Abstract
Purpose: The aim of the present study was to correlate etiological factors with noncarious cervical lesions in a group of patients from Craiova. Material and Methods: The study was conducted between November 2015 and May 2016 on 50 patients, aged 18-56 years, who addressed to the Oral Rehabilitation Clinic, from the University of Medicine and Pharmacy of Craiova. Patients were divided into two groups: the study group consists of patients who had noncarious cervical dental lesions (NCCLs) and the control group with patients who did not have noncarious cervical lesions. Each patient underwent a clinical examination and completed a questionnaire, referring to eating habits, oral hygiene, vicious habits and personal impressions about the appearance and functionality of his teeth, highlighting the factors involved in the noncarious dental lesions etiology. Results: The study group consisted of 64% women and 36% men. Noncarious cervical lesions were higher in men (72.22%) compared to women (56.25%). Regarding on the tooth brushing method, it has been noted that 34% of patients used a vertical tooth brushing method, 52% were using a circular brushing method, while 14% were practicing a horizontal tooth brushing method. Cervical sensitivity has been detected in 48% of the patients, against 52% who showed no sensitivity. 62% of the participants did not have bruxism, while nighttime/daytime bruxism was found in 38% of the patients. Conclusions: There are several etiological factors correlated with noncarious cervical lesions, among which are: tooth brushing method, bruxism, eating behaviors.
Keywords: Noncarious dental lesions, etiology, cervical lesions, abfraction, erosive tooth wear
Introduction
Noncarious cervical lesions (NCCLs) develop following normal and/or pathological function in time, and manifest as abfraction, abrasion, erosion or structural and composition degradation of teeth tissues [1]. Clinical appearance of NCCLs can vary depending on the type and severity of the etiological factors involved [2]. Published data supports the fact that abfraction, such as any NCCLs, have a complex etiology. Of the many possible etiological factors associated with NCCLs, occlusal stress forces have mostly studied during the recent years [1]. The interaction between chemical, biological, and behavioral factors is critical and helps to explain why some individuals exhibit more than one type of cervical lesional mechanism compared to others [1].
ETW (erosive tooth wear) occurs as a loss of the normal tooth surface structure and morphology. Typical changes of ETW on occlusal surfaces are represented by cupping of the cusps and flattening of the occlusal planes. With the advance of ETW, all the occlusal normal morphology can disappear leading sometimes to the formation of hollowed out surfaces [2]. Typical signs of ETW on smooth surfaces are flattening of the surface, and an intact rim along the gingival margin may be present. Concavities may become present, which are normally more wide than deep [3].
In a study published in 2016, Nascimento et al [1] shows the scheme of pathodynamic mechanisms of tooth surface lesions, as proposed by Grippo (Fig. 1):
Figure 1.
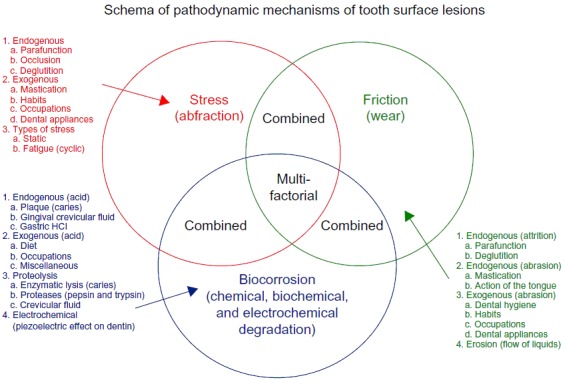
Scheme of pathodynamic mechanisms involved in NCCLs as proposed by Grippo et al. [1,12]
NCCLs are relatively common clinical conditions that can adversely affect structural integrity, retention of the dental plaque, pulpal vitality, tooth sensitivity, and overall morphology [1,6,7,8]. A dental practice-based study revealed that NCCLs are the main reason besides than caries that lead to placement of restorations on previously unre-stored permanent tooth surfaces [9].
The aim of the present study was to correlate etiological factors to noncarious cervical lesions (NCCL) in a group of patients from Craiova.
