Abstract
Background:
Aspirin-exacerbated respiratory disease (AERD) is characterized by asthma, recurrent nasal polyposis, and respiratory reactions upon ingestion of cyclooxygenase-1 inhibitors. Increased numbers of platelet-leukocyte aggregates are present in the sinus tissue and blood of patients with AERD, compared to aspirin-tolerant patients, and platelet activation may contribute to aspirin-induced reactions.
Objective:
We sought to determine whether treatment with prasugrel, which inhibits platelet activation by blocking the P2Y12 receptor, would attenuate the severity of sinonasal and respiratory symptoms induced during aspirin challenge in subjects with AERD.
Methods:
40 subjects with AERD completed a 10-week, double-blinded, placebo-controlled crossover trial of prasugrel. All subjects underwent oral aspirin challenges after 4 weeks of prasugrel and after 4 weeks of placebo. The primary outcome was a change in the provocative dose of aspirin that would elicit an increase in total nasal symptom score of 2 points (PD2). Changes in lung function, urinary eicosanoids, plasma tryptase, platelet-leukocyte aggregates, and platelet activation were also recorded.
Results:
Prasugrel did not significantly change the mean PD2 (79 ±15 on placebo and 139 ±32 on prasugrel, P = 0.10), platelet-leukocyte aggregates, or increases in urinary leukotriene E4 (LTE4) and prostaglandin D2 metabolite (PGD-M) levels during aspirin-induced reactions in the study population as a whole. Five subjects (“responders”) reacted to aspirin while on placebo but did not develop any reaction to aspirin challenge after the prasugrel arm. In contrast to prasugrel nonresponders (35 subjects), the prasugrel responders had smaller reaction-induced increases in TNSS, did not show significant aspirin-induced increases in urinary LTE4, PGD-M, or thromboxane B2 levels, and failed to display increases in serum tryptase levels during the aspirin reactions on the placebo arm, all of which were observed in the nonresponders.
Conclusion:
In the overall study population, prasugrel did not attenuate aspirin-induced symptoms, possibly because it failed to decrease the frequencies of platelet-adherent leukocytes, or to diminish aspirin-induced mast cell activation. In a small subset of patients with AERD who had higher baseline platelet activation and milder upper respiratory symptoms during aspirin reactions, P2Y12 antagonism with prasugrel completely inhibited all aspirin-induced reaction symptoms, suggesting a contribution from P2Y12 signaling in this subset.
Clinical Implications:
P2Y12 antagonism with prasugrel prevents aspirin-induced reactions in only a subset of AERD patients with a component of P2Y12-dependent mechanisms of reaction to aspirin.
Capsule summary:
Platelet activation increased in AERD. In a randomized placebo-controlled cross-over trial, platelet inhibition through P2Y12 antagonism with prasugrel neither prevented nor lessened aspirin-induced reactions in most patients with AERD, though did identify a small subset of responders who may have a distinct P2Y12-dependent mechanism of reaction to aspirin.
Keywords: Aspirin-exacerbated respiratory disease, double-blinded randomized placebo controlled crossover trial, prasugrel, Samter’s triad, N-ERD, leukotrienes, P2Y12
Introduction
Aspirin-exacerbated respiratory disease (AERD) is a distinctive clinical syndrome affecting ~7% of adults with asthma and ~14% of those with severe asthma.1 AERD is characterized clinically by asthma, rhinosinusitis with nasal polyposis, respiratory tissue eosinophilia, and respiratory reactions to ingestion of aspirin and other inhibitors of cyclooxygenase (COX)-1. Biochemically, AERD is marked by over-activity of the 5-lipoxygenase (5-LO)/leukotriene C4 synthase (LTC4S) pathway, resulting in generation of high levels of leukotriene (LT)C4 and, therefore, high urinary levels of LTE4, the stable end-product of cysteinyl leukotriene (cysLT) metabolism. CysLTs are powerful bronchoconstrictors, and facilitate vascular leak and tissue eosinophilia. They play a major pathogenic role in AERD, particularly in clinical reactions to aspirin challenge/desensitization.2
Although eosinophils3 and mast cells,4 which express both 5-LO and LTC4S, likely account for the majority of cysLTs released in AERD during clinical reactions, platelets may also play a role in cysLT overproduction. Platelets lack 5-LO, but can convert leukocyte-derived LTA4 to LTC4 due to their expression of LTC4S.5 This conversion requires platelet activation and CD62P expression, resulting in CD62P-dependent adherence of platelets to 5-LO expressing leukocytes (platelet-leukocyte aggregates). We have previously shown that both peripheral blood and sinonasal tissue of individuals with AERD contain higher numbers of platelet-leukocyte aggregates than do aspirin-tolerant patients, and their numbers correlate with basal urinary LTE4 levels.6 Moreover, mouse studies implicate platelet-leukocyte aggregates in cysLT generation during reactions to aspirin.7 These observations suggest that agents capable of interfering with the formation of platelet-leukocyte aggregates could have therapeutic efficacy in AERD. Based on these findings, we hypothesized that antiplatelet therapy may be efficacious in AERD, particularly if it interferes with the transcellular generation of LTC4.
Many stimuli that activate platelets elicit their release of adenosine diphosphate (ADP), which signals through the type 12 purinergic (P2Y12) receptor to amplify CD62P expression and the interaction of platelets with leukocytes. Thienopyridine drugs (prasugrel, clopidogrel) that block P2Y12 receptors inhibit platelet activation in cardiovascular disease.8 This double-blind placebo-controlled trial aimed to test the hypothesis that blockade of P2Y12 receptors with prasugrel would attenuate the severity of sinonasal and respiratory symptoms that occur during aspirin challenges in subjects with AERD by blocking platelet activation and reducing cysLT generation by decreasing the frequency of platelet-leukocyte aggregates.
