Abstract
Objective
We aimed to determine if a decline in walking speed during the year prior to disease onset is associated with concurrent changes in cartilage, bone marrow lesions (BMLs), or effusion in adults who develop common knee osteoarthritis (KOA), accelerated KOA (AKOA), or no KOA.
Methods
We identified 3 groups from the Osteoarthritis Initiative based on annual radiographs from baseline to 48 months: 1) AKOA; 2) common KOA; 3) no KOA. We used the cartilage damage index (CDI) to assess tibiofemoral cartilage damage and used a semi-automated program to measure BML and effusion volume. Walking speed was assessed as an individual’s habitual walking speed over 20 meters. One-year change in walking speed and structural measures were calculated as index visit minus the year prior visit. Logistic regression models were used to determine if change in walking speed (exposure) was associated with change in each structural measure (outcome) for the overall group and then separately for AKOA, common KOA, and no KOA.
Results
Adults who slowed their walking speed are almost twice as likely to present with increased BML volume, with a statistically significant association (OR=3.04, 95%CI=1.03,8.95) among adults with AKOA. Adults with AKOA who slowed their walking speed were ~3.4x (95%CI=1.10,10.49) more likely to present with increased effusion volume. Walking speed change was not significantly associated with CDI change.
Conclusion
A change in an easily assessable clinical examination (i.e. 20m walk test) is associated with concurrent worsening in BML and effusion volume in adults who developed AKOA.
INTRODUCTION
Walking speed is an easily accessible clinical measure that reflects physical function in individuals with or at risk for knee osteoarthritis (OA).(1) Walking speed decline is a clinically relevant impairment that is a risk factor for developing radiographic knee OA and receiving a knee replacement.(2, 3) While walking speed decline is a prognostic marker of knee OA, it remains unclear how it relates to structural changes prior to the onset of radiographic OA. Understanding whether early changes in walking speed are associated with commonly used sensitive measures of knee health that are related to OA onset and progression (e.g., changes in bone marrow lesions [BMLs],(4) effusion,(5) cartilage(6)) may provide a better understanding of the early link to physical function and joint health decline.
While knee OA is commonly considered a slowly progressive disorder, some individuals develop an accelerated form of the disease that progresses from a normal joint [Kellgren-Lawrence Grade (KL) 0–1] to advanced-stage disease (KL 3–4) within 4 years.(7–9) Individuals with accelerated knee OA present with earlier worsening of magnetic resonance (MR)-based structural measures (e.g., effusion) and poorer patient-reported and physical function measures (e.g., walking speed) compared to individuals with a more gradual onset of knee OA (common knee OA).(8, 10) Hence, the association between walking speed decline and early structural changes may be more pronounced among adults with accelerated knee OA than those with common knee OA.
Therefore, we aimed to determine if a decline in walking speed during the year prior to disease onset is associated with concurrent worsening of tibiofemoral effusion, BMLs, and articular cartilage in three groups: adults who develop accelerated knee OA, common knee OA, or no knee OA. This information may help clarify the relationship between a decline in knee structure and physical function in individuals with incident knee OA. We hypothesize that an association exists between a change in walking speed and a change in structural features due to two possibilities: 1) a decline in walking speed may alter loading at the knee and result in structural alterations, or 2) alterations in structural features may lead to a decline in gait speed in an attempt to avoid pain or protect the joint from further damage. Either way, the results of this study may demonstrate that a clinically feasible physical function test may be a proxy for early structural changes and hence help identify individuals with early evidence of structural changes in a knee.
PATIENTS AND METHODS
Study Design
To determine the association between changes in walking speed and changes in MR-based knee structural measures, we conducted a longitudinal analysis of data from the Osteoarthritis Initiative (OAI). The OAI is a multicenter (Memorial Hospital of Rhode Island, The Ohio State University, University of Maryland and Johns Hopkins University, and the University of Pittsburgh) cohort study that recruited 4,796 adults with or at risk for symptomatic knee osteoarthritis between February 2004 and May 2006.(11) MR images and walking speed were obtained at the initial baseline study visit, as well at the first 4 annual follow-up visits. Institutional review boards at all OAI clinical sites and the OAI coordinating center (University of California, San Francisco) approved the OAI study. Participants provided informed consent prior to participation.
Participant Selection
We identified 3 groups within the OAI based on radiographs obtained at baseline and the first four annual follow-up visits.(9) All groups had at least one knee with no radiographic knee OA at baseline (Kellgren-Lawrence [KL] < 1). Individuals with incident accelerated knee OA were defined as having one knee that developed advanced-stage knee OA (KL Grade = 0/1 to 3/4, definitive osteophyte and joint space narrowing) within 48 months (n = 125).(9) Individuals with incident common knee OA had no knee OA in both knees at baseline and were defined as having a more gradual onset of knee OA, with one knee increasing in KL grade within 48 months (i.e. KL = 0 to 1, 0 to 2, 1 to 2; n = 187). Individuals with no knee OA were defined as having no knee OA in both knees at baseline and had no change in KL grade in either knee from baseline to the 48-month follow-up (n = 1,325). To match people with common and no knee OA, we first identified those with one or no missing MR images. Next, we used SAS to assign each male and female a random number from a uniform distribution and used this number to randomly match people with common or no knee OA to those in the accelerated knee OA group stratified by sex (125 participants/group).
Index Knee
For individuals with accelerated knee OA or common knee OA, the index knee was defined as the knee that first met the definition for incident accelerated knee OA or common knee OA. The index knee for individuals with no knee OA was side-matched to that person’s matched member of the accelerated knee OA group.
Index Visits
For the individuals with accelerated knee OA and common knee OA, the index visit was defined as the visit that the index knee met the criteria for accelerated OA or common OA. For individuals with no knee OA, the index visit was the same as their matched member of the accelerated knee OA group.
