Abstract
Background
Hepatocyte is particularly vulnerable to apoptosis, a hallmark of many liver diseases. Although pro-apoptotic mechanisms have been extensively explored, less is known about the hepatocyte-specific anti-apoptotic molecular events and it lacks effective approach to combat hepatocyte apoptosis. We investigated the anti-apoptotic effect and mechanism of farnesoid X receptor (FXR), and strategies of how to target FXR for inhibiting apoptosis implicated in liver fibrosis.
Methods
Sensitivity to apoptosis was compared between wild type and Fxr−/− mice and in cultured cells. Cell-based and cell-free assays were employed to identify the binding protein of FXR and to uncover the mechanism of its anti-apoptotic effect. Overexpression of FXR by adenovirus-FXR was employed to determine its anti-fibrotic effect in CCl4-treated mice. Specimens from fibrotic patients were collected to validate the relevance of FXR on apoptosis/fibrosis.
Findings
FXR deficiency sensitizes hepatocytes to death receptors (DRs)-engaged apoptosis. FXR overexpression, but not FXR ligands, inhibits apoptosis both in vitro and in vivo. Apoptotic stimuli lead to drastic reduction of FXR protein levels, a prerequisite for DRs-engaged apoptosis. Mechanistically, FXR interacts with caspase 8 (CASP8) in the cytoplasm, thus preventing the formation of death-inducing signaling complex (DISC) and activation of CASP8. Adenovirus-FXR transfection impedes liver fibrosis in CCl4-treated mice. Specimens from fibrotic patients are characterized with reduced FXR expression and compromised FXR/CASP8 colocalization.
Interpretation
FXR represents an intrinsic apoptosis inhibitor in hepatocytes and can be targeted via restoring its expression or strengthening FXR/CASP8 interaction for inhibiting hepatocytes apoptosis in liver fibrosis.
Fund
National Natural Science Foundation of China.
Keywords: Apoptosis, Liver fibrosis, FXR, Caspase 8, Transactivation independent
Highlights
-
•
FXR physically interacts with CASP8 in cytoplasm.
-
•
FXR inhibits death receptors-engaged apoptosis independent of transactivation.
-
•
Reduction of cytosolic FXR is a prerequisite initiating apoptosis cascade.
-
•
Forced overexpression of FXR impedes liver fibrosis.
Research in context.
Evidence before this study
Hepatocytes apoptosis represents a hallmark of the pathogenesis of many liver diseases, and inhibition of hepatocellular apoptosis has been suggested be to a plausible treatment therapy for liver diseases.
As a nuclear transcription receptor, ligands-bound FXR translocates into the nucleus to elicit its canonical role on gene transcription.
FXR plays pivotal roles in maintaining homeostasis of bile acids, lipids and glucose, thus it is generally regarded as a therapeutic target for metabolic diseases.
Added value of this study
Cytosolic FXR is an intrinsic apoptosis inhibitor via physically interacting with CASP8, thus inhibiting DRs-engaged apoptosis.
Reduction of cytosolic FXR is a prerequisite initiating apoptosis cascade. Forced overexpression of FXR, but not FXR agonists, impedes hepatocyte apoptosis and liver fibrosis.
Implications of all the available evidence
Restoring the expression of FXR via preventing its degradation as well as strengthening FXR/CASP8 interaction would be attractive strategies for the treatment of liver fibrosis.
Alt-text: Unlabelled Box
1. Introduction
The liver is an organ of immense complexity and functionally indispensable for its essential roles in controlling endogenous metabolic homeostasis, xenobiotic metabolism and innate immunity. Due to its unique function and environment, hepatocyte, the predominant liver cell, is continuously exposed to high levels of toxic endobiotics, xenobiotics, viruses, and inflammatory triggers [1,2]. To maintain homeostasis, the liver has developed a sophisticated system ensuring efficient removal of damaged or virus-infected hepatocytes mainly via death receptors (DRs)-engaged apoptosis. However, excessive apoptosis and massive loss of hepatocytes may result in irreversible liver damage. Indeed, accumulating evidence indicate that excessive hepatocytes apoptosis represents a hallmark of the pathogenesis of many liver diseases [3,4]. Enhanced hepatocytes apoptosis amplifies inflammatory damage and promotes the development of fibrosis and ultimately liver cancer [5,6]. Thus, inhibition of hepatocellular apoptosis has been suggested to be a plausible treatment therapy for liver injury, especially liver fibrosis.
In contrast to the extensive knowledge in signals triggering cell death, the molecular events underlying how hepatocytes survive from such a hostile environment remain poorly understood. To combat against cell death from various damaging factors, the liver must have developed an efficient protective system to maintain homeostasis. Previously identified anti-apoptotic proteins, mainly including XIAP, Bcl-XL, Mcl-1, and receptor interacting protein 1 (RIP1) [[7], [8], [9], [10], [11]], are ubiquitously expressed in cells other than hepatocytes. In view that excessive apoptosis of hepatocytes is implicated in many forms of liver diseases which are extremely in short of effective therapeutic treatments, it is urgent to uncover liver specific and/or dominant molecular signals in balancing pro-apoptotic and anti-apoptotic events.
Farnesoid X receptor (FXR), highly expressed in hepatocytes, is conventionally recognized as a member of nuclear receptor (NR) superfamily of ligand-activated transcription factors that controls the metabolism of bile acids, lipids, glucoses, and amino acids [12,13]. Thereafter, FXR is increasingly regarded as a potential drug target for treatment of a number of diseases, including obesity [14], cholestasis [15,16] and septic shock [17,18]. Moreover, FXR was shown to influence viral hepatitis, alcohol-induced liver disease, nonalcoholic steatohepatitis (NASH), cholestasis, and ischemia/reperfusion injury, and even hepatocellular carcinoma [19], all of which are characterized by enhanced hepatocyte apoptosis. Thus, besides its well-documented roles in metabolic control, FXR may also act as a cell protector for hepatocytes [20], although the molecular basis underlying how FXR protects against liver injury remains elusive. Previous efforts mainly focused on how FXR functions as a ligand-dependent transcriptional factor in controlling the metabolic homeostasis of bile acids and lipids and its potential benefit in the therapy of various liver diseases. These findings indicated FXR agonism to be a promising therapeutic strategy of liver diseases [21]. Numerous efforts in the identification of various kinds of FXR agonists culminated in the successful launch of obeticholic acid (OCA) as a clinical treatment for primary biliary cholangitis (PBC) [19,22], and it is now in the phase 3 clinical trials for NASH. However, in addition to its side effects including pruritus and increased serum lipids [23], recent findings indicated that OCA is not efficacious against liver fibrosis in PBC patients [24,25], despite improvement in NASH patients [23,26]. These results indicate that FXR agonists alone might not be sufficiently effective against liver fibrosis, at least in the case of PBC.
Here, we report that cytosolic FXR is an intrinsic apoptosis inhibitor in hepatocytes via physically interacting with caspase 8 (CASP8). Under un-liganded conditions, FXR naturally associates with CASP8 in the cytoplasm and precludes its recruitment to DISC for activation and thereby inhibits apoptotic signal transduction. Activation of DRs reduces cytosolic FXR levels and facilitates CASP8-mediated apoptotic cell death. Forced overexpression of FXR, but not FXR agonists, attenuates both acute liver injury and chronic liver fibrosis in mice. Moreover, we show that the identification of cytosolic FXR as an apoptosis inhibitor via interacting with CASP8 is clinically translatable since fibrotic patients are characterized with reduced FXR levels and compromised FXR/CASP8 colocalization. Since apoptosis is a hallmark in most forms of liver injury, this study provides a mechanistic rationale for restoring cytosolic FXR levels or strengthening FXR/CASP8 interaction, but not FXR agonism, as a promising approach to inhibit hepatocyte apoptosis for the therapy of diverse liver diseases.
