Abstract
Objective
The Patient Reported Outcomes Measurement Information System (PROMIS) is a calibrated item bank used to assess patient reported outcomes across multiple domains. The purpose of this study was to describe the performance of selected PROMIS measures in rheumatoid arthritis (RA) patients with active disease, initiating a disease-modifying antirheumatic drug (DMARD).
Methods
Participants in an ongoing prospective observational study completed eight PROMIS measures before and after DMARD initiation. Linear regression models were performed to identify cross-sectional associations between baseline PROMIS measures and disease activity, measured using the Clinical Disease Activity Index (CDAI). Paired t-tests were performed to evaluate responsiveness after 12-weeks of DMARD treatment. Associations between changes in PROMIS measures and changes in CDAI were assessed using linear regression.
Results
Among the 156 participants who completed the first study visit, the mean baseline CDAI was 25.5 ± 14.0. Baseline scores for PROMIS measures of physical health, pain and sleep were associated with baseline CDAI (p ≤ 0.05). Among the 106 participants with 12-week data, all PROMIS scores improved after DMARD initiation (p ≤ 0.05). With the exception of Depression, changes in all assessed PROMIS measures were correlated with changes in CDAI (standardized beta’s from |0.23 – 0.38|).
Conclusion
These data provide support for the utility of PROMIS measures for the assessment of physical and mental health in individuals with active RA. All PROMIS measures improved significantly after DMARD initiation, with the magnitudes of association between changes in PROMIS measures and changes in CDAI in the low to moderate range.
Keywords: Patient Reported Outcomes, Outcome Measures, Disease Activity, Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic illness that can significantly impact daily life when not aggressively managed. To evaluate disease activity, physicians rely heavily on assessment of swollen joints, measurement of blood inflammatory markers, and radiographs. Physicians cannot, however, gain a full understanding of disease activity and its effects without direct feedback from patients. Hallmark symptoms of RA, such as pain and fatigue, are necessarily evaluated through patient self-report. Additionally, other factors, including patient’s physical function, are often assessed through patient report (1).
The importance of patient reported outcomes (PROs) has been recognized in multiple realms – clinical trials, clinical care, and insurance authorizations (2). Recommendations from the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) incorporate the use of composite indices (e.g., the Clinical Disease Activity Index (CDAI), Disease Activity Score in 28-joints (DAS-28), and Simplified Disease Activity Index (SDAI)), which include patient global assessment, for reporting disease activity in all clinical trials (3). In addition, the Outcome Measures in Rheumatology (OMERACT) group is composing guidelines for the assessment of additional PROs within clinical trials (4). For the treatment of RA, an international task force recommended that physicians rely on composite measures of disease activity to evaluate a patient’s progress towards a treatment target and that they incorporate the patient perspective in developing a management strategy (5). Insurance companies are also pushing physicians to be patient-centered in their care as good outcomes are, in part, being defined as “value-adding activities” for patients. Physician recognition of what patients consider value-adding activities can come from PROs (6).
Although the value of PROs is widely recognized, researchers and clinicians are often faced with the conundrum of deciding which measure(s) to use. This study focused on one option for PRO assessment, the Patient-Reported Outcomes Measuremesnt Information System (PROMIS). PROMIS was developed to provide a standardized set of assessments of PROs, which allows for comparability across diseases and direct translation from research to clinical settings (7). Assessments are administered as fixed item short forms or via computer adaptive testing (CAT) (8).
The successful implementation of PROMIS measures for RA patients in research and clinical care settings requires establishment of their validity and ability to detect changes in symptoms. Bartlett et. al. provided preliminary evidence of the reliability and construct validity of PROMIS measures to assess RA symptoms in a general RA clinic cohort, finding that PROMIS domain measures correlated well with established measures for assessment of disease activity and RA symptoms in cross-sectional analyses (9, 10). In addition, Katz et al. reported on the performance of the static 29-item PROMIS profile in a large population of individuals with rheumatic disease, including RA (11). The findings presented in this paper aim to: 1) provide additional evidence for the feasibility of using PROMIS in a research setting, 2) examine the distribution of PROMIS scores within an RA cohort with mostly moderate to high levels of disease activity, and 3) provide the first evidence demonstrating the responsiveness of PROMIS measures to changes in RA disease activity, associated with starting a disease-modifying antirheumatic drug (DMARD).
PATIENTS AND METHODS
Study population
Data for this study were from the first 156 participants enrolled in the ongoing multi-site, prospective, observational Central Pain in Rheumatoid Arthritis (CPIRA) study, which began enrollment in January 2014. All data obtained on or before September 16, 2016 were included in this set of analyses. Participants were recruited from five academic medical centers. Inclusion criteria required participants to have active disease necessitating a start or switch to a new DMARD based on physician judgment. Subjects starting hydroxychloroquine or subjects switching from one TNF-α inhibitor to another were not eligible for inclusion. All participants had to meet 2010 ACR criteria for a diagnosis of RA (12). No subjects could be taking more than 10 mg of prednisone or chronic opioid pain medications. Those using central acting pain medications (e.g., tricyclic antidepressants, serotonin norepinephrine reuptake inhibitors, anticonvulsants) had to be on stable doses for the past three months and planning to continue the same usage for the study duration. Patients with fibromyalgia were included in the study population. Patients with peripheral neuropathy or severe peripheral vascular disease were excluded. Subjects with a diagnosis of another autoimmune disease were excluded. Written informed consent was obtained from all participants. This study was approved by the institutional review boards at each study site.
