Summary
Background
Many RAS family small GTPases are expressed in platelets, including RAC, RHOA, RAP, and H-/N-/RRAS1, but most of their signaling and cellular functions remain poorly understood. Like RRAS1, TC21/RRAS2 reverses HRAS-induced suppression of integrin activation in CHO cells. However, a role for TC21 in platelets has not been explored.
Objectives
To determine TC21 expression in platelets, TC21 activation in response to platelet agonists, and roles of TC21 in platelet function in vitro and in vivo thrombosis.
Results
We demonstrate that TC21 is expressed in human and murine platelets and is activated in response to agonists for the glycoprotein VI (GPVI)/FcRγ immunoreceptor tyrosine-based activation motif (ITAM)-containing collagen receptor, in a Src-dependent manner. GPVI-induced platelet aggregation, integrin αIIbβ3 activation, and α- and dense-granule secretion, as well as phosphorylation of Syk, PLCγ2, AKT, and ERK, were inhibited in TC21-deficient platelets ex vivo. In contrast, these responses were normal in TC21-deficient platelets following stimulation with P2Y, PAR4 and CLEC-2 receptor agonists, indicating that the function of TC21 in platelets is GPVI/FcRγ-ITAM-specific. TC21 was required for GPVI-induced activation of Rap1b. TC21-deficient mice did not show a significant delay in injury-induced thrombosis compared to WT controls; however, thrombi were unstable. Hemostatic responses showed similar effects.
Conclusions
TC21 is essential for GPVI/FcRγ-mediated platelet activation and for thrombus stability in vivo via control of Rap1b and integrins.
Keywords: Blood Platelets, Embolism and Thrombosis, Monomeric GTP-Binding Proteins, Ras Proteins, Receptors, Collagen
Introduction
Platelets rely on fast, highly coordinated cytosolic signal transduction to mediate rapid responses to pro-thrombotic stimuli. These “inside-out” signaling responses lead to activation of αllbβ3 integrin adhesion receptors, which bind fibrinogen, allowing platelets to maintain hemostasis in response to vascular injury on a time scale of seconds to minutes[1]. The RAS family small GTPase Rap1b is a proximal player in integrin activation via recruiting talin[2–9]. Many pathways of Rap1b activation in platelets have been studied; however, the molecular basis of platelet activation remains incompletely understood. Previous studies have shown that other RAS small GTPases may have some functions in platelets, but the RAS isotypes involved in platelet functional responses were not identified[10, 11]. Much of the knowledge regarding RAS small GTPases and integrins comes from over-expression studies. HRAS was found to suppress activation of chimeric, ectopically expressed integrins in CHO cells, whereas RRAS1 and RRAS2/TC21 can reverse suppression by HRAS[7, 8, 12, 13]. Proteomic analysis of palmitoylated proteins demonstrated moderate expression of several Ras isotypes in platelets, including HRAS, NRAS and RRAS1[14].
RRAS2, also known as TC21 and encoded by the RRAS2 gene, shares features with both oncogenic RAS and RRAS sub-groups. RRAS2/TC21 can stimulate the RAL, PI3K and RAF/MEK/ERK pathways, is strongly tumorigenic, and somatic mutations which render it insensitive to inactivation by GAPs are prevalent in many human tumors. TC21 also regulates cell migration and survival, resembling functions of RRAS1[15–19]. TC21, like RRAS1, can also reverse the suppression of activation of chimeric integrins by HRAS[13]. Rras2−/− mice are lymphopenic due to poor survival and homeostatic proliferation of peripheral T and B cells, reflecting a TC21-dependent TCR recycling mechanism: TC21 constitutively associates with the TCR and BCR immunoreceptor tyrosine-based activation motif (ITAM) and recruits PI3K to these sites, required for its activation[20, 21]. Despite these defects, Rras2−/− knockout mice appear grossly normal (viable and fertile), but have a delay in mammary gland development due to a reduction in terminal end buds and ductal branching[20]. We have found that TC21 is highly expressed in murine and human platelets. In this study we have investigated roles for TC21 in inside-out integrin signaling, platelet aggregation, hemostasis and thrombosis.
Materials and methods
Reagents
2MeSADP, sodium citrate, fucoidan, acetyl salicylic acid, apyrase (type VII), and bovine serum albumin (fraction V) were purchased from Sigma. AYPGKF was custom synthesized by Invitrogen. CRP was purchased from Dr. Richard Farndale at the University of Cambridge. Convulxin was purified as described[22]. Type I collagen was from Chrono-log (Havertown PA). Phycoerythrin-conjugated JON/A antibodies, FITC conjugated anti-P-selectin antibodies, and FITC-conjugated anti-GPVI antibodies were from Emfret Analytics (Wurzburg, Germany). Mouse anti-TC21 antibodies were purchased from Abnova (Walnut, CA). OXSI-2 (2,3-dihydro-3-[(1-methyl-1H-indol-3-yl) methylene]-2-oxo-1H-indole-5-sulfonamide) was from Tocris Biosciences (Minneapolis, MN). IV.3 [CD32] antibodies were purchased from Stemcell Technologies (Cambridge, MA) and goat anti-mouse IgG (Fab′2) antibodies were from Santa Cruz Biotechnologies (Santa Cruz, CA). FcR gamma chain antibodies were from Sigma (St. Louis, MO). Anti-phospho-Syk (Tyr525/Tyr526), anti-phospho-PLCγ2 (Tyr 759), anti-phospho-AKT (Ser473), anti-phospho-ERK (Thr202/Tyr204), and anti-β-actin antibodies were purchased from Cell Signaling Technology (Beverly, MA). PP2 and PP3 were from Enzo Life Sciences. Human fibrinogen was from Enzyme Research Laboratories. PPACK (Enzo Life Sciences, Farmingdale, NY) was from Glycotech (Gaithersburg, MD). RAF-1 and RALGDS GST-RBD proteins were prepared as described[23, 24]. All other reagents were reagent-grade, and deionized water was used throughout.
Animals
Rras2−/− mice were a gift of Balbino Alarcón, Universidad Autónoma de Madrid, Madrid, Spain. These mice were backcrossed 10 times against a C57Bl/6 background. WT C57Bl/6 mice were purchased from Charles River Laboratories. All mice were maintained housed in a pathogen-free facility, and all animal procedures were approved by the Temple University Institutional Animal Care and Use Committee. Age- and gender-matched wild type animals were used as controls.
Preparation of human and murine platelets
Human and murine platelets were isolated from blood as described[25]. Approval was obtained from the institutional review board of Temple University. Informed consent was provided prior to blood donation, in accordance with the Declaration of Helsinki.
Platelet aggregation and secretion
Platelet ATP release and aggregation were performed simultaneously using a lumi-aggregometer as described[25].
Flow cytometry
Washed murine platelets were labeled with phycoerythrin-conjugated JON/A antibodies or FITC-conjugated α-P-selectin antibodies and detected as described[26].
Western blotting
Platelets were stimulated with agonists for the appropriate time, and protein levels and phosphorylation events were assessed as described[27].
GTP-TC21 and GTP-Rap1b pull down assays
Washed platelets (0.5 ml, 2 × 109/ml) were stimulated with different agonists in an aggregometer and reactions were stopped by adding 250 μL of 3X HEPES lysis buffer (55 mM HEPES, 174 mM NaCl, 30% glycerol, 6 mM EGTA, 6 mM MgCl2, 3% NP40, pH 7.5) with 10 μM GTP and 20 μg GST-RBD of RAF-1 or RALGDS[23, 24]. Platelet lysates were clarified by centrifugation at 13000 × g for 5 min at 4°C. 50 μL supernatants were separated for whole cell lysate samples, and 40 μL glutathione-agarose beads were added to the remaining supernatant and kept on a rocker for 2 h at 4°C. Bead-bound complexes were washed 3 times with 1X HEPES wash containing 10 μM GTP, eluted with SDS sample buffer, and analyzed by Western blotting.
Immunofluorescence microscopy
Platelet spreading and clot retraction
These experiments were performed as described[25].
FeCl3–induced in vivo thrombosis and bleeding times
Adult mice (10–12 weeks old) were anesthetized by intraperitoneal injection of pentobarbital (40 mg/kg), and FeCl3-induced thrombosis was assessed as described[30]. Bleeding times following tail clip were assessed in 6–8-week-old male and female mice as described[30].
