Abstract
Photobiomodulation (PBM), also known as low-level laser (light) therapy, was discovered over 50 years ago, but only recently has it been making progress towards wide acceptance. PBM originally used red and near-infrared (NIR) lasers, but now other wavelengths and non-coherent light emitting diodes (LEDs) are being explored. The almost complete lack of side-effects makes the conduction of controlled clinical trials relatively easy. Laboratory research has mainly concentrated on mammalian cells (normal or cancer) in culture, and small rodents (mice and rats) as models of different diseases. A sizeable body of work was carried out in the 1970s and 1980s in Russia looking at various bacterial and fungal cells. The present review will cover some of these studies and a recent number of papers that have applied PBM to so-called “model organisms”. These models include flies (Drosophila), worms (C. elegans), fish (zebrafish), and caterpillars (Galleria). Much knowledge about the genomics and proteomics, and many reagents for these organisms already exist. They are inexpensive to work with and have lower regulatory barriers compared to vertebrate animals. Other researchers have studied different models (snails, sea urchins, Paramecium, toads, frogs and chickens). Plants may respond to NIR light differently from visible light (photosynthesis and photomorphogenesis) but PBM in plants has not been much studied. Veterinarians routinely use PBM to treat non-mammalian patients. The conclusion is that red or NIR light does indeed have significant biological effects conserved over many different kingdoms, and perhaps it is true that “all life-forms respond to light”.
Keywords: Non-mammalian lifeforms, model organisms, photobiomodulation, low-level laser therapy, insects, worms and snails, aquatic creatures, birds
Graphical Abstract
Photobiomodulation (red or near infrared light) has been shown to have beneficial effects on many different life-forms. Although many pre-clinical studies have been carried out in mice or rats, and clinical studies in humans, it turns out that all life-forms from bacteria and fungi, to paramecia, worms, insects, fish, amphibians, reptiles and birds respond. Even plants respond to NIR light differently to photosynthesis with visible light.
INTRODUCTION
Photobiomodulation (PBM) previously known as “low-level laser therapy” (LLLT) was discovered in 1967 by Endre Mester in Hungary. However, light therapy for a variety of diseases had been practiced for thousands of years first using sunlight, and then by electric lamps since their discovery in the 19th C by Davy (arc lamp) and Edison (incandescent bulb). Niels Finsen was awarded the Nobel Prize for physiology or medicine in 1904 for his work on treating cutaneous tuberculosis with blue light and smallpox with red light. In the 21st C it was realized that light-emitting diodes was equally as effective as lasers in most applications of PBM. The mechanisms of action of PBM have been intensively investigated starting with the work of Tina Karu in Russia (1). Karu was the first to attribute the effects of red and near-infrared light to photon absorption by the electron transport chain in mitochondria, and by unit IV cytochrome c oxidase (CCO) in particular (2). Since CCO has absorption peaks in the red (600–700nm) and near-infrared (NIR, 760–900 nm) regions of the spectrum (depending on its precise oxidation state), red and NIR light have been the most often employed wavelengths for PBM (3, 4). In recent years, it has become apparent that there are other chromophores operating in different regions of the spectrum (5). The most important alternative chromophore appears to be transient receptor potential (TRP) ion channels that can be activated by light or heat (6). An example is TRPV1 that can be activated by blue or green light (7, 8) and by longer wavelength NIR light (980 nm) (9) that targets water in the form of nanostructured exclusion zone water clusters (10). Regardless of whether CCO is activated by red/NIR light, or TRP ion channels are activated by blue/green/980 nm light, the cellular results have some aspects in common, but also display some differences. Both pathways give rise to increased ATP and a brief burst of reactive oxygen species (5). However, the CCO pathway is more likely to lead to release of nitric oxide and the TRP pathway is more likely to give rise to changes in cytosolic and mitochondrial calcium levels.
The secondary effects of PBM can be short term in nature, such as increased blood flow, greater oxygen consumption, improved tissue ATP production etc. They can also be more long term in nature such as increased antioxidant defenses, activation of transcription factors and alteration of the expression pattern of cytokines and growth factors.
One pervasive observation in the field of PBM, is called the biphasic dose response curve otherwise known as the Arndt-Schulz law (11, 12). This states that as the dose of light (J/cm2) is gradually increased, the beneficial response reaches a maximum at some point. When the dose is further increased beyond that maximum, the response declines until the baseline is reached again (13). If the dose of light is increased even more than that, a negative response starts to occur (inhibition) that might end up in cellular damage and even cell death.
Since many (if not most) of the mechanisms proposed to explain the cellular effects of PBMT are common to a lot of different life forms on earth, it has recently become possible to use a range of non-mammalian hosts, that have become popular in biomedical research laboratories throughout the world in recent years. These include worms, flies, fish, frogs and other “model organisms”.
MODEL ORGANISMS
The use of animal models has played a major role in the advancement of biomedicine and has been a cornerstone of medical progress for many centuries. Mammals such as mice, rats, rabbits and monkeys have traditionally been the preferred models for biomedical research due to their close evolutionary relationship with humans. However, the use of mammals in research has raised ethical and budgetary concerns, and has caused many laboratories and funding agencies to adopt the principle first elaborated by W. M. S. Russell and R. L. Burch as the “Three Rs”: Replacement, Reduction, and Refinement” (14). The Three Rs principle encourages the use of alternative model systems in addition to cell culture, which is already widely used in PBM research. These laboratory model systems include three important animal species, Danio rerio (zebrafish), Drosophila melanogaster (fruit fly) and Caenorhabditis elegans (nematode worm) that have now become widespread in biomedical research. These three model organisms have a rapid rate of reproduction with a relatively short generation time. They are easily grown in the laboratory (with no need of expensive animal housing) and do not require burdensome and time-consuming IACUC protocols. So far they have largely escaped the attention of animal rights protesters. Because they are living animals they are considerably more powerful than cell culture systems. In addition, thanks to the vast number of molecular and genetic tools available to study Drosophila, C. elegans and zebrafish, coupled with similarities in development and behavior, these organisms have come to serve as powerful alternatives to mammalian models to investigate genetics, diseases, and possible therapeutics.
Table 1 shows a comparison of the advantages and limitations of these three model organisms in comparison with the traditional laboratory mouse which is still the most widely used species in all of biomedical research.
Table 1.
Advantages of model organisms compared to the mouse.
| Mouse | Zebrafish | Drosophila | C. elegans | |
|---|---|---|---|---|
| Generation time | 10 weeks | 4–8 weeks | 12 days | 3 days |
| Numer of offspring | 6–8 pups/2 months | 200 eggs/week | 120 eggs/day | 140 eggs/day |
| Lifespan | 2–3 years | 2–3 years | 40–50 days | 14–21 days |
| Regulatory and ethics constraints | yes | no | no | no |
| Expense of housing | high | moderate | low | low |
| Number of neurons | > 70,000,000 | ~ 10,000,000 | > 100,000 | 302 |
| Fully annotated genome | yes | In progress/duplicate genes | yes | yes |
| Availability of mutants and transgenics | yes | growing | yes | yes |
| Homolgy with humans | > 90% | 84% | 77% | 65% |
| Easy behavioral assays | no | yes | yes | yes |
| High throughput screening | no | yes | yes | yes |
C. elegans
C. elegans is a transparent, free-living, non-parasitic nematode. C. elegans larvae at hatching are 0.25 millimeters long and adults are 1 millimeter long with a maximum diameter of ~80 μm. This nematode can survive on a lawn of Escherichia coli bacteria growing on agar medium in Petri dishes, which makes it very easy and cost-effective to grow in the laboratory. C. elegans has a short life cycle (3.5 days to obtain fertile adults at 20°C) and adult hermaphrodites are highly prolific reproducers (>140 eggs per adult per day) (15). Males are rare in laboratory populations (1 out of 1000 individuals) and the hermaphrodites reproduce by self-fertilization, creating genetic clones of themselves.
The anatomical simplicity of C. elegans and lack of a central nervous system may be considered to be drawback in a model used to study mammalian biomedical responses. However, this simplicity has led to one of the key advantages: every single neuron has been identified and the connections between them (the connectome) are known (16). This is not the case for any other organism. In addition, with the aid of genetically encoded fluorescent proteins, processes such as axon growth, embryogenesis and metabolic processes can easily be studied in the living worm. Although the adult hermaphrodite has only 959 somatic cells and its entire nervous system contains a mere 302 neurons, C. elegans is still a sophisticated multicellular animal with different organs and tissues including muscle, skin, intestine, reproductive system, and glands.
Drosophila melanogaster
The fruit fly (Drosophila melanogaster) has been instrumental in important advances biomedicine (17). Many of the genes found to be critical for fly development were shown to also be important for human development. Drosophila is relatively inexpensive and easy to culture and there are very few ethical and regulatory restrictions on its use in the laboratory. The short life cycle (approximately 12 days to obtain a fertile adult fruit fly) and the large brood size (120 eggs per adult per day) (18)
The adult fly has organs that are functionally equivalent to the mammalian heart, lung, kidney, gut, and reproductive tract. The Drosophila genome was fully sequenced in 2000 and Reiter et al reported that 77% of 929 genes known to be involved in human diseases displayed a match within the fruit fly genome (19)
Danio rerio
The zebrafish (Danio rerio) is a small freshwater teleost fish widely used in developmental research. Zebrafish embryos are transparent and develop outside the mother, making it relatively easy to study different stages of development using optical imaging techniques. Zebrafish genome contains numerous duplicate genes (20) and this feature often hinders the generation of knockout strains, as approaches that disrupt one gene copy likely will not disrupt the second copy. The zebrafish genome has been fully sequenced and its annotation is an ongoing project. A recent study indicated that 84% of the genes known to be associated with human disease have a counterpart in the zebrafish genome (21).
