Abstract
We have previously shown that antimicrobial photodynamic therapy (aPDT) mediated by different photosensitizers can be potentiated by a variety of inorganic salts. Potassium thiocyanate potentiated aPDT mediated by methylene blue (MB), while potassium selenocyanate potentiated aPDT mediated by MB, Rose Bengal and the anionic porphyrin TPPS4. However, the mechanisms of action that were proposed were fundamentally different.
In the present study we compare these two salts (KSCN and KSeCN) with different light-activated photosensitizers (PS) and different oxidative reactions for killing Gram-positive and Gram-negative bacteria. Overall KSeCN was more powerful than KSCN, and worked with a wider range of PS, while KSCN only worked with phenothiazinium salts. KSeCN produced killing when cells were added after light suggesting production of a semi-stable species called selenocyanogen, (SeCN)2. We tested three different oxidative reactions that can all potentially kill bacteria: lead tetraacetate; Fenton reagent (hydrogen peroxide and ferrous sulfate); hydrogen peroxide and horseradish peroxidase (H2O2/HRP). In every case KSeCN was substantially more effective (several logs) than KSCN in potentiating the bacterial killing. We conclude that (SeCN)2 is the mediator for aPDT using KSeCN, while sulfur trioxide radical anion is the mediator for KSCN using phenothiaziums. For H2O2/HRP with KSCN, hypothiocyanite (OSCN-) is proposed to be the antibacterial agent in the literature, while hyposelenocyanite is said not to exist. Lead tetracetate is known to produce (SeCN)2 from KSeCN as well as the analogous (SCN)2 from KSCN. The mediators from Fenton reaction are unclear (pseudohalogen radical ions?) Both KSCN (which occurs naturally in the human body) and KSeCN may be clinically applicable.
Keywords: Antimicrobial photodynamic therapy, potassium thiocyanate, potassium selenocyanate, phenothiazinium dyes, lead tetraacetate, horseradish peroxidase/hydrogen peroxide, Fenton reagent
Introduction
The ever-increasing risk of multi-drug resistant pathogens has been called “a global health crisis” and has been forecast to cost the world $100 trillion and cause ten million extra deaths by 2050 if nothing is done about it [1]. This alarming prospect has spurred a search for alternative antimicrobial technologies to which bacteria will not be able to develop resistance, and which are unaffected by the existing antibiotic-resistance status [2]. One of the best candidate technologies for this role (especially for localized infections) is antimicrobial photodynamic therapy (aPDT) (sometimes called antimicrobial photodynamic inactivation) [3]. aPDT involves the combination of non-toxic photosensitizers (PS) with harmless visible light to produce reactive oxygen species (ROS) by the long-lived triplet state interacting with ambient oxygen [4]. The photochemical reactions are divided into Type I involving electron transfer to produce superoxide, hydrogen peroxide and hydroxyl radicals, and Type II involving energy transfer to produce singlet oxygen. Both types of ROS can damage biomolecules (lipids, proteins and nucleic acids) to kill cells. aPDT relies on cationic PS that bind and penetrate Gram-negative bacterial cells, while Gram-positive bacterial cells are susceptible to killing by all water-compatible PS regardless of overall charge [5]. The use of aPDT for localized infections relies on designing PS that selectively bind to microbial cells, together with the use of topical administration into the infected area, and a short drug-light interval (because uptake into mammalian cells is a slower process than binding to bacteria).
We have recently reported that a surprisingly wide variety of inorganic salts can potentiate the aPDT killing of microbial cells [6]. This strategy can be applied to Gram-positive bacteria, Gram-negative bacteria and also to fungi, and is effective in animal models of localized infections [7, 8]. A variety of different salts can be employed, including potassium iodide [6, 7, 9–11], potassium bromide [12], sodium azide [13, 14], potassium thiocyanate [15] and potassium selenocyanate [16]. Potassium iodide, potassium bromide and potassium thiocyanate are non-toxic, potassium selenocyanate has low toxicity, while sodium azide has moderate toxicity. Thiocyanate and selenocyanate are known to be representative members of a class of polyatomic inorganic anions called “pseudohalides” [17]. Other members of this class are cyanide, isocyanide, cyanate, isocyanate, and azide. We had previously shown that aPDT mediated by MB could be potentiated by addition of 10 mM KSCN [15]. We have also previously reported [16] that KSeCN (at concentrations up to 100 mM) could potentiate aPDT mediated not only by MB, but also by RB and also by TPPS4. We recently reported [9] that the supposedly anionic porphyrin TPPS4 behaved more like a cationic porphyrin, and functioned as a surprisingly effective antimicrobial PS especially when potentiated by KI. Considering the similarities between the chemical behaviors of thiocyanate and selenocyanate, we wanted to compare the relative activities of these two salts in potentiating aPDT bacterial killing, and investigate whether the mechanisms of action were similar or different.
Material and Methods
Compounds and Light Sources
Methylene blue (MB), toluidine blue O (TBO), 1,9-dimethyl-methylene blue (DMMB) Rose Bengal (RB), 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin dihydrochloride (TPPS4), potassium selenocyanate (KSeCN), potassium thiocyanate (KSCN), lead tetraacetate {Pb(OAc)4 ferrous sulfate (FeSO4), hydrogen peroxide (H2O2) and horseradish peroxidase were all from Sigma-Aldrich (St. Louis, MO, USA). Pb(OAc)4 solution was prepared freshly each day. All PS stock solution (2.5 mM) were stored at 4°C in the dark for no more than 1 week. KSeCN, KSCN, Pb(OAc)4, FeSO4, HRP, H2O2 were freshly dissolved in distilled H2O each time. The light source we used for red and green light was a white lamp with a band-pass filter probe 660±15 nm or 540±15 nm (Lumacare, Newport Beach, CA, USA). For blue light we used an Omnilux Clear-U light-emitting diode (LED) array (Photo Therapeutics, Inc., Carlsbad, CA) at 415±15nm. The power densities were between 12–16 mW/cm2 measured with a power meter (Coherent, Santa Clara, California). The time to deliver 10 J/cm2 was 10–13 minutes.
