Abstract
Currently incurable, Charcot-Marie-Tooth (CMT) disease is the most commonly inherited neurological disorder, which affects a small percentage of the population. The most common cause of CMT is the duplication of a region on the short arm of chromosome 17, which includes the gene PMP22. We report a thirty-seven-year-old man with CMT disease having sleep, memory and attention disorders characterized by brief retrograde amnesia at early age. The patient has no genetic disease in the family, but was diagnosed with diabetes mellitus, which emphasizes the sensory loss and prolonged infections. Diabetes mellitus emphasizes the sensory symptomatology and predisposes to the development of infections with delayed healing.
Keywords: Charcot-Marie-Tooth, inherited neuromuscular disorder, sleep disorders
Introduction
Sjogren Syndrome (SS) is a systemic autoimmune disease, referred more specific as an autoimmune exocrinopathy. This is due to its hallmark clinical feature, which consists mainly of dryness of the mouth and eyes as a result of the functional impairment of the salivary and lacrimal glands. Classically, SS is viewed either as a primary form (pSS), i.e. independent of other autoimmune disease, or a secondary form (sSS) when co-existence of other immune-mediated diseases is confirmed [1].Diagnosis of SS resides in the existence of a series of both subjective and objective items.In today’s clinical practice, physicians commonly use the revised American European Group Criteria (AECG) in order to establish a correct diagnosis of either pSS or sSS [2].Items enlisted in the AECG also consist of several exclusion criteria, which include hepatitis C virus (HCV) infection. Exclusion of HCV infection from pSS diagnosis is a highly debated topic, mainly because HCV is viewed as an ethiopathogenic factor in the disease onset. Although the pathogenic effect through which HCV initiates the autoimmune response is not clear, the main body of evidence suggests molecular mimicry through sialotropism as the most plausible mechanism. In studies on transgenic mice for HCV envelope proteins (E 1,2), sialadenitis occurred in 84% of the mice compared to 0% of the HCV core protein transgenic mice [3].
Also, histological features positively correlated with the level of expression of E1,2; this suggested a causal effect of the infection with HCV in the initiation of autoimmune activity. Studies on large series of HCV patients revealed a significant overlap between SS diagnostic criteria and extrahepatic manifestations of HCV infection: xerostomia 18%, xerophthalmia 17%, positive ocular tests 38%, positive gland biopsy 25%, rheumatoid factor (RF) positivity 40%, andoccurrence of antinuclear antibodies 18% [4]. The subset of patients with HCV-induced SS are considered a distinct form from those with pSS, with some large studies onHCV-SS patients that hint to various epidemiological, clinical and serological differences [5,6].We report the cases of two female patients diagnosed with HCV chronic infection, whom developed casual sicca symptoms, parotidomegaly and were later diagnosed with HCV-induced SS. First patient developed only the histopathological criteria of pSS and co-existence of mixed cryoglobulinemia (MC), which is viewed as a HCV-related marker. The second patient developed both histopathological and serological (anti-Ro and anti-La autoantibodies) SS-related markers.
Patient A
First patient is a 61 year old woman, diagnosed with HCV chronic infection 20 years ago. She underwent treatment with interferon (IFN) after initial diagnosis, for 6 months, and combined treatment 12 years later, with peg-IFN and ribavirin, without sustained virusological response. In the course of the disease, the general health of the patient declined. She started to fell a quasi-permanent pain in both lower limbs, accompanied by a discrete swelling of ankles, with a diffuse brownish pigmentation on both calves. On clinical examination, palpable purpura of the lower limbs (Fig. 1) and perimaleolar edema was noted. In the clinical context of HCV infection, cryoglobulinemicvasculitis was suggested. Further test confirmed the diagnosis. The laboratory panel showed polyclonal hypergammaglobulinemia, low C4 levels, presence of cryoglobulins, and also positive RF activity. The urine analysis showed microscopic hematuria and a 24 hour-proteinuria of 2.73g. In addition, a neurological exam coupled with electromyography and a skin biopsy was performed. At this stage the patient is diagnosed with HCV-related MC, with cutaneous, neurological and renal involvement. One year ago, during a regular check-up, the patient added that throughout the last few years she started to feel intense dryness of the mouth and eyes, while also noticing a slight swelling of both parotid glands. Consecutively, an ultrasound (US) examination of the parotid glands was performed. The US features were marked by intenseparenchymal inhomogeneity (PIH) of both parotid glands with large anechoic regions, PIH score 4 using Salaffi F et al semiquantitative scoring system [7] (Fig. 2). Histopathological analysis of the tissue obtained from minor salivary gland biopsy (SGB) confirmed the presence of lymphocytic sialadenitis, with a focus score of 2.