Material and Method
The study was conducted between November 2015 and May 2016 on a 50 patients study group, aged 18-56 years, who addressed to the Oral Rehabilitation Clinic, from the UMF Craiova and it was approved by the Ethics Committee of UMF Craiova. Patients were divided into two groups: the study group consists of patients who had noncarious cervical dental lesions (NCCLs) and the control group with patients who did not have noncarious cervical lesions. Each patient underwent an endooral and exooral clinical examination and completed a questionnaire, referring to eating habits, oral hygiene, vicious habits and personal impressions about the appearance and functionality of his teeth, highlighting the factors involved in the noncarious dental lesions etiology.
A database was created using Microsoft Office Word 2007 software, and the results have been processed using the same program.
Results and Discussions
The subjects studied (50 patients), were divided into two groups: the study group, which gathered the patients with noncarious cervical dental lesion (62% of the participants) and the control group, that of the patients without noncarious lesions (38% of participants). According to the patients age, 40% of the participants were aged 18-30 years (65% of them had noncarious cervical dental lesions) and 60% of the participants were aged 31-56 years (60% patients among them had noncarious cervical dental lesions) (Table 1).
Table 1.
Patients distribution according to the their age
| Age (18-56 years) | Study Group | Control Group | Total |
| 18-30 years | 13 | 7 | 20 |
| 31-56 years | 18 | 12 | 30 |
| Total | 31 | 19 | 50 |
Literature shows that NCCLs are more prevalent in the adult population, with the incidence increasing from 3% to 17% between 20 years and 70 years of age [1,10,7,11].
64% of study participants were women, and 36% were men. Noncarious cervical dental lesion frequency was greater in males (72.22%) compared to women (56.25%) (Table 2).
Table 2.
Patients distribution according to sex
| Sex | Study Group | Control Group | Total |
| Men | 13 | 5 | 18 |
| Women | 18 | 14 | 32 |
| Total | 31 | 19 | 50 |
Depending on the patients origin, 80% of the patients from this study came from the urban areas, while 20% came from rural areas (Table 3).
Table 3.
Patients distribution depending on their origin area
| Origin area | Study Group | Control Group | Total |
| Urban | 23 | 17 | 40 |
| Rural | 8 | 2 | 10 |
| Total | 31 | 19 | 50 |
Erosive tooth wear prevalence has been studied all over the world and the results varied widely [3]. In many regions, including Europe, the prevalence of the condition is high [4]. For instance, a recent European study indicated that around 30% of the population attending general practices, aged 18-35 years, has at least one tooth with advanced erosive tooth wear [5].
One of the causes of ETW (erosive tooth wear) is regurgitation of stomach contents into the mouth, but for the risk to be significant, frequent regurgitation over an extended period of time is needed. The term biocorrosion has also been proposed to include all forms of chemical, biochemical, and electrochemical degradation [1,12]. Vomiting due to occasional stomach disorders or to morning sickness in pregnancy is not considered a cause for concern. A high prevalence of ETW has been identified in groups of people in which frequent and persistent regurgitation is a symptom of an underlying medical condition such as gastro-esophageal reflux disorder, rumination, and eating disorders with frequent vomiting (e.g., bulimia nervosa) [1].
Some medications (e.g., acidic saliva stimulants or preparations containing acetylsalicylic acid) and dietary supplements (e.g., vitamin C tablets) are potentially erosive if they are in the form of chewable tablets or effervescent drinks. Other medications have the side effect of reducing salivary flow, which could indirectly enhance ETW from other agents [1].
Data obtained from the patients enrolled in this study supports data from the literature, considering that a small percentage of patients (21%) of those with noncarious dental lesions said they had gastroesophageal reflux or sometimes took potential erosive medication.
ETW (erosive tooth wear) lesions occur on both permanent and deciduous teeth, can extend into dentine and must be distinguished mainly from attrition, which is caused by the action of antagonistic teeth (e.g., grinding) and leads to matching facets; lesions are typically flat, sharp bordered, and glossy [3].
Smooth surface lesions must be mainly distinguished from abrasion, which is predominantly caused by traumatic oral hygiene habits and may vary in appearance depending on the causative impact. The main difficulty in the clinical assessment is the interaction of the various causative factors, especially when the respective injuries act for a long time [3]. Fig. no 1 demonstrates that 10% of the study patients used to consume candies or soft drinks once a week, 14% were eatting sweets twice a week, 6% of them were eatting candies 4 times a week and only 2% of the participants were using carbonated drinks or sweets four times a week. We noticed that most of the patients in the study are eatting sweets at least 2-3 times a day (36%), while 30% only one once a day. 2% of the patients specified that they never use sweets or soft drinks (Fig. 2).
