Abstract
Background
Physical function is integral to healthy aging; however, limited research has examined the association of venous thromboembolism(VTE) with subsequent physical function.
Objectives
To prospectively evaluate the relationship between VTE and decline in physical function among 80,836 women from the Nurses’ Health Study(NHS), ages 46–72 in 1992, and 84,304 women from the Nurses’ Health Study II(NHS II), ages 29–48 in 1993.
Methods
Physical function was measured by the Medical Outcomes Short Form-36 physical function scale, administered every four years. We compared change in physical function for women with versus without an incident VTE in each 4-year follow-up period using multivariable linear regression.
Results
We observed a decline in physical function over four years comparing women with versus without incident VTE in both older (NHS) and younger (NHS II) women (multivariable-adjusted mean difference NHS:−6.5 points [95% CI:−7.4,−5.6] per 4-years, NHS II:−3.8 [95% CI:−5.6,−2.0]). This difference appeared greater among women specifically reporting a pulmonary embolism (NHS:−7.4 [95% CI:−8.7,−6.1], NHS II:−4.8 [95% CI:−6.8,−2.8]), and was equivalent to 6.2 years of aging. While longer-term slopes of physical function decline following a VTE were not different from the slopes of decline in women without a VTE, absolute level of physical function of women with VTE was worse at the end of follow-up compared to women without VTE.
Conclusions
In this prospective cohort, incident VTE was strongly associated with an acute decline in physical function. These results suggest it may be clinically important to consider approaches to ameliorating functional deficits shortly after VTE diagnosis.
Keywords: Cohort Studies, Epidemiology, Physical and Rehabilitation Medicine, Pulmonary Embolism, Venous Thromboembolism
INTRODUCTION
Venous thromboembolism (VTE) includes both deep vein thrombosis (DVT) and pulmonary embolism (PE). Approximately 2 million Americans are diagnosed with DVT and 500,000–600,000 are diagnosed with PE every year.[1] While much research has examined quality of life measures after other cardiovascular outcomes, relatively little research has investigated quality of life after VTE especially over the longer term. Physical function, which is the ability to perform basic and instrumental activities of daily living, is a vital component of healthy aging and quality of life. Poor physical function is related to hospitalization [2], long-term nursing home care [3, 4], and increased mortality [4, 5] among older adults. Understanding how VTE affects physical function may improve our ability to intervene to improve quality of life after a VTE event.
To examine the relationship between VTE and subsequent physical function, we analyzed data from the Nurses’ Health Study and Nurses’ Health Study II, two large cohorts of female health professionals, with repeated measures of physical function and self-reported VTE over 8–20 years of follow-up.
METHODS
Study population
The Nurses’ Health Study (NHS) began in 1976, when 121,700 female nurses, aged 30–55 years, completed a mailed questionnaire about their health and lifestyle. The Nurses’ Health Study II (NHS II) began in 1989 when 116,430 female nurses, aged 25–42 (i.e. younger than those enrolled in NHS), completed and returned a similar questionnaire. Both cohorts use identical methods for data collection and follow-up, including biennial mailed questionnaires to update health and lifestyle information. The physical function domain of the Medical Outcomes Study Short Form-36 (SF-36) was included on the questionnaires beginning in 1992 in the NHS and 1993 in NHS II. To date, the follow-up rate in both cohorts is approximately 90%. All participants provided informed consent and study protocols were approved by the institutional review boards at Brigham and Women’s Hospital and the Harvard T.H. Chan School of Public Health.
From the population of all NHS and NHS II participants, women were excluded from this analysis if they died prior to the first measure of physical function, did not complete the first physical function assessment in 1992/1993, or had a history of VTE prior to the first physical function assessment. Thus, we included 80,836 women from NHS and 84,304 women from NHS II in this analysis.
Assessment of VTE
Assessment of VTE in the NHS and NHS II cohorts has been previously described.[6–8] Initial identification of VTE events is based on participant self-reports on the biennial questionnaires. Since cohort inception, NHS biennial questionnaires have included a specific question asking participants whether they had a physician-diagnosed PE within the preceding two years since the last questionnaire. NHS participants who report a physician-diagnosed PE are sent a follow-up letter requesting medical records from the facility where they were diagnosed. A detailed review of records is then performed. Cases reviewed validate approximately 95% of nurses’ self-reported VTE.[9] Incident cases for which the medical record includes imaging that was diagnostic for PE, are considered confirmed. Imaging is considered diagnostic if a radiologist noted a ventilation/perfusion lung scan that indicated a high probability of PE, a filling defect on a contrast-enhanced computed tomographic scan of the pulmonary arteries, or a filling defect on a catheter-based pulmonary angiogram. In NHS, physician-diagnosed DVT is identified when participants write in this diagnosis on a blank line reserved for “other conditions”. Although DVT was not confirmed by medical records review, in a validation study of 101 self-reported cases of DVT, it was found that 94% of cases were confirmed in medical records, 2% were probably, and only 4% were not confirmed. [9]
On NHS II questionnaires, a single question asks participants whether they had a physician-diagnosed VTE (“DVT/PE”) in the past two years. NHS II participants reporting VTE then received a follow-up letter requesting re-confirmation and identification of their specific diagnosis; those reporting PE were also asked to provide access to medical records for review. Otherwise, confirmation methods for PE in NHS II are identical to those in NHS. Given the severity of diagnosis, followed by extensive treatment, therapeutic monitoring, and clinical follow-up required of patients with VTE, cases are likely reported with high accuracy in this population of nurses with high health literacy and we are likely not missing cases that participants neglected to report. Since the inception of both cohorts, PE events have been sub-coded according to whether they were provoked by cancer, surgery or trauma. For our analysis, we included participants diagnosed with concurrent PE and DVT in the PE analysis. All cases included in this analysis were the participant’s first diagnosis of VTE.