2. Materials and methods
2.1. Animals
Specific pathogen free male C57BL/6 J mice (8-weeks-old, 18–22 g) were obtained from Comparative Medicine Centre of Yangzhou University, China. The animal studies were approved by the Animal Ethics Committee of China Pharmaceutical University. FXR knock out (Fxr−/−) mice and wild-type (WT) mice on a C57BL/6 J genetic background were raised and maintained in the National Cancer Institute, and mouse handling was in accordance with an animal study protocol approved by the National Cancer Institute Animal Care and Use Committee. All mice were kept at a temperature of 25 ± 2 °C and a relative humidity of 50 ± 10% with 12-hour light/dark cycles for 1 week before experiments and allowed water and standard chow ad libitum.
2.2. LPS-induced fulminant hepatic failure
Fulminant hepatic failure was induced using a previously described method [27] with minor modifications. Briefly, mice were given an intraperitoneal (i.p) injection of D-galactosamine (GalN, Sigma, 800 μg/g), followed by an i.p injection of lipopolysaccharide (LPS, Sigma, 100 ng/g) before fasted for 8 h. Mice were killed 5 h after LPS injection. To determine the effects of FXR on hepatocyte apoptosis, mice were pretreated with vehicle, GW4064 (Medchem Express, 30 mg/kg/daily, i.p) or CDCA (Sigma, 50 mg/kg/daily, i.p) for 5 days before the injection of GalN/LPS. Separately, mice were injected intravenously with 109pfu of adenoviruses (amplified by Biowit Technologies) Ad-Ctrl or Ad-FXR daily for a consecutive 3 days [28] and subjected to GalN/LPS treatment 3 days after final virus delivery. To compare the apoptotic sensitivity between WT and Fxr−/− mice, they were injected with GalN (400 μg/g) and LPS (50 ng/g) as described above. Mice were fasted for 8 h before sacrifice.
2.3. CCl4-induced liver fibrosis
To investigate the effect of FXR overexpression in liver fibrosis, mice were injected with CCl4 (0.1 ml/kg, i.p) twice a week for 6 weeks [29]. From the 3rd week, mice were intravenously administered with Ad-Ctrl or Ad-FXR (109pfu/mouse) twice per week for 4 weeks. Mice were fasted for 8 h before sacrifice.
2.4. TNFα-induced cell apoptosis
Apoptosis of hepatocytes was induced by actinomycin D (ActD, MedChem Express, 0.2 μM)/tumor necrosis factor alpha (TNFα, Peprotech, 20 ng/ml) for 12 h as previous described [30].
To investigate the effect of FXR depletion on apoptosis, HepG2 cells were transfected with FXR-specific siRNA (Dharmacon) or negative control siRNA (Santa Cruz) using Lipofectamine RNAiMAX (Invitrogen).
To investigate the effect of FXR activation on apoptosis, cells were treated with CDCA (5–50 μM) and GW4064 (1–5 μM) for 12 h. To investigate the effect of FXR overexpression on apoptosis, cells were transfected with Ad-Ctrl or Ad-FXR (20 MOI) in the presence or absence of Z-guggulsterone (GS, Santa Cruz,10 μM), a FXR antagonist [31].
2.5. Human specimens
112 patients who were pathologically diagnosed with liver fibrosis were enrolled in this study. Their ages ranged from 22 to 66, with a median age of 41. The available clinical characteristics of these patients are summarized in Supplementary Table S1. Percutaneous liver biopsies were performed using a biopsy gun with a 16 g needle (Bard-Magnum Biopsy Instrument, Covington, GA, USA). Histological scoring was performed by experienced hepato-pathologists according to the Guideline of Prevention and Treatment for Chronic Hepatitis B (2nd Version). Blood was obtained from each patient at the time of liver biopsy, processed to plasma and stored frozen at −80 °C. In addition, 17 healthy age-matched controls (F0) from blood bank donors without clinical signs or symptoms of liver disease, and no history of chronic illnesses, were analyzed. The study was approved by The Ethics Committee of The First Affiliated Hospital of Anhui Medical University (PJ2016-10-11) and all patients gave written informed consent prior to participation.
2.6. Statistical analysis
Data were analyzed using GraphPad Prism (Graphpad Software, Inc., San Diego, CA, USA) and are presented as the mean ± standard error of mean (SEM). A two-tailed Student's t-test was applied for comparison of two groups and a one-way ANOVA with Tukey post hoc analysis was applied for comparison of multiple groups. P values below 0.05 were considered statistically significant.
2.7. Supplemental methods
Serum Biochemical Analysis; Histology Analysis; Primary Hepatocytes and Hepatic Stellate Cell (HSCs) Isolation; Determination of Apoptosis by Flow Cytometry; RT-PCR; Western Blot; Co-immunoprecipitation; Confocal Microscopy; GST Pull-down Assay; Biolayer Interferometry Assay; Microscale Thermophoresis Assay; Cross-linking Analysis of PPI; Caspase Assay, and ELISA.
3. Results
3.1. FXR deficiency sensitizes hepatocytes to apoptosis
To dissect the role of FXR in apoptosis, Fxr−/− mice were compared with wild-type (WT) littermates after treatment with D-galactosamine (GalN)/lipopolysaccharide (LPS). Serum ALT and AST levels were much higher in Fxr−/− mice than that in the WT group (Fig. 1A). H&E and TUNEL staining confirmed that GalN/LPS treatment induced more severe apoptosis of hepatocytes in Fxr−/− mice (Fig. 1B and C). In line with the histological findings, increased cleavage of BID and PARP and higher enzymatic activities of CASP3, CASP8, and CASP9 were observed in Fxr−/− mice than that in WT littermates (Supplementary Fig. S1A and B). To confirm this finding, adeno-associated virus (AAV) transfected Fxr shRNA was injected via tail vein to directly knock down the hepatic expression of FXR. Liver specific knock down of FXR by AAV shRNA resulted in increased apoptotic cell death of hepatocytes, as supported from the analysis of serum aminotransferases, caspase activities, and liver histology (Supplementary Fig. S1D–G).
Fig. 1.
FXR Deficiency Sensitizes Hepatocytes to Apoptosis.
(A) Serum ALT and AST levels of WT or Fxr−/− mice were treated with GalN/LPS (n = 6).
(B) H&E staining of liver sections of WT or Fxr−/− mice were treated with GalN/LPS.
(C) TUNEL staining of liver sections of WT or Fxr−/− mice were treated with GalN/LPS.
(D) Cell viability of primary hepatocytes isolated from WT or Fxr−/− mice and treated with ActD/TNFα (n = 6).
(E) Cell viability of HepG2 cells transfected with Ctrl or Fxr siRNA and treated with ActD/TNFα (n = 6).
(F) Cell apoptosis analysis of HepG2 cells transfected with Ctrl or Fxr siRNA and treated with ActD/TNFα (n = 6).
(G) Loss of FXR sensitized hepatocytes to FasL- and TRAIL- induced apoptosis (n = 6).
Results are mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ## P < 0.01, as assessed with Student's t-test or ANOVA. Scale bars 100 μm.
