Abstract
Purpose. Paper intended to present experimental evidences that adrenaline has a direct effect of inducing platelets aggregation in the concentration range 1-8µM.Material/Methods. Platelet rich plasma from patients of Colentina Clinical Hospital, following an informed consent. The platelet rich plasma (PRP) was prepared by centrifuging the anticoagulated sample at 200 G for 10 minutes. Aggregation was evaluated by optical aggregometry, classical method of Born, using Helena PACKS-4 Aggregometer.Results. The curves transmission light-time followed the structure: a lag-time, a first phase aggregation, more or less linear, defined by a “Slope 1”, a second wave of aggregation defined by “slope 2” and a “saturation” phase. Slope 1 increases with the concentration of adrenaline. The second slopes of the aggregation curves, maximum aggregation and areas under curves depended linear on adrenaline concentration. Conclusions.Adrenaline, in concentrations in the 1-8µM, induce aggregation of human platelets from platelet rich plasma. Linear regression models for slope and area were practically identical suggesting a rather unique than biphasic mechanism of action of adrenaline during the time course of aggregation.
Keywords: Adrenaline, Platelet Aggregation, DLVO
Introduction
Platelets aggregate at sites of vascular lesion, leading to the formation of thrombi that stop bleedings but also might occlude atherosclerotic arteries, resulting in reduced blood flow and being strongly linked with cardiac and cerebrovascular diseases. Platelet aggregation is a fundamental mechanism for life saving from blood loss and alterations of its normal course and it is implicated in all cardiovascular diseases, mainly after stroke or transient ischaemic accidents [1,2].
Platelet aggregation, the process by which platelets adhere to each other in contact with denudated sub-endothelial tissue, has been considered a key factor in the physiopathological chain of hemostatic plug formation and thrombosis [3,4,5,6,7].
Several excellent reviews discuss the role of platelets in haemostasis and thrombosis [8,9,10,11].
Excessive accumulation of platelets at sites of atherosclerotic plaque ulcerations is one of the main events accelerating arterial thrombus formation, and resulting in acute myocardial infarction, ischemic stroke and sudden death [12]. Since thrombosis leads to a higher morbidity and mortality than any other pathology, the plateletsare a major target for therapeutic intervention. Many factors contribute to the platelet-activating properties of ulcerated atheromatousplaques, including its high concentrationof fibrillar collagens [13,14,15], the presence of tissue factor [16], as well as the direct platelet-activating effects of high shear stress caused by arterial narrowing [17,18,19,20].
Many epidemiologic studies have demonstrated that coronary atherosclerosis and acute myocardial infarction occur more frequently in men who are subject to acute and recurrent stress [21,22,23]. Such individuals have been found to produce high levels of sympathetic catecholamines [24].
In vitro a number of investigators have found that sympathetic catecholamines will increase the "stickiness" of platelets and will cause platelet aggregation [25,26,27]. A number of investigators have postulated that the precipitating event in acute myocardial infarction may be the formation of a platelet thrombus intravascularly, that forms at or travels to, and occludes a segment of a coronary artery already narrowed by atherosclerosis [28,29].
Not much progress has been made in the understanding of platelet aggregation until the development of the platelet aggregometer in 1962 by Born [30] and independently by O’Brien[31], which measures platelet aggregation as a function of optical density of platelet suspension, as determined by means of a turbidometric method.
After this, optical aggregometry has been used worldwide in fundamental, clinical and epidemiological applications [32]. It was shown that adrenaline stimulated a cyclooxygenase independent pathway resulting in potentiation of platelet aggregation. Contrary to other reports, adrenaline also stimulated the cyclooxygenase pathway to an extent sufficient to generate TXB2 when platelets were under the inhibitory influence of acetylsalicylic acid [33].