Physical function
Information on physical function was collected from the SF-36 questionnaire, a widely used and validated instrument.[10] The physical function domain of the SF-36 is a consistent and reliable predictor of morbidity and mortality in a variety of populations.[5, 11, 12] The physical function component of the SF-36 was administered to participants starting in 1992 and every four years thereafter in NHS and in 1993, 1997, and 2001 in NHS II. It is comprised of 10 questions regarding physical limitations in performing the following activities: bathing/dressing yourself, walking one block, walking several blocks, walking more than one mile, bending/kneeling, climbing stairs, lifting groceries, moderate activities, and vigorous activities. Each question has the same three response choices; each answer of “Yes, limited a lot” is assigned one point, an answer of “Yes, limited a little” is assigned two points, and an answer of “No, not limited at all” is assigned three points. A raw score is derived from the set of 10 questions and ranges from a minimum of 10 points to a maximum of 30 points. The raw score is then transformed to a 100-point scale, with a score of 100 considered highest physical function.[10]
Statistical analysis
We investigated the association between an incident VTE event and the change in physical function score over a four-year period using multivariable linear regression. There were a total of five, four-year time periods between physical function classifications in NHS and two, four-year time periods in NHS II. We applied an unstructured correlation matrix in the multiple regression procedure to account for within-person repeated measures of health and lifestyle variables as covariates, as well as physical function. Models were adjusted for all of the potential clinical confounders that we previously found on univariate analysis to be associated with VTE in our cohorts[6]: age (continuous), aspirin use, body mass index (<25 kg/m2, 25–<30 kg/m2, ≥30 kg/m2), cancer (excluding non-melanoma skin cancer), coronary heart disease (history of myocardial infarction or angina), diabetes, hypertension, non-aspirin nonsteroidal anti-inflammatory drug use, physical activity (measured as average metabolic equivalents/week), post-menopausal status (including the use of oral contraceptives or post-menopausal hormone replacement), rheumatologic disease (rheumatoid arthritis or systemic lupus erythematosus) and smoking status (never, past, current). Data on covariates was obtained from the 1992 (NHS) or 1993 (NHS II) questionnaire and updated every four years. Very few data on covariates were missing. However, when data were missing, we carried forward responses from the previous questionnaire cycle. If data remained missing after two cycles, missing indicator variables were created and included in models as covariates. For these analyses of four-year physical function, we removed women who had previously been diagnosed with VTE from the at-risk group to enable comparisons between women with VTE during each four-year period to women with no history of VTE up until that time-period.
When evaluating the association between VTE and trajectories of physical function decline, we focused our analysis on NHS, where we had 20 years of follow-up and six repeated measures of physical function (i.e. five, four-year time periods). In contrast, in NHS II, only eight years of follow-up and three measures of physical function (i.e. two four-year time periods) were available, which limited our ability to measure longer-term trends. We used linear mixed effects models to estimate mean differences in rate of physical function decline over the entire follow-up period. Models were adjusted for the potential confounders mentioned above. Models included random varying intercepts and slopes to allow for individual physical function trajectories over time. In these models, women contributed follow-up time for “no VTE” at each follow-up interval, and subsequently began contributing time to the “VTE” category if they had an incident event.
We performed pre-defined subgroup analyses of women with DVT-only and women with PE (with or without DVT). We recognized that a change in physical function associated with VTE could be secondary to confounding by factors that are risk factors for VTE. To assess potential sources of bias or confounding, we therefore performed several sensitivity analyses. First, we analyzed the subgroup of women who did not have PE provoked by cancer, surgery, or trauma, to remove potential effects of these provoking factors on physical function. We also conducted an analysis in which we excluded women from the analysis during the follow-up period when they reported a diagnosis that could confound the relationship between VTE and physical function: incident cancer, coronary heart disease, congestive heart failure, stroke, transient ischemic attack, hip replacement, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, ulcerative colitis/Crohn’s, rheumatologic disease, and gout. We performed this analysis to ensure that we addressed potential confounding to the best of our capabilities and to that a change in the slope of physical function after VTE was unlikely to be related to another incident diagnosis. Lastly, since physical function is highly correlated with physical activity, we compared multivariable models both including and excluding physical activity. All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC). All statistical tests were two sided and P < 0.05 was considered statistically significant.
