Abstract
Background
Secukinumab has demonstrated sustained improvement in the signs and symptoms of psoriatic arthritis (PsA) over 2 years in the FUTURE 2 study (NCT01752634). This post hoc analysis assessed the ability of secukinumab to achieve Psoriatic Arthritis Disease Activity Score (PASDAS)-based remission or low disease activity (LDA) through 2 years among patients with PsA in the FUTURE 2 study.
Methods
PASDAS (cut-off scores: remission ≤ 1.9; LDA > 1.9 and < 3.2; Moderate Disease Activity ≥ 3.2 and < 5.4; and high disease activity [HDA] ≥ 5.4) was assessed in the overall population (tumour necrosis factor inhibitor [TNFi]-naïve and TNFi-experienced), in patients stratified by prior TNFi use and by disease duration at weeks 16, 52 and 104. The impact of secukinumab on individual PASDAS core components and on the relationship between PASDAS states and patient-reported outcomes (PROs), including physical function, health-related quality of life (HRQoL) and work productivity, were also assessed. Data for the approved doses of secukinumab (300 and 150 mg) are reported. PASDAS scores and core components were reported as observed, and PROs were analysed using mixed models for repeated measures.
Results
In the overall population, PASDAS remission and LDA were achieved in 15.6% and 22.9%, respectively, of patients treated with secukinumab 300 mg and in 15.2% and 19.2%, respectively, in the secukinumab 150 mg group versus 2.3% and 13.8%, respectively, with placebo at week 16. In the TNFi-naïve group, a higher proportion of patients achieved remission + LDA at week 16 with secukinumab 300 and 150 mg (46.2% and 42.9%, respectively) versus placebo (17.5%), with corresponding responses in TNFi-experienced patients being 22.6% and 19.4% versus 13.3%. Remission/LDA responses with secukinumab were sustained through 2 years. Patients achieving remission/LDA reported greater improvements in PROs than patients in HDA through 2 years.
Conclusions
Secukinumab-treated patients achieved higher PASDAS-defined remissions or LDA compared with placebo at week 16, which were sustained through 2 years. Remission/LDA was achieved by both TNFi-naïve and TNFi-experienced patients treated with secukinumab, with higher rates in TNFi-naïve patients. Secukinumab-treated patients achieving remission/LDA reported significantly greater improvements in PROs, including physical function and different dimensions of health-related quality of life and work, than patients in HDA.
Trial registration
ClinicalTrials.gov, NCT01752634. Registered on December 19, 2012.
EUDRACT, 2012-004439-22. Registered on December 12, 2012.
Keywords: Psoriatic arthritis, Secukinumab, PASDAS, Remission, Interleukin-17A, FUTURE 2 study
Introduction
Psoriatic arthritis (PsA) is an inflammatory musculoskeletal disease comprising several domains, including peripheral arthritis, axial disease, enthesitis, dactylitis, and skin and nail disease [1, 2]. Different tools, such as Disease Activity Score (DAS) and American College of Rheumatology (ACR) response criteria, which primarily focus on peripheral joint manifestations and were validated in patients with rheumatoid arthritis, have been used for measuring joint responses in PsA. However, outcome measures borrowed from rheumatoid arthritis, such as ACR response criteria and DAS28-CRP, do not reflect the variety of disease manifestations [3, 4]. One of the overarching principles of the treat-to-target recommendations for patients with PsA by international task forces [5], the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) [6], and the European League Against Rheumatism (EULAR) [7] is to achieve the lowest possible level of disease activity across all domains of disease. Disease remission and low/minimal disease activity are defined by the minimal disease activity (MDA), which measures all domains of the disease, or Disease Activity Index for Psoriatic Arthritis (DAPSA), which measures peripheral arthritis and acute-phase reactants [4, 8, 9].
Another measure, the Psoriatic Arthritis Disease Activity Score (PASDAS), was developed by the GRAPPA Composite Exercise Project and provides different validated thresholds to identify remission, low disease activity (LDA), moderate disease activity (MoDA) and high disease activity (HDA) [10]. PASDAS assesses multiple manifestations of PsA, distinguishes treatment effects, performs better than traditional joint-only indices, and can be used as a treatment target in PsA randomized controlled trials (RCTs) [10–12].
Secukinumab, a fully human IgG1 monoclonal antibody that selectively neutralizes IL-17A, has substantial efficacy in the treatment of moderate to severe psoriasis, PsA and ankylosing spondylitis, demonstrating rapid onset of action and sustained responses with a favourable safety profile [13–19]. In the placebo-controlled, double-blind, phase III FUTURE 2 trial (NCT01752634), secukinumab treatment resulted in significant improvements in key clinical domains of PsA compared with placebo, and these improvements were sustained through week 104 [15, 16].
In the present study, we explored the ability of secukinumab-treated patients to achieve remission or LDA using PASDAS scores at weeks 16, 52 and 104 in the FUTURE 2 study. This post hoc analysis also determined the impact of secukinumab on individual components of PASDAS and the relationship of PASDAS states with patient-reported outcomes (PROs), including health related-quality of life (HRQoL), physical function, work productivity and activity impairments.
Methods
Study design and patients
FUTURE 2 is a phase III multicentre RCT designed to evaluate the efficacy and safety of subcutaneous secukinumab treatment in patients with active PsA. Details of the study design, inclusion and exclusion criteria, and 104-week efficacy and safety results have been reported previously [15, 16]. Briefly, patients were randomized (1:1:1:1) to receive subcutaneous (s.c.) secukinumab 300, 150 or 75 mg or placebo at baseline; weeks 1, 2, 3 and 4; and every 4 weeks thereafter. Placebo-treated patients were re-randomized to receive secukinumab 300 or 150 mg at either week 16 or week 24, based on clinical responses [15]. Data only with the approved doses of secukinumab (i.e., 300 and 150 mg) and placebo are reported herein.
The study was conducted in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonisation good clinical practice guidelines, and all applicable laws and regulations. All centres received approval from independent ethics committees or institutional review boards (IRBs). Patients provided written informed consent before starting the study-related procedures. The details of the study were mentioned in the earlier FUTURE 2 publications [15, 16].