Other studies suggest that adrenaline does not modify platelet membrane fluidity, as studied with the lipophilic fluorescent probe trimethylammonium-diphenylhexatriene. It has no direct effect on fibrinogen binding to intact platelets, intracellular Ca2+ levels measured by quin2, or protein phosphorylation. Adrenaline potentiates the action of all types of aggregating agents on aggregation, secretion, intracellular Ca2+ levels, membrane fluidity, fibrinogen binding, or protein phosphorylation. These effects are mediated by alpha 2-adrenergic agonists and inhibited by alpha 2-adrenergic antagonists. This study shows that adrenaline alone does not induce modifications of morphology, metabolism, or function of intact and functional washed human platelets and that it cannot be considered per se as an aggregating agent. However, adrenaline interacts with alpha 2-adrenergic receptors on human platelets and potentiates biochemical and aggregatory responses induced by other platelet agonists [34].
The aim of this study was to determine the dependence between the concentration of adrenaline and the extent of the platelet aggregation process.
Material and Method
Reagents
Adrenaline reagent:was purchased from Helena Laboratories and was of analytical grade.
Preparation for use: it was prepared a stock solution by reconstituting one vial with 1.0mL of distilled water. It was stirred gently until completely dissolved. After reconstitution it was obtained a stock solution of adrenaline bitartrate 3mM.
Storage and stability: The Adrenaline Reagent was stored in dry form at 2 to 80 C and was stable until the expiration date on the vial. The reconstituted reagent was stable for 1 week at 2-80C.
Signs of deterioration: when dry, unreconstitued regent was not uniformly white in appearance, it was not been used.
Specimen Collection and Handling
Specimen. Plasma obtained from whole blood collected with 3.2% sodium citrate as an anticoagulant was the specimen of choice.
Specimen collection. The blood was purchased from patients from internal medicine ward of Colentina Clinical Hospital, who gave informed consent before participating in this study. Blood was collected in evacuated test tubes.
Specimen preparation
1.The platelet rich plasma (PRP) was prepared by centrifuging the anticoagulated sample at 200 G for 10 minutes. The PRP was removed from the cells with a plastic Pasteur pipette and placed in a plastic tube labelled “PRP”. The tube was capped and maintained at room temperature. 30 minutes after PRP was removed the test begun.
2.The platelet poor plasma (PPP) was prepared by centrifuging the remaining blood samples at 3000 G for 15 minutes at room temperature. The PPP was removed, placed in a plastic tube, labelled PPP and covered. It was stored at room temperature.
Storage and stability
Plasma as well as whole blood was stored at room temperature (15-250 C). The samples were covered to maintain the pH. Test were performed within maximum three hours after sample collection.
Method
Platelet aggregation test was performed using Born turbidimetric light transmission method [30] using Helena PACKS-4 Aggregometer. The initial absorbance is caused by light scattered by the floating platelets in the solution. This absorbance is nearly proportional to the number of platelets. Platelet poor plasma (PPP) made from the same sample simulated 100% aggregation. Absorbance caused by factors other than platelets was determined by measuring the absorbance of PPP.
The aggregation capacity of the platelets was determined by the amount of aggregation induced when a known amount of reagent is added to PRP. The absorbance of the unreacted PRP mixed with the aggregation reagent represented 0% aggregation and the absorbance of the PPP control represented 100% aggregation (no floating platelets). As platelets aggregated, the number of floating platelets decreased, reducing the light absorbed by the PRP. It was measured human platelet aggregation by the absorbance method using up to four channels simultaneously. The absorbance curve for each channel was displayed during data acquisition. The absorbance data was displayed and stored at the conclusions of the measurements, and was printed for permanent records.
The employed Helena PACKS-4 Aggregometerand the tests were done at 1000rpm. Patient results were compared to normal ranges run under the same conditions.
The following steps were employed:
1.The blood specimens were collected and prepared according to directions in “Specimen Collection and Handling” section.
2.The aggregation agents were reconstituted according to the directions in “Reagent” section.
3.The Adrenaline Reagent used was obtained by diluting the 3 mM stock solution with saline solution (0.9% NaCl). Final concentrations in sample when using diluted reconstituted reagent: 1-8µM.
4.The employed aggregometer was prepared for use as recommended in the Operator’s Manual.
5.A volume of 450µL PPP was pipetted in a cuvette. This was the blank used to set the 100% aggregation.
6.A volume of 450µL PRP was pipetted in a cuvette with a stir bar. The cuvette was incubated at 370C for one to three minutes.