Procedures
Subjects were evaluated before starting the new DMARD and 12 to 24 weeks after taking their first dose of medication. Because the onset of action of methotrexate is 3–6 weeks, subjects starting methotrexate were eligible for enrollment if they had taken one dose before the baseline evaluation (13). Since not all participants had completed follow-up visits at the time of this analysis, follow-up data are presented on a subset of the baseline cohort.
RA patients were registered as study participants at the time of their baseline visit in the PROMIS Assessment Center (www.assessmentcenter.net). Participants answered questionnaires using a desktop or tablet computer. At the baseline and follow-up study visits, subjects completed the PROMIS Global Health v1.1 and the PROMIS Pain Intensity 3a short forms (14). Subjects also completed the following PROMIS physical and mental health domains, assessed using CATs: Pain Interference, Pain Behavior, Sleep Disturbance, Sleep Related Impairment, Fatigue, Anxiety, and Depression (15–20). CATs use Item Response Theory to provide tailored and precise assessment, across the continuum of experience (21). Answering questionnaires took participants typically between 5–10 minutes. Research coordinators measured height and weight at each visit. Trained research coordinators also performed swollen and tender joint counts (28 joints) at both visits and provided global assessments on a 0–100 NRS of the patients’ health with respect to their RA. These coordinators were trained in the joint examination during a one-day orientation session at the beginning of the study. In addition, each coordinator was provided with a training video to review the joint count. Additional training in the joint examination was provided at each site, supervised directly by the site principal investigators, who are all board-certified rheumatologists.
The presence of fibromyalgia symptoms and the diagnosis of fibromyalgia was assessed using the 2010 modified ACR Preliminary Diagnostic Criteria for Fibromyalgia (22). Patients also provided their own global assessment of disease activity using a 0–100 NRS, using the anchors of 0 being “very well” and 100 being “very poorly”. Medication information was obtained from participants at both visits. Use of a DMARD at baseline was defined as having taken the DMARD within the six weeks prior to the baseline visit. Serological status of subjects was obtained from chart review from each participants’ electronic medical records.
Scoring
Instrument scores were calculated by the PROMIS Assessment Center and reported as T-scores standardized to a general population mean of 50 and standard deviation of 10. High scores indicated more of the concept measured. Higher physical and mental global health scores indicated better health; whereas, higher scores on the pain, sleep, anxiety and depression measures indicated worse outcomes. The CDAI score was calculated as the sum of the swollen and tender joints (0–28 for swelling and 0–28 for tenderness), patient global score (0–10), and assessor global score (0–10) (23). Scores ≤ 10 indicated low disease activity, scores from 10 to 22 indicated moderate disease activity, and scores > 22 indicated high disease activity (24).
Statistical Analysis
We created histograms of the distributions of scores for all PROMIS measures and compared means in our population to the general population mean of 50. Multivariable linear regression models were used to compute adjusted mean PROMIS scores according to CDAI category. Multivariable adjusted linear regression models were also used to determine baseline associations between each PROMIS measure, the CDAI score and its components. All multivariable models were adjusted for site, gender, race, age, seropositive status, and RA disease duration. Among participants with follow-up data, paired t-tests were used to assess for change in CDAI and PROMIS measure scores with DMARD treatment. We used multivariable linear regression models to evaluate associations between changes in PROMIS scores and changes in CDAI and its components. Associations were presented as standardized betas. Approximate p-values (e.g., P ≤ 0.05; P ≤ 0.01; P ≤ 0.001) were reported rather than exact p-values according to the recommendations of Boos and Stefanski (24). To assess for evidence of a floor effect, a subgroup analysis was performed among individuals with moderate to high CDAI at baseline.
RESULTS
Participant Characteristics
One hundred fifty-six RA patients were enrolled in CPIRA at the time of this analysis (Table 1). The majority of participants were female (82.1%) and Caucasian (95.5%). Mean age was 54.6 ± 13.6 years, and mean disease duration was 10.0 ± 12.6 years. Overall, the population was overweight with a mean BMI of 31.1 ± 16.4.
Table 1.
Baseline characteristics of RA patients initiating a new DMARD (N = 156)
| Female | 82.1% |
| Age, years (SD) | 54.6 (13.6) |
| Caucasian | 95.5% |
| RA Disease Duration, years (SD) | 10.0 (12.6) |
| Seropositive | 81.4% |
| CDAI (SD), 0–100 | 25.5 (14.0) |
| Swollen Joints, 0–28 (SD) | 5.8 (5.6) |
| Tender Joints, 0–28 (SD) | 11.8 (9.2) |
| Patient Global, 0–10 (SD) | 4.1 (2.3) |
| Assessor Global, 0–10 (SD) | 3.8 (2.3) |
| Average Pain Rating, 0–10 (SD) | 5.2 (2.2) |
| Pain Catastrophizing Score, 0–52 (SD) | 18.5 (13.6) |
| Medication Use | |
| DMARDs | 60.9% |
| Non-biologic DMARDs1 | 44.2% |
| Biologic DMARDs1 | 26.3% |
| Corticosteroids | 43.0% |
| Mean prednisone dose, mg (SD)2 | 6.7 (3.0) |
| NSAID use | 46.2% |
Percentages reflect a denominator of the whole population (n = 156).