Cells and culture conditions
The bacterial strains were: methicillin-resistant Staphylococcus aureus (MRSA) USA 300, Escherichia coli (E. coli) K-12 (ATCC 33780) A single colony of bacteria was grown overnight in 5 mL of brain heart infusion (BHI) broth in a shaker incubator (New Brunswick Scientific) then refreshed in BHI for 2-3 h at 37 °C to mid-log phase. Cell concentration was estimated by measuring the optical density (OD) at 600 nm (OD of 0.6 = 108 cells/mL). The bacterial suspension was centrifuged, washed, and resuspended in pH7.4 PBS to arrest microbial growth and 108 cells/mL used for experiments.
aPDT experiments
aPDT experiments were carried out either using 1 mL of cell suspension in wells of a 24-well plate, or using 100 μL of cell suspension in wells of a 96 well plate. The initial studies used aPDT with suspensions of bacteria (108 cells/mL) and one of three different PS (10 μM MB, 200 nM RB and 200 nM TPPS4 for Gram-positive MRSA cells; 10 μM MB, 10 μM RB or 200 nM TPPS4 for Gram-negative E. coli cells) incubated with KSCN or KSeCN at 10 mM in pH 7.4 PBS in the dark at room temperature for 30 min. Cells were then irradiated with 10 J/cm2 of red light (660 nm) for MB, 10 J/cm2 of green light (540 nm) for RB and 10 J/cm2 of blue light (415 nm) for TPPS4. At the end of illumination CFUs were determined according to the method of Jett et al [18]. The aliquots were serially diluted tenfold in PBS to give dilutions of 10−1 to 10−5 times in addition to the original concentration and 10 μL aliquots of each of the dilutions were streaked horizontally on square BHI agar plates. Plates were incubated for 12–18 h at 37 °C in the dark to allow colony formation. Each experiment was performed at least three times. The appropriate controls were conducted, bacterial cells with PS in the dark, bacterial cells with salt in the dark, bacterial cells with light and salt (no PS). No significant killing was observed in any of these experiments. There was zero microbial toxicity with either KCSN and KSeCN alone at concentrations up to 400 mM.
The next experiment employed 10 μM concentrations of MB, TBO, or DMMB irradiated by 10 J/cm2 of red light with increasing concentrations of KSCN or KSeCN. Then we used the same concentrations of MB, RB, TPPS4 and same light as before, except that a range of concentrations of KSCN or KSeCN were added before light.
For experiments where cells were added after light, we used 10 mM of either salt (KSCN or KSeCN) plus 10 μM MB concentration irradiated with 10 J/cm2 660 nm. After the end of illumination then immediately or at different time points we added an equal volume of E. coli or MRSA suspension in PBS (108 cells/mL) and mixed them thoroughly. After 30 min incubation, the aliquots were serially diluted as before.
Experiments with lead tetraacetate
Suspensions of bacteria (108 CFU/ml) in PBS were mixed with KSCN or KSeCN (10 mM) and then a range of concentrations of Pb(OAc)4 (up to 10 mM) were added, mixed and incubated at room temperature for 30 min. Alternatively a solution of 6 mM Pb(OAc)4 was mixed with 10 mM KSCN or KSecN and after different times an equal volume of bacterial suspension was added. After 30 min incubation at room temperature, the aliquots were serially diluted as before.
Horseradish peroxide/hydrogen peroxide treatment.
Bacteria (108 CFU/ml) were suspended in PBS mixed with 10 mM H2O2 and different concentrations of either KSCN and KSeCN. HRP (final concentration of 2.5 U/mL) was added and the mixture was incubated for 60 min before serial dilution.
Fenton reagent treatment. Bacteria (108 CFU/ml) were suspended in PBS mixed with either 11 mM H2O2 (for MRSA) or 9 mM H2O2 (for E. coli) and different concentrations of either KSCN and KSeCN. Next a solution of FeSO4 (final concentration 11 mM for MRSA, or 9 mM for E. coli) was added and the mixture incubated for 60 min before serial dilution.
Results
aPDT experiments
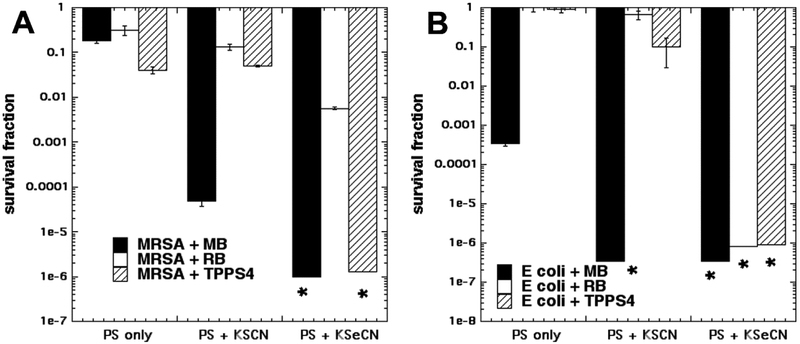
Therefore, the first task here was to ask whether KSCN could potentiate aPDT mediated by other PS such as RB and TPPS4. Figure 1 shows the results. The choice of aPDT dose (combination of PS concentration and light fluence) in a salt potentiation experiment should be selected to kill only a relatively small number of bacterial cells (about 1–2 logs) in order that a larger degree of potentiation can be observed. Fig 1A shows the results with Gram-positive MRSA. aPDT alone. For MB we used 10 μM and 10 J/cm2 of 660 nm light, while for RB we used 200 nM and 10 J/cm2 540 nm light and for TPPS4 we used 200 nM and 10 J/cm2 of 415 nm light. KSCN at 10 mM did give 3 logs of potentiation of MRSA killing but with neither RB nor with TPPS4 did we see any potentiation with addition of 10 mM KSCN. By contrast with KSeCN both MB and TPPS4 gave eradication and RB gave 2 logs of potentiation.
Figure 1. Comparison of KSCN and KSeCN to potentiate aPDT killing using three different PS.
Cells were incubated for 30 min with KSCN or KSeCN at 100 mM plus MB at 10 μM or RB at 200 nm for MRSA or 10 μM for E. coli, and then irradiated with 10 J/cm2 of light (660 nm for MB; 540 nm for RB; 415 nm for TPPS4). (A) Gram-positive MRSA; (B) Gram-negative E. coli. * indicates eradication.