To further validate the role of FXR in apoptosis, primary hepatocytes from WT and Fxr−/− mice were isolated and treated with actinomycin D/tumor necrosis factor alpha (ActD/TNFα). Primary hepatocytes from Fxr−/− mice were more sensitive to ActD/TNFα-induced apoptosis than those from WT mice (Fig. 1D). Likewise, HepG2 cells transfected with FXR short interfering RNA (siRNA) exhibited an exacerbated apoptosis and lower cell viability than that with control siRNA upon ActD/TNFα treatment (Fig. 1E and F), which was also supported by the assessment of cleavage of BID and PARP and caspase activities (Supplementary Fig. S1H–J). In addition to TNFα triggered receptor, engagement of others DRs, such as Fas and TRAIL is also dominant in triggering hepatocytes apoptosis. Thus, we explored the effect of FXR on apoptosis triggered by ligands of Fas and TRAIL receptor. The results showed that FXR deficiency also rendered hepatocytes more susceptible to FasL and TRAIL induced apoptotic cell death (Fig. 1G). Together, these results indicate that FXR is important in protecting against DRs-engaged apoptotic cell death.
3.2. FXR overexpression but not FXR agonists inhibits apoptosis
Because FXR is conventionally recognized as a ligand-activated transcription factor, we asked whether FXR agonists could protect against apoptosis of hepatocytes. To this end, the synthetic FXR agonist GW4064 and the natural endogenous FXR agonist chenodeoxycholic acid (CDCA) were used to test their effects on apoptosis. However, neither GW4064 nor CDCA showed protective effects against apoptotic cell death of hepatocytes both in vivo and in vitro. Additionally, no anti-apoptotic effect was observed for other FXR agonists, including OCA, Px-102, Tropifexor and WAY-362450 (Supplementary Fig. S2). We next tested whether forced overexpression of FXR could protect hepatocytes against apoptosis. Mice transfected with Ad-FXR showed reduced serum ALT and AST levels upon GalN/LPS treatment (Fig. 2A). Histological assessment by H&E and TUNEL staining indicated that the number of apoptotic cells was significant lower in Ad-FXR transfected mice than that in the Ad-Ctrl infected mice (Fig. 2B and C). In agreement, lower caspase activities and less cleavage of BID and PARP were observed in Ad-FXR transfected mice (Supplementary Fig. S3A–C). The anti-apoptotic effect of FXR was also observed in cultured HepG2 cells with Ad-FXR infection (Fig. 2E, E, and Supplementary Fig. S3D–F). Similar results were observed in FasL and TRAIL-induced hepatocyte apoptosis (Supplementary Figs. S2H–K and S3I–L).
Fig. 2.
FXR overexpression but not activation alleviates apoptosis.
(A) Serum ALT and AST levels of mice transfected with Ad-FXR and treated with GalN/LPS (n = 6).
(B) H&E staining of liver sections from mice transfected with Ad-FXR and treated with GalN/LPS.
(C) TUNEL staining of liver sections from mice transfected with Ad-FXR and treated with GalN/LPS.
(D) Cell viability of HepG2 cells transfected with Ad-FXR and treated with ActD/TNFα (n = 6).
(E) Cell apoptosis of HepG2 cells transfected with Ad-FXR and treated with ActD/TNFα (n = 6).
(F) Cell viability of HepG2 cells transfected with Ad-FXR and treated with ActD/TNFα in the presence or absence of GS (n = 6).
(G) Cell apoptosis analysis of HepG2 cells transfected with Ad-FXR and treated with ActD/TNFα in the presence or absence of GS (n = 6).
Results are mean ± SEM, **P < 0.01, ***P < 0.001, # P < 0.05, ### P < 0.001, as assessed with ANOVA. Scale bars 100 μm.
The finding that forced overexpression of FXR but not agonist treatment protected against hepatocellular apoptosis hints that FXR may protect against apoptotic cell death in a transcriptional-independent manner. Transfection of Ad-FXR resulted in obviously enhanced expression of FXR protein in both the cytoplasm and nucleus of HepG2 cells (Supplementary Fig. S4A), and increased transcriptional activity was observed (Supplementary Fig. S4B). Since Ad-FXR transfection also induced a slight transcriptional activation of FXR, a FXR antagonist was employed to exclude the possible involvement of FXR transactivation. GS treatment significantly suppressed FXR transactivation induced by Ad-FXR transfection, but had negligible influence on its anti-apoptotic effect (Fig. 2F, G, and Supplementary Fig. S4B–C). These results demonstrate that FXR protein level but not FXR transcriptional activity is responsible for its anti-apoptotic effect.
3.3. FXR physically interacts with CASP8
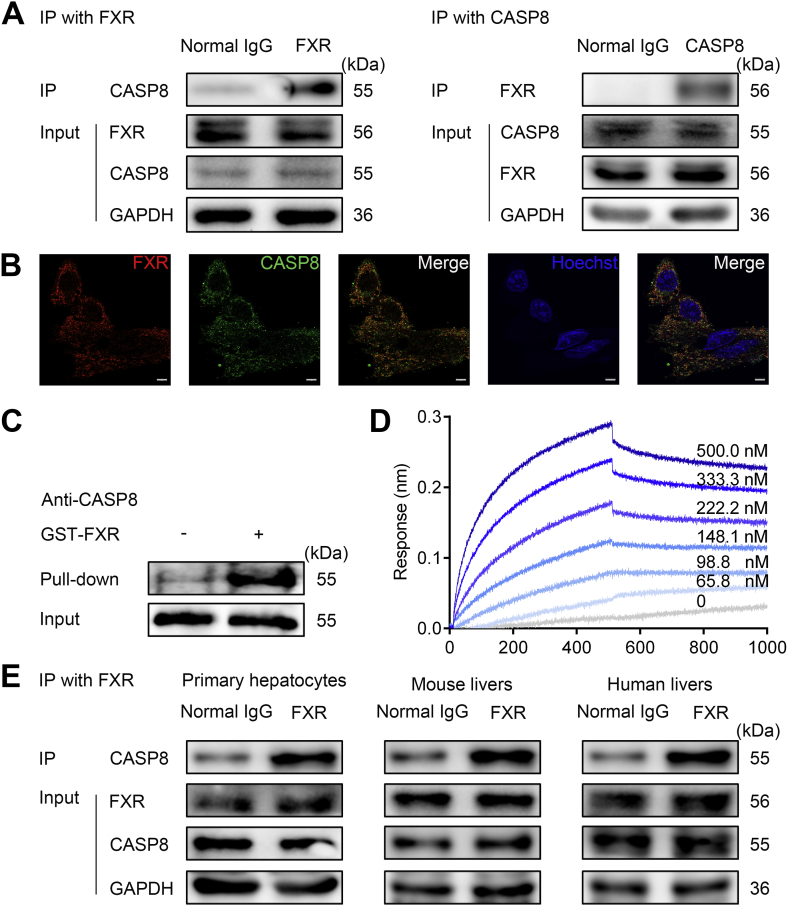
While FXR is characterized as a ligand-activated NR, the current results indicate that FXR may protect against hepatocyte apoptosis independent of its transcriptional activity. Apoptosis of hepatocytes induced by TNFα, FasL and TRAIL is initiated by the formation of the DISC, which is required for activation of pro-caspase 8. Activated CASP8 can cleave multiple intercellular substrates, such as downstream effector CASP3, CASP9 and BID to execute apoptosis [32,33]. We tested whether FXR may interrupt the formation of DISC and the activation of CASP8. FXR overexpression significantly repressed CASP8 activation (Supplementary Fig. S3A and E) but had little effect on the mRNA and protein levels of DISC components (Supplementary Fig. S5A–B), further supporting the view that FXR protection against apoptosis is independent of transcription. We assumed that FXR protein may directly bind to the members of DISC and interfere with DISC assembly. Co-immunoprecipitation (Co-IP) experiments were performed to investigate the interaction of FXR with members of DISC complex including Fas-associated protein with death domain (FADD), RIP1, and CASP8. In contrast to normal IgG, immunoprecipitations of FXR demonstrated binding of FXR to DISC members in hepatocyte lysates. Silence of CASP8 but not FADD impaired these interactions, suggesting that FXR may directly interact with CASP8 (Supplementary Fig. S5C). To validate the direct interaction of FXR with CASP8, Co-IP assays, confocal microscopy analysis and GST pull-down analysis were employed. All the results support a physical interaction between FXR and CASP8 in the resting conditions (Fig. 3A–C). In particular, the confocal analysis clearly showed that under resting conditions FXR is abundantly localized in the cytoplasm where it physically interacts with CASP8 (Fig. 3B). The direct interaction between FXR and CASP8 was further validated by cell-free assays including biolayer interferometry (BLI) analysis (Fig. 3D) and microscale thermophoresis (MST) analysis (Supplementary Fig. S5D) using recombinant proteins. Moreover, the Co-IP analysis supported a physical and natural interaction of FXR with CASP8 in primary mouse hepatocytes, mouse livers and, more importantly, in the human liver biopsies from patients with liver fibrosis (Fig. 3E).