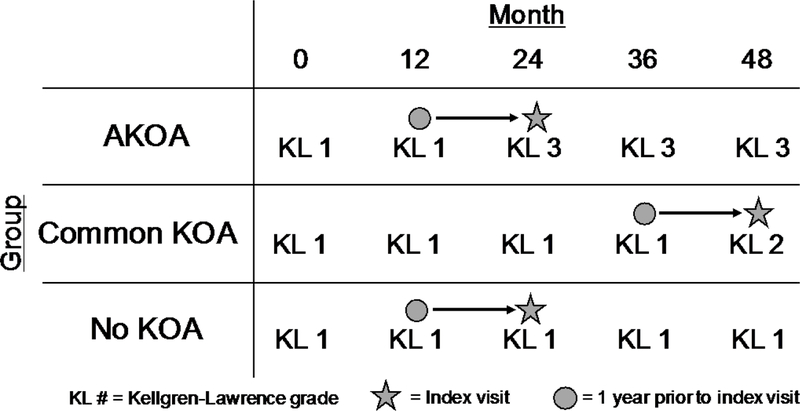
For this study, we assessed walking speed and the MR-based knee structure measures at the index visit and the visit in the year prior to the index visit (Figure 1).
Figure 1.
Walking speed and structural measures were assessed at the visit in the year prior to the index visit, as well as the index visit. One-year change in walking speed and structural measures were calculated as the index visit minus the year prior visit.
Knee Radiographs
To determine group assignment, we used readings of bilateral weight-bearing, fixed-flexion posteroanterior knee radiographs obtained at baseline and each of the annual follow-up visits.(9) Central readers blinded on group assignment scored the KL Grade of each knee (KL = 0 to 4) The intrarater reliability agreement for the KL grades was good (weighted κ = 0.70 to 0.80). These data are publicly available (files: kXR_SQ_BU##_SAS [versions 0.6, 1.6, 3.5, 5.5, and 6.3]).(12)
Magnetic Resonance Imaging Acquisition
MR images were acquired annually with one of four identical Siemens (Erlangen, Germany) Trio 3-Tesla MR systems at each clinical site using the OAI MR imaging protocol.(12, 13) BML and effusion quantitative measurements were performed using a sagittal intermediate-weighted, turbo spin echo, fat-suppressed MR sequence with the following parameters: field of view=160mm, slice thickness=3mm, skip=0mm, flip angle=180 degrees, echo time=30ms, recovery time=3200ms, 313x448 matrix, x resolution=0.357mm, y resolution=0.511mm, and total slice number=37. Cartilage was quantified using a 3-dimensional dual-echo steady-state sequence with the following parameters: field of view=140mm, slice thickness=0.7mm, skip=0mm, flip angle=25 degrees, echo time=4.7ms, recovery time=16.3ms, 307x384 matrix, x resolution=0.365mm, y resolution=0.456mm, and total slice number=160.
Magnetic Resonance Imaging Outcomes
For BML, effusion, and cartilage processing, the readers were unaware of group assignment. Additionally, during the processing of all MRI measures the readers had both time points on screen and were unblinded to the order of time as this is the standard method used to maximize the sensitivity to change.(14, 15)
Bone Marrow Lesion Volume
One reader (ACS) measured tibiofemoral BML volume with a semi-automated segmentation method.(16, 17) The only manual step required the reader to identify crude boundaries of the tibia and femur in each slice of the MR images. The boundary furthest from the articular surfaces was marked just prior to the epiphyseal line or at the edge of the bone and soft tissue. The program then automatically identified the precise bone boundaries and performed a thresholding and curve evolution process twice to segment areas of high signal intensity, which may represent a BML. We eliminated false-positive regions by operationally defining a BML based on 2 criteria: 1) the distance between a BML to the articular surface should be <10 mm, and 2) a BML needed to span more than one MR image. BML volume was expressed as a total tibiofemoral BML volume. A previous study used a similar total tibiofemoral BML volume and observed a significant association between change in BML volume and change in knee pain severity.(17) The study principal investigator (JBD) reviewed all measurements with both timepoints on screen simultaneously. Our reader demonstrated excellent intra-reader reliability (ICC3,1=0.91).
Effusion Volume
We used a customized semi-automatic software to measure knee effusion/synovitis, which reflects effusion and synovitis volume but for simplicity is referred to as effusion volume. Two readers (JBD and FA) used the software to mark the first and last MR slice that included bone, the proximal border of the patella, and the apex of the fibular head on a central slice. The software then automatically segmented effusion between these limits based on an existing threshold. The senior reader (JBD) then manually adjusted the threshold to change the effusion boundaries and removed areas of high signal intensity that were not effusion (e.g., subchondral cysts, blood vessels). The effusion volume measurement was a total tibiofemoral effusion volume. The senior reader demonstrated excellent intra-reader reliability (ICC3,1=0.96).
Cartilage Damage Index
To quantify change in tibiofemoral cartilage damage we used the validated cartilage damage index (CDI).(18, 19) One reader (JED) manually marked the bone-cartilage boundary on specific knee slices that are automatically selected based on the width of the knee. The reader then measured cartilage thickness at predefined informative locations, which the software automatically located. The software then computed the CDI for the medial femur, lateral femur, medial tibia, and lateral tibia by summing the products of cartilage thickness, cartilage length (anterior-posterior), and voxel size from 9 informative locations in each compartment. All measurements were reviewed by study principal investigator. Our reader demonstrated excellent intra-reader reliability (ICC3,1=0.86 to 0.99).
Walking Speed Assessment
To assess habitual walking speed, participants performed two trials of a timed 20-meter walk at their usual, comfortable walking pace.(1, 2) The participants began each trial in a stationary, standing position and timing began when the participant took their first step at the starting line and ended when they passed a cone positioned 20-meters away. Participants were instructed to maintain their usual walking pace for three steps past the cone to ensure the participants were not decelerating at the end of each trial. The time needed to complete the 20-meters was converted to walking speed (i.e. meters/second [m/s]) and averaged across the two trials.