Methods
Study design
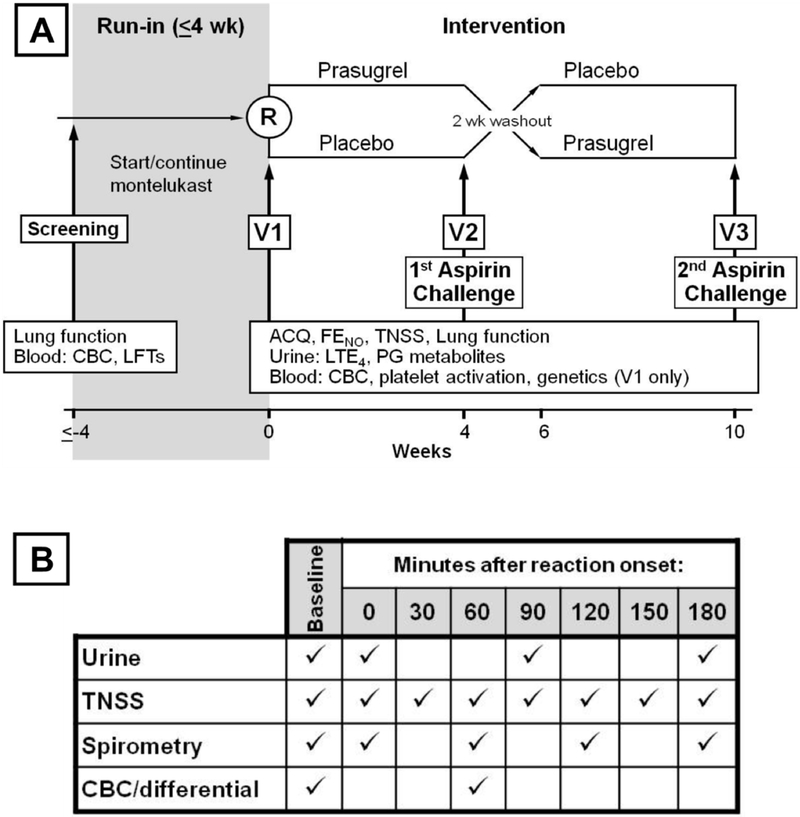
Participants enrolled in a 10-week, double-blinded, placebo-controlled crossover trial of prasugrel. Each treatment arm lasted 4 weeks, with a 2-week washout phase in-between. An oral aspirin challenge was conducted at the end of each treatment arm (Figure 1). In a separate continuation study, patients continued onto high-dose aspirin (650 mg twice daily).
FIG 1. Detailed Schema of the trial.
A, Study schema showing timeline and schedule of assessments. B, Table of time-points when assessments were collected on the day of the aspirin challenge.
Participants
Subjects with AERD had a history of physician-diagnosed asthma, nasal polyposis, and at least one clinical reaction to aspirin, or another non-selective COX inhibitor, with features of lower and/or upper airway involvement. They had refractory AERD with respiratory symptoms, which were not adequately responsive to other standard therapies, including corticosteroids and leukotriene modifiers, thus qualifying for aspirin desensitization and treatment with high-dose aspirin. To ensure the successful and safe completion of the protocol, subjects had stable asthma, defined as a post-bronchodilator FEV1 of 70% of predicted or better, no increase in baseline dose of oral glucocorticoids for at least 3 months, and no history of hospitalization or emergency room visits due to asthma for at least 6 months prior to enrolling in the study.
Subjects were excluded if they had current severe gastro-esophageal reflux disease, or a history of peptic ulcer disease or a gastrointestinal bleed. All subjects were between the ages of 18 and 65 years old, and were not pregnant, breastfeeding, or smoking while participating in the trial.
This was a single-site study, conducted at the Asthma Research Center, Brigham and Women’s Hospital. The Institutional Review Board of Brigham and Women’s Hospital/Partners Healthcare approved this study, and all subjects gave written informed consent. Patients were recruited from August 2012 through April 2016, with the last patient’s final visit in December 2016. The trial ended when recruitment goals were met. Compliance with montelukast and daily study drug administration was confirmed with both diary cards and pill counts.
Clinical Procedures
After a 4-week run-in period on stable asthma treatment and montelukast 10 mg daily, subjects were randomized to begin taking either prasugrel (5 mg (for patients <60kg) or 10mg (≥ 60kg) daily, following a 60mg loading dose) or equivalent placebo for 4-weeks, followed by a 4-dose aspirin challenge (40.5mg, 81mg, 162mg, and 325mg) with 90-minute intervals,9 which was terminated when patients experienced either an increase in the total nasal symptom score (TNSS) of at least two points above baseline, or a decline in FEV1 of at least 15% from baseline, or reached a dose of 325mg of aspirin without any reaction symptoms. The TNSS questionnaire is a 9-symptom patient recorded outcome measurement on a total scale of 0–40, which includes assessment of nasal congestion, runny nose, sneezing, nasal itching, and eye and throat symptoms (see Supplemental Figure E1 for the full questionnaire). Baseline assessments were collected the morning of the aspirin challenge.
Patients were observed for a 3-hour period following the onset of their reaction and challenge termination, with serial measurements of lung function, TNSS, and collection of blood and urine samples. Subjects were discharged to home to continue daily montelukast and washout the study drug from the first treatment phase. At the end of the 2-week washout period, subjects crossed over to the alternate treatment for 4 weeks and returned for the second aspirin challenge, which proceeded as above.
Laboratory Procedures
Blood was drawn into heparinized tubes, kept at room temperature, and assayed within 1 hour of collection. For aspirin-challenge visits, blood was drawn in the morning prior to administration of aspirin, and 1 hour after the onset of the aspirin-induced reaction. If no reaction occurred, blood was drawn again 3 hours after the 325mg dose of aspirin. Platelet-rich plasma (PRP) was obtained from the top layer of blood samples after a 20-minute centrifuge at 200g.
To monitor platelet activation and quantify platelet-leukocyte aggregates, we incubated 10 μL of PRP with directly-conjugated antibodies specific for CD61 and CD62P and 20 μL of whole blood with antibodies specific for CD61, CD45, CD14 and CCR3, or appropriate isotype controls for 20 minutes (CCR3 antibody from BioLegend, San Diego CA, all others from BD Biosciences, San Jose CA. We lysed the red blood cells with FACS™ Lysing Solution (BD Biosciences, San Jose CA) then fixed the cells in 1% paraformaldehyde. At least 20,000 CD45+ cells for the whole blood analyses or 30,000 platelets for the PRP analyses were recorded for each sample in a BD FACSCanto flow cytometer. Analyses completed with FlowJo Version 10 (TreeStar, Ashland OR). CD45+ leukocytes were classified as eosinophils (CCR3+ granulocytes), neutrophils (CCR3- granulocytes), monocytes (CD14+), and lymphocytes. The presence of adherent platelets was determined by the relative expression of CD61 on each cell type. An aliquot of plasma from each blood draw was also analyzed at Virginia Commonwealth University for total tryptase levels.
Urinary eicosanoids were measured by gas chromatography-mass spectrometry (GCMS) at Vanderbilt University as previously described.6 For the aspirin-challenge visits, urine samples were collected in the morning before the administration of aspirin, and again at the onset of, 90, and 180 minutes after the aspirin-induced reaction. For those visits where no reaction was elicited, urine was collected 180 minutes after the 325mg dose of aspirin.
