Abstract
Background
Patients with chronic kidney disease (CKD) and type 2 diabetes mellitus (DM) have increased risk of endothelial dysfunction, cardiovascular disease, and mortality. Several studies have separately analyzed endothelial function in these populations. However, data of patients with both CKD and DM are scarce. The aim of this study was to evaluate whether the presence of DM has any additional effect on the endothelial dysfunction of CKD patients.
Methods
We measured endothelial progenitor cells (EPCs), stromal-derived factor 1 alpha (SDF-1α), serum and urinary nitric oxide (NO), flow-mediated dilation (FMD), and pulse wave velocity (PWV) in 37 CKD patients with DM (CKD-DM group) and in 37 without DM (CKD group).
Results
CKD-DM group had a higher prevalence of obesity (P < 0.01), previous myocardial infarction (P = 0.02), myocardial revascularization (P = 0.04), and a trend for more peripheral artery disease (P = 0.07). Additionally, CKD-DM group had higher EPC (P = 0.001) and PWV (P < 0.001) values. On the other hand, no difference in SDF-1α and serum or urinary NO and FMD was observed between the groups.
Conclusions
Endothelial dysfunction is frequent in CKD patients, and an additive effect of diabetes cannot be implicated, suggesting the predominant role of uremia in this condition.
1. Introduction
The Global Burden Disease 2010 study had highlighted chronic kidney disease (CKD) as an important cause for global mortality [1]. It is estimated that 10–15% of the adult population has CKD at various stages of severity [2]. This rate has grown [3] in parallel with the increasing incidence and prevalence of type 2 diabetes mellitus (DM) [3, 4], one of the main causes of CKD [4].
It is well known that patients with CKD and DM have higher mortality rates compared to their counterparts without DM [2, 5]. Cardiovascular disease (CVD) is the most important cause of mortality in CKD as well as in DM patients [6, 7]. Endothelial dysfunction, the initial lesion of atherosclerosis [8, 9], is an early marker of CVD frequently observed both in CKD [10, 11] and DM patients [12]. Several factors are associated with endothelial dysfunction in these populations [13, 14], such as uremic toxins and hyperglycemia, that are related to the depletion of endothelial nitric oxide (NO) [12, 14–16]. Moreover, uremic toxins stimulate the expression of adhesion molecules, which are also associated with endothelial dysfunction [14].
The evaluation of endothelial function includes the measurement of endothelial progenitor cells (EPCs), which have been shown to take part in the endogenous vascular repair system. The EPC count is considered to be a predictor of endothelial dysfunction and cardiovascular outcomes [17, 18] in populations with known high cardiovascular risk, who have reduced number or impaired function of EPC. Several studies have been demonstrated that EPC number was reduced in patients with isolated CKD and DM, compared to healthy controls [19, 20]. Ozuyaman et al. [21] demonstrated that EPC mobilization and function require NO. Among other factors and chemokines, stromal cell-derived factor-1 alpha (SDF-1α) is the most potent chemokine that mobilizes EPC from bone marrow to the injured vessel sites [22, 23]. The levels of SDF-1α are associated with increased CVD risk, both in general [24] and CKD patients [25].
Endothelial dysfunction can also be quantified by the degree of flow-mediated dilation (FMD) of the brachial artery, a widely used noninvasive technique [26]. The reduction of FMD occurs early in the development of atherosclerosis [27]. Several studies have shown that FMD is impaired in CKD [28, 29] as well as in DM patients [20, 30].
Data regarding endothelial dysfunction in patients with concomitant DM and CKD is scarce. Therefore, we aimed to evaluate the impact of DM on the endothelial function of patients with CKD.
2. Materials and Methods
2.1. Study Subjects
In this case-control study, 74 patients with CKD were recruited: 37 patients with DM (CKD-DM group) and 37 patients without DM (CKD or control group), from the outpatients CKD clinic of the Federal University of São Paulo, Brazil.
The inclusion criteria were age ≥ 18 years and CKD stages 3a–4. Regarding diabetic patients, only those on insulin therapy were included. Exclusion criteria were type 1 or secondary forms of DM; use of oral hypoglycemic agents, erythropoietin, or estrogen supplementation; malignancy in the last 5 years; hepatic insufficiency, New York Heart Association class III/IV heart failure, acute myocardial infarction, or peripheral arterial disease, decompensated in the last 6 months; acute infectious disease in the last 30 days; and pregnant or breastfeeding and regular smokers. Regular smokers were considered to be those consumers of at least one daily cigarette for at least six months.