Galleria melonella
Larvae of the greater wax moth Galleria mellonella have recently been used as model hosts for studying pathogenic microorganisms as an alternative to vertebrate animals (22). The model has been used to study microbial virulence and pathogenesis by such species as Candida albicans, Cryptococcus neoformans and Enterococcus faecalis (22), and has been used to study new therapies such as antimicrobial photodynamic inactivation (23, 24).
MECHANISMS OF PHOTOBIOMODULATION, MITOCHONDRIA AND PLASTIDS
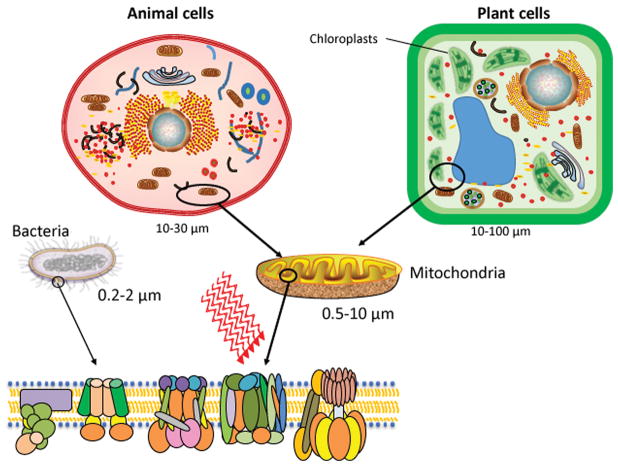
It is widely believed that mitochondria act as the most important photoreceptor in mammalian cells (5). Pioneering studies by Karu and Pasarella (25, 26) established that cytochrome c oxidase (unit IV in the mitochondrial respiratory chain) absorbed red and near-infrared light, leading to increased electron transport, raised mitochondrial membrane potential and more oxygen consumption. Since mitochondria are critically important to PBM we will take a little time to discuss them in further detail. Lynn Margulis in 1971 proposed a theory that endosymbiosis played a major role in the evolution of plant and animal cells (27, 28). The idea was that mitochondria originated when a primitive eukaryotic cell engulfed an aerobic bacterial cell and the combination became stable (this was possibly a unique event), and in similar fashion, a primitive plant cell engulfed a photosynthetic blue-green algal cell that became a stable chloroplast. This theory was originally controversial, but began to be accepted when Schwartz and Dayhoff (29) compared protein and nucleic acid sequence data and produced evolutionary trees. The electron transport chains in bacteria are located in the plasma membrane, and are more complicated than those found in mammalian mitochondria. For instance, Paracoccus denitrificans is a soil bacterium classified as a Gram-negative facultative anaerobe. When this bacterium grows aerobically, its electron transport chain possesses four complexes that correspond to those in the mitochondrial chain. However, when this bacterium grows anaerobically with nitrate as its electron acceptor, the chain is structured quite differently. Electron transport chains in other bacteria vary in their cytochromes and can be branched. Electrons often enter at several points and leave via different terminal oxidases (30). Figure 1 shows similarities between electron transport chains in animal and plant mitochondria and in bacteria.
Figure 1.
Mechanisms of PBM in animal cells, plant cells and bacteria.
In plants, there exist several different organelles including mitochondria, and a range of different plastids. The most common plastid is the chloroplast containing chlorophyll for photosynthesis, but other plastids called chromoplasts, gerontoplasts, amyloplasts, proteinoplasts and elaioplasts also exist (31). All plastids, including chloroplasts, develop from undifferentiated proplastids and contain the same basic DNA. In addition, mature plastids are able to change from one type to another, as happens during the ripening of fruit, where chloroplasts (green chlorophyll) change into chromoplasts (red/yellow carotenes). Raghavendra and Padmasree discussed (32) how, although chloroplasts and mitochondria had been traditionally considered to be autonomous organelles, they might not be as independent as they were once thought to be. The mutually beneficial interactions between mitochondria and chloroplasts, and also including the peroxisomes and the cytosol, appear to be in a delicate metabolic equilibrium. Mitochondria appear to be the key players because they function during both photorespiration and dark respiration.
The number of mitochondria inside different eukaryotic cells varies markedly from a single mitochondrion in some single-celled organisms (33), up to millions in a single oocyte of Xenopus laevis (34). Mitochondrial biogenesis is highly responsive to cellular demands for energy and also to environmental stimuli (35). The mechanistic target of rapamycin (mTOR) pathway regulates mitochondrial biogenesis to co-ordinate energy homeostasis with cell growth (36). PGC-1α (peroxisome proliferator activated receptor γ coactivator 1α) is a master transcriptional coactivator regulating oxidative metabolism in skeletal muscle (37, 38). PGC-1α interacts with Tfam (transcription factor A, mitochondrial) which is involved in the replication and transcription of mtDNA and the transcription of nuclear-encoded mitochondrial components.
The fission of mitochondria is correlated with mtDNA replication, and is controlled by Drp1 (a member of the dynamin family of large GTPases). Drp1 controls the final part of mitochondrial fission, pinching off the membrane stalk between two emerging daughter mitochondria. Several mitochondrial outer membrane proteins, including mitochondrial fission protein 1 (Fis1), mitochondrial fission factor (Mff) and mitochondrial dynamics of 51 kDa protein (MiD51), also known as mitochondrial elongation factor 1 (MEIF1), have been reported to promote mitochondrial fission by recruiting Drp1 (39, 40).
Mitochondrial fusion, on the other hand, is designed to avert and repair damage produced in mitochondria or mtDNA, by mixing the contents together so the undamaged structures predominate. The mitochondrial membrane fusion (MMF) family contains mitofusins, Mfn1 and Mfn2, GTPases localized to the outer membrane, and OPA1, a GTPase in the inner membrane that mediates inner-membrane fusion. Mutations in Mfn2 or OPA1 cause neurodegenerative disease.
There have been several recent reports that PBM has significant effects on mitochondrial dynamics including both fission and fusion (41–43).
PHOTOBIOMODULATION IN NON-MAMMALIAN HOSTS AND MODEL ORGANISMS
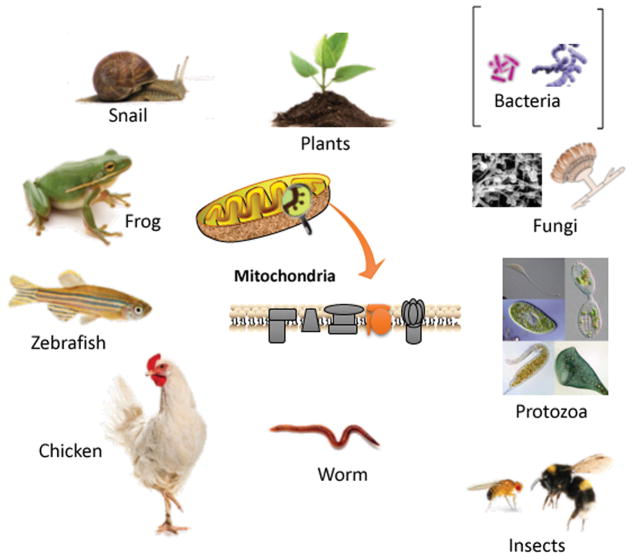
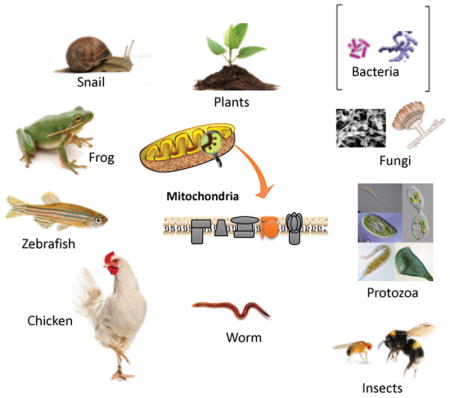
Table 2 presents a list of the non-mammalian organisms and model organisms that have been used in PBM studies. This is also graphically illustrated in Figure 2. The range of lifeforms that have been studied is impressive, but as yet, there are not many examples of each individual type of lifeform. Therefore, we have no idea as yet if the parameters described are the best for that particular indication or creature. The organisms range from the simplest prokaryotic unicellular bacteria (both Gram-positive and Gram-negative), to unicellular eukaryotic organisms such as fungi and paramecia. They go on to include, insects, aquatic organisms, worms and snails, amphibians and birds. Not only have laboratory studies used non-mammalian models to study scientific questions, but veterinarians have also employed PBM in their clinical practice, in the case of aquatic species (44) and birds (45) (of course vets employ PBM on the vast majority of mammalian patients they encounter daily) (46).
Table 2.
Non-mammalian animals and model organisms used for PBM.
| Lifeform | Latin name | Function | Device/wavelength/dose | Author (year) | Reference |
|---|---|---|---|---|---|
| Bacteria | Various species Escherichia coli |
Growth rate, mutagenesis | Laser, mainly 632.8 nm, 0.04–0.4 J/cm2 | Karu et al (1996) Nussbaum et al (2002) |
(47) (55) |
| Fungi, yeasts | Various species Saccharomyces, Candida |
Growth rate, metabolic activity | Laser, mainly 632.8 nm, 0.04–0.4 J/cm2 | Karu et al (1996) | (47) |
| Protozoa | Paramecium primaurelia | Increased swimming speed, ATP, fission rate, release of NO and stored calcium | Laser; 808 nm or 980 nm; 0.1; 0.5; 1; and 1.5 W; 6.4; 32; 64; and 96 J/cm2 | Amaroli et al (2015–2017) | (70–73, 118, 119) |
| Plants | Avena sativa cv Seger | Shorter mesocotyl; longer coleoptile; more vertical growth | LED/880 nm or 935 nm/0.6 mW/cm2 | Johnson et al (1996) | (79) |
| Bumblebee | Bombus terrestris audax | Reversed loss of ATP and mobility, caused by pesticide exposure | LED/670nm/12 hours daily for 10 days | Powner et al (2016) | (83) |
| Fruitfly | Drosophila melanogaster | Increased lifespan, ATP and mobility | LED/670nm/ | Begum et al (2015) | (120) |
| Sea urchin larvae | Paracentrotus lividus | Earlier development of fertilized eggs from irradiated sperm (192 J/cm2) | Laser/808 nm/3 W/64 or 192 J/cm2 | Amaroli et al (2017) | (84) |
| Zebrafish embryo | Danio rerio | Delayed hatching, increased mortality, teratogenicity, behavioral changes | Laser/685 nm, 30mW, 830 nm, 90mW/cm2 | Campagnaro (2016) | (85) |
| Aquatic species in veterinary practice | Fish, reptiles, dolphins | Ameliorations of skin lesions, stimulated wound healing, stomatitis, spondylitis | Various clinical laser systems | Stremme (2017) | (44) |
| Frog | Rana cameroni | Gastrocnemius muscle electrophysiology, sciatic nerve irradiated | Laser/904 nm, 220 nsec/up to 25.7 J/cm2 | Comelekoglu et al (2002) | (90) |
| Toad | Bufo viridis | Repair of muscle injury, enhancement of angiogenesis, and regeneration of nerves | Laser/632 nm/6 mW | Bibikova and Oron (1993–1995) | (86–89) |
| Green iguana | Iguana iguana | Full thickness wound healing was stimulated by 10 J/cm2 better than control silver sulfadiazine | Laser/660 nm/5 or 10 J/cm2 | Cusack et al (2018) | (91) |
| Earth worm | Dendrobaena veneta | Improved healing and regeneration after amputation | Laser/808 nm/1 W/64 J/cm2 | Amaroli et al (2017, 2018) | (95, 97) |
| Round worm (nematode) | Caenorhabditis elegans | Increased lifespan mediated by HSF1 and HSP70 expression | LED/1072 nm/600 Hz/5 mW/cm2 | Duggett (2013) | (98) |
| Flat worm | Dugesia tigrina | Improved healing and regeneration after amputation. Increased stem cell proliferation | LED/880 nm/1 mW/m2/0.01 J/cm2 | Wu et al (2011) | (92) |
| Snail | Ariophanta laevipes | Improved cognitive function and behavioral response as measured by T-maze | Laser/630 nm/< 5 mW | Pereira (2017) | (100) |
| Snail | Helix pomatia | Single sub-esophageal neurons irradiated and action potential measured | Laser/632 nm/10 mW | Balaban et al (2017) | (105) |
| Chicken | Gallus gallus domesticus | Fertilized eggs hatched with bigger embryos | LED/635 nm/continuous | Buzza et al (2017) | (112) |
| Birds in veterinary practice | Various species of birds | Multiple diseases and conditions | Clinical therapeutic lasers | Ness and Meyer (2017) | (45) |
MRH was supported by US-NIH grants R01AI050875 and R21AI121700.
Figure 2.
The range of non-mammalian lifeforms which have been treated with PBM for various conditions.
Bacteria
Most investigations of red-to-NIR light irradiation of bacteria have been published in the Russian language. However, many of the findings have been summarized in English reviews authored by original investigators including many original papers (47, 48).
Tiina Karu was one of the first scientists to seriously study the phenomenon of PBM at a molecular and cellular level. There is a large body of work from Tiina Karu in Russia reporting on PBM effects on various microorganisms such as bacteria and fungi (49, 50). Karu group’s investigations on bacterial photobiomodulation focused mostly on the Escherichia coli WP2 trp− strain. Most of the experiments were done with He-Ne laser (632.8 nm), but some experiments have also been reported with many other wavelengths in the 300–800 nm range, and also with 890, 950, 1066 and 1286 nm. The principal finding was that PBM causes a transient acceleration of cell division after moving the cells from the buffer in which they were irradiated to nutritive medium (latent period). The difference between irradiated and non-irradiated groups was notably diminished in cultures with only a short latent period.
In the Anaerobacter polyendosporus, PBM increased the number of germinated and outgrown endospores. A biphasic dose response was noted, the effect peaking at 300 J/m2 (47).
It is noteworthy that the studies on the effects of PBM on E. coli were mostly limited to strains with impaired rates of growth (47, 51). Some experiments with wild type E coli failed to affect bacterial growth (52). Also, it has been reported that the PBM effects do not occur in the absence of oxygen. If anaerobically grown cells were exposed to oxygen for 24 hours, they again became sensitive to growth stimulation by PBM (53).
Some researchers have also used light in order to inhibit bacterial growth. For example, in one study, the effects of wavelengths of 660 nm, 830 nm and 904 nm were studied in Staphylococcus aureus, E. coli, and Pseudomonas aeruginosa and all of these wavelengths had inhibitory effects on bacterial growth (54). In another study, 810 nm light had an inhibitory effect on P. aeruginosa growth and a stimulatory effect on E. coli growth (55). In yet another series of experiments with P. aeruginosa, E. coli, and S. aureus, differing results were obtained with different wavelengths and doses (56).
In some experiments, red light has protected bacteria against ultraviolet radiation (57–59). Also, red light has been used for “mutagenic irradiation”, meaning that it is possible to induce higher mutation rates in bacterial cultures by using the appropriate parameters of light irradiation (60, 61). This effect can be utilized in production of bacteria with specific metabolic traits. It is relatively easy to select the desired mutant form thousands of possible candidates.
Which mechanisms could explain the PBM effects in bacteria, especially E. coli? It has been shown that the PBM effects can be modified by quenchers and enhancers of singlet oxygen (1O2), and it was therefore suggested that PBM might generate singlet oxygen via excitation of cellular chromophores via a photodynamic effect (ie energy transfer between triplet states), and the damage caused by modest amounts of singlet oxygen could trigger the accelerated cellular metabolism of bacteria (53). Other groups had also suggested earlier that PBM using red-to-near-IR spectrum light happens in E. coli due to the absorption by the terminal respiratory chain oxidase complexes cyt bd and cyt bo, which have comparable functions to cytochrome c oxidase in eukaryotic cells (62). It has also been claimed that transient heating of microenvironment of these molecules might be involved (63). Most studies have been done with facultative anaerobes, but the Polo paper (53) also studied oxygen-free cell culture environment, suggesting that the PBM sensitivity began only after the bacteria had been for a few hours in oxygenated conditions “Moreover, cytochromes b and d are not expressed when E. coli is grown under anaerobic conditions, which would explain the lack of detectable photoresponse when the irradiations are carried out in the absence of oxygen, while the photostimulation reappears after the anaerobically grown cells are exposed to oxygen for a few hours, i.e. a time interval required for activating the normal oxygen-dependent metabolism.” (53)
Fungi
The effects of PBM on fungi have not been much investigated. Some studies have been conducted in Troitsk, Russia by the Karu group. The original Russian language papers are difficult to access, but many experiments were described in two English review articles by the original researchers (47, 64). The majority of studies have been carried out on yeasts rather than on filamentous fungi presumably because of the relative ease of culture (54–56, 65, 66).
PBM of various strains showed a slight effect on the growth curves of yeast cultures. For example, irradiated Endomyces magnusii reached the stationary phase of growth 1.5 hours earlier than a non-irradiated culture (47). In Torulopsis sphaerica, the logarithmic phase of the growth occurred 1.5–1.8 times sooner than in the non-irradiated group.
The research was mainly undertaken with yeast strain T. sphaerica since it appeared to have higher sensitivity to PBM effects compared to other strains the group had been studying (E. magnusii, Candida maltosa, S. cerevisiae and Saccharomycodes ludwigii).
It was reported that He-Ne laser increased total cellular protein synthesis, and the biphasic dose response showed a peak at 460 J/m2 (0.046 J/cm2). The effects also included increased NADH dehydrogenase and cytochrome c oxidase activities and decreased activities of acid phosphatase and catalase. After these initial observations, ultrathin cellular sections of successive generations of T. sphaerica were prepared and examined using quantitative electron microscopy in order to analyze the structural changes in the yeast mitochondria. The findings showed that PBM with He-Ne laser was able to modify the ultrastructure so that giant mitochondrion could be observed in budding yeast cells after mitosis. PBM with the optimal dose increased mean mitochondrial profile area, while a higher dose led to an opposite effect, i.e. reduced mitochondrial area. PBM also increased the relative surface area of mitochondrial cristae by 25% according to the morphometric analysis. Moreover, PBM increased the contact between mitochondria and the endoplasmic reticulum, which could reflect the increased mitochondrial capacity to uptake Ca2+.
Some experiments with Candida albicans have also been published in other countries, and basically the results have been slight to moderate stimulation of growth (67, 68).
Paramecium
Paramecium sp are eukaryotic unicellular freshwater organisms with a characteristic slipper-like shape and are covered with beating cilia (69). Paramecia feed on microorganisms (bacteria, algae, and yeasts) and must therefore move around to force organisms, along with some water, through the oral groove into the mouth. The food then passes into the gullet. Moreover, paramecia can respond to stimuli by changing the coordination, direction and frequency of the ciliary beating, so they can “swim” away from danger.
Amaroli et al (70) used a flat-top hand-piece that could emit two different laser wavelengths, 808 nm and 980 nm (separately) at 0.1; 0.5; 1; and 1.5 W to generate 6.4; 32; 64; and 96 J/cm2 and examined swimming speed, intracellular calcium [Ca2+]i and nitric oxide (NO) immediately afterwards. Swimming speed was increased in a dose-dependent manner by both wavelengths with 96 J/cm2 giving the best effect with 808 nm, and 32 J/cm2 giving the best effect with 980 nm. After 64 J/cm2 of 980 nm the Paramecia circled and showed avoidance behavior, while after 96 J/cm2 was delivered the swimming abruptly stopped suggesting that the Paramecia were dead. NO was released by high doses of both wavelengths and [Ca2+]i levels were lowered by the two highest doses of 980 nm (64 and 96 J/cm2). In another study using this Paramecium model the same group has shown that 808 nm laser (1W; 64J/cm2) increased cellular respiration in P. primaurelia inducing a 40% increase in oxygen consumption (71). A third study (72) showed the same parameters (808 nm, 1W, 64 J/cm2) produced an increased cellular binary fission rate rhythm faster than the control cells. Yet a fourth study (73) used a different laser (1064 nm, 10 Hz, 100 msec pulse duration) delivering fluences from 30 – 90 J/cm2. The best results were achieved with the lowest dose (30 J/cm2 that increased ATP synthesis and fission rate rhythm. All higher doses induced adverse effects.
Plants
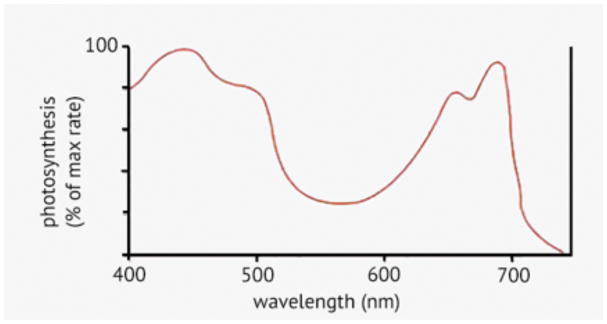
It is intrinsically difficult to study PBM in plants. This is because many wavelengths of visible light are absorbed by plants in order to carry out their normal physiological functions, the most important of which are photosynthesis and photomorphogenesis. Therefore, the default expectation is that plants will be stimulated by light. Pioneering studies in photosynthesis by William A Arnold (74) and KJ McCree (75) established the action spectrum for photosynthesis, which has a broad double peak in the blue (440 nm and a shoulder at 490 nm) and a double peak in the red (660 nm and 690 nm). The action spectrum drops off sharply above 700 nm (Figure 3).
Figure 3.
Action spectrum for photosynthesis.
Robert Emerson (Arnold’s PhD supervisor) discovered the so-called “Emerson Enhancement Effect” that led to the realization that there were far-red absorbing chlorophyll derivatives (710–725 nm) that could mediate “uphill energy transfer” to photosystem II (PSII) and thereby enhance plant growth (76). Pettal et al reported the existence of even longer far-red wavelength chromophores (up to 780 nm) that could in some circumstances stimulate PSII (77).
It should not be forgotten that plant cells contain two different light-responsive organelles i.e. chloroplasts and mitochondria at the same time. Chloroplasts and mitochondria were traditionally considered to be autonomous organelles but they are not as independent as they were once thought to be. Mitochondrial metabolic processes such as oxidative electron transport and phosphorylation, continue to be active in the light and are essential for sustaining photosynthetic carbon assimilation (78). The mutually beneficial interaction between mitochondria and chloroplasts is intriguing. The organelles within plant cells, including not only mitochondria and chloroplasts but also the peroxisomes and cytosol, appear to be in a delicate metabolic equilibrium. Disturbance of any of these compartments perturbs the metabolism of whole cell.
However, it is generally accepted that near-infrared light (> 800 nm) does not normally activate photosynthesis or indeed affect plant cells, possibly because plant biologists are largely unaware of the science of photobiomodulation. Johnson et al (79) addressed this issue by comparing oat seedlings grown either in the dark or under continuous illumination with NIR LEDs (880 nm or 935 nm). The power density was given in μmol/m2/sec but it was approximately equal to 0.6 mW/cm2. Illumination was constant from planting to harvest at 120 hours. They found the NIR illuminated seedlings had shorter mesocotyls (white part immediately after roots) and longer coleoptiles (part between mesocotyl and emerging leaf). Furthermore, the illuminated seedlings demonstrated shorter overall length but more vertical growth (gravitropism). The emerging leaves were longer. The authors attributed their observations to the presence of small amounts of red light impurities in their NIR LED emission spectra, presumably because they were unaware of PBM effects. They cautioned against the use of NIR imaging systems to monitor plant growth, because the imaging light could affect the growth.
Algae are metabolically similar to plants, but we have been unable to find any references to PBM in algae.
Some non-peer-reviewed comments on the website (“Hunker” https://www.hunker.com/) include statements such as “According to Texas A&M University, infrared light plays a part in the blooming of flowering plants. Plants grown indoors may grow well under fluorescent lights, but will not bloom until appropriate levels of infrared radiation have been introduced. This can be done using special horticultural lights, or simply by adding incandescent light bulbs” and “According to Planta, increased infrared waves can affect the speed at which plant stems grow. A short exposure to far infrared light increased the space between nodes when the exposure occurred at the end of an eight-hour light period.”
Insects
Bumblebees play an important role in pollination throughout the world, which is vital for ensuring that crops (and particularly fruit trees) continue to be productive. However the overuse of neonicotinoid insecticides has posed a threat to the worlds bee population (80). Neonicotinoids function as postsynaptic acetylcholine receptor agonists in insects, over stimulating neurons and depolarizing the mitochondria (81). This neuronal damage to the insect brain results in immobility and subsequent death (82). Powner and coworkers in London reasoned (83) that using PBM to stimulate the bees’ mitochondria might overcome the inhibition caused by neonicotinoids. The experiment comprised four groups with a 10-day treatment period and a 22 day recovery period: (a) controls, (b) neonicotinoid alone, (c) PBM alone, (d) PBM + neonicotinoid. Imidacloprid was administered in 50% sucrose solution, and 670 nm LED light was delivered for 15 min twice a day with a power density of 40mW/cm2 from two light sources at either end of the 3L container. Imidacloprid caused the ATP levels to drop by 25%, but these were almost restored to normal by PBM. In the imidacloprid group 70 % of the bees had died by day 10, but in the PBM + imidacloprid group only 30% had died. All the imidacloprid group had died by day 20, but 30% of PBM + imidacloprid group were alive, followed by 40% of controls and 70% of PBM alone bees. Not only could PBM extend the lifespan of neonicotinoid treated bees but could also extend the lifespan of normal healthy bees.
Begum and colleagues studied whether PBM could extend the lifespan of aged Drosophila fruit flies. Seven-week old flies were either exposed to 670 nm LED light at 40 mW/cm2 for 20 minutes once a day or else to normal room lighting (2 mW/cm2). The survival of PBM flies was on average 10% greater than controls, and PBM treated flies contained 70% more ATP and 10% less complement component C3 (a marker of systemic inflammation) compared to controls. Moreover, PBM treated flies had increased mobility (climbing and distance traveled).
Aquatic organisms
Amaroli et al (84) studied the effects of PBM on the reproductive processes of sea urchins (Paracentrotus lividus). The justification for this study was the possible use of PBM in assisted reproduction and artificial insemination protocols in human fertility clinics. They irradiated P. lividus gametes, zygotes, embryos, and larvae and the fertilization rate and the early developmental stages were investigated. They used an 808 laser with a flat top hand-piece delivering a fluence of either 64 J/cm2 at 1 W or 192 J/cm2 at 3 W. The only difference was earlier development of fertilized eggs from irradiated sperm at the higher dose response (192 J/cm2). They concluded that even higher fluences may be worth testing.
Milene Borges Campagnaro is a dentist who completed her MSc thesis in the Postgraduate Program in Dentistry at Catholic University of Rio Grande do Sul in Brazil in 2016 (85). The title was “Embryotoxic and teratogenic effects of LLLT: a study in zebrafish”. She used two different lasers, NIR (830 nm, 90 mW, CW) and red (685 nm, 30 mW, CW) and looked at early stages (first five days) of development in the zebrafish embryos. Each laser was applied at a range of doses (0.5 J, 1 J, 2 J, 4 J, 8 J). The results showed that the PBM groups displayed a delay in egg hatching (eclosion), a higher mortality rate and some teratogenic changes were observed in the animals, as well as behavioral alterations, when compared to the control group. The heartbeat was higher in both IR and R groups, with the IR group showing the highest values. The cartilage and bone formation did not show any visual alterations. By and large this study must be considered negative, which might be due to the applied power being excessive.
Donald Stremme, a practicing veterinarian wrote a chapter entitled “ Laser Therapy for Aquatic Species” (44). Among the conditions that he discussed having treated were fish suffering from head and lateral line erosion (HLLE) disease and chronic ulcerative dermatopathy, long term lesions in a stingray, stomatitis in a reptile, spondylitis in various aquatic species, and improved bone healing in a dolphin with a fractured jaw.
Amphibians and Reptiles
Bibikova and Oron carried out a series of studies (86–89) on the use of PBM to regenerate a muscle injury in the European green toad (Bufo viridis). The motivations was that while PBM was known to help with muscle injury in small mammals which have a high rate of metabolism, would it also be active in amphibians which have a much lower metabolic rate. They first used a 6 mW HeNe laser to irradiate the cold-injured gastrocnemius muscle for 2.3 min every alternate day starting on the fourth day post-injury (88). At 9 days post-injury, mono-nucleated cells populated 70% of the injured area, but decreased more rapidly in the PBM-treated muscle than in the control. The fraction of the myotubes in the PBM muscles at 9 days was higher and at 30 days most of the injured zone was filled with mature muscle fibers. In the next study (88) they compared the HeNe laser with a pulsed Ga-As 904 nm laser (2.8 Hz, 200 nsec, average power 0.005 mW, peak power 9W). Both lasers stimulated muscle healing in the toads equally well and a single exposure on the 9th day was as good as five consecutive exposures every other day. In the 3rd paper (86) they looked at angiogenesis in toad muscles treated with the HeNe laser. The surface density of capillaries in the injured zone was 2.3-fold higher in the laser-irradiated muscles than in the control muscles at 9 days after injury, but the capillaries further increased in the controls between 9 and 14 days, while it declined in the PBM muscles. The surface-to-volume ratios indicated a straighter, rather than a convoluted appearance in the PBM muscles suggest a more rapid normalization. Finally in the 4th paper they looked at muscle regeneration following nerve damage (89). This time the muscles were surgically denervated rather than cold-injured. HeNe laser was delivered (6.0 mW, 31.2 J/cm2) at 7 days post-denervation, and allowed better muscle regeneration with PBM.
Comelekoglu et al reported an electrophysiological study using frogs (Rana cameroni ) (90). The skin was removed from the frogs and the sciatic nerve was exposed. Standardized needle electromyography and nerve conduction measurements were carried out using monopolar needle implanted into the sciatic notch. They used a Ga-As 904 nm laser (spot size: 0.28 cm2, pulse duration 220 nsec; peak power 27 W; fluence 0.0003–7.2 J). The pulse repetition rate was varied from: 1–1000 Hz to give average power values: 0.024–6 mW. The study failed to detect any significant effects of the laser on action potentials.
Cusack et al reported on a wound healing study in the green iguana (Iguana iguana) (91). Full thickness punch biopsy wounds were made, and wound healing was stimulated by 10 J/cm2 660 nm laser better compared to the control treatment of silver sulfadiazine.
Worms and snails
Wu and Persinger (92) asked whether PBM could influence the regeneration of amputated planarian worms. The fact that planarian worms can regenerate their whole body if they are cut in half has led them to be widely studies as models of stem cell mediated tissue repair (93). In fact, a planarian can regenerate its entire body from a piece that is less than 1% of the original body weight (94). Wu et al exposed whole and amputated worms to either ambient laboratory lighting, darkness, white (indium–gallium/yttrium–aluminum–garnet) LED, red (630 nm) LED, or near-infrared (880 nm) LED continuously illuminated for 3 days. LED irradiation used a power density of 1.2 mW/m2 (0.12 μW/cm2) for 24 h/day to deliver 0.01 J/cm2 in total fluence each day. Compared to the other groups, amputated planarians exposed to NIR (880 nm) PBM displayed increased mobility by the 3rd day of exposure. Higher densities of stem cells were measured in these worms 84 h post injury.
Amaroli and coworkers studied the earthworm Dendrobaena veneta (95). While earthworms can still regenerate themselves after amputation (96), they are not as efficient at doing so as are planarian worms. These workers excised a piece from the caudal section of the worm and irradiated the “stump” with a CW 808-nm diode laser using a flat-top hand-piece at a power of 1 W to deliver a fluence of 64 J/cm2. After 24 h the worms were examined with histology and gross inspection. Immediately after PBM the worms showed a clear muscular contraction at the wound site and internalization of the alimentary tract only in the irradiated earthworms. At 24 h PBM treated worms had healed much better than controls, and there was a smaller coelomic plug in the irradiated samples due to muscular contraction reducing the leakage of coelomic fluid and of coelomocytes from the wound. Immunofluorescence showed less acetylcholinesterase staining in PBM treated wounds indicating an anti-inflammatory effect. In a follow-up study (97) using this model they confirmed their earlier findings of improved wound healing by PBM due to “effects on muscular and blood vessel contraction, decrement of bacteria load, reduction of inflammatory processes and tissue degeneration”.
Natalie Duggett carried out her PhD research at Durham University finishing in 2013 (98). The title of her thesis was “Photobiomodulation in Animal Models of Ageing and Alzheimer’s Disease”. Duggett used 1072 nm LEDs at 5 mW/cm2 to irradiate C. elegans and found the lifespan was increased by 16% after a 12-minute exposure to PBM. Control experiments established that the increase in lifespan was due to direct effects of the NIR light on the worms, and not on the bacterial lawn upon which they fed. Biochemical studies established the importance of pathways involving heat shock factor 1 (HSF1) and heat shock protein 70, that can function to prevent cell stress and lessen cell death.
On the other hand, De Magalhaes Filho and colleagues explored the effects of visible light on C. elegans longevity (99). On the surface, it may appear that their results were is contradiction with those of Duggett as they found that light reduced the lifespan of C. elegans. However, De Magalhaes Filho found the biggest effects with blue light (470 nm), a moderate effect with green light (530 nm), and only small effects with yellow (600 nm) and red light (675 nm), and did not test NIR light. They showed that visible light created photo-oxidative stress along with a general unfolded-protein response that decreased the lifespan of the worms. Long-lived mutants were more resistant to light stress, as well as wild-type worms that had been given antioxidants.
Contzen Pereira published (100) an interesting study in terrestrial pulmonates, a broad group of creatures including land snails and slugs. These mollusks are characterized by possession of a vascularized lung instead of a mantle cavity with gills. Snails are known to possess extraocular photosensitive neurons on the dorsal surface of the subesophageal ganglia that are proposed to be internal photoreceptors based on their electrophysiological properties (101). Based on the slow response time, Gotow and Nishi proposed that these neurons could not be involved in vision or image-formation and that therefore “we suggest that simple photoreceptors operate in the general potentiation of synaptic transmission and subsequent motor output; i.e., they perform a new photosensory function” (102). Pereira aimed to study the effect of light on cognition and behavioral response in the land snail Ariophanta laevipes (100). Snails were separately exposed to a 5 mW 630 nm laser held 1 cm above the brain region of the snail for 1 min 40 sec twice a day at 12 hr intervals (9am and 9pm) for a period of 5 days. Cognitive abilities of the snails were improved as measured by the time taken to complete the T-maze pre-and post PBM for 5 days. Memory-formation and retention and learning ability was improved by PBM.
Workers in Russia had tested various laser wavelengths on single isolated photosensitive neurons taken from snails (103). The preparation of the neurons was described here (104). Balaban et al (105) used a 10 mW HeNe (632 nm) laser focused into a small spot. They used an intracellular glass microelectrode filled with 2 M KCl to record the membrane potential threshold potential, resting potential, and neuronal spiking activity. When the spontaneously active neurons generating spikes every 7–10 min were irradiated in between the spikes, the depolarization of membrane and generation of action potentials occurred as a function of light intensity. The probability of spike generation increased until the intensity reached 1 W/cm2. The depolarization had a threshold at 0.1 W/cm2, then increased with increasing the intensity, and reached a plateau at 0.7 W/cm2 (depolarization rate 0.18 mV/s).
Birds
The effect of light is very important in the poultry rearing and in egg production (106). “Photostimulation” (provision of ambient white fluorescent or incandescent lighting) is generally considered to be the cue to initiate puberty in chickens, although the response to light is readily modified by feeding (107). At photostimulation, light energy passes through the skull, and reaches the hypothalamus. When the hypothalamus receives a photostimulatory signal (sufficient number of hours of light above a certain threshold of intensity), hormones are produced that affect ovarian function (108). One of the first responses in the ovaries of the bird after photostimulation, is that the very small ovarian follicles begin to increase in size.
It has also been reported that illumination during the egg incubation period produced broiler chickens with improved health, productivity, and behavior (109). Conversely, eggs incubated in the dark hatched into chickens with a greater level of composite physical asymmetry considered to be an indicator of developmental stress. Sindhuraker and Bradley (110, 111) found that 24-hour white light illumination of incubated eggs led to chickens that could walk 2 days earlier than eggs hatched in the dark.
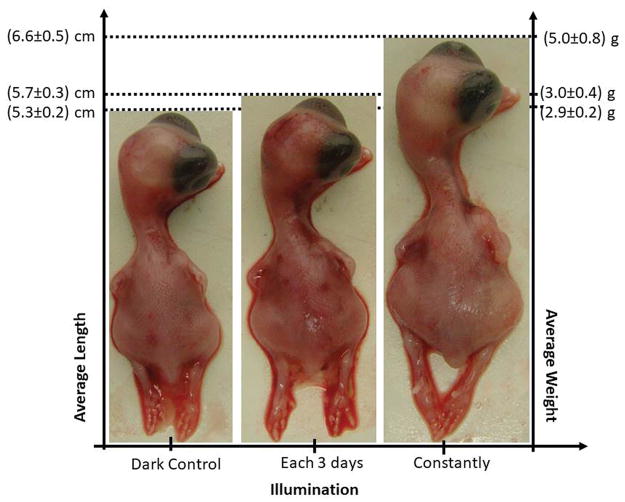
Buzza et al (112) in Sao Paulo Brazil, decided to test whether red light delivered during the egg incubation period could affect the hatched chickens better than bright white light. They found that the best results were achieved with low power densities (0.014 mW/cm2) delivered continuously 24 hours per day for 10 days (a dose of 1.2 J/cm2 per day). Newly hatched chickens treated with PBM had a 25% increase in average size and > 70% increase in average weight compared to the controls. Figure 4 shows an example comparing the hatched chicks (controls, PBM every 3 days, PBM every day). There was no difference between a 630 nm laser and a 635 nm LED.
Figure 4.
PBM delivered continuously to eggs during the incubation period produces significantly larger chickens. Personal communication from Hilde Buzza, Sao Paolo, Brazil. Similar to a figure in (112).
As mentioned above veterinarians are more likely than human physicians to employ PBM for many of the conditions they encounter daily in their practice. The book chapter “Laser Therapy for Birds” (44) listed the following conditions : Deep wounds; Feather disorders; Lacerations; Petagium restriction; Pododermatitis; Self-mutilation; Surgical incisions; Thermal burns; Arthritis; Articular gout; Edema; Fractures; Neuropathic pain; Muscular sprains and strains; Splayed legs; Wing tip trauma; Ascites; Gastrointestinal disorders; Renal disease.
CONCLUSIONS AND FUTURE DIRECTIONS
It certainly seems to be the case that all life forms can respond to PBM type interventions in one way or another. The reason for this is probably that all life forms rely on cytochromes for one function or another and most of them contain a respiratory chain either in the mitochondria, or in the plasma membrane in the case of bacteria.
During the preparation of this review article several points occurred to us that are worth mentioning. The first point is that the almost complete lack of studies of PBM on plant cells, is truly remarkable. The obvious explanation is that everyone assumes that red light drives photosynthesis, and cannot therefore carry out PBM. However, even with NIR light (wavelengths above 800 nm) which cannot carry out photosynthesis, but can stimulate mitochondria (at least in mammalian cells) there are virtually no papers. The one paper we could find attributed their results to “a small impurity of red light in their NIR LEDs” (79). Another point concerns PBM stimulation of bacteria. Despite the number of papers published there has not been much investigation of the molecular mechanisms involved. Considering the vast array of mutants now available for many bacterial species, this seems to be a missed opportunity. Reversal of chronological aging has become something of a “hot topic” in recent years. Much research into age-reversal utilizes model organisms such as Drusophila (113), C. elegans (114) or honey bees (115). In a similar vein, the study of epigenetics has become popular (116, 117) and there are few studies looking at the effects of PBM on epigenetics.
Considering the dramatic effects of red light PBM on hatching of larger chickens from eggs (112), there would appear to be many opportunities for PBM in poultry rearing and egg production, especially that now this is often conducted indoors under artificial lighting. Although much of the livestock industry is concerned with mammalian species and this may be off-topic, it is worth pointing out that there would appear to be large numbers of unexplored opportunities for PBM in agricultural food production.
Footnotes
This article is part of a Special Issue celebrating Photochemistry and Photobiology’s 55th Anniversary.
Conflict of Interests
The authors declare no conflict of interests.
References
- 1.Karu TI. Molecular mechanism of the therapeutic effect of low-intensity laser irradiation. Dokl Akad Nauk SSSR. 1986;291:1245–1249. [PubMed] [Google Scholar]
- 2.Karu TI. Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. 2010;62:607–610. doi: 10.1002/iub.359. [DOI] [PubMed] [Google Scholar]
- 3.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Freitas LF, Hamblin MR. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron. 2016:22. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamblin MR. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem Photobiol. 2017 doi: 10.1111/php.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeronimo R, Moraes MN, de Assis LVM, Ramos BC, Rocha T, Castrucci AML. Thermal stress in Danio rerio: a link between temperature, light, thermo-TRP channels, and clock genes. J Therm Biol. 2017;68:128–138. doi: 10.1016/j.jtherbio.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation (blue and green light) encourages osteoblastic-differentiation of human adipose-derived stem cells: role of intracellular calcium and light-gated ion channels. Sci Rep. 2016;6:33719. doi: 10.1038/srep33719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Red (660 nm) or near-infrared (810 nm) photobiomodulation stimulates, while blue (415 nm), green (540 nm) light inhibits proliferation in human adipose-derived stem cells. Sci Rep. 2017;7:7781. doi: 10.1038/s41598-017-07525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Huang YY, Wang Y, Lyu P, Hamblin MR. Photobiomodulation of human adipose-derived stem cells using 810nm and 980nm lasers operates via different mechanisms of action. Biochim Biophys Acta. 2016 doi: 10.1016/j.bbagen.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai B, Yoo H, Pollack GH. Effect of radiant energy on near-surface water. J Phys Chem B. 2009;113:13953–13958. doi: 10.1021/jp908163w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang YY, Chen AC, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy. Dose Response. 2009;7:358–383. doi: 10.2203/dose-response.09-027.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang YY, Sharma SK, Carroll JD, Hamblin MR. Biphasic dose response in low level light therapy - an update. Dose Response. 2011;9:602–618. doi: 10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommer AP, Pinheiro AL, Mester AR, Franke RP, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg. 2001;19:29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 14.Russell WM, Burch RL. The Principles of Humane Experimental Technique. Methuen and Co. Ltd; 1959. [Google Scholar]
- 15.Muschiol D, Schroeder F, Traunspurger W. Life cycle and population growth rate of Caenorhabditis elegans studied by a new method. BMC Ecol. 2009;9:14. doi: 10.1186/1472-6785-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnatkevic Iute A, Fulcher BD, Pocock R, Fornito A. Hub connectivity, neuronal diversity, and gene expression in the Caenorhabditis elegans connectome. PLoS Comput Biol. 2018;14:e1005989. doi: 10.1371/journal.pcbi.1005989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckingham KM, Armstrong JD, Texada MJ, Munjaal R, Baker DA. Drosophila melanogaster--the model organism of choice for the complex biology of multi-cellular organisms. Gravit Space Biol Bull. 2005;18:17–29. [PubMed] [Google Scholar]
- 18.Szabad J, Fajszi C. Control of female reproduction in Drosophila: genetic dissection using gynandromorphs. Genetics. 1982;100:61–78. doi: 10.1093/genetics/100.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milan DJ, Peterson TA, Ruskin JN, Peterson RT, MacRae CA. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation. 2003;107:1355–1358. doi: 10.1161/01.cir.0000061912.88753.87. [DOI] [PubMed] [Google Scholar]
- 21.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Junqueira JC. Galleria mellonella as a model host for human pathogens: recent studies and new perspectives. Virulence. 2012;3:474–476. doi: 10.4161/viru.22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chibebe J, Junior, Fuchs BB, Sabino CP, Junqueira JC, Jorge AO, Ribeiro MS, Gilmore MS, Rice LB, Tegos GP, Hamblin MR, Mylonakis E. Photodynamic and Antibiotic Therapy Impair the Pathogenesis of Enterococcus faecium in a Whole Animal Insect Model. PLoS ONE. 2013;8:e55926. doi: 10.1371/journal.pone.0055926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chibebe J, Junior, Sabino CP, Tan X, Junqueira JC, Wang Y, Fuchs BB, Jorge AO, Tegos GP, Hamblin MR, Mylonakis E. Selective photoinactivation of Candida albicans in the non-vertebrate host infection model Galleria mellonella. BMC Microbiol. 2013;13:217. doi: 10.1186/1471-2180-13-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J Photochem Photobiol B. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Karu TI. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem Photobiol. 2008;84:1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x. [DOI] [PubMed] [Google Scholar]
- 27.Margulis L. Symbiosis and evolution. Sci Am. 1971;225:48–57. doi: 10.1038/scientificamerican0871-48. [DOI] [PubMed] [Google Scholar]
- 28.Margulis L. The origin of plant and animal cells. Am Sci. 1971;59:230–235. [PubMed] [Google Scholar]
- 29.Schwartz RM, Dayhoff MO. Origins of prokaryotes, eukaryotes, mitochondria, and chloroplasts. Science. 1978;199:395–403. doi: 10.1126/science.202030. [DOI] [PubMed] [Google Scholar]
- 30.Alberts B, Johnson A, Lewis J. The Evolution of Electron-Transport Chains. New York: 2002. [Google Scholar]
- 31.Cooper GM. Chloroplasts and Other Plastids. Sunderland, MA: 2000. [Google Scholar]
- 32.Raghavendra AS, Padmasree K. Beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Trends Plant Sci. 2003;8:546–553. doi: 10.1016/j.tplants.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Gray MW, Lang BF, Burger G. Mitochondria of protists. Annu Rev Genet. 2004;38:477–524. doi: 10.1146/annurev.genet.37.110801.142526. [DOI] [PubMed] [Google Scholar]
- 34.Marinos E. The number of mitochondria in Xenopus laevis ovulated oocytes. Cell Differ. 1985;16:139–143. doi: 10.1016/0045-6039(85)90527-5. [DOI] [PubMed] [Google Scholar]
- 35.Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- 36.Wei Y, Zhang YJ, Cai Y, Xu MH. The role of mitochondria in mTOR-regulated longevity. Biol Rev Camb Philos Soc. 2015;90:167–181. doi: 10.1111/brv.12103. [DOI] [PubMed] [Google Scholar]
- 37.Wu Z, Boss O. Targeting PGC-1 alpha to control energy homeostasis. Expert Opin Ther Targets. 2007;11:1329–1338. doi: 10.1517/14728222.11.10.1329. [DOI] [PubMed] [Google Scholar]
- 38.Mormeneo E, Jimenez-Mallebrera C, Palomer X, De Nigris V, Vazquez-Carrera M, Orozco A, Nascimento A, Colomer J, Lerin C, Gomez-Foix AM. PGC-1alpha induces mitochondrial and myokine transcriptional programs and lipid droplet and glycogen accumulation in cultured human skeletal muscle cells. PLoS One. 2012;7:e29985. doi: 10.1371/journal.pone.0029985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Liu L, Wu S, Xing D. Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation-induced apoptosis. FASEB J. 2016;30:466–476. doi: 10.1096/fj.15-274258. [DOI] [PubMed] [Google Scholar]
- 41.Kim HB, Baik KY, Choung PH, Chung JH. Pulse frequency dependency of photobiomodulation on the bioenergetic functions of human dental pulp stem cells. Sci Rep. 2017;7:15927. doi: 10.1038/s41598-017-15754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME, Zhu L, Liu TC, Cohen RM, Zhang Q. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin K, Zhu R, Wang S, Zhao RC. Low-Level Laser Effect on Proliferation, Migration, and Antiapoptosis of Mesenchymal Stem Cells. Stem Cells Dev. 2017;26:762–775. doi: 10.1089/scd.2016.0332. [DOI] [PubMed] [Google Scholar]
- 44.Stremme DW. In: Laser Therapy for Aquatic Species. Riegel RJ, Godbold JC Jr, editors. New York: 2017. [Google Scholar]
- 45.Ness RD, Mayer J. In: Laser Therapy for Birds. Riegel RJ, Godbold JC Jr, editors. New York: 2017. [Google Scholar]
- 46.Riegel RJ, Godbold JC., Jr . Laser Therapy in Veterinary Medicine. John Wiley and Sons; 2017. [Google Scholar]
- 47.Karu T. Activation of metabolism of nonphotosynthesizing microorganisms with monochromatic visible (laser) light: A critical review. Lasers in the Life Sciences. 1996;7:11–34. [Google Scholar]
- 48.Tiphlova O, Karu T. Action of low-intensity laser radiation on Escherichia coli. Crit Rev Biomed Eng. 1991;18:387–412. [PubMed] [Google Scholar]
- 49.Karu TI, Tiphlova OA, Letokhov VS, Lobko YV. Stimulation of E. coli growth by laser and incoherent red light. Il Nuoyo Cimento. 1983;2:1138–1144. [Google Scholar]
- 50.Fedoseyeva GE, Karu TI, Lyapunova TS, Pomoshnikova NA, Meissel MN. The activation of yeast mitochondria with He-Ne radiation 1. Protein synthesis in various cultures. Lasers Life Sci. 1988;2:137–146. [Google Scholar]
- 51.Bertoloni G, Sacchetto R, Baro E, Ceccherelli F, Jori G. Biochemical and morphological changes in Escherichia coli irradiated by coherent and non-coherent 632.8 nm light. J Photochem Photobiol B. 1993;18:191–196. doi: 10.1016/1011-1344(93)80062-e. [DOI] [PubMed] [Google Scholar]
- 52.Roos C, Santos JN, Guimarães OR, Geller M, Paoli F, Fonseca AS. The effects of a low-intensity red laser on bacterial growth, filamentation and plasmid DNA. Laser Physics. 2013;23:075602. [Google Scholar]
- 53.Polo L, Presti F, Schindl A, Schindl L, Jori G, Bertoloni G. Role of ground and excited singlet state oxygen in the red light-induced stimulation of Escherichia coli cell growth. Biochem Biophys Res Commun. 1999;257:753–758. doi: 10.1006/bbrc.1999.0426. [DOI] [PubMed] [Google Scholar]
- 54.de Sousa NT, Gomes RC, Santos MF, Brandino HE, Martinez R, de Jesus Guirro RR. Red and infrared laser therapy inhibits in vitro growth of major bacterial species that commonly colonize skin ulcers. Lasers Med Sci. 2016;31:549–556. doi: 10.1007/s10103-016-1907-x. [DOI] [PubMed] [Google Scholar]
- 55.Nussbaum EL, Lilge L, Mazzulli T. Effects of low-level laser therapy (LLLT) of 810 nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure. J Clin Laser Med Surg. 2003;21:283–290. doi: 10.1089/104454703322564497. [DOI] [PubMed] [Google Scholar]
- 56.Nussbaum EL, Lilge L, Mazzulli T. Effects of 630-, 660-, 810-, and 905-nm laser irradiation delivering radiant exposure of 1–50 J/cm2 on three species of bacteria in vitro. J Clin Laser Med Surg. 2002;20:325–333. doi: 10.1089/104454702320901116. [DOI] [PubMed] [Google Scholar]
- 57.Kohli R, Gupta PK, Dube A. Helium-neon laser preirradiation induces protection against UVC radiation in wild-type E. coli strain K12AB1157. Radiat Res. 2000;153:181–185. doi: 10.1667/0033-7587(2000)153[0181:hnlpip]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 58.Lage C, Teixeira PC, Leitao AC. Non-coherent visible and infrared radiation increase survival to UV (254 nm) in Escherichia coli K12. J Photochem Photobiol B. 2000;54:155–161. doi: 10.1016/s1011-1344(00)00010-5. [DOI] [PubMed] [Google Scholar]
- 59.Kohli R, Gupta PK. Irradiance dependence of the He-Ne laser-induced protection against UVC radiation in E. coli strains. J Photochem Photobiol B. 2003;69:161–167. doi: 10.1016/s1011-1344(03)00018-6. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Y, Wen J, Caiyin Q, Lin L, Hu Z. Mutant AFM 2 of Alcaligenes faecalis for phenol biodegradation using He-Ne laser irradiation. Chemosphere. 2006;65:1236–1241. doi: 10.1016/j.chemosphere.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 61.Lu W, Wen J, Jia X, Sun B, Chen Y, Liu M. Effect of He–Ne laser irradiation on hydrogen production by Enterobacter aerogenes. International Journal of Hydrogen Energy. 2008;33:34–42. [Google Scholar]
- 62.Karu T. Proceedings of the 2nd International Conference on Bioelectromagnetism (Cat. No.98TH8269); 1998. pp. 125–126. [Google Scholar]
- 63.Karu TI. Comparison of the effects of visible femtosecond laser pulses and continuous wave laser radiation of low average intensity on the clonogenicity of Escherichia coli. J Photochem Photobiol B. 1991;10:339–344. doi: 10.1016/1011-1344(91)80019-e. [DOI] [PubMed] [Google Scholar]
- 64.Manteifel VM, Karu TI. Structure of Mitochondria and Activity of Their Respiratory Chain in Successive Generations of Yeast Cells Exposed to He-Ne Laser Light. Biology Bulletin. 2005;32:556–566. [PubMed] [Google Scholar]
- 65.de Sousa NT, Guirro RR, Santana HF, Silva CC. In vitro analysis of bacterial morphology by atomic force microscopy of low level laser therapy 660, 830 and 904 nm. Photomed Laser Surg. 2012;30:281–285. doi: 10.1089/pho.2011.3160. [DOI] [PubMed] [Google Scholar]
- 66.Nussbaum EL, Lilge L, Mazzulli T. Effects of 810 nm laser irradiation on in vitro growth of bacteria: comparison of continuous wave and frequency modulated light. Lasers Surg Med. 2002;31:343–351. doi: 10.1002/lsm.10121. [DOI] [PubMed] [Google Scholar]
- 67.Carneiro VSM, Araújo NC, Menezes RFd, Moreno LM, Ad Santos-Neto P, Gerbi MEM. SPIE BiOS. 2016:7. [Google Scholar]
- 68.Kim KS, Kim SK, Lee PY, Song YH, Kim KB, Jeon EH, Ahn TY. Effects of low incident energy levels of infrared laser irradiaton on the proliferation of candida albicans. Part iii A study on the interirradiation interval. Laser Therapy. 1995;7:151–156. [Google Scholar]
- 69.Tassin AM, Lemullois M, Aubusson-Fleury A. Paramecium tetraurelia basal body structure. Cilia. 2015;5:6. doi: 10.1186/s13630-016-0026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Amaroli A, Benedicenti A, Ferrando S, Parker S, Selting W, Gallus L, Benedicenti S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem Photobiol. 2016;92:854–862. doi: 10.1111/php.12644. [DOI] [PubMed] [Google Scholar]
- 71.Amaroli A, Ravera S, Parker S, Panfoli I, Benedicenti A, Benedicenti S. The protozoan, Paramecium primaurelia, as a non-sentient model to test laser light irradiation: The effects of an 808nm infrared laser diode on cellular respiration. Altern Lab Anim. 2015;43:155–162. doi: 10.1177/026119291504300305. [DOI] [PubMed] [Google Scholar]
- 72.Amaroli A, Parker S, Dorigo G, Benedicenti A, Benedicenti S. Paramecium: a promising non-animal bioassay to study the effect of 808 nm infrared diode laser photobiomodulation. Photomed Laser Surg. 2015;33:35–40. doi: 10.1089/pho.2014.3829. [DOI] [PubMed] [Google Scholar]
- 73.Amaroli A, Benedicenti A, Ravera S, Parker S, Selting W, Panfoli I, Benedicenti S. Short-pulse neodymium:yttrium-aluminium garnet (Nd:YAG 1064nm) laser irradiation photobiomodulates mitochondria activity and cellular multiplication of Paramecium primaurelia (Protozoa) Eur J Protistol. 2017;61:294–304. doi: 10.1016/j.ejop.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Choules L, Govindjee Stories and photographs of William A. Arnold (1904–2001), a pioneer of photosynthesis and a wonderful friend. Photosynth Res. 2014;122:87–95. doi: 10.1007/s11120-014-0013-9. [DOI] [PubMed] [Google Scholar]
- 75.McCree KJ. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agricultural Meteorology. 1971;9:191–216. [Google Scholar]
- 76.Wilhelm C, Jakob T. Uphill energy transfer from long-wavelength absorbing chlorophylls to PS II in Ostreobium sp. is functional in carbon assimilation. Photosynth Res. 2006;87:323–329. doi: 10.1007/s11120-005-9002-3. [DOI] [PubMed] [Google Scholar]
- 77.Pettai H, Oja V, Freiberg A, Laisk A. Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta. 2005;1708:311–321. doi: 10.1016/j.bbabio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Roberts WG, Klein MK, Loomis M, Weldy S, Berns MW. Photodynamic therapy of spontaneous cancers in felines, canines, and snakes with chloro-aluminum sulfonated phthalocyanine. J Natl Cancer Inst. 1991;83:18–23. doi: 10.1093/jnci/83.1.18. [DOI] [PubMed] [Google Scholar]
- 79.Johnson CF, Brown CS, Wheeler RM, Sager JC, Chapman DK, Deitzer GF. Infrared light-emitting diode radiation causes gravitropic and morphological effects in dark-grown oat seedlings. Photochem Photobiol. 1996;63:238–242. doi: 10.1111/j.1751-1097.1996.tb03020.x. [DOI] [PubMed] [Google Scholar]
- 80.Goulson D. An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology. 2013;50:977–987. [Google Scholar]