DNA from peripheral blood leukocytes was used to sequence the P2RY12 locus (gene encoding the P2Y12 receptor) in 28 subjects. DNA was extracted with an AllPrep DNA/RNA Mini Kit (Qiagen, Valencia CA). Five overlapping PCR amplicons, covering the entire gene, were sequenced using the Illumina MiSeq platform to generate 300 base paired-end reads which were aligned to the reference human genome (GRCh37/hg19) using Bowtie2 (v2.2.6). SAM tools (v1.4) was used to call genetic variants from sorted and indexed BAM files. Association testing of genotype with response to prasugrel was performed by logistic regression on selected SNPs.
Outcomes
Because all subjects received montelukast throughout the study (which substantially attenuates aspirin-induced decreases in lung function), we used changes in nasal symptoms as the main indicator of reaction. The primary outcome compared the effect of 4 weeks of prasugrel vs. placebo on the provocative dose that caused an increase in TNSS of 2 (PD2) points during an aspirin challenge. PD2 is modeled after the aspirin PD20 (the provocative dose causing a 20% fall in baseline FEV1) established in the 2007 EAACI guidelines,10 and was calculated as follows (see detailed description of each variable in Supplemental Figure E2):
Participants who did not develop any clinically apparent reaction to aspirin during an aspirin challenge (a fall in FEV1 of less than 15% and no significant development of any naso-ocular or non-respiratory symptoms, as quantified by the lack of increase in TNSS) were designated as having a “negative challenge” and were assigned a PD2 of 650, which is double the maximum dose of aspirin given during either challenge.
Secondary outcomes included differences in provocative dose of aspirin, maximum increase in TNSS, change in urinary eicosanoids, and platelet activation during aspirin challenge while on prasugrel versus placebo. Additional pre-defined outcomes included the effect of prasugrel on chronic asthma control, urinary eicosanoid metabolites, platelet activation, and fractional exhaled nitric oxide (FeNO) in patients with AERD.
Sample size
The study was powered to detect an effect of prasugrel on the PD2 during aspirin desensitization. The sample size of 40, using a 2-sided paired t-test comparing the mean PD2 between treatment with prasugrel and placebo, provided 80% power to detect a 1.74 (0.8 after log2 transformation) fold change in the PD2, at an 0.05 level of significance.
Randomization
Randomization consisted of 3 randomly-selected blocks for each stratum of dose (5 mg or 10 mg) with a block size of 5 patients randomly assigned to treatment or control groups for each randomly selected block. We used permuted block randomization with SAS software Version 9.2 proc plan, ensuring equal numbers of AERD subjects were randomized to each group. The BWH Investigational Drug Services (IDS) pharmacy conducted the randomization and kept the records to maintain the blind of the study. IDS pharmacy also prepared the placebo and prasugrel via encapsulation.
Blinding
All participants, clinical care providers, and research staff were kept blinded as to intervention, and all analyses of mechanistic data (flow cytometric measurements of platelet activation, urinary eicosanoid measurements, etc.) were completed prior to unblinding of research investigators.
Statistical methods
For the crossover study, we compared the difference in PD2 between the two (prasugrel vs. placebo) aspirin challenges using a two-sided paired t-test as the main comparison, and used the same analysis for the change in maximum reduction in FEV1 during aspirin reaction. We used mixed model diagnostics such as residual plots for goodness-of-fit, and before undertaking the primary analysis, we checked for a period effect. We compared the levels of urinary LTE4 and PG metabolites measured during the reaction to aspirin for subjects in both treatment arms, each separately by using a paired t-test. We compared both the pre-aspirin baseline and the aspirin reaction-induced percentages of activated platelets and platelet-leukocyte aggregates for subjects pretreated with prasugrel and placebo, each by using a two-sided paired t-test. For the additional post hoc analyses done on the “responder” vs “nonresponder” subgroups, the False Discovery Rate method was used to correct for the multiple comparison testing, with a rate greater than 0.05 considered significant.11 The raw p-values are presented, and the significant values using the False Discovery Rate are identified in Figures 6 and 7. Data are presented ±standard error (SE) unless otherwise specified.
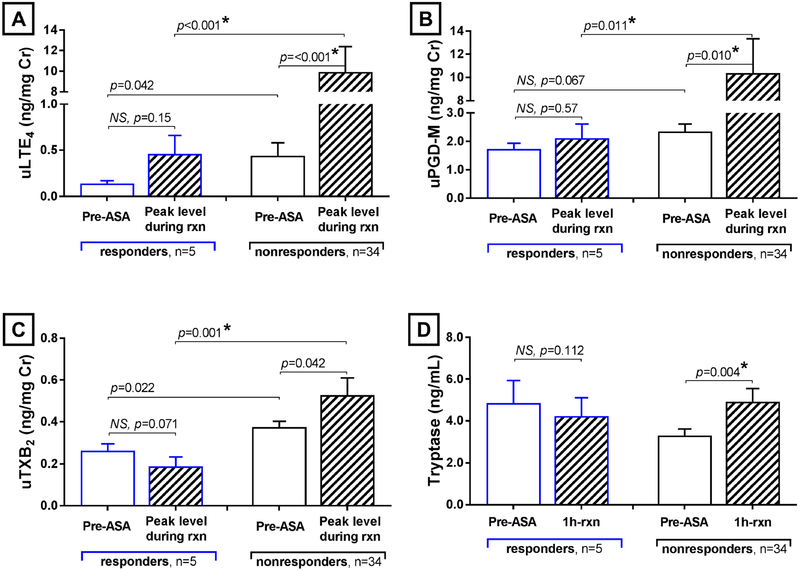
FIG 6. Responder vs non-responder differences in aspirin-induced urinary eicosanoid and plasma tryptase levels.
Placebo arm urinary levels of LTE4 (A), PGD-M (B), and TXB2 (C) before aspirin administration (white columns) and at the aspirin reaction-induced peak (striped columns) are shown for the responders (blue lines) and the nonresponders. Placebo arm plasma tryptase levels (D) before aspirin administration and 1 hour after the onset of reaction are shown for the same patient subgroups. Data are expressed as mean +SE. P-values with a * are significant using a False Discovery Rate of 0.05 to correct for multiple comparisons.