7.The PPP cuvette was inserted into the appropriate channel and the instrument was set to 100% aggregation.
8.The PRP cuvette was inserted into the appropriate channel.
9.A volume of 50µL of aggregating reagent dilutions were added to de PRP cuvette and the aggregation percent was recorded (instrument set 0% when aggregating agent is added and the channel activated).
Results and Discussions
First question addressed by the present paper was to evaluate the direct effect of adrenaline alone, not in association with other aggregants. Some authors considered that adrenaline does not induce modifications of morphology, metabolism, or function of intact and functional washed human platelets and that it cannot be considered per se as an aggregating agent [35].
It was reported for example the potentiation of adrenaline by ADP [36]. In this respect, our result was clear: adrenaline had a proagregant activity. This was an expected result since adrenaline was proved to have also and effect on the sedimentation of erythrocytes, most probable following an effect on their aggregation [37].
The second problem concerned concentrations required for obtaining aggregation. In a study on a large interval of adrenaline concentrations (0.25-16µM), was found that there areunder-threshold concentrations of adrenaline (0.03-1µM) where antiggregant effect appears only after association with collagen or serotonin [38]. At higher concentrations adrenaline alone is sufficient to promote aggregation. It is to underline that threshold is modified in different pathologic situations. For example it was found a decreased threshold of aggregation to low-dose adrenaline, which effect was interpreted as sign of platelet hyperaggregability in patients with thrombosis [39], mainly in sticky platelet syndrome (SPS), an autosomal dominant platelet disorder associated with arterial and venous thromboembolic events [40,41,42,43,44,45].(
Concentrations used in this study were 1-8µmol/l which means concentrations greater than the threshold. So that it was expected a correlation of our results with previous reported results, for adrenaline or combinations of adrenaline with other antiaggregantsat concentrations higher than 1µmol/l.
Form of curves.
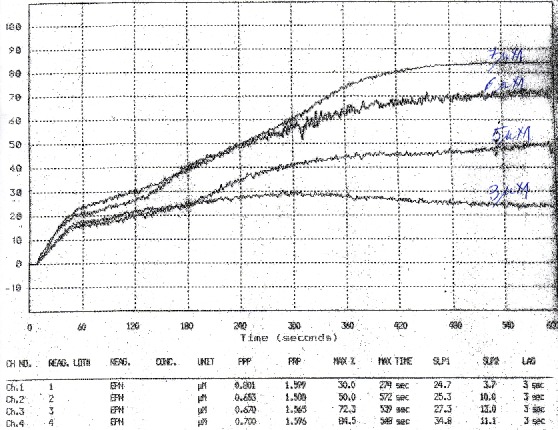
The curves transmission light-time followed as a rule, the structure: a lag-time, a first phase aggregation, more or less linear, defined by a “slope 1”, a second wave of aggregation, following the release of ADP from platelets and “enhancement” of aggregation, defined by “slope 2” and a “saturation” phase.
This is a possible standard behavior. As it can be seen for example in Fig. 1 these components are not clear separated or even are not present in some curves. The soft of aggregometer is identifying these phases in the curves and calculates associated parameters but the results have to be validated each time by visual inspections and considerations concerning phenomena in the back of obtained curves.
Figure 1.

Slope 1 as a function of adrenaline concentration in PRP

The primary “endpoint” considered in this study was the “area under aggregation curve” (AU-AGC). This parameter is largely used in pharmacokinetics and its application was recently extended in clinical studies for comparison of curves of pharmacodynamic effects [46].
Slope 1. Slope 1 increased with concentration of adrenaline as can be seen in Fig. 2 for the mean values corresponding to 8 patients. Dependence could be considered linear but the correlation for regression is small enough. Alternatively, results could be interpreted as different behavior for 1-3µmol/l adrenaline and 4-8µmol/l but there are not enough points to establish clear models of evolution.
Figure 2.

Slope 2 as a function of adrenaline concentration in PRP
Slope 2. The aggregation by adrenaline or ADP of human platelets occurs in two phases, and it has been suggested that the second phase is brought about by ADP released from the platelets [47].