Fig. 3.
FXR physically interacts with CASP8.
(A) Protein-protein interaction (PPI) between FXR and CASP8 in HepG2 cells Co-IP assay.
(B) HepG2 cells were fixed and stained with FXR (red), CASP8 (green), and Hoechst (blue) and visualized using confocal microscopy.
(C) GST Pull-down analysis of the PPI between FXR and CASP8.
(D) BLI analysis by ForteBio about the association between recombinant FXR and CASP8.
(E) FXR interacts with CASP8 in primary hepatocytes, mouse livers and human livers by Co-IP analysis.
Scale bars 5 μm.
3.4. FXR inhibits apoptosis via interaction with CASP8
To prove that FXR elicits its anti-apoptotic effect via interacting with CASP8, CASP8 siRNA and inhibitor was used to determine their influences on anti-apoptotic effect of FXR. HepG2 cells treated with CASP8 siRNA or inhibitor (Supplementary Fig. S6A–E) largely abrogated the anti-apoptotic effects of FXR, supporting that FXR inhibits apoptosis via CASP8. We next tested whether association of FXR may directly inhibit the CASP8 activity. Unexpectedly, the association of FXR and CASP8 had little influence on the enzymatic activity of recombinant CASP8 (Supplementary Fig. S6F), suggesting that FXR may not directly inhibit CASP8 activity upon binding. Because CASP8 activation depends on DISC assembly to cleave pro-caspase 8 to the activated protein, we asked whether FXR interaction may prevent pro-caspase 8 recruitment to DISC, thereby inhibiting its activation. CASP8 contains a C-terminal catalytic protease domain (CPD) and N-terminal tandem death effector domain (DED), which is recruited to FADD upon apoptotic stimulation [34]. Homologous modeling and molecular docking analysis (Supplementary Fig. S6G) showed that the DED of CASP8 may interact with the FXR ligand binding domain (LBD), which was confirmed by GST pull-down analysis (Supplementary Fig. S6H). Thus, it is likely that FXR may occupy the DED of CASP8. Forced expression of FXR by Ad-FXR transfection restored the association between FXR and CASP8, and thus precluded the recruitment of CASP8 to FADD and suppressed the activation of CASP8 (Supplementary Fig. S6I–K).
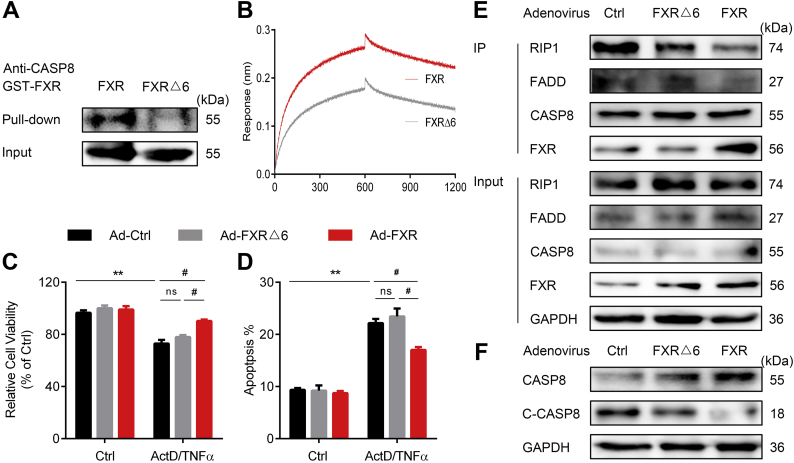
Molecular docking (Supplementary Fig. S7A) and cross-linking mass spectrometry (Supplementary Fig. S7B) were employed to predict and validate the exact binding sites of FXR to CASP8. The results indicated that D363, E364, S371, K374, R440, and E443 of FXR LBD interact with the DED of CASP8. To further validate these findings, we constructed mutant FXR recombinant proteins with D363A, E364A, S371A, K374A, R440A, and E443A. Mutation of these sites impaired not only the binding of FXR with CASP8 as demonstrated by GST pull-down, BLI (Fig. 4A and B) and Co-IP assay (Supplementary Fig. S7C and D), but also the protective role of FXR against apoptosis as demonstrated by cell viability and cell apoptosis analysis (Fig. 4C and D). Further results demonstrated that mutation of these sites failed to prevent the assembly of DISC and activation of CASP8 (Fig. 4E and F). Together, these results support that FXR physically interacts via its LBD to the DED of CASP8, thereby preventing the recruitment of pro-CASP8 to FADD and inhibiting apoptosis signal transduction. Of note, mutation of these sites has little influence on the transcriptional activity of FXR, as supported from nearly identical activity compared to WT FXR in upregulating SHP and BSEP, and downregulating CYP7A1 (Supplementary Fig. S7E). Together, these results strongly support that FXR in the cytoplasm functions as an intrinsic apoptosis inhibitor via interacting with CASP8 and this function is independent of its canonical transcriptional activity.
Fig. 4.
FXR Inhibits Apoptosis via Interaction with CASP8.
(A) GST Pull-down analysis of the PPI between FXRΔ6 and CASP8.
(B) BLI analysis of the PPI between FXRΔ6 and CASP8.
(C) Cell viability analysis about the effect of FXRΔ6 against cell apoptosis (n = 6).
(D) Apoptosis analysis by flow cytometry analysis of the effect of FXRΔ6 against cell apoptosis (n = 6).
(E) DISC assembly analyzed by Co-IP.
(F) Cleavage of CASP8 analyzed by western blot.
Results are mean ± SEM, ** P < 0.01, # P < 0.05 as assessed with ANOVA.
3.5. FXR reduction is a prerequisite for DISC assembly
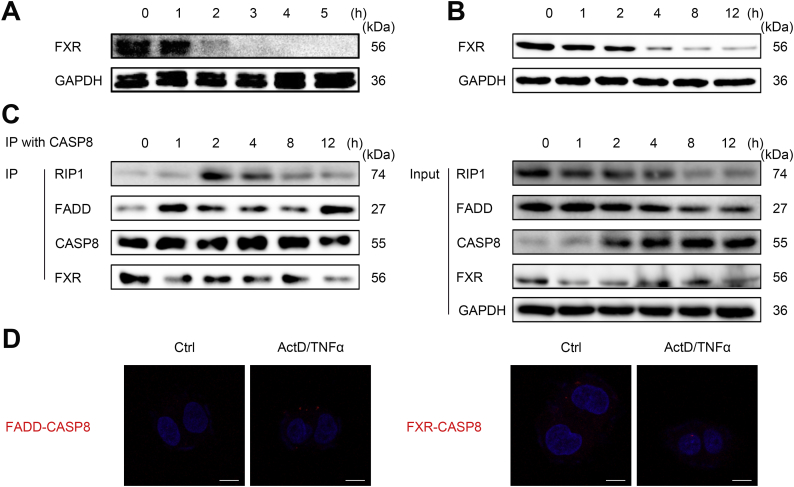
Since FXR physically interacts with CASP8 in the cytoplasm under resting conditions, we asked what would happen when hepatocytes are challenged by apoptotic stimulus. It was of interest to note that, upon apoptotic stimulation, FXR protein levels were dramatically decreased (Fig. 5A and B), resulting in impaired binding of FXR-CASP8 and enhanced binding of FADD-CASP8 (Fig. 5C and D). Association between FXR and CASP8 inhibited recruitment of CASP8 to FADD/RIP1 and disrupted DISC assembly and CASP8 activation. Collectively, these results indicate that cytosolic FXR represents an intrinsic apoptosis-inhibitory signal; upon apoptosis stimulation, downregulation of FXR is a prerequisite step conferring DISC assembly for ultimately activating apoptotic signal in hepatocytes.