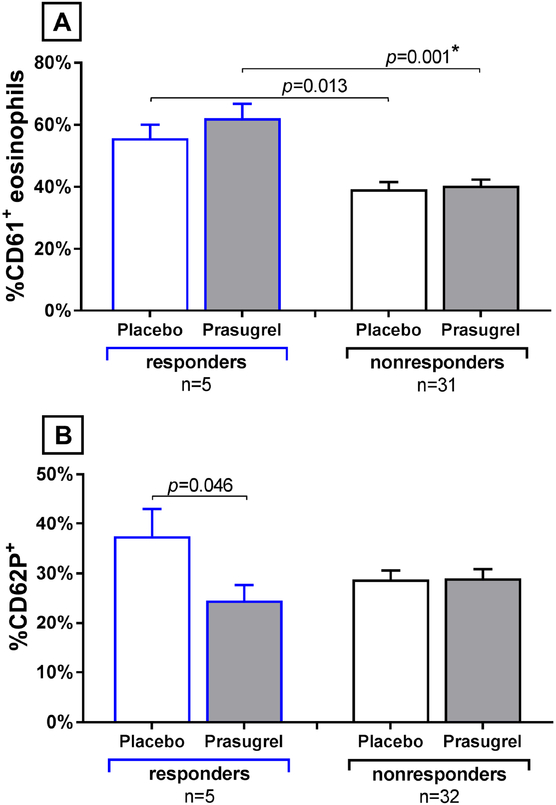
FIG 7. Responder vs non-responder platelet activation and platelet-leukocyte aggregates.
(A) Percentages of eosinophils with adherent platelets before aspirin administration are shown for each treatment arm for the responders (n=5, blue lines) and the non-responders. (B) Percentages of platelets that expressed surface CD62P are shown for the two patient subgroups, at the same timepoints. Data are expressed as mean + SE. P-values with a * are significant using a False Discovery Rate of 0.05 to correct for multiple comparisons.
Results
Patients
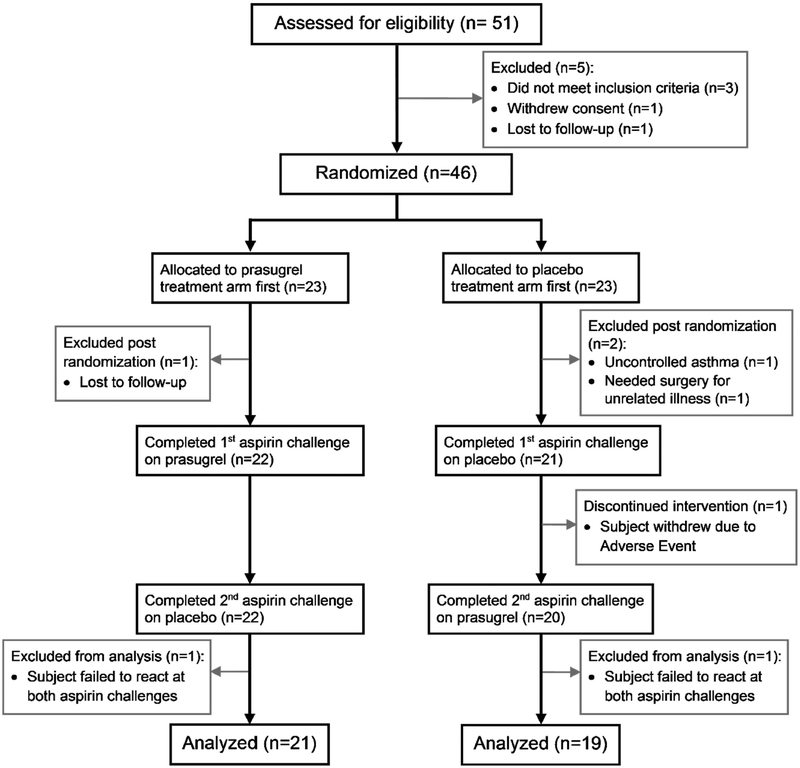
A total of 51 potential participants were screened, 46 of whom underwent randomization per protocol, and 40 of whom completed the trial and were analyzed (Figure 2). The baseline characteristics of the patients who underwent randomization are summarized in Table 1. Thirty-nine out of forty patients had confirmed (diary cards and pill counts) compliance with both daily montelukast and daily study drug of over 90%.
FIG 2.
Patient flow through the study.
TABLE I.
Patients’ baseline demographics and characteristics. Data are mean ±SE.
| AERD (n = 44) |
|
|---|---|
| Age (y) | 47 ± 10 |
| Sex (female) | 24 (55%) |
| Race | |
| White | 40 (91%) |
| Black | 1 (2%) |
| Asian | 0 |
| Other | 3 (7%) |
| Ethnicity | |
| Hispanic | 3 (7%) |
| FEV1 (L) | 3.15 ± 0.11 |
| FEV1 predicted (%) | 93.7 ± 2.0 |
| FVC (L) | 4.22 ± 0.16 |
| FeNO (ppb) | 45 ± 4 |
| Peripheral Eosinophil Count (/μL) | 423 ± 53 |
| Peripheral Basophil Count (/μL) | 47 ± 8 |
| ACQ-7 | 0.65 ± 0.09 |
| TNSS | 4.5 ± 0.7 |
| Low-dose ICS (≤ 200 mcg*) | 15 (34%) |
| Medium-dose ICS (201–500 mcg *) | 18 (41%) |
| High-dose ICS (>500 mcg*) | 4 (9%) |
| Oral Glucocorticoid Use | 2 (5%) |
| Long-acting Beta Agonist Use | 28 (64%) |
| Long-acting Muscarinic Antagonist Use | 0 |
= Fluticasone Propionate dry powder equivalent
Primary Outcome
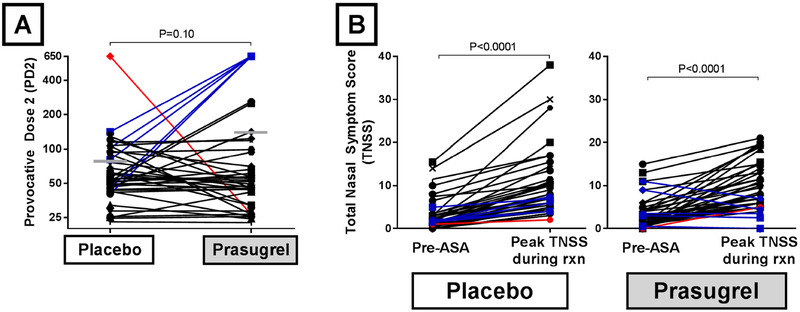
The mean PD2 on the prasugrel arm of 79 ± 15 and the mean PD2 on the placebo arm of 139 ± 32 (P=0.10) (Figure 3A). Of the 40 AERD patients who completed the cross-over trial, 5 did not develop any clinically apparent reaction to aspirin during the arm when they had been treated with prasugrel (prasugrel “responders”), and were assigned a PD2 of 650. The clinical reactions of the 5 responders during the placebo-arm challenge showed significantly smaller increases in TNSS, compared to that of the 35 nonresponders (maximum TNSS increase of 3.4 ±0.8 for 5 responders, vs 7.5 ±0.9 for 35 nonresponders, P=0.003), though the average fall in FEV1 was similar (9.9 ±3.5% vs 12.8 ±2.0, P=0.50). There was also 1 patient who did not develop any clinically apparent reaction to aspirin during the arm when they had been treated with placebo, but did develop a reaction during the prasugrel arm. For the entire study population, the mean difference in maximum increase in TNSS for patients on the prasugrel arm compared to the placebo arm was 0.5 ±1.0 (P=0.32), with mean increase in TNSS on the prasugrel arm of 6.5 ±0.8 and the mean increase in TNSS on the placebo arm of 7.0 ±0.8 (Figure 3B). The administered aspirin dose that provoked a reaction for patients on the prasugrel arm was 0.2 ±0.2-fold higher (P=0.38) than the administered provocative aspirin dose for patients on the placebo arm.
