Abstract
ABSTRACT: Laryngeal cancer represents the malignant degeneration, of epithelial nature or from the connective tissue, which starts in the constitutive elements of the larynx. Cancer localization in the larynx represents between 1% and 3% of all malignant tumors and approximately 50% of ear, nose and throat (ENT) tumors. Squamous cell carcinoma is the most common form (around 95%) of laryngeal cancer, with high incidence in human males from southern and central Europe, Romania occupying one of the top places. Material and Methods. Our study consisted of 490 patients diagnosed with laryngeal cancer from 2010 to 2016. They have been clinically, histologically, immunohistochemically, genetically, therapeutically and prognostically analyzed. Suspended microlaringoscopy has been the standard, allowing tumor extension evaluation and biopsy. All specimens were microscopically analyzed in standard or special histology stainings. For unclear histology specimens, immunohistochemical stainings were performed. Results and discussions. Histological types have been represented as follows: 31 carcinomas in situ, 17 microinvasive carcinoma, 205 poorly differentiated carcinomas, 138 moderately differentiated carcinomas, 63 well differentiated carcinomas, 8 papillary carcinomas, 1 leiomyosarcoma, 1 chondrosarcoma, 6 basaloid squamous cell carcinomas, 4 verrucous carcinoma, 1 malignant melanoma. Conclusions. The study brings to light the importance of integrated clinical, morphological and genetic evaluation of laryngeal cancer, regarding tumoral invasion grading and establishing an adequate surgical and oncologic treatment. The importance of immunohistochemistry in laryngeal cancer concerns prognosis factors which correlate with the evolution and histopathological degree of the lesion. The analysis of tumor invasion can lead to the development of therapeutic conduct and the establishment of prognostic markers.
Keywords: laryngeal cancer, carcinoma, immunohistochemistry, histology
Introduction
Cancers of the oral cavity, pharynx and larynx, taken together, are the seventh most frequent type of cancer in the world, with around 600,000 new cases in 2012 (about 5% of incident cancer cases) [1]. Laryngeal cancer is the most common ENT cancer, with an incidence of 10/100,000 in Europe and a 4.3/100,000 mortality rate [2] in more recent years (2010-2011), second only to pulmonary cancer within the respiratory tract cancers. A decline in laryngeal cancer rates in Europe was observed between the 1980s and the 2000s. However, male laryngeal mortality rose up in the early 1990s in countries from central and Eastern Europe, such as Romania and leveled off thereafter [2,3].
Within our ENT Clinic, laryngeal cancer represents over 50% of all diagnosed and treated cancers, with a growing incidence. Central roles for laryngeal cancer development are attributed to smoking, alcohol consumption and certain viral infections (such as HPV) [4, 5, 6, 7]. We have to note that laryngeal cancer incidence is significantly lower in women, due to various reasons, including, but no limited to a lower alcohol intake and smoking, or biologically higher natural estrogen levels, which have been proposed as an explanation for lower risk of head and neck cancers compared to men [8].
Survival rates depend on tumor location, size, histopathological type, tumor grading, presence of metastasis but also on the accuracy of the diagnosis and treatment. Our study highlights the need and importance of a multidisciplinary approach of malignant laryngeal lesions, focusing on correlating clinical and morphologic information with histological and immunohistochemical analysis [6] of p53 tumor protein, PCNA and Ki-67 nuclear proliferation antigens, and epidermal growth factor receptor (EGFR) and CD44 antigen.
Material and Methods
Our study consisted of patients diagnosed with laryngeal cancer, between 2010 and 2016, shared in a retrospective group (2010-2014) and a prospective analysis group (2014-2016). The differences between the two groups are a longer follow up for the retrospective one, and that the prospective group specimens were also subjected to a thoroughly genetic analysis of blood samples (for DNA isolation and purification), tumor biopsies, oncological borderlines and matching healthy tissue. Since the genetic study is not the subject of our current report, did not influence inclusion, diagnostic workup or the applied treatment, we considered all patients as a single homogenous group.
The documentation, composition of the study lots was done by establishing an experimental model, by elaborating the working protocol and by creating an electronic database, criteria for inclusion and exclusion. The study was approved by the Ethics Committee of the University of Medicine and Pharmacy of Craiova. Written informed consent was obtained from the patients for study inclusion, sample collection, data publication and any accompanying images. For the retrospective analysis group the tumor tissues and other biological samples were retrieved from the department of anatomical pathology (bio-archive).