Clinical Data
Demographic and other participant characteristics were acquired based on a standard protocol. We extracted OAI baseline age, body mass index (BMI), index knee Western Ontario & McMaster Universities Osteoarthritis Index (WOMAC) pain score, self-reported Physical Activity Scale for the Elderly (PASE) score, frequent knee pain, and injury between the two study visits. The data are publicly available (Files: allclinical0#; version 0.2.2, 1.2.1, 3.2.1, 5.2.1, and 6.2.1).(13)
Statistical Analysis
Data Analysis
As cartilage thickness is largely dependent on an individual’s height,(20) we normalized the CDI of each tibiofemoral compartment (i.e. medial femur, lateral femur, medial tibia, and lateral tibia) to participant height. One-year change in BML, effusion, and CDI for each compartment was calculated as “index” visit minus “year prior index” visit. If individuals were missing a timepoint of the structural measures, we used the most proximate visit (e.g. if missing “year prior index” visit, we used the “two years prior index” visit; if missing the “index” visit we used the “year following index” visit) to calculate an annual rate of change over two years (n=13). Total tibiofemoral CDI change was calculated as the sum of the change for each individual compartment CDI. Total tibiofemoral change for BML, effusion, and CDI were then separated into tertiles, and converted to a dichotomous variable to compare the worst tertile (i.e. highest BML and effusion, lowest CDI) to the combination of the other two tertiles to facilitate the interpretation of the odds ratio. These binary change variables were used in our statistical analysis to compare individuals with the greatest increase in BML/effusion volume and greatest decrease in CDI to individuals with no change/decrease in BML/effusion volume and no change/increase in CDI, respectively.
Walking speed change was calculated as “index” visit walking speed minus “year prior to index” visit walking speed. Based on a previous study detecting an increase in risk of knee OA in individuals decreasing their walking speed,(2) we dichotomized walking speed change as: 1) slower/decline in walking speed: walking speed change < −0.1m/s, 2) no change/increase in walking speed: walking speed change > −0.1m/s. This dichotomous variable allowed us to compare individuals with declining walking speed to individuals with no change/increase in walking speed.
Primary Analysis - Association Between Change in Walking Speed and Change in Structure
Three logistic regression models were used to determine if the change in walking speed (predictor) was associated with the change in BML volume, effusion volume, and CDI (outcomes) for the overall group. Additionally, we separately explored these relationships for people with accelerated knee OA, common knee OA, or no knee OA. As a post hoc analysis, we replicated these analyses using a linear regression with each structural feature as a continuous variable for the overall group and separated for accelerated knee OA, common knee OA, and no knee OA.
Sensitivity Analysis
We conducted three sensitivity analysis using the same logistic regression models above on three subsets: 1) individuals who developed accelerated knee OA within 1 year, 2) individuals who had no radiographic knee OA bilaterally at baseline (KL = 0/1), 3) excluding the 13 individuals with missing structural data that we imputed by calculating an annual rate of change over two years.
Covariates for all analyses included baseline age, body mass index (BMI), WOMAC pain, and PASE score. We used baseline covariates because the covariate means were stable throughout the study period and to prevent the loss of participants due to missing self-reported data between the two time points. We ran sensitivity analyses that used the “frequent knee pain” variable as a covariate instead of WOMAC pain and a sensitivity analysis that included injury between the two visits as a covariate. All analyses were performed with SAS Enterprise 7.15 (Cary, NC, USA).
RESULTS
The group demographics are described in Table 1. Due to missing MR or walking speed data, our final analyses included 106 individuals with accelerated knee OA, 121 individuals with common knee OA, and 119 individuals with no knee OA.
Table 1.
Group Demographics.
| Variable | Overall n=346 | Accelerated Knee OA n=106 | Common Knee OA n=121 | No Knee OA n=119 |
|---|---|---|---|---|
| Age (years), mean (SD) | 60.6 (8.6) | 64.5 (8.4) | 59.4 (8.4) | 58.3 (7.8) |
| BMI (kg/m2), mean (SD) | 28.2 (4.6) | 29.7 (4.5) | 28.0 (4.5) | 27.1 (4.6) |
| WOMAC Pain, mean(SD) | 2.0 (2.8) | 3.3 (3.5) | 1.8 (2.3) | 1.1 (2.1) |
| PASE, mean (SD) | 162.8 (84.0) | 148.1 (89.1) | 161.6 (80.1) | 177.3 (81.6) |
| Female, n(%) | 214 (62%) | 66 (62%) | 75 (62%) | 73 (61%) |
OA = osteoarthritis, PASE = Physical Activity Scale for The Elderly, WOMAC = Western Ontario and McMaster’s Osteoarthritis Index
Primary Analyses
BML Volume and Walking Speed
Overall, adults who slowed their walking speed over one year had almost twice the odds of presenting with increased BML volume (adjusted odds ratio [OR]=1.8, 95% confidence interval [CI]=1.00, 3.20, Table 2). Specifically, adults with accelerated knee OA who slowed their walking speed had 3x the odds of increasing BML volume (OR=3.0, 95% CI=1.03, 8.95). However, in individuals who develop common knee OA or no knee OA, walking speed change was not significantly associated with a change in BML volume.
Table 2.