FIG 3. Effect of prasugrel on PD2 and TNSS.
(A) The primary outcome, change in the provocative dose of aspirin that would elicit an increase on the total nasal symptom score of 2 points (PD2) is shown, with horizontal bars representing the mean PD2 for each treatment arm. (B) The change in TNSS from the pre-aspirin baseline to the maximum TNSS recorded during each aspirin challenge is shown. 34 subjects reacted to aspirin at both challenges (black lines), 1 subject reacted to aspirin only on the prasugrel arm (red line), and 5 subjects reacted only on the placebo arm (blue lines).
Secondary Outcomes
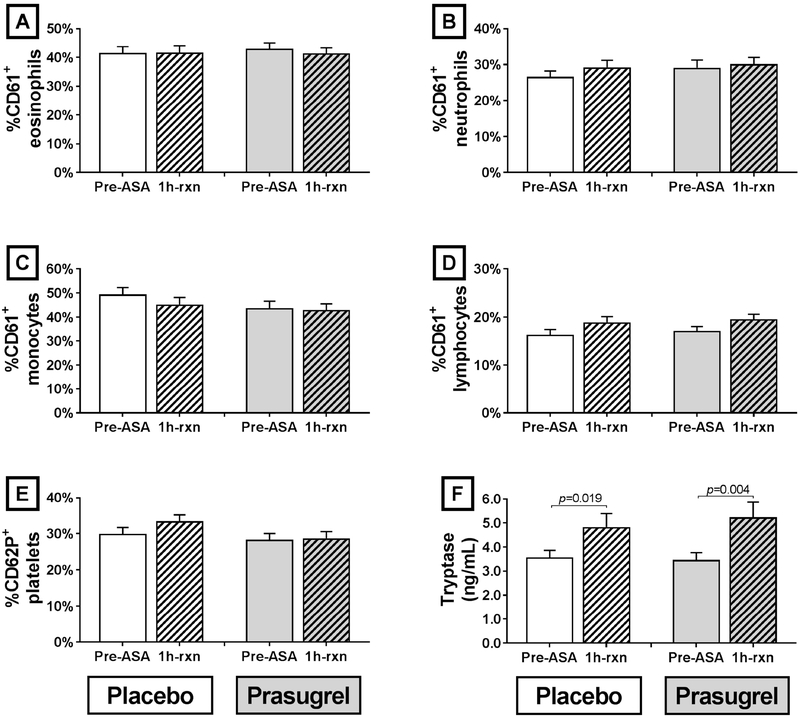
Treatment with prasugrel did not alter baseline percentages of CD62P+ platelets compared with placebo in all 40 AERD patients who completed the trial, and did not change the percentages of any leukocyte subset with adherent platelets (Figure 4, A-E). In the 40 patients as a group, neither the percentage of CD62P+ platelets nor the percentages of leukocytes with adherent platelets changed one hour into the aspirin-induced reactions, and prasugrel did not alter these percentages when compared with placebo (Figure 4, A-E). Plasma tryptase levels rose significantly during the aspirin-induced reactions (increase of 44 ±12% for placebo arm, P=0.02, and 60 ±21% for prasugrel arm, P=0.004) though treatment with prasugrel did not alter either the pre-aspirin levels of tryptase, nor the aspirin-induced increases (Figure 4, F).
FIG 4. Platelet-leukocyte aggregation, platelet activation, and plasma tryptase.
Percentages of leukocytes with adherent platelets (as determined by staining with CD61) before aspirin administration (solid columns) and 1 hour after the onset of reaction (striped columns) are shown for each treatment arm for eosinophils (A), neutrophils (B), monocytes (C), and lymphocytes (D). Percentages of platelets that expressed surface CD62P (E) and total plasma tryptase levels (F) are shown at the same timepoints. Data are expressed as mean +SE.
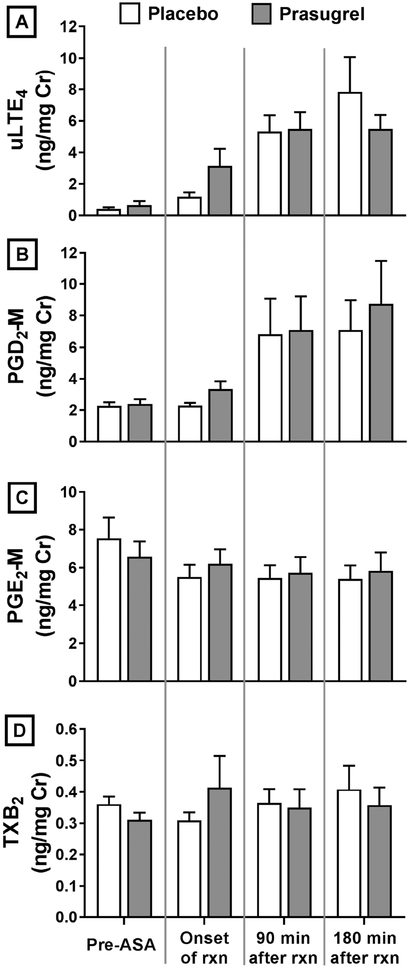
Prasugrel did not alter baseline levels of urinary eicosanoids measured prior to aspirin administration (Figure 5). Urinary eicosanoid levels for patients on prasugrel treatment increased during the aspirin-induced reactions to the same extent as those on placebo treatment.