Of people taking prednisone.
The majority of participants were seropositive (81.4%) and had high disease activity, indicated by a mean CDAI score of 25.5 ± 14.0. The average numbers of swollen and tender joints were 5.8 ± 5.6 and 11.8 ± 9.2, respectively. The mean patient global score was 4.1 ± 2.3. The mean assessor global score was 3.8 ± 2.3. Within the study population, 33.3% of participants had fibromyalgia defined by the 2010 modified ACR Preliminary Diagnostic Criteria for Fibromyalgia.
At baseline, 60.9% of participants were taking one or more DMARDs. Forty-four percent were on non-biologic DMARDs, and 26.3% were on biologic DMARDs. Slightly less than half (43.0%) of participants were using corticosteroids at their baseline visit. Among corticosteroid users, the mean prednisone dose was 6.7 ± 3.0 milligrams per day. Forty-six percent of participants used NSAIDs on a regular basis.
Means and Distributions of Baseline PROMIS Measures
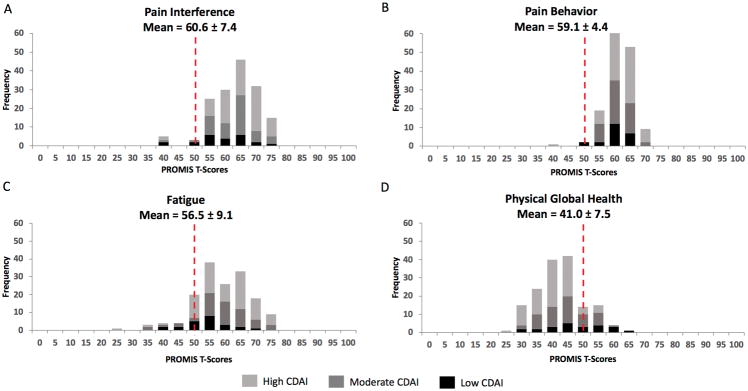
All PROMIS T-scores exhibited a normal distribution. The PROMIS measures with the greatest shifts in distribution from the general population (mean of 50 ± 10) were Pain Interference (60.6 ± 7.4), Pain Behavior (59.1 ± 4.4), Fatigue (56.5 ± 9.1) and Physical Global Health (41.0 ± 7.5) (Figure 1). The means for Sleep Disturbance and Sleep-Related Impairment were close to one half of a SD above the general population mean of 50 ± 10, at 54.2 ± 9.1 and 55.1 ± 10.0, respectively (Supplementary Figure 1). The distributions of the Depression, Pain Intensity 3a, Anxiety and Mental Global Health measures were similar to that of the general population, with means of 53.7 ± 8.8, 51.5 ± 6.0, 50.5 ± 9.2 and 47.9 ± 8.4, respectively (Supplementary Figure 2). Participants with fibromyalgia had significantly worse scores on all assessed PROMIS measures (P < 0.0001) (Supplementary Table 1).
Figure 1.
Distributions of PROMIS scores for study participants at baseline (N = 156).
Distributions of PROMIS Pain Interference (A), Pain Behavior (B), Fatigue (C) and Physical Global Health (D) T-Scores for study participants showing scores by CDAI level at baseline (N = 156). Dotted line represents the general population mean score of 50.
Cross-Sectional Associations between Baseline PROMIS and Disease Activity Measures
In multivariable analyses, mean scores for all PROMIS measures were significantly worse (p < 0.001) across categories of increasing disease activity (Table 2). Mean physical global health scores ranged from 45.3 among those with low disease activity to 38.8 among those with high disease activity, whereas mental global health scores ranged from 51.2 among those with low disease activity to 46.1 among those with high disease activity. Of the individual PROMIS domains, Sleep Disturbance showed the widest range in scores (7.9 points), whereas Pain Behavior showed the smallest difference (2.1 points).
Table 2.