In Fig 1B for Gram-negative E. coli we used the same aPDT parameters except for the concentration of RB being raised to 10 μM. It can be seen that that the only killing with aPDT alone (no added salt) was in the case of MB where about 3 logs were achieved. When KSCN was added the only significant potentiation was seen with MB, where eradication was achieved, and no significant potentiation effect with RB and TPPS4. By contrast with KSeCN, excellent potentiation was observed with all three PS giving eradication (up to 6 logs extra killing)
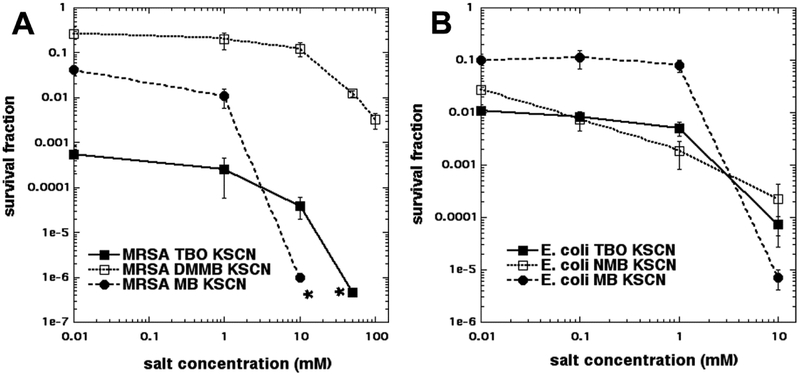
Since we had confirmed our previous result [15] that KSCN could potentiate MB-mediated aPDT, we wanted to test the hypothesis that there was something special about the phenothiazinium PS MB that did not apply with RB and TPPS4. This “something special” was proposed to be the ability to carry out a mixture of Type I and Type II photochemistry, as opposed to the predominantly Type II photochemistry seen with RB and TPPS4. Therefore, we selected another two different phenothiazinium dyes, toluidine blue (TBO) and dimethylmethylene blue (DMMB). We had previously published that aPDT mediated by these dyes could be paradoxically potentiated (rather than inhibited) by the addition of potassium azide, a well-known singlet oxygen quencher [13, 14]. It had become apparent that the concentration of salt required to achieve the maximum amount of potentiation may indeed be higher than the previously employed concentration of 10 mM. Therefore, in these studies we plotted the killing curves achieved with a fixed aPDT dose and increasing salt concentrations up to 100 mM. Figure 2A shows the results for MRSA, and it can be seen that the aPDT killing produced by all three phenothiazinium dyes was indeed able to be potentiated by KSCN, although MB still showed the biggest increase. In Figure 2B it can be seen that the results were broadly similar with E. coli with all 3 dyes giving potentiation and MB again giving the biggest increase.
Figure 2. Effect of increasing KSCN concentration on aPDT with three phenothiazinium dyes.
Cells were incubated for 30 min with MB, TBO, or DMMB at 10 μM, plus a range of concentrations of KSCN, and then irradiated with 10 J/cm2 of 660 nm light. (A) Gram-positive MRSA; (B) Gram-negative E. coli. * indicates eradication. The concentration of 0.01 mM salt was actually zero, but zero cannot be used on a logarithmic axis.
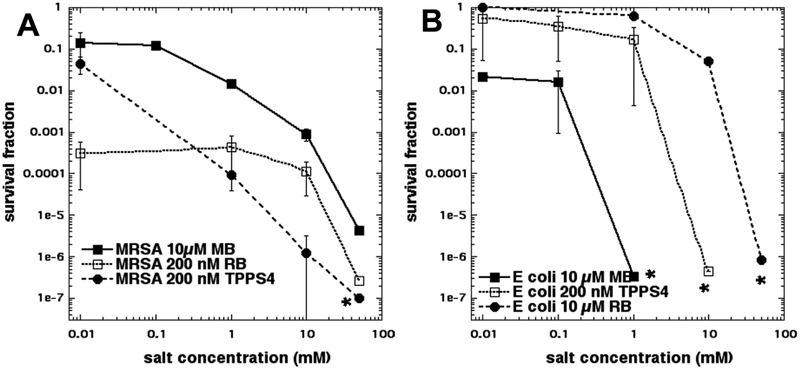
In order to compare the amount of potentiation found with KSCN with that given by KSeCN we carried out a similar set of experiments (increasing the salt concentration) with the 3 PS compounds used in Figure 1 (MB, RB, TPPS4). Figure 3A shows the results with MRSA where it can be seen that all 3 PS were significantly potentiated by KSeCN with TPPS4 giving the overall biggest effect at the lowest salt concentration. In Figure 3B the analogous results with E. coli are presented. Again, all three PS demonstrated a large degree of potentiation (up to 6 logs). The bigger potentiation seen with E. coli (compared to MRSA) is due to the basic aPDT parameters being less effective in the case of Gram-negative bacteria. Broadly speaking, comparing Figures 2 and 3 it can be appreciated that KSeCN was substantially more effective overall in producing potentiation of aPDT killing, than was KSCN.
Figure 3. Effect of increasing KSeCN concentration on aPDT with three photosensitizers.
Cells were incubated for 30 min with MB at 10 μM, or RB at 200 nm for MRSA or 10 μM for E. coli, or TPPS4 at 200 nM, plus KSeCN at increasing concentrations, and then irradiated with 10 J/cm2 of light (660 nm for MB; 540 nm for RB; 415 nm for TPPS4). (A) Gram-positive MRSA; (B) Gram-negative E. coli. * indicates eradication. The concentration of 0.01 mM salt was actually zero, but zero cannot be used on a logarithmic axis.
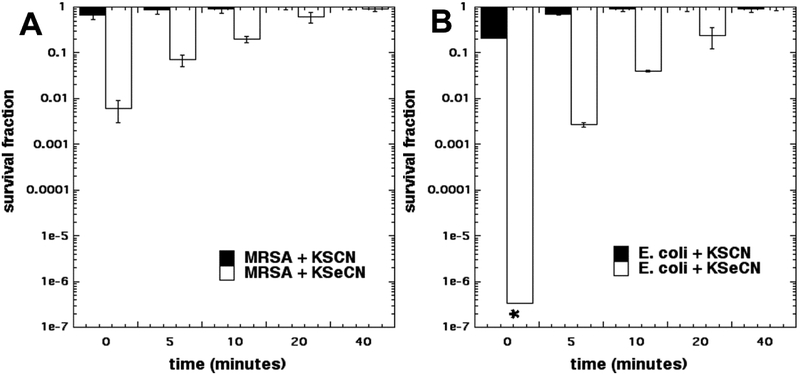
Next we wanted to investigate whether any bactericidal compound was formed by the action of aPDT-induced ROS (singlet oxygen) on the salt. We had previously shown [16] that in the case of KSeCN this was indeed the case, but that the antibacterial activity observed when the cells were added after light, decayed in a short period of time [16]. For this experiment we only used MB, as the PS as this was the only one that had been shown to be potentiated by both salts (KSCN and KSeCN) (Fig 1). In Figure 4A it can be seen that in the case of MRSA there was some killing (2 logs) when cells were added immediately to an irradiated mixture of MB and KSeCN (as soon as the light was switched off), but this bactericidal activity soon started to decay and was gone after 20 minutes. No significant killing was seen when cells were added to an irradiated mixture of MB and KSCN at any time. In Figure 4B it can be seen that E. coli was even more sensitive to the antibacterial substance produced by PDT treatment of KSeCN, than was MRSA. When cells were added immediately (t=0 min), eradication was achieved (>6 logs). The antibacterial activity against E. coli decayed on a similar time scale to that against MRSA and was all gone after 40 minutes. There was no killing observed when E. coli cells were added to MB + KSCN after the light (even immediately).