Figure 2.
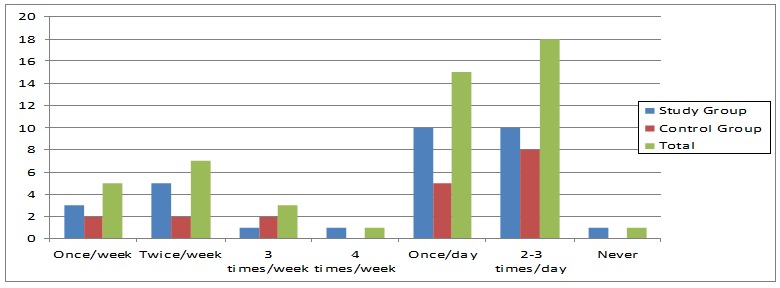
Sweets and carbonated drinks consumption frequency
Patients with high risk of tooth erosive lesions are recommended moderate consumption and reduced frequency of beverages and harmful foods intake, which should be consumed only during main meals. Their contact with the teeth surface should be reduced as much as possible. The acids from our diet act by the contact time with the oral cavity, while teeth are subjected to acid challenge, as well as by their clearance possibilities [14,15].
Drinking habits such as sucking small and rare sips or keeping the drink inside the oral cavity for a few seconds should be discouraged [16]. Using a straw is indicated since it avoids anterior teeth direct contact with the beverage, which can leak directly to the pharynx [14,15]. The temperature of the fizzy drinks also influences the erosive potential [16] because‚ cold as ice’ drinks intake (4°C) reduce its erosive effect.
Foods and beverages composition determines how erosive they are. Analyses of many products have identified a low pH and a high buffer capacity as the major risk factors, and the calcium concentration as the major protective factor in determining the erosive potential. For instance, the pH of yoghurts is about 4.0, yet they are not erosive because they have high calcium concentrations [3]. The buffer capacity of the product (determined by the type of acid and the pH) affects the resistance of the product to being neutralized by saliva. If the product contains substances, such as gums, which adhere to tooth surfaces, they may retain the product at the tooth surface and hence prolong the erosive challenge [3]. Studies have shown that milk and yoghurt consumption is related to a lower prevalence of ETW [13].
As for the teethbrushing, 34% of the studied patients used a vertical brushing method, 52% used circular brushing method, while 14% use a type of horizontal toothbrushing (Fig. 3).
Figure 3.
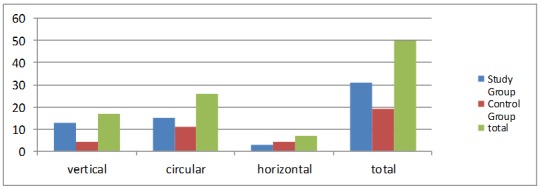
Patients distribution deppening on the toothbrushing method
As for the western population, the main abrasive agent is the toothpaste, which affects far more the dentine than the enamel. Clinical data from several in vitro studies [17,18,19] support the affirmation that tooth brushing with toothpaste is the main agent for dentin abrasion. The brush itself is only a transport vehicle since teethbrushing without toothpaste has no effect on the tooth enamel and a tiny effect on dentin [20]. However, the toothbrush design and in particular the arrangement of the filaments, the density and texture, can influence the abrasion degree [21]. Tissue loss caused by toothbrushing is time dependent and it seems to deppend on many factors, including frequency, action time and the brushing force [19,22]. Dentine damage predilection areas seem to be correlated with the toothbrushing habits: the most affected tooth surfaces are the ones receiving the most attention during toothbrushing.
Nighttime/ daytime bruxism was found in 38% of the patients, while 62% of the participants had no bruxism. As for the study group, bruxism has been found in 45% of the cases; the control group showed that 26% of the participants had bruxism (Table 4).
Table 4.