Association Between Longitudinal Walking Speed Change and Knee Structure Change.
| BML Change (Decrease/No Change = Reference) | ||||
| Group | Walking Speed Change | Decrease/No Change | Increase | Adjusted* Odds Ratio |
| n (%) | n (%) | OR (95%Cl) | ||
| Overall | Slower | 35 (58%) | 25 (42%) | 1.79 (1.00, 3.20) |
| Faster/No Change | 200 (70%) | 86 (30%) | Reference | |
| Accelerated Knee OA |
Slower | 7 (30%) | 16 (70%) | 3.04 (1.03, 8.95) |
| Faster/No Change | 46 (55%) | 37 (45%) | Reference | |
| Common Knee OA |
Slower | 15 (68%) | 7 (32%) | 1.17 (0.41, 3.32) |
| Faster/No Change | 72 (73%) | 27 (27%) | Reference | |
| No Knee OA |
Slower | 13 (87%) | 2 (13%) | 0.60 (0.12, 2.92) |
| Faster/No Change | 82 (79%) | 22 (21%) | Reference | |
|
Effusion Change (Decrease/No Change = Reference) | ||||
| Decrease/No Change | Increase | |||
| n (%) | n (%) | |||
| Overall | Slower | 36 (60%) | 24 (40%) | 1.48 (0.83, 2.66) |
| Faster/No Change | 195 (68%) | 91 (32%) | Reference | |
| Accelerated Knee OA |
Slower | 6 (26%) | 17 (74%) | 3.39 (1.10, 10.49) |
| Faster/No Change | 41 (49%) | 42 (51%) | Reference | |
| Common Knee OA |
Slower | 17 (77%) | 5 (23%) | 0.57 (0.19, 1.73) |
| Faster/No Change | 67 (68%) | 32 (32%) | Reference | |
| No Knee OA |
Slower | 13 (87%) | 2 (13%) | 0.80 (0.16, 4.02) |
| Faster/No Change | 87 (84%) | 17 (16%) | Reference | |
|
CDI Change (Increase/No Change = Reference) | ||||
| Decrease | Increase/No Change | |||
| n (%) | n (%) | |||
| Overall | Slower | 18 (30%) | 42 (70%) | 0.89 (0.48, 1.66) |
| Faster/No Change | 97 (34%) | 189 (66%) | Reference | |
| Accelerated Knee OA |
Slower | 12 (52%) | 11 (48%) | 0.81 (0.30, 2.16) |
| Faster/No Change | 51 (61%) | 32 (39%) | Reference | |
| Common Knee OA |
Slower | 4 (18%) | 18 (82%) | 0.75 (0.22, 2.55) |
| Faster/No Change | 22 (22%) | 77 (78%) | Reference | |
| No Knee OA |
Slower | 2 (13%) | 13 (87%) | 0.49 (0.10, 2.35) |
| Faster/No Change | 24 (23%) | 80 (77%) | Reference | |
CDI = cartilage damage index, BML = bone marrow lesion, OR = odds ratio, 95% CI = 95% confidence intervals, OA = osteoarthritis,
Adjusted for baseline age, body mass index, WOMAC pain, and physical activity
Effusion Volume and Walking Speed
Adults with accelerated knee OA who slowed their walking speed had 3.4 greater odds of presenting with increased effusion volume (OR=3.4, 95% CI=1.10, 10.49, Table 2). However, in individuals who develop common knee OA or no knee OA, walking speed change was not significantly associated with a change in effusion volume.
CDI and Walking Speed
Walking speed change was not significantly associated with CDI change (Table 2).
The results of our post hoc linear regressions using the continuous structural variables agreed with our primary results (Supplemental Table).
Sensitivity Analyses
Neither of the sensitivity analyses that included “frequent knee pain” or injury as a covariate significantly altered the odds ratios observed in our primary results.
Individuals Who Developed Accelerated Knee OA Within One Year
Similar trends with stronger odds ratios were observed when limiting our analysis to individuals with accelerated knee OA who progressed from KL 0/1 to KL 3/4 within one year and their matched individuals in the common knee OA and no knee OA groups (Table 3). Adults with accelerated knee OA who slowed their walking speed had 9.3x (95% CI=1.52, 56.50) and 6.4x (95% CI=1.04, 39.38) greater odds of presenting with increased BML and effusion volume, respectively. CDI change and walking speed change were not significantly associated in any of the groups.
Table 3.
Association Between Longitudinal Walking Speed Change and Knee Structure Change Among Individuals Who Developed Accelerated Knee Osteoarthritis Within One Year./Table_Caption>
| BML Change (Decrease/No Change = Reference) | ||||
| Group | Walking Speed Change | Decrease/No Change | Increase | Adjusted* Odds Ratio |
| n (%) | n (%) | OR (95%CI) | ||
| Overall | Slower | 35 (58%) | 25 (42%) | 1.79 (1.00, 3.20) |
| Faster/No Change | 200 (70%) | 86 (30%) | Reference | |
| Accelerated Knee OA |
Slower | 7 (30%) | 16 (70%) | 3.04 (1.03, 8.95) |
| Faster/No Change | 46 (55%) | 37 (45%) | Reference | |
| Common Knee OA |
Slower | 15 (68%) | 7 (32%) | 1.17 (0.41, 3.32) |
| Faster/No Change | 72 (73%) | 27 (27%) | Reference | |
| No Knee OA |
Slower | 13 (87%) | 2 (13%) | 0.60 (0.12, 2.92) |
| Faster/No Change | 82 (79%) | 22 (21%) | Reference | |
|
Effusion Change (Decrease/No Change = Reference) | ||||
| Decrease/No Change | Increase | |||
| n (%) | n (%) | |||
| Overall | Slower | 36 (60%) | 24 (40%) | 1.48 (0.83, 2.66) |
| Faster/No Change | 195 (68%) | 91 (32%) | Reference | |
| Accelerated Knee OA |
Slower | 6 (26%) | 17 (74%) | 3.39 (1.10, 10.49) |