From a clinical point of view, the entire database includes personal data, the main signs and symptoms that led to a doctor presentation suggestive of laryngeal neoplasia (dysphonia, dysphagia, dyspnea, swelling cervical), their age (history of the disease and the presence of associated pathologies), the stage and extension of the primary tumor assessed by ENT clinical examination, fibroscopic examination and suspended micro-laryngoscopy.
The convexity of the vocal cords was appreciated more precisely by means of a stroboscope, and the limitation of the movement, a figurative immobility or a total immobility were noted as signs of a neoplastic process.
Micro-laryngoscopy allowed us a more accurate study of the surface and extension of a laryngeal-headed tumor through indirect palpation to assess the depth and eventual infiltration of neighboring tissues. For targeted and precise biopsies we used some filters in narrow band imaging (NBI) with magnifying endoscopy.
For tumoral extension evaluation all the patients were subjected to computer tomography which properly evaluates preepiglottic and paraglottic spaces, but can also detect distant metastases.
After complete clinical and paraclinical workup, various types of surgeries were performed: cordectomy, extended fronto-lateral cordectomy, partial supraglottic horizontal laringectomy, total laringectomy. The type of surgery has been elected in accordance to UICC (Union for International Cancer Control) recommendations. The surgical procedures were classified as curative, palliative or strictly diagnostic.
All resected specimens were subjected to microscopical study using classic histopathology techniques and immunohistochemical stainings. The biological material has been included in paraffin and then cut with microtome, obtaining sections with a 3 microns thickness. For the classic histopathology study was used: hematoxylin-eosin staining.
For the immunohistochemical study, out of the material included in paraffin histological serial sections were cut and collected onto slides coated with poly-L-lysine and then kept in a thermostat at 37°C for 24 hours to increase the adhesion of biological material to the slides Following dewaxing and hydration of histological sections, the biological material was incubated for 30 minutes in a 1% solution of hydrogen peroxide (hydrogen peroxide). Then the sections were washed in tap water before being microwaved in citrate pH 6 solution for 20 minutes for antigen retrieval. After boiling, the sections were cooled and then washed in phosphate buffer saline (PBS), followed by blocking of endogenous peroxidase phase in 2% skimmed milk for 30 minutes. Then, the sections were incubated with primary antibody overnight (16 hours) at 4°C, and the next day, the signal was amplified for 30 minutes using a secondary antibody with peroxidase on a polymeric backbone (EnVision, Dako). The signal was detected with 3,3'-diaminobenzidine (DAB) (Dako).
Immunohistochemical markers used in the present study were: p53, PCNA, EGFR, CD44, EMA, and Ki-67.
Table 1.
Immunohistochemical markers used in the study
Antigen> |
Reactivity |
Source |
Dilution |
EGFR |
cell membrane and vascular endothelium | Sygma |
1/1000 |
PCNA |
the cell nucleus | DAKO |
1/100 |
P53 |
the cell nucleus | P53 |
1/100 |
KI67 |
the cell nucleus | DAKO |
1/100 |
EMA |
the cell membrane | DAKO |
1/100 |
CD44 |
the cell membrane> | DAKO |
1/100 |
Expression variations of these cytokines were analyzed, taking into consideration the number of immuno-positive cells and their intensity-with subsequent statistical processing.
Results
Our study consisted of 490 patients diagnosed in our ENT Clinic with laryngeal cancer, between 2010 and 2016. The retrospective group analysis included 382 patients diagnosed with laryngeal cancer, between 2010 and 2014, their data being stored in an electronic database containing clinical data and investigations, operating and oncological protocols followed. Long term follow-up was available for most of the patients in this group. The biological samples and tumor fragments have already been processed histologically and immunohistochemically and the data have been retrieved from the anatomical pathology’s bioarchive. For those patients some data from the follow-up period were also available. It worth noted that 5-year survival rate was over 80% for patients who received radical surgery and respected the full adjuvant oncologic protocol.
The prospective group included other 108 patients diagnosed between 2015 and 2016. These patients have been thoroughly investigated-clinical examination, fibroscopy, imaging (CT/RMN, echography), and blood tests. Upon completion of these investigations, suspended micro-laryngoscopy has been performed, under general anesthesia, with tumor biopsy.
From an epidemiological point of view we encountered a greater percentage of patients coming from a rural areas (66.5%) compared to patients from an urban environment (33.5%), as well as an overwhelming percentage of male patients (roughly 97%), mostly aged between 51 to 70 years old (around 71% of patients in our study). We must underline here that most of the patients were diagnosed in advanced stages-stage IV (75%), stage III (23%). This implied lymph node metastasis as well, (correlated with the pattern of tumoral growth) but also infected tumor necrosis. For this reason most of the patients received radical or palliative surgery as first therapeutic step in the oncologic treatment protocol.