Figure 1.
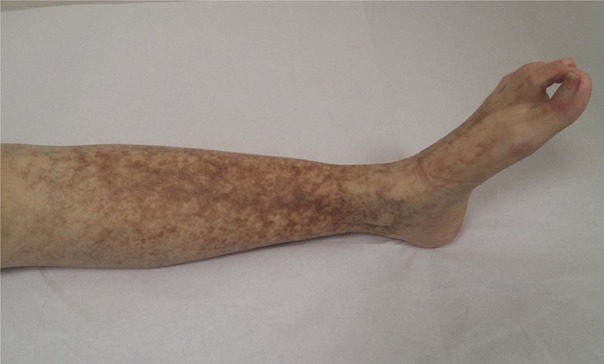
Intense brownish pigmentation on lower limb of Patient A suggestive of palpable purpura in the setting of cryoglobulinemicvasculitis
Figure 2.
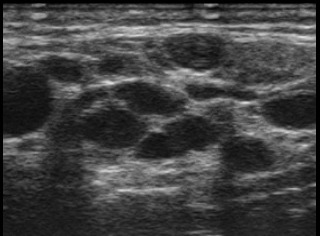
Parotid gland US features of patient A. B-mode US image shows severe PIH, marked by multiple well-defined anechoic areas, of over 6 mm in diameter, which corresponds to PIH score of 4
Immunological panel showed negative antinuclear antibodies (ANA). The patient was diagnosed with HCV related SS and associated MC.
Patient B
The second patient is 57 year old woman, diagnosed with HCV infection 7 years ago. At the time of diagnosis, laboratory work-up showed elevated liver enzymes, aspartate aminotransferase (AST) 50U/L (N<31U/L), positive anti-HCV antibodies, detection of HCV-RNA by polymerase chain reaction (PCR), and an increased erythrocyte sedimentation rate (ESR) of 20mm/1h, 40mm/2h (N<14/1h, <30/2h). She underwent antiviral treatment with pegylated IFN in combination with ribavirin, for a period of one year, without sustained virusological response. Two years later she was diagnosed with autoimmune thyroiditis (AIT), with high level of anti-thyroid peroxidase antibodies, of 250 UI/ml (N <34UI/ml) and secondary hypothyroidism. After a course of 5 years, the patient started to complain of dry eyes which prompted the use of artificial tears. Also, she noticed a stiff, growing mass on the left mandible and sought medical advice in the maxillofacial surgery department. She was diagnosed with left parotid gland tumor and advised to perform a computed tomography (CT) scan of the head and neck coupled with sialotomography which revealed severe structural changes of both parotid glands(Fig. 3a-3c). A minor SGB was performed and the histopathological analysis revealed the presence of a lymphocytic infiltrate, with a focus score of 3. Subsequently, she was referred to our department with suspected diagnosis of SS. Upon admission, we performed an ANA panel test, which revealed intensively positive (titer ≥1:160) anti-Ro and anti-La autoantibodies. Cryoglobulins were absent. Schirmer test was also positive, 3/4mm after 5min. Ultrasound features were assessed in B-mode, color and power Doppler(Fig. 3d, 3e). PIH of parotid gland showed a high score of 4. On power Doppler mode, vascularity pattern was increased, scoring 2 on a semi-quantitative scale [8]. Final diagnosis was HCV associated SS in which the development of AIT may be traced back to multiple factors, either HCV on itself, IFN-therapy induced or as systemic manifestation of SS.
Figure 3.