FIG 5. Urinary eicosanoid levels.
Baseline pre-aspirin and aspirin-induced reaction levels of urinary eicosanoid levels analyzed by gas chromatography-mass spectrometry are shown for both treatment arms. (A) LTE4, (B) PGD2-M, (C) PGE-M, and (D) TXB2. Data are expressed as mean +SE.
Characteristics of Responder/Nonresponder Subgroups
The 5 prasugrel “responders” did not differ from the 35 nonresponders in terms of baseline demographics, ACQ, FEV1, TNSS, FeNO, or pre-aspirin differential cell counts during either the prasugrel or placebo treatment arms, and their PD2 during the placebo-arm challenge also did not differ (Supplemental Table EI). The 5 responders had lower pre-aspirin baseline urinary levels of LTE4 (0.14 ± 0.03 vs 0.44 ± 0.14 ng/mg Cr, P=0.042) and TXB2 (0.26 ± 0.03 vs 0.38 ± 0.03 ng/mg Cr, P=0.022) while on placebo compared to the 35 nonresponders, and tended to show reduced PGD-M levels (1.73 ±0.20 vs 2.35 ±0.26 ng/mg Cr, P=0.067). The responders showed significantly lower peak levels of urinary LTE4 (0.46 ±0.20 vs 9.91 ±2.50 ng/mg Cr, P=0.0009), PGD-M (2.11 ±0.50 vs 10.37 ±2.97 ng/mg Cr, P=0.011), and TXB2 (0.19 ± 0.04 vs 0.53 ± 0.08 ng/mg Cr, P=0.001) in response to aspirin challenge (Figure 6, A-C). Compared to baseline pre-challenge levels, the responders showed no significant aspirin-induced increases in urinary LTE4 (Figure 6, A) or PGD-M (Figure 6, B). Furthermore, although there was a clear aspirin-induced rise in plasma tryptase one hour into the aspirin-induced reactions in the 35 nonresponders (increase of 52 ±14% for placebo arm, P=0.004), the 5 responders trended toward a slight decrease in plasma tryptase levels (change of −9 ±4%, P=0.112) during their placebo arm aspirin challenge with a significance difference in aspirin-induced change in tryptase between the two groups (P=0.001) (Figure 6, D). The maximum rise in TNSS during the placebo-arm challenges correlated with the fold increase in urinary LTE4 (r=0.58, P=0.001), with a trend toward rise in TNSS correlations with the increase in urinary PGD-M and serum tryptase.
Peripheral blood absolute eosinophil count decreased in the 35 nonresponders during the aspirin-induced reaction while on the placebo arm (−155 ±41 cell/μL), but did not change in the responders (+40 ±68 cell/μL, P=0.043). While their baseline eosinophil numbers did not differ, the 5 responders had significantly higher percentages of eosinophils with platelets attached than did the nonresponders at the pre-aspirin baselines while on the prasugrel (62 ±5% vs 40 ±5%, P=0.001) arm (Figure 7A). The 5 responders also had a trend toward prasugrel-induced decrease in pre-aspirin baseline activated platelets (CD62P+ platelets) while on the prasugrel treatment arm compared to the placebo arm (reduction from 38 ±5% on placebo to 25 ±3% on prasugrel, P=0.046), though this difference was not significant when corrected for multiple comparison testing. In contrast, prasugrel treatment did not lead to a decrease in platelet activation for the nonresponders (Figure 7B).
The urine collected 3 hours after the 325 mg dose of aspirin from the patients who did not manifest any reaction is not biologically equivalent in timepoint or dose to that collected during the aspirin-induced reactions. However, we did analyze these samples for eicosanoid levels from the 4 patients for whom they were collected. The change in urinary LTE4 and urinary PGD-M (pre-aspirin baseline to 3-hour post-325mg) during their prasugrel arm did not differ from the change (pre-aspirin baseline to 3-hour post-reaction) measured during their placebo arm, nor did the magnitude of the mediator production (Supplemental Fig. E3).
We conducted a post hoc analysis of the P2RY12 sequencing of the 5 responders and 23 nonresponders for whom we had sufficient genomic DNA. Of the 26 selected SNPs, 19 of which were previously associated with P2RY12 gene expression, 2 were exonic, 23 intronic, and 1 up-stream of the gene. No variant was predicted to disrupt amino acid composition and none were associated with drug response (Supplemental Table E2).
Safety
The number of total and severe adverse events was similar between the placebo arm and the prasugrel arm (Supplemental Table E3). Prasugrel was associated with a higher likelihood of bruising (P=0.006). No patients withdrew participation due to any prasugrel-related adverse events. One patient withdrew participation following the first aspirin challenge, which had been during the placebo arm, due to a systemic reaction to the aspirin challenge that included vomiting.
Discussion
Aberrantly increased production of cysLTs, eosinophilic respiratory tract inflammation and persistent mast cell activation are all steady-state features of AERD that are further amplified during reactions to drugs that block COX-1. These reactions are thought to involve a prominent contribution from cysLTs and mast cell-derived mediators. We6 and others12 have demonstrated increased numbers of platelet-leukocyte complexes in the blood of patients with AERD, and also observed such complexes in sinonasal tissues.6 The CD62P-dependent adherence of platelets to leukocytes has several physiologic consequences, including transcellular conversion of LTA4 to LTC4, by platelets.5,13 Adherent platelets accounted for the majority of LTC4S activity of blood granulocyte fractions from subjects with AERD.6 Platelets were also necessary for the surge of cysLT generation induced by aspirin challenges of AERD-like mice.6,7 We aimed to determine whether a similar platelet-dependent mechanism contributed to the aspirin-induced symptoms in patients with AERD. A previous study suggested that 6 days of treatment with clopidogrel, a thienopyridine antagonist of the P2Y12 receptor, reduced the numbers of platelet-monocyte aggregates in the blood of healthy patients.14 We therefore conducted this crossover study to determine whether prasugrel could block cysLT-dependent reactions to aspirin challenge and inhibit the formation of platelet-leukocyte aggregates in well-characterized subjects with AERD.
Reactions to aspirin in AERD frequently result in sharp reductions in FEV1, along with sinonasal congestion and ocular symptoms. These reductions in part reflect the actions of cysLTs at the type 1 cysLT receptor (CysLT1R). To ensure safety of the challenges in this study, all subjects were treated with montelukast, an inhibitor of CysLT1R, during the challenges. CysLT1R blockade attenuates the decrease in lung function induced by aspirin, but does not tend to eliminate the upper respiratory tract symptoms indicative of a reaction.15 We therefore used TNSS, rather than a change in FEV1, as the basis for assessing reactions, and calculated the PD2 for aspirin on prasugrel vs. placebo as the primary outcome based on TNSS.