Figure 4. Bacterial killing when cells were added at different times after end of illumination of MB plus salts.
10 μM MB was mixed with 100mM KSCN or KSeCN, and irradiated with 10 J/cm2 of 660 nm light and then cells were added and incubated for 30 min. * indicates eradication.
Studies with lead tetraacetate
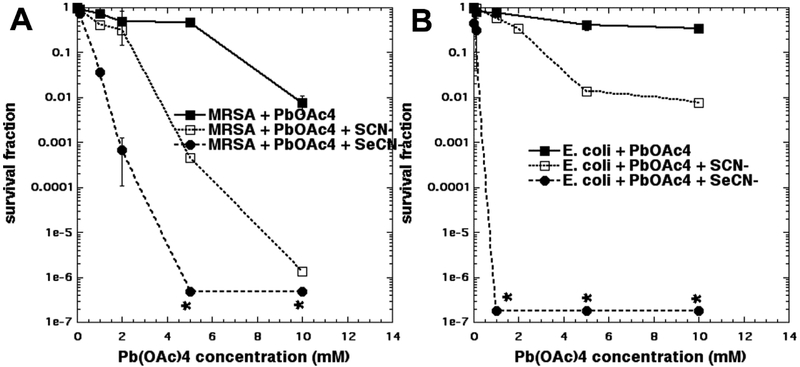
We had previously shown that lead tetraacetate, Pb(OAc)4 combined with KSeCN, could synergistically kill bacteria [16]. The reason for choosing Pb(OAc)4 was that it is mentioned as a literature synthetic procedure for the production of selenocyanogen (SeCN)2 from KSeCN [19, 20]. The production of (SeCN)2 was our principal hypothesis to explain why KSeCN potentiated aPDT. Therefore, it was of interest to compare the action of KSCN with KSeCN in the potentiation of bacterial killing produced by Pb(OAc)4. Figure 5A shows the results with MRSA. Both salts potentiated the bacterial killing, but KSeCN was significantly better than KSCN. For instance, at 2 mM Pb(OAc)4 KSeCN gave 3 logs of killing, while KSCN gave no killing, and at 5 mM Pb(OAc)4 KSeCN gave eradication, while KSCN gave 3 logs of killing, and Pb(OAc)4 gave no killing. For E. coli shown in Fig 5B the situation was even more clear-cut. With KSeCN the lowest concentration of Pb(OAc)4 tested (1 mM) gave eradication, and no killing was seen with KSCN or Pb(OAc)4 alone. The highest concentration of Pb(OAc)4 (10 mM) only gave 2 logs of killing with KSCN vs no killing with Pb(OAc)4 alone.
Figure 5. Effects of KSCN or KSeCN on Pb(OAc)4 mediated bacterial killing.
10 mM KSeCN or KSeCN or nothing was added to bacterial suspensions and increasing amounts of Pb(OAc)4 was added and incubated for 30 min. (A) MRSA; (B) E. coli. * indicates eradication.
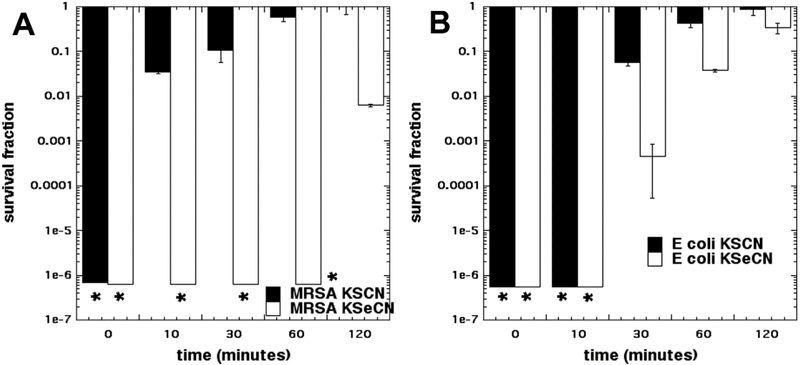
Next we wished to see if the product of reaction between Pb(OAc)4 and KSeCN or KSCN lost its antibacterial activity with time. The experiments shown in Figure 5 had been carried out by mixing a suspension of bacterial cells with KSeCN or KSCN and then adding a solution of Pb(OAc)4 and incubating for 30 min. However in Figure 6 we changed the order and mixed Pb(OAc)4 with KSeCN or KSCN and then added the cells either immediately, or after waiting periods of time up to 120 minutes. Figure 6A shows that Pb(OAc)4 with both KSeCN and KSCN killed MRSA when added immediately, but the antibacterial activity decayed more slowly with KSeCN (2 logs of killing remaining after 2 hours) and faster with KSCN (2 logs of killing after only 10 minutes). Similar results were found with E. coli in Figure 5B, where again the antibacterial activity produced with KSCN decayed faster than that produced from KSeCN. It should be noted that more killing was obtained with 10 mM Pb(OAc)4 and KSeCN when cells were added immediately after mixing (Fig 6B), than when 10 mM Pb(OAc)4 was added to cells + KSeCN (Fig 4B). The explanation for this observation is presumably that in the latter case part of the Pb(OAc)4 reacted with the bacteria leaving less reagent available to react with the KSeCN.
Figure 6. Bacterial killing when cells were added at different times after mixing Pb(OAc)4 with salts.
6mM Pb(OAc)4 and 10 mM KSCN or KSeCN were mixed, and after different times an equal volume of bacterial suspension was added and incubated for 30 min. (A) MRSA; (B) E. coli. * indicates eradication.