RESULTS
Over 20 years of follow-up from 1992 to 2012, 2,304 women from NHS developed a VTE; this includes 996 women who developed a DVT-only and 1,308 women who developed a PE (with or without DVT). Over eight years of follow-up in the NHS II from 1993 to 2001, 620 women developed a VTE; this includes 323 women who developed DVT-only and 297 women who developed a PE (with or without DVT). The overall cumulative incidence rate of VTE was 130/100,000 person-years in NHS and 100/100,000 person-years in NHS II. Table 1 presents participant characteristics at the first measure of physical function, separately for women who developed a VTE during follow-up and women who did not develop a VTE. In both cohorts, women who developed VTE had higher BMI and slightly higher prevalence of hypertension, coronary heart disease, and rheumatological disease at baseline (1992/1993) compared to women who did not develop a VTE. Women who subsequently developed a VTE also reported more aspirin at baseline compared to women who did not develop a VTE. Finally, women who subsequently developed a VTE had worse physical function scores in 1992/1993 (NHS: physical function score in 1992 for VTE=81.5 vs. no VTE=85.2, NHS II: physical function score in 1993 for VTE=83.5 vs. no VTE=91.2).
Table 1.
Age-adjusted baseline characteristics of women who developed venous thromboembolism (VTE) and women who did not develop VTE from the Nurses’ Health Studies, 2002/2003*
| Nurses’ Health Study†
|
Nurses’ Health Study II ‡
|
|||
|---|---|---|---|---|
| No VTE§ (n=78,532) | VTE§ (n=2,304) | No VTE¶ (n=83,684) | VTE¶ (n=620) | |
| Mean Age, years (SD) | 58.7 (7.2) | 59.7 (6.8) | 38.5 (4.6) | 39.7 (4.5) |
| Mean BMI, kg/m2 | 26.0 (5.0) | 27.7 (5.5) | 25.2 (5.6) | 28.3 (7.1) |
| Race,% | ||||
| White | 98 | 98 | 97 | 97 |
| Black | 1 | 1 | 2 | 2 |
| Asian | 1 | 1 | 1 | 1 |
| Mean Physical Activity METs/week**, (SD) | 19.0 (23.2) | 17.6 (25.9) | 21.1 (28.0) | 21.5 (26.7) |
| Smoking,% | ||||
| Never | 44 | 42 | 66 | 64 |
| Past | 41 | 46 | 23 | 23 |
| Current | 15 | 12 | 11 | 13 |
| Hypertension,% | 34 | 37 | 7 | 16 |
| Coronary heart disease††, % | 7 | 9 | 1 | 3 |
| Rheumatological disease§§, % | 8 | 11 | 2 | 5 |
| Type 2 Diabetes, % | 5 | 6 | 1 | 3 |
| Cancer, % | 10 | 10 | 2 | 3 |
| Aspirin use, % | 13 | 15 | 9 | 12 |
| NSAID use, % | 41 | 48 | 21 | 29 |
| Mean Baseline Physical Function Score (SD) | 85.2 (18.6) | 81.5 (20.5) | 91.2 (14.6) | 83.5 (21.0) |
| Mean time to event, years (SD) | 11.2 (5.5) | 3.9 (2.5) | ||
Abbreviations: BMI, body mass index; kg, kilograms; m, meters; METs, metabolic equivalent of task; NSAID, nonsteroidal anti-inflammatory drug; SD, standard deviation; VTE, venous thromboembolism
All variables expect age were age adjusted.
Characteristics measured in 1992
Characteristics measured in 1993
No VTE was defined as no report of VTE through the end of follow-up in 2012. VTE was defined by participants’ report of physician-diagnosed VTE from 1992 to 2012.
No VTE was defined as no report of VTE through the end of follow-up in 2001. VTE was defined by participants’ report of physician-diagnosed VTE from 1993 to 2001.
Physical activity is assessed across 6 different types of exercise and activity is summarized as weekly expenditure of metabolic equivalents.
Coronary heart disease includes any history of myocardial infarction or angina.
Rheumatological disease includes any history of systemic lupus erythematosis or rheumatoid arthritis.