| Faster/No Change | 41 (49%) | 42 (51%) | Reference | |
| Common Knee OA |
Slower | 17 (77%) | 5 (23%) | 0.57 (0.19, 1.73) |
| Faster/No Change | 67 (68%) | 32 (32%) | Reference | |
| No Knee OA |
Slower | 13 (87%) | 2 (13%) | 0.80 (0.16, 4.02) |
| Faster/No Change | 87 (84%) | 17 (16%) | Reference | |
|
CDI Change (Increase/No Change = Reference) | ||||
| Decrease | Increase/No Change | |||
| n (%) | n (%) | |||
| Overall | Slower | 18 (30%) | 42 (70%) | 0.89 (0.48, 1.66) |
| Faster/No Change | 97 (34%) | 189 (66%) | Reference | |
| Accelerated Knee OA |
Slower | 12 (52%) | 11 (48%) | 0.81 (0.30, 2.16) |
| Faster/No Change | 51 (61%) | 32 (39%) | Reference | |
| Common Knee OA |
Slower | 4 (18%) | 18 (82%) | 0.75 (0.22, 2.55) |
| Faster/No Change | 22 (22%) | 77 (78%) | Reference | |
| No Knee OA |
Slower | 2 (13%) | 13 (87%) | 0.49 (0.10, 2.35) |
| Faster/No Change | 24 (23%) | 80 (77%) | Reference | |
CDI = cartilage damage index, BML = bone marrow lesion, OR = odds ratio, 95% CI = 95% confidence intervals, OA = osteoarthritis,
Adjusted for baseline age, body mass index, WOMAC Pain, and physical activity
Individuals with No Radiographic OA Bilaterally at Baseline
Similar trends with stronger odds ratios were observed when limiting our analysis to individuals without radiographic knee OA at baseline (KL 0/1) and their matched individuals in the common knee OA and no knee OA groups (Table 4). Adults with accelerated knee OA who slowed their walking speed had 5.7x (95%CI=1.00, 32.39) the odds of presenting with increased BML volume. However, due to the loss of power with this sensitivity analysis the association between declining walking speed and increasing effusion volume had wide confidence intervals that crossed 1 (OR = 6.38; 95%CI=0.96, 42.33). CDI change and walking speed change were not significantly associated in any of the groups.
Table 4.
Association Between Longitudinal Walking Speed Change and Knee Structure Change in Individuals with no Radiographic Knee OA Bilaterally at baseline (KL = 0/1).
| BML Change (Decrease/No Change = Reference) | ||||
| Group | Walking Speed Change | Decrease/No Change | Increase | Adjusted* Odds Ratio |
| n (%) | n (%) | OR (95%CI) | ||
| Overall | Slower | 10 (40%) | 15 (60%) | 3.67 (1.47, 9.19) |
| Faster/No Change | 85 (69%) | 38 (31%) | Reference | |
| Accelerated Knee OA |
Slower | 3 (25%) | 9 (75%) | 5.68 (1.00, 32.39) |
| Faster/No Change | 19 (54%) | 16 (46%) | Reference | |
| Common Knee OA |
Slower | 6 (50%) | 6 (50%) | 2.11 (0.47, 9.57) |
| Faster/No Change | 29 (73%) | 11 (27%) | Reference | |
| No Knee OA |
Slower | 1 (100%) | 0 (0%) | Unable to Calculate |
| Faster/No Change | 37 (77%) | 11 (23%) | ||
|
Effusion Change (Decrease/No Change = Reference) | ||||
| Decrease/No Change | Increase | |||
| n (%) | n (%) | |||
| Overall | Slower | 14 (56%) | 11 (44%) | 1.95 (0.78, 4.85) |
| Faster/No Change | 85 (69%) | 38 (31%) | Reference | |
| Accelerated Knee OA |
Slower | 4 (33%) | 8 (67%) | 6.37 (0.96, 42.33) |
| Faster/No Change | 19 (54%) | 16 (46%) | Reference | |
| Common Knee OA |
Slower | 9 (75%) | 3 (25%) | 0.49 (0.10, 2.34) |
| Faster/No Change | 27 (68%) | 13 (32%) | Reference | |
| No Knee OA |
Slower | 1 (100%) | 0 (0%) | Unable to Calculate |
| Faster/No Change | 39 (81%) | 9 (19%) | ||
|
CDI Change (Increase/No Change = Reference) | ||||
| Decrease | Increase/No Change | |||
| n (%) | n (%) | |||
| Overall | Slower | 9 (36%) | 16 (64%) | 1.34 (0.52, 3.43) |
| Faster/No Change | 40 (33%) | 83 (67%) | Reference | |
| Accelerated Knee OA |
Slower | 7 (58%) | 5 (42%) | 1.37 (0.31, 5.98) |
| Faster/No Change | 19 (54%) | 16 (46%) | Reference | |
| Common Knee OA |
Slower | 2 (17%) | 10 (83%) | 0.74 (0.11, 4.85) |
| Faster/No Change | 9 (23%) | 31 (77%) | Reference | |
| No Knee OA |
Slower | 0 (1%) | 1 (100%) | Unable to Calculate |
| Faster/No Change | 12 (25%) | 36 (75%) | ||
CDI = cartilage damage index, BML = bone marrow lesion, OR = odds ratio, 95% CI = 95% confidence intervals, OA = osteoarthritis,
Adjusted for baseline age, body mass index, WOMAC pain, and physical activity
Excluding the Individuals that were Included after Imputing Missing Structural Data
Similar trends with stronger odds ratios were observed when excluding the individuals that were included after imputing their missing structural data (Table 5). Adults with accelerated knee OA who slowed their walking speed had 3.6x (95%CI=1.11, 11.62) odds of presenting with increased effusion volume. However, due to the loss of power with this sensitivity analysis, the association between declining walking speed and increasing BML volume had wide confidence intervals that crossed 1 (OR = 3.04; 95%CI=0.99, 9.30). CDI change and walking speed change were not significantly associated in any of the groups.