Outcomes
PASDAS is a composite score that includes physician and patient global assessment of skin and joint disease by visual analogue scale (VAS), the physical component summary score of the Medical Outcomes Study Short Form Health Survey (SF-36 PCS), swollen joint count based on 66 joints (SJC66), tender joint count based on 68 joints (TJC68), Leeds Enthesitis Index enthesitis count, tender dactylitis count and CRP level [6, 11]. The continuous PASDAS score [11] was calculated for each patient with evaluable data at each point of interest. Disease activity states are defined as follows:
Remission: PASDAS score ≤ 1.9
LDA: 1.9 < PASDAS score < 3.2
MoDA: 3.2 ≤ PASDAS score < 5.4
HDA: PASDAS score ≥ 5.4
The proportions of patients in remission, LDA, MoDA and HDA were assessed at weeks 16, 52 and 104 in the overall population, and patients stratified by prior TNF inhibitor treatment (TNFi-naïve or TNFi-experienced) or by time since diagnosis (≤ 2 versus > 2 years). Shifts in disease activity in patients in each PASDAS state at week 16 were evaluated for secukinumab 300 and 150 mg treatment to assess sustainability of responses at weeks 52 and 104.
PASDAS states and individual core components
The impact of secukinumab treatment on individual PASDAS components was calculated among patients categorized in each disease activity state at weeks 16, 52 and 104.
PASDAS states and patient-reported outcomes
The relationship between PASDAS disease activity and PROs was assessed at weeks 16, 52 and 104 using data pooled across the treatment groups. HRQoL was assessed using SF-36 PCS (a component of the PASDAS score) and SF-36 MCS scores, Psoriatic Arthritis Quality of Life questionnaire (PsAQoL), Dermatology Life Quality Index (DLQI), physical function by Health Assessment Questionnaire Disability Index (HAQ-DI), fatigue by Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-Fatigue) and work productivity by the Work Productivity and Activity Impairment (WPAI) questionnaire.
Statistical analysis
The percentages of patients in each PASDAS disease activity state in the overall population (TNFi-naïve and TNFi-experienced) and by prior TNFi treatment status and disease duration at baseline were reported using mutually exclusive categories at the group level according to as-observed analyses, including shifts in disease activity states from week 16 to weeks 52/104.
Median, first (Q1) and third quartiles (Q3) were computed for each of the individual core PASDAS components at weeks 16, 52 and 104 for each disease activity state in patients with data at weeks 16/52 and 16/104.
Additionally, SF-36 PCS and MCS scores, PsAQoL, DLQI, HAQ-DI, FACIT-Fatigue and WPAI were assessed at weeks 16, 52 and 104 using mixed models for repeated measures (MMRM) analyses with analysis visit, PASDAS disease activity state at the analysis visit (remission, LDA, MoDA and HDA), and randomization stratum (TNFi-naïve or TNFi-experienced) as factors and weight and baseline score as continuous covariates. PASDAS disease activity state and baseline score by analysis visit were included as interaction terms. An unstructured covariance structure was used for MMRM analysis. Least-squares mean (LSM) estimates and SEs along with P values were provided at each assessment for patients in each disease activity state. Analysis data were pooled across treatment arms (secukinumab + placebo) pertaining to relationship between PASDAS disease activity states and PROs. All statistical analyses were performed using SAS version 9.4 or higher software (SAS Institute, Cary, NC, USA).
Results
Patients
Demographic and baseline characteristics were well balanced across treatment groups in FUTURE 2 [15]; the parameters relevant to PASDAS are shown in Table 1. Mean (SD) PASDAS scores at baseline were 5.9 (0.9), 6.0 (1.0) and 5.8 (1.0) in the secukinumab 300 mg, secukinumab 150 mg and placebo groups, respectively. At baseline, > 60% of patients were TNFi-naïve across the groups (Table 1). Retention rates of enrolled patients at week 104 were 86% (86/100) and 76% (76/100) with secukinumab 300 and 150 mg, respectively. Discontinuations due to lack of efficacy at week 104 were 3% (3/100) and 7% (7/100) in the secukinumab 300 and 150 mg groups, respectively [16].
Table 1.
Demographics and baseline disease characteristics relevant to Psoriatic Arthritis Disease Activity Score
| Characteristic mean (SD) unless otherwise stated | Secukinumab 300 mg s.c. (N = 100) |
Secukinumab 150 mg s.c. (N = 100) |
Placebo (N = 98) |
|---|---|---|---|
| Age, years | 46.9 (12.6) | 46.5 (11.7) | 49.9 (12.5) |