Fig. 5.
FXR downregulation is a prerequisite for DISC assembly.
(A) Western blot analysis of FXR expression in the liver of GalN/LPS-treated mice.
(B) Western blot analysis of FXR expression in ActD/TNFα-treated HepG2 cells.
(C) Cytosolic FXR protein levels control DISC assembly by Co-IP.
(D) Cytosolic FXR protein levels control DISC assembly by in situ PLA Duolink kit analysis.
Results are mean ± SEM, **P < 0.01, ***P < 0.001 as assessed with ANOVA. Scale bars 5 μm.
3.6. FXR overexpression protects against chronic liver fibrosis
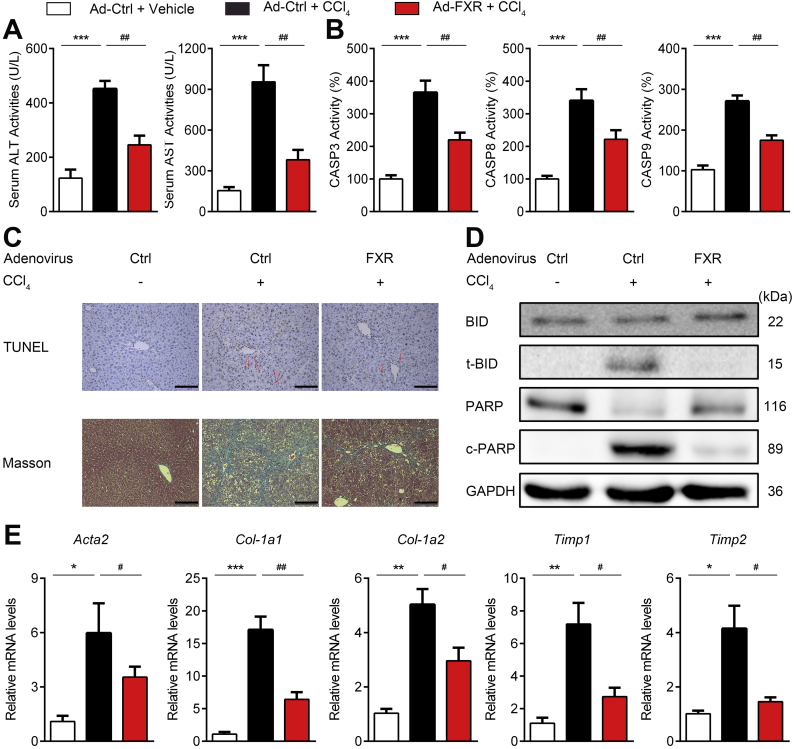
Apoptosis of hepatocytes is a hallmark for both acute liver damage and chronic liver diseases. Additionally, hepatocytes apoptosis is regarded as an important causal factor of fibrosis [35] and anti-apoptosis is regarded as a plausible treatment for liver fibrosis [36]. More importantly, hepatocellular CASP8 was demonstrated as an essential modulator of fibrosis [37]. Since our results indicated that FXR could attenuate apoptosis via interaction with CASP8, it is reasonable to predict that hepatic levels of FXR would be a key determinant in the pathological development of liver fibrosis. To test this hypothesis, mice were injected with AAV-ctrl shRNA or AAV-Fxr shRNA to knock down hepatic FXR and then subjected to CCl4-induced liver fibrosis. Compared to mice injected with AAV-ctrl shRNA, mice injected with AAV-Fxr shRNA exhibited aggravated hepatic apoptosis and injury as evidenced by serum aminotransferase levels, histological analysis, and caspase activities (Supplementary Fig. S8A–C). Moreover, the mRNA levels of Acta2 (encoding αSMA), col1a1, col1a2, Timp1 and Timp2 indicated enhanced fibrosis in AAV8-Fxr shRNA injected mice (Supplementary Fig. S8D). These results indicated that reduction of hepatic FXR levels may aggravate the pathological development of liver fibrosis via sensitizing hepatocytes to apoptosis. To further validate this point, we tested whether the forced overexpression of FXR would hamper the process of liver fibrosis. To this end, mice were treated with CCl4 to induce fibrosis and treated with Ad-ctrl or Ad-FXR. As expected, Ad-FXR transfection significantly reduced serum aminotransferases (Fig. 6A) and apoptosis (Fig. 6B–D). As revealed by Masson-trichrome staining and Sirius red staining of liver sections, fibrosis was obviously observed in CCl4-treated mice, while the fibrosis was alleviated in Ad-FXR transfected mice (Fig. 6C and Supplementary Fig. S9A). In accordance with the histological evidence, the mRNA expression levels of Acta2, col1a1, col1a2, Timp1 and Timp2 were increased in livers of CCl4-treated mice, and all these increases were significantly reversed by transfection with Ad-FXR (Fig. 6E), confirming the protective role of FXR against liver fibrosis. The apoptosome released from apoptotic hepatocytes represents an important causal factor in activating hepatic stellate cells (HSCs) for fibrotic development. Indeed, co-culture of apoptosis-triggered hepatocytes dramatically increased the fibrotic biomarkers of HSCs. In contrast, enforced overexpression of FXR into hepatocytes largely abolished such an effect (Supplementary Fig. S9C), supporting that FXR may impede hepatic fibrosis via protecting against hepatocytes apoptosis.
Fig. 6.
FXR overexpression protects against chronic liver fibrosis.
Mice received Ad-ctrl or Ad-FXR were treated with CCl4 for 6 weeks to induce fibrosis (n = 6).
(A) Serum ALT and AST levels.
(B) Activities of CASP 3, 8, and 9.
(C) TUNEL staining and Masson's trichrome staining of liver sections.
(D) Western blotting analysis of apoptotic proteins.
(E) RT-PCR analysis of mRNA levels of fibrotic markers.
Results are mean ± SEM, ***P < 0.001, ###P < 0.001, ##P < 0.01, as assessed with ANOVA. Scale bars 100 μm.