Blood flow over collagen
Blood was collected from 12–14-week-old mice via cardiac puncture into tubes containing 400 mM PPACK and 5 U/ml heparin as anti-coagulant. Blood was flowed over surfaces coated with 50 μg/ml type I collagen at 500 sec−1 for 3 min using a parallel plate flow chamber kit from Glycotech. The chamber was flushed with PBS and images were obtained using a Nikon Eclipse TE300 at 400X magnification. Thrombus area was quantified using ImageJ. At least 5 randomly chosen images were used for analysis per experiment.
Statistical analysis
Each experiment was performed at least 3 times unless otherwise indicated. All statistical tests were carried out using Prism software (version 3.0). Data are presented as means + s.e.m. Statistical significance was determined by Student’s t test and analysis of variance.
Results
Expression of the small GTPase TC21 in platelets and involvement in GPVI-induced platelet functional responses
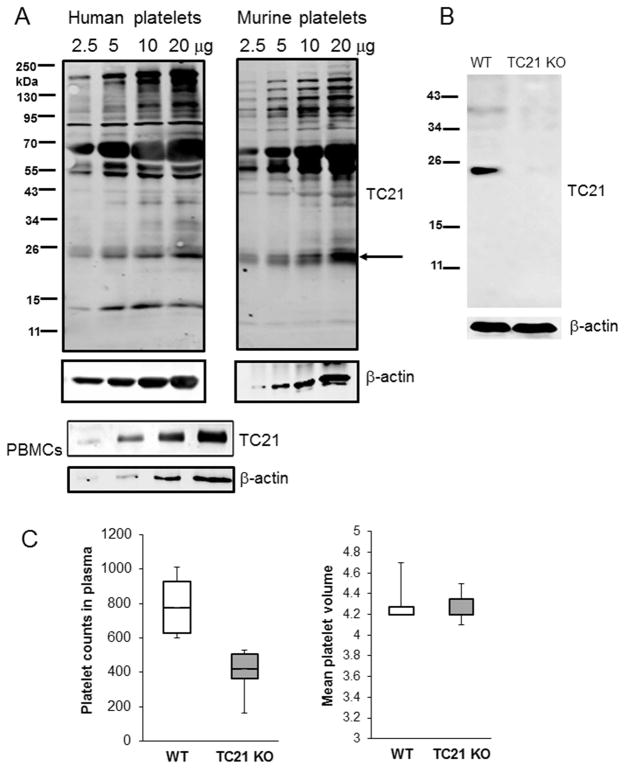
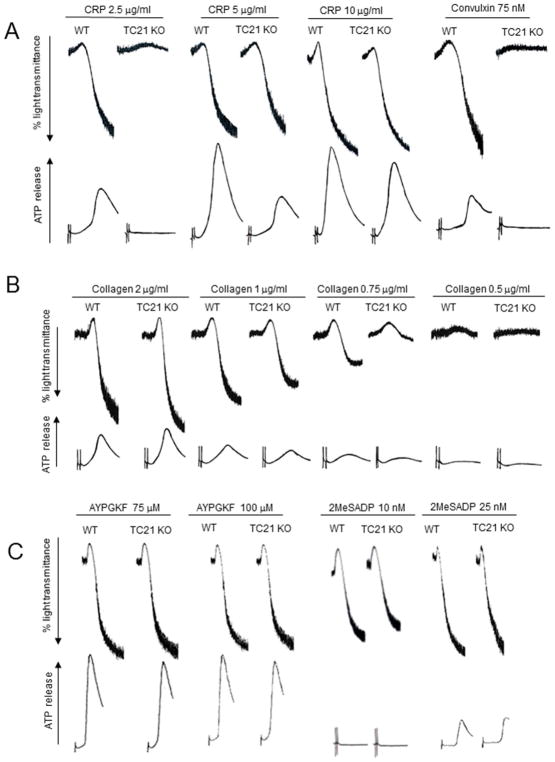
Early studies showed that “RAS” (isotype not specified) can be activated in platelets, and RAS proteins appear to be important for platelet functions[10, 11, 31]. RRAS1 is also expressed in human platelets[14], but its functions are unclear. Western blot analysis with TC21 mouse monoclonal antibodies confirmed prior proteomic studies[32], and demonstrated that TC21 is expressed in both human and murine platelets, at levels similar to peripheral blood mononuclear cells, in which TC21 plays important function roles[21, 33, 34] (Fig. 1A). There are currently no selective pharmacological inhibitors of TC21. To evaluate the functions of TC21 in platelets, we investigated agonist-induced platelet responses ex vivo in platelets isolated from TC21-null (Rras2−/−) mice[21]. TC21 is expressed in wild type (WT) murine platelets but not in TC21-null murine platelets (Fig. 1B). TC21-null mice had decreased circulating platelet counts relative to WT, indicating potential defects in either platelet production or platelet lifespan; however, mean platelet volumes were in the normal range (Fig. 1C). Ex vivo platelet aggregation and dense granule secretion induced by the glycoprotein VI (GPVI)-specific agonists collagen-related peptide (CRP) or convulxin were inhibited in TC21-null platelets compared with WT platelets (Fig. 2A). These defects were restored with higher concentrations of CRP, suggesting compensatory mechanisms in the absence of TC21 with higher doses of agonist. Similarly, collagen-induced aggregation and dense granule secretion were inhibited with low concentrations of agonist (0.5 – 0.75 μg/ml), but not at higher concentrations (Fig. 2B). However, platelet aggregation and secretion in response to the PAR4-activating peptide, AYPGKF or P2Y receptor agonist, 2MeSADP were similar in TC21-null and WT platelets (Fig. 2C), indicating that G protein-coupled receptor agonists induce normal platelet aggregation and secretion in the absence of TC21. Thus, TC21 is required for platelet aggregation and dense granule secretion downstream of GPVI receptor pathways.
Fig. 1. Expression of TC21/RRas2 in human and murine platelets.
(A) Whole cell lysates (WCL) of washed human (2×108/ml) and murine platelets (1.5×108/ml) and PBMCs were separated by SDS-PAGE and immunoblotted with TC21 antibodies. HEMAVET analysis showed no detectable levels of leukocytes or erythrocytes. (B) Western blot of WCL samples from wild type (WT) and TC21-null (TC21 KO) C57Bl/6 mouse platelets, using TC21 antibodies. HEMAVET analysis showed no detectable levels of leukocytes or erythrocytes. (C) Platelet counts in plasma (left) and mean platelet volume (right) were assessed by HEMAVET analysis. n = 6.
Fig. 2. TC21 is required for GPVI-induced platelet aggregation and dense granule secretion.
TC21-null (TC21 KO) and wild type (WT) murine platelets isolated from whole blood were re-suspended in phosphate buffer and apyrase at physiological pH and placed under stirring conditions in a lumiaggregometer in the presence of luciferin-luciferase reagent, then stimulated with CRP (2.5, 5, 10 μg/ml) and convulxin (75 nM) (A), Collagen (0.5, 0.75, 1, 2 μg/ml) (B), or AYPGKF (75, 100 μM) and 2MeSADP (25 nM) (C) for 3.5 min. Representative aggregation (top) and dense granule secretion (bottom) tracings from three independent experiments are shown.