Studies with horseradish peroxidase/hydrogen peroxide.
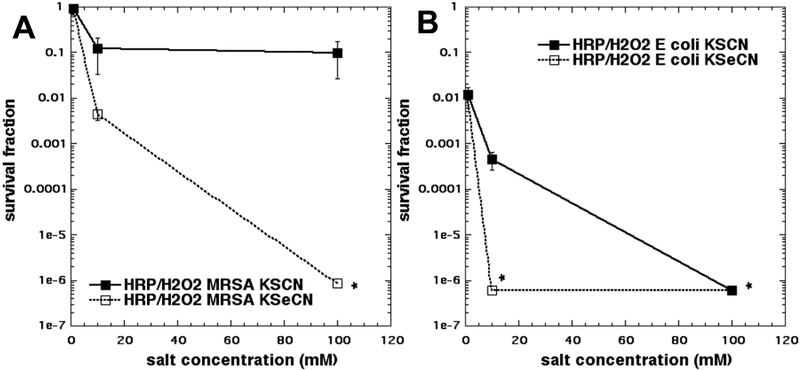
It has been reported many times that various peroxidase enzymes (such as lactoperoxidase, myeloperoxidase, and horseradish peroxidase) in combination with hydrogen peroxide, can oxidize thiocyanate anion to an alternative pseudohalide anion called hypothiocyanite, [OSCN]− (an analogue of hypochlorite [OCI]−) [21]. [OSCN]− is a well-known antibacterial substance that is proposed to be responsible for one of the naturally-occurring antimicrobial immune systems operating in the human oral cavity and respiratory tract [22]. It is formed by the action of lactoperoxidase on thiocyanate which occurs naturally in the saliva and bronchial fluid [23]. We were not able to trace any publication dealing with the action of any peroxidase on selenocyanate. One paper did state “the corresponding selenium species (hyposelenocyanite) is not known” [24]. For the present studies we chose to use horseradish peroxidase despite reports of its “low catalytic turnover” [25] solely because of its easy availability (as compared to lactoperoxidase and meyloperoxidase). Figure 7A shows that KSeCN gave much higher killing than KSCN against MRSA, with eradication achieved with 100 mM KSeCN as compared to only one log of killing with KSCN. Figure 7B shows similar findings with E. coli, except that the overall killing effects were much higher. With 10 mM salts 3 logs of killing were obtained with KSCN compared to eradication with KSeCN. At 100 mM concentration. eradication was seen with both salts. There was no bacterial killing with peroxidase and H2O2 without any added salt (data not shown).
Figure 7. Effects of KSCN or KSeCN on potentiating bacterial killing mediated by horseradish peroxidase/hydrogen peroxide.
Cells were mixed with 10 mM H2O2 and different concentrations of KSCN or KSeCN, and then 2.5 IU/mL of HRP were added and incubated for 60 min. (A) MRSA; (B) E. coli. * indicates eradication.
Studies with Fenton reagent
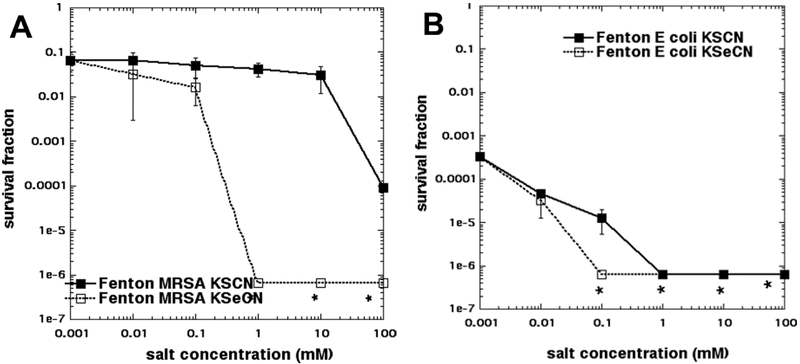
We were not able to trace any papers describing the use of thiocyanate to potentiate the antimicrobial effects of the Fenton reaction (ferrous salts plus hydrogen peroxide), although the use of iodide to potentiate Fenton-mediated bacterial killing was well-described by Klebanoff in 1982 [26]. Nevertheless, by making an analogy with peroxidase/peroxide systems, we thought it would be interesting to compare KSCN with KSeCN for potentiation of the Fenton reaction. Figure 8A shows that the concentration of Fenton reagent chosen only killed 1 log of MRSA, but when the concentration of KSeCN reached 1 mM there was eradication (5 logs more killing), but KSCN at 1 mM had no effect. It was not until the KSCN concentration reached 100 mM that we found 2–3 logs more killing. In Figure 8B we see that the effects of the two salts were not so different for E. coli. First of all there was more killing with Fenton reagent alone (3.5 logs) and secondly there was only a difference in killing (6 logs for KSeCN and 5 logs for KSCN) at 0.1 mM salt concentration. It may have been possible to have seen a bigger difference between the two salts if lower concentrations of Fenton reagent had been used.
Figure 8. Effects of KSCN or KSeCN on potentiating bacterial killing mediated by Fenton reagent.
Cells were mixed with 11 mM H2O2 (for MRSA) or 9 mM H2O2 (for E. coli) and different concentrations of KSCN or KSeCN, and then 11 mM FeSO4 (for MRSA) or 9 mM FeSO4 (for E. coli) were added and incubated for 60 min. (A) MRSA; (B) E. coli. * indicates eradication.
Discussion
The present study has discovered some fascinating mechanistic differences between the two different pseudohalide salts, KSCN and KSeCN when it comes to potentiating the effects of a range of potential oxidative killing approaches against Gram-positive and Gram-negative bacteria. We progressed into this area of research via the exploration of the mechanisms of aPDT. It is well known that aPDT can produce two different kinds of ROS called Type I (superoxide, hydrogen peroxide, hydroxyl radicals) and Type II (singlet oxygen) depending on the PS structure, redox potential, and surrounding oxygen concentration [27]. In our original publication on thiocyanate and MB we postulated a two-step process involving a Type II process by which singlet oxygen oxidized [SCN] to sulfite and cyanide, and sulfite underwent a one-electron oxidation process to produce sulfur trioxide radical anion (SO3−•) which had been unambiguously identified by ESR spin-trapping [15]. However, we cannot just assume that the SO3−• was the only species responsible for the bacterial killing. Next, we reported that KSeCN could potentiate the aPDT killing mediated by three different PS (MB, RB and TPPS4). The mechanism of action was attributed to the formation of (SeCN)2, based on the production of a substance that could kill bacteria when they were added immediately after the light, but which rapidly decayed over a period of 20–40 minutes. This hypothesis was bolstered by the finding that the bacterial killing caused by Pb(OAc)4 was potentiated by KSeCN, and that Pb(OAc)4 is reported in the literature as a synthetic reagent to produce (SeCN)2 from KSeCN [19, 20]. Moreover, the antibacterial killing produced by KSeCN and Pb(OAc)4 decayed over time in a similar manner to that found with aPDT (MB + KSeCN).