- 81.Tomizawa M, Casida JE. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–268. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 82.Moffat C, Pacheco JG, Sharp S, Samson AJ, Bollan KA, Huang J, Buckland ST, Connolly CN. Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris) FASEB J. 2015;29:2112–2119. doi: 10.1096/fj.14-267179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Powner MB, Salt TE, Hogg C, Jeffery G. Improving Mitochondrial Function Protects Bumblebees from Neonicotinoid Pesticides. PLoS One. 2016;11:e0166531. doi: 10.1371/journal.pone.0166531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amaroli A, Gambardella C, Ferrando S, Hanna R, Benedicenti A, Gallus L, Faimali M, Benedicenti S. The Effect of Photobiomodulation on the Sea Urchin Paracentrotus lividus (Echinodermata) Using Higher-Fluence on Fertilization, Embryogenesis, and Larval Development: An In Vitro Study. Photomed Laser Surg. 2017;35:127–135. doi: 10.1089/pho.2016.4136. [DOI] [PubMed] [Google Scholar]
- 85.Campagnaro MB. Embryotoxic and teratogenic effects of LLLT: a study in zebrafish. Pontifical Catholic University of Rio Grande do Sul; 2016. http://hdl.handle.net/10923/8520. [Google Scholar]
- 86.Bibikova A, Belkin V, Oron U. Enhancement of angiogenesis in regenerating gastrocnemius muscle of the toad (Bufo viridis) by low-energy laser irradiation. Anat Embryol (Berl) 1994;190:597–602. doi: 10.1007/BF00190110. [DOI] [PubMed] [Google Scholar]
- 87.Bibikova A, Oron U. Promotion of muscle regeneration in the toad (Bufo viridis) gastrocnemius muscle by low-energy laser irradiation. Anat Rec. 1993;235:374–380. doi: 10.1002/ar.1092350306. [DOI] [PubMed] [Google Scholar]
- 88.Bibikova A, Oron U. Attenuation of the process of muscle regeneration in the toad gastrocnemius muscle by low energy laser irradiation. Lasers Surg Med. 1994;14:355–361. doi: 10.1002/lsm.1900140408. [DOI] [PubMed] [Google Scholar]
- 89.Bibikova A, Oron U. Regeneration in denervated toad (Bufo viridis) gastrocnemius muscle and the promotion of the process by low energy laser irradiation. Anat Rec. 1995;241:123–128. doi: 10.1002/ar.1092410116. [DOI] [PubMed] [Google Scholar]
- 90.Comelekoglu U, Bagis S, Buyukakilli B, Sahin G, Erdogan C. Electrophysiologic effect of gallium arsenide laser on frog gastrocnemius muscle. Lasers Surg Med. 2002;30:221–226. doi: 10.1002/lsm.10027. [DOI] [PubMed] [Google Scholar]
- 91.Cusack LM, Mayer J, Cutler DC, Rissi DR, Divers SJ. Gross and histologic evaluation of effects of photobiomodulation, silver sulfadiazine, and a topical antimicrobial product on experimentally induced full-thickness skin wounds in green iguanas (Iguana iguana) Am J Vet Res. 2018;79:465–473. doi: 10.2460/ajvr.79.4.465. [DOI] [PubMed] [Google Scholar]
- 92.Wu HP, Persinger MA. Increased mobility and stem-cell proliferation rate in Dugesia tigrina induced by 880nm light emitting diode. J Photochem Photobiol B. 2011;102:156–160. doi: 10.1016/j.jphotobiol.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 93.Lobo D, Beane WS, Levin M. Modeling planarian regeneration: a primer for reverse-engineering the worm. PLoS Comput Biol. 2012;8:e1002481. doi: 10.1371/journal.pcbi.1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wenemoser D, Reddien PW. Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev Biol. 2010;344:979–991. doi: 10.1016/j.ydbio.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amaroli A, Ferrando S, Hanna R, Gallus L, Benedicenti A, Scarfi S, Pozzolini M, Benedicenti S. The photobiomodulation effect of higher-fluence 808-nm laser therapy with a flat-top handpiece on the wound healing of the earthworm Dendrobaena veneta: a brief report. Lasers Med Sci. 2017 doi: 10.1007/s10103-016-2132-3. [DOI] [PubMed] [Google Scholar]
- 96.Molnar L, Pollak E, Skopek Z, Gutt E, Kruk J, Morgan AJ, Plytycz B. Immune system participates in brain regeneration and restoration of reproduction in the earthworm Dendrobaena veneta. Dev Comp Immunol. 2015;52:269–279. doi: 10.1016/j.dci.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 97.Amaroli A, Ferrando S, Pozzolini M, Gallus L, Parker S, Benedicenti S. The earthworm Dendrobaena veneta (Annelida): A new experimental-organism for photobiomodulation and wound healing. Eur J Histochem. 2018;62:2867. doi: 10.4081/ejh.2018.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Duggett NA. Photobiomodulation in Animal Models of Ageing and Alzheimer’s Disease. Durham University; Durham, UK: 2013. [Google Scholar]
- 99.De Magalhaes Filho CD, Henriquez B, Seah NE, Evans RM, Lapierre LR, Dillin A. Visible light reduces C. elegans longevity. Nat Commun. 2018;9:927. doi: 10.1038/s41467-018-02934-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pereira C. Improved cognitive functions and behavioural response after exposure to low-level near-infrared laser in snails (Ariophanta laevipes) J Entomol Zool Stud. 2017;5:169–176. [Google Scholar]
- 101.Kartelija G, Nedeljkovic M, Radenovic L. Photosensitive neurons in mollusks. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:483–495. doi: 10.1016/s1095-6433(02)00351-3. [DOI] [PubMed] [Google Scholar]
- 102.Gotow T, Nishi T. A new photosensory function for simple photoreceptors, the intrinsically photoresponsive neurons of the sea slug onchidium. Front Cell Neurosci. 2009;3:18. doi: 10.3389/neuro.03.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Proldiorov AM, Bunkin FV, Sitnikov GA, Logachev FA, Sudakov KV, Osipovsky SA, Savransky W, Shutygina AR. Reactions of central neurons of edible snail upon the irradiation with laser light with various wavelengths. Doklady Akad Nauk USSR. 1980;251:766–768. [PubMed] [Google Scholar]
- 104.Balaban PM. Postsynaptic mechanism of withdrawal reflex sensitization in the snail. J Neurobiol. 1983;14:365–375. doi: 10.1002/neu.480140504. [DOI] [PubMed] [Google Scholar]
- 105.Balaban P, Esenaliev R, Karu T, Kutomkina E, Letokhov V, Oraevsky A, Ovcharenko N. He-Ne laser irradiation of single identified neurons. Lasers Surg Med. 1992;12:329–337. doi: 10.1002/lsm.1900120315. [DOI] [PubMed] [Google Scholar]
- 106.Robinson FE, Melnychuk VL, Muller LD, Oosterhoff HH, Wautier TA, WJL 45th Poultry Breeders Roundtable Proceedings; St. Louis, MO. 1996. pp. 110–122. [Google Scholar]
- 107.Robinson FE, Zuidhof MJ, Renema RA. Reproductive efficiency and metabolism of female broiler breeders as affected by genotype, feed allocation, and age at photostimulation. 1. Pullet growth and development. Poult Sci. 2007;86:2256–2266. doi: 10.1093/ps/86.10.2256. [DOI] [PubMed] [Google Scholar]
- 108.Renema RA, Robinson FE, Oosterhoff HH, Feddes JJ, Wilson JL. Effects of photostimulatory light intensity on ovarian morphology and carcass traits at sexual maturity in modern and antique egg-type pullets. Poult Sci. 2001;80:47–56. doi: 10.1093/ps/80.1.47. [DOI] [PubMed] [Google Scholar]
- 109.Archer GS, Shivaprasad HL, Mench JA. Effect of providing light during incubation on the health, productivity, and behavior of broiler chickens. Poult Sci. 2009;88:29–37. doi: 10.3382/ps.2008-00221. [DOI] [PubMed] [Google Scholar]
- 110.Sindhurakar A, Bradley NS. Light accelerates morphogenesis and acquisition of interlimb stepping in chick embryos. PLoS One. 2012;7:e51348. doi: 10.1371/journal.pone.0051348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sindhurakar A, Bradley NS. Kinematic analysis of overground locomotion in chicks incubated under different light conditions. Dev Psychobiol. 2010;52:802–812. doi: 10.1002/dev.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Buzza HH, Zangirolami AC, Kurachi C, Bagnato VS. Photostimulation effects on chicken egg development: Perspectives on human newborn treatment. J Biophotonics. 2017 doi: 10.1002/jbio.201700046. [DOI] [PubMed] [Google Scholar]
- 113.Harvanek ZM, Mourao MA, Schnell S, Pletcher SD. A computational approach to studying ageing at the individual level. Proc Biol Sci. 2016:283. doi: 10.1098/rspb.2015.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Akhoon BA, Pandey S, Tiwari S, Pandey R. Withanolide A offers neuroprotection, ameliorates stress resistance and prolongs the life expectancy of Caenorhabditis elegans. Exp Gerontol. 2016;78:47–56. doi: 10.1016/j.exger.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 115.Munch D, Baker N, Rasmussen EM, Shah AK, Kreibich CD, Heidem LE, Amdam GV. Obtaining specimens with slowed, accelerated and reversed aging in the honey bee model. J Vis Exp. 2013 doi: 10.3791/50550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zannas AS. Editorial Perspective: Psychological stress and epigenetic aging - what can we learn and how can we prevent? J Child Psychol Psychiatry. 2016;57:674–675. doi: 10.1111/jcpp.12535. [DOI] [PubMed] [Google Scholar]
- 117.Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY) 2018;10:573–591. doi: 10.18632/aging.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amaroli A, Ravera S, Parker S, Panfoli I, Benedicenti A, Benedicenti S. Effect of 808 nm Diode Laser on Swimming Behavior, Food Vacuole Formation and Endogenous ATP Production of Paramecium primaurelia (Protozoa) Photochem Photobiol. 2015;91:1150–1155. doi: 10.1111/php.12486. [DOI] [PubMed] [Google Scholar]
- 119.Amaroli A, Ravera S, Parker S, Panfoli I, Benedicenti A, Benedicenti S. 808-nm laser therapy with a flat-top handpiece photobiomodulates mitochondria activities of Paramecium primaurelia (Protozoa) Lasers Med Sci. 2016;31:741–747. doi: 10.1007/s10103-016-1901-3. [DOI] [PubMed] [Google Scholar]
- 120.Begum R, Calaza K, Kam JH, Salt TE, Hogg C, Jeffery G. Near-infrared light increases ATP, extends lifespan and improves mobility in aged Drosophila melanogaster. Biol Lett. 2015:11. doi: 10.1098/rsbl.2015.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]