal glycemic control and metabolic syndrome.
Diabetic ketoacidosis is a severe metabolic complication of diabetes, with a high mortality rate in the absence of a diagnosis. Its occurrence during pregnancy associates a high degree of maternal-fetal morbidity [28].
Diabetic ketoacidosis during pregnancy occurs more frequently in women with insulin-dependent diabetes than in women with type 2 diabetes or gestational diabetes [28].
Insulin therapy is the reference element within this entity, and in the presence of gestation, rapid correction of metabolic abnormalities and, consequently, hyperosmolarity by the administration of hypotonic fluids and insulin is to be avoided, in order to reduce the risk of brain edema precipitation [28,29].
Early and constant glycemic control is also essential in preventing this accident with remarkable maternal and fetal morbidity, which is also confirmed in the case presented in this paper.
Conclusion
Maternal diabetes, especially pregestational, may cause in addition to congenital malformations, metabolic and organic maternal imbalances with consequences difficult to evaluate. Maternal-fetal and obstetric management in this context involves completing the pregnancy by caesarean section after 37 gw. The favorable maternal-fetal outcome in this case was confirmed by the results of the prenatal screening and morphological examination on the one hand, and the maintenance of an effective and constant glycemic control throughout pregnancy, maternal blood pressure control, prevention and treatment of diabetic neuropathy, prevention of diabetic retinopathy and short-to-medium control in the recurrence of ischio-rectal tumor.
References
- 1.Ivaniševic M. Ultrasonic Surveillance of the Diabetic Fetus. In: Djelmiš J, Desoye G, Ivaniševic M, editors. Diabetology of Pregnancy. Frontiers in Diabetes. 17. Basel: Karger; 2005. pp. 230–253. [Google Scholar]
- 2.Pennison EH, Egerman RS. Perinatal outcomes in gestational diabetes: a comparison of criteria for diagnosis. Am J Obstet Gynecol. 2001;184(6):1118–1121. doi: 10.1067/mob.2001.114918. [DOI] [PubMed] [Google Scholar]
- 3.Sacks DA, Greenspoon JS, Abu-Fadil S, Henry HM, Wolde-Tsadik G, Yao JF. Toward universal criteria for gestational diabetes: the 75-gram glucose tolerance test in pregnancy. Am J Obstet Gynecol. 1995;172(2 Pt 1):607–614. doi: 10.1016/0002-9378(95)90580-4. [DOI] [PubMed] [Google Scholar]
- 4.Bar J, Hod M. Hypertensive disorders and diabetic pregnancy. In: Hod M, Jovanovic L, Di Renzo GC, De Leiva A, Langer O, editors. Textbook of Diabetes and Pregnancy. 2. London: Informa Healthcare; 2008. pp. 308–317. [Google Scholar]
- 5.Sermer M, Naylor CD, Gare DJ. Impact of increasing carbohydrate intolerance on maternal-fetal outcomes in 3637 women without gestational diabetes. The Toronto Tri-Hospital Gestational Diabetes Project. Am J Obstet Gynecol. 1995;173(1):146–156. doi: 10.1016/0002-9378(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 6.Hanson U, Persson B. Epidemiology of pregnancy-induced hypertension and preeclampsia in type 1(insulin-dependent) diabetic pregnancies in Sweden. Acta Obstet Gynecol Scand. 1998;77(6):620–624. doi: 10.1034/j.1600-0412.1998.770608.x. [DOI] [PubMed] [Google Scholar]
- 7.Berceanu C, Vladareanu S, Bratila E. Prenatal diagnosis and types of structural congenital malformations in diabetic pregnancy-a tertiary multicentric study. J Matern Fetal Neonatal Med. 2016;29(S1):1–313. [Google Scholar]
- 8.Goldenberg RL, McClure EM, Harrison MS, Miodovnik M. Diabetes during Pregnancy in Low-and Middle-Income Countries. Am J Perinatol. 2016;33(13):1227–1235. doi: 10.1055/s-0036-1584152. [DOI] [PubMed] [Google Scholar]
- 9.Lloreda-García JM, Sevilla-Denia S, Rodríguez-Sánchez A, Muñoz-Martínez P, Díaz-Ruiz M. Perinatal outcome of macrosomic infants born to diabetic versus non-diabetic mothers. Endocrinol Nutr. 2016;63(8):409–413. doi: 10.1016/j.endonu.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Castiglioni MT, Valsecchi L, Cavoretto P, Pirola S, Di Piazza L, Maggio L, Caretto A, Garito TS, Rosa S, Scavini M. The risk of preeclampsia beyond the first pregnancy among women with type 1 diabetes parity and preeclampsia in type 1 diabetes. Pregnancy Hypertens. 2014;4(1):34–40. doi: 10.1016/j.preghy.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Garabedian C, Vambergue A, Salleron J, Deruelle P. Prediction of macrosomia by serial sonographic measurements of fetal soft-tissues and the liver in women with pregestational diabetes. Diabetes Metab. 2013;39(6):511–518. doi: 10.1016/j.diabet.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Bratila E, Comandasu DE, Iacob G, Teodorescu C, Cirstoiu M, Bohiltea R, Berceanu C, Mehedintu C. Gestational diabetes and pregnancy morbidity and outcome. J Matern Fetal Neonatal Med. 2016;29(S1):1–317–1–317. [Google Scholar]