TC21 regulates platelet GPVI-mediated integrin activation and α-granule secretion
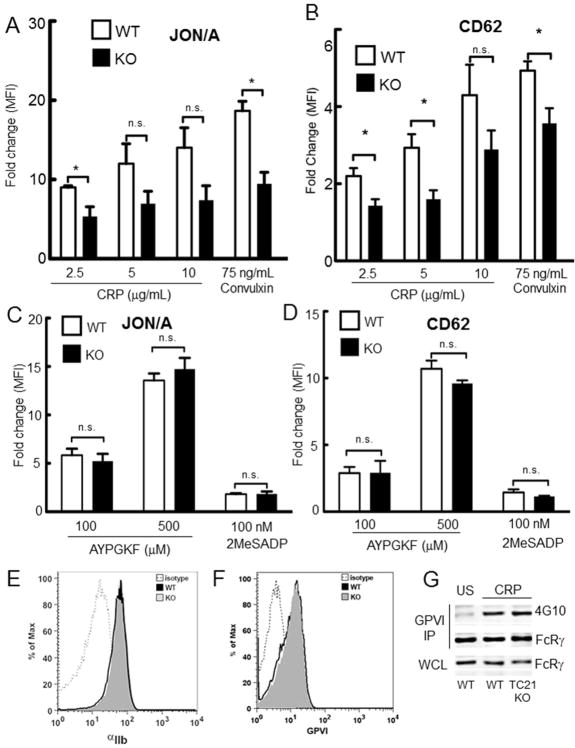
Platelet aggregation results from the activation of integrin αIIbβ3, mediated by inside-out signaling downstream of ligation of signaling receptors[1]. We analyzed the agonist-induced activation of integrin αIIbβ3 by measuring binding of the conformation-dependent antibody JON/A to stimulated platelets using flow cytometry[35]. Consistent with a defect in GPVI-mediated platelet aggregation, we found that JON/A binding induced by different concentrations of CRP and convulxin was significantly inhibited in TC21-null platelets compared with WT platelets (Fig. 3A). Thus, TC21 is required for GPVI-mediated inside-out signaling leading to integrin activation and platelet aggregation. P-selectin surface expression induced by CRP and convulxin was also significantly blocked in TC21-null platelets (Fig. 3B), indicating defects in α-granule secretion [36]. However, at both low and high concentrations, AYPGKF- and 2MeSADP-induced JON/A binding and P-selectin expression in TC21-null platelets were similar to WT platelets (Fig. 3C,D), indicating that G protein-coupled receptor agonists cause integrin αIIbβ3 activation and α-granule secretion independently of TC21. Surface expression of integrin αIIbβ3 and GPVI in TC21-null platelets were comparable to WT (Fig. 3E, F). Expression of FcRγ chain was also similar to WT, and FcRγ chain phosphorylation in response to platelet stimulation with CRP was also similar to WT (Fig. 3G). Thus, TC21 is selectively required for GPVI-mediated activation of αIIbβ3 integrin, dense and α-granule secretion, and aggregation responses in platelets.
Fig. 3. TC21 is required for GPVI-induced platelet αIIbβ3 integrin activation and α-granule secretion.
Washed platelets from TC21-null (KO) and wild type (WT) murine platelets were stimulated with the indicated doses of CRP or convulxin (A,B), or AYPGKF or 2MeSADP (C,D) for 15 min in the presence of phycoerythrin-labeled JON/A antibodies (A, C) or FITC-labeled α-P-selectin antibodies (CD62) (B,D) and labeling was assessed by flow cytometry. Mean fluorescence intensities (MFI) are shown as fold change from unstimulated control, and are representative data from three independent experiments, + s.e.m. *, p < 0.05; n.s, not significant. (E, F) Washed platelets were labeled with antibodies to GPVI (E) or αIIb integrin (F) and fluorescence intensities were detected by FACS. (G) Washed platelets from TC21-null and WT mice were either kept unstimulated (US) or treated with 2.5 μg/ml CRP for 60 sec, lysed, and subject to immunoprecipitation (IP) with α-GPVI antibodies. IP fractions were separated by SDS-PAGE and western blotting with the indicated antibodies. The FcRγ chain band migrated at ~14 kDa.
Inhibition of GPVI-mediated downstream signaling events in platelets in the absence of TC21
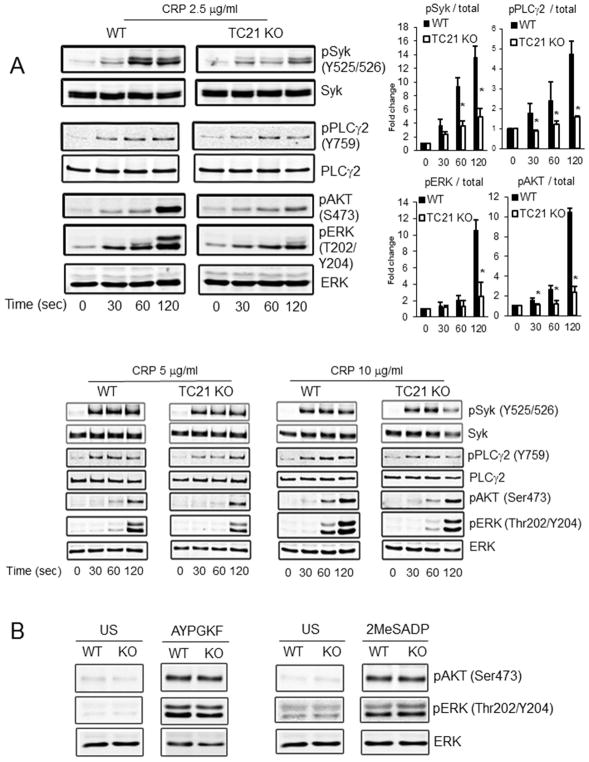
Many signaling players in the GPVI integrin activation pathway have been investigated in platelets[37, 38]. To determine whether TC21 regulates these signaling events, we assessed GPVI-mediated signaling in lysates of TC21-null or WT platelets stimulated with GPVI agonists, by immunoblotting with phospho-specific antibodies: Syk (Y525/526), PLCγ2 (Y759), AKT (S473) and ERK1/2 (T202/Y204). CRP treatment of WT platelets at 2.5 μg/ml caused a rapid signaling response including phosphorylation of each of these targets. In contrast, CRP-induced phosphorylation of Syk, PLCγ2, AKT and ERK1/2 were all reduced in TC21-null platelets (Fig. 4A). Similar results were observed with CRP stimulation at 5 μg/ml, with repression of GPVI signaling responses in TC21-null platelets. However, at 10 μg/ml CRP stimulation, GPVI signaling responses were similar between TC21-null and WT platelets (Fig. 4A). When platelets from TC21-null mice were stimulated with AYPGKF or 2MeSADP, phosphorylation of AKT and ERK were comparable to those in WT platelets (Fig. 4B). These results demonstrate that TC21 regulates GPVI downstream signaling responses, and suggest a receptor-proximal role, upstream of Syk, for TC21 in GPVI-mediated signaling phosphorylation events.
Fig. 4. GPVI-mediated downstream signaling events are inhibited in TC21-null platelets.
Washed platelets isolated from TC21-null (TC21 KO) and WT mice were stimulated for the indicated times with CRP (A) at the indicated concentrations or (B) AYPGKF (100 μM) or 2MeSADP (25 nM) (60 sec each), lysed, and lysates were immunoblotted with antibodies as shown. US, unstimulated. Phospho-protein/total protein ratios from six independent experiments are shown to the right in panel A for 2.5 μg/ml CRP treatment. Representative blots are shown. *, p < 0.03.
TC21 activation induced by GPVI receptor agonists requires Src kinases
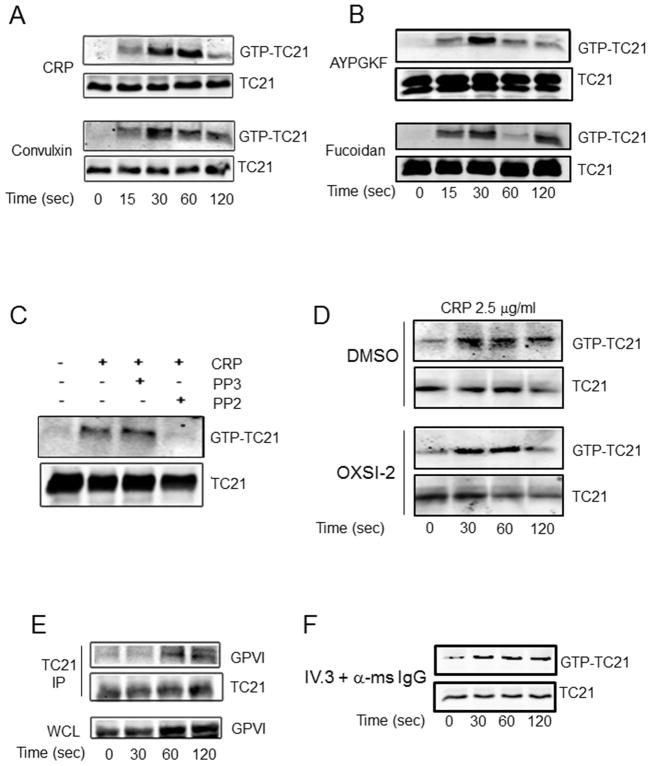
The above results demonstrate a requirement for TC21 expression in GPVI signaling in platelets, and suggest that the molecular function of TC21 may be an important factor in these responses. Therefore we assessed TC21 activation in human platelets following GPVI agonist stimulation, using a standard pull-down assay for GTP-bound RAS proteins, employing the RAS-binding domain of RAF-1 fused to GST (GST-RBD) as bait bound to glutathione-sepharose beads[23]. This GST fusion protein is predicted to bind to activated, GTP-bound TC21[15, 19], such that immunoblotting of the precipitated fractions indicates a ratio of activated TC21 compared with total from the whole platelet lysate. TC21 was precipitated by the GST-RBD in lysates from human platelets stimulated with CRP and convulxin (GPVI agonists), but not from unstimulated platelets, confirming the utility of this approach for assessing TC21 cellular activation in platelets. CRP and convulxin – structurally distinct GPVI agonists – each caused rapid activation of TC21 in human platelets, as early as 30 sec and persisting for up to 120 sec (Fig. 5A). Thus, GPVI signaling activates TC21 in platelets. Interestingly, TC21 was also activated rapidly upon platelet stimulation with PAR4 agonist AYPGKF, and CLEC-2 agonist fucoidan (Fig. 5B).