The hystopathological types of tumors are exposed in 2.
Table 2.
Histological types encountered in the study
Histopathological type |
Number of cases |
In situ carcinomas |
35 |
Microinvasive carcinomas |
19 |
Poorly differentiated carcinomas |
202 |
Moderately differentiated carcinomas |
144 |
Well differentiated carcinomas |
68 |
Verrucous carcinomas |
3 |
Basaloid carcinomas |
8 |
Papillary carcinomas |
8 |
Chondrosarcoma |
1 |
Leiomyosarcoma |
1 |
Malignant melanoma |
1 |
Total |
490 |
In situ carcinoma consists of neoplastic lesions that haven’t penetrated the basal membrane-without invasion. Architectural abnormalities concern the entire thickness of the epithelium with high cellular atypia (1).
Figure 1.
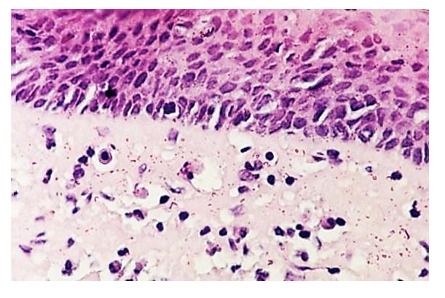
Carcinoma in situ, H&E stain, x200
Our study revealed 35 such cases. We’ve encountered 202 cases of poorly differentiated carcinoma-the most frequent in our study. Its appearance under the microscope consisted of large areas of epithelial tumor cells, of various dimensions sometimes with areas of necrosis and peritumoral inflammation (2).
Figure 2.
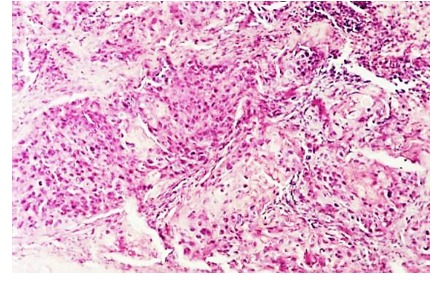
Poorly differentiated carcinoma, H&E stain, x100
Within our study we recorded 68 cases of well differentiated epidermoid carcinoma. It develops in the surface by invading and replacing the normal epithelium and invading deep tissues by breaking the basal membrane (3).
Figure 3.
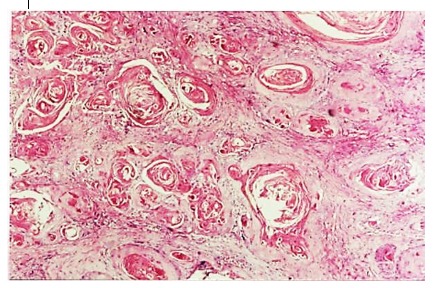
Well differentiated carcinoma with keratin pearls formation, H&E stain, x100
Our study has come across 144 cases of moderately differentiated carcinoma in which epithelial beads are rare, sometimes vaguely sketched, while atypical mitosis is more common and the stroma is poorer (4).
Figure 4.
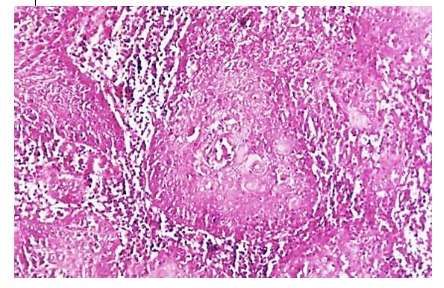
Moderately differentiated carcinoma, H&E stain, x100
Verrucous carcinoma (5) is an uncommon variant of squamous cell carcinoma. It appears as an exophytic polyp, strain, with filiform extensions in any of the three areas of the larynx, but especially in the glottis.
Figure 5.
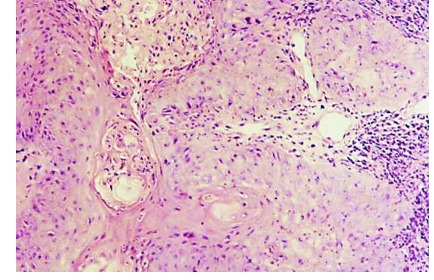
Verrucous carcinoma, H&E stain, x100
Basaloid carcinoma represents an aggressive type of squamous cell carcinoma, of which we have encountered 8 cases. It is a biphasic variant of squamous carcinoma, made up of a basalloid type and a squamous, conventional one (6).
Figure 6.