In these conditions it was expected that the slopes in the second phasewould be greater than those in the first phase, i.e. slope 2>slope 1. This was not the case in our experiments, the mean slopes being practically equal. It is to note that, in case of slope 2 the dependence on adrenaline concentration was more clear linear, with a correlation coefficient much better than in case of slope 1.
AU-AGC
The most significant parameter was considered to be area under curve. As can be seen in Fig. 3, AU-AGC depended linear on adrenaline concentration. It is to note that automatic calculation performed by the software of the aggregometer considers as interval of integration time from zero to the end of measuring transmission of light T. In cases when aggregation reach its saturation in a short time t0, the area from t0to Thas no significance it concerns aggregation process and sensibility of AU-AGC parameter is decreased.
Figure 3.

AU-AGC as a function of adrenaline concentration in PRP
On other hand, if analysis refers to a longer interval of concentrations, obtained curves are both “short time” and “long time” and comparison have to take into consideration longest time interval. In our evaluations integral was in all cases from 0 to 10 minutes.
Since dependence of AU-AGC on concentration (Fig.3) looked similar to dependence of slope 2 on concentration, it was performed a normalization of AU-AGC to maximum value of slope 2 and the two parameters were represented in the same figures (Fig.4). It can be seen that similarity is real. The regression lines were practically parallel (slopes 3.86 and 3.69) which means a common mechanism in both linear and saturation parts of the sedimentation curves.
Figure 4.

Variation of AU-AGC and slope 2 as a function of adrenaline concentration in PRP, following normalization
Linear dependence on concentration was not reported as a characteristic until now but such result was obtained also in other studies though not observed by authors [48]. We represented the maximum platelet aggregation (MPA) estimated from curves obtained at different concentrations and obtained, in domain similar with our concentrations, an excellent linear regression.
On other hand a „saturation” or even a reverse of effect can appear with farther increasing of adrenaline concentration. If we consider that adrenaline influences the aggregation at the levelof repulsion electric forces [37], the biphasic dependence [49] of platelet electrophoretic mobility response to ADP or noradrenaline have to lead finally to a significant change in the dependence of aggregation on adrenaline concentration.
Conclusion
Adrenaline, in concentrations in the 1-8µM, induce aggregation of human platelets from platelet rich plasma.
The second slopes of the aggregation curves and areas under curves depended linear on adrenaline concentration.
Linear regression models for slope and area were practically identical suggesting a rather unique than biphasic mechanism of action of adrenaline during the time course of aggregation.
Acknowledgments
This paper was supported by the Sectorial Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/137390/.
References
- 1.Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood. 2006;108(6):1903–1910. doi: 10.1182/blood-2006-04-011551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. doi: 10.1182/blood-2006-12-027698. [DOI] [PubMed] [Google Scholar]
- 4.Conti CR, Mehta JL. Acute myocardial ischemia: role of atherosclerosis, thrombosis, platelet activation, coronary vasospasm, and altered arachidonic acid metabolism. Circulation. 1987;75(6 Pt 2):V84–V95. [PubMed] [Google Scholar]
- 5.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326(4):242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 6.Handin RI. Platelets and coronary artery disease. N Engl J Med. 1996;334(17):1126–1127. doi: 10.1056/NEJM199604253341710. [DOI] [PubMed] [Google Scholar]
- 7.Liu M, Wallmon A, Olsson-Mortlock C. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77(3):700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 8.Brass LF. Thrombin and platelet activation. CHEST. 2003;124:185–255. doi: 10.1378/chest.124.3_suppl.18s. [DOI] [PubMed] [Google Scholar]