| Female, n (%) | 49 (49.0) | 45 (45.0) | 59 (60.2) |
| Time since diagnosis of PsA in years | 7.4 (7.5) | 6.5 (8.2) | 7.3 (7.8) |
| TNFi-naïve, n (%) | 67 (67.0) | 63 (63.0) | 63 (64.3) |
| Psoriasis ≥ 3% of BSA, n (%) | 41 (41.0) | 58 (58.0) | 43 (43.9) |
| Physician’s global VAS | 55.0 (14.7) | 56.7 (16.6) | 55.0 (16.0) |
| Patient’s global VAS | 60.7 (18.9) | 62.0 (19.5) | 57.6 (19.8) |
| SF-36 PCS | 36.9 (8.0) | 36.2 (8.1) | 37.4 (8.8) |
| Dactylitis counta | 3.6 (3.5) | 4.5 (5.1) | 2.7 (2.2) |
| Enthesitis countb | 2.8 (1.7) | 3.2 (16) | 3.1 (1.7) |
| TJC (78 joints) | 20.2 (13.3) | 24.1 (19.4) | 23.4 (19.0) |
| SJC (76 joints) | 11.2 (7.8) | 11.9 (10.1) | 12.1 (10.7) |
| PASDAS score | 5.9 (0.9); n = 98 |
6.0 (1.0); n = 99 |
5.8 (1.0); n = 98 |
Abbreviations: BSA Body surface area, SJC Swollen joint count, TJC Tender joint count, VAS Visual analogue scale
n, number of patients in each treatment group providing data; N, number of randomized patient
aThe dactylitis count is the number of fingers and toes with dactylitis, with a range of 0–20 and if dactylitis is present with any finger or toe, the patient is counted as a patient with dactylitis
bEnthesitis was evaluated by Leeds Enthesitis Index, a validated enthesitis index. If enthesitis is present in any of the 6 sites (lateral epicondyle humerus L + R, proximal Achilles L + R and medial condyle femur L + R), the patient is counted as a patient with enthesitis
PASDAS states
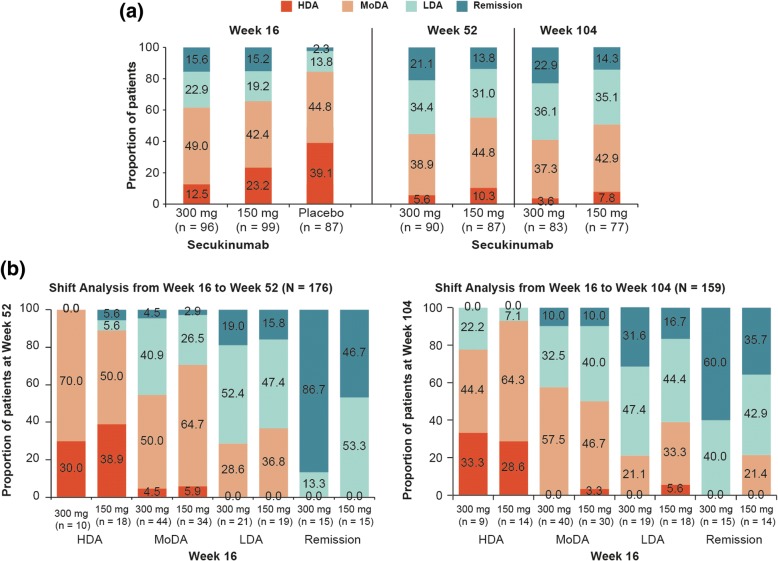
In the overall population, a higher proportion of secukinumab 300 mg (38.5% [37/96]) and 150 mg (34.3% [34/99])-treated patients achieved PASDAS remission + LDA at week 16 versus placebo (16.1% [14/87]). At week 104, 22.9% (19/83) and 14.3% (11/77) patients achieved remission, and 36.1% (30/83) and 35.1% (27/77) patients reached LDA with secukinumab 300 and 150 mg, respectively. The proportions of patients in PASDAS remission, LDA, MoDA and HDA at weeks 16, 52 and 104 are depicted in Fig. 1a. The shift analysis from weeks 16 to 104 showed that the majority of secukinumab 300 mg-treated patients who achieved remission at week 16 maintained remission (60%) or were in LDA (40%) at week 104, and 79% of patients in LDA at week 16 maintained or improved their status to remission at week 104; all patients in MoDA at week 16 either maintained or improved their status. In contrast, patients treated with secukinumab 150 mg who achieved remission at week 16 maintained remission (35.7%) or were in LDA (42.9%) or MoDA (22%) at week 104, patients in LDA at week 16 maintained or improved their status (60%) or lost their status (40%), and 97% of patients in MoDA maintained or improved their status at week 104 (Fig. 1b).
Fig. 1.
a Proportion of patients achieving Psoriatic Arthritis Disease Activity Score (PASDAS) remission, LDA, MoDA and HDA at weeks 16, 52 and 104 in overall population. Data were reported using mutually exclusive categories at group level and as observed analysis. LDA: 1.9 < PASDAS score < 3.2; remission: PASDAS score ≤ 1.9. Secukinumab 300 and 150 mg data are reported (approved doses). n = number of patients in the treatment group with evaluation. b Shift analysis of PASDAS disease activity states from week 16 to week 52 or 104. Data were reported using mutually exclusive categories at group level and as observed analysis. n = number of patients in each PASDAS state at week 16; N = total number of patients with non-missing PASDAS scores at week 16 and weeks 52/104. HDA High disease activity, LDA Low disease activity, MoDA Moderate disease activity
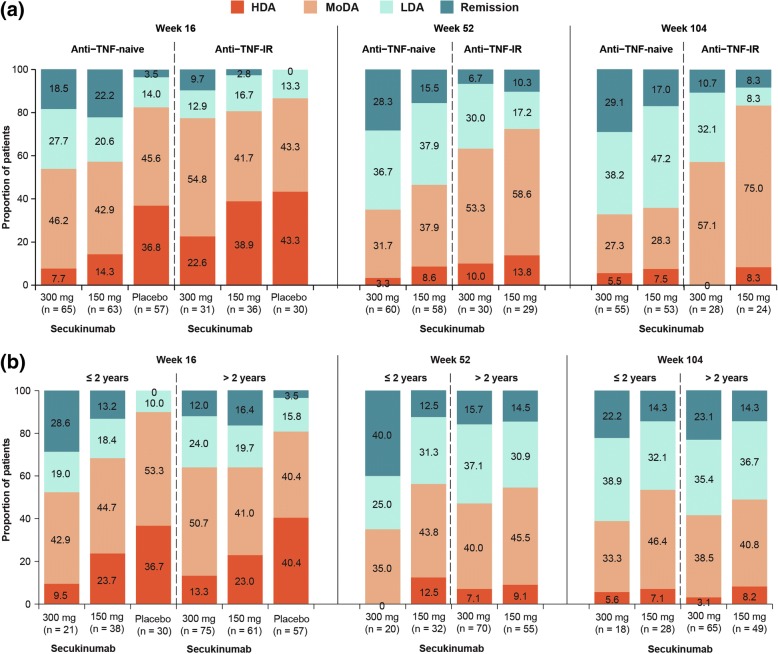
A higher proportion of TNFi-naïve and TNF-experienced patients achieved remission or LDA at week 16 versus placebo with generally higher response rates in the TNFi-naïve subgroup. In TNFi-naïve patients, remission + LDA was achieved by 46.2% (30/65) patients with secukinumab 300 mg and 42.9% (27/63) with 150 mg versus placebo (17.5% [10/57]), with responses in TNFi-experienced patients being 22.6% (7/31) for secukinumab 300 mg and 19.4% (7/36) for 150 mg versus 13.3% (4/30) for placebo. These response rates were sustained at weeks 52 and 104 in both TNFi-naïve and TNFi-experienced subgroups (Fig. 2a). Regardless of time since PsA diagnosis (≤ 2 years versus > 2 years), a higher proportion of secukinumab-treated patients achieved PASDAS remission or LDA than placebo at week 16. This proportion of patients achieving remission/LDA increased further at weeks 52 and 104, regardless of time since PsA diagnosis (Fig. 2b).
Fig. 2.
Proportion of patients achieving Psoriatic Arthritis Disease Activity Score (PASDAS) remission, LDA, MoDA and HDA at weeks 16, 52 and 104 by (a) Tumour necrosis factor inhibitor (TNFi) status and (b) time since psoriatic arthritis diagnosis. HDA High disease activity, LDA Low disease activity, MoDA Moderate disease activity. n = number of patients in the treatment group with evaluation
PASDAS states and core components
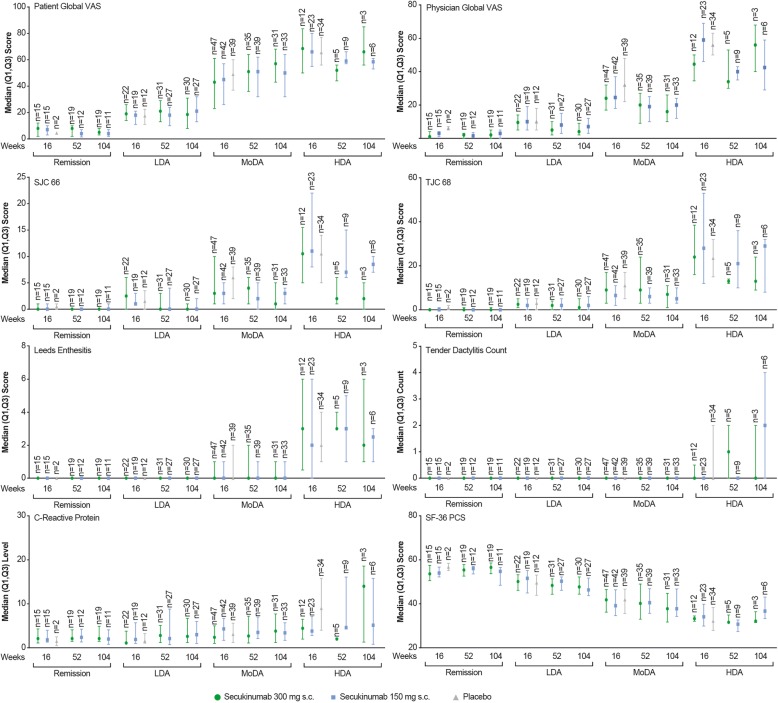
The median (Q1, Q3) scores of PASDAS core components in each PASDAS state at weeks 16, 52 and 104 are shown in Fig. 3. The most improved individual components in patients achieving PASDAS remission and LDA were dactylitis, enthesitis, SF-36 PCS, physician global VAS and SJC at weeks 16, 52 and 104. For dactylitis and enthesitis core components, median improvements were numerically similar in patients reaching PASDAS remission and LDA.
Fig. 3.
Scores of Psoriatic Arthritis Disease Activity Score (PASDAS) components at weeks 16, 52 and 104. The median value is denoted by symbol in the figure, and the upper and lower error bars represent third (Q3) and first (Q1) quartiles, respectively. n = number of patients in respective disease states at assessment. HDA High disease activity, LDA Low disease activity, MoDA Moderate disease activity, SF-36 MCS Short Form Health Survey Mental Component Summary, SJC Swollen joint count, TJC Tender joint count, VAS Visual analogue scale
PASDAS states and PROs
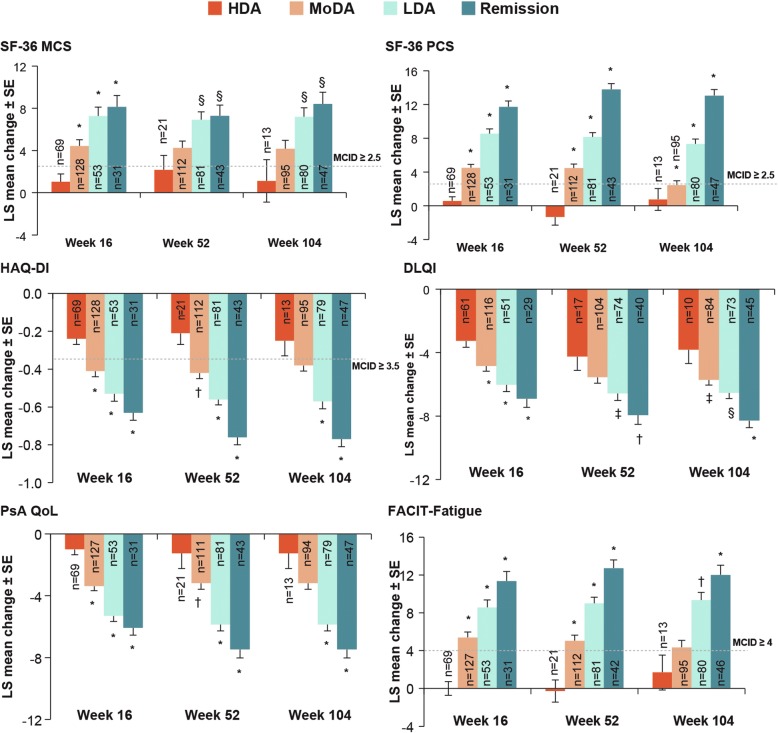
At week 16, LSM changes from baseline in SF-36 PCS and MCS, PsAQoL, DLQI, HAQ-DI and FACIT-Fatigue scores were significantly higher (P < 0.0001) among patients reaching PASDAS remission and LDA than those in HDA (Fig. 4). Similarly, higher LSM changes in these PROs were reported by patients in PASDAS remission and LDA versus those in HDA at weeks 52 and 104 (Fig. 4).