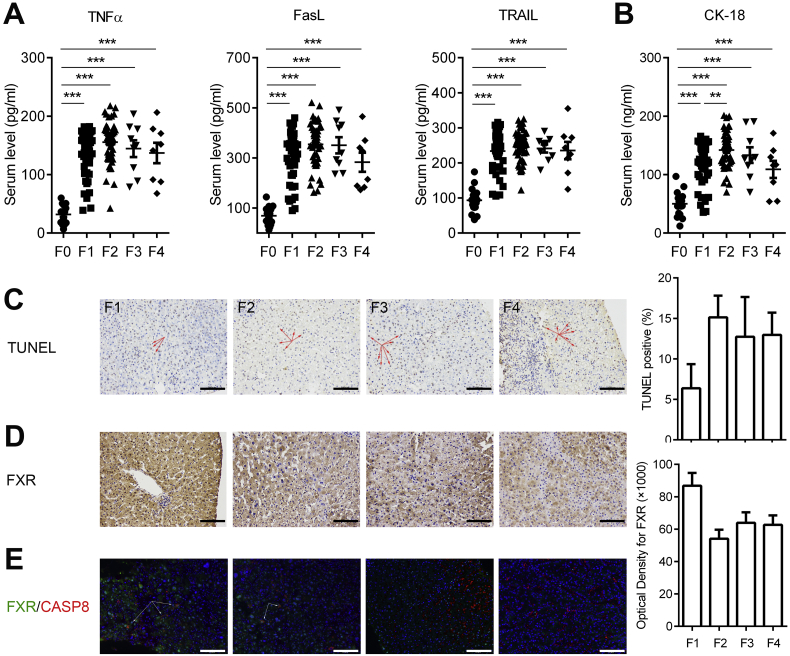
To provide a translational link to human beings, we collected serum and liver biopsy samples from patients with liver fibrosis. The serum levels of TNFα, FasL, and TRAIL were all increased in patients with liver fibrosis in comparison with those from healthy controls (Fig. 7A), suggesting that the hepatocytes in fibrotic livers are likely subjected to DRs-engaged apoptotic stress. Actually, apparent apoptotic cell death of fibrotic livers was witnessed from the increased serum apoptotic biomarker CK-18 and the positive TUNEL staining of fibrotic liver biopsies (Fig. 7B and C). Immunohistochemical staining of fibrotic livers indicated a clear trend of gradual loss of cytoplasmic FXR from stage-1 to stage-4 liver fibrosis (Fig. 7D), and also the decreased co-localization of FXR with CASP8 in the cytoplasm (Fig. 7E). These results indicate that, in the process of hepatic fibrosis development, the hepatic cytosol FXR may be gradually reduced accompanying with fibrosis progression, rendering hepatocytes more susceptible to DRs engaged apoptotic cell death.
Fig. 7.
Compromised FXR/CASP8 interaction and enhanced apoptosis in liver fibrosis patients.
(A) Serum TNFα, TRAIL, and FasL levels of fibrotic patients.
(B) Serum CK-18 levels of fibrotic patients.
(C) TUNEL staining of liver sections of fibrotic patients.
(D) Reduced FXR expression in liver biopsies from fibrosis patients detected by FXR IHC.
(E) Reduced FXR/CASP8 interaction in liver biopsies from fibrosis patients detected by Perkin Elmer Opal Kit.
Results are mean ± SEM, **P < 0.01, ***P < 0.001, as assessed with ANOVA. Scale bars 100 μm.
4. Discussion
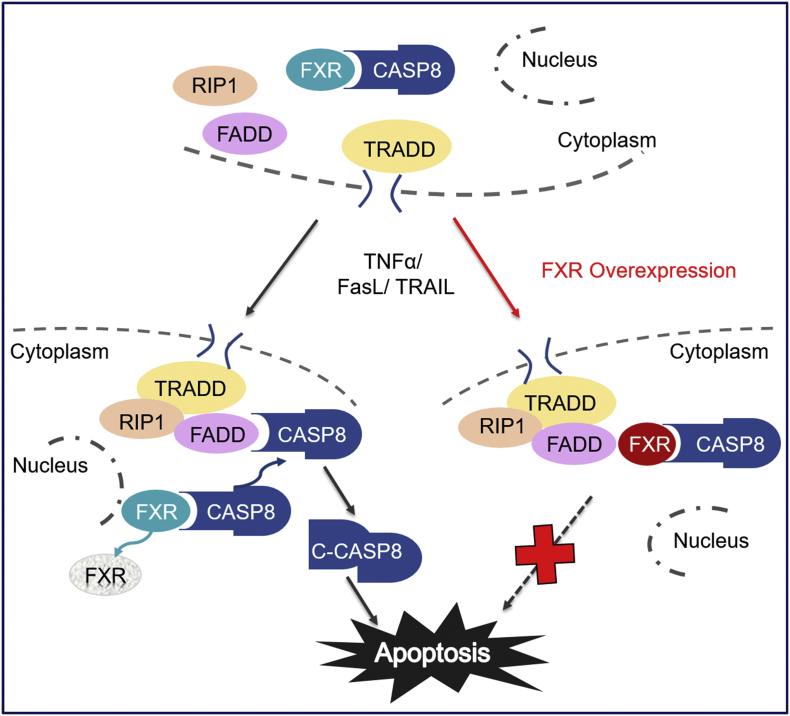
The hepatocytes are continuously exposed to high apoptotic stress including toxic endobiotics and xenobiotics, viruses, inflammatory triggers, and the high expression levels of DRs [1,2]. It is reasonable to expect that hepatocytes should have developed a powerful apoptosis inhibitory system to combat against DRs-engaged apoptotic challenge and thereby maintaining functional homeostasis of the liver. The present study shows that FXR, which is highly expressed in hepatocytes, acts as an intrinsic apoptotic inhibitor combating against DRs engaged apoptosis. Under un-liganded conditions, FXR physically interacts with CASP8, precluding its recruitment to the DISC and thereby blocking apoptotic signal cascade. Upon excessive apoptotic challenge, FXR is rapidly reduced, conferring CASP8 recruitment to DISC for activation and initiation of apoptotic signals. Forced FXR overexpression attenuates both acute liver injury and chronic liver fibrosis via inhibiting hepatocytes apoptosis. Surprisingly, FXR agonists are not effective against DRs engaged apoptotic cell death, supporting that FXR combats apoptosis in a non-genomic manner (Fig. 8).
Fig. 8.
Proposed mechanism for cytosolic FXR inhibiting death receptors engaged apoptosis. FXR physically interacts with CASP8 in the cytoplasm. Apoptotic stimulation leads to rapid FXR downregulation and DISC formation. Enhanced association between FXR and CASP8 precludes DISC formation and CASP8 activation, thereafter preventing apoptosis.
DRs-engaged apoptosis is the major form causing hepatocytes loss in many forms of liver diseases [3]. Upon binding with their cognate ligands, DRs, including Fas, TRAIL-R1/2, and TNFR1, activate the same extrinsic apoptotic signaling pathway involving FADD interaction with CASP8 to form a protein complex defined as DISC which facilitates CASP8 activation [38]. In hepatocytes, activated CASP8 cleaves the BH3-only protein Bid generating truncated Bid (t-Bid) which translocates to mitochondria and, in concert with active Bax and Bak, results in mitochondrial outer membrane permeablization (MOMP) [39]. There have been identified two anti-apoptotic proteins, Bcl-xL and Mcl-1, which inhibit the activation of Bax and Bak blocking MOMP mediated intrinsic apoptotic signaling. XIAP can inhibit caspase 9 and effector caspases 3, 6, 7 [40,41], and depletion of XIAP switches hepatocytes to CASP8 dependent but Bid independent apoptosis as seen in type I cells [39]. Altogether, these findings indicate that CASP8 activation is a pivotal step in initiating apoptotic signaling pathway in hepatocytes. In this regard, CASP8 activation should be precisely regulated to maintain apoptosis to anti-apoptosis balance. Cellular CASP8 inhibitory protein (cFLIP), which is also recruited to the DISC, is the best defined regulator in controlling CASP8 activation. Because cFLIP, together with Bcl-xL and Mcl-1, are ubiquitously expressed in many types of cells, they might not be sufficient for precise regulation of apoptotic balance in hepatocytes existed in a unique environment with high apoptotic stress. The present identification of cytosolic FXR as an inhibitor of apoptosis in hepatocytes via natural interaction with CASP8 may thus shed new insights in delineating the molecular events of the intrinsic hepatoprotective system. Under un-liganded conditions, FXR interacts via its LBD to the DED of CASP8, which is the same binding domain of CASP8 interacts with FADD. Thus, FXR competitively inhibits FADD association with CASP8 to preclude DISC assembly. Unlike cFLIP, FXR cannot directly inhibit the catalytic activity of CASP8, but retard CASP8 autoactivation on the DISC platform. Therefore, cytosolic FXR may coordinate with cFLIP to limit CASP8 overactivation and thus enhance the apoptotic threshold of hepatocytes. Of note, high levels of TNFα, TRAIL and FasL induced a fast reduction of cytosolic FXR before the initiation of apoptotic signal cascade, while forced overexpression of FXR could largely inhibit DRs-engaged apoptosis. These facts indicate that reduction of cytosolic FXR levels is a prerequisite to activate the apoptotic cascade and that the balance between FXR and DRs could be an important determinant for cell fate decision of hepatocytes. Because FXR is predominantly expressed in hepatocytes, the identification of FXR as an intrinsic apoptosis inhibitor is important in understanding how hepatocytes survive from the uniquely hostile environment.