Patients distribution by the presence or absence of the bruxism
| Daytime / nighttime bruxism | Study Group | Control Group | Total |
| Present | 14 | 5 | 19 |
| Absent | 17 | 14 | 31 |
| Total | 31 | 19 | 50 |
The few clinical studies available were not able to confirm a positive association between occlusal loading and abfraction lesions [1,23,24,25]. The role of occlusal loading in NCCLs appears to be part of a multifacto¬rial event that may not necessarily follow the proposed, classic abfraction mechanism [12,26]. Overall, there is a weak association between NCCLs and occlusal factors (interference in excursive movements, force, premature contacts, type of guidance, and slide of centric occlusion to maximum intercuspation) and it has been argued that an occlusal load that is far from the cervical defect site cannot be considered as the cause of abfraction lesions [27].
Optical coherence tomography studies suggest that dentin demineralization promotes the formation of NCCLs from an early stage, whereas occlusal stress is an etiological factor that contributes to the progression of these lesions [28].
For the prevention and progression of the initial abfraction lesions, use of occlusal night guards has been recommended, which reduce nocturnal bruxism and axial forces acting on the teeth. However, the use of these occlusal night guards to reduce bruxism is still controversial, even if there were studies supporting their effectiveness [29]. When performed correctly, they have the potential to reduce nonaxial tooth loading. Although they provide a conservative treatment for the abfraction lesions, some authors cannot find a clear demonstration of the effectiveness of their use [30,31]. Regarding the abfraction lesions development mechanism, occlusal night guards should be considered as a good treatment strategy due to its conservative nature.
Cervical sensitivity (hypersensitivity/ hyperesthesia) was detected in 48% of the subjects, against 52% that showed no sensitivity. Among the patients with cervical sensitivity, 79% are in the study group, as opposed to only 21% from the control group. In the study group, most of the patients (61%) showed hypersensitivity or hyperesthesia at the cervical lesions of the teeth affected by abfraction. 39% of the participants did not show any teeth sensitivity (Table 5).
Table 5.
Patients distribution by the presence of the cervical sensitivity
| Hypersensitivity / hyperesthesia | Study Group | Control Group | Total |
| absent | 12 | 14 | 26 |
| present | 19 | 5 | 24 |
| total | 31 | 19 | 50 |
Dental sensitivity issue is frequently related to the noncariogenic dental lesions. Dentinal hypersensitivity is a short, sharp pain response to a stimulus. Tooth sensitivity may be a temporary symptom associated with early stages of abfraction lesions [1]. It is expected that the chronic nature of abfraction, which is accompanied by the natural process of dentinal remineralization, will slowly relieve tooth sensitivity. If sensitivity persists, the exposed dentin may require therapeutic treatment to relieve or eliminate the discomfort [1,32,33]. The various clinical manifestations of abfraction appear to be dependent on the type and severity of the etiological factors involved [1,2].
Regarding the distribution of treated or untreated noncarious cervical lesions, the study group has revealed that 62% of the patients did not have any kind of treatment, and 31.2% of all the cervical lesions had been treated before this study. Depending on the affected areas, 71.5% of the lesions were detected in the anterior teeth, while 28.5% were found in the posterior teeth (Fig.4).
Figure 4.
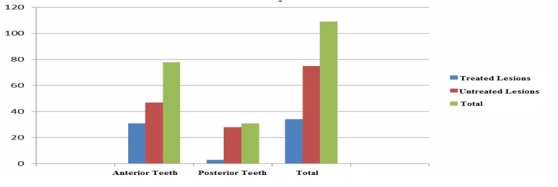
Study group distribution by the presence of treated or untreated noncarious cervical lesions
The prevalence of NCCLs is greater in incisors and premolars than in canines and molars. Mandibular premolars are affected by NCCLs more often and more severely than maxillary premolars. Abfraction lesions and other NCCLs, such as erosion, may also affect the whole dentition in severe cases where aging is associated with other pathological factors [1].
The observation that premolar teeth of patients aged older than 40 years are the most common sites of restorations placed due to NCCLs highlights the importance of preventive interventions at an earlier age in order to avoid the need for future restorative or any other irreversible treatment [1,9].
72% of the study participants had no fillings or prosthetic restorations, compared to 28% of those who had. 78.5% of the patients with incorrectly adjusted prosthetic restorations or fillings were in the study group and only 21.5% were in the control group (Fig. 5).
Figure 5.