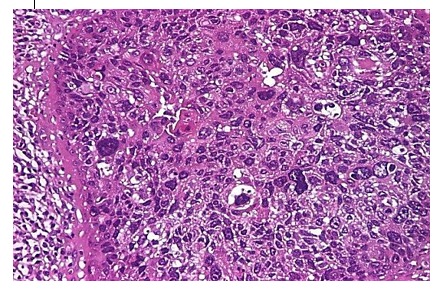
Basaloid carcinoma, H&E stain, x100
Macroscopically, it has the appearance of a tumour mass with central ulceration and extensive submucosal infiltration. We also report one hyoid bone chondrosarcoma, which it is a very rare malignant tumor (less than 25 published cases available in PubMed), that exhibits chondrocytes with irregular hyperchromic nuclei, reduced mitotic activity and bone metaplasia areas (7). Our study also revealed a patient with larygho-tracheo-bronchial metastasis of malignant melanoma (8).
Figure 7.
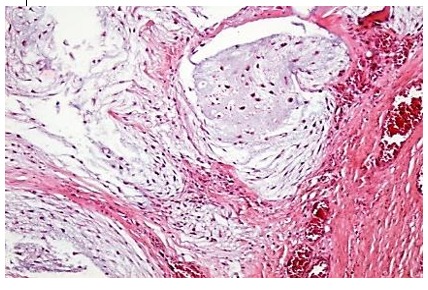
Chondrosarcoma, H&E stain, x 100
Figure 8.
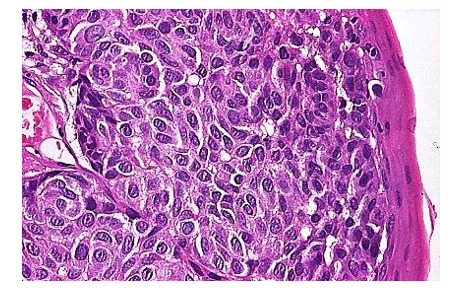
Malignant melanoma, H&E stain, x 200
Since the modern concept of oncogenesis is based on the interaction between factors that modulate growth and cellular differentiation, oncogenes and tumor suppressor genes we have studied a few of the most frequently used IHC markers. EGFR expression has been studied in 45 of laryngeal cancer cases from our research, without finding any correlation regarding tumor location, invasion, metastasis or patient age. Its presence has, however, been associated with the degree of differentiation, EGFR being expressed in well and moderately differentiated carcinoma (9,10).
Figure 9.
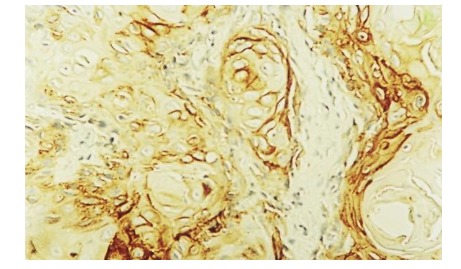
EGFR strong immunoexpression in epithelial cells-G1 carcinoma, IHC anti-EGFR antibody, x200
Figure 10.
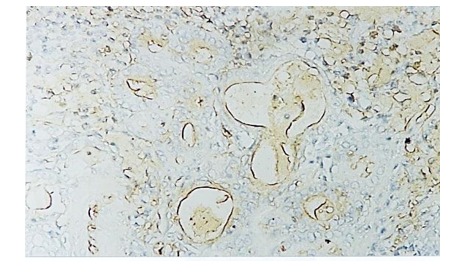
Moderately differentiated carcinoma-EGFR expression in epithelial cells and stroma, IHC anti-EGFR, x 200
High levels of EGFR were also encountered in patients with a bad prognosis or tumor relapse. In the case of patients explored for EGFR, PCNA (proliferating cell nuclear antigen) has also been studied. PCNA was found to be in direct relationship with tumor aggressiveness. We have noticed a consistency between the degree of tumor differentiation and PCNA, during the course of the study. Results showed a significantly higher PCNA index in cases which had an unfavorable evolution. Therefore, PCNA testing could be considered a marker for a tumors malignant predisposition or for a more likely development of neck metastases (11,12). During the course of our study, we found that the p53 protein (13) has been less commonly met than PCNA, even though there has been a positive correlation between the expressions of the two tumor markers-both p53 and PCNA have been expressed better in high malignancy tumors and in advanced stage patients. Within our study, CD 44 has been correlated with p53 expression, PCNA expression and laryngeal cancer malignancy degree. There has not been a significant difference for CD44 expression between in situ carcinomas and invasive carcinomas (14). Ki-67 and EMA have been used for cellular proliferation detection, with Ki-67 being of greater significance, since EMA has had more diffuse, unspecific reactions.
Figure 11.