Adjusted means (95% confidence intervals) of PROMIS T-scores by CDAI category1
| PROMIS Measures2 | Low CDAI ≤ 10 N = 23 |
Moderate CDAI 10–22 N = 51 |
High CDAI >22 N = 82 |
|---|---|---|---|
| Physical Global Health (95% CI)*** | 45.3 (44.4, 46.1) | 42.6 (41.9, 43.3) | 38.8 (38.3, 39.3) |
| Mental Global Health (95% CI)** | 51.2 (50.6, 51.8) | 49.3 (48.7, 49.9) | 46.1 (45.6, 46.5) |
| Pain Intensity 3a (95% CI)*** | 48.5 (47.8, 49.2) | 50.7 (50.2, 51.2) | 52.9 (52.5, 53.3) |
| Pain Interference (95% CI)** | 57.2 (56.5, 57.9) | 59.6 (59.0, 60.1) | 62.2 (61.8, 62.6) |
| Pain Behavior (95% CI)* | 57.7 (56.9, 58.5) | 58.7 (58.1, 59.3) | 59.8 (59.3, 60.2) |
| Sleep Disturbance (95% CI)*** | 48.9 (48.2, 49.7) | 52.6 (51.9, 53.2) | 56.8 (56.4, 57.2) |
| Sleep-Related Impairment (95% CI)** | 50.0 (48.9, 51.0) | 53.1 (52.3, 53.9) | 57.8 (57.2, 58.4) |
| Fatigue (95% CI)** | 52.9 (52.1, 53.7) | 55.0 (54.4, 55.6) | 58.4 (57.9, 58.9) |
| Anxiety (SD)** | 50.5 (49.2, 51.8) | 52.4 (51.6, 53.2) | 55.3 (54.7, 56.0) |
| Depression (SD)* | 47.7 (46.5, 48.8) | 49.2 (48.5, 49.8) | 52.1 (51.5, 52.7) |
Multivariable models adjusted for study site, gender, race, age, seropositivity, and RA disease duration to predict means by CDAI category and to determine trend across categories.
Differences between groups were all statistically significant at P < 0.001.
indicates P ≤ 0.05;
indicates P ≤ 0.01;
indicates P ≤ 0.001
Global Health and Pain Intensity 3a scores were collected using short forms. All other instruments were collected using computerized adaptive tests. Lower Global Health scores indicate worse global health. For all other measures, high scores indicate worse symptoms. All PROMIS scores range from about 20–80 and are standardized to a general population mean (SD) of 50 (10).
In multivariable models examining the relationship between PROMIS measures and CDAI scores, significant associations (β’s ranging from |0.21 – 0.34|; p < 0.05) were found with Physical Global Health, Pain Intensity 3a, Pain Interference, Pain Behavior, Sleep Disturbance, and Sleep-Related Impairment (Table 3). Tender joints, patient global, and assessor global were also significantly associated (β’s ranging from |0.18 – 0.43|; p ≤ 0.05) with these PROMIS measures. The swollen joint component of CDAI was only significantly associated (β = 0.18; p < 0.05) with Sleep Disturbance. The directionality of all associations was such that increases in CDAI or the components of the CDAI were associated with worsening PROMIS scores.
Table 3.
Standardized betas showing associations between PROMIS T-scores and disease activity measures at baseline (n = 156) 1
| PROMIS Measure | CDAI | Swollen Joints | Tender Joints | Patient Global | Assessor Global |
|---|---|---|---|---|---|
| Physical Global Health2 | −0.25** | 0.019 | −0.21* | −0.43*** | −0.28** |
| Mental Global Health2 | −0.14 | 0.073 | −0.12 | −0.30** | −0.24** |
| Pain Intensity 3a2 | 0.30*** | 0.063 | 0.25* | 0.38*** | 0.27*** |
| Pain Interference | 0.27** | 0.016 | 0.25* | 0.35*** | 0.27** |
| Pain Behavior | 0.21* | 0.018 | 0.22* | 0.25** | 0.18* |
| Sleep Disturbance | 0.34*** | 0.18* | 0.27** | 0.30*** | 0.23** |
| Sleep –Related Impairment | 0.27** | 0.045 | 0.26** | 0.29*** | 0.26** |
| Fatigue | 0.17 | −0.065 | 0.23* | 0.26** | 0.16 |
| Anxiety | 0.14 | −0.040 | 0.12 | 0.28*** | 0.18* |
| Depression | 0.026 | −0.10 | 0.021 | 0.17* | 0.14 |
Multivariable models adjusted for study site, gender, race, age, seropositivity, and RA disease duration to predict means by CDAI category and to determine trend across categories.
Standardized betas reflect the amount of standardized deviations a dependent variable changes per standard deviation change in the independent variable.
indicates P ≤ 0.05;
indicates P ≤ 0.01;
indicates P ≤ 0.001.
Global Health and Pain Intensity 3a scores were collected using short forms. All other instruments were collected using computerized adaptive tests. Lower Global Health scores indicate worse global health. For all other measures, high scores indicate worse symptoms. All PROMIS scores range from about 20–80 and are standardized to a general population mean (SD) of 50 (10).
Changes in PROMIS Measures and CDAI from Baseline to 12-Weeks Post DMARD Initiation
At the time of this analysis, 106 subjects had data from a follow-up visit. With 12–24 weeks of DMARD treatment, disease activity significantly decreased with a mean (SD) decrease in CDAI of 10.8 (13.1). The 52 subjects with high baseline disease activity experienced an average improvement in CDAI score (SD) of 17.6 (14.6). The 38 participants with moderate baseline disease activity experienced an average improvement in CDAI score of 5.8 (6.6), and 16 participants with low baseline disease activity experienced an average improvement in CDAI score of 0.8 (6.3).
All PROMIS measures improved significantly (p < 0.05) with DMARD treatment (Table 4). The greatest improvement was seen in the Pain Intensity 3a scores (6.0 points), and the smallest improvement was seen in Mental Global Health scores (1.7 points). In a sensitivity analysis examining only individuals with moderate to high CDAI at baseline, the results were similar.
Table 4.