All 40 subjects experienced characteristic reactions on at least one of the two challenges, and most reacted to both challenges, confirming their diagnosis of AERD. Although treatment with prasugrel increased PD2, this did not reach significance (Fig. 3, P=0.10). Moreover, in the population of patients as whole, prasugrel did not alter the baseline TNSS or ACQ scores, baseline urinary eicosanoids, FeNO, or changes in eicosanoids with aspirin challenges. In general, the maximum fall in FEV1 during aspirin-induced reactions correlated with the maximum rise in TNSS (Supplemental Fig. E4, r=0.50, P=0.001). Of the 74 aspirin-induced reactions in the study, 28 of those involved a fall in FEV1 of 15% of more (38% of the reactions), which is consistent with previous reports of the rates of lower-respiratory symptoms.15 As previously reported, CD62P+-free platelets (Fig. 4A) and platelet-leukocyte aggregates (Fig. 4B–D) were readily detectable in the blood of all subjects and did not change with aspirin challenges.6,12 As expected, the mean urinary levels of LTE4 rose sharply during reactions for the group as a whole (Figure 5), along with the metabolite levels of PGD2, the dominant COX product of mast cells, and plasma tryptase levels, which are all potential or verified indices of mast cell activation.
Five of the 40 subjects reacted while on placebo but failed to exhibit any signs or symptoms of aspirin-induced reaction (neither TNSS increase of ≥2 points nor FEV1 decrease of ≥15%) while receiving prasugrel. Although the PD2 and the change in FEV1 in responders were similar to the remainder of the cohort, the severity of upper respiratory symptoms, as measured by maximum increase in TNSS, was significantly milder in responders than in the nonresponders (Supplemental Table EI). Several features suggest that responders could represent an endotype of AERD patients with mild upper respiratory reactions. First, the baseline fraction of blood eosinophils with adherent platelets in the responder subgroup significantly exceeded those in the nonresponders. This did not change as a result of prasugrel treatment (Figure 7A). Second, in marked contrast to the nonresponders, responders failed to display significant aspirin-induced increases in urinary LTE4, PGD-M, and TXB2 levels, or aspirin-induced increases in plasma tryptase levels (all of which reflect mast cell activation) (Figure 6)16,17 during reactions on the placebo arm. Finally, the numbers of blood eosinophils significantly declined during reactions on the placebo arm in the 35 nonresponders (presumably reflecting their recruitment to the respiratory tissue)18,19 but not in the responders. Both LTE420 and PGD221 recruit eosinophils to the respiratory tissue. Thus, the lack of change in blood eosinophils during the reactions in the responder group could reflect low release of these lipid mediators by tissue mast cells. Collectively, our findings strongly suggest a small group of subjects with AERD whose relatively low levels of mast cell mediator release reveal a P2Y12-- mediated component of their aspirin-induced reactions. This work, in conjunction with previous reports of a highly PGD2-driven subgroup, confirms that multiple endotypes exist within the AERD phenotype of asthma.17
Our previous studies, and those of others, demonstrated that baseline blood platelet activation in AERD exceeds that found in aspirin-tolerant controls by several fold.6,22 Given that P2Y12 receptors are required for autocrine activation of platelets in response to several classical agonists, it is surprising that prasugrel failed to alter platelet CD62P levels in the entire cohort, and did not change the percentages of platelet-leukocyte aggregates. Thus, the aberrant levels of steady-state platelet activation in AERD may be driven by mechanisms that do not require P2Y12-dependent amplification. Since P2Y12 receptors are also expressed by M2-type macrophages,23 dendritic cells,24 and eosinophils,25 it is possible that cell types other than platelets contribute to the reactions in the responder group. Polymorphic variants of the P2RY12 gene have been associated with the risk of airway obstruction in house dust mite -sensitized children,26 and with indices of platelet and eosinophil activation in subjects with AERD.27 Although our identification of a “responder” subgroup of AERD suggests a potential genetic mechanism, we did not identify any P2RY12 variants that distinguished the two groups.
Because the mechanism by which platelet activation occurs in AERD does not appear to require the P2Y12 receptor amplification loop (except for the “responder” subgroup), our study can neither verify nor refute the potential role of platelets in facilitating leukocyte adhesion and recruitment or transcellular synthesis of LTC4 in AERD. Determining the mechanism responsible for AERD-associated platelet activation will be necessary to address this role using a different target for intervention. Although prasugrel treatment is not effective for most patients with AERD, this study uncovered a potential subset of patients with AERD whose aspirin-induced reactions may involve P2Y12 receptors, potentially due to reduced mast cell-mediator release. Future larger studies will be necessary to validate this observation and reveal mechanism.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institutes of Health (NIH grant nos U19AI095219, K23HL111113, K23AI118804, K23AI125785, R01HL128241, T32AI00730, AI078908, HL117945, and AI52353), and by generous contributions from the Vinik and Kaye Families.
Registration
Abbreviations:
- AERD
Aspirin Exacerbated Respiratory Disease
- TNSS
Total Nasal Symptom Score
- BWH
Brigham and Women’s Hospital
- PD2
Increase in TNSS of 2 points
- LTE4
Leukotriene E4
- PG
Prostaglandin
- COX
Cyclooxygenase
- ACQ
Asthma Control Questionnaire
- FeNO
Fractional Exhaled Nitric Oxide
- EP2
E prostanoid 2
- LTA4
Leukotriene A4
- CysLT
Cysteinyl leukotriene
- LTC4
Leukotriene C4
- FEV1
Forced expiratory volume in 1 second
- 5-LO
5-lipoxygenase
- P2Y12 receptor
Type 12 purinergic receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: T Laidlaw has served on scientific advisory boards for GlaxoSmithKline and Allakos and has received consultancy fees from Knopp Biosciences and Sanofi-Genzyme. K Cahill has served on scientific advisory boards for Teva. J Cardet, K Murphy, J Cui, B Dioneda, P Kothari, B Raby, and J Boyce have no conflicts of interest to disclose. E Israel has received consultancy fees from AstraZeneca, Philips Respironics, Regeneron Pharmaceuticals, Birk Rock Bio, Nuvelution Pharmaceuticals, Vitaeris Inc, Sanofi, and Merck.