FXR is conventionally recognized as a ligand-activated NR. Upon binding with ligands, NRs translocate from the cytoplasm to the nucleus and to target sites in the genome, exerting genomic actions by regulating its target genes. In this study, we uncovered that FXR in the cytoplasm of hepatocytes functions as an intrinsic apoptosis inhibitor via interacting with CASP8. Forced overexpression, but not FXR ligands, protects against DRs-engaged apoptosis supporting that the anti-apoptotic effect of FXR is independent of its canonical transcriptional activity. In line with our study, no significant improvement of caspase-cleaved keratin-18 levels was observed for NASH patients after treatment with OCA [26]. Previous studies demonstrated that FXR is important in the pathological development of many liver diseases including alcohol hepatitis, NASH, viruses induced hepatitis, cholesterol liver diseases, and liver cancer. However, the exact molecular mechanisms of how FXR protects against diverse pathological factors-induced liver injury have not been fully addressed, and in most cases, presumably ascribed to the transcriptional activity of FXR on the regulation of bile acids and lipids homeostasis. Of interest, it was previously shown that FXR agonists were effective against intrinsic apoptosis induced by serum deprivation and fasting [42]. However, we found that FXR agonists cannot protect cells against DRs-engaged extrinsic apoptosis. Thus, it is reasonable to presume that both genomic and non-genomic functions of FXR may coordinate with each other to protect against diverse factors induced liver injury. More work is needed to delineate how the pleiotropic functions of FXR are tuned to maintain homeostasis of hepatocytes. Notably, non-genomic functions of some NRs have been witnessed. Nur77 was reported to interact with Bcl-2 and induce conformational change of Bcl-2, resulting in conversion of Bcl-2 from a protector to a killer [43]. ERβ elicited its anti-apoptosis and anti-inflammasome activity via interacting with a protein network in the cytoplasm [44]. Interaction between vitamin D receptor (VDR)/RXR with p62 was demonstrated as a crucial determinant of HSC activation and fibrosis [45]. A non-canonical, transcription-independent function of NOTCH1 in regulating adherens junctions and vascular barrier function is revealed recently [46]. Together, all these findings indicate that, although NRs are usually considered to be mainly located in the nucleus to elicit their canonical transcriptional activity, some NRs in unliganded conditions may also locate and function in the cytoplasm in a non-canonical manner via PPI.
Increased serum levels of TNFα, FasL and TRAIL, enhanced apoptotic cell death, reduced FXR levels and compromised FXR/CASP8 interaction were found in fibrotic patients, suggesting that our findings are clinically relevant. Excessive DRs-engaged apoptotic death of hepatocytes, which is aggravated by gradual loss of FXR, may represent a hallmark in facilitating fibrotic development. In both GalN/LPS-induced acute hepatic failure and CCl4-induced chronic fibrosis, drastic reduction of hepatic FXR levels was noted. As a support, previous studies also showed that the age-dependent decline in FXR expression and activity is a major factor in the development of fatty liver observed in aging mice [47]. Together, these findings indicate that reduced levels of FXR may render hepatocytes more susceptible to apoptotic stresses and therefore it is reasonable to expect that restoring FXR levels would be a promising therapeutic strategy for many liver diseases. Indeed, we found that forced overexpression of FXR protected against both acute liver injury and chronic liver fibrosis. Due to the explicit knowledge on its genomic actions, previous efforts mainly focused on the development of FXR agonists. Among which, OCA was approved by FDA and EMEA as a breakthrough drug. Although OCA may be effective against liver fibrosis in NASH [23,26], it had little effect on liver fibrosis in PBC patients [24,25], which may be partially explained by our findings that FXR agonists are not effective against DRs-engaged hepatocytes apoptosis, a key event in the pathological development of liver fibrosis. In contrast to FXR agonists, forced overexpression of FXR combats against hepatocytes apoptosis both in vitro and in vivo. We thus propose that, in addition to the development of more efficient FXR agonists, future efforts can be directed to design drug candidates that recovers FXR protein levels by preventing its degradation or strengthens FXR/CASP8 interaction for the therapy of liver diseases in which apoptosis of hepatocytes is a causal event. Future studies in delineating the exact mechanism of FXR degradation in conditions of liver fibrosis are thus warranted to exploit the strategy of restoring FXR protein levels for combating apoptosis triggered fibrotic events [19]. Indeed, both high throughput screening and rational design of compounds targeting FXR degradation and FXR/CASP8 interaction are now undergoing in our lab.
In summary, this study uncovers cytosolic FXR as an apoptosis inhibitor via precluding CASP8 recruitment to the DISC in hepatocytes (Fig. 8), shedding insights in delineating molecular events involved in controlling anti-apoptotic and pro-apoptotic homeostasis of hepatocytes. Moreover, it suggests a mechanistic basis for targeting cytosolic FXR protein levels and FXR/CASP8 interaction as a promising strategy for the therapy of liver diseases.
Funding sources
This research was supported by National Natural Science Foundation of China (grants 81430091, 81720108032, 81421005, and 91429308 to H.H.; 81530098 and 81421005 to G.W.; and 81603194 to H.W.); the Project for Major New Drug Innovation and Development (grants 2015ZX09501010 and 2017ZX09101003-002-003) to H.H.; Overseas Expertise Introduction Project for Discipline Innovation (G20582017001) to H.H.; China Postdoctoral Science Foundation (grants 2016M600455 and 2017T100423) to H.W.; and the National Cancer Institute Intramural Research Program to F.J.G..
Declaration of interests
H.W. receives grants from National Natural Science Foundation of China and China Postdoctoral Science Foundation. F.J.G receives grants from National Cancer Institute Intramural Research Program. G.W. receives grants from National Natural Science Foundation of China. H.H. receives grants from National Natural Science Foundation of China, the Project for Major New Drug Innovation and Development, and Overseas Expertise Introduction Project for Discipline Innovation. H.W., J.Z., Y.G., S.C., N.H., G.W., and H.H. have pending patents (CN108079316A and CN107812199A).
Author contributions
H.W., J.Z., Y.G., S.C., T.Y., performed the experiments with assistance from C.G., N.H., L.C., Y.C. and Q.Z.; H.W., J.Z. and H.H. analyzed data; H.H. and G.W. supervised the project. H.W., H.H., G.W., X.Z., and F.J.G. wrote and revised the manuscript.
Footnotes
Supplementary data to this article can be found online. https://doi.org/10.1016/j.ebiom.2018.10.028
Contributor Information
Guangji Wang, Email: gjwang@cpu.edu.cn.
Haiping Hao, Email: haipinghao@cpu.edu.cn.