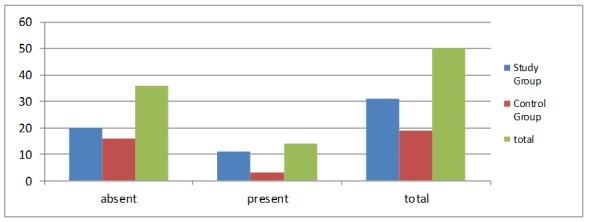
Patients distribution by the presence of prosthetic restorations or fillings
It should be noted that when restoring noncarious cervical lesions, clinicians do not treat their etiology, and they replace the lost dental tissue. Some dentists recommend periodic inspection and expectation, while others recommend early intervention [31,34,35,36,37]. There are no specific generally accepted guidelines stating that all lesions should be restored. A good, logic clinical thinking would suggest that they should be restored when the clinical consequences (ie, dentin hypersensitivity) have developed, or most likely will develop in the future. Patients’ aesthetic requirements may also influence the restoration decision and a risk-benefit analysis should be done in these situations. Cervical restorations may increase the plaque amount and may lead to tooth decay and periodontal disease [31,38,39].
Conclusion
1. Noncarious cervical lesions (NCCLs) develop as a result of normal or pathological wear and cause abfraction, abrasion, and erosion or chemical degradation of dental tissues.
2. Noncarious lesions incidence in this study was 62% versus 38% of the patients in the control group. Most patients (61%) from the study group showed hypersensitivity or hyperesthesia of the teeth affected by abfraction lesions and 39% of the participants showed no teeth sensitivity.
3. Evidence supports that NCCLs have a multifactorial etiology involving toothbrushing, erosive food, bruxism.
4. Clinical appearance of NCCLs can vary depending on the type and severity of the etiological factors involved.
5. There are several mechanisms able to compensate the dental tissue loss, among which the most important are: treating digestive disorders, reducing the frequency and action time of acid challenges, emphasizing salivary defense factors, enhancing the effect of the neutralizing acids, using protective dental night guard, improving personal care and hygiene etc.
Acknowledgments
This study was financially supported by the project named "Program of Excellence in doctoral and postdoctoral research in multidisciplinary chronic diseases" contract no. POSDRU/159/1.5/S/133377financed from the European Social Fund through Sectorial Operational Human Resources Development Program 2007-2013.
References
- 1.Nascimento MM, Dilbone DA, Pereira PNR, Duarte WR, Geraldeli S, Delgado AJ. Abfraction lesions: etiology, diagnosis and treatment options, Clinical. Cosmetic and Investigational Dentistry. 2016;8:79–87. doi: 10.2147/CCIDE.S63465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett DW, Shah P. A critical review of non-carious cervical (wear) lesions and the role of abfraction, erosion, and abrasion. J Dent Res. 2006;85(4):306–312. doi: 10.1177/154405910608500405. [DOI] [PubMed] [Google Scholar]
- 3.Carvalho TS, Colon P, Ganss C, Huysmans MC, Lussi A, Schlueter N, Schmalz G, Shellis RP, Tveit AB, Wiegand A. Consensus report of the European Federation of Conservative Dentistry: erosive tooth wear-diagnosis and management. Clin Oral Invest. 2015;19(7):1557–1561. doi: 10.1007/s00784-015-1511-7. [DOI] [PubMed] [Google Scholar]
- 4.Jaeggi T, Lussi A. Prevalence, incidence and distribution of erosion. In: Lussi A, Ganss C, editors. Erosive tooth wear-from diagnosis to therapy, Monographs in Oral Science. 1. Basel: Karger; 2014. pp. 55–73. [Google Scholar]
- 5.Bartlett D, Lussi A, West NX, Bouchard P, Sanz M, Bourgeois D. Prevalence of toothwear on buccal and lingual surfaces and possible risk factors in young European adults. J Dent. 2013;41(11):1007–1013. doi: 10.1016/j.jdent.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 6.Michael JA, Townsend GC, Greenwood LF, Kaidonis JA. Abfraction: separating fact from fiction. Aust Dent J. 2009;54(1):2–8. doi: 10.1111/j.1834-7819.2008.01080.x. [DOI] [PubMed] [Google Scholar]