Fig. 5. TC21 activation by GPVI receptor agonists downstream of Src kinases.
Washed platelets were stimulated with 2.5 μg/ml CRP or 75 ng/ml CVX (A), or 500 μM AYPGKF or 100 μg/ml fucoidan (B) as shown, lysed, and GTP-bound TC21 (upper panels) was extracted from platelet lysates (lower panels, total TC21) using GST-RAF-1-RBD coupled to Gluthatione-sepharose beads. (C) TC21 activation induced by 5 μg/ml CRP for 60 sec was evaluated in the presence of 10 μM PP2 or PP3 as indicated. (D) Washed platelets were incubated with 1 μm OXSI-2 or DMSO vehicle for 5 min, then stimulated with CRP as shown and evaluated for total and GTP-bound TC21 as in (A). (E) TC21 was immunoprecipitated from IgG-sepharose pre-cleared lysates of GPVI-stimulated platelets, with 2 μg of α-TC21 antibodies. Washed immunoprecipitate fractions were subject to western blotting with α-TC21 and α-GPVI antibodies as indicated. (F) FcγRIIa was cross-linked with 2.5 μg/ml IV.3 antibodies followed by 50 μg/ml anti-mouse IgG(F(ab)) for the indicated times. GTP-bound, activated TC21 was precipitated from platelet lysates and detected by western blotting as above. All data representative of three independent experiments each.
Although signaling through multiple receptors activated TC21 in platelets, GPVI platelet response was selectively impaired by TC21 deletion (Figs. 2–4). As GPVI-coupled Fc receptors harbor ITAM motifs subject to tyrosine phosphorylation by Src family kinases (SFKs), leading to downstream signaling events[37, 38], we investigated whether TC21 activation by GPVI requires SFK activation, using the SFK inhibitor, PP2, and control analogue, PP3. TC21 activation by CRP was completely blocked in the presence of PP2, but not PP3, demonstrating that GPVI-FcRγ–mediated TC21 activation requires SFK activity (Fig. 5C). We evaluated CRP-induced TC21 activation in murine platelets and found similar activation profiles as in human platelets (Fig. 5D, left panels, cf. Fig. 5A). TC21 was upstream of Syk activation induced by GPVI stimulation (Fig. 4); however, TC21 could also be downstream of Syk in a putative feedback loop. Therefore, we tested CRP-induced TC21 activation under conditions of Syk inhibition in WT murine platelets, and found that TC21 activation was unaffected by OXSI-2 Syk inhibitor (Fig. 5D). Thus, TC21 is upstream of Syk in the GPVI pathway and is not subject to negative feedback effects. However, negative feedback downstream of Syk at later time points could not be ruled out. Based on the ability of TC21 to interact with ITAM receptors in other cells, we considered whether TC21 may associate with the GPVI/FcRγ complex. GPVI was detected in TC21 immunoprecipitate fractions after CRP stimulation, indicating that TC21 physically associates with the GPVI complex upon receptor ligation (Fig. 5E). To investigate further a role for TC21 in ITAM signaling in platelets, we assessed TC21 activation following crosslinking of the FcγRIIa receptor, which is not expressed in murine platelets. This ITAM receptor drives platelet signaling through similar pathways as GPVI/FcRγ, to affect aggregation and thrombus formation[39, 40]. Cross-linking FcγRIIa in human platelets induced rapid activation of TC21 (Fig. 5F), suggesting a conserved ITAM-mediated mechanism for TC21 activation in platelets.
TC21 is required for GPVI-induced Rap1b activation in platelets
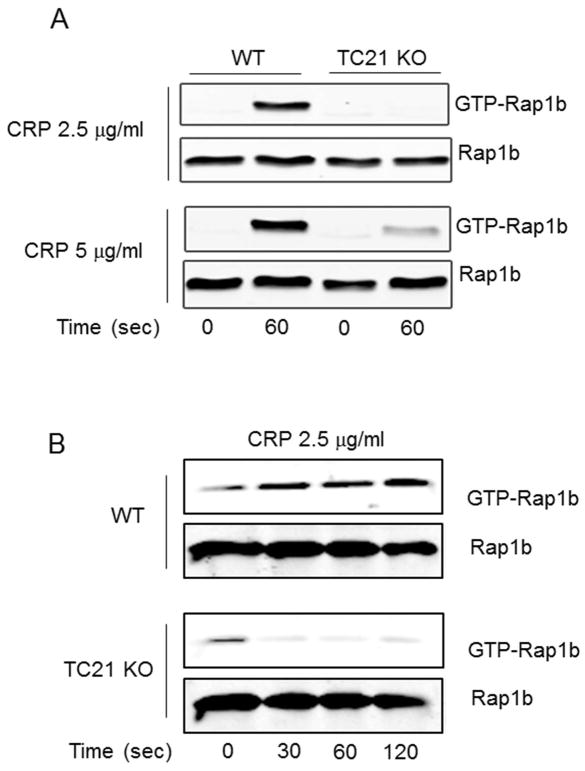
The RAS family small GTPase Rap1b plays an essential, proximal role in the activation of αIIbβ3 integrin in platelets, by stimulating the recruitment of talin and co-factors to the integrin tails[9]. As shown in Fig. 6, Rap1b activation induced by CRP was abolished in TC21-null platelets, compared to robust Rap1b activation in WT platelets. Thus, TC21 regulates GPVI-mediated integrin activation and platelet activation through crosstalk signaling required for activation of Rap1b.
Fig. 6. TC21 is required for GPVI-mediated Rap1b activation.
GTP-Rap1b pulldown (upper panels) with GST-RALGDS-RBD from lysates (total Rap1b, lower panels) of WT or TC21-null (KO) platelets stimulated with CRP at the indicated concentrations and for the indicated times. (A) 60 sec stimulation with 2.5 or 5 μg/ml CRP; (B) time course with 2.5 μg/ml CRP.
CLEC-2-mediated platelet aggregation and downstream signaling events in TC21-deficient platelets
To confirm that the block in integrin-mediated platelet aggregation and secretion responses in the TC21-null platelets was GPVI-specific, we assessed aggregation and secretion in response to fucoidan, a sulfated polysaccharide which stimulates platelet activation via Syk and PLCγ2, but through the CLEC-2 receptor, which harbors a hemi-ITAM motif[41], independently from the GPVI/FcRγ receptor complex at low concentrations[42]. Fucoidan-induced platelet aggregation, αIIbβ3 integrin activation, and α-granule secretion were normal in both WT and TC21-null platelets (Fig. S1A–C). In addition, fucoidan treatment induced Syk, PLCγ2, AKT and ERK1/2 phosphorylation in TC21-null platelets to similar levels as in WT platelets (Fig. S1D). These results indicate that TC21 is not important for platelet aggregation or Syk and AKT phosphorylation downstream of the CLEC-2 receptor, and confirm that TC21 selectively functions downstream of the GPVI/FcRγ receptor complex in platelet activation.