Appendix A. Supplementary data
The label for co-first author was corrected to be same in the main body of the MS.
References
- 1.Strnad P., Tacke F., Koch A., Trautwein C. Liver - guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14:55–66. doi: 10.1038/nrgastro.2016.168. [DOI] [PubMed] [Google Scholar]
- 2.Heymann F., Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. doi: 10.1038/nrgastro.2015.200. [DOI] [PubMed] [Google Scholar]
- 3.Malhi H., Guicciardi M.E., Gores G.J. Hepatocyte death: A clear and present danger. Physiol Rev. 2010;90:1165–1194. doi: 10.1152/physrev.00061.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Macdonald S., Andreola F., Bachtiger P. Cell death markers in patients with cirrhosis and acute decompensation. Hepatology. 2018;67:989–1002. doi: 10.1002/hep.29581. [DOI] [PubMed] [Google Scholar]
- 5.Luedde T., Kaplowitz N., Schwabe R.F. Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuchida T., Friedman S.L. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14:397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed] [Google Scholar]
- 7.Vince J.E., Wong W.W., Khan N. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi N., Vereecke L., Bertrand M.J. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 9.Czabotar P.E., Lessene G., Strasser A., Adams J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 10.Newton K., Dugger D.L., Maltzman A. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Luo A., Shrivastava I. Regulation of XIAP turnover reveals a role for USP11 in promotion of tumorigenesis. EBioMedicine. 2017;15:48–61. doi: 10.1016/j.ebiom.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 13.Massafra V., Milona A., Vos H.R. Farnesoid X receptor activation promotes hepatic amino acid catabolism and ammonium clearance in mice. Gastroenterology. 2017;152:1462–1476. doi: 10.1053/j.gastro.2017.01.014. [e1410] [DOI] [PubMed] [Google Scholar]
- 14.Ryan K.K., Tremaroli V., Clemmensen C. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modica S., Petruzzelli M., Bellafante E. Selective activation of nuclear bile acid receptor FXR in the intestine protects mice against cholestasis. Gastroenterology. 2012;142:355–365. doi: 10.1053/j.gastro.2011.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Baghdasaryan A., Claudel T., Gumhold J. Dual farnesoid X receptor/TGR5 agonist INT-767 reduces liver injury in the Mdr2−/− (Abcb4−/−) mouse cholangiopathy model by promoting biliary HCO(−)(3) output. Hepatology. 2011;54:1303–1312. doi: 10.1002/hep.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hao H., Cao L., Jiang C. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metab. 2017;25:856–867. doi: 10.1016/j.cmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X., Cao L., Jiang C. PPARalpha-UGT axis activation represses intestinal FXR-FGF15 feedback signalling and exacerbates experimental colitis. Nat Commun. 2014;5:4573. doi: 10.1038/ncomms5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., He Q., Wang G., Xu X., Hao H. FXR modulators for enterohepatic and metabolic diseases. Expert Opin Ther Pat. 2018 doi: 10.1080/13543776.2018.1527906. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y.D., Chen W.D., Moore D.D., Huang W. FXR: A metabolic regulator and cell protector. Cell Res. 2008;18:1087–1095. doi: 10.1038/cr.2008.289. [DOI] [PubMed] [Google Scholar]
- 21.Neuschwander-Tetri B.A. Targeting the FXR nuclear receptor to treat liver disease. Gastroenterology. 2015;148:704–706. doi: 10.1053/j.gastro.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Samur S., Klebanoff M., Banken R. Long-term clinical impact and cost-effectiveness of obeticholic acid for the treatment of primary biliary cholangitis. Hepatology. 2017;65:920–928. doi: 10.1002/hep.28932. [DOI] [PubMed] [Google Scholar]
- 23.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): A multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nevens F., Andreone P., Mazzella G. A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N Engl J Med. 2016;375:631–643. doi: 10.1056/NEJMoa1509840. [DOI] [PubMed] [Google Scholar]
- 25.Kowdley K.V., Luketic V., Chapman R. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology. 2018;67:1890–1902. doi: 10.1002/hep.29569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mudaliar S., Henry R.R., Sanyal A.J. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. [e571] [DOI] [PubMed] [Google Scholar]
- 27.Amir M., Zhao E., Fontana L. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013;20:878–887. doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y.Q., Lee F.Y., Barrera G. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osawa Y., Oboki K., Imamura J. Inhibition of cyclic adenosine monophosphate (cAMP)-response element-binding protein (CREB)-binding protein (CBP)/beta-catenin reduces liver fibrosis in mice. EBioMedicine. 2015;2:1751–1758. doi: 10.1016/j.ebiom.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walter D., Schmich K., Vogel S. Switch from type II to I Fas/CD95 death signaling on in vitro culturing of primary hepatocytes. Hepatology. 2008;48:1942–1953. doi: 10.1002/hep.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y., Wang H., Cheng X. Farnesoid X receptor activation promotes cell proliferation via PDK4-controlled metabolic reprogramming. Sci Rep. 2016;6:18751. doi: 10.1038/srep18751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter M.E. Programmed cell death: Apoptosis meets necrosis. Nature. 2011;471:310–312. doi: 10.1038/471310a. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y., Yu M., Hu X. STAT1 mediates transmembrane TNF-alpha-induced formation of death-inducing signaling complex and apoptotic signaling via TNFR1. Cell Death Differ. 2017;24:660–671. doi: 10.1038/cdd.2016.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan J., Najafov A., Py B.F. Roles of caspases in necrotic cell death. Cell. 2016;167:1693–1704. doi: 10.1016/j.cell.2016.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canbay A., Friedman S., Gores G.J. Apoptosis: The nexus of liver injury and fibrosis. Hepatology. 2004;39:273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 36.Eguchi A., Du Jeu X.D., Johnson C.D., Nektaria A., Feldstein A.E. Liver Bid suppression for treatment of fibrosis associated with non-alcoholic steatohepatitis. J Hepatol. 2016;64:699–707. doi: 10.1016/j.jhep.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatting M., Zhao G., Schumacher F. Hepatocyte caspase-8 is an essential modulator of steatohepatitis in rodents. Hepatology. 2013;57:2189–2201. doi: 10.1002/hep.26271. [DOI] [PubMed] [Google Scholar]
- 38.Mattisson I.Y., Bjorkbacka H., Wigren M. Elevated markers of death receptor-activated apoptosis are associated with increased risk for development of diabetes and cardiovascular disease. EBioMedicine. 2017;26:187–197. doi: 10.1016/j.ebiom.2017.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jost P.J., Grabow S., Gray D. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deveraux Q.L., Takahashi R., Salvesen G.S., Reed J.C. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 41.Srinivasula S.M., Hegde R., Saleh A. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature. 2001;410:112–116. doi: 10.1038/35065125. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y.D., Yang F., Chen W.D. Farnesoid X receptor protects liver cells from apoptosis induced by serum deprivation in vitro and fasting in vivo. Mol Endocrinol. 2008;22:1622–1632. doi: 10.1210/me.2007-0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin B., Kolluri S.K., Lin F. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 44.Han S.J., Jung S.Y., Wu S.P. Estrogen receptor beta modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163:960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duran A., Hernandez E.D., Reina-Campos M. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell. 2016;30:595–609. doi: 10.1016/j.ccell.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Polacheck W.J., Kutys M.L., Yang J. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature. 2017;552:258–262. doi: 10.1038/nature24998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiong X., Wang X., Lu Y. Hepatic steatosis exacerbated by endoplasmic reticulum stress-mediated downregulation of FXR in aging mice. J Hepatol. 2014;60:847–854. doi: 10.1016/j.jhep.2013.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The label for co-first author was corrected to be same in the main body of the MS.