- 7.Levitch LC, Bader JD, Shugars DA, Heymann HO. Non-carious cervical lesions. J Dent. 1994;22(4):195–207. doi: 10.1016/0300-5712(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 8.LittleStar ML, Summitt JB. Non-carious cervical lesions: an evidenced-based approach to their diagnosis. Tex Dent J. 2003;120(10):972–980. [PubMed] [Google Scholar]
- 9.Nascimento MM, Gordan VV, Qvist V, Bader JD, Rindal DB, Williams OD, Gewartowski D, Fellows JL, Litaker MS, Gilbert GH. Dental Practice-Based Research Network Collaborative Group. Restoration of noncarious tooth defects by dentists in The Dental Practice-Based Research Network. J Am Dent Assoc. 2011;142(12):1368–1375. doi: 10.14219/jada.archive.2011.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grippo JO. Abfractions: a new classification of hard tissue lesions of teeth. J Esthet Dent. 1991;3(1):14–19. doi: 10.1111/j.1708-8240.1991.tb00799.x. [DOI] [PubMed] [Google Scholar]
- 11.Mayhew RB, Jessee SA, Martin RE. Association of occlusal, periodontal, and dietary factors with the presence of non-carious cervical dental lesions. Am J Dent. 1998;11(1):29–32. [PubMed] [Google Scholar]
- 12.Grippo JO, Simring M, Coleman TA. Abfraction, abrasion, biocorrosion, and the enigma of noncarious cervical lesions: a 20-year perspective. J Esthet Restor Dent. 2012;24(1):10–23. doi: 10.1111/j.1708-8240.2011.00487.x. [DOI] [PubMed] [Google Scholar]
- 13.Salas MM, Nascimento GG, Vargas-Ferreira F, Tarquinio SB, Huysmans MC, Demarco FF. Diet influenced tooth erosion prevalence in children and adolescents: results of a meta-analysis and meta-regression. J Dent. 2015;43(8):865–875. doi: 10.1016/j.jdent.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Zero DT, Lussi A. Etiology of enamel erosion-intrinsic and extrinsic factors. In: Addy M, Embery G, Edgar WM, editors. Tooth wear and sensitivity. 1. London: Martin Dunitz; 2000. pp. 121–140. [Google Scholar]
- 15.Lussi A. Dental Erosion-From Diagnosis to Therapy. In: Whitford GM, editor. Monographs in Oral Science. 1. Augusta; 2006. [Google Scholar]
- 16.Amaechi BT, Higham SM. In vitro remineralisation of eroded enamel lesions by saliva. J Dent. 2001;29(5):371–376. doi: 10.1016/s0300-5712(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 17.Bartlett D, Smith BGN. Definition, classification and clinical assessment of atrition, erosion and abrasion of enamel and dentine. In: Addy M, Embery G, Edgar WM, editors. Tooth wear and sensitivity. 1. London: Martin Dunitz; 2000. pp. 87–92. [Google Scholar]
- 18.Addy M, Hunter ML. Can toothbrushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53(Suppl 3):177–186. doi: 10.1111/j.1875-595x.2003.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 19.Hunter ML, West NX, Hughes JA, Newcombe RG, Addy M. Erosion of deciduous and permanent dental hard tissue in the oral environment. J Dent. 2000;28(4):257–263. doi: 10.1016/s0300-5712(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 20.Absi EG, Addy M, Adams D. Dentine hypersensitivity. The effects of toothbrushing and dietary compounds on dentine in vitro: a SEM study. J Oral Rehabil. 1992;19(2):101–110. doi: 10.1111/j.1365-2842.1992.tb01086.x. [DOI] [PubMed] [Google Scholar]
- 21.Phaneuf EA, Harrington JH, Dale PP, Shklar G. Automatic toothbrush: a new reciprocating action. J Am Dent Assoc. 2012;65:12–25. doi: 10.14219/jada.archive.1962.0187. [DOI] [PubMed] [Google Scholar]
- 22.Addy M, Hughes J, Pickles MJ, Joiner A, Huntington E. Developement of a method in situ to study toothpaste abrasion of dentine: comparation of 2 products. J Clin Periodontal. 2002;29(10):896–900. doi: 10.1034/j.1600-051x.2002.291004.x. [DOI] [PubMed] [Google Scholar]