- 13.Correa A. Pregestational Diabetes Mellitus and Congenital Heart Defects. Circulation. 2016;133(23):2219–2221. doi: 10.1161/CIRCULATIONAHA.116.022960. [DOI] [PubMed] [Google Scholar]
- 14.Pridjian G. Pregestational diabetes. Obstet Gynecol Clin North Am. 2010;37(2):143–158. doi: 10.1016/j.ogc.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Cyganek K, Skupien J, Katra B, Hebda-Szydlo A, Janas I, Trznadel-Morawska I, Witek P, Kozek E, Malecki MT. Risk of macrosomia remains glucose-dependent in a cohort of women with pregestational type 1 diabetes and good glycemic control. Endocrine. 2017;55(2):447–455. doi: 10.1007/s12020-016-1134-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metzger BE. Biphasic effects of maternal metabolism on fetal growth. Quintessential expression of fuel-mediated teratogenesis. Diabetes. 1991;40(Suppl 2):99–105. doi: 10.2337/diab.40.2.s99. [DOI] [PubMed] [Google Scholar]
- 17.Binbir B, Yeniel AO, Ergenoglu AM, Kazandi M, Akercan F, Sagol S. The role of umbilical cord thickness and HbA1c levels for the prediction of fetal macrosomia in patients with gestational diabetes mellitus. Arch Gynecol Obstet. 2012;285(3):635–639. doi: 10.1007/s00404-011-1961-3. [DOI] [PubMed] [Google Scholar]
- 18.Kanda E, Matsuda Y, Makino Y, Matsui H. Risk factors associated with altered fetal growth in patients with pregestational diabetes mellitus. J Matern Fetal Neonatal Med. 2012;25(8):1390–1394. doi: 10.3109/14767058.2011.636096. [DOI] [PubMed] [Google Scholar]
- 19.Weintrob N, Karp M, Hod M. Short-and long-range complications in offspring of diabetic mothers. J Diabetes Complications; 1996;10(5):294–301. doi: 10.1016/1056-8727(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 20.Zawiejska A, Wender-Ozegowska E, Pietryga M, Brazert J. Maternal endothelial dysfunction and its association with abnormal fetal growth in diabetic pregnancy. Diabet Med. 2011;28(6):692–698. doi: 10.1111/j.1464-5491.2011.03249.x. [DOI] [PubMed] [Google Scholar]
- 21.Miodovnik M, Rosenn BM, Khoury JC, Grigsby JL, Siddiqi TA. Does pregnancy increase the risk for development and progression of diabetic nephropathy? . Am J Obstet Gynecol . 1996;174(4):1180–1191. doi: 10.1016/s0002-9378(96)70660-9. [DOI] [PubMed] [Google Scholar]
- 22.Sibai BM, Caritis S, Hauth J. Risks of preeclampsia and adverse neonatal outcomes among women with pregestational diabetes mellitus. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 2000;182(2):364–369. doi: 10.1016/s0002-9378(00)70225-0. [DOI] [PubMed] [Google Scholar]
- 23.Berger H, Gagnon R, Sermer M. Diabetes in Pregnancy. J Obstet Gynaecol Can. 2016;38(7):667–679. doi: 10.1016/j.jogc.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Park FJ, Leung CH, Poon LC, Williams PF, Rothwell SJ, Hyett JA. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. Aust N Z J Obstet Gynaecol. 2013;53(6):532–539. doi: 10.1111/ajo.12126. [DOI] [PubMed] [Google Scholar]
- 25.Gutaj P, Wender-Ozegowska E, Brazert J. Maternal lipids associated with large-for-gestational-age birth weight in women with type 1 diabetes: results from a prospective single-center study. Arch Med Sci. 2017;13(4):753–759. doi: 10.5114/aoms.2016.58619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Djelmis J, Bukovic D, Pfeifer D, Ivanisevic M. Ponderal index and disproportionate fetal growth in IDDM pregnancies. Coll Antropol. 1998;22(2):491–495. [PubMed] [Google Scholar]
- 27.Meizner I, Mashiach R. Sonography in diabetic pregnancies. In: Hod M, Jovanovic L, Di Renzo GC, De Leiva A, Langer O, editors. Textbook of Diabetes and Pregnancy. London: Informa Healthcare; 2008. pp. 253–258. [Google Scholar]
- 28.Yogev Y, Ben-Haroush A, Hod M. Diabetic ketoacidosis in pregnancy. In: Hod M, Jovanovic L, Di Renzo GC, De Leiva A, Langer O, editors. Textbook of Diabetes and Pregnancy. 2. London: Informa Healthcare; 2008. pp. 333–337. [Google Scholar]
- 29.Morrison FJR, Movassaghian M, Seely EW et al. Fetal Outcomes After Diabetic Ketoacidosis During Pregnancy. Diabetes Care. 2017;40(7):e77–e79. doi: 10.2337/dc17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]