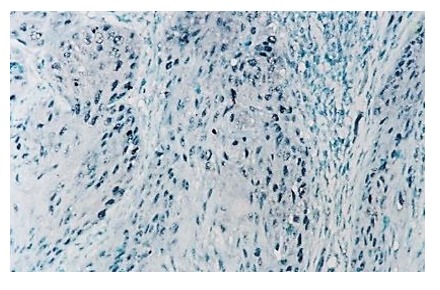
Laryngeal squamos carcinoma-PCNA is positive in 40% of tumor cells, IHC anti-PCNA, x100
Figure 12.
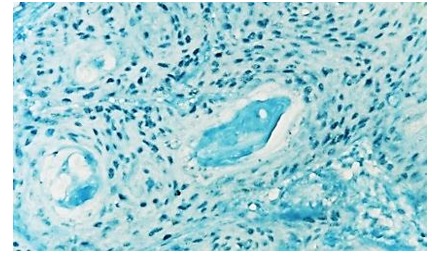
Laryngeal squamos carcinoma-PCNA is positive in 60% of tumor cells, IHC anti-PCNA, x100
Figure 13.
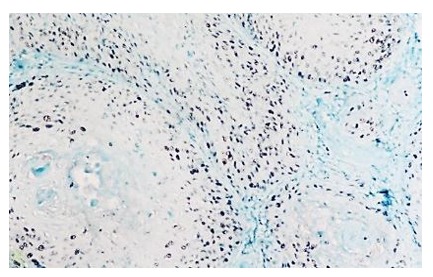
Laryngeal squamos carcinoma-p53 positive in 20% of tumor cells, IHC anti p53, x100
Figure 14.
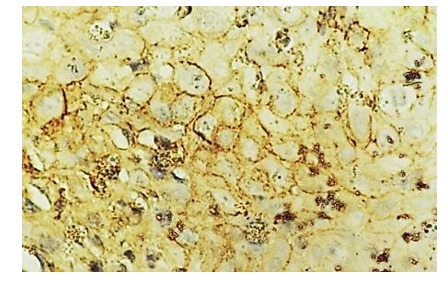
Laryngeal squamos carcinoma-CD44 positive in tumoral cell membrane, IHC anti-CD44, x200
Discussions
Squamous cell carcinoma of the larynx continues to be the most frequent head and neck cancer in many countries [9]. The illness has a significant higher incidence in males (97% in our study, most patients aged over 40 years) mostly due to tobacco and alcohol abuse habits, smoking and alcohol consumption being major risk factors for the larynx cancer [10]. We also remark a higher incidence in rural areas (66.5%) versus urban (33.5%). Head and neck cancer incidence rates seem to increase among females [11,12] somehow in direct relation with the use of tobacco and alcohol, also increasing in females. However, laryngeal cancer mortality in women remains substantially lower when compared to men [3].
The factors determining the therapeutic approach and majorly influencing the results are related to the tumor, patient and therapeutic resources/options (side effects, complications, partial/total surgery with reconstruction) [13,14]. The tumor-related factors are: tumor location, size, degree of differentiation, TNM stage, factors that sometimes are very difficult to evaluate preoperatively. Patient-related factors include age, gender, tobacco and alcohol addiction, social situation, occupation, toxic inhalation exposure, co-morbidities, education (psychological capacity for cooperation and willingness to rehabilitate). Factors related to cure are taken into account through the statistical outlook of healing rate, side effects, complications, and the possibility of partial, total or reconstructive surgery.
Since the results are highly-dependent on those factors an individualized treatment approach becomes mandatory. Currently, laryngeal cancer treatment protocols are complex, including surgery, radiotherapy, chemotherapy and immunotherapy. From this point of view, the best results for the patient can only be obtained through close collaboration between surgeon, radiologist, pathologist, genetic specialist, oncologist and radiotherapist, but not only.
The treatment should simultaneously address laryngeal tumor but also the tumoral extension in the cervical region. Current surgical options include laser endoscopic surgery, conservative surgery, partial laryngectomy and total laryngectomy. Surgical techniques depend on the location and extension of the primary tumor, as well as the presence of lymph nodes or distant metastases.
The oncologic results obtained with the therapeutic sequences used along our study period, justify us to conclude that the best therapeutic sequence (and most appropriate for our areal) is primary surgery followed by adjuvant radiotherapy.
More recently, there have been major changes in treatment paradigms for advanced laryngeal cancer with a major increase in the number of patients treated with radiotherapy and chemo-radiotherapy [9].