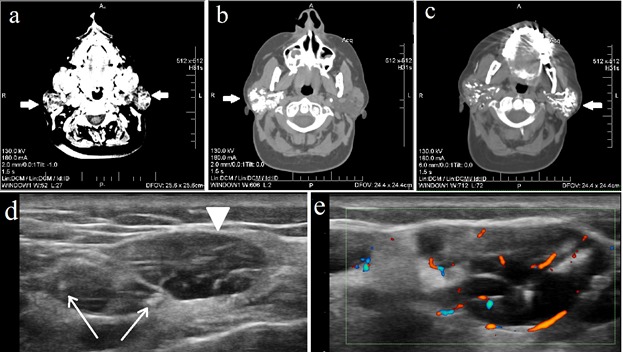
Imaging features in patient B. Head and neck CT scan: (a) native CT scan in transverse plane shows both parotid glands (arrows) increased in size and displaying structural changes with a cystic-like pattern; (b-c) sialotomography images after administration of contrast agent in which both right (b) and left (c) parotid glands display dilated canalicular ducts. Parotid gland US exam: (e) B-Mode US longitudinal scan image displays severe PIH, with large anechoic areas, some measuring over 1 cm (arrowhead), frequently confluent, multiple cysts and calcifications (arrows), severely modified glandular architecture; (f) Power Doppler US image which shows increased vascularity pattern
Discussion
It is well established that HCV associates a wide variety of extrahepaticmanifestations. Among these, cryoglobulinemia has a relative high prevalence, estimated at 40-60% in most case series [9,10]. Although circulating cryoglobulins are frequently detected, an overt form of MC is rarer, being present in 5-10% of the cases [9], in which case the main target organs are the kidney, nerves, skin and joints. Other surrogate markers of positive cryoglobulinemia are low C3 levels, polyclonal or monoclonal (mostly IgM) hypergammaglobulinemia and positive RF activity, the latter being present in 70% of the cases. The core pathophysiological phenomenon in HCV-SS is the viral-induced sialadenitis. Experimental studies on mice established that sialotropism and molecular mimicry through expression of envelope proteins are the main mechanism of disease onset. Studies on transgenic mice for HCV envelope protein demonstrated the capacity of E1 and E2 envelope proteins to recruit lymphocytes, with the occurrence of sialadenitis [3].
SS-related markers that can occur in HCV infection are sicca symptoms, with a prevalence of 18%, sialadenitis with positive biopsy (i.e. lymphocytic infiltrate) in 25% percent of cases, RF activity (40% prevalence) and positive ANA. This overlap of extrahepatic manifestations with SS-features can lead to a false diagnosis of pSS. Although casual xerostomia is frequently noted, the presence of SS-specific antibodies (anti-Ro/SSA, anti-La/SSB) is rarely seen in HCV-SS patients, having a very low prevalence of 3-4% [4]. Another shared complication between HCV infection and pSS is the development of lymphoma. Thus, HCV infection may raise the risk of lymphoma, either by-itself as an extrahepatic complication through lymphotropism (5-fold increased risk) [11], through induced SS, or by the additive risk of both. A large study on 483 HCV-SS patients conducted by Ramos-Casals et al. offered statistical data regarding clinical and immunological aspects. Also they established some differentiating features between pSS and HCV-SS, listed in Table 1 [12]. One major aspect is the immunologic pattern seen in HCV-SS patients, with a predominance of cryoglobulinemic-related markers (mixed cryoglobulins, RF, hypocomplementemia) over SS-related markers (anti-Ro/SS-A and anti-La/SS-B autoantibodies).
Table 1.
Features related to HCV-SS in comparison to pSS
| Higher mean age on diagnosis |
| Lower frequency of parotidomegaly |
| Liver involvement in majority of patients |
| Clinical features of cryoglobulinemia Cutaneous vasculitis Peripheral neuropathy Renal involvement |
| Immunological patterns Higher frequency of cryoglobulins Higher frequency of RF activity Higher frequency of hypocomplementemia Lower frequency of positive anti-Ro and anti-La antibodies |
Referring to our two patients, the above mentioned balance between HCV-markers and SS-markers was tipped differently in each case. Clinical profile of patient A was clearly dominated by HCV-markers, with overt MC involving the kidneys, nerves and skin. Although she developed sialadenitis, with positive histopathological SS criteria, the ANA were absent, a common feature in HCV-SS. Nevertheless, her clinical profile fulfilled the established SS criteria. In contrast, patient B developed an atypical form, in which HCV-related markers (cryoglobulinemia, RF, low C3) were absent, while all pSS criteria were met, especially highly intense anti-Ro and anti-La antibodies. This is a very rare occurrence of HCV-SS dominated by SS-related markers. Also, the development of AIT in this patient should be placed into clinical context and her medication history. Thus three factors, i.e. HCV infection, induced SS or IFN therapy may trigger the onset of AIT. Also, an additional distinction between the two cases is the onset of SS. An asymmetrical, more severe glandular involvement was seen in patient B, probably due to the predominance of SS-markers. Also, the interval between HCV infection and SS onset was shorter (5y compared to 19y for patient A) in her case.