PROMIS T-score means pre and post-DMARD treatment (N = 106)
| Pre-DMARD Treatment | Post-DMARD Treatment | |
|---|---|---|
| Physical Global Health (SD) 1 | 41.4 (7.3) | 45.1 (8.7)*** |
| Mental Global Health (SD) 1 | 48.0 (8.2) | 49.7 (9.0)* |
| Pain Intensity 3a (SD) 1 | 51.5 (6.0) | 45.5 (7.7)*** |
| Pain Interference (SD) | 60.6 (7.3) | 55.5 (8.0)*** |
| Pain Behavior (SD) | 59.2 (4.7) | 54.8 (8.0)*** |
| Sleep Disturbance (SD) | 55.2 (8.5) | 50.9 (8.8)*** |
| Sleep-Related Impairment (SD) | 55.2 (9.8) | 52.0 (10.5)*** |
| Fatigue (SD) | 56.8 (8.6) | 52.3 (8.8)*** |
| Anxiety (SD) | 54.3 (8.8) | 51.2 (9.5)*** |
| Depression (SD) | 50.8 (9.7) | 48.5 (9.2)** |
indicates P ≤ 0.05;
indicates P ≤ 0.01;
ndicates P ≤ 0.001 from paired T-tests.
Global Health and Pain Intensity 3a scores were collected using short forms. All other instruments were collected using computerized adaptive tests. Lower Global Health scores indicate worse global health. For all other measures, high scores indicate worse symptoms. All PROMIS scores range from about 20–80 and are standardized to a general population mean (SD) of 50 (10).
Changes in all PROMIS measures, except Depression, were significantly associated (β’s ranging from |0.23 – 0.38|; p ≤ 0.05) with changes in CDAI and changes in tender joint count (β’s ranging from |0.24 – 0.36|; p ≤ 0.05) (Table 5). Changes in all PROMIS measures, except Mental Global Health, were significantly associated with changes in patient global assessment (β’s ranging from |0.21 – 0.35|; p ≤ 0.05). Changes in Physical Global Health, Mental Global Health, Pain Interference, Sleep Disturbance, Sleep-Related Impairment and Fatigue were significantly associated with changes in assessor global (β’s ranging from |0.22 – 0.34|; p ≤ 0.05). Changes in PROMIS measures were not significantly associated with changes in swollen joint count. A sensitivity analysis among those with moderate to high baseline CDAI showed similar results, with some associations being slightly stronger in the subgroup of individuals with moderate to high disease activity (Supplementary Table 2).
Table 5.
Standardized betas showing associations between changes in PROMIS T-scores and changes in disease activity measures (n = 106)1
| PROMIS Measure | CDAI | Swollen Joints | Tender Joints | Patient Global | Assessor Global |
|---|---|---|---|---|---|
| Physical Global Health2 | −0.29** | −0.12 | −0.30** | −0.21* | −0.24* |
| Mental Global Health2 | −0.34** | −0.20 | −0.33** | −0.18 | −0.22* |
| Pain Intensity 3a2 | 0.27* | 0.12 | 0.24* | 0.23* | 0.20 |
| Pain Interference | 0.33** | 0.12 | 0.31** | 0.30** | 0.28** |
| Pain Behavior | 0.27* | 0.059 | 0.28** | 0.28** | 0.20 |
| Sleep Disturbance | 0.38*** | 0.12 | 0.36*** | 0.35** | 0.34** |
| Sleep –Related Impairment | 0.33** | 0.11 | 0.34** | 0.27** | 0.30** |
| Fatigue | 0.33** | 0.086 | 0.35** | 0.29** | 0.27* |
| Anxiety | 0.23* | 0.048 | 0.24* | 0.24* | 0.16 |
| Depression | 0.088 | −0.039 | 0.067 | 0.24* | 0.10 |
Multivariable models adjusted for study site, gender, race, age, seropositivity, and RA disease duration to predict means by CDAI category and to determine trend across categories.
Standardized betas reflect the amount of standardized deviations a dependent variable changes per standard deviation change in the independent variable.
indicates P ≤ 0.05;
indicates P ≤ 0.01;
indicates P ≤ 0.001.
Global Health and Pain Intensity 3a scores were collected using short forms. All other instruments were collected using computerized adaptive tests. Lower Global Health scores indicate worse global health. For all other measures, high scores indicate worse symptoms. All PROMIS scores range from about 20–80 and are standardized to a general population mean (SD) of 50 (10).
DISCUSSION
To our knowledge, this is the first prospective longitudinal study to examine changes in PROMIS measures among RA patients starting a DMARD. This study is also unique in that it provides data regarding PROMIS measures among RA patients with moderate to high disease activity, whereas previous studies included RA patients on established DMARD regimens, with lower average disease activity (9, 11).
At baseline, mean PROMIS Physical Global Health, Pain Interference and Pain Behavior scores differed by approximately one standard deviation from general population norms, indicating that these measures are able to differentiate RA patients with active disease from the general population. In contrast, mean PROMIS Pain Intensity and Depression T-scores were similar to population norms. This may be due to habituation, meaning that patient perception of their symptoms may change over time as they habituate to the new reality of their chronic illness. In other words, a rating of 0 may equate to baseline symptoms instead of a complete lack of symptoms (9). Further research is needed to examine the role of habituation in the assessment of pain and depression in patients with active RA. In the meantime, investigators wanting to compare symptoms of pain and depression between RA patients and the general population should consider including other assessments of these domains and/or supplement with related PROMIS measures (e.g., Pain Interference and Pain Behavior).