REFERENCES
- 1.Rajan JP, Wineinger NE, Stevenson DD, White AA. Prevalence of aspirin-exacerbated respiratory disease among asthmatic patients: A meta-analysis of the literature. J Allergy Clin Immunol 2015;135(3):676–81 e1. [DOI] [PubMed] [Google Scholar]
- 2.Israel E, Fischer AR, Rosenberg MA, Lilly CM, Callery JC, Shapiro J, et al. The pivotal role of 5-lipoxygenase products in the reaction of aspirin-sensitive asthmatics to aspirin. Amer Rev Resp Dis 1993;148(6 Pt 1):1447–51. [DOI] [PubMed] [Google Scholar]
- 3.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest 1998;101(4):834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoshida S, Amayasu H, Sakamoto H, Onuma K, Shoji T, Nakagawa H, et al. Cromolyn sodium prevents bronchoconstriction and urinary LTE4 excretion in aspirin-induced asthma. Ann Allergy Asthma 1998;80(2):171–6. [DOI] [PubMed] [Google Scholar]
- 5.Maclouf J, Antoine C, Henson PM, Murphy RC. Leukotriene C4 formation by transcellular biosynthesis. Ann N Y Acad Sci 1994;714:143–50. [DOI] [PubMed] [Google Scholar]
- 6.Laidlaw TM, Kidder MS, Bhattacharyya N, Xing W, Shen S, Milne GL, et al. Cysteinyl leukotriene overproduction in aspirin-exacerbated respiratory disease is driven by platelet-adherent leukocytes. Blood 2012;119(16):3790–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Laidlaw TM, Katz HR, Boyce JA. Prostaglandin E2 deficiency causes a phenotype of aspirin sensitivity that depends on platelets and cysteinyl leukotrienes. Proc Natl Acad Sci U S A 2013;110(42):16987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damman P, Woudstra P, Kuijt WJ, de Winter RJ, James SK. P2Y12 platelet inhibition in clinical practice. J Thromb Thrombolysis. 2012;33(2):143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macy E, Bernstein JA, Castells MC, Gawchik SM, Lee TH, Settipane RA, et al. Aspirin challenge and desensitization for aspirin-exacerbated respiratory disease: a practice paper. Ann Allergy Asthma 2007;98(2):172–4. [DOI] [PubMed] [Google Scholar]
- 10.Nizankowska-Mogilnicka E, Bochenek G, Mastalerz L, Swierczynska M, Picado C, Scadding G, et al. EAACI/GA2LEN guideline: aspirin provocation tests for diagnosis of aspirin hypersensitivity. Allergy. 2007;62(10):1111–8. [DOI] [PubMed] [Google Scholar]
- 11.Osborne JA. Estimating the False Discover Rate using SAS. Proceedings of the Thirty-First Annual SAS Users Group International Conference. 2006;190–31:1–10. [Google Scholar]
- 12.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E, et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137(2):400–11. [DOI] [PubMed] [Google Scholar]
- 13.Maugeri N, Evangelista V, Celardo A, Dell’Elba G, Martelli N, Piccardoni P, et al. Polymorphonuclear leukocyte-platelet interaction: role of P-selectin in thromboxane B2 and leukotriene C4 cooperative synthesis. Thromb Haemost 1994;72(3):450–6. [PubMed] [Google Scholar]
- 14.Klinkhardt U, Graff J, Harder S. Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and P-selectin expression in a human ex vivo in vitro model. Clin Pharmacol Ther 2002;71(3):176–85. [DOI] [PubMed] [Google Scholar]
- 15.Stevenson DD, Simon RA, Mathison DA, Christiansen SC. Montelukast is only partially effective in inhibiting aspirin responses in aspirin-sensitive asthmatics. Ann Allergy Asthma 2000;85(6 Pt 1):477–82. [DOI] [PubMed] [Google Scholar]
- 16.Bochenek G, Nagraba K, Nizankowska E, Szczeklik A. A controlled study of 9alpha,11beta-PGF2 (a prostaglandin D2 metabolite) in plasma and urine of patients with bronchial asthma and healthy controls after aspirin challenge. J Allergy Clin Immunol 2003;111(4):743–9. [DOI] [PubMed] [Google Scholar]
- 17.Cahill KN, Bensko JC, Boyce JA, Laidlaw TM. Prostaglandin D(2): a dominant mediator of aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2015;135(1):245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2016;137(5):1566–76 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupczyk M, Kurmanowska Z, Kuprys-Lipinska I, Bochenska-Marciniak M, Kuna P. Mediators of inflammation in nasal lavage from aspirin intolerant patients after aspirin challenge. Respir Med 2010;104(10):1404–9. [DOI] [PubMed] [Google Scholar]
- 20.Gauvreau GM, Parameswaran KN, Watson RM, O’Byrne PM. Inhaled leukotriene E(4), but not leukotriene D(4), increased airway inflammatory cells in subjects with atopic asthma. Am J Respir Crit Care Med 2001;164(8 Pt 1):1495–500. [DOI] [PubMed] [Google Scholar]
- 21.Hirai H, Tanaka K, Yoshie O, Ogawa K, Kenmotsu K, Takamori Y, et al. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. J Exp Clin Med 2001;193(2):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsui C, Kajiwara K, Hayashi H, Ito J, Mita H, Ono E, et al. Platelet activation markers overexpressed specifically in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol 2015. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 2006;177(10):7303–11. [DOI] [PubMed] [Google Scholar]
- 24.Ben Addi A, Cammarata D, Conley PB, Boeynaems JM, Robaye B. Role of the P2Y12 receptor gin the modulation of murine dendritic cell function by ADP. J Immunol 2010;185(10):5900–6. [DOI] [PubMed] [Google Scholar]
- 25.Muniz VS, Baptista-Dos-Reis R, Benjamim CF, Mata-Santos HA, Pyrrho AS, Strauch MA, et al. Purinergic P2Y12 Receptor Activation in Eosinophils and the Schistosomal Host Response. PLoS One 2015;10(10):e0139805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bunyavanich S, Boyce JA, Raby BA, Weiss ST. Gene-by-environment effect of house dust mite on purinergic receptor P2Y12 (P2RY12) and lung function in children with asthma. Clin Exp Allergy 2012;42(2):229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Losol P, Palikhe NS, Lee JW, Palikhe S, Kim MA, Yang EM, et al. Association of P2RY12 polymorphisms with eosinophil and platelet activation in patients with aspirin-exacerbated respiratory disease. Ann Allergy Asthma 2015;114(5):423–4 e1. [DOI] [PubMed] [Google Scholar]
- 28.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119(19):2553–2560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.