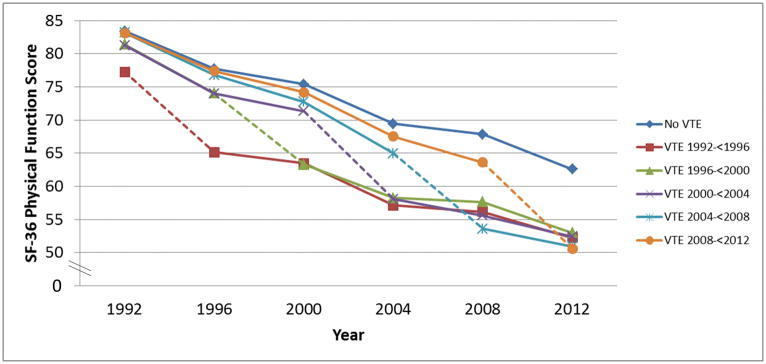
When we investigated change in physical function over four years comparing women with an incident VTE during those four years to women without an incident VTE, we first looked at each time-period separately, as diagnosis and treatment of VTE has changed substantially over time. For women who had an incident VTE in the first time-period (1992–<1996), we observed a significantly worse decline in function (−4.9 points [95% confidence interval (CI): −6.8, −3.1]) compared to women without an incident VTE in that time-period. Similar associations were found for women with incident VTE in all subsequent time-periods compared to women without VTE (Incident VTE in: 1996–<2000 −5.3 points [95% CI : −7.3, −3.4]; 2000–<2004 −6.7 [95 % CI: −8.4, −5.0]; 2004–<2008 −7.0 points [95% CI: −8.7, −5.3], 2008–<2012 −5.8 points 95% CI: [−5.8 95% CI: −7.4, −4.1]) (Figure 1). The overall pooled estimate for change in physical function over four years comparing women with an incident VTE to women without an incident VTE during those four years was (NHS: −6.5 points [95% CI: −7.4, −5.6]) (Table 2). For context, among women in NHS, one year of age was associated with a 1.2 point decline in physical function score. Thus, the overall mean difference of 6.5 points we observed in women who had a VTE, compared to those who did not have a VTE, was equivalent to the physical function decline associated with 5.4 years of aging. When we looked at the specific activities for which women with VTE reported impairment, the most common limitations were with vigorous activities, climbing several flights of stairs, walking more than one mile, and bending/kneeling.
Figure 1.
Long-term decline in physical function score* associated with developing venous thromboembolism (VTE), separated according to the follow-up period in which the VTE occurred.
* Least square mean physical function scores are presented, after adjusting age (continuous), aspirin use, body mass index (<25 kg/m2, 25 – <30 kg/m2, ≥30 kg/m2), cancer (excluding non-melanoma skin cancer), coronary heart disease (history of myocardial infarction or angina), diabetes, hypertension, non-aspirin nonsteroidal anti-inflammatory drug use, physical activity (measured as average metabolic equivalent of tasks/week), post-menopausal status (including the use of oral contraceptives or post-menopausal hormone replacement), rheumatologic disease (rheumatoid arthritis or systemic lupus erythematosus) and smoking status (never, past, current). Covariates were updated at the beginning of each 4-year time period and we stopped updating covariates after VTE diagnosis. Participants were not censored after VTE. Physical function determined by score on Medical Outcomes Short Form-36 Physical Function Scale; scores can range from 0–100, with higher scores indicating better function. Dotted line indicates time period in which VTE occurred.
Table 2.
Short-term change in physical function score* associated with developing venous thromboembolism (VTE) in the 4-year period
| Type of Event | Nurses’ Health Study | Nurses’ Health Study II | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| No VTE | VTE in 4-year period† | No VTE | VTE in 4-year period† | |||
| Mean difference | 95% CI | Mean difference | 95% CI | |||
| Any Venous Thromboembolism | Cases: n=2304 | Cases: n=620 | ||||
| Age adjusted mean difference in physical function over 4 years | 0.0 (Reference) | −7.3 | −8.2, −6.3 | 0.0 (Reference) | −4.1 | −5.9, −2.3 |
| Multivariable adjusted‡ mean difference in physical function over 4 years | 0.0 (Reference) | −6.5 | −7.4, −5.6 | 0.0 (Reference) | −3.8 | −5.6, −2.0 |
|
| ||||||
| Deep Vein Thrombosis | Cases: n=996 | Cases: n=323 | ||||
| Age adjusted mean difference in physical function over 4 years | 0.0 (Reference) | −5.9 | −7.2, −4.6 | 0.0 (Reference) | −3.3 | −5.5, −1.0 |
| Multivariable adjusted‡ mean difference in physical function over 4 years | 0.0 (Reference) | −5.6 | −6.9, −4.3 | 0.0 (Reference) | −3.0 | −5.2, −0.7 |
|
| ||||||
| Pulmonary Embolism | Cases: n=1308 | Cases: n=297 | ||||
| Age adjusted mean difference in physical function over 4 years | 0.0 (Reference) | −8.5 | −9.8, −7.2 | 0.0 (Reference) | −5.2 | −7.2, −3.2 |
| Multivariable adjusted ‡ mean difference in physical function over 4 years | 0.0 (Reference) | −7.4 | −8.7, −6.1 | 0.0 (Reference) | −4.8 | −6.8, −2.8 |
Abbreviations: BMI, body mass index; kg, kilograms; m, meters; METs, metabolic equivalent of task; VTE, venous thromboembolism
Physical function decline determined by score on Medical Outcomes Short Form-36 Physical Function Scale at the start and finish of a 4-year follow-up period. Scores can range from 0–100, with higher scores indicating better function.
Women censored in time period after VTE
Adjusted for age (continuous), aspirin use, BMI (<25 kg/m2, 25–<30 kg/m2, ≥30 kg/m2), cancer (excluding non-melanoma skin cancer), coronary heart disease (history of myocardial infarction or angina), diabetes, hypertension, non-aspirin nonsteroidal anti-inflammatory drug use, physical activity (measured as average METs/week), post-menopausal status (including the use of oral contraceptives or post-menopausal hormone replacement), rheumatologic disease (rheumatoid arthritis or systemic lupus erythematosus) and smoking status (never, past, current). Covariates determined from the start of each 4-year time period.
When we examined the slope of physical function decline in four-year periods occurring after a four-year period in which a VTE event occurred, the slope of decline was not significantly different than that of women without a VTE (Figure 1). Specifically, the overall mean difference in the rate of decline in physical function score per four-year period was −0.2 points (95% CI: −0.5, 0.2) comparing women who had had a VTE to those without VTE (Supplementary Table 1). Nonetheless, due to the decline that occurred during the four-year time period when a VTE occurred, women with a VTE had a lower physical function score at the end of follow-up compared to women without a VTE (Figure 1).
When we divided women according to whether their VTE was DVT-only or PE (with or without DVT), we found a significant decrement in physical function over four-years for both groups compared to women with no VTE (Table 2). However, women reporting a PE appeared to have greater decline in their physical function (multivariable adjusted mean difference for NHS subjects with PE compared to women without a VTE = −7.4 [95% CI: −8.7, −6.1] compared to −5.6 [95%CI: −6.9, −4.3] for DVT). This mean difference of 7.4 points we observed in women who had a PE, compared to those who did not have a VTE, was equivalent to the physical function decline associated with 6.2 years of aging and the mean difference of 5.6 points we observed in women who had a DVT, compared to those who did not have a VTE, was equivalent to the physical function decline associated with 4.7 years of aging.
When we limited our analysis to the subgroup of NHS subjects who did not have a PE provoked by cancer, surgery, or trauma, the decrease in physical function score over four-years in this subgroup of women with PE (multivariable adjusted mean difference compared to women without a VTE = −5.3 [95% CI: −7.5, −3.0]) was similar to the decrease in the cohort overall. Similarly, results did not substantially change when we excluded women who reported incident cancer, coronary heart disease, congestive heart failure, stroke, transient ischemic attack, hip replacement, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, ulcerative colitis/Crohn’s disease, rheumatologic disease, and gout (multivariable adjusted mean difference compared to women without a VTE = −6.8 [95% CI: −7.9, −5.8]) Results also did not change when we performed our analysis without adjustment for physical activity (data not shown).
Findings were generally consistent in the younger women in NHS II, though, the mean differences between subjects with and without VTE were smaller in magnitude than those we found in the older (Table 2). In NHS II, the multivariable adjusted mean difference in physical function decline over four-years comparing women with versus without an incident VTE was −3.8 points (95% CI: −5.6, −2.0). When we divided women according to DVT-only or PE with or without DVT, we again found that women specifically reporting a PE appeared to have a greater decline in physical function (multivariable adjusted mean difference = −4.8 [95% CI: −6.8, −2.8] compared to −3.0 [95% CI: −5.2, −0.7] for DVT). Additionally, when we limited the analysis in NHS II to the subgroup of women who did not have PE provoked by cancer, surgery, or trauma, the decrease in physical function score over four-years in this subgroup of women with PE (multivariable adjusted mean difference= −4.1 [95% CI: −9.6, 1.5]) was similar to the decrease in the cohort overall, although here, the difference was not statistically significant, likely due to the small number of younger women who had a PE that was not provoked by cancer, surgery, or trauma.
DISCUSSION
In this large, prospective study of women, VTE was associated with an acute, marked decline in physical function. On average, after controlling for potential confounding factors, the four-year decline in physical function was 6.5 points greater in older women at the time of their VTE than in women without VTE, and 3.8 points greater in younger women at the time of their VTE than in women without VTE. The decrease appeared worse if the VTE event was a PE. In subsequent time periods after a venous thromboembolism event physical function returned to the expected rate of decline associated with aging, the steep decline occurring at the time of the VTE resulted in worse absolute physical function among women with VTE at the end of follow-up.