Tumor extension analysis, clinically evidenced by tumor size and the presence of adenopathies, was in large terms consistent with the type and degree of histological differentiation in our study. A histological classification and characterization of neoplasms is extremely important for a reliable prognosis to be established and it can provide the basis for clinical management of patients. Furthermore, immunohistochemical markers and genetic studies may lead to the development of adapted therapeutic behaviors and the establishment of new prognostic factors.
Our histopathological study of laryngeal cancer revealed an increased number of epidermoid carcinomas (over 95%) and a limited number of special histopathological types. Depending on the degree of differentiation, poorly differentiated G3 carcinoma was most frequently encountered.
The changes in the peritumoral stroma were evaluated in correlation with clinical evolution. We have found that a stroma rich in lymphoplasmocytes infiltration with the presence of giant Langerhans cells can be considered a favorable prognostic factor. In contrast, the presence of necrosis in both the tumor and the peritumoral areas was an unfavorable prognostic factor. The presence of metastases positively correlates with differentiation degree and necrosis, while inflammatory reactive lymph nodes were a favorable prognostic factor.
The histopathological examination of the primary tumor should include, besides the histopathological type, the degree of tumor differentiation, the degree of peritumoral inflammatory infiltrate, vascular invasion and perineural invasion [15].
The importance of immunohistochemistry in laryngeal cancer targets prognostic factors-PCNA, p53, Ki67 [16,17] that correlate with the histopathological degree of the lesion and evolution. CD44-the adhesion molecule that is present in both normal epithelium and dysplasia could play an important role in the prognosis of laryngeal cancer.
EGFR (epidermal growth factor receptor) was found to be significantly elevated in tumor cells compared to normal mucosa. We have found no correlation with patient age, tumor location, with invasion degree and metastasis presence. The presence of EGFR expression could be correlated with the degree of differentiation of the tumor. EGFR was well represented in the epithelium of the well differentiated carcinomas, moderate and weaker in the poorly differentiated. Overexpression of EGFR has been associated with an increased risk of death from disease [18]. High levels of EGFR were highlighted in patients with tumor recurrence and those with poor prognosis [19]. The distribution of EGFR was different in tumor stroma versus epithelial cells. Unlike well-differentiated carcinomas, where EGFR was more strongly positive in the epithelial cell membrane, the distribution of EGFR in the tumor stroma was richer in poorly differentiated carcinomas.
PCNA was found positively both in the thickness of the dysplastic epithelium and in the carcinoma islets. We have noticed a correlation between the degree of tumor differentiation and the PCNA index. PCNA testing may be considered a marker of malignancy potential in the choice of therapeutic conduct [18,20,1].
P53 is a phosphoprotein acting as a suppressive tumor factor that regulates growth and cell division. Mutations occurring at the p53 gene are the most common changes in human cancer [16,22]. P53 expression is very suggestive for the presence of a laryngeal carcinoma. High risk p53 mutations have been associated with poor disease specific survival [23]. In our study, expression of the p53 protein was less common than in the case of PCNA. However, there was a positive correlation between the expressions of the two tumor markers: both p53 and PCNA were better expressed in high malignancy of the larynx cancer [24].
CD44 is an ineffective membrane glycoprotein with various functions in cell-cell and cell-substrate interactions, being an adhesion molecule. It has been suggested that it could be a determinant of cancer metastasis and invasion capabilities. Its implication in the development and progression of laryngeal tumors makes it an important prognostic factor [25] and can help predict benign to precancerous tumor transformation.CD44 expression positively correlated with expression of p53 protein, PCNA expression, and aggressivity of laryngeal cancer.
The presence of immune compromised irregularity has been associated with poorly differentiated carcinoma pattern, increased mitotic index and high frequency of lymph node invasion and metastasis. In addition, irregular immunomodulation has been associated with a low survival tendency and an aggressive growth pattern.
EMA (epithelial membrane antigen) has little value in the diagnosis of laryngeal cancer due to the non-specific diffusion reaction. For proliferation detection, Ki-67 is a more appropriate marker [26]. High Ki-67 expression can be correlated with cervical lymph node metastasis, therefore making it a valuable predictor in laryngeal squamous carcinoma prognosis [27].
Conclusion
Therapy needs to address the laryngeal tumor and cervical lymph nodes, current treatment consisting of complex protocols: surgery, radiotherapy, chemotherapy and immunotherapy. Our histopathological study of laryngeal cancer identified a large number of epidermoid carcinoma compared to a reduced number of special hisptopathological types. Regarding the degree of differentiation, type G3 poorly differentiated carcinoma, was most common. All changes that have occurred at peritumoral stroma level have been evaluated and correlated with the clinical evolution. A favorable prognosis has been associated with a stroma that is rich in lymfoplasmocytary infiltrate, with the presence of giant Langerhans cells. Tumoral and peritumoral necrosis represents an unfavorable prognosis factor. Primary tumor histopathological examination will have to specify, apart from the histopathological type, all of the following: degree of tumoral differentiation, vascular invasion in the tumoral stroma and perineural invasion status.