All patients underwent an assessment of their medical history, physical examination, laboratory tests, and endothelial evaluation, including EPC number, SDF-1α, serum and urinary NO levels, and FMD.
The study was reviewed and approved by the Ethics Advisory Committee of the Federal University of São Paulo (approval number: 569.458). All patients gave written informed consent.
2.2. Laboratory Tests
After a 12-hour overnight fast, blood samples were collected to measure serum creatinine, glucose, glycosylated hemoglobin (HbA1c), total HDL and LDL cholesterol, triglycerides, potassium, sodium, intact parathyroid hormone (iPTH—chemiluminescent microparticle immunoassay performed at ARCHITECT i4000, Abbott), ionized calcium, phosphate, alkaline phosphatase, bicarbonate, and blood count. Serum SDF-1α was determined by enzyme immunoassay (ELISA, Elabscience, Wuhan, Hubei, China). Nitric oxide was quantified in serum and 24-hour urine sample by chemiluminescence, using nitric oxide analyzer (NOATM 280, Sievers Instruments Inc., Boulder, CO, USA). Albuminuria was measured in 24-hour urine sample. The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.
2.3. Flow Cytometry Analysis of Circulating EPCs
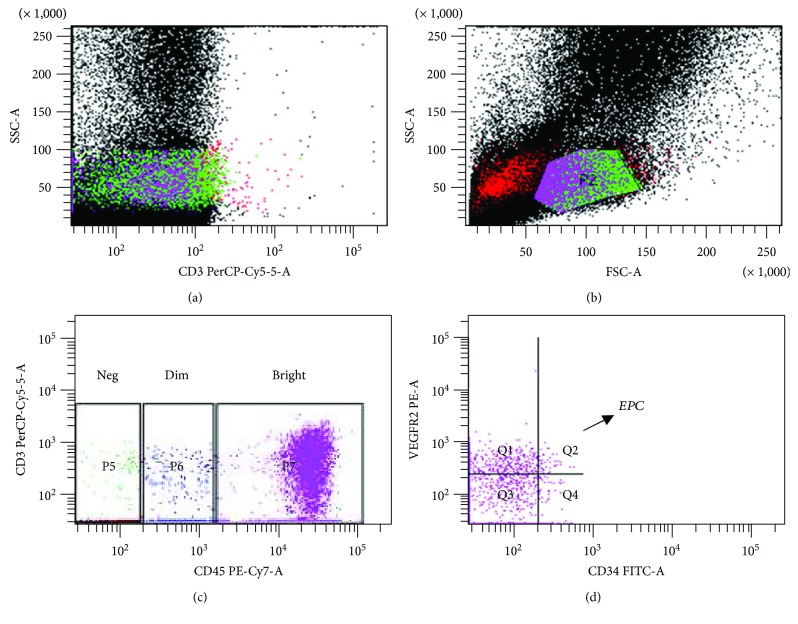
Ten milliliters of peripheral blood was collected in an EDTA tube for EPC analysis. The blood samples were processed within 4 hours after collection. Mononuclear cells were separated using Ficoll–Hypaque density gradient centrifugation (Sigma-Aldrich, St. Louis, USA) and washed using phosphate-buffered saline (PBS) (Sigma-Aldrich, St. Louis, USA). An automatic cell counter was used to ensure that in each analyzed tube there were 1,000,000 cells. Subsequently, the samples were exposed to the following antibodies: CD45-PE-Cy7 (BD Biosciences, San Diego, USA), CD34-FITC (BD Biosciences, San Diego, USA), and VEGFR2-PE (BD Biosciences, San Diego, USA). Isotype-stained samples were used as negative control. After incubation in the dark, excess antibody was removed by washing with PBS. Lastly, the cells were washed with PBS buffered with sodium azide and analyzed by flow cytometry. To facilitate lymphocyte gate demarcation, CD3-PerCP or CD3-APC lymphocyte markers (BD Biosciences, San Diego, USA) were used in most samples. After demarcation of this gate, EPