Abstract
It is difficult to obtain in vivo digestion kinetics data of high protein ingredients using chickens. Collecting kinetics data requires repeated sampling of digesta from the small intestine during the digestion process, which is not easily accomplished due to the anatomical structure of chicken digestive tract. An in vitro technique is proposed for measuring the digestion kinetics of protein sources fed to chickens. The method has a 30 min gastric and 3 h intestinal phase. Five hundred milligram crude protein (CP) equivalent of each meal sample (CP = % N × 6.25) was digested with pepsin (28,260 units) in 50 mL polyethylene centrifuge tubes for 30 min in a shaking water bath (150 strokes/min; 30 mm stroke length) at 41 °C. The 6.5 mL pancreatin was selected as the enzyme concentration for the intestinal phase, during which time 500 μL aliquots were collected at 0, 15, 30, 45, 60, 90, 120, 150, 180 and 240 min. Samples were diluted 1:820 with HCl and sodium acetate buffer, and then mixed with ninhydrin reagent (2:1) at 100 ± 2 °C for 15 min and spectrometric readings taken at 568 nm. To validate the assay, 5 replications of soybean meal (SBM), corn gluten meal (CGM), corn distillers dried grains with solubles (CDDGS), porcine meal (PCM), fish meal (FM) and casein (CA) were digested. The digestion data were modeled with PROC NLIN procedure, and the intra coefficient of variation (CV) assessed using PROC MEANS of SAS 9.4. The digestion values at 180 min were SBM 95 ± 4, FM 93 ± 3, PCM 68 ± 4, CGM 82 ± 3 and CDDGS 70 ± 2. Intra CV for SBM, CGM, CDDGS, PCM and FM were 5%, 5%, 12%, 10% and 2%, respectively. The estimated fractional digestion rates for SBM, CGM, CDDGS, FM and PCM were 0.023, 0.013, 0.009, 0.024 and 0.013, respectively. In conclusion, the proposed in vitro technique estimated the rate and extent of the digestion of CP for the meals with low intra CV.
Keywords: Protein digestion rate, Soybean meal, Corn gluten meal, Corn distillers dried grains with solubles, Fish meal, Porcine meal
1. Introduction
Broiler chickens have been extensively selected for rapid growth and as a consequence the ability of the birds to deposit body protein has increased dramatically (Zuidhof et al., 2014). Concurrently, the quality of protein in broiler diets has increased in importance, with quality being defined by amino acid digestibility and balance (Ravindran and Bryden, 1999). It is a general consensus among poultry nutritional researchers that the jejunum and proximal ileum are the major sites for amino acid absorption. However, little information can be found pertaining to how much protein from common ingredients gets digested in the proximal and distal portions of the small intestine.
In vivo assays are considered to be the gold standard for assessing ingredient nutritional quality in poultry (Fuller, 1991). In vivo estimation of protein quality of a feed ingredient is normally achieved by feeding the ingredient to the intended animal while assessing the extent to which nutrients are absorbed by the terminal intestine. Protein quality can also be evaluated using in vitro chemical methods (Boisen and Eggum, 1991). In vitro assays are less expensive, can evaluate more ingredients, and are less time consuming than in vivo assays. Historically, the focal point of assessing protein quality for chickens has been based on the extent of digestion, and as a result, little data are available on the rate at which proteins are digested and absorbed.
The rate of digestion of protein along the digestive tract has been known to have significant biological effects in other species (Boirie et al., 1997, Ørskov and McDonald, 1979) and the same could be true for poultry. In vivo protein nutritional research in humans suggested that the sequential breakdown of proteins having different digestion rates modulated tissue protein synthesis and deposition (Boirie et al., 1997). The sequential breakdown of protein into intermediate peptides is considered to be the rate limiting step for the digestion and absorption of soybean meal (SBM) protein in poultry diets (Sklan and Hurwitz, 1980), which might also be the case for other protein sources commonly fed to poultry. Therefore, the degradation kinetics and bioavailability of proteins are both important factors, which could be considered when trying to maximize yield in poultry production.
Extensive research is available on the extent of digestibility for various high protein ingredients estimated using in vivo and in vitro procedures. However, information on the degradation characteristics of feed ingredients (in vivo or in vitro) for poultry is scarce and this type of research is often limited to human research (Dangin et al., 2001, Koopman et al., 2009). Most in vivo techniques used to evaluate protein degradation in humans and other animal research require the use of expensive isotope labeling of pure proteins and tracers (Boirie et al., 1997). Less expensive and time consuming in vitro methods have been used to obtain protein digestion data in ruminant species (Boila et al., 1980) and may have value for poultry. Currently, there is no in vitro method which estimates protein degradation kinetics for poultry.
The purpose of this research was to develop an in vitro protein digestibility assay for poultry specific, which could predict the degradation kinetics of high protein feed ingredients commonly fed to poultry. A multi-enzymatic digestion technique using gastric and intestinal digestion phases was defined and validated. The digestive tract transit time in broiler chickens has been reported to be 2 to 3.5 h (Hughes, 2008, Svihus et al., 2002), so the optimum enzyme to substrate concentration that resulted in the most effective degree of digestion within 3 h was used as a criterion for the assay. The condition for the colorimetric assay used to evaluate the degree of digestion was optimized. The effectiveness of the in vitro digestions technique on a variety of high protein ingredients was tested. This in vitro protein digestibility assay was developed to predict the rapidly and slowly undigested protein fractions of ingredients, as well as the rate and extent of digestion of the proteins.
2. Material and methods
The following methods illustrate the stages which were involved in the development of the proposed in vitro assay. The first stage describes an appropriate colorimetry assay for identifying changes in a protein sample due to hydrolysis of peptide bonds. The chemical composition of the reagent, its shelf life and wavelength sensitivity during reactions were evaluated and optimized for the in vitro assay. The second stage involved the establishment of the conditions for the in vitro assay gastric and intestinal digestion phase. The composition of the buffers which were compatible to the enzymes used in the gastric and intestinal phase was identified. The optimal units of pepsin for the gastric phase were elucidated using a dose response study over a 30 min digestion time frame. Selection of enzyme dose in the pancreatin for the intestinal phase was based on a dose response study building on the gastric phase results. The final stage of the research provides validation data for high protein ingredients using the colorimetry procedure and the two-stage in vitro digestion assay.
The reagents used in this study were obtained from the following sources. Tin (II) chloride dehydrate (CAS 107-21-1), benzoic acid (CAS 65-85-0), glacial acetic acid (CAS 64-19-7), trichloroacetic acid solution (Sigma T0699), hemoglobin (Sigma H2625), pepsin (P7125-100 g; CAS 9001-75-6), Nα-benzoyl-L-arginine ethyl ester (Sigma B4500), Trizma Base (Sigma T1503), N-benzoyl-L-tyrosine ethyl ester (Sigma B6125), methanol (Sigma M1775), N-succinyl-Ala-Ala-Ala-p-nitroanilide (Sigma S4760) and ninhydrin (CAS 485-47-2) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Ethylene glycol (CAS 107-21-1), sodium hydroxide (CAS 1310-73-2), sodium acetate trihydrate (CAS 6131-90-4), calcium chloride (CAS 10035-04-8), guar gum (CAS 9000-30-0) and hydrochloric acid (7647-01-0) were obtained from Fisher Scientific (Pittsburgh, PA, USA). The liquid bovine pancreatin (62,500 USP trypsin units/mL) was purchased from RENCO (10 London Street, Eltham 4322, New Zealand).
2.1. Colorimetry assay
2.1.1. Ninhydrin reagent composition
A 4 mol/L sodium acetate buffer was prepared by dissolving 544 g of sodium acetate trihydrate in 100 mL of warm glacial acetic acid and then Millipore water was added to make a total volume of 1,000 mL. The tin (II) chloride solution was prepared by adding 1.2 g tin (II) chloride to 12 mL of ethylene glycol and then vortexing to dissolve all the tin (II) chloride. To prepare the ninhydrin reagent, 9.75 g of ninhydrin were dissolved in 366 mL ethylene glycol, and then 122 mL 4 mol/L sodium acetate buffer were added. This solution was mixed for 5 min with a magnetic stir bar before the addition of 12 mL of tin (II) chloride solution and mixing for another 5 min.
2.1.2. Validation of ninhydrin reagent
The absorbance spectrum for SBM, casein (CA), corn distiller's dried grains with solubles (CDDGS) and corn gluten meal (CGM) were determined as follows. All samples were ground to pass through a 0.5 mm screen using a Retsch Ultra Centrifugal Mill ZM 200 (Haan, Germany). The CP contents of all the meals were determined as N × 6.25 using the Dumas method, where N contents were determined using a Leco nitrogen analyzer (Model 601–500–100, Serial # 3211, Leco Corporation, St. Joseph, MA, USA). Samples (500 mg CP equivalent) were weighed and placed in individual 100 mL Pyrex glass bottles (No.14395) after 6 mol/L hydrochloric acid was added at 4 mL per 100 mg of sample weight. The samples were gently mixed by swirling, capped and placed in an oven at 110 °C for 24 h. After 24 h hydrolysis, samples were allowed to cool to room temperature and then filtered through Whatman Grade 601 filter paper. An aliquot of the sample was collected after filtering and the pH was adjusted to 7 ± 0.5 with sodium hydroxide. The filtered sample was diluted with Millipore water to give 0.36 mg CP per mL based on the initial 500 mg CP of the sample that was hydrolyzed.
Each sample (100 μL) was mixed with 1,900 μL of Millipore water and 1,000 μL of ninhydrin reagent in disposable glass culture tubes (borosilicate glass 16 × 100 mm, No. 14-961-29). A blank sample with 2,000 μL of Millipore water and 1,000 μL of ninhydrin reagent was prepared. Glass marbles were placed on top of each tube and the tubes were placed in a boiling water bath for 10 min. The tubes were allowed to cool for 5 min before 200 μL of sample were pipetted in triplicate into a 96 well plate (Falcon 353910 U-Bottom well). The samples were read from 200 to 999 nm at 1 nm wavelength interval using a microplate reader (Epoch 2, BioTeck, USA) set at 22 °C.
The concentration detection limits for the ninhydrin reagent with a lysine standard were identified as follows. The lysine standard was prepared and diluted over the range from 0.25 to 410 μg/mL. One milliliter of each dilution was mixed with 500 μL of ninhydrin reagent in disposable glass culture tubes. A blank tube was prepared by replacing the diluted sample with Millipore water. Marbles were placed on the tubes before being placed in a boiling water bath according to the process described previously. A 200 μL volume of sample was pipetted into a 96 well plate and read at maximum absorbance (OD value) identified during the previous spectrum scan of the samples.
The shelf life of the ninhydrin reagent was evaluated over 304 d. A CA standard was prepared from the hydrolyzed CA sample. A fresh batch of ninhydrin reagent was prepared on the morning of day 1 and placed in a dark glass bottle wrapped in aluminum foil. At 16:00 the CA standard was reacted with the reagent as outlined in the absorbance spectrum test above, and then the ninhydrin reagent was placed on a shelf for storage at room temperature (22 ± 3 °C). This test was repeated on days 10, 14, 120, and 304 after the first test was conducted.
2.2. In vitro digestion assay
The in vitro assay method had a 30 min gastric and 3 h intestinal phase mimicking digestion in chickens based on previous research (Svihus et al., 2002, Hughes, 2008). The ratio of optimum enzyme to substrate was verified for the gastric phase and the intestinal phase using enzyme dose response assays with SBM as the model protein. Soybean was selected as the model protein because it is the most widely used protein source in poultry diets worldwide and its volume in production accounts for more than 69% of the world's total protein source for animal feed (USDA, 2016).
2.2.1. Buffer compositions
Multiple buffer compositions were evaluated in preliminary studies to test their interaction with the colorimetry reagent and their impact on the stability of enzymes. Sodium acetate buffers with pH 12.5 and 6.5 were the most suitable for maintaining enzyme activity of the glycerol based pancreatin and compatibility with the ninhydrin reagent used in this study. To prepare 1 L of a 10 mmol/L HCl solution, 833 μL of concentrated HCL were mixed with 999.167 mL of Millipore water. A 0.1 mol/L calcium chloride solution was prepared by dissolving 33.3 g of calcium chloride in 300 mL Millipore water. A benzoic acid solution was prepared by dissolving 5.8 g of benzoic acid into 2 L of Millipore water. For the sodium acetate buffers preparation, 27.2 g of sodium acetate trihydrate were dissolved in 500 mL benzoic acid solution. The pH was adjusted to 12.5 or 6.5 using saturated sodium hydroxide solution (50%; wt/wt) and then the volume of the solution was made up to 2 L with Millipore water followed by the addition of 8 mL 0.1 mol/L calcium chloride solution. All buffers were stored in the refrigerator until use.
2.2.2. Pepsin dose response assay
Pepsin activity was determined using the Sigma enzymatic assay for pepsin (3.4.23.1). One unit of pepsin was defined as a change in ΔA280 of 0.001 per min at pH 2.0 and 37 °C measured as trichloroacetic acid soluble products using hemoglobin as the substrate. Pepsin was dissolved in 10 mmol/L HCl solution to give 9,420, 14,130, 18,840, 28,260 or 32,970 units per mL of freshly prepared solution, which was used on the day of preparation. The pepsin dose response assay was carried out in 50 mL polyethylene screw cap centrifuge tubes (VWR 21008-178).
A 500 mg CP (N × 6.12) equivalent of SBM sample was placed in centrifuge tubes with 50 mg of guar gum and 8.5 mL of 10 mmol/L HCl solution. The tubes were vortexed to evenly mix and saturate the sample with the 10 mmol/L HCl solution. After mixing, 1.5 mL pepsin solution with either 14, 130, 21,195, 28,260, 42,390 or 49,455 units of pepsin were added to 6 replicate tubes plus 3 blank tubes per enzyme concentration. All tubes were vortexed and 0.5 mL sample was taken for electrophoresis, and another 0.5 mL was placed in 20 mL of sodium acetate buffers (pH 6.5) for colorimetric evaluation. The tubes were placed in a shaking water bath (150 strokes/min; 30 mm stroke length) at 41 °C for 30 min. After the gastric phase digestion, 0.5 mL sample was taken for electrophoresis. Another 0.5 mL of sample was placed in 20 mL of sodium acetate buffer for colorimetric evaluation.
The samples for electrophoresis were placed in a boiling water bath for 15 min immediately after collection to denature the pepsin and then samples were centrifuged at 2,140 × g (Beckman Allegra 6 model, Beckman Coulter, Inc. California, USA) for 1 min at 21 °C. An aliquot was taken from the supernatant of all samples and used for electrophoresis. All samples were analyzed in a non-reducing condition using an Agilent 2100 Bioanalyzer system (Agilent Technologies, Lexington, USA) and the Protein 230 Chip assay following the manufacture's protocol.
The samples for colorimetric analysis were vortexed before centrifuging at 2,568 × g for 10 min at 21 °C. A 100 μL aliquot of the sample was diluted with 1,900 μL of Millipore water in disposable glass culture tubes and then 1 mL of ninhydrin reagent was added. A marble was placed on top of the tubes before heat treatment. The cool reaction mixture (approximately 2 mL) was read in 4.5 mL disposable plastic cuvettes (Cat. No. 14,955,129 Fisherbrand) using a Genesys 20 spectrophotometer UV–Vis (Termo Fisher Scientific Inc., Waltham, USA).
2.2.3. Pancreatin dose response assay
The pancreatin used was a liquid bovine enzyme (65,000 trypsin units/mL) from RENCO (New Zealand). The activity of trypsin (30,667 BAEE units/mL), chymotrypsin (2,157 BTEE units/mL) and elastase (7 units/mL) were determined using Sigma EC 3.4.21.4, EC 3.4.21.1 and EC 3.4.21.36 assays, respectively. Six pancreatin levels (1, 3, 5, 6.5, 7.5 and 9 mL) were evaluated in the intestinal phase for the enzyme dose response assay. Six replicate tubes and 3 blank tubes per enzyme level were used during the pancreatin dose response assay.
All samples were digested for 30 min using the selected pepsin concentration identified in the pepsin dose response assay. A 500 μL volume of 4.9 mol/L sodium hydroxide solution was added to each tube immediately after gastric digestion. Sodium acetate buffer (pH 12.5) was added to each tube and the pH was adjusted to 7.5. The selected volume of pancreatin solution was added to the respective tubes to bring the final volume of the tubes up to 26.5 mL. All tubes were vortexed and 0.5 mL sample was taken for electrophoresis. Another 0.5 mL was taken from the tubes for colorimetric evaluation and placed in 10 mL 10 mmol/L HCl solution, the mixture was then vortexed followed by the addition of 10 mL of sodium acetate buffers (pH 6.5). Three marbles were placed in each tube and the tubes were placed in a shaking water bath (150 strokes/min; 30 mm stroke length) at 41 °C for 180 min. During the intestinal digestion phase, a 0.5 mL aliquot was taken for colorimetry assay evaluation at 15, 30, 40, 60, 90, 120, 150 and 180 min. At 180 min of digestion, a 0.5 mL aliquot was taken from each tube for electrophoresis.
2.2.4. In vitro assay validation
The assay intra-variability was evaluated using high protein feed ingredients. The ingredients selected for the validation study were SBM, CGM, CDDGS, PCM, and FM; CA was used as a control because it represents a pure protein source. All samples were ground to pass through a 0.5 mm screen before proximate analysis. The moister content of all meal samples were determined using method 990.03 (AOAC International, 2006). Protein sources were analyzed for N using a Leco nitrogen analyzer (Model 601–500–100, Serial # 3211, Leco Corporation, St. Joseph, MA, USA) according to the combustion method 990.03 (AOAC International, 2006) using 6.25 as the conversion factor to calculate CP. Soybean meal was analyzed for trypsin inhibitor activity following method 22-40 (AOAC International, 2006). All meals were analyzed for protein dispersibility index (PDI) as outlined by Johnson (1970) and protein solubility in 0.2% potassium hydroxide solution (Araba and Dale, 1990). The calcium and magnesium contents of all meal samples were analyzed using inductively coupled plasma optical emission spectrometry (ICP-OES) after total acid digestion with HCl.
A subset of each meal sample was hydrolyzed with 6 mol/L HCL as outlined in the validation of ninhydrin reagent section. The protein content of the samples was calculated as N × 6.25, and then 500 mg CP equivalent of each ground sample were placed in 5 replicate tubes. The samples were digested using the optimum pepsin and pancreatin concentrations identified during the 2 dose response assays. The 0.5 mL aliquots for the colorimetry assay were only collected during the intestinal digestion phase at 0, 15, 30, 40, 60, 90, 120, 150 and 180 min.
2.3. Calculations and statistics
The protein digestibility of the samples was calculated using the OD of the digested sample and the OD of the totally hydrolyzed sample as follows:
where OD = the absorbance at 568 nm.
The absolute percentage of CP digested per minute was calculated using the following rate formula:
where and represent different time points during the 180 min digestion period.
All the protein digestibility data were fitted to the following modified 2 tail compartmental statistical models proposed by Ørskov and McDonald (1979) using the PROC NLIN procedure of SAS 9.4: , where P = CP digested at a specific time point, A = rapidly digested CP fraction, B = slowly digested CP fraction, kd = the rate at which B is digested over time (fractional rate per min). This constant was set to negative since the data represented increasing protein digestion over time, and t = time. The undigested fraction of the proteins UD was calculated as 100 − (A + B) and the potential digestibility (PD) of the protein was calculated as (A + B).
The spectrum scan data were analyzed for the maximum inflection point using the PROC REG procedure. Correlation analysis was performed between calculated digestibility and the models predicted digestibility using PROC CORR, and the means of the kinetic constants were compared using the PROC MIXED procedure of SAS 9.4 with probability of P ≤ 0.05 considered significant. If significant differences were found between means, LSD means statement was used to separate treatment means.
3. Results
3.1. Validation of ninhydrin reagent
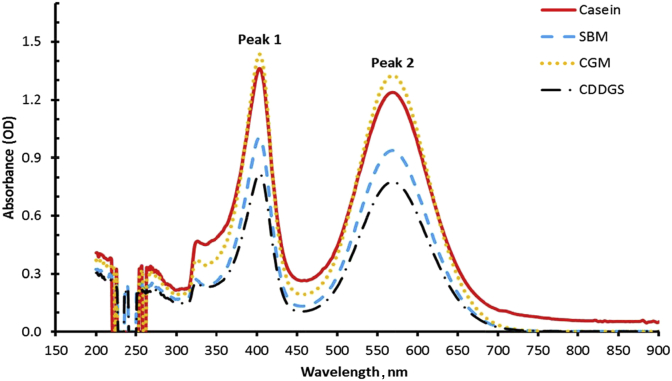
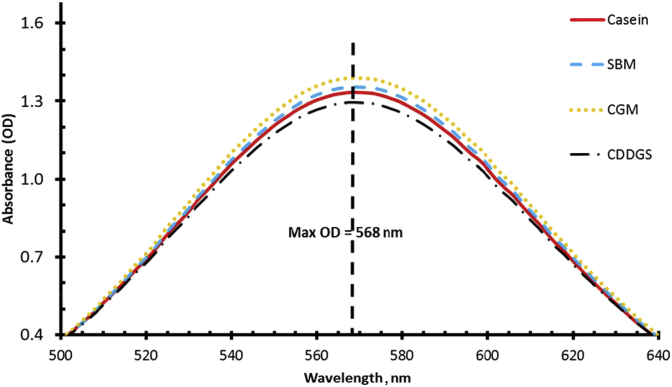
Two major peaks were identified after a full spectrum scan of the reactions between the ninhydrin reagent and the samples as illustrated in Fig. 1. The first peak span was from 300 to 450 nm while the second peak was from 500 to 650 nm. The reaction was monitored for 30 min during which time there was no change in the OD reading of the second peak. However, the first peak OD decreased with time and by 30 min it was no longer present. Evaluation of the second peak data from 500 to 630 nm (Fig. 2) using the PROC REG function of SAS 9.4 revealed that the inflection point for all the samples was 568.
Fig. 1.
Absorbance spectrum from 150 to 950 nm for ninhydrin reagent reaction with casein, soybean meal (SBM), corn gluten meal (CGM), and corn distiller's dried grain with solubles (CDDGS) hydrolyzed with 6 mol/L HCl at 100 °C for 24 h.
Fig. 2.
Maximum absorbance spectrum of the ninhydrin reagent reaction with casein, soybean meal (SBM), corn gluten meal (CGM), and corn distillers dried grain with solubles (CDDGS) with 6 mol/L HCl at 100 °C for 24 h.
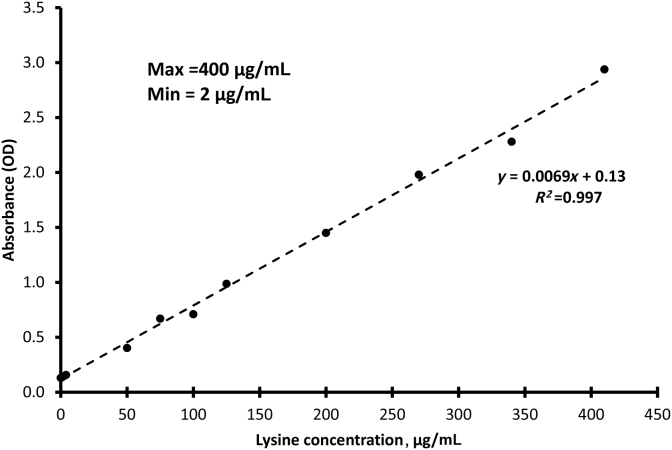
The relationship of the ninhydrin reaction with the lysine standard was used to determine the detection limits of the reaction (Fig. 3). Below 2 μg/mL lysine, the absorbance values did not show a linear trend, and above 400 μg/mL, the detector of the spectrophotometer was saturated. The R2 value of the point between the lysine standard concentrations and OD values obtained at each concentration was 0.97 for the range from 2 to 400 μg/mL of lysine. This inferred that the OD reading of the sample was a good predictor of the amount of free amino and carboxyl group present in the reaction.
Fig. 3.
Relationship between the concentrations of lysine standard and absorbance values when reacted with ninhydrin reagent.
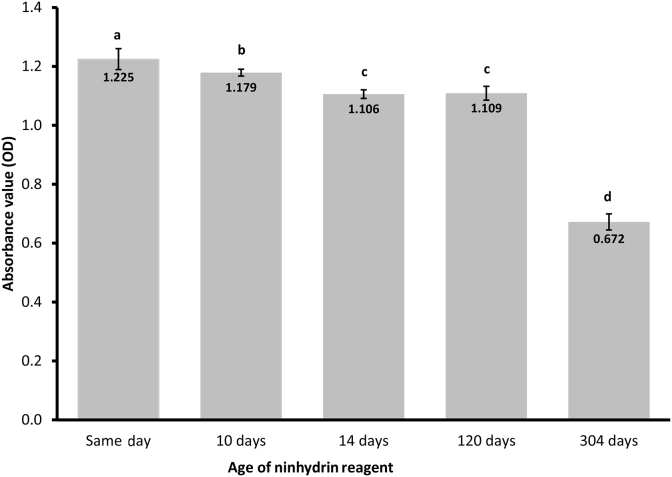
Aging the ninhydrin reagent in dark bottles shielded from light, kept the reagent relatively stable up to 120 days (Fig. 4). It took 14 d for the reagent to stabilize and during which time there was a 0.119 OD reduction in the absorbance reading.
Fig. 4.
Effects of ninhydrin reagent storage time on the absorbance reading of hydrolyzed casein. a–d Means ± standard deviation with different letters are significantly different (P < 0.05) and n = 6.
3.2. Enzyme dose response assay
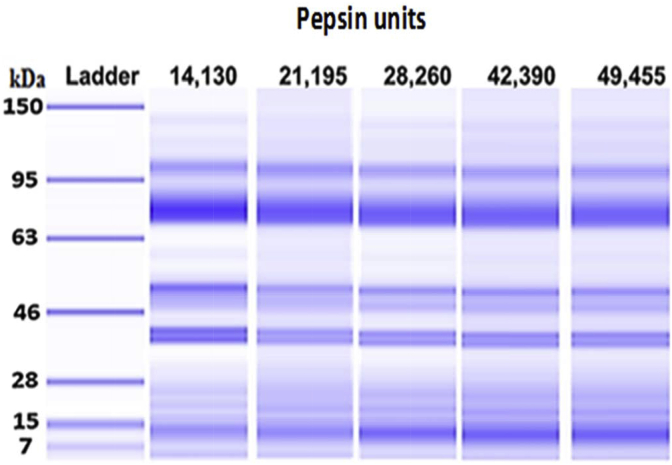
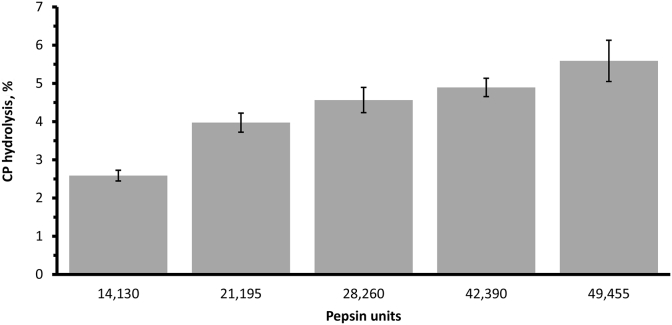
Increasing the concentration of pepsin from 14,130 to 49,455 units reduced the polypeptides between 46 and 28, 63 to 46 and 95 to 63 kDa according to the ladder standards (Fig. 5). This reduction resulted in an increase in the concentration of peptides between 12 and 7 kDa and it confirmed that hydrolysis had taken place. The colorimetry assay data presented in Fig. 6 had a similar trend to what was observed for the peptide concentration between 7 and 12 kDa. By dividing the units of pepsin used in the assay by the percentage CP hydrolyzed (Fig. 6), the CP hydrolyzed per unit of enzyme can be calculated. This resulted in 0.188%, 0.189%, 0.163%, 0.116% and 0.114% hydrolyzed CP per unit of enzyme for the 5 enzyme concentrations, respectively.
Fig. 5.
Effects of pepsin concentration (units) on the molecular weight distribution of peptide from soybean meal digested for 30 min at 41 °C. The ladder represents protein and peptide fragments with molecule weights measured in kilo-Daltons (kDa). Each mean represents 6 replicates per treatment.
Fig. 6.
Effects of pepsin concentration (units) on CP hydrolysis (%) from soybean meal digested for 30 min at 41 °C. Each mean represents 6 replicates per treatment.
The pepsin concentration of an in vitro assay can be selected based on a number of criteria. In this study, taking the cost of the pepsin into consideration, having a minimum of 4% CP hydrolysis in the gastric phase and the ability of the selected pepsin concentration to produce a typical digestion curve (Fig. 7) in the intestinal phase were the basic criteria for this assay. The 28,260 units of pepsin were selected because the units of pepsin below that level did not achieve 4% CP hydrolysis during the 30 min of the assay. Concentration above 28,260 units gave a substantial reduction in the percentage of CP hydrolysis per unit of enzyme, which indicated that those levels of enzyme might not be economical since the concentrations were almost doubled. In a preliminary study, the 4 lowest pepsin concentrations were used to digest a sample of SBM, then a standard 7 mL volume of pancreatin was used for the intestinal phase of the digestion. The digestion of the samples was monitored over a 3-h period. The 28,260 units of pepsin gave a time-dependent digestion curve, which we assumed to be the case in vivo, while the 42,390 units curve was very steep (data not shown). Therefore, the 28,260 units of pepsin was selected as the standard pepsin concentration because the pepsin efficiency measured as percentage CP hydrolyzed per unit of enzyme was drastically reduced after 28,260 units of pepsin. The shape of the digestion curved from the preliminary data gave a gradual digestion with time and the extent of hydrolysis for the SBM by 28,260 units were above 4%.
Fig. 7.
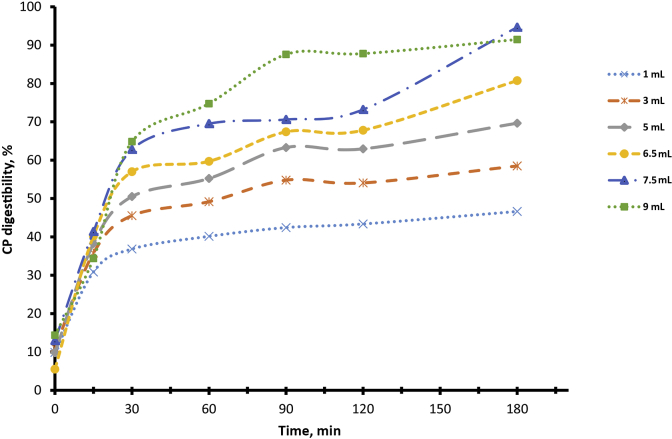
Effects of pancreatin concentrations (1 mL = 30,667 BAEE units of trypsin; 2,157 BTEE units of chymotrypsin, and 7 units of elastase) on the digestibility of soybean meal CP over 180 min of the intestinal phase at 41 °C after predigesting with 28,260 units of pepsin. Each time point represents 6 replicates per treatment.
The criteria for the selection of the pancreatin was based on the extent of hydrolysis which mimic that of in vivo SBM CP digestion by poultry. The 7.5 and 9 mL pancreatin gave the highest degree of hydrolysis which was above 90% at the end of the intestinal incubation time (180 min). Both the 7.5 and 9 mL pancreatin treatments also had the steepest digestion curve over time. The 1, 3 and 5 mL volumes were only able to hydrolyze less than 60% of the CP in the SBM samples after 180 min incubation. The digestion curve from the 6.5 mL volume of pancreatin was more gradual over time (Fig. 7). Approximately 81% of the CP in the SBM sample was hydrolyzed by the 6.5 mL volume of pancreatin at the end of the 180 min intestinal digestion phase.
A preliminary literature search suggested that SBM samples from 4 different countries had an average in vivo CP digestibility of 82% (Ravindran et al., 2014). The percentage of CP hydrolyzed by the 6.5 mL of pancreatin was similar to the 82%, and the digestion curve was more gradual over time, which is assumed to be the case for protein digestion in vivo. The shape of the curve also provided the opportunity to obtained relevant digestion kinetic data from the assay. Based on the criteria listed above, the 6.5 mL of pancreatin (199,335.5 BAEE units of trypsin; 14,020.5 BTEE units chymotrypsin, and 445.5 units elastase) was selected as the optimum enzyme dosage for the intestinal phase of the assay.
3.3. In vitro assay validation
The composition and chemical properties of the feed ingredients used in this assay are shown in Table 1. These data are presented in order to give the reader a clearer overview of the status of the ingredients that were used. Ingredient composition (mineral, CP and DM contents) was similar to values previously reported for samples used as poultry feed ingredients (National Research Council, 1994).
Table 1.
Feed ingredient composition and chemical properties as fed (%).
| Item | Meals |
|||||
|---|---|---|---|---|---|---|
| CA | FM | PCM | SBM | CGM | CDDGS | |
| Dry mater | 98.0 | 89.2 | 95.4 | 89.2 | 90.6 | 97.7 |
| Crude protein | 90.2 | 67.2 | 62.0 | 45.3 | 62.1 | 28.3 |
| Calcium | ND | 3.54 | 4.32 | 0.50 | 0.10 | 0.06 |
| Magnesium | ND | 0.33 | 0.22 | 0.29 | 0.05 | 0.34 |
| Trypsin inhibitor, TIU/g | ND | ND | ND | 4,335 | ND | ND |
| Protein dispersability index | ND | 32 | 25 | 15 | 15 | 2 |
| Protein solubility | ND | 45 | 39 | 78 | 24 | 28 |
CA = casein; FM = fish meal; PCM = porcine meal; SBM = soybean meal; CGM = corn gluten meal; CDDGS = corn distillers' dried grain with solubles; ND = not determined.
The rapidly digested CP fraction (A) was higher (P ≤ 0.05) for FM and PCM than SBM and CDDGS, while other protein fractions were intermediate (Table 2). The coefficient of variation (CV) for fraction (A) of the samples was numerically higher for CDDGS, CGM and SBM than that for FM, PCM and CA. The CV for fraction (B) which represents the proportion of the proteins digested over time was higher (P ≤ 0.05) for CA, FM, SBM and CGM than that for PCM, while CDDGS was similar to all samples evaluated. The CV for fraction (B) was higher for CDDGS when compared to the other ingredients.
Table 2.
Digestion kinetic constant of meals generated with the in vitro digestion data1.
| Item | A2, % |
B2, % |
Kd2, h−1 |
Adr2, %/min |
UD2, % |
PD2, % |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | CV | Mean | CV | Mean | CV | Mean | CV | Mean | CV | Mean | CV | |
| CA | 16.9a | 16 | 71.9a | 5 | 0.018b | 18 | 0.443b | 3.5 | 13.0b | 27 | 87.0a | 5 |
| FM | 13.2ab | 9 | 70.6a | 4 | 0.024a | 20 | 0.463b | 4.0 | 16.1b | 9 | 83.9a | 2 |
| PCM | 13.9ab | 11 | 55.2b | 7 | 0.013bc | 20 | 0.340c | 3.8 | 30.9a | 23 | 69.1b | 10 |
| SBM | 6.5c | 30 | 78.8a | 5 | 0.023a | 13 | 0.507a | 5.0 | 14.6b | 15 | 85.4a | 5 |
| CGM | 10.3bc | 20 | 72.7a | 7 | 0.013bc | 20 | 0.433b | 5.0 | 17.1b | 27 | 82.9a | 5 |
| CDDGS | 8.1c | 24 | 66.8ab | 12 | 0.009c | 12 | 0.346c | 4.1 | 25.1ab | 31 | 74.9ab | 12 |
| SEM | 1.2 | 3.6 | 0.001 | 0.008 | 3.5 | 3.5 | ||||||
| ANOVA | ||||||||||||
| P-value | <0.0001 | 0.0023 | <0.0001 | <0.0001 | 0.0039 | 0.0039 | ||||||
CV = coefficient of variation; CA = casein; FM = fish meal; PCM = porcine meal; SBM = soybean meal; CGM = corn gluten meal; CDDGS = corn distillers' grain with solubles.
a–c Means within a column with different superscripts are significantly different (P < 0.05).
Data were fitted to the model proposed by Ørskov and McDonald (1979): A + B (1 − e-kd×t).
A = rapidly digested CP fraction; B = slowly digested CP fraction; kd = the rate at which the B fraction is digested over time; UD = undigested fraction calculate as 100 − (A + B); PD = potential digestible fraction calculated as A + B; adr = absolute digestion rate (percentage of protein digested per min from 0 to 180 min); SEM = standard error of means where n = 6.
The SBM and FM samples had the highest (P ≤ 0.05) fractional digestion rate (rate at which faction [B] was digested over time; kd) compared to all other samples. The CDDGS had a higher (P ≤ 0.05) fractional digestion rate compared to CA, but all other samples were intermediate. The CV for the fractional digestion rate was the lowest for CDDGS and SBM. The trend observed for the absolute digestion rate (adr), which was calculated by dividing the extent of digestion by the total digestion time, was different from that of the fractional digestion rate. The SBM had a higher (P ≤ 0.05) absolute digestion rate compared to all other samples. The absolute digestion rates for PCM and CDDGS were similar, but lower (P ≤ 0.05) than those of all the other ingredients. The CV for the absolute digestion rate of the samples was similar.
The undigested protein fraction was calculated as the difference between the total protein content of the sample and the total protein digested. There was more (P ≤ 0.05) undigested protein in the PCM sample than all other samples except for the CDDGS which was intermediate. Numerically, lower CV was seen for the undigested protein of FM and SBM compared to the other samples.
The potential digestibility (PD) of samples equals the sum of fraction (A and B) and values were higher (P ≤ 0.05) for Ca, FM, SBM and CGM than those for PCM. The value for CDDGS was intermediate and not different from any of the protein sources tested. The coefficients of variation for the potential digestibility of the samples were generally low, but PCM and CDDGS values were twice those of the other values. The actual CP digestibility values calculated using the OD values of the samples at 180 min of intestinal digestion expressed as a percentage of the OD values after 24 h acid hydrolysis of the samples ranged from 68% to 90%. After modeling the data, the predicted CP digestibility of the samples ranged from 60% to 84%. The correlation R2 value between meal actual CP digestibility and the model's predicted CP digestibility were above 0.9 for all the meals evaluated (Table 3, Table 4).
Table 3.
Actual and predicted digestibly coefficient of meals over 180 min.1
| Time, min | Actual coefficient |
Predicted coefficient 2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA | SBM | FM | CDDGS | CGM | PCM | CA | SBM | FM | CDDGS | CGM | PCM | |
| 0 | 12 ±3.1 | 5 ± 2.9 | 9 ± 3.1 | 7 ± 1.3 | 4 ± 1.8 | 7 ± 2.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15 | 27 ± 6.5 | 31 ± 6.1 | 30 ± 5.1 | 15 ± 6.4 | 27 ± 2.5 | 28 ± 2.6 | 19 | 25 | 25 | 9 | 15 | 12 |
| 30 | 50 ± 2.7 | 52 ± 1.6 | 54 ± 3.0 | 29 ± 1.2 | 38 ± 3.0 | 34 ± 3.3 | 33 | 43 | 43 | 18 | 27 | 22 |
| 45 | 56 ± 4.0 | 59 ± 2.3 | 58 ± 3.1 | 31 ± 1.9 | 43 ± 5.0 | 38 ± 2.8 | 45 | 55 | 55 | 25 | 37 | 31 |
| 60 | 67 ± 2.4 | 67 ± 2.5 | 64 ± 8.6 | 37 ± 6.8 | 49 ± 6.9 | 45 ± 3.0 | 54 | 64 | 64 | 31 | 45 | 37 |
| 90 | 69 ± 7.1 | 67 ± 3.9 | 66 ± 3.7 | 38 ± 6.8 | 50 ± 3.3 | 45 ± 2.2 | 66 | 75 | 74 | 42 | 57 | 48 |
| 120 | 69 ± 2.1 | 71 ± 1.3 | 71 ± 1.8 | 49 ± 3.0 | 62 ± 1.8 | 48 ± 2.9 | 74 | 80 | 79 | 49 | 66 | 55 |
| 180 | 93 ± 3.2 | 95 ± 3.9 | 93 ± 3.2 | 70 ± 1.8 | 82 ± 3.0 | 68 ± 3.7 | 82 | 84 | 83 | 60 | 75 | 62 |
CA = casein; SBM = soybean meal; FM = fish meal; CDDGS = corn distillers' dried grain with solubles; CGM = corn gluten meal; PCM = porcine meal.
Means ± standard error of means where n = 6.
Model = A + B (1 − e-kd×t) where A, B and kd are (CA = 16.93, 71.9 and 0.018; SBM = 6.5, 78.8 and 0.023; FM = 13.2, 70.6 and 0.024; CDDGS = 8.1, 66.8 and 0.009; CGM = 10.3, 72.7 and 0.013; PM = 13.9, 55.2 and 0.013), respectively.
Table 4.
Pearson correlation coefficients between model predicted and actual digestibility of meals over 180 min of digestion.
| Model predicted digestibility | Actual in vitro digestibility |
|||||
|---|---|---|---|---|---|---|
| CA | SBM | FM | CDDGS | CGM | PM | |
| CA | 0.97 <0.011 |
|||||
| SBM | 0.97 <0.011 |
|||||
| FM | 0.97 <0.011 |
|||||
| CDDGS | 0.97 <0.011 |
|||||
| CGM | 0.97 <0.011 |
|||||
| PM | 0.95 <0.011 |
|||||
CA = casein; SBM = soybean meal; FM = fish meal; CDDGS = corn distillers' dried grain with solubles; CGM = corn gluten meal; PCM = porcine meal.
P-value.
4. Discussion
4.1. Colorimetry assay
The oxidative deamination of an amino acid to form Ruhemann's purple is a complex reaction with a wide absorbance spectrum (Bottom et al., 1978). The nitrogen from the amino acids is incorporated in the bluish-violet pigment after reacting with ninhydrin in the presence of tin (II) chloride dehydrate as a reducing agent (Bottom et al., 1978). The full spectrum scan of this reaction reveals that all the samples tested had maximum OD reading at 568 nm, so this OD was chosen as the OD for the colorimetry assay. Identifying this OD provides an opportunity for the assay to increase its sensitivity and precision in detecting the amino and carboxyl end of peptide bonds as they are broken during hydrolysis. It is possible that the first peak identified during the spectrum scan was as a result of intermediate products of the reaction.
An ethylene glycol sodium acetate base was chosen for the ninhydrin reagent because it provided a stable reagent and it is easy to make. The reagent does not require a nitrogen atmosphere and similarly it is not required for storage, unlike dimethyl sulfoxide base reagents (Moore, 1968). The ninhydrin reagent is susceptible to light during storage, and in this study, it took up to 14 days for the reagent to stabilize and provide a constant OD reading. If the reagent is stored in a dark sealed bottle, it can be stored up to 120 d and still give good OD readings. Even though there was a reduction in the OD readings of the reagent over time, this would only be of significance if OD values from different digestion runs were being compared directly. The reagent is very sensitive in detecting α amino acids, and ammonia, so proper precaution must be taken to prevent amino acid or ammonia contamination of solutions used to make the reagent and buffers.
Due to the sensitivity of the reagent, the relationship between the concentrations of free α amino and carboxyl group in solution with the OD reading is linear from 2 to 400 μg. The maximum concentration from that range was at the upper limit of the detector in the spectrophotometer used in the study. This close relationship makes it possible to track changes in the hydrolysis of the CP samples over time as more free α amino and carboxyl groups are exposed. In theory, the OD intensity is directly proportional to the degree of hydrolysis, which has occurred as seen in Fig. 3. If the OD from the total hydrolysis of an ingredient is known, the degree of hydrolysis can be calculated using the OD values. The very low detection limit of the reagent means any small change in the concentration of amino acids or available amino acids, and carboxyl side group will induce a large change in OD reading. This can produce large variation in the reading of a sample if pipetting is not accurate; therefore, it is advisable to read samples in triplicate when using the reagent as outlined in this colorimetry assay. Proper controls and blank samples should be run with every batch of samples that goes into the water bath in order to generate a correction factor for any change in temperature of the water bath during the assay.
4.2. Enzyme dose response assay
One of the most important elements of an enzymatic in vitro assay is the enzyme to substrate ratio at a known enzyme activity (Boisen and Eggum, 1991). The pepsin dose response assay suggested that the greatest change in the degree of hydrolysis over the 30 min was between 14,130 and 28,260 units of pepsin to 500 mg of CP. When the pepsin concentration increased above 28,260 units, the equivalent change in the degree of hydrolysis per unit of enzyme addition was reduced. Using a pepsin concentration which maximizes the hydrolysis achieved per unit of enzyme can help to develop the most economical assay.
Electrophoresis data of the gastric phase sample presented in Fig. 5 suggests that the 2 tertiary structures normally seen in proteins extracted from soybean seed were subdivided into 5 major peptides and many smaller groups in the meal. The major shift in these peptides of the meal due to pepsin concentration was seen at the lower molecular weight 11S globulins 38 to 39 kDa compared to the 7S fractions 62 to 90 kDa. Similar results were observed by Yang et al. (2016) after peptic digestion of SBM isolated CP. The authors suggested that 11S glycinin was more susceptible to pepsin digestion because of its lower surface hydrophobicity and less β-sheet secondary structures (Yang et al., 2016). Nevertheless, electrophoresis and the colorimetry assay both show that hydrolysis had taken place after pepsin digestion of the SBM.
The digestion kinetic data obtained is dependent to a large extent on the shape of the digestion curve during the intestinal phase. The pancreatin concentration which produced a curve fitting the model proposed by Ørskov and McDonald (1979) and gave a value approximating in vivo CP digestion for SBM at the end of the digestion period were considered to be key criteria for the pancreatin concentration selection. Colorimetric testing of samples from 7.5 to 9 mL pancreatin after 180 min gave OD values which were higher than the OD values of SBM sample totally hydrolyzed (Data not shown). This suggested that at those higher concentrations of pancreatin, there might have been auto-hydrolysis of enzyme after 180 min of digestion. Even after 240 min of intestinal digestion, the 6.5 mL pancreatin did not produce OD values which were higher than those of the total hydrolysis sample. The 1, 3 and 5 mL enzyme concentrations gave final digestibility values below the average of 82% in vivo digestibility for SBM (Ravindran et al., 2014). The 6.5 mL pancreatin was selected as the enzyme concentration for the intestinal phase, based on the shape of its digestion curve, the extent of digestion mimicking SBM in vivo digestion and the stability of the enzyme after 180 min during the intestinal digestion phase.
4.3. In vitro assay validation
Based on fractional digestion rates (kd) values, SBM and FM can be classified as rapidly digested protein sources and CDDGS slowly digested. The kd value represented the rate at which faction (B) of the proteins were digested over time assuming that the process followed the first order of kinetics. The absolute digestion rate (adr) is a different kind of measurement which assumed that the rate of digestion is linear. The data presented in Fig. 7 suggested that the rate at which the protein was digested followed the first order of kinetics which is typical of most biological reactions and therefore is a true representation of that process.
The animal based protein ingredients tend to have higher fraction (A). It is possible that this difference relates to a higher proportion of peptides or free amino acids in animal than plant based ingredients. Another reason for the difference between fraction (A) of plant and animal ingredients might relate to the nature of the proteins in these meals. Plants tend to store protein in vacuoles in cells which are often surrounded by a fiber matrix (Staswick, 1994), while animal proteins do not have a fiber matrix associated with the protein and there are also free amino acids and peptides present in extracellular space of animal tissue. These factors could have made the animal based proteins more susceptible to enzymatic hydrolysis than the plant proteins. Predicting fraction (A) produced higher variability in the plant based ingredients compared to animal based ingredients, but the reason for this is still to be determined.
The potential digestibility was quite similar for all the ingredients except for PCM, which was lower than all the other samples. It is possible that the PCM meal has a higher elastin and collagen content, which would require more elastase to hydrolyze this meal than the 445.5 units present in pancreatin that was used. Porcine meal also tends to have high levels of arginine (Wang and Parsons, 1998), which could mean that more carboxypeptidase B is needed to break arginine bonds present in small peptides. Most likely, the processing conditions during the rendering process could have damaged the PCM proteins, which makes them more resistant to digestion (Wang and Parsons, 1998).
The CDDGS potential digestibility values were the second lowest of all ingredients evaluated, but they were in the range for in vivo values previously reported for CDDGS in broilers (Adedokun et al., 2015). Corn products like CDDGS are known to contain zein which is a prolamine that is insoluble in water and resistant to most proteolytic enzymes except alcalase (Shukla and Cheryan, 2001). The level of zein present in the protein fraction could reduce the protein digestibility of CDDGS during gastric and pancreatic digestion. Apart from the zein content of the CDDGS, the drying process used during the postharvest of corn has been shown to reduce its protein digestible (Barrier-Guillot et al., 1993).
4.4. Assay advantages and disadvantages
The in vitro assay presented in this work for measuring CP digestibility is not the first of its kind. Other 2 stages in vitro methods have been previously described for measuring CP digestibility in poultry (Clunies and Leeson, 1984, Ravindran and Bryden, 1999). The main problems with those assays lie in the length of the 4 h gastric digestion period, which is not representative of poultry in vivo digestion, the use of just a single enzyme and the lack of information pertaining to the activity of the major enzymes in the pancreatin used. All in vitro assays will suffer from various degrees of uncertainty due to the complexity of simulating the mechanism which are involved in the digestion process of proteins. However, in vitro assays based on enzymatic digestion can provide meaningful characterization of feed ingredients (Ravindran and Bryden, 1999).
One of the major disadvantages of the current assay is that it requires a minimum of 3 people to collect the samples during the intestinal phase. The sequential timing of sample collection is affected by the length of time required for sample collection and processing. For example, the lowest sample interval that was achieved in the assay was 15 min with 4 people conducting the assay with 30 digestion tubes. Due to the sensitivity of the ninhydrin reagent, proper pipetting skills are needed, and all buffers and solution used in the assay must be free of ammonia, peptide, proteins and ammo acids. During the color development stage of the assay, the water bath should always be at boiling to obtain consistent sample color development.
Most in vitro digestion methods suffer from some degrees of imprecision. The assay presented in this study has the following advantages, many samples can be analyzed in a short time frame, it is relatively inexpensive and easy to perform in a basic animal nutrition lab, and no special training is needed to use required equipment. The assay can be easily transferred to an automated platform for running the entire assay. This would significantly reduce the number of personnel needed to collect the kinetic data. The level of precision between sample collection time intervals would be increased, and the timing interval could also be reduced below 15 min.
The digestibility assay was able to generate kinetic data for all the ingredients tested because their digestion curves over time all followed the first order kinetics plot. The model developed from the digestion constant was able to predict the actual in vitro digestion of the meals over time with a high degree of accuracy. However, it should be noted that the digestion constants generated for each meal only represent that specific sample and may not predict the response of other samples of the same ingredient. Based on the correlation coefficients in Table 2, it is safe to say that the constants generated from the model reflected the digestion characteristic of those samples tested.
4.5. Implication on future research
This study provides a basic assay, which can be used to generate kinetic data for high protein poultry feed ingredients in a short time frame. It is well known that protein digestion rate modulates tissue protein synthesis and deposition, but this process is still unknown in poultry due to the lack of kinetic data for high protein ingredients. Data from this assay can be used to develop diets for studying the metabolic response of poultry to specific ingredient digestion characteristics. More research is needed to test the assay inter-variability and to develop more precise digestion constants for each high protein ingredient, which would be representative of the ingredient and not the sample.
5. Conclusions
A multi-enzymatic in vitro protein digestion technique mimicking the chicken digestive tract was defined and validated. The effectiveness of the in vitro digestion technique was tested on a variety of high protein ingredients. The in vitro protein digestibility assay predicted the rapidly, slowly and undigested protein fraction of ingredients, as well as the rate and extent of digestion of the proteins. The in vitro assay described in this study can be used to study the digestion kinetic of high protein ingredients fed to poultry.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to acknowledge the National Science and Engineering Research Council (NSERC), Industrial Research Chair Program for financial support for this project (Grant No. IRCSA 452664-12). Funding for this program was derived from Aviagen, Canadian Poultry Research Council, Chicken Farmers of Saskatchewan, NSERC, Ontario Poultry Industry Council, Prairie Pride Natural Foods Ltd., Saskatchewan Egg Producers, Saskatchewan Hatching Egg Producers, Saskatchewan Turkey Producers, Sofina Foods Inc. and the University of Saskatchewan.
Footnotes
Presented at Annual Poultry Science Meeting, Louisiana, USA, July 12, 2016.
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Contributor Information
Dervan D.S.L. Bryan, Email: dervan.bryan@usask.ca.
Henry L. Classen, Email: hank.classen@usask.ca.
References
- Adedokun S.A., Jaynes P., Payne R.L., Applegate T.J. Standardized ileal amino acid digestibility of corn, corn distillers' dried grains with solubles, wheat middlings, and bakery by-products in broilers and laying hens. Poult Sci. 2015;94:2480–2487. doi: 10.3382/ps/pev226. [DOI] [PubMed] [Google Scholar]
- AOAC International . 18th ed. AOAC Int.; Gaithersburg, MD: 2006. Official methods of analysis. [Google Scholar]
- Araba M., Dale N.M. Evaluation of protein solubility as an indicator of over processing soybean meal. Poult Sci. 1990;69:76–83. [Google Scholar]
- Barrier-Guillot B., Jondreville C., Chagneau A.M., Larbier M., Leuillet M. Effect of heat drying temperature on the nutritive value of corn in chickens and pigs. Anim Feed Sci Technol. 1993;41:149–159. [Google Scholar]
- Boila R.J., Erfle J.D., Sauer F.D. Evaluation of the two-stage technique for the in vitro estimation of the dry matter digestibility of corn silage. Can J Anim Sci. 1980;60:367–378. [Google Scholar]
- Boirie Y., Dangin M., Gachon P., Vasson M.-P., Maubois J.-L., Beaufrère B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci. 1997;94:14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisen S., Eggum B.O. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr Res Rev. 1991;4:141–162. doi: 10.1079/NRR19910012. [DOI] [PubMed] [Google Scholar]
- Bottom C.B., Hanna S.S., Siehr D.J. Mechanism of the ninhydrin reaction. Biochem Educ. 1978;6:4–5. [Google Scholar]
- Clunies M., Leeson S. In vitro estimation of dry matter and crude protein digestibility. Poult Sci. 1984;63:89–96. [Google Scholar]
- Dangin M., Boirie Y., Garcia-Rodenas C., Gachon P., Fauquant J., Callier P. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280:E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- Fuller M.F. C.A.B. International; Wallingford, England: 1991. In vitro digestion for pigs and poultry. [Google Scholar]
- Hughes R.J. Relationship between digesta transit time and apparent metabolisable energy value of wheat in chickens. Br Poult Sci. 2008;49:716–720. doi: 10.1080/00071660802449145. [DOI] [PubMed] [Google Scholar]
- Johnson D. Functional properties of oilseed proteins. J Am Oil Chem Soc. 1970;47:402–407. [Google Scholar]
- Koopman R., Crombach N., Gijsen A.P., Walrand S., Fauquant J., Kies A.K. Ingestion of a protein hydrolysate is accompanied by an accelerated in vivo digestion and absorption rate when compared with its intact protein. Am J Clin Nutr. 2009;90:106–115. doi: 10.3945/ajcn.2009.27474. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968;243:6281–6283. [PubMed] [Google Scholar]
- National Research Council . 9 rev ed. NRC, National Academy Press; Washington, DC: 1994. Nutrient requirements of poultry. [Google Scholar]
- Ørskov E.R., McDonald I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J Agric Sci. 1979;92:499–503. [Google Scholar]
- Ravindran V., Abdollahi M.R., Bootwalla S.M. Nutrient analysis, metabolizable energy, and digestible amino acids of soybean meals of different origins for broilers. Poult Sci. 2014;93:2567–2577. doi: 10.3382/ps.2014-04068. [DOI] [PubMed] [Google Scholar]
- Ravindran V., Bryden W.L. Amino acid availability in poultry—in vitro and in vivo measurements. Aust J Agric Res. 1999;50:889–908. [Google Scholar]
- Shukla R., Cheryan M. Zein: the industrial protein from corn. Ind Crop Prod. 2001;13:171–192. [Google Scholar]
- Sklan D., Hurwitz S. Protein digestion and absorption in young chicks and turkeys. J Nutr. 1980;110:139–144. doi: 10.1093/jn/110.1.139. [DOI] [PubMed] [Google Scholar]
- Staswick P.E. Storage proteins of vegetative plant tissues. Annu Rev Plant Biol. 1994;45:303–322. [Google Scholar]
- Svihus B., Hetland H., Choct M., Sundby F. Passage rate through the anterior digestive tract of broiler chickens fed on diets with ground and whole wheat. Br Poult Sci. 2002;43:662–668. doi: 10.1080/0007166021000025037. [DOI] [PubMed] [Google Scholar]
- USDA . U S Dep Agric Foreign Agric Serv; 2016. Major protein meals: world supply and distribution.https://apps.fas.usda.gov/psdonline/app/index.html#/app/downloads [Google Scholar]
- Wang X., Parsons C.M. Effect of raw material source, processing systems, and processing temperatures on amino acid digestibility of meat and bone meals. Poult Sci. 1998;77:834–841. doi: 10.1093/ps/77.6.834. [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang Z., Wang R., Sui X., Qi B., Han F. Secondary structure and subunit composition of soy protein in vitro digested by pepsin and its relation with digestibility. BioMed Res Int. 2016;2016:1–11. doi: 10.1155/2016/5498639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 20051. Poult Sci. 2014;93:2970–2982. doi: 10.3382/ps.2014-04291. [DOI] [PMC free article] [PubMed] [Google Scholar]