In conclusion, the frequent occurrence of casual sicca symptoms in HVC patients can sometimes lead to an apparently true pSS. HCV-SS may be a masked clinical entity, but should be considered even in the presence of all pSS diagnostic criteria. We described two clinical scenarios in which HCV induced the onset of SS, but with different immunological patterns, a classical form marked by overt MC and an atypical form dominated by SS features.
Abbreviations
SS-Sjogren Syndrome
pSS-primarySjogren Syndrome
sSS-secondarySjogren Syndrome
AECG-American European Consensus Group
HCV-hepatitis C virus
RF-rheumatoid factor
MC-mixed cryoglobulinemia
IFN-interferon
US-ultrasound
PIH-parenchymal inhomogeneity
SGB-salivary gland biopsy
ANA-antinuclear antibodies
AST-aspartate aminotransferase
PCR-polymerase chain reaction
ESR-erythrocyte sedimentation rate
AIT-autoimmune thyroiditis
CT-computed tomography
Acknowledgments
All authors equally contributed in the research and drafting of this paper.
The authors declare that they have no conflict of interests.
References
- 1.Bijlsma JWJ, da Silva J, Hachulla E, Doherty M, Cope A, Liotée F. In: EULAR Textbook on Rheumatic Diseases. Bijlsma JWJ, Hachulla E, editors. London: BMJ Group; 2012. [Google Scholar]
- 2.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koike K, Moriya K, Ishibashi K, Yotsuyanagi H, Shintani Y, Fujie H, Kurokawa K, Matsuura Y, Miyamura T. Sialadenitis histologically resembling Sjögren's Syndrome in mice transgenic for hepatitis C envelope genes. Proc Natl Acad Sci USA. 1997;94(1):233–236. doi: 10.1073/pnas.94.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramos-Casals M, Garcia-Carrasco M, Cervera R, Font J. Sjogren’s syndrome and hepatitis C virus. Clin Rheumatol. 1999;18(2):93–100. doi: 10.1007/s100670050064. [DOI] [PubMed] [Google Scholar]
- 5.Ramos-Casals M, Loustaud-Ratti V, De Vita S, Zeher M, Bosch JA, Toussirot E, Medina F, Rosas J, Anaya JM, Font J. Sjogren syndrome associated with hepatitis C virus: a multicenter analysis of 137 cases. Medicine (Baltimore) 2005;84(2):81–89. doi: 10.1097/01.md.0000157397.30055.c9. [DOI] [PubMed] [Google Scholar]
- 6.Ramos-Casals M, Jara LJ, Medina F, Rosas J, Calvo-Alen J, Mañá J, Anaya JM, Font J. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257(6):549–557. doi: 10.1111/j.1365-2796.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- 7.Salaffi F, Argalia G, Carotti M, Giannini FB, Palombi C. Salivary gland ultrasonography in the evaluation of primary Sjögren’s syndrome. Comparison with minor salivary gland biopsy. J Rheumatol. 2000;27(5):1229–1236. [PubMed] [Google Scholar]
- 8.Martinoli C, Derchi LE, Solbiati L, Rizzatto G, Silvestri E, Giannoni M. Color Doppler sonography of salivary glands. AJR Am J Roentgenol. 1994;163(4):933–941. doi: 10.2214/ajr.163.4.8092039. [DOI] [PubMed] [Google Scholar]
- 9.Cacoub P, Poynard T, Ghillani P, Charlotte F, Olivi M, Piette JC, Opolon P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheum. 1999;42(10):2204–2212. doi: 10.1002/1529-0131(199910)42:10<2204::AID-ANR24>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 10.Donada C, Crucitti A, Donadon V, Tommasi L, Zanette G, Crovatto M, Santini GF, Chemello L, Alberti A. Systemic manifestations and liver disease in patients with chronic hepatitis C and type II or III mixed cryoglobulinaemia. J Viral Hepat. 1998;5(3):179–185. doi: 10.1046/j.1365-2893.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95(9):745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos-Casals M, Theander E, Tzioufas AG. Digestive involvement in primary Sjogren syndrome. In: Font J, Ramos-Casals M, Rodes J, editors. Digestive involvement of autoimmune diseases. Amsterdam: Elsevier; 2008. pp. 71–81. [Google Scholar]