The decline in physical function we observed is clinically significant. Prior studies have indicated that a change of 4 to 5 points on the SF-36 physical function scale is a clinically important difference - indicating that the decrease in physical function we observed among older women with VTE is clinically important. [13] In addition, it has been demonstrated that a one point lower score on the physical function scale is associated with a 7–12% increased risk of being unable to work.[14] Moreover, the overall mean difference of 6.5 points we observed in women who had a VTE was equivalent to the physical function decline associated with 5.4 years of aging, which may have substantial impact on a woman’s health and independence.
To our knowledge, our study is the first large, longitudinal analysis of women to indicate a potential deleterious effect of VTE on physical function. Prior studies have had modest sample sizes (n=100 to 392) and only measured physical function at one time point.[15–23] Two studies have used the SF-36 to measure physical function and found that patients with a history of PE reported significantly worse physical function compared to general population norms.[17, 19] One larger study (n=27,145), an analysis of women who survived to age 80 in the Women’s Health Initiative, found that women who reported a prior VTE scored, on average, 12 points lower on the SF-36 physical function score than women who reported no cardiovascular disease.[24] However, unlike our study, this analysis relied on a single physical function measurement, so could not assess the temporal association of the physical function decline and VTE. Despite the different designs, the results of our study are generally compatible with this previous work, which supports the robustness of our findings.
In addition to investigating overall VTE, we were also able to divide women according to whether their VTE was a DVT-only or a PE. As anticipated, we found a greater decline in physical function associated with PE than DVT-only. Patients with a DVT may have post-thrombotic syndrome with associated leg pain and swelling following the event [25] and prior work has found that post-thrombotic syndrome is significantly related to decreased quality of among patients with DVT [22]. Patients with PE can experience these symptoms plus complications including difficulty breathing or decreased cardiopulmonary reserve.[20, 23] This may result in more severe physical function deficits. We also found that decreases in physical function associated with VTE were greater among the older women enrolled in NHS than the younger women in NHS II. This might be expected given the strong effect of aging on physical function and the more limited functional reserve among older individuals.
The fact that we saw a greater decrease in physical function associated with the more severe form of VTE (PE) than the less severe form (DVT) supports the hypothesis that the decrease in physical function was related to the VTE event. However, it is possible that decreased physical function after VTE is, at least in part, due to factors other than VTE, such as comorbid illness or hospitalization. However, we found little attenuation in the association of VTE with decreased physical function when we limited our analysis to the subgroup of women who did not have PE provoked by cancer, surgery, or trauma. While these are among the strongest provoking factors for VTE, we recognize that ours was not an exhaustive list of all potential confounders. However, VTE cases have been coded using this definition of provoking factors since cohort inception (1976 in NHS and 1989 in NHS II), and updating our definitions of provoking factors would have introduced inconsistency into our case definition. We therefore felt it was important to keep the coding and definitions of unprovoked cases consistent throughout follow-up. In order to further address the concern that the observed association may be due to confounding, we conducted a number of sensitivity analyses excluding women with a wide range of chronic diseases and still found a similar association.
Our study had numerous strengths including multiple measures of physical function over 10 to 20 years, large sample size, and the ability to control for multiple potential confounders. Potential limitations also need to be considered. Our study population was homogenous, comprised of predominately white, female nurses, so it is possible our findings may not be generalizable to men or other racial or ethnic groups. However, the homogeneity and the uniform health access of this population reduces confounding by many factors and provides strong internal validity. There is also potential for misclassification of VTE. However, we would expect bias towards the null if women who experienced a VTE were classified as not having a VTE, or vice versa. Thus, our findings are unlikely to overestimate the true association between VTE and physical function. Additionally, the overall cumulative incidence rates of VTE observed in these cohorts are similar to rates found in other population-based cohort studies.[26] We also did not have access to data on recurrent VTE and were thus unable to assess the effect of recurrence on physical function. Instead, we removed subjects with VTE from the at-risk group, which allowed us to analyze the association between first VTE event and physical function. There is also a potential for measurement error in physical function. However, the SF-36 is well-validated [10] and has been found to correlate well with in-person physical performances measurements such as grip strength, longer timed-up-and-go, chair rises, three-minute walk test, and the six-minute walk test. [27–29] Lastly, in this analysis, we focused on physical function. However, we acknowledge that VTE may be associated with other quality of life measures from the SF-36 as well, and we plan to investigate these in future analyses.
In summary, VTE is strongly associated with a decline in physical function and this decline is greatest in the years immediately following the VTE event, for both older and younger women. A prior clinical trial found that early initiation of an exercise training and behavioral weight loss program was both safe and effective in improving physical activity and fitness following VTE, which could help to ameliorate functional deficits after diagnosis. [30] Given the importance of physical function to healthy aging and quality of life, the results of this analysis highlight the importance of both preventing VTE and intervening early after VTE diagnosis to improve long-term health outcomes.