Fig. 4.
Mean change in patient-reported outcome scores by Psoriatic Arthritis Disease Activity Score (PASDAS) states at weeks 16, 52 and 104. Data are from mixed model for repeated measures analysis. *P < 0.0001; †P < 0.001; §P < 0.01; ‡P < 0.05 versus HDA. n = number of patients with measurements at baseline and post-baseline visits; N = number of patients in each group of the specified analysis set. DLQI Dermatology Life Quality Index, FACIT-Fatigue Functional Assessment of Chronic Illness Therapy–Fatigue, HAQ-DI Health Assessment Questionnaire Disability Index, HDA High disease activity, LDA Low disease activity, LS Least squares, PsAQoL Psoriatic arthritis-specific quality of life, SF-36 MCS Short Form Health Survey Mental Component Summary, SF-36 PCS Short Form Health Survey Physical Component Summary. Week 16: N = 32 (remission), N = 53 (LDA), N = 128 (MoDA) and N = 69 (HDA); week 52: N = 44 (remission), N = 81 (LDA), N = 112 (MoDA) and N = 21 (HDA); week 104: N = 47 (remission), N = 81 (LDA), N = 95 (MoDA) and N = 13 (HDA). Analytic data were pooled across treatment arms
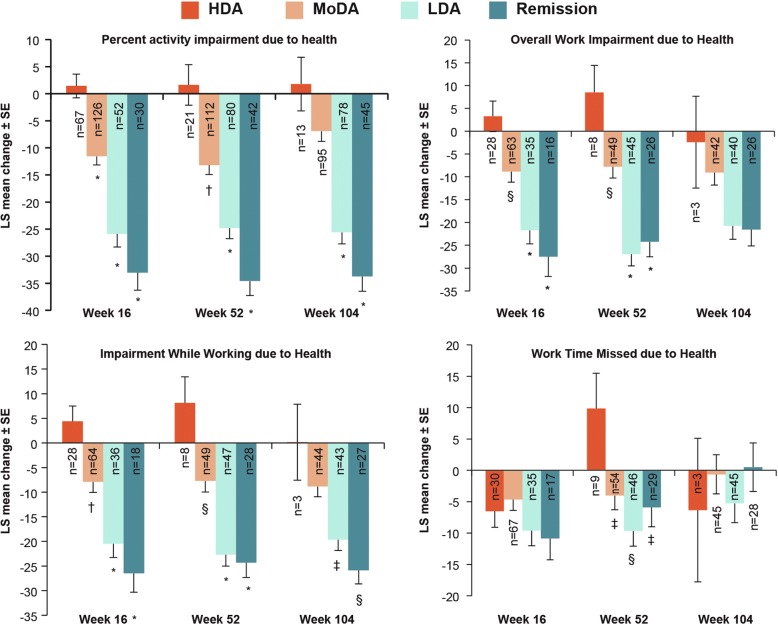
As shown in Fig. 5, a decrease (P < 0.0001) from baseline to week 16 in the percentage of activity impairment due to health, overall work impairment due to health, and impairment while working due to health were reported by patients in PASDAS remission and LDA compared with those in HDA. Similar reductions in percentage of activity impairment due to health (P < 0.0001) and impairment while working due to health (P < 0.05) were reported by patients in PASDAS remission and LDA than HDA at weeks 52 and 104; percentage of overall work impairment due to health was reduced (P < 0.0001) at week 52. The percentage of work time missed due to health decreased (P < 0.01) from baseline to week 52 among patients in remission and LDA compared with HDA at week 52.
Fig. 5.
Mean change in work productivity and activity impairment outcome measures by Psoriatic Arthritis Disease Activity Score (PASDAS) states at weeks 16, 52 and 104. Data are from mixed model for repeated measures analysis. *P < 0.0001; †P < 0.001; §P < 0.01 and ‡P < 0.05 versus HDA. n = number of patients with measurements at both baseline and post-baseline visits; N = number of patients in each group of the specified analysis set. HDA High disease activity, LDA Low disease activity, LS Least squares, MoDA Moderate disease activity. Week 16: N = 32 (remission), N = 53 (LDA), N = 128 (MoDA) and N = 69 (HDA); week 52: N = 44 (remission), N = 81 (LDA), N = 112 (MoDA) and N = 21 (HDA); week 104: N = 47 (remission), N = 81 (LDA), N = 95 (MoDA) and N = 13 (HDA). Analytic data were pooled across treatment arms
Discussion
PASDAS is a novel composite index which assesses multiple facets of PsA, including tender and swollen joints, dactylitis, enthesitis, and HRQoL, and offers both a treatment target and a disease activity state assessment across important clinical domains with validated cut points. PASDAS distinguishes treatment effect, performs better than traditional joint-only indices, and could be used as a treatment target in RCTs and longitudinal observational studies in PsA. There are limited data available on PASDAS in two TNFi clinical trials and in two ixekizumab trials. A study on a golimumab data set (GO-REVEAL trial) showed that PASDAS was able to distinguish treatment effect, having larger effect size at week 24 [20]. Another study using data on certolizumab pegol (RAPID-PsA trial) also showed that PASDAS demonstrated good responsiveness and discriminative ability at week 12, supporting further exploration of its use in PsA clinical trials [21]. Assessment of PASDAS-defined LDA and very low disease activity (VLDA) was also reported with ixekizumab data from SPIRIT-P1 and SPIRIT-P2 trials [22] and showed that the proportions of patients achieving PASDAS LDA and PASDAS VLDA were greater with ixekizumab than with placebo [22], further confirming the validity of PASDAS as a measure that can differentiate treatment effect. Therefore, in the present study, the ability of secukinumab to reach PASDAS-related remission or LDA was evaluated using data from the FUTURE 2 trial. To the best of our knowledge FUTURE 2 is the first trial to report detailed PASDAS-based analysis in a mixed TNFi-naïve and TNFi-experienced PsA population treated with an IL-17A inhibitor.