Table 5.
Association Between Longitudinal Walking Speed Change and Knee Structure Change Excluding Individuals with Imputed Missing Structural Data.
| BML Change (Decrease/No Change = Reference) | ||||
| Group | Walking Speed Change | Decrease/No Change | Increase | Adjusted* Odds Ratio |
| n (%) | n (%) | OR (95%CI) | ||
| Overall | Slower | 34 (57%) | 26 (43%) | 1.95 (1.09, 3.49) |
| Faster/No Change | 192 (70%) | 81 (30%) | Reference | |
| Accelerated Knee OA |
Slower | 7 (30%) | 16 (70%) | 3.04 (0.99, 9.30) |
| Faster/No Change | 39 (54%) | 33 (46%) | Reference | |
| Common Knee OA |
Slower | 14 (64%) | 8 (36%) | 1.50 (0.54, 4.21) |
| Faster/No Change | 71 (73%) | 26 (27%) | Reference | |
| No Knee OA |
Slower | 13 (87%) | 2 (13%) | 0.60 (0.12, 2.92) |
| Faster/No Change | 82 (79%) | 22 (21%) | Reference | |
|
Effusion Change (Decrease/No Change = Reference) | ||||
| Decrease/No Change | Increase | |||
| n (%) | n (%) | |||
| Overall | Slower | 37 (62%) | 23 (38%) | 1.44 (0.80, 2.61) |
| Faster/No Change | 189 (69%) | 84 (31%) | Reference | |
| Accelerated Knee OA |
Slower | 6 (26%) | 17 (74%) | 3.59 (1.11, 11.62) |
| Faster/No Change | 35 (49%) | 37 (51%) | Reference | |
| Common Knee OA |
Slower | 17 (77%) | 5 (23%) | 0.59 (0.20, 1.79) |
| Faster/No Change | 66 (68%) | 31 (32%) | Reference | |
| No Knee OA |
Slower | 14 (93%) | 1 (7%) | 0.40 (0.05, 3.44) |
| Faster/No Change | 88 (85%) | 16 (15%) | Reference | |
|
CDI Change (Increase/No Change = Reference) | ||||
| Decrease | Increase/No Change | |||
| n (%) | n (%) | |||
| Overall | Slower | 19 (32%) | 41 (68%) | 0.98 (0.53, 1.81) |
| Faster/No Change | 91 (33%) | 182 (67%) | Reference | |
| Accelerated Knee OA |
Slower | 12 (52%) | 11 (48%) | 0.84 (0.31, 2.30) |
| Faster/No Change | 45 (63%) | 27 (37%) | Reference | |
| Common Knee OA |
Slower | 5 (23%) | 17 (77%) | 1.00 (0.32, 3.13) |
| Faster/No Change | 22 (23%) | 75 (77%) | Reference | |
| No Knee OA |
Slower | 2 (13%) | 13 (87%) | 0.49 (0.10, 2.35) |
| Faster/No Change | 24 (23%) | 80 (77%) | Reference | |
CDI = cartilage damage index, BML = bone marrow lesion, OR = odds ratio, 95% CI = 95% confidence intervals, OA = osteoarthritis,
Adjusted for baseline age, body mass index, frequent pain, and physical activity
DISCUSSION
Individuals with accelerated knee OA who slowed their walking speed had 3.0x and 3.4x greater odds of demonstrating an increase in BML and effusion volume, respectively, when compared to individuals who did not decrease their walking speed. However, there was not a significant association between change in walking speed and cartilage damage in individuals developing accelerated knee OA. Additionally, individuals with no knee OA or common knee OA demonstrated no significant associations between a change in walking speed and any of our knee structural measures. These findings build upon a growing body of work that indicates a stark difference between individuals that develop accelerated and common knee OA,(8, 10, 21–24) which highlights the potential need for future studies to separately analyze these individuals. These results are important as they indicate that a one-year change in an easy, clinically accessible examination (i.e. 20m walk) is associated with concurrent worsening in BML and effusion volume in adults developing accelerated knee OA.
Walking speed has been labelled a “functional vital sign”(25) because this physical function measure has been linked to the prediction of falls,(26) hospitalization,(27) and mortality(28) in older individuals. In knee OA specifically, declining walking speed is associated with decreased knee confidence,(29) radiographic development of disease,(29) and likelihood to undergo a knee replacement.(3) This is the first study linking declining walking speed with concurrent worsening of specific knee structural measures in individuals with knee OA. The mechanisms leading to this association between walking speed and knee structure are unknown, but we foresee two possibilities: 1) declining walking speed is creating altered knee loading(30–32) that leads to worsening knee structure, or 2) the worsening knee structure is leading to a protective gait strategy that decreases walking speed to minimize loading of the joint. Future work is needed to tease out the causality of this association. Understanding this causality may lead to the development of two treatment possibilities: interventions targeting the maintenance of walking speed to prevent pathologic joint loading created by slower walking speed or interventions that decrease BML and effusion volume to prevent the decline in walking speed.
Even though walking speed decline was associated with worsening BML and effusion volume in individuals that developed accelerated knee OA, there was no significant association between walking speed decline and knee articular cartilage in any group. While we observed no significant association between walking speed and articular cartilage, prior cross-sectional studies and prognostic studies have suggested a link between walking speed and cartilage health.(33, 34) Specifically, slower walking speed is significantly associated with both worse cartilage composition(33) (i.e. T1rho relaxation times) and serum biomarkers of cartilage metabolism(34) (i.e. ratio of type II collagen degradation to synthesis) in individuals at high risk of knee OA (i.e. young adults with a history of an anterior cruciate ligament reconstruction). Additionally, habitual walking speed is associated with acute femoral cartilage deformation following a 30-minute treadmill walk,(35) indicating that walking speed may play a role in cartilage loading that is important in the maintenance of cartilage health. Our analysis determined the association between concurrent change in walking speed and change in cartilage structure, which is a more robust analysis technique than previous cross-sectional studies.(33–35) However, this difference in analysis may be one reason why we did not observe a significant association between changes in walking speed and articular cartilage.