In the present study we confirmed that that KSCN could indeed potentiate the aPDT killing mediated by MB and red light. Moreover, we showed that there was no potentiation of aPDT killing caused by two other different PS that have more purely Type II mechanisms (RB and TPPS4). In order to confirm our hypothesis that a mixture of Type I and Type II photochemistry was necessary, we then used two additional phenothiazinium dyes, namely TBO and DMMB. We had previously shown that these dyes showed “paradoxical potentiation” of aPDT killing by addition of sodium azide [13, 14]. This remarkable result indicated that they were able to produce both singlet oxygen and Type I ROS when photoactivated, and suggested they could in theory produce SO3−• as previously shown for MB [15]. The fact that TBO and DMMB could both be potentiated by addition of KSCN, while RB and TPPS4 could not, provided further experimental verification of the mechanistic hypothesis. In order to be absolutely confident in the difference between Type I and Type II PS it will be necessary to use a much wider variety of PS structures. These structures could include fullerenes [28] and curcumin [29] that have predominantly Type I photochemistry, and phenalenones that have predominantly Type II photochemistry [30].
Therefore, the equations to describe the KSCN potentiation of aPDT killing mediated by MB, TBO, and DMMB are proposed to be:
| (1) |
| (2) |
In the previous study [15] we were able to measure the production of HCN by a Prussian blue test, and also the production of sulfite by a test involving bleaching of Malachite green.
The equation for the KSeCN potentiation of aPDT by MB, RB, and TPPS4 is proposed to be:
| (3) |
It is not completely clear exactly why KSCN is oxidized by 1O2 via one reaction pathway, and why KSeCN is oxidized by 1O2 via a different reaction pathway. The different mechanisms are presumably due to the relative redox potentials of KSCN and KSeCN.
Since the literature suggested that Pb(OAc)4 was able to produce [SeCN]2 from KSeCN, and KSeCN could potentiate the bactericidal killing effect of Pb(OAc)4 we asked whether a similar phenomenon could be observed using KSCN. The literature was no help as we could not trace any studies where Pb(OAc)4 had been used as an antibacterial agent. There are however a few references to the fact that Pb(OAc)4 can oxidize [SCN]− to thiocyanogen [SCN]2 [31, 32]. In one of these references [31], [SCN]2 is described as “a yellow, volatile oil, mp about −3°C, which polymerizes irreversibly at room temperature to insoluble, brick-red parathiocyanogen (NCS)x”.
We were not able to find any references to the antibacterial effects of [SCN]2, which is in stark contrast to hypothiocyanite, where there must be literally dozens of references to its antibacterial effects [33–36]. The oxidizing agents that are reported to be able to produce [OSCN] −from [SCN]− are not restricted to combinations of peroxidase enzymes and hydrogen peroxide (although these are in a clear majority), but include such other agents as small-molecule chloramines. It was reported that [36], while the reaction product between taurine chloramine (TauCl) and [SCN]− was previously thought to be either ClSCN or (SCN)2 [37], that in their study it was identified as [OSCN] −. This uncertainty only goes to underline how confusing the exact identity of these reactive intermediates can be. It is known that [OSCN] − is unstable, but when it was prepared from HRP and H2O2 in our hands it was not clear how the reaction should be quenched, in order to be able to study how long the antibacterial activity lasted for.
At the present time, it is not clear what the identity of the intermediate species is in the reaction caused by the combination of HRP/H2O2and KSeCN. The literature maintains that [OSeCN] − does not exist [24], although exactly how it is possible to prove for certain that an unstable molecule does not exist is not completely clear. Nevertheless, the dramatic and consistent superiority of KSeCN over KSCN in every single oxidative approach tested is remarkable. In some cases, KSeCN was six orders of magnitude better than KSCN, while 2 or 3 orders of magnitude better was quite common. If we assume that the reactive species in the case of Pb(OAc)4 was comparable with both salts i.e. (SCN)2 or (SeCN)2, then is it possible that the reactivity of (SeCN)2 towards bacteria is higher than that of (SCN)2, or is the case that the rate of production of (SCN)2 is slower than that of (SeCN)2 so there is simply less of it?
The mechanism of the potentiation by Fenton reaction is still unclear. One can propose that HO• (produced during the Fenton reaction) and possessing a standard redox potential (E0’) of 2.0V can oxidize SCN- to SCN• (E0’ = 1.63V) and can also oxidize SeCN- to SeCN• (E0’ = 1.27V) [38]. In the presence of the respective salts the radicals would add to the anions to form the pseudohalogen radical anions, HO• (E0’ = 1.3V) and (SeCN)2•− (E0’ = 1.0V) [39]. Both these redox potentials indicate sufficiently strong oxidizing agents to oxidize many biological components of bacterial cells (amino acids and lipids). It is at present not clear exactly why SeCN- is better at bacterial killing than SCN- with Fenton reagent. It may the case that less reactive radicals can actually penetrate better into bacteria and therefore do more serious damage deeper inside the cells, compared to highly reactive radicals that expend themselves at the surface. This would explain why the order of reactivity (E0’) is HO• > (SCN)2•−> (SeCN)2•−, while the order of bacterial killing efficiency is exactly the opposite (SeCN)2•− > (SCN)2•−> HO•. This would be an example of the “weak means strong” argument.
Further studies using methodologies such as spin-trapping and electron spin resonance analysis, or the use of model substrates together with LC-MS analysis of trapped products will be necessary to attempt to firmly identify the reactive species we have proposed to be involved in this study.
Table 1 summarizes our present hypotheses concerning the mechanisms of action of the two different salts (KSCN and KSeCN) in potentiating four different oxidative antibacterial techniques (aPDT, Pb(OAc)4, HRP/H2O2, Fenton). As can be seen there are at least six different species proposed to be involved: (1) SO3−•; (2) [SCN]2; (3) [SeCN]2; (4) [OSCN]−; (5) (SCN)2•−; (6) (SeCN)2•− with the possibility of more as yet undiscovered.