Sustained clinical benefit with secukinumab was previously demonstrated through 104 weeks in the treatment of moderate to severe PsA in the phase III FUTURE 2 trial [15, 16], which included the more stringent clinical end points such as ACR70 responses, PASI 90 response, resolution of enthesitis and dactylitis, and high retention rate as a surrogate marker [15, 16, 23]. Results of this post hoc analysis using PASDAS scores showed that higher proportions of patients treated with secukinumab 300 and 150 mg achieved PASDAS remission or LDA at week 16 than those who received placebo in the overall population at a group level with responses sustained through week 104. Shift analysis of PASDAS states from weeks 16 to 52 and from weeks 16 to 104 confirmed that at an individual level, a majority of patients meeting either PASDAS remission or LDA either maintained or improved their status over time. These data illustrate that secukinumab can meet more stringent treatment goals in line with EULAR and GRAPPA recommendations [6, 7]. Results of this post hoc analysis complement and extend previous reports from the FUTURE 2 trial which have shown that patients treated with secukinumab achieved and sustained remission or LDA defined by other composite indices such as MDA/VLDA and DAPSA up to 2 years [24, 25]. At week 16, in the overall population, the proportions of patients treated with secukinumab 300/150 mg achieving remission were 14%/10% (DAPSA-REM) and 8%/6% (VLDA), respectively, and in those achieving LDA the proportions were 42%/44% (DAPSA REM/LDA) and 28%/23% (MDA) [24, 25], respectively. These results were sustained through week 104.
Secukinumab (300 and 150 mg)-treated patients achieving remission and LDA had improved median scores across all PASDAS core components relating to physician and patient global VAS, SF-36 PCS, SJC 66, TJC 68, dactylitis and enthesitis in contrast to PASDAS HDA. Among these, physician and patient global VAS, TJC 68, SJC 66, dactylitis and enthesitis were most improved.
In the subgroup analysis by previous TNFi use, the proportion of secukinumab-treated patients achieving remission and LDA at week 16 was higher in both TNFi-naïve and TNFi-experienced patients than in patients receiving placebo, with responses in secukinumab groups sustained or increased at weeks 52 and 104. The proportion of secukinumab-treated patients achieving remission and LDA was generally greater in the TNFi-naïve subgroup than in the TNFi-experienced subgroup through week 104. In a recent cross-sectional analysis of a longitudinal cohort, of 79 patients with PsA receiving their first biologic DMARD (32: etanercept, 24: adalimumab, 18: golimumab, and 5: ustekinumab) for at least 6 months, 12.6% achieved remission (PASDAS ≤ 1.9) and 41.8% were in PASDAS LDA [26]. Our study showed in 128 TNFi-naïve patients treated with secukinumab (300 or 150 mg) that 16–28% of patients achieved remission and 37–38% achieved LDA at week 52.
At week 16, a higher proportion of patients treated with secukinumab achieved PASDAS remission or LDA versus placebo, regardless of time since PsA diagnosis (≤ 2 years versus > 2 years). A numerically higher proportion of secukinumab-treated patients reached PASDAS remission or LDA at weeks 52 and 104 than at week 16, regardless of time since PsA diagnosis. Although PASDAS includes SF-36 PCS as one of its core components, patients achieving PASDAS remission and LDA also reported greater improvements in other PROs (HRQoL, mental health, physical function, fatigue and work productivity) through week 104, confirming that these stringent goals translate into improved patient quality of life and function as well as for society owing to higher workforce productivity.
The PASDAS has certain limitations in that, being a complex composite index, it requires complex mathematical calculations, which are time-consuming, although this has been overcome with an application allowing for an easy calculation. PASDAS thus may be more appropriately used in RCTs [10]. It does not include a measure for axial involvement and patient pain, both important manifestations of PsA. Moreover, PASDAS-based publications on RCT data are sparse to place it in context with other composite indices used in PsA. Another limitation of this study was that patients in HDA were not specifically treated to reach the preferable REM/LDA states. Furthermore, while PASDAS change from baseline was an exploratory end point in the FUTURE 2 study, the cut points related to the different disease activity states were recently validated, and this post hoc analysis was generated following the more recently validated cut-offs. These data will require confirmation in new RCTs. Also, there is a lack of assessment in relation to structural outcomes as per the FUTURE 2 study protocol. Further, while we appreciate the fact that SF-36 PCS is one of the core components of PASDAS and therefore a question of circularity in looking at the relationship of PASDAS states and PROs could be raised, we still think it is relevant to do this analysis and see how PASDAS states translate to patient well-being and thus outcomes reported by them.
Conclusions
In summary, this post hoc analysis of FUTURE 2 data showed that secukinumab treatment resulted in PASDAS remission or LDA at week 16 with responses sustained or further improved through week 104 at the group and individual levels. Improvement or sustainability of MoDA, LDA and remission states were more frequently achieved with secukinumab 300 mg than 150 mg. PASDAS remission/LDA was associated with significantly greater improvements in HRQoL, physical function, fatigue and work productivity. These results extend the previous findings of maintenance of other stringent clinical efficacy end points, including VLDA and MDA, in the FUTURE 2 trial, demonstrating that secukinumab treatment can result in sustained PASDAS-defined remission or LDA, thus demonstrating the potential utility of PASDAS as an outcome measure in RCTs in PsA.
Acknowledgements
The authors thank the patients who participated in this study; the study investigators; and John Gallagher, medical consultant for Novartis Pharma AG, Basel, Switzerland. Scientific review support was provided by Neeta Pillai, Novartis Healthcare Pvt. Ltd., Hyderabad, India. Manuscript writing support was provided by Santoshkumar Tota, Novartis Healthcare Pvt. Ltd., Hyderabad, India.
Funding
Novartis Pharma AG (Basel, Switzerland).
Availability of data and materials
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com.
Trial details are available at https://www.clinicaltrialsregister.eu/ctr-search/search?query=2012-004439-22 and https://clinicaltrials.gov/ct2/show/NCT01752634.