- 9.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357(24):2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 10.Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. 2008;28(3):403–412. doi: 10.1161/ATVBAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 11.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99(12):1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell MJ, Westein E, Nesbitt WS, Giuliano S, Dopheide SM, Jackson SP. Identification of a 2-stage platelet aggregation process mediating shear-dependent thrombus formation. Blood. 2007;109(2):566–576. doi: 10.1182/blood-2006-07-028282. [DOI] [PubMed] [Google Scholar]
- 13.Badimon L. Atherosclerosis and thrombosis: lessons from animal models. ThrombHaemost. 2001;86(1):356–365. [PubMed] [Google Scholar]
- 14.Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987;67(1):9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- 15.Van Zanten GH, de Graaf S, Slootweg PJ, et al. Increased platelet deposition on atherosclerotic coronary arteries. J Clin Invest. 1994;93(2):615–632. doi: 10.1172/JCI117014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox JN, Smith KM, Schwartz SM et al. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci USA. 1989;86(8):2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kroll MH, Hellums JD, McIntire LV, et al. Platelets and shear stress. Blood. 1996;88(5):1525–1541. [PubMed] [Google Scholar]
- 18.Ikeda Y, Murata M, Goto S. von Willebrand factordependent shear-induced platelet aggregation: basic mechanisms and clinical implications. Ann N Y AcadSci. 1997;811:325–336. doi: 10.1111/j.1749-6632.1997.tb52012.x. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri ZM. von Willebrand factor. J Clin Invest. 1997;100(11 Suppl):S41–S46. [PubMed] [Google Scholar]
- 20.Andrews RK, Lopez JA, Berndt MC. Molecular mechanisms of platelet adhesion and activation. Int J Biochem Cell Biol. 1997;29(1):91–105. doi: 10.1016/s1357-2725(96)00122-7. [DOI] [PubMed] [Google Scholar]
- 21.Russek HI, Zohman BL. Relative significance of heredity, diet, and occupational stress in coronary heart disease of young adults: Based on analysis of 100 patients between the ages of 25 and 40 years and a similar group of 100 normal subjects. Am J Med Sci. 1958;235(3):266–277. doi: 10.1097/00000441-195803000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Pearson H, Joseph J. Stress and occlusive coronary artery disease. Lancet. 1963;1(7278):415–418. doi: 10.1016/s0140-6736(63)92304-3. [DOI] [PubMed] [Google Scholar]
- 23.Wolf S. Psychosocial forces in myocardial infarction and sudden death. Circulation. 1969;40(suppl IV):74–74. [Google Scholar]
- 24.Nestel PJ, Verghese A, Lovell RRH. Catecholamine secretion and sympathetic nervous responses to emotion in men with and without angina pectoris. AMJ. 1967;73(2):227–234. [Google Scholar]
- 25.O'brien JR. Some effects of adrenaline and antiadrenaline compounds platelets in vitro and in vivo. Nature. 1963;200:763–764. doi: 10.1038/200763a0. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JR, Sharp AA. Platelet clumping in vitro. Br J Haematol. 1964;10:78–93. doi: 10.1111/j.1365-2141.1964.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 27.Macmillan DC. Secondary clumping effect in human citrated platelet rich plasma produced by adenosine diphosphate and adrenaline. Nature. 1966;211(5045):140–144. doi: 10.1038/211140a0. [DOI] [PubMed] [Google Scholar]
- 28.Mustard JF, Packham MA. Platelet function and myocardial infarction. Circulation. 1969;9-40(Suppl 4):20–20. [Google Scholar]