Another reason for our lack of statistical significance is that the change in walking speed may be eliciting subtle changes in the cartilage composition that may precede changes in cartilage thickness.(36) Declines in cartilage thickness may be a downstream event that occur later in the structural progression of knee OA following significant increases in BML and effusion volume. Therefore, further studies are needed to determine if changes in walking speed are associated with more sensitive cartilage compositional metrics in individuals with knee OA. Since pain may influence the decline in walking speed and early cartilage damage is typically not painful,(37) this may be another reason why there appears to be no association between change in walking speed and change in cartilage damage. Future studies should explore whether the change in walking speed contributes to future loss of articular cartilage.
While these results indicate that a decline in walking speed is associated with concurrent worsening of BML and effusion volume in individuals with accelerated knee OA, there are some limitations we must acknowledge. We only included individuals that completed the walking speed assessment and MR protocol at their index and year prior visit. Thus, the individuals with potentially the largest change in outcomes may have been omitted from our analysis due to their inability to complete the study protocol. However, we expanded our analysis to the most proximate visit in the individuals with missing data (n=13) to reclaim some of these excluded individuals. Due to our analysis using concurrent changes in walking speed and knee structure, we are unable to determine if one outcome is generating the change in the other outcome. Future research is needed to determine if slower walking speed is creating the alterations in knee structure, or if early decline in knee structure are leading to declines in walking speed. Previous investigations have determined that individuals with accelerated knee OA oftentimes have self-reported and MR-detected knee injuries,(38) which may influence the change in the BML, effusion, and cartilage structure.
In conclusion, these results highlight a significant link between a decline in a clinically accessible physical function measure (i.e. walking speed) and specific changes in knee structure in individuals that develop accelerated knee OA. Specifically, walking speed decline was associated with concurrent worsening BML and effusion volume over the year prior to the development of accelerated knee OA, but not in individuals with incident common KOA or no knee OA. Additionally, cartilage structure changes were not associated with walking speed decline in any group. Future studies are needed to determine if interventions that target the declining walking speed will also create concurrent improvements in knee structure outcomes, and vice versa.
Supplementary Material
SIGNIFICANCE & INNOVATION.
Understanding whether early changes in walking speed are associated with sensitive measures of knee structure may provide a better understanding of the early link to physical function and joint health decline.
Individuals with accelerated knee osteoarthritis present with earlier worsening of knee structure as well as poorer patient-reported and physical function measures compared to individuals with a more common, gradual onset of knee osteoarthritis.
Individuals with accelerated knee osteoarthritis who slowed their walking speed were 3.0 and 3.4 times more likely to demonstrate an increase in BML and effusion volume, respectively.
Cartilage structure changes were not associated with walking speed decline in individuals with accelerated or common knee osteoarthritis.
ACKNOWLEDGEMENTS
We thank Fatimah Al Eid for her assistance with the quantitative effusion volume measurements.
Grant Support: These analyses were financially supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR065977. The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners. This work was also supported in part by the Houston Veterans Affairs Health Services Research and Development Center of Excellence (HFP90–020). The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors have no other conflicts of interest regarding this work.
REFERENCES
- 1.White DK, Niu J, Zhang Y. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis Care Res 2013;65(2):187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herzog MM, Driban JB, Cattano NM, Cameron KL, Tourville TW, Marshall SW, et al. Risk of Knee Osteoarthritis Over 24 Months in Individuals Who Decrease Walking Speed During a 12-Month Period: Data from the Osteoarthritis Initiative. J Rheumatol. 2017;44(8):1265-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeni JA, Jr., Axe MJ, Snyder-Mackler L Clinical predictors of elective total joint replacement in persons with end-stage knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driban JB, Lo GH, Lee J, Ward RJ, Miller E, Pang J, et al. Quantitative bone marrow lesion size in osteoarthritic knees correlates with cartilage damage and predicts longitudinal cartilage loss. BMC Musculoskelet Disord. 2011;12(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252(3):772-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein JS, Jose J, Baraga MG, Subhawong TK. Baseline Cartilage Thickness and Meniscus Extrusion Predict Longitudinal Cartilage Loss by Quantitative Magnetic Resonance Imaging: Data From the Osteoarthritis Initiative. J Comput Assist Tomogr. 2016;40(6):979-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Driban JB, Eaton CB, Lo GH, Ward RJ, Lu B, McAlindon TE. Association of knee injuries with accelerated knee osteoarthritis progression: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2014;66(11):1673-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driban JB, Price LL, Eaton CB, Lu B, Lo GH, Lapane KL, et al. Individuals with incident accelerated knee osteoarthritis have greater pain than those with common knee osteoarthritis progression: data from the Osteoarthritis Initiative. Clin Rheumatol. 2016;35(6):1565-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driban JB, Stout AC, Lo GH, Eaton CB, Price LL, Lu B, et al. Best performing definition of accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Ther Adv Musculoskelet Dis. 2016;8(5):165-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Driban JB, Ward RJ, Eaton CB, Lo GH, Price LL, Lu B, et al. Meniscal extrusion or subchondral damage characterize incident accelerated osteoarthritis: Data from the Osteoarthritis Initiative. Clin Anat 2015;28(6):792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging--the osteoarthritis initiative. Nat Rev Rheumatol. 2012;8(10):622-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Osteoarthritis Initiative [Accessed 7 Dec 2016]. Available from: http://oai.epi-ucsf.org/.