The importance of immunohistochemistry in laryngeal cancer concerns prognosis factors which correlate to the evolution and histopathological degree of the lesion. The analysis can lead to the development and modulation of therapeutic conduct.
Author Contribution
M.S. Ciolofan and A.N. Vlăescu share first authorship.
Acknowledgments
This paper is supported by the Sectoral Operational Programme Human Resources Development (SOPHRD), ”Program of excellence in doctoral and postdoctoral research multidisciplinary chronic diseases” financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/133377.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in Globocan 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Chatenoud L, Garavello W, Pagan E, Bertuccio P, Gallus S, La Vecchia C, Negri E, Bosetti C. Laryngeal cancer mortality trends in European countries. Int. J. Cancer. 2006;138(4):833–842. doi: 10.1002/ijc.29833. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Garavello W, Levi F, Lucchini F, Negri E, La Vecchia C. Trends in laryngeal cancer mortality in Europe. Int. J. Cancer. 2006;119(3):673–681. doi: 10.1002/ijc.21855. [DOI] [PubMed] [Google Scholar]
- 4.Chen WC, Chuang HC, Lin YT, Huang CC, Chien CY. Clinical impact of human papillomavirus in laryngeal squamous cell carcinoma: a retrospective study. PeerJ. 2017;5:e3395–e3395. doi: 10.7717/peerj.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseini S, Makvandi M, Samarbafzade A. Frequency of Human Papillomavirus (HPV) 16 and 18 Detection in Paraffin-Embedded Laryngeal Carcinoma Tissue. Asian Pac J Cancer Prev. 2017;18(4):889–893. doi: 10.22034/APJCP.2017.18.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bossi P, Resteghini C, Paielli N, Licitra L, Pilotti S, Perrone F. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget. 2016;7(45):74362–74379. doi: 10.18632/oncotarget.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lampri ES, Chondrogiannis G, Ioachim E, Varouktsi A, Mitselou A, Galani A, Briassoulis E, Kanavaros P, Galani V. Biomarkers of head and neck cancer, tools or a gordian knot? Int J Clin Exp Med. 2015;8(7):10340–10357. [PMC free article] [PubMed] [Google Scholar]
- 8.Hashim D, Sartori S, Vecchia CL, Serraino D, Maso LD, Negri E, Smith E, Levi F, Boccia S, Cadoni G, Luu HN, Lee YA, Hashibe M, Boffetta P. Hormone factors play a favorable role in female head and neck cancer risk. Cancer Med. 2017;6(8):1998–2007. doi: 10.1002/cam4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patrick S. Management of Advanced Laryngeal Cancer. Rambam Maimonides Med J. 2014;5(2):e0015–e0015. doi: 10.5041/RMMJ.10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anantharaman D, Marron M, Lagiou P, Samoli E, Ahrens W, Pohlabeln H, Slamova A, Schejbalova M, Merletti F, Richiardi L, Kjaerheim K, Castellsague X, Agudo A, Talamini R, Barzan L, Macfarlane TV, Tickle M, Simonato L, Canova C, Conway DI, McKinney PA, Thomson P, Znaor A, Healy CM, McCartan BE, Hashibe M, Brennan P, Macfarlane GJ. Population attributable risk of tobacco and alcohol for upper aerodigestive tract cancer. Oral Oncol. 2011;47(8):725–731. doi: 10.1016/j.oraloncology.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Hashibe MP, Brennan P, Chuang SC, Boccia S, Castellsague X, Chen C, Curado MP, Dal Maso L, Daudt AW, Fabianova E, Fernandez L, Wünsch-Filho V, Franceschi S, Hayes RB, Herrero R, Kelsey K, Koifman S, La Vecchia C, Lazarus P, Levi F, Lence JJ, Mates D, Matos E, Menezes A, McClean MD, Muscat J, Eluf-Neto J, Olshan AF, Purdue M, Rudnai P, Schwartz SM, Smith E, Sturgis EM, Szeszenia-Dabrowska N, Talamini R, Wei Q, Winn DM, Shangina O, Pilarska A, Zhang ZF, Ferro G, Berthiller J, Boffetta P. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18(2):541–550. doi: 10.1158/1055-9965.EPI-08-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosetti CE, Negri E, Franceschi S, Conti E, Levi F, Tomei F, La Vecchia C. Risk factors for oral and pharyngeal cancer in women: a study from Italy and Switzerland. Br. J. Cancer. 2000;82(1):204–207. doi: 10.1054/bjoc.1999.0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motta G, Esposito E, Motta S, Tartaro G. Testa D. CO2 laser surgery in the treatment of glottic cancer. Head Neck. 2005;27(7):566–574. doi: 10.1002/hed.20135. [DOI] [PubMed] [Google Scholar]