Supplementary Material
Essentials.
The association of venous thromboembolism (VTE) with subsequent physical function remains unclear.
We prospectively evaluated this relationship among women from the Nurses’ Health Studies.
We found a decline in physical function over four years in women with incident VTE.
This decline was somewhat greater among women specifically reporting a pulmonary embolism.
Acknowledgments
We thank the participants and staff of the Nurses’ Health Study and the Nurses’ Health Study II for their valuable contributions. This work was supported by the National Institutes of Health (Grants UM1 CA186107, UM1 CA176726, T32 HL098048, and R01 HL116854).
Footnotes
ADDENDUM
K. A. Hagan, F. Grodstein, and C. Kabrhel contributed to the analysis concept and design. K. A. Hagan performed statistical analyses and drafted the manuscript. All authors (K. A. Hagan, L. B. Harrington, J. Kim, E. B. Rimm, F. Grodstein, C. Kabrhel) contributed to the interpretation of data, provided substantial scientific contributions to the revision of the manuscript, and approved the final version of the manuscript.
Disclosure of Conflict of Interests
C. Kabrhel reports grants from Janssen, Diagnostica Stago and Siemens Healthcare Diagnostics, outside the submitted work. The other authors state that they have no conflict of interest.
References
- 1.Anderson FA, Jr, Zayaruzny M, Heit JA, Fidan D, Cohen AT. Estimated annual numbers of US acute-care hospital patients at risk for venous thromboembolism. Am J Hematol. 2007;82:777–82. doi: 10.1002/ajh.20983. [DOI] [PubMed] [Google Scholar]
- 2.Motl RW, McAuley E. Physical activity, disability, and quality of life in older adults. Phys Med Rehabil Clin N Am. 2010;21:299–308. doi: 10.1016/j.pmr.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 3.Beswick AD, Rees K, Dieppe P, Ayis S, Gooberman-Hill R, Horwood J, Ebrahim S. Complex interventions to improve physical function and maintain independent living in elderly people: a systematic review and meta-analysis. Lancet. 2008;371:725–35. doi: 10.1016/S0140-6736(08)60342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 5.Cesari M, Onder G, Zamboni V, Manini T, Shorr RI, Russo A, Bernabei R, Pahor M, Landi F. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. doi: 10.1186/1471-2318-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varraso R, Kabrhel C, Goldhaber SZ, Rimm EB, Camargo CA., Jr Prospective study of diet and venous thromboembolism in US women and men. Am J Epidemiol. 2012;175:114–26. doi: 10.1093/aje/kwr377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA., Jr Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ. 2011;343:d3867. doi: 10.1136/bmj.d3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabrhel C, Varraso R, Goldhaber SZ, Rimm EB, Camargo CA. Prospective study of BMI and the risk of pulmonary embolism in women. Obesity (Silver Spring) 2009;17:2040–6. doi: 10.1038/oby.2009.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pun VC, Hart JE, Kabrhel C, Camargo CA, Jr, Baccarelli AA, Laden F. Prospective Study of Ambient Particulate Matter Exposure and Risk of Pulmonary Embolism in the Nurses’ Health Study Cohort. Environ Health Perspect. 2015;123:1265–70. doi: 10.1289/ehp.1408927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305:160–4. doi: 10.1136/bmj.305.6846.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Haley SM, McHorney CA, Ware JE., Jr Evaluation of the MOS SF-36 physical functioning scale (PF-10): I. Unidimensionality and reproducibility of the Rasch item scale. J Clin Epidemiol. 1994;47:671–84. doi: 10.1016/0895-4356(94)90215-1. [DOI] [PubMed] [Google Scholar]
- 13.Wyrwich KW, Tierney WM, Babu AN, Kroenke, Wolinsky FD. A Comparison of Clinically Important Differences in Health-Related Quality of Life for Patients with Chronic Lung Disease, Asthma, or Heart Disease. Health Serv Res. 2005;40:577–91. doi: 10.1111/j.1475-6773.2005.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjorner JB, Lyng Wolden M, Gundgaard J, Miller KA. Benchmarks for interpretation of score differences on the SF-36 health survey for patients with diabetes. Value Health. 2013;16:993–1000. doi: 10.1016/j.jval.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Tavoly M, Utne KK, Jelsness-Jorgensen LP, Wik HS, Klok FA, Sandset PM, Ghanima W. Health-related quality of life after pulmonary embolism: a cross-sectional study. BMJ Open. 2016;6:e013086. doi: 10.1136/bmjopen-2016-013086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hogg K, Kimpton M, Carrier M, Coyle D, Forgie M, Wells P. Estimating quality of life in acute venous thrombosis. JAMA Intern Med. 2013;173:1067–72. doi: 10.1001/jamainternmed.2013.563. [DOI] [PubMed] [Google Scholar]