Regulation of hemostasis and thrombosis in vitro and in vivo by TC21
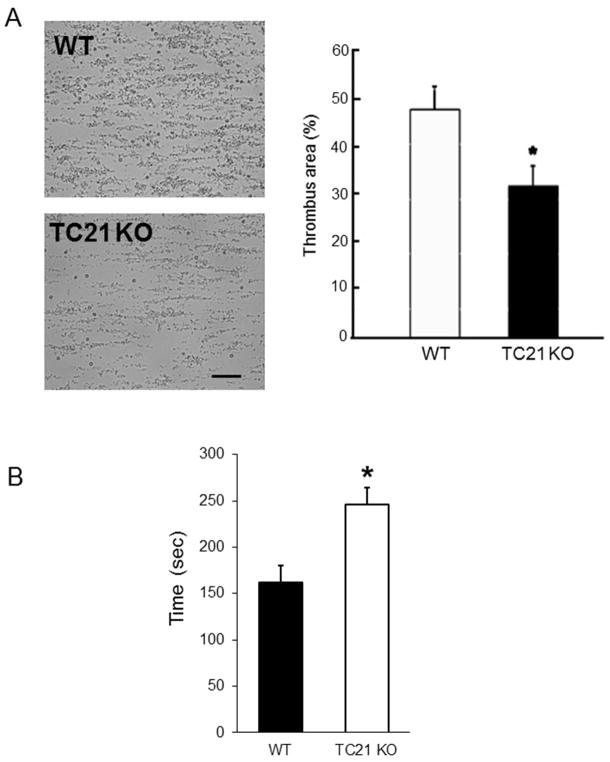
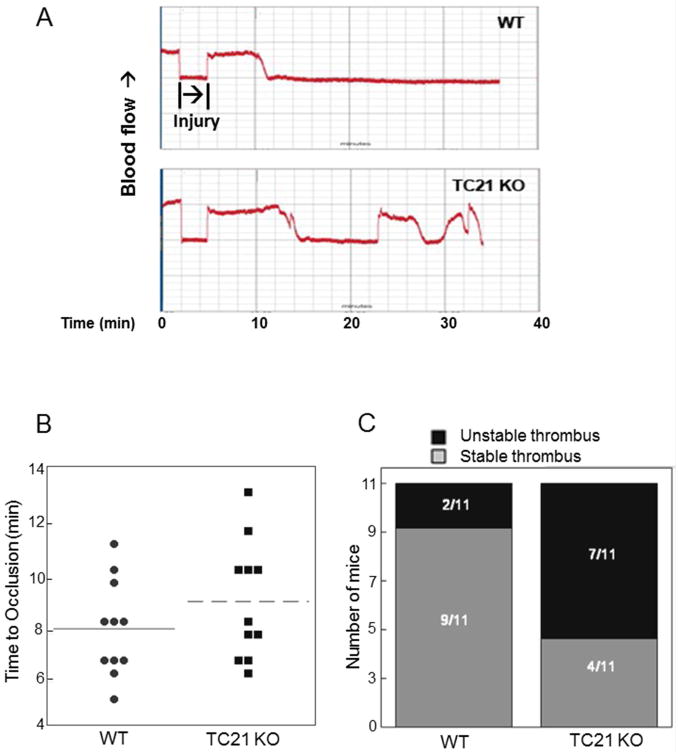
We next assessed the contributions of TC21 to thrombus formation and stability in vitro and in vivo. We first observed thrombus formation in vitro in whole blood flowing over collagen-coated surfaces. As shown in Fig. 7A, thrombus formation over collagen-coated surfaces was significantly decreased in blood from TC21-null mice compared to WT. Based on these results, we investigated putative roles for TC21 in hemostasis and injury-induced thrombus formation and stability in vivo. We first measured bleeding times following tail clip, and found significantly increased bleeding times in TC21 KO mice (Fig. 7B). We measured time to occlusion in the carotid artery following 7.5% ferric chloride-induced injury for 90 sec, in TC21-null and WT mice. As shown in Fig. 8A, in vivo thrombosis at the site of injury appeared slightly delayed, as observed by increased time to occlusion, in TC21-null compared to WT mice. The average time to occlusion after injury in TC21-deficient mice was 10.5 min, compared to 8 min in WT mice (Fig. 8B). However, this delay in initial time to occlusion was not significant. In contrast, embolization of the thrombi was observed in 64% of TC21-null mice, compared with 18% for WT mice (n = 11), indicating unstable thrombi after arterial injury in these mice (Fig. 8C). This result is consistent with prolonged bleeding in the tail clip thrombosis model in TC21-null mice. Thus, the small GTPase TC21 is required in platelets for rapid thrombus formation and maintenance of thrombus stability in response to arterial injury in mice.
Fig. 7. TC21 regulates thrombus formation over collagen surfaces and in vivo hemostasis.
(A) Representative images of thrombus formation on collagen surfaces in blood from WT and TC21-null (TC21 KO) mice in a flow chamber. Percentages of the imaging area covered by platelets are shown to the right, + s.e.m. *, p < 0.05. (n=5). Bar, 50 μm. (B) Bleeding times following tail clip in WT and TC21-null mice. *, p < 0.002. (n = 20).
Fig. 8. Delay and instability of in vivo arterial thrombus formation in TC21-null mice.
(A) Representative Doppler tracings of blood flow in the carotid artery following FeCl3-induced injury in TC21-null (TC21 KO) and WT mice. (B) Time to occlusion and (C) stability of thrombus formation as determined by lack of restored blood flow in Doppler tracings, n = 11 each.
TC21 effects in outside-in signaling in platelets
To obtain a broader picture of the contributions of TC21 to platelet responses, we analyzed platelet spreading on immobilized fibrinogen and thrombin-induced clot retraction ex vivo. TC21-null platelets spread to a similar degree as WT platelets (Fig. S2A). Similarly, thrombin-induced clot retraction with TC21-deficient platelets proceeded at the same rate as with WT platelets ex vivo (Fig. S2B). Thus, TC21 is not required for outside-in signaling in platelets. Fibrin has also been shown to activate GPVI by direct binding to the receptor[43, 44]; however, we did not observe differences in spreading on fibrin between WT and TC21-null platelets, whereas TC21-null platelets did not spread efficiently on collagen or CRP compared to WT (Fig. S3), indicating that fibrin signaling in platelets does not require TC21.
Discussion
This study is the first to demonstrate a functional role for a proto-oncogenic RAS small GTPase - TC21/RRAS2 - in platelet functional responses, hemostasis and thrombosis. TC21-deficient mice showed prolonged times to cessation of bleeding and a tendency to rebleed in a tail clip hemostasis model, reduced platelet deposition on collagen surfaces in whole blood under flow, as well as thrombus embolization in the carotid artery following injury, indicating a role for TC21 in both thrombus formation and thrombus stability. Thrombus instability was due to a defect in activation of αIIbβ3 integrins in TC21-deficient platelets downstream of impaired platelet signaling responses. TC21-dependent platelet responses were selective for the GPVI/FcRγ collagen receptor, and TC21 was activated by GPVI stimulation upstream of Syk, pointing to TC21 activation as a receptor-proximal event. TC21 was also required for downstream GPVI-mediated Rap1b activation, demonstrating RAS GTPase crosstalk and a RAS/Rap1b linkage in GPVI-mediated integrin activation in platelets.
Prolonged bleeding in TC21 knockout mice in a tail clip hemostasis model, and thrombus embolization in a vascular thrombosis model, together suggest that TC21 plays an important role in the strength of platelet adhesion and aggregation. This was supported by a defect in TC21-deficient platelet thrombus formation in vitro in blood flowing over collagen surfaces. In addition, decreased platelet count in TC21-deleted mice may also contribute to impaired thrombosis and hemostasis. Many hereditary platelet functional disorders such as Bernard-Soulier syndrome, von Willebrand disease and Glanzmann’s thrombasthenia are the result of compromised platelet adhesive capacity, leading to complications of either spontaneous bleeding or thrombosis defects[45–48]. Thus, it is conceivable that somatic or inherited mutations in TC21 may correlate with bleeding tendencies in humans, but this merits further investigation.
The role of TC21 in platelet aggregation and thrombus stability correlated with a role in activation of the αIIbβ3 integrin fibrinogen receptor, indicating that TC21-mediated thrombus stability is due to a requirement for TC21 in platelet integrin activation. RAS family proteins including HRAS, RRAS1 and TC21 have been associated with regulation of integrin activation in other contexts, and these studies indicated a positive role for TC21 in integrin activation[12, 13, 49]. However, these studies represented ectopic expression of both integrins and RAS proteins in heterologous cells. Thus, an integrin regulatory function for RAS proteins had not previously been recognized in platelets. Many studies have characterized upstream regulators of RAS as playing roles in platelet integrin activation and function, including CALDAG-GEFs which are important for platelet adhesion and aggregation[50–54], as well as an early report identifying the GRB2 RAS activation complex in these processes[55]. However, many of these GEF proteins also stimulate activation of Rap1, an integrin-proximal player essential for integrin activation[9, 56]. Hence, a direct role for proto-oncogenic RAS in platelet integrin activation was not demonstrated. To our knowledge this study is the first to demonstrate a functional role for a RAS protein (other than Rap1), TC21, in regulation of native integrins in platelets, which is required for thrombus stability in vitro and in vivo.