- 14.Weiss BG, Bertlich M, Canis M, Ihler F. Transoral laser microsurgery or total laryngectomy for recurrent squamous cell carcinoma of the larynx: Retrospective analysis of 199 cases. Head Neck. 2017;39(6):1166–1176. doi: 10.1002/hed.24737. [DOI] [PubMed] [Google Scholar]
- 15.Varshney S, Singh J, Saxena RK, Kaushal A, Pathak VP. Verrucous carcinoma of larynx. Indian Journal of Otolaryngology andurgery. 2004;56(1):54–56. doi: 10.1007/BF02968777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarafoleanu D, Postelnicu V, Iosif C, Manea C, Sarafoleanu C. The role of p53, PCNA and Ki-67 as outcome predictors in the treatment of laryngeal cancer. Journal of Medicine and Life. 2009;2(2):219–226. [PMC free article] [PubMed] [Google Scholar]
- 17.Sapci T, Filizel F, Karavus A, Akbulut UG, Karavus M. The prognostic significance of proliferating cell nuclear antigen (pcna) in laryngeal cancer. Indian Journal of Otolaryngology and Head & Neck Surgery. 1998;50(4):354–361. doi: 10.1007/BF03000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradford CR, Kumar B, Bellile E, Lee J, Taylor J, D'Silva N, Cordell K, Kleer C, Kupfer R, Kumar P, Urba S, Worden F, Eisbruch A, Wolf GT, Teknos TN, Prince ME, Chepeha DB, Hogikyan ND, Moyer JS, Carey TE. Biomarkers in Advanced Larynx Cancer. The Laryngoscope. 2014;124(1):179–187. doi: 10.1002/lary.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurizi M, Almadori G, Ferrandina G, Distefano M, Romanini ME, Cadoni G, Benedetti-Panici P, Paludetti G, Scambia G, Mancuso S. Prognostic significance of epidermal growth factor receptor in laryngeal squamous cell carcinoma. Br J Cancer. 1996;74(8):1253–1257. doi: 10.1038/bjc.1996.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 21.Kupisz K, Stepulak A, Zdunek M, Klatka J. Preliminary results of prognostic significance of proliferating cell nuclear antigen expression in advanced primary larynx carcinomas and lymph node metastases. Archives of Medical Science. 2010;6(1):65–70. doi: 10.5114/aoms.2010.13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kropveld A, Slootweg P, Blankenstein M. Ki-67 and p53 in T2 laryngeal cancer. Laryngoscope. 1998;108(10):1548–1552. doi: 10.1097/00005537-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Scheel A, Bellile E, McHugh JB, Walline HM, Prince ME, Urba S, Wolf GT, Eisbruch A, Worden F, Carey TE, Bradford C. Classification of TP53 Mutations and HPV Predict Survival in Advanced Larynx Cancer. The Laryngoscope. 2016;126(9):E292–E299. doi: 10.1002/lary.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu X, You Y, Huang J, Wang X, Zhu H, Wang Z. LAMP3 and TP53 overexpression predicts poor outcome in laryngeal squamous cell carcinoma. International Journal of Clinical and Experimental Pathology. 2015;8(5):5519–5527. [PMC free article] [PubMed] [Google Scholar]
- 25.Güler G, Saraç S, Üner A, Karabulut E, Ayhan A, Hiroshi O. Prognostic Value of CD44 Variant 6 in Laryngeal Epidermoid Carcinomas. Arch Otolaryngol Head Neck Sur. 2002;128(4):393–397. doi: 10.1001/archotol.128.4.393. [DOI] [PubMed] [Google Scholar]
- 26.Debashri M, Kaushik S, Chhanda D, Uttara Ch, Arunabho S. Ki67, p27 and p53 expression in squamous epithelial lesions of larynx. Indian J Otolaryngol Head Neck Surg. 2013;65(2):126–133. doi: 10.1007/s12070-012-0590-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y, Shao Y, Li H, Xue W, Quan F, Wu S. Ki-67 is overexpressed in human laryngeal carcinoma and contributes to the proliferation of HEp2 cells. Oncology Letters. 2016;12(4):2641–2647. doi: 10.3892/ol.2016.4980. [DOI] [PMC free article] [PubMed] [Google Scholar]