Study sponsor statement
This study was sponsored by Novartis Pharma AG, Basel, Switzerland, and was designed by the scientific steering committee and Novartis personnel. Medical writing support was funded by Novartis.
Abbreviations
- ACR
American College of Rheumatology
- CPDAI
Composite Psoriatic Disease Activity Index
- CRP
C-reactive protein
- DAPSA
Disease Activity Index for Psoriatic Arthritis
- DAS
Disease Activity Score
- DAS-28 CRP
28-joint Disease Activity Score based on C-reactive protein
- DLQI
Dermatology Life Quality Index
- DMARD
Disease-modifying anti-rheumatic drug
- EULAR
European League Against Rheumatism
- FACIT
Functional Assessment of Chronic Illness Therapy
- GRAPPA
Group for Research and Assessment of Psoriasis and Psoriatic Arthritis
- HAQ-DI
Health Assessment Questionnaire Disability Index
- HDA
High disease activity
- HRQoL
Health-related quality of life
- IL
Interleukin
- IRB
Institutional review board
- LDA
Low disease activity
- LS
Least squares
- MoDA
Moderate disease activity
- NSAID
Non-steroidal anti-inflammatory drug
- PASDAS
Psoriatic Arthritis Disease Activity Score
- PASI
Psoriasis Area Severity Index
- PRO
Patient-reported outcome
- PsA
Psoriatic arthritis
- PsAQoL
Psoriatic Arthritis Quality of Life questionnaire
- Q
Quartile
- RCT
Randomized controlled trial
- REM
Remission
- s.c.
Subcutaneous
- SF-36 MCS
Short Form Health Survey Mental Component Summary
- SF-36 PCS
Short Form Health Survey Physical Component Summary
- SJC
Swollen joint count
- TJC
Tender joint count
- TNF
Tumour necrosis factor
- VAS
Visual analogue scale
- WPAI
Work Productivity and Activity Impairment questionnaire
Authors’ contributions
All authors had access to the data, contributed to its interpretation and collaborated in the development of the manuscript. The initial draft of the manuscript was written by a medical writer employed by the study sponsor. All authors critically reviewed and provided feedback on subsequent versions. All authors made the decision to submit the manuscript for publication and vouch for the accuracy and completeness of the data and fidelity of this report to the study protocol. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki. The institutional review board (IRB) at each participating centre approved the protocol. Further information related to ethics approval can be found at https://www.clinicaltrialsregister.eu/ctr-search/search?query=2012-004439-22. All centres received approval from independent ethics committees or IRBs.
Consent for publication
Patients provided written informed consent before starting the study-related procedures.
Competing interests
LCC is funded by a National Institute for Health Research Clinician Scientist award. The present research was supported by the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. LCC has received grant/research support from AbbVie, Janssen, Lilly, Novartis and Pfizer and has served as a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Pfizer, UCB, MSD, Boehringer Ingelheim, Novartis, Lilly, Janssen, Sun Pharma, Prothena and Galapagos. DDG has received grants and/or personal fees from Amgen, AbbVie, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, Novartis, Pfizer and UCB. PN has received research grants for clinical trials and honoraria for lectures and advice from Novartis, AbbVie, Roche, Pfizer, Bristol-Myers Squibb, Janssen and Celgene. OF has received fees for speaking or consulting from Bristol-Myers Squibb, AbbVie, Pfizer Inc., UCB Pharma Ltd., Lilly, Novartis, Celgene and Janssen Research. OF has received grants from Bristol-Myers Squibb, AbbVie, Novartis and Pfizer Inc. AK has served as a consultant for Novartis. TKK has received fees for speaking and/or consulting from AbbVie, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celltrion, Eli Lilly, Epirus, Janssen, Merck-Serono, MSD, Mundipharma, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz and UCB. LG has received research grants from AbbVie, Bristol-Myers Squibb, Celgene, Janssen, Novartis, MSD, Roche and UCB. VS has served as a consultant for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Celltrion, CORRONA, Genentech/Roche, GSK, Janssen, Lilly, Merck, Novartis, Pfizer, Regeneron, Sanofi and UCB. LR has served as a consultant for Novartis through employment at RTI Health Solutions and is an employee of RTI Health Solutions. LP is a shareholder of Novartis and an employee of Novartis. KD is a shareholder of Novartis and an employee of Novartis. SMJ is an employee of Novartis. CG is a shareholder of Novartis and Bristol-Myers Squibb and is an employee of Novartis.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
L. C. Coates, Phone: +44-(0)-1865 227 374, Email: laura.coates@ndorms.ox.ac.uk
D. D. Gladman, Email: dafna.gladman@utoronto.ca
P. Nash, Email: drpnash@tpg.com.au
O. FitzGerald, Email: oliver.fitzgerald@ucd.ie
A. Kavanaugh, Email: akavanaugh@ucsd.edu
T. K. Kvien, Email: t.k.kvien@medisin.uio.no
L. Gossec, Email: les.gossec@gmail.com
V. Strand, Email: vibekestrand@me.com
L. Rasouliyan, Email: lrasouliyan@rti.org
L. Pricop, Email: luminita.pricop@novartis.com
K. Ding, Email: kevin.ding@novartis.com
S. M. Jugl, Email: steffen.jugl@novartis.com
C. Gaillez, Email: corine.gaillez@novartis.com
References
- 1.Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis. 2005;64(Suppl 2):ii14–ii17. doi: 10.1136/ard.2004.032482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen CF, Mussani F, Chandran V, Eder L, Thavaneswaran A, Gladman DD. Patients with psoriatic arthritis have worse quality of life than those with psoriasis alone. Rheumatology (Oxford) 2012;51:571–576. doi: 10.1093/rheumatology/ker365. [DOI] [PubMed] [Google Scholar]
- 3.Her M, Kavanaugh A. A review of disease activity measures for psoriatic arthritis: what is the best approach? Expert Rev Clin Immunol. 2014;10:1241–1254. doi: 10.1586/1744666X.2014.943663. [DOI] [PubMed] [Google Scholar]