Table 1.
Proposed mechanisms of action of potentiation of different antibacterial oxidative reactions by addition of KSCN or KSeCN.
| Oxidant | KSCN | KSeCN |
|---|---|---|
| aPDT | SO3•−;
only phenothiazinium PS |
(SCN)2; any PS that
produces 1O2 |
| Pb(OAc)4 | (SCN)2 (literature) | (SeCN)2 (literature) |
| Hrp/H2O2 | [OSCN]− (literature) | ?? [OSeCN]− said not to
exist (literature) |
| Fenton (FeSO4/H2O2) | ?? (SCN)2•− | ?? (SeCN)2•− |
Antimicrobial PDT is the term used to application of aPDT in a living animal model of localized infection [40]. The procedure is normally carried out by adding the PS solution into/onto the infected tissue and then shining light after a short drug-light interval. This set-up is ideal for potentiation of the effect by addition of a solution of an inorganic salt to the PS solution. The only caveat is that with some PS (for instance MB), there can occur a slow precipitation by addition of a 100 mM concentration of KI (a salting out effect). This does not happen with a KI concentration of only 10 mM, nor indeed does it occur with 100 mM concentrations of KSCN or KSeCN.
The concentrations of salts chosen to potentiate aPDT and other antimicrobial oxidative treatments do depend on the type of oxidative antimicrobial technique. In the case of aPDT the salt is reacting with (or trapping) a very short-lived ROS intermediate (1O2 or HO•) and requires a high concentration to achieve this efficiently. On the other hand, with Pb(OAc)4 there is a simple stoichiometric reaction between the two salts and the concentration of the two species should be the same. The situation with HRP/H2O2 appears to resemble aPDT to some extent since a salt concentration of 100 mM gave more potentiation than 10 mM, in at least two instances. The situation with Fenton reagent is complex. First of all E. coli is significantly more sensitive to killing by Fenton reagent than is MRSA. This finding is in agreement with a paper we published in 2012 [41], where we showed that two Gram-positive species (S. aureus and E. faecalis) were significantly more resistant to Fenton reagent than three Gram-negative species (E. coli, P. aeruginosa, and P. mirabilis). Moreover in that study we showed that addition of sodium azide (500 μM) to the Fenton reagent potentiated the bacterial killing by 2–3 logs [41]. In the present study, a concentration as low as 100 μM of either salt was enough to show potentiation of Fenton-mediated killing against E. coli, while MRSA required 1 mM of KSeCN and 100 mM of KSCN.
It is perhaps not surprising, that selenocyanate has not been much studied in the biological arena in the past. It is generally considered to be a foreign inorganic chemical, and inorganic chemicals are mostly studied by biologists as potentially toxic materials. Unlike its close relative thiocyanate, selenocyanate does not occur naturally in the human body, and may therefore be considered potentially troublesome. However, selenium is known to be an essential trace element for humans, and is a chemical constituent of more than twenty selenoproteins that play important roles in reproduction, thyroid hormone metabolism, DNA synthesis, and protection from oxidative stress [42]. In the USA almost 20% of the population regularly take a dietary supplement containing selenium [43]. Among other selenium compounds, selenocyanate has been suggested to function as a low-toxicity inorganic selenium species that could be suitable for dietary supplementation [44]. Anan et al [45] showed using mass spectrometry, that SeCN− was produced in human hepatoma HepG2 cells that were exposed to sodium selenite. It was proposed that selenite was metabolized to SeCN− to temporarily reduce its toxicity, and that SeCN− could function as an intrinsic selenium pool in cultured cells. One interesting question that would need further experimentation to answer is, are these salts (SCN- and SeCN-) bound to or taken up by bacterial cells? This is non-trivial to answer and would probably need a radioactively labeled salt to do it accurately.
Thiocyanate has been proposed as an ingredient of various antimicrobial treatments. For instance, nebulized SCN− has been tested in a mouse model of P. aeruginosa lung infection [46], and also in a mouse model of cystic fibrosis [47]. An antimicrobial mouth-rinse was devised to take advantage of naturally occurring salivary peroxidases, which contained 4 mM H2O2 and 1 mM KSCN at pH 5.5 [48]. Tonoyan et al described an antibacterial system containing hydrogen peroxide, thiocyanate, and iodide that produced antibacterial reactive oxygen and iodine species under the action of peroxidases [49, 50]. Can selenocyanate improve upon these antimicrobial applications of thiocyanate? In order to progress SeCN further in the aPDT field it will be necessary to carry out studies in animal models of localized infections as we have previously done with potassium iodide [7, 11].