In cross-sectional analyses, all of the evaluated PROMIS instruments, including Pain Intensity and Depression, differentiated between groups of RA patients with different levels of disease activity. Pain Intensity and Depression scores were significantly higher among those with higher disease activity compared to those with lower disease activity, despite similar mean scores in the total cohort compared to general population norms. This observation underscores the distinction between comparing subgroups within a population vs. comparing two different populations. Even though RA patients may have a different frame of reference than the general population for gauging symptoms of pain severity and depression, these measures were able to differentiate between RA patients with different disease activity levels.
Congruent with the finding that baseline PROMIS scores were associated with baseline CDAI categories, the baseline scores for Physical Global Health, Pain Intensity 3a, Pain Interference, Pain Behavior, Sleep Disturbance, and Sleep-Related Impairment were correlated with each component of the CDAI, except swollen joint count. The absence of association between PROMIS measures and swollen joint count may be due to multiple factors, including the relative insensitivity of the swollen joint count as an independent measure of inflammatory disease activity (25, 26) and the multifaceted nature of these PROs. The patient-reported measures of disease activity likely capture more intangible aspects of the patient experience, which may variably reflect actual inflammation, depending on individual circumstances (27–29).
One of the most novel findings of this study was the observation that all PROMIS measures improved with DMARD treatment. In our study, the largest change was seen with Pain Intensity, whereas the smallest change was seen in the Mental Global Health scores. The changes in the physical health domains were larger than those for the mental health domains. This observation is consistent with reports for legacy instruments assessing PROs in clinical trials (30). A meta-analysis of the effect of TNF-α inhibitor therapy in chronic illnesses, including RA, reported that although depression and anxiety improved with treatment, effect sizes were small (31). It is still unclear whether the improvements observed in this study were clinically meaningful. Research is underway to determine the minimal clinically important differences in PROMIS scores for RA patients.
Changes in all PROMIS measures, except Depression, were associated with changes in CDAI, though the magnitudes of correlation were generally low. In a subgroup analysis, including only those with baseline moderate to high disease activity, some correlations were slightly stronger, suggesting a possible mild floor effect among individuals with low disease activity at baseline. Of the physical health domains, the strongest associations with changes in disease activity were noted for the measures of sleep and fatigue (PROMIS Sleep Disturbance, Sleep-Related Impairment, and Fatigue). These findings highlight the important relationship between sleep, fatigue and disease activity in RA and are consistent with reports of significant reductions in sleep problems and fatigue in clinical trials of RA patients treated with DMARDs (32–34).
The lack of association between changes in depression and changes in CDAI is notable in the context of growing interest in the impact of inflammation on depressive symptoms. Others have reported that depression and inflammatory disease activity have a reciprocal relationship. Whereas depressive symptoms decrease with effective treatment of inflammatory disease activity, prevalent depression also decreases the likelihood of response to DMARDs (35). Multiple factors, including the complex relationship between depression, pain and inflammation, the absence of severe depressive symptoms at baseline, and the small magnitude of change in Depression scores, may have limited our ability to detect associations in this study.
The strengths of our study include the large sample size of RA patients with active disease and the comprehensive assessment of these patients after initiation of a DMARD. Limitations of this study include the absence of a comparison group of RA patients who were not starting DMARDS. In addition, we were not able to examine associations between PROMIS measures and serum markers of inflammation. Although blood samples were obtained from these subjects, these measures have not been assayed, as this study is ongoing. We also did not include assessments of physical function, which could be an important determinant of irreversible components of disease. Other studies, however, have shown that PROMIS measures of physical function are valid and responsive in RA (36, 37).
Another limitation may be generalizability as only 60.9% of participants were taking a DMARD at the time of the baseline study visit. The relatively low number on DMARDs at baseline reflects the inclusion criterion requiring patients to have active disease, necessitating a start or switch to a new DMARD. Many subjects had previously been taking DMARDs but were off of their DMARD for at least six weeks prior to the study visit for various reasons (e.g., insurance changes, infection/other comorbidities, history of remission) and, as a result, were experiencing increased disease activity, requiring initiation of a new DMARD.
This study contributes new information regarding the role of PROMIS measures in the longitudinal assessment of RA patients with active disease, treated with DMARDs. The PROMIS Global Health, Pain Intensity, Pain Interference, Pain Behavior, Sleep Disturbance, Sleep-Related Impairment, Fatigue, Anxiety, and Depression measures were all able to differentiate between RA patients with different levels of disease activity. The PROMIS physical global health, pain, and sleep measures were correlated with CDAI and each of its components, except swollen joint count. With respect to statistical significance, all the aforementioned PROMIS measures improved with initiation of a DMARD. However, from a clinical standpoint, it is not known whether these changes were meaningful. While the majority of improvements in PROMIS measures were associated with improvements in CDAI, the magnitudes of association were not strong. Further research is needed to determine minimal clinically important changes in these measures for RA patients and to clarify the effects of baseline RA disease activity on the responsiveness of these measures.