- 29.Jorgensen L, Haerem J, Chandler AB et al. The pathology of acute coronary death. ActaAnaesthesiolScandSuppl. 1968;29:193–201. doi: 10.1111/j.1399-6576.1968.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 30.Born GV. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature. 1962;194:927–929. doi: 10.1038/194927b0. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien JR. Platelet aggregation. II. Some results from a new method of study. J ClinPathol. 1962;5(15):452–455. doi: 10.1136/jcp.15.5.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Born G, Patrono C. Antiplatelet drugs. British Journal of Pharmacology. Br J Pharmacol. 2006;147(Suppl 1):S241–S251. doi: 10.1038/sj.bjp.0706401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron HA, Ardlie NG. The facilitating effects of adrenaline on platelet aggregation. Prostaglandins Leukot Med. 1982;9(1):117–128. doi: 10.1016/0262-1746(82)90077-4. [DOI] [PubMed] [Google Scholar]
- 34.Lanza F, Beretz A, Stierlé A et al. Epinephrine potentiates human platelet activation but is not an aggregating agent. Am J Physiol. 1988;255(6 Pt 2):H1276–H1288. doi: 10.1152/ajpheart.1988.255.6.H1276. [DOI] [PubMed] [Google Scholar]
- 35.Lanza F, Beretz A, Stierlé A et al. Epinephrine potentiates human platelet activation but is not an aggregating agent. Am J Physiol. 1988;255(6 Pt 2):H1276–H1288. doi: 10.1152/ajpheart.1988.255.6.H1276. [DOI] [PubMed] [Google Scholar]
- 36.Alarayyed NA, Graham BR, Prichard BNC et al. The potentiation of adrenaline-induced in vitro platelet aggregation by ADP, collagen and serotonin and its inhibition by naftopidil and doxazosin in normal human subjects. Br J ClinPharmacol. 1995;39(4):369–374. doi: 10.1111/j.1365-2125.1995.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voicu VA, Mircioiu C, Jiquidi M, Gref R, Olteanu M. Studies concerning some effects of drugs, colloid vectors for drugs and decorporators on some physicochemical parameters of blood. In: Sohns T, Voicu VA, editors. NBC Risks. Current Capabilities and Future Perspectives for Protection. 1. Amsterdam: Kluwer Academic; 1999. pp. 311–330. [Google Scholar]
- 38.Alarayyed NA, Graham BR, Prichard BNC et al. The potentiation of adrenaline-induced in vitro platelet aggregation by ADP, collagen and serotonin and its inhibition by naftopidil and doxazosin in normal human subjects. Br J ClinPharmacol. 1995;39(4):369–374. doi: 10.1111/j.1365-2125.1995.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chelsea H, Sumire K, Tcherniantchouk O. Decreased threshold of aggregation to low-dose epinephrine is evidence of platelet hyperaggregability in patients with thrombosis. Hematol Rep. 2014;6(3):5326–5326. doi: 10.4081/hr.2014.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen EF. Sticky platelet syndrome. Semin Thromb Hemost. 1999;25(4):361–365. doi: 10.1055/s-2007-994939. [DOI] [PubMed] [Google Scholar]
- 41.Bick RL. Sticky platelet syndrome: a common cause of unexplained arterial and venous thrombosis. Clin Appl Thromb Hemost. 1998;4(2):77–81. [Google Scholar]
- 42.Mammen EF, Barnhart MI, Selik NR, et al. Sticky platelet syndrome: a congenital platelet abnormality predisposing to thrombosis? Folia HaematolInt Mag KlinMorpholBlutforsch. 1988;115:361–365. [PubMed] [Google Scholar]
- 43.Frenkel EP, Mammen EF. Sticky platelet syndrome and thrombocythemia. HematolOncolClin North Am. 2003;17(1):63–83. doi: 10.1016/s0889-8588(02)00096-5. [DOI] [PubMed] [Google Scholar]
- 44.Kubisz P, Stasko J, Holly P. Sticky platelet syndrome. Semin Thromb Hemost. 2013;39:674–683. doi: 10.1055/s-0033-1353394. [DOI] [PubMed] [Google Scholar]
- 45.Mammen EF. Ten years’ experience with the sticky platelet syndrome. SeminThrombHemost. 2013;39(6):674–683. [Google Scholar]
- 46.Mircioiu C, Borisova SA, Voicu VA. Biopharmaceutic Metrics Applied in Comparison of Clusters of time Courses of Effect in Clinical Trials. Journal of Applied Biopharmaceutics and Pharmacokinetics. 2013;1(1):37–44. [Google Scholar]
- 47.MacMillan DC. Secondary clumping effect in human citrated platelet-rich plasma produced by adenosine diphosphate and adrenaline. Nature. 1966;211(5045):140–144. doi: 10.1038/211140a0. [DOI] [PubMed] [Google Scholar]
- 48.Muhammad SR, Idrees FB, Muhammad A, et al. Dose response relationships of different reagents for platelet aggregation. Pak J Physiol. 2005;1((1-2):11–14. [Google Scholar]
- 49.Hampton JR. Platelet abnormalities induced by the administration of oestrogens. J Clin Path. 1969;3:75–80. [Google Scholar]