- 4.Lubrano E, Perrotta FM, Kavanaugh A. An overview of low disease activity and remission in psoriatic arthritis. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S51–S54. [PubMed] [Google Scholar]
- 5.Smolen JS, Schöls M, Braun J, Dougados M, FitzGerald O, Gladman DD, et al. Treating axial spondyloarthritis and peripheral spondyloarthritis, especially psoriatic arthritis, to target: 2017 update of recommendations by an international task force. Ann Rheum Dis. 2018;77(1):3–17. doi: 10.1136/annrheumdis-2017-211734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Laura Acosta-Felquer M, Armstrong AW, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol. 2016;68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 7.Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75:499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- 8.Chandran V, Maharaj AB. Assessing disease activity in psoriasis and psoriatic arthritis: impact on management and therapy. Expert Rev Clin Immunol. 2016;12:573–582. doi: 10.1586/1744666X.2016.1146133. [DOI] [PubMed] [Google Scholar]
- 9.Smolen JS, Schoels M, Aletaha D. Disease activity and response assessment in psoriatic arthritis using the Disease Activity index for Psoriatic Arthritis (DAPSA): a brief review. Clin Exp Rheumatol. 2015;33(5 Suppl 93):S48–S50. [PubMed] [Google Scholar]
- 10.Helliwell PS, FitzGerald O, Fransen J, Gladman DD, Kreuger GG, Callis-Duffin K, et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project) Ann Rheum Dis. 2013;72:986–991. doi: 10.1136/annrheumdis-2012-201341. [DOI] [PubMed] [Google Scholar]
- 11.Coates LC, Helliwell PS. Defining low disease activity states in psoriatic arthritis using novel composite disease instruments. J Rheumatol. 2016;43:371–375. doi: 10.3899/jrheum.150826. [DOI] [PubMed] [Google Scholar]
- 12.Helliwell PS, Waxman R. Modification of the Psoriatic Arthritis Disease Activity Score (PASDAS) Ann Rheum Dis. 2018;77:467–468. doi: 10.1136/annrheumdis-2016-211010. [DOI] [PubMed] [Google Scholar]
- 13.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 14.Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17a in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 15.McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386:1137–1146. doi: 10.1016/S0140-6736(15)61134-5. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Mease PJ, Ritchlin CT, Rahman P, Gottlieb AB, Kirkham B, et al. Secukinumab sustains improvement in signs and symptoms of psoriatic arthritis: 2 year results from the phase 3 FUTURE 2 study. Rheumatology (Oxford) 2017;56:1993–2003. doi: 10.1093/rheumatology/kex301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nash P, Mease PJ, McInnes IB, Rahman P, Ritchlin CT, Blanco R, et al. Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo-controlled trial (FUTURE 3) Arthritis Res Ther. 2018;20:47. doi: 10.1186/s13075-018-1551-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17a inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 19.Baraliakos X, Kivitz AJ, Deodhar AA, Braun J, Wei JC, Delicha EM, et al. Long-term effects of interleukin-17A inhibition with secukinumab in active ankylosing spondylitis: 3-year efficacy and safety results from an extension of the phase 3 MEASURE 1 trial. Clin Exp Rheumatol. 2018;36:50–55. [PubMed] [Google Scholar]
- 20.Helliwell PS, Kavanaugh A. Comparison of composite measures of disease activity in psoriatic arthritis using data from an interventional study with golimumab. Arthritis Care Res (Hoboken) 2014;66:749–756. doi: 10.1002/acr.22204. [DOI] [PubMed] [Google Scholar]
- 21.Helliwell P, Mease PJ, Nurminen T, FitzGerald O. Further analysis of Psoriatic Arthritis Disease Activity Score (PASDAS) and Composite Psoriatic Disease Activity Index (CPDAI) using data from a placebo-controlled trial of certolizumab pegol in psoriatic arthritis. Ann Rheum Dis. 2014;73:740. [Google Scholar]
- 22.Coates LC, Husni ME, Lespessailles E, Kerr L, Gallo G. Ixekizumab makes very low disease activity and remission with psoriatic arthritis disease activity score possible in active psoriatic arthritis patients for up to 1 year: SPIRIT-P1 AND SPIRIT-P2 trials. Ann Rheum Dis. 2018;77:375. [Google Scholar]
- 23.Mease PJ, Kavanaugh A, Reimold A, Tahir H, Rech J, Hall S, et al. Secukinumab provides sustained improvements in the signs and symptoms of active psoriatic arthritis through 3 years: efficacy and safety results from a phase 3 trial [abstract 961]. Arthritis Rheumatol. 2016;68(suppl 10).
- 24.Coates LC, Mease PJ, Gossec L, Kirkham B, Sherif B, Gaillez C, et al. Minimal Disease Activity among Active Psoriatic Arthritis Patients Treated with Secukinumab: 2-year Results from the FUTURE 2 Study. Arthritis Care Res (Hoboken) 2018;70:1529–1535. doi: 10.1002/acr.23537. [DOI] [PubMed] [Google Scholar]
- 25.Coates LC, Nash P, Kvien T, Gossec L, Mease PJ, Rasouliyan L, et al. Secukinumab Provides Sustained Minimal Disease Activity (MDA) and Remission Related to Disease Activity Index for Psoriatic Arthritis (DAPSA): 2-Year Results from a Phase 3 Study [abstract 622]. Arthritis Rheumatol. 2017;69(suppl 10).
- 26.Lubrano E, De Socio A, Perrotta FM. Comparison of composite indices tailored for psoriatic arthritis treated with csDMARD and bDMARD: a cross-sectional analysis of a longitudinal cohort. J Rheumatol. 2017;44:1159–1164. doi: 10.3899/jrheum.170112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Novartis is committed to sharing with qualified external researchers access to patient-level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial, in line with applicable laws and regulations. The trial data availability is according to the criteria and process described at www.clinicalstudydatarequest.com.
Trial details are available at https://www.clinicaltrialsregister.eu/ctr-search/search?query=2012-004439-22 and https://clinicaltrials.gov/ct2/show/NCT01752634.