Supplementary Material
SIGNIFICANCE AND INNOVATIONS.
This is the first study to assess 8 PROMIS computerized adaptive tests (CATs) and 2 short forms (yielding a total of 10 different scores) of physical and mental health in RA patients starting a disease-modifying antirheumatic drug (DMARD) for active disease.
PROMIS measures can detect change in symptoms of global health, pain, sleep, fatigue, and emotional health among RA patients with active disease, starting a new DMARD.
Changes in PROMIS measures of physical global health, mental global health, pain, sleep, fatigue and anxiety were significantly associated with changes in disease activity after 12 weeks of DMARD treatment.
Acknowledgments
Funding: This study was funded by NIAMS R01 AR064850. This work was also conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health. Additional funding sources for the Johns Hopkins site include RDRCC P30 AR053503 and P30 AR07254 and the Camille Julia Morgan Arthritis Research and Education Fund. The Boston University site also gets funding from P60 AR47785, R01 AR062506 and K24 AR070892.
We would like to thank the study coordinators at all of our sites, including Malini Moni and Grazyna Purwin (Johns Hopkins), Alieysa Patel and Melanie Woods (University of Michigan), Joyce Goggins (Boston University), Laurie Hope and Kelly Reckley (University of Pittsburgh), and Cassandra Corrigan, Agnes Zak, Josh Colls, and Dee Luo (Brigham and Women’s Hospital). We would like to acknowledge Chang Xu for her programming assistance. We would also like to acknowledge all of our referring physicians and patient participants.
Footnotes
Disclosures: Dr. Lee reports a research grant from Pfizer and stock in Express Scripts. Dr. Bolster reports receiving research funding from Amgen and Eli Lilly.
References
- 1.Her M, Kavanaugh A. Patient-reported outcomes in rheumatoid arthritis. Curr Opin Rheumatol. 2012;24:327–34. doi: 10.1097/BOR.0b013e3283521c64. [DOI] [PubMed] [Google Scholar]
- 2.Wahl ER, Yazdany J. Challenges and opportunities in using patient-reported outcomes in quality measurement in rheumatology. Rheum Dis Clin North Am. 2016;42:363–75. doi: 10.1016/j.rdc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aletaha D, Landewe R, Karonitsch T, Bathon J, Boers M, Bombardier C, et al. Reporting disease activity in clinical trials of patients with rheumatoid arthritis: EULAR/ACR collaborative recommendations. Arthritis Rheum. 2008;59:1371–7. doi: 10.1002/art.24123. [DOI] [PubMed] [Google Scholar]
- 4.Tugwell P, Boers M, Brooks P, Simon L, Strand V, Idzerda L. OMERACT: an international initiative to improve outcome measurement in rheumatology. Trials. 2007;8:38. doi: 10.1186/1745-6215-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75:3–15. doi: 10.1136/annrheumdis-2015-207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Tuyl LH, Michaud K. Patient-Reported Outcomes in Rheumatoid Arthritis. Rheum Dis Clin North Am. 2016;42:219–37. doi: 10.1016/j.rdc.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna D, Krishnan E, Dewitt EM, Khanna PP, Spiegel B, Hays RD. The future of measuring patient-reported outcomes in rheumatology: Patient-Reported Outcomes Measurement Information System (PROMIS) Arthritis care & research. 2011;63(Suppl 11):S486–90. doi: 10.1002/acr.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett SJ, Orbai AM, Duncan T, DeLeon E, Ruffing V, Clegg-Smith K, et al. Reliability and validity of selected PROMIS measures in people with rheumatoid arthritis. PloS one. 2015;10:e0138543. doi: 10.1371/journal.pone.0138543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bingham CO, 3rd, Bartlett SJ, Merkel PA, Mielenz TJ, Pilkonis PA, Edmundson L, et al. Using patient-reported outcomes and PROMIS in research and clinical applications: experiences from the PCORI pilot projects. Qual Life Res. 2016;25:2109–16. doi: 10.1007/s11136-016-1246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz P, Pedro S, Michaud K. Performance of the Patient-Reported Outcomes Measurement Information System 29-Item Profile in Rheumatoid Arthritis, Osteoarthritis, Fibromyalgia, and Systemic Lupus Erythematosus. Arthritis care & research. 2017;69:1312–21. doi: 10.1002/acr.23183. [DOI] [PubMed] [Google Scholar]
- 12.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 13.Methotrexate [package insert] Mp. Feucht, Germany: EXCELLA GmbH; 2016. [Google Scholar]
- 14.Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. 2009;18:873–80. doi: 10.1007/s11136-009-9496-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amtmann D, Cook KF, Jensen MP, Chen WH, Choi S, Revicki D, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150:173–82. doi: 10.1016/j.pain.2010.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Revicki DA, Chen WH, Harnam N, Cook KF, Amtmann D, Callahan LF, et al. Development and psychometric analysis of the PROMIS pain behavior item bank. Pain. 2009;146:158–69. doi: 10.1016/j.pain.2009.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the Patient-Reported Outcomes Measurement Information System (PROMIS) Med Care. 2007;45:S22–31. doi: 10.1097/01.mlr.0000250483.85507.04. [DOI] [PubMed] [Google Scholar]