TC21 signaling leading to integrin activation in platelets was selectively downstream of the GPVI collagen receptor, and TC21 appears to propagate signaling proximal to the receptor. TC21 was activated by GPVI agonists (CRP and convulxin), indicating receptor-mediated stimulation of a RasGEF promoting GTP loading of TC21. The notion of TC21 as a receptor-proximal signal transducer is consistent with our findings that GPVI-induced TC21 activation required Src family kinase activity, and was upstream of PLCγ2, AKT, and ERK[37, 57–59]. TC21 was activated by GPVI stimulation under conditions of Syk inhibition, placing TC21 upstream of Syk with minimal negative feedback signaling from Syk to TC21. Moreover, TC21 associated with GPVI following CRP agonist stimulation, indicating that TC21 forms a molecular complex with GPVI upon ligation. Thus, a GPVI→Src→TC21→Syk pathway regulates platelet activation. However, precisely how TC21 functions upstream of Syk in a Src-dependent manner remains to be fully elucidated. Fibrin can activate GPVI by direct binding[43, 44]; however, the platelet signaling responses of this interaction are poorly understood. We did not observe fibrin-dependent spreading or clotting defects in TC21-null platelets, suggesting that collagen-mediated GPVI signaling through TC21 represents a distinct pathway from fibrin-GPVI signaling. As TC21 was also activated by AYPGKF and fucoidan, it appears that TC21 can be activated by multiple upstream signaling pathways in platelets. Furthermore TC21 was also activated in human platelets by crosslinking the FcγRIIa receptor, which is similar to the GPVI/FcRγ receptor, signaling via ITAM motifs in the Fc chains. This pathway has parallels to roles of TC21 in lymphocytes, where TC21 associates with the BCR (B cells) and TCR (T cells) via interaction with ITAM motifs in each receptor, both of which propagate signaling through Syk pathways[21, 33, 60, 61]. The GPVI/FcRγ receptor complex also contains ITAM motifs (in the FcRγ chain)[38, 62], suggesting a possible conserved mechanism of TC21-ITAM motif association in receptor signaling in hematopoietic cells. Many RAS family GEFs stimulate RAS activation as a result of recruitment to receptor phosphotyrosines, typically via adapter proteins - such a mechanism has been shown to connect the cell adhesion receptor CEACAM3 with activation of RAC via direct binding of the RACGEF, VAV, to phosphotyrosines in the receptor ITAM[56, 63] - suggesting the potential for a conserved mechanism of ITAM-GEF-RAS complex formation. However, Syk is generally considered to bind directly to ITAM motifs following phosphorylation by Src family kinases in many cells including platelets[64, 65]; thus, the role of TC21 in mediating this connection is unknown. Which GEFs mediate GPVI-induced TC21 activation in platelets, and their mechanism of action, also remain to be determined.
Our results demonstrate cross talk between two RAS GTPases, TC21/RRAS2 and Rap1b, in platelet signaling and functional responses. TC21 was required for GPVI-mediated Rap1b activation, which is a requisite downstream signaling event leading to αIIbβ3 integrin activation in platelets, as well as for activation of other integrins through a common mechanism[9, 51]. Thus, we predict that in the absence of TC21, talin recruitment to integrin tails is compromised, preventing allosteric activation of the integrin ligand-binding domain[9, 66, 67]. The molecular mechanisms by which TC21 regulates Rap1b activation in platelets remain to be explored. However, TC21 may be involved in integrin regulation in other contexts with varied physiological effects, e.g., Schwann cell migration and tumor metastasis, which have been associated with increased TC21 activity[68–71].
Recent interest in hemostasis and thrombosis research has been focused on identifying pharmacological targets such as regulators of integrin activation, which strike a balance between minimal bleeding events and maintenance of hemostasis[72, 73]. GPVI-deficient mice do not demonstrate impaired hemostasis[74]; however, the hemostasis defect observed in TC21-deficient mice may also be due to lower platelet count. Deficiency of TC21 is compatible with life, suggesting that targeting a TC21 pathway in platelets may have few unwanted effects. Thus, the TC21 pathway may be a viable target for intervention in pathological conditions driven by thrombus instability, such as atherosclerosis and stroke.
Supplementary Material
Essentials.
RAS proteins are expressed in platelets but their functions are largely uncharacterized.
TC21/RRas2 is required for glycoprotein VI (GPVI)-induced platelet responses and for thrombus stability in vivo.
TC21 regulates platelet aggregation by control of αIIbβ3 integrin activation, via crosstalk with Rap1b.
This is the first indication of functional importance of a proto-oncogenic RAS protein in platelets.
Acknowledgments
We thank Balbino Alarcón, Universidad Autónoma de Madrid, Madrid, Spain, for providing the Rras2−/− mice. We thank Xiaoxuan Fan for technical assistance. The project was supported by NIH grants HL137207 to L. Goldfinger, HL93231, HL137207 and HL132171 to S. Kunapuli, HL114405 and GM105671 to M. Holinstat, and AHA grants 16GRNT27260319 to L. Goldfinger and 12SDG8980013 to S. Kim.
The abbreviations used are
- CLEC-2
C-type lectin receptor 2
- CRP
collagen-related peptide
- GPVI
glycoprotein VI
- GST
glutathione-S-transferase
- ITAM
immunoreceptor tyrosine-based activation motif
- PAR
protease-activated receptor
- SFK
Src family kinase
Footnotes
Addendum
L. Goldfinger and S. Kunapuli contributed to the conception and design of the work. S. Janapati, C. Dangelmaier, J. Wurtzel, B. Manne, D. Bhavanasi, J. Kostyak, and S. Kim performed experiments and acquired data. L. Goldfinger, S. Kim, M. Holinstat and S. Kunapuli were involved in the analysis and interpretation of data. M. Holinstat provided key reagents. S. Kunapuli and L. Goldfinger supervised the experiments and analysis. S. Janapati and L. Goldfinger wrote the manuscript.
Disclosure of Conflict of Interests
The authors state that they have no conflicts of interest.
References
- 1.Plow EF, Ginsberg MH, Furie B, Shattil SJ. Hematology:Basic Principles and Practice. Churchill Livingstone Inc; 1995. The molecular basis of platelet function; pp. 1524–35. [Google Scholar]
- 2.Katagiri K, Hattori M, Minato N, Irie S, Takatsu K, Kinashi T. Rap1 is a potent activation signal for leukocyte function-associated antigen 1 distinct from protein kinase C and phosphatidylinositol-3-OH kinase. Mol Cell Biol. 2000;20:1956–69. doi: 10.1128/mcb.20.6.1956-1969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reedquist KA, Ross E, Koop EA, Wolthuis RM, Zwartkruis FJ, van Kooyk Y, Salmon M, Buckley CD, Bos JL. The small GTPase, Rap1, mediates CD31-induced integrin adhesion. Journal of Cell Biology. 2000;148:1151–8. doi: 10.1083/jcb.148.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai A, Nosaka Y, Kanda E, Yamamoto K, Miyasaka N, Miura O. Rap1 is activated by erythropoietin or interleukin-3 and is involved in regulation of beta1 integrin-mediated hematopoietic cell adhesion. Journal of Biological Chemistry. 2001;276:10453–62. doi: 10.1074/jbc.M004627200. [DOI] [PubMed] [Google Scholar]
- 5.de Bruyn KM, Rangarajan S, Reedquist KA, Figdor CG, Bos JL. The small GTPase Rap1 is required for Mn(2+)- and antibody-induced LFA- 1- and VLA-4-mediated cell adhesion. Journal of Biological Chemistry. 2002;277:29468–76. doi: 10.1074/jbc.M204990200. [DOI] [PubMed] [Google Scholar]
- 6.Sebzda E, Bracke M, Tugal T, Hogg N, Cantrell DA. Rap1A positively regulates T cells via integrin activation rather than inhibiting lymphocyte signaling. Nat Immunol. 2002;3:251–8. doi: 10.1038/ni765. [DOI] [PubMed] [Google Scholar]
- 7.Hughes PE, Oertli B, Han J, Ginsberg MH. R-Ras Regulation of Integrin Function. Methods Enzymol. 2001;333:163–71. doi: 10.1016/s0076-6879(01)33054-9. [DOI] [PubMed] [Google Scholar]