- 17.van Es J, den Exter PL, Kaptein AA, Andela CD, Erkens PM, Klok FA, Douma RA, Mos IC, Cohn DM, Kamphuisen PW, Huisman MV, Middeldorp S. Quality of life after pulmonary embolism as assessed with SF-36 and PEmb-QoL. Thromb Res. 2013;132:500–5. doi: 10.1016/j.thromres.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Chow V, Ng AC, Seccombe L, Chung T, Thomas L, Celermajer DS, Peters M, Kritharides L. Impaired 6-min walk test, heart rate recovery and cardiac function post pulmonary embolism in long-term survivors. Respir Med. 2014;108:1556–65. doi: 10.1016/j.rmed.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Kaptein AA, Huisman MV. Quality of life in long-term survivors of acute pulmonary embolism. Chest. 2010;138:1432–40. doi: 10.1378/chest.09-2482. [DOI] [PubMed] [Google Scholar]
- 20.Klok FA, van der Hulle T, den Exter PL, Lankeit M, Huisman MV, Konstantinides S. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014;28:221–6. doi: 10.1016/j.blre.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Kahn SR, Hirsch AM, Akaberi A, Hernandez P, Anderson DR, Wells PS, Rodger MA, Solymoss S, Kovacs MJ, Rudski L, Shimony A, Dennis C, Rush C, Geerts WH, Aaron SD, Granton JT. Functional and Exercise Limitations After a First Episode of Pulmonary Embolism: Results of the ELOPE Prospective Cohort Study. Chest. 2017;151:1058–68. doi: 10.1016/j.chest.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SR, Shbaklo H, Lamping DL, Holcroft CA, Shrier I, Miron MJ, Roussin A, Desmaris S, Joyal F, Kassis J, Solymoss S, Desjardins L, Johris M, Ginsbery JS. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thomb Haemost. 2008;6:1005–12. doi: 10.1111/j.1538-7836.2008.03002.x. [DOI] [PubMed] [Google Scholar]
- 23.Kahn SR, Akaberi A, Granton JT, Anderson DR, Wells PS, Rodger MA, Solymoss S, Kovas MJ, Rudski L, Shimony A, Dennie C, Rush C, Hernandez P, Aaron SD, Hirsch AM. Quality of Life, Dyspnea, and Functional Exercise Capacity Following a First Episode of Pulmonary Embolism: Results of the ELOPE Cohort Study. Am J Med. 2017;130:990.e9–990.e21. doi: 10.1016/j.amjmed.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 24.Stefanick ML, Brunner RL, Leng X, Limacher MA, Bird CE, Garcia DO, Hogan PE, LaMonte MJ, Mackey RH, Johnson KC, LaCroix A, Robinson JG, Seguin RA, Tindle HA, Wassertheil-Smoller S. The Relationship of Cardiovascular Disease to Physical Functioning in Women Surviving to Age 80 and Above in the Women’s Health Initiative. J Gerontol A Biol Sci Med Sci. 2016;71(Suppl 1):S42–53. doi: 10.1093/gerona/glv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kahn SR. The post thrombotic syndrome. Thromb Res. 2011;127(Suppl 3):S89–92. doi: 10.1016/S0049-3848(11)70024-X. [DOI] [PubMed] [Google Scholar]
- 26.Martinez C, Cohen AT, Bamber L, Rietbrock S. Epidemiology of first and recurrent venous thromboembolism: a population-based cohort study in patients without active cancer. Thromb Haemost. 2014;112:255–63. doi: 10.1160/TH13-09-0793. [DOI] [PubMed] [Google Scholar]
- 27.Syddall HE, Martin HJ, Harwood RH, Cooper C, Aihie Sayer A. The SF-36: a simple, effective measure of mobility-disability for epidemiological studies. J Nutr Health Aging. 2009;13:57–62. doi: 10.1007/s12603-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alison JA, Kenny P, King MT, McKinley S, Aitken LM, Leslie GD, Elliott D. Repeatability of the Six-Minute Walk Test and Relation to Physical Function in Survivors of Critical Illness. Phys Ther. 2012;92:1556–63. doi: 10.2522/ptj.20110410. [DOI] [PubMed] [Google Scholar]
- 29.Moriello C, Mayo NE, Feldman L, Carli F. Validating the Six-Minute Walk Test as a Measure of Recovery After Elective Colon Resection Surgery. Arch Phys Med Rehabil. 2008;89:1083–9. doi: 10.1016/j.apmr.2007.11.031. [DOI] [PubMed] [Google Scholar]
- 30.Lakoski SG, Savage PD, Berkman AM, Penalosa L, Crocker A, Ades PA, Kahn SR, Cushman M. The safety and efficacy of early-initation exercise training after acute venous thromboembolism: a randomized clinical trial. J Thromb Haemost. 2015;13:1238–44. doi: 10.1111/jth.12989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.