- 14.Collins JE, Losina E, Nevitt MC, Roemer FW, Guermazi A, Lynch JA, et al. Semiquantitative Imaging Biomarkers of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 2016;68(10):2422-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felson DT, Nevitt MC. Blinding images to sequence in osteoarthritis: evidence from other diseases. Osteoarthritis Cartilage. 2009;17(3):281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pang J, Driban JB, Destenaves G, Miller E, Lo GH, Ward RJ, et al. Quantification of bone marrow lesion volume and volume change using semi-automated segmentation: data from the osteoarthritis initiative. BMC Musculoskelet Disord. 2013;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Driban JB, Price L, Lo GH, Pang J, Hunter DJ, Miller E, et al. Evaluation of bone marrow lesion volume as a knee osteoarthritis biomarker--longitudinal relationships with pain and structural changes: data from the Osteoarthritis Initiative. Arthritis Res Ther. 2013;15(5):R112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang M, Driban JB, Price LL, Lo GH, Miller E, McAlindon TE. Development of a Rapid Cartilage Damage Quantification Method for the Lateral Tibiofemoral Compartment Using Magnetic Resonance Images: Data from the Osteoarthritis Initiative. Biomed Res Int. 2015;2015:634275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang M, Driban JB, Price LL, Harper D, Lo GH, Miller E, et al. Development of a rapid knee cartilage damage quantification method using magnetic resonance images. BMC Musculoskelet Disord. 2014;15:264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connolly A, FitzPatrick D, Moulton J, Lee J, Lerner A. Tibiofemoral cartilage thickness distribution and its correlation with anthropometric variables. Proc Inst Mech Eng H. 2008;222(1):29-39. [DOI] [PubMed] [Google Scholar]
- 21.Davis J, Eaton CB, Lo GH, Lu B, Price LL, McAlindon TE, et al. Knee symptoms among adults at risk for accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2017;36(5):1083-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Driban JB, McAlindon TE, Amin M, Price LL, Eaton CB, Davis JE, et al. Risk factors can classify individuals who develop accelerated knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res. 2018;36(3):876-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driban JB, Eaton CB, Amin M, Stout AC, Price LL, Lu B, et al. Glucose homeostasis influences the risk of incident knee osteoarthritis: Data from the osteoarthritis initiative. J Orthop Res. 2017;35(10):2282-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Driban JB, Stout AC, Duryea J, Lo GH, Harvey WF, Price LL, et al. Coronal tibial slope is associated with accelerated knee osteoarthritis: data from the Osteoarthritis Initiative. BMC Musculoskelet Disord. 2016;17:299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guimaraes RM, Isaacs B. Characteristics of the gait in old people who fall. Int Rehabil Med 1980;2(4):177-80. [DOI] [PubMed] [Google Scholar]
- 27.Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2005;60(10):1304-9. [DOI] [PubMed] [Google Scholar]
- 28.Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA. Improvement in usual gait speed predicts better survival in older adults. J Am Geriatr Soc. 2007;55(11):1727-34. [DOI] [PubMed] [Google Scholar]
- 29.Colbert CJ, Song J, Dunlop D, Chmiel JS, Hayes KW, Cahue S, et al. Knee confidence as it relates to physical function outcome in persons with or at high risk of knee osteoarthritis in the osteoarthritis initiative. Arthritis Rheum. 2012;64(5):1437-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chung MJ, Wang MJ. The change of gait parameters during walking at different percentage of preferred walking speed for healthy adults aged 20–60 years. Gait Posture. 2010;31(1):131-5. [DOI] [PubMed] [Google Scholar]
- 31.Chiu MC, Wang MJ. The effect of gait speed and gender on perceived exertion, muscle activity, joint motion of lower extremity, ground reaction force and heart rate during normal walking. Gait Posture. 2007;25(3):385-92. [DOI] [PubMed] [Google Scholar]
- 32.Cook TM, Farrell KP, Carey IA, Gibbs JM, Wiger GE. Effects of restricted knee flexion and walking speed on the vertical ground reaction force during gait. J Orthop Sports Phys Ther. 1997;25(4):236-44. [DOI] [PubMed] [Google Scholar]
- 33.Pfeiffer S, Harkey MS, Stanley LE, Blackburn JT, Padua DA, Spang JT, et al. Associations between Slower Walking Speed and T1rho Magnetic Resonance Imaging of Femoral Cartilage following Anterior Cruciate Ligament Reconstruction. Arthritis Care Res 2017; Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pietrosimone B, Troy Blackburn J, Harkey MS, Luc BA, Hackney AC, Padua DA, et al. Walking Speed As a Potential Indicator of Cartilage Breakdown Following Anterior Cruciate Ligament Reconstruction. Arthritis Care Res 2016;68(6):793-800. [DOI] [PubMed] [Google Scholar]
- 35.Harkey MS, Blackburn JT, Davis H, Sierra-Arevalo L, Nissman D, Pietrosimone B. The association between habitual walking speed and medial femoral cartilage deformation following 30 minutes of walking. Gait Posture 2018;59:128-33. [DOI] [PubMed] [Google Scholar]
- 36.Guermazi A, Alizai H, Crema MD, Trattnig S, Regatte RR, Roemer FW. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthritis Cartilage. 2015;23(10):1639-53. [DOI] [PubMed] [Google Scholar]
- 37.Eckstein F, Collins JE, Nevitt MC, Lynch JA, Kraus VB, Katz JN, et al. Brief Report: Cartilage Thickness Change as an Imaging Biomarker of Knee Osteoarthritis Progression: Data From the Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 2015;67(12):3184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis JE, Harkey MS, Ward RJ, Mackay JW, Bing LU, Price L, et al. Characterizing the distinct structural changes associated with self‐reported knee injury among individuals with incident knee osteoarthritis: Data from the osteoarthritis initiative. Clin Anat 2018;31(3):330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.