- 23.Wood ID, Kassir AS, Brunton PA. Effect of lateral excursive movements on the progression of abfraction lesions. Oper Dent. 2009;34(3):273–279. doi: 10.2341/08-100. [DOI] [PubMed] [Google Scholar]
- 24.Pintado MR, Delong R, Ko CC, Sakaguchi RL, Douglas WH. Correlation of noncarious cervical lesion size and occlusal wear in a single adult over a 14-year time span. J Prosthet Dent. 2000;84(4):436–443. doi: 10.1067/mpr.2000.109477. [DOI] [PubMed] [Google Scholar]
- 25.Estafan A, Furnari PC, Goldstein G, Hittelman EL. In vivo correlation of noncarious cervical lesions and occlusal wear. J Prosthet Dent. 2005;93(3):221–226. doi: 10.1016/j.prosdent.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Sarode GS, Sarode SC. Abfraction: a review. J Oral Maxillofac Pathol. 2013;17(2):222–227. doi: 10.4103/0973-029X.119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levrini L, Di Benedetto G, Raspanti M. Dental wear: a scanning electron microscope study. Biomed Res Int. 2014;(2014):340425–340425. doi: 10.1155/2014/340425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada I, Shimada Y, Ikeda M, Sadr A, Nakashima S, Tagami J, Sumi Y. Clinical assessment of non carious cervical lesion using swept-source optical coherence tomography. J Biophotonics. 2015;8(10):846–854. doi: 10.1002/jbio.201400113. [DOI] [PubMed] [Google Scholar]
- 29.Pegoraro LF, Scolaro JM, Conti PC, Telles D, Pegoraro TA. Noncarious cervical lesions in adults: prevalence and occlusal aspects. The Journal of the American Dental Association. 2005;136(12):1694–1700. doi: 10.14219/jada.archive.2005.0113. [DOI] [PubMed] [Google Scholar]
- 30.Litonjua LA, Andreana S, Cohen RE. Toothbrush abrasions and noncarious cervical lesions: evolving concepts. Compendium of Continuing Education in Dentistry. 2005;26(11):767–776. [PubMed] [Google Scholar]
- 31.Michael JA, Townsend GC, Greenwood LF, Kaidonis JA. Abfraction: separating fact from fiction. Australian Dental Journal. 2009;54(1):2–8. doi: 10.1111/j.1834-7819.2008.01080.x. [DOI] [PubMed] [Google Scholar]
- 32.Jena A, Shashirekha G. Comparison of efficacy of three different desensitizing agents for in-office relief of dentin hypersensitivity: a 4 weeks clinical study. J Conserv Dent. 2015;18(5):389–393. doi: 10.4103/0972-0707.164052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baysan A, Lynch E. Treatment of cervical sensitivity with a root sealant. Am J Dent. 2003;16(2):135–138. [PubMed] [Google Scholar]
- 34.Wood I, Jawad Z, Paisley C, Brunton P. Non-carious cervical tooth surface loss: a literature review. Journal of Dentistry. 2008;36(10):759–766. doi: 10.1016/j.jdent.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Lee WC, Eakle WS. Stress-induced cervical lesions: review of advances in the past 10 years. Journal of Prosthetic Dentistry. 1996;75(5):487–494. doi: 10.1016/s0022-3913(96)90451-5. [DOI] [PubMed] [Google Scholar]
- 36.Spreafico R. Composite resin rehabilitation of eroded dentition in a bulimic patient: a case report. The European Journal of Esthetic Dentistry. 2010;5(1):28–48. [PubMed] [Google Scholar]
- 37.Kuroe K, Caputo AA, Ohata N, Itoh H. Biomechanical effects of cervical lesions and restoration on periodontally compromised teeth. Quintessence International. 2001;32(2):111–118. [PubMed] [Google Scholar]
- 38.Aw TC, Lepe X, Johnson GH, Mancl L. Characteristics of noncarious cervical lesions: a clinical investigation. Journal of the American Dental Association. 2001;133(6):725–733. doi: 10.14219/jada.archive.2002.0268. [DOI] [PubMed] [Google Scholar]
- 39.Wood ID, Kassir ASA, and Brunton PA. Effect of lateral excursive movements on the progression of abfraction lesions. Operative Dentistry. 2009;34(3):273–279. doi: 10.2341/08-100. [DOI] [PubMed] [Google Scholar]