- 19.Pilkonis PA, Choi SW, Reise SP, Stover AM, Riley WT, Cella D. Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS(R)): depression, anxiety, and anger. Assessment. 2011;18:263–83. doi: 10.1177/1073191111411667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilkonis PA, Yu L, Dodds NE, Johnston KL, Maihoefer CC, Lawrence SM. Validation of the depression item bank from the Patient-Reported Outcomes Measurement Information System (PROMIS) in a three-month observational study. J Psychiatr Res. 2014;56:112–9. doi: 10.1016/j.jpsychires.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Witter JP. The Promise of Patient-Reported Outcomes Measurement Information System-Turning Theory into Reality: A Uniform Approach to Patient-Reported Outcomes Across Rheumatic Diseases. Rheum Dis Clin North Am. 2016;42:377–94. doi: 10.1016/j.rdc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 23.Aletaha D, Nell VP, Stamm T, Uffmann M, Pflugbeil S, Machold K, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther. 2005;7:R796–806. doi: 10.1186/ar1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52:2625–36. doi: 10.1002/art.21235. [DOI] [PubMed] [Google Scholar]
- 25.Pincus T, Richardson B, Strand V, Bergman MJ. Relative efficiencies of the 7 rheumatoid arthritis Core Data Set measures to distinguish active from control treatments in 9 comparisons from clinical trials of 5 agents. Clin Exp Rheumatol. 2014;32:S-47–54. [PubMed] [Google Scholar]
- 26.Ward MM. Clinical measures in rheumatoid arthritis: which are most useful in assessing patients? The Journal of rheumatology. 1994;21:17–27. [PubMed] [Google Scholar]
- 27.Radner H, Yoshida K, Tedeschi S, Studenic P, Frits M, Iannaccone C, et al. Different rating of global rheumatoid arthritis disease activity in rheumatoid arthritis patients with multiple morbidities. Arthritis Rheumatol. 2017;69:720–7. doi: 10.1002/art.39988. [DOI] [PubMed] [Google Scholar]
- 28.Nikiphorou E, Radner H, Chatzidionysiou K, Desthieux C, Zabalan C, van Eijk-Hustings Y, et al. Patient global assessment in measuring disease activity in rheumatoid arthritis: a review of the literature. Arthritis Res Ther. 2016;18:251. doi: 10.1186/s13075-016-1151-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker JF, Conaghan PG, Emery P, Baker DG, Ostergaard M. Relationship of patient-reported outcomes with MRI measures in rheumatoid arthritis. Ann Rheum Dis. 2017;76:486–90. doi: 10.1136/annrheumdis-2016-209463. [DOI] [PubMed] [Google Scholar]
- 30.Wells G, Li T, Maxwell L, Maclean R, Tugwell P. Responsiveness of patient reported outcomes including fatigue, sleep quality, activity limitation, and quality of life following treatment with abatacept for rheumatoid arthritis. Ann Rheum Dis. 2008;67:260–5. doi: 10.1136/ard.2007.069690. [DOI] [PubMed] [Google Scholar]
- 31.Abbott R, Whear R, Nikolaou V, Bethel A, Coon JT, Stein K, et al. Tumour necrosis factor-alpha inhibitor therapy in chronic physical illness: A systematic review and meta-analysis of the effect on depression and anxiety. J Psychosom Res. 2015;79:175–84. doi: 10.1016/j.jpsychores.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Pope J, Bingham CO, 3rd, Fleischmann RM, Dougados M, Massarotti EM, Wollenhaupt J, et al. Impact of certolizumab pegol on patient-reported outcomes in rheumatoid arthritis and correlation with clinical measures of disease activity. Arthritis Res Ther. 2015;17:343. doi: 10.1186/s13075-015-0849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strand V, Kremer J, Wallenstein G, Kanik KS, Connell C, Gruben D, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis research & therapy. 2015;17:307. doi: 10.1186/s13075-015-0825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells G, Li T, Tugwell P. Investigation into the impact of abatacept on sleep quality in patients with rheumatoid arthritis, and the validity of the MOS-Sleep questionnaire Sleep Disturbance Scale. Ann Rheum Dis. 2010;69:1768–73. doi: 10.1136/ard.2009.119727. [DOI] [PubMed] [Google Scholar]
- 35.Kekow J, Moots R, Khandker R, Melin J, Freundlich B, Singh A. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology (Oxford) 2011;50:401–9. doi: 10.1093/rheumatology/keq327. [DOI] [PubMed] [Google Scholar]
- 36.Hays RD, Spritzer KL, Fries JF, Krishnan E. Responsiveness and minimally important difference for the patient-reported outcomes measurement information system (PROMIS) 20-item physical functioning short form in a prospective observational study of rheumatoid arthritis. Ann Rheum Dis. 2015;74:104–7. doi: 10.1136/annrheumdis-2013-204053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahl E, Gross A, Chernitskiy V, Trupin L, Gensler L, Chaganti K, et al. Validity and Responsiveness of a 10-Item Patient-Reported Measure of Physical Function in a Rheumatoid Arthritis Clinic Population. Arthritis care & research. 2017;69:338–46. doi: 10.1002/acr.22956. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.