Acknowledgements
This work was supported by US-NIH grants R01AI050875 and R21AI121700. Research carried out at the Jagiellonian University (T.S) was supported by a grant from the National Science Centre (2011/03/B/NZ1/00007). Liyi Huang was supported by National Natural Science Foundation of China (81260239, 81472002), Guangxi Scientific and Technological Project (1355005-1-2), Guangxi Natural Science Foundation (2016GXNSFAA380312)
References
- 1.O’Neill J The Review on Antimicrobial Resistance Chaired by Jim O’Neill (2015) [Google Scholar]
- 2.Bouvet-Gerbettaz S, Merigo E, Rocca JP, Carle GF, and R N, Lasers Surg Med 41, 291–7(2009) [DOI] [PubMed] [Google Scholar]
- 3.R Hamblin M, Curr Opin Microbiol 33, 67–73 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castano AP, Demidova TN, and Hamblin MR, Photodiagnosis Photodyn Ther 1, 279–293 (2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma SK, Mroz P, Dai T, Huang YY, St Denis TG, and Hamblin MR, Isr J Chem 52, 691–705 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamblin MR, Expert Rev Anti Infect Ther 1-11 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vecchio D, Gupta A, Huang L, Landi G, Avci P, Rodas A, and Hamblin MR, Antimicrob Agents Chemother 59, 5203–12 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Dai T, Wang M, Vecchio D, Chiang LY, and Hamblin MR, Nanomedicine (Lond) 10, 603–14 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L, El-Hussein A, Xuan W, and Hamblin MR, J Photochem Photobiol B 178, 277–286 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang L, Szewczyk G, Sarna T, and Hamblin MR, ACS Infect Dis 3, 320–328 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen X, Zhang X, Szewczyk G, El-Hussein A, Huang YY, Sarna T, and Hamblin MR, Antimicrob Agents Chemother 61, (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu X, Huang YY, Kushida Y, Bhayana B, and Hamblin MR, Free Radic Biol Med 95, 74–81 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang L, St Denis TG, Xuan Y, Huang YY, Tanaka M, Zadlo A, Sarna T, and Hamblin MR, Free Radic Biol Med 53, 2062–71 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kasimova KR, Sadasivam M, Landi G, Sarna T, and Hamblin MR, Photochem Photobiol Sci 13, 1541–8 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Denis TG, Vecchio D, Zadlo A, Rineh A, Sadasivam M, Avci P, Huang L, Kozinska A, Chandran R, Sarna T, and Hamblin MR, Free Radic Biol Med 65C, 800–810 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang L, Xuan W, Zadlo A, Kozinska A, Sarna T, and Hamblin MR, J Biophotonics (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holub AM, Kohler H, and Skopenko VV, Chemistry of Pseudohalides TOPICS IN INORGANIC AND GENERAL CHEMISTRY MONOGRAPH (Book 21) 2000, London, UK: Elsevier Science Ltd. [Google Scholar]
- 18.Jett BD, Hatter KL, Huycke MM, and Gilmore MS, Biotechniques 23, 648–50 (1997) [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann HP and Kögler F, Eur J Inorg Chem 59, 178 (1926) [Google Scholar]
- 20.Challenger FC, Peters AT, and Halevy J, J Chem Soc 1648–1655 (1926) [Google Scholar]
- 21.Modi S, Deodhar SS, Behere DV, andMitra S, Biochemistry 30, 118–24 (1991) [DOI] [PubMed] [Google Scholar]
- 22.Chandler JD and Day BJ, Free Radic Res 49, 695–710 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gau J, Furtmuller PG, Obinger C, Arnhold J, and Flemmig J, Biochem Biophys Rep 4, 257–267 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cupp-Sutton KA and Ashby MT, Antioxidants (Basel) 5, (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adak S, Mazumdar A, and Banerjee RK, J Biol Chem 272, 11049–56 (1997) [DOI] [PubMed] [Google Scholar]
- 26.Klebanoff SJ, J Exp Med 156, 1262–7 (1982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Silva Baptista M, Cadet J, Di Mascio P, Ghogare AA, Greer A, Hamblin MR, Lorente C, Nunez SC, Ribeiro MS, Thomas AH, Vignoni M, and Yoshimura TM, Photochem Photobiol (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin R, Wang M, Huang YY, Landi G, Vecchio D, Chiang LY, and Hamblin MR, Free Radic Biol Med 79, 14–27 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahl TA, Bilski P, Reszka KJ, and Chignell CF, Photochem Photobiol 59, 290–4 (1994) [DOI] [PubMed] [Google Scholar]
- 30.Tabenski I, Cieplik F, Tabenski L, Regensburger J, Hiller KA, Buchalla W, Maisch T, and Spath A, Photochem Photobiol Sci 15, 57–68 (2016) [DOI] [PubMed] [Google Scholar]
- 31.Van-Nostrand, Thiocyanic Acid, in Enclyopedia of Chemistry 2005, John Wiley and Sons. [Google Scholar]
- 32.Madan RL and Tuli GD, Inorganic Chemistry: Questions and Answers (Success Guides) 2010, Sahibabad, India: S. Chand Publishing. [Google Scholar]
- 33.Schlorke D, Atosuo J, Flemmig J, Lilius EM, and Arnhold J, Free Radic Res 50, 1287–1295 (2016) [DOI] [PubMed] [Google Scholar]
- 34.Cegolon L, Salata C, Piccoli E, Juarez V, Palu G, Mastrangelo G, and Calistri A, Int J Hyg Environ Health 217, 17–22 (2014) [DOI] [PubMed] [Google Scholar]
- 35.Lorentzen D, Durairaj L, Pezzulo AA, Nakano Y, Launspach J, Stoltz DA, Zamba G, McCray PB Jr., Zabner J, Welsh MJ, Nauseef WM, and Banfi B, Free Radic Biol Med 50, 1144–50 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xulu BA and Ashby MT, Biochemistry 49, 2068–74 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calvo P, Crugeiras J, Rios A, and Rios MA, J Org Chem 72, 3171–8 (2007) [DOI] [PubMed] [Google Scholar]
- 38.D.A. Armstrong and I.T.G.o.R.E. Potentials”, The IUPAC Technical Report PAC-REP-14-05-02, (2015)
- 39.Sykes AG, Advances in Inorganic Chemistry 1989, London, UK: Academic Press. [Google Scholar]
- 40.Dai T, Huang YY, and Hamblin MR, Photodiagnosis Photodyn Ther 6, 170–188 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, and Hamblin MR, Lasers Surg Med 44, 490–9 (2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunde RA, Selenium, in Modern Nutrition in Health and Disease. 11th ed, Ross AC, et al. , Editors. 2012, Lippincott Williams & Wilkins: Philadelphia, PA: p. 225–37. [Google Scholar]
- 43.Bailey RL, Gahche JJ, Lentino CV, Dwyer JT, Engel JS, Thomas PR, Betz JM, Sempos CT, and Picciano MF, J Nutr 141, 261–6 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi K, Suzuki N, and Ogra Y, Int J Mol Sci 18, (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anan Y, Kimura M, Hayashi M, Koike R, and Ogra Y, Chem Res Toxicol 28, 1803–14 (2015) [DOI] [PubMed] [Google Scholar]
- 46.Chandler JD, Min E, Huang J, Nichols DP, and Day BJ, Br J Pharmacol 169, 1166–77 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chandler JD, Min E, Huang J, McElroy CS, Dickerhof N, Mocatta T, Fletcher AA, Evans CM, Liang L, Patel M, Kettle AJ, Nichols DP, and Day BJ, Am J Respir Cell Mol Biol 53, 193–205 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansson-Rahemtulla B, Pruitt KM, Tenovuo J, and Le TM, J Dent Res 62, 1062–6 (1983) [DOI] [PubMed] [Google Scholar]
- 49.Tonoyan L, Boyd A, Fleming GTA, Friel R, Gately CM, Mc Cay PH, and O’Flaherty V, Toxicol In Vitro 50, 264–273 (2018) [DOI] [PubMed] [Google Scholar]
- 50.Tonoyan L, Fleming GTA, Mc Cay PH, Friel R, and O’Flaherty V, Front Microbiol 8, 680 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]