- 8.Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Schwartz MA, Ginsberg MH. Suppression of integrin activation: A novel function of a Ras/Raf-initiated MAP kinase pathway. Cell. 1997;88:521–30. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Lim CJ, Watanabe N, Soriani A, Ratnikov B, Calderwood DA, Puzon-McLaughlin W, Lafuente EM, Boussiotis VA, Shattil SJ, Ginsberg MH. Reconstructing and deconstructing agonist-induced activation of integrin alphaIIbbeta3. Curr Biol. 2006;16:1796–806. doi: 10.1016/j.cub.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Shock DD, He K, Wencel-Drake JD, Parise LV. Ras activation in platelets after stimulation of the thrombin receptor, thromboxane A 2 receptor or protein kinase C. Biochemical Journal. 1997;321:525–30. doi: 10.1042/bj3210525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulasne D, Bori T, Watson SP. Regulation of RAS in human platelets. Evidence that activation of RAS is not sufficient to lead to ERK1-2 phosphorylation. Eur J Biochem. 2002;269:1511–7. doi: 10.1046/j.1432-1033.2002.02798.x. [DOI] [PubMed] [Google Scholar]
- 12.Kinbara K, Goldfinger LE, Hansen M, Chou FL, Ginsberg MH. Ras GTPases: integrins’ friends or foes? Nat Rev Mol Cell Biol. 2003;4:767–76. doi: 10.1038/nrm1229. [DOI] [PubMed] [Google Scholar]
- 13.Sethi T, Ginsberg MH, Downward J, Hughes PE. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf initiated integrin suppression pathway. Molecular Biology of the Cell. 1999;10:1799–809. doi: 10.1091/mbc.10.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dowal L, Yang W, Freeman MR, Steen H, Flaumenhaft R. Proteomic analysis of palmitoylated platelet proteins. Blood. 2011;118:e62–73. doi: 10.1182/blood-2011-05-353078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosario M, Paterson HF, Marshall CJ. Activation of the Raf/MAP kinase cascade by the Ras-related protein TC21 is required for the TC21-mediated transformation of NIH 3T3 cells. EMBO J. 1999;18:1270–9. doi: 10.1093/emboj/18.5.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosario M, Paterson HF, Marshall CJ. Activation of the Ral and phosphatidylinositol 3′ kinase signaling pathways by the ras-related protein TC21. Mol Cell Biol. 2001;21:3750–62. doi: 10.1128/MCB.21.11.3750-3762.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Self AJ, Caron E, Paterson HF, Hall A. Analysis of R-Ras signalling pathways. Journal of Cell Science. 2001;114:1357–66. doi: 10.1242/jcs.114.7.1357. [DOI] [PubMed] [Google Scholar]
- 18.Marte BM, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Current Biology. 1997;7:63–70. doi: 10.1016/s0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Movilla N, Crespo P, Bustelo XR. Signal transduction elements of TC21, an oncogenic member of the R-Ras subfamily of GTP-binding proteins. Oncogene. 1999;18:5860–9. doi: 10.1038/sj.onc.1202968. [DOI] [PubMed] [Google Scholar]
- 20.Larive RM, Abad A, Cardaba CM, Hernandez T, Canamero M, de Alava E, Santos E, Alarcon B, Bustelo XR. The Ras-like protein R-Ras2/TC21 is important for proper mammary gland development. Mol Biol Cell. 2012;23:2373–87. doi: 10.1091/mbc.E12-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delgado P, Cubelos B, Calleja E, Martinez-Martin N, Cipres A, Merida I, Bellas C, Bustelo XR, Alarcon B. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat Immunol. 2009;10:880–8. doi: 10.1038/ni.1749. [DOI] [PubMed] [Google Scholar]
- 22.Francischetti IM, Saliou B, Leduc M, Carlini CR, Hatmi M, Randon J, Faili A, Bon C. Convulxin, a potent platelet-aggregating protein from Crotalus durissus terrificus venom, specifically binds to platelets. Toxicon: official journal of the International Society on Toxinology. 1997;35:1217–28. doi: 10.1016/s0041-0101(97)00021-4. [DOI] [PubMed] [Google Scholar]
- 23.de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–5. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 24.Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, Andre P, Conley PB, Bergmeier W. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler Thromb Vasc Biol. 2012;32:434–41. doi: 10.1161/ATVBAHA.111.239194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim S, Dangelmaier C, Bhavanasi D, Meng S, Wang H, Goldfinger LE, Kunapuli SP. RhoG Protein Regulates Glycoprotein VI-Fc Receptor gamma-Chain Complex-mediated Platelet Activation and Thrombus Formation. J Biol Chem. 2013;288:34230–8. doi: 10.1074/jbc.M113.504928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel JL, Dangelmaier CA, Mada S, Buitrago L, Jin J, Langdon WY, Tsygankov AY, Kunapuli SP, Sanjay A. Cbl-b is a novel physiologic regulator of glycoprotein VI-dependent platelet activation. J Biol Chem. 2010;285:17282–91. doi: 10.1074/jbc.M109.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S, Jin J, Kunapuli SP. Relative contribution of G-protein-coupled pathways to protease-activated receptor-mediated Akt phosphorylation in platelets. Blood. 2006;107:947–54. doi: 10.1182/blood-2005-07-3040. [DOI] [PubMed] [Google Scholar]
- 28.Mao G, Songdej N, Voora D, Goldfinger LE, Del Carpio-Cano FE, Myers RA, Rao AK. Transcription Factor RUNX1 Regulates Platelet PCTP (Phosphatidylcholine Transfer Protein): Implications for Cardiovascular Events: Differential Effects of RUNX1 Variants. Circulation. 2017;136:927–39. doi: 10.1161/CIRCULATIONAHA.116.023711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao GF, Goldfinger LE, Fan DC, Lambert MP, Jalagadugula G, Freishtat R, Rao AK. Dysregulation of PLDN (pallidin) is a mechanism for platelet dense granule deficiency in RUNX1 haplodeficiency. J Thromb Haemost. 2017;15:792–801. doi: 10.1111/jth.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bynagari-Settipalli YS, Lakhani P, Jin J, Bhavaraju K, Rico MC, Kim S, Woulfe D, Kunapuli SP. Protein kinase C isoform epsilon negatively regulates ADP-induced calcium mobilization and thromboxane generation in platelets. Arterioscler Thromb Vasc Biol. 2012;32:1211–9. doi: 10.1161/ATVBAHA.111.242388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen MY, Hsiao G, Fong TH, Chen HM, Chou DS, Lin CH, Sheu JR, Hsu CY. Amyloid beta peptide-activated signal pathways in human platelets. Eur J Pharmacol. 2008;588:259–66. doi: 10.1016/j.ejphar.2008.04.040. [DOI] [PubMed] [Google Scholar]
- 32.Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, Sickmann A. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114:e10–9. doi: 10.1182/blood-2009-02-203828. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Martin N, Fernandez-Arenas E, Cemerski S, Delgado P, Turner M, Heuser J, Irvine DJ, Huang B, Bustelo XR, Shaw A, Alarcon B. T cell receptor internalization from the immunological synapse is mediated by TC21 and RhoG GTPase-dependent phagocytosis. Immunity. 2011;35:208–22. doi: 10.1016/j.immuni.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alarcon B, Martinez-Martin N. RRas2, RhoG and T-cell phagocytosis. Small GTPases. 2012;3:97–101. doi: 10.4161/sgtp.19138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bergmeier W, Schulte V, Brockhoff G, Bier U, Zirngibl H, Nieswandt B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry. 2002;48:80–6. doi: 10.1002/cyto.10114. [DOI] [PubMed] [Google Scholar]
- 36.Israels SJ, Gerrard JM, Jacques YV, McNicol A, Cham B, Nishibori M, Bainton DF. Platelet dense granule membranes contain both granulophysin and P-selectin (GMP-140) Blood. 1992;80:143–52. [PubMed] [Google Scholar]
- 37.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–62. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 38.Jung SM, Moroi M. Platelet glycoprotein VI. Adv Exp Med Biol. 2008;640:53–63. doi: 10.1007/978-0-387-09789-3_5. [DOI] [PubMed] [Google Scholar]
- 39.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121:1858–67. doi: 10.1182/blood-2012-07-443325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomiyama Y, Kunicki TJ, Zipf TF, Ford SB, Aster RH. Response of human platelets to activating monoclonal antibodies: importance of Fc gamma RII (CD32) phenotype and level of expression. Blood. 1992;80:2261–8. [PubMed] [Google Scholar]
- 41.Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, Watson SP, Kunapuli SP. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2) J Biol Chem. 2013;288:7717–26. doi: 10.1074/jbc.M112.424473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alshehri OM, Montague S, Watson S, Carter P, Sarker N, Manne BK, Miller JL, Herr AB, Pollitt AY, O’Callaghan CA, Kunapuli S, Arman M, Hughes CE, Watson SP. Activation of glycoprotein VI (GPVI) and C-type lectin-like receptor-2 (CLEC-2) underlies platelet activation by diesel exhaust particles and other charged/hydrophobic ligands. Biochem J. 2015;468:459–73. doi: 10.1042/BJ20150192. [DOI] [PubMed] [Google Scholar]
- 43.Alshehri OM, Hughes CE, Montague S, Watson SK, Frampton J, Bender M, Watson SP. Fibrin activates GPVI in human and mouse platelets. Blood. 2015;126:1601–8. doi: 10.1182/blood-2015-04-641654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mammadova-Bach E, Ollivier V, Loyau S, Schaff M, Dumont B, Favier R, Freyburger G, Latger-Cannard V, Nieswandt B, Gachet C, Mangin PH, Jandrot-Perrus M. Platelet glycoprotein VI binds to polymerized fibrin and promotes thrombin generation. Blood. 2015;126:683–91. doi: 10.1182/blood-2015-02-629717. [DOI] [PubMed] [Google Scholar]
- 45.Baker EK, Tozer EC, Pfaff M, Shattil SJ, Loftus JC, Ginsberg MH. A genetic analysis of integrin function: Glanzmann thrombasthenia in vitro. Proceeding of the National Academy of Science of the United States of America. 1997;94:1973–8. doi: 10.1073/pnas.94.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.French DL, Coller BS. Hematologically important mutations: Glanzmann thrombasthenia. Blood Cells, Molecules, and Diseases. 1997;23:39–51. doi: 10.1006/bcmd.1997.0117. [DOI] [PubMed] [Google Scholar]
- 47.Nurden AT, Didry D, Rosa JP. Molecular defects of platelets in Bernard-Soulier syndrome. Blood Cells. 1983;9:333–58. [PubMed] [Google Scholar]
- 48.Peyvandi F, Kunicki T, Lillicrap D. Genetic sequence analysis of inherited bleeding diseases. Blood. 2013;122:3423–31. doi: 10.1182/blood-2013-05-505511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oertli B, Han J, Marte BM, Sethi T, Downward J, Ginsberg M, Hughes PE. The effector loop and prenylation site of R-Ras are involved in the regulation of integrin function [In Process Citation] Oncogene. 2000;19:4961–9. doi: 10.1038/sj.onc.1203876. [DOI] [PubMed] [Google Scholar]
- 50.Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, Leavitt AD, Shattil SJ. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A. 2002;99:12819–24. doi: 10.1073/pnas.202380099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tao L, Zhang Y, Xi X, Kieffer N. Recent advances in the understanding of the molecular mechanisms regulating platelet integrin alphaIIbbeta3 activation. Protein & cell. 2010;1:627–37. doi: 10.1007/s13238-010-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Canault M, Ghalloussi D, Grosdidier C, Guinier M, Perret C, Chelghoum N, Germain M, Raslova H, Peiretti F, Morange PE, Saut N, Pillois X, Nurden AT, Cambien F, Pierres A, van den Berg TK, Kuijpers TW, Alessi MC, Tregouet DA. Human CalDAG-GEFI gene (RASGRP2) mutation affects platelet function and causes severe bleeding. J Exp Med. 2014;211:1349–62. doi: 10.1084/jem.20130477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cifuni SM, Wagner DD, Bergmeier W. CalDAG-GEFI and protein kinase C represent alternative pathways leading to activation of integrin alphaIIbbeta3 in platelets. Blood. 2008;112:1696–703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasvolsky R, Feigelson SW, Kilic SS, Simon AJ, Tal-Lapidot G, Grabovsky V, Crittenden JR, Amariglio N, Safran M, Graybiel AM, Rechavi G, Ben-Dor S, Etzioni A, Alon R. A LAD-III syndrome is associated with defective expression of the Rap-1 activator CalDAG-GEFI in lymphocytes, neutrophils, and platelets. J Exp Med. 2007;204:1571–82. doi: 10.1084/jem.20070058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saci A, Liu WQ, Vidal M, Garbay C, Rendu F, Bachelot-Loza C. Differential effect of the inhibition of Grb2-SH3 interactions in platelet activation induced by thrombin and by Fc receptor engagement. Biochem J. 2002;363:717–25. doi: 10.1042/0264-6021:3630717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mitin N, Rossman KL, Der CJ. Signaling interplay in Ras superfamily function. Curr Biol. 2005;15:R563–74. doi: 10.1016/j.cub.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Clark EA, Shattil SJ, Ginsberg MH, Bolen J, Brugge JS. Regulation of the protein tyrosine kinase pp72SYK by platelet agonists and the integrin αIIbβ3. Journal of Biological Chemistry. 1994;269:28859–64. [PubMed] [Google Scholar]
- 58.Yanaga F, Poole A, Asser U, Blake R, Schieven GL, Clark EA, Che-Leung L, Watson SP. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fcy-IIA receptor. Biochemical Journal. 1995;311:471–8. doi: 10.1042/bj3110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Poole A, Gibbins JM, Turner M, van Vugt M, van de Winkel J, Saito T, Tybulewicz VLJ, Watson SP. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO Journal. 1997;16:2333–41. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geahlen RL. Syk and pTyr’d: Signaling through the B cell antigen receptor. Biochim Biophys Acta. 2009;1793:1115–27. doi: 10.1016/j.bbamcr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Palacios EH, Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23:7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 62.Ezumi Y, Kodama K, Uchiyama T, Takayama H. Constitutive and functional association of the platelet collagen receptor glycoprotein VI-Fc receptor gamma-chain complex with membrane rafts. Blood. 2002;99:3250–5. doi: 10.1182/blood.v99.9.3250. [DOI] [PubMed] [Google Scholar]
- 63.Schmitter T, Pils S, Sakk V, Frank R, Fischer KD, Hauck CR. The granulocyte receptor carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3) directly associates with Vav to promote phagocytosis of human pathogens. J Immunol. 2007;178:3797–805. doi: 10.4049/jimmunol.178.6.3797. [DOI] [PubMed] [Google Scholar]
- 64.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1456–67. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 65.Bergmeier W, Stefanini L. Platelet ITAM signaling. Curr Opin Hematol. 2013;20:445–50. doi: 10.1097/MOH.0b013e3283642267. [DOI] [PubMed] [Google Scholar]
- 66.Tadokoro S, Shattil S, Eto K, Tai V, Liddington R, de pereda J, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003 doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 67.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Rangwala F, Fulkerson PC, Ling B, Reed E, Cox AD, Kamholz J, Ratner N. Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells. Oncogene. 2004;23:368–78. doi: 10.1038/sj.onc.1207075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luo H, Hao X, Ge C, Zhao F, Zhu M, Chen T, Yao M, He X, Li J. TC21 promotes cell motility and metastasis by regulating the expression of E-cadherin and N-cadherin in hepatocellular carcinoma. Int J Oncol. 2010;37:853–9. doi: 10.3892/ijo_00000736. [DOI] [PubMed] [Google Scholar]
- 70.Jeong HW, Nam JO, Kim IS. The COOH-terminal end of R-Ras alters the motility and morphology of breast epithelial cells through Rho/Rho-kinase. Cancer Res. 2005;65:507–15. 65/2/507 [pii] [PubMed] [Google Scholar]
- 71.Keely PJ, Rusyn EV, Cox AD, Parise LV. R-Ras signals through specific integrin alpha cytoplasmic domains to promote migration and invasion of breast epithelial cells. Journal of Cell Biology. 1999;145:1077–88. doi: 10.1083/jcb.145.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen B, Zhao X, O’Brien KA, Stojanovic-Terpo A, Delaney MK, Kim K, Cho J, Lam SC, Du X. A directional switch of integrin signalling and a new anti-thrombotic strategy. Nature. 2013;503:131–5. doi: 10.1038/nature12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Estevez B, Shen B, Du X. Targeting Integrin and Integrin Signaling in Treating Thrombosis. Arterioscler Thromb Vasc Biol. 2014 doi: 10.1161/ATVBAHA.114.303411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lockyer S, Okuyama K, Begum S, Le S, Sun B, Watanabe T, Matsumoto Y, Yoshitake M, Kambayashi J, Tandon NN. GPVI-deficient mice lack collagen responses and are protected against experimentally induced pulmonary thromboembolism. Thrombosis research. 2006;118:371–80. doi: 10.1016/j.thromres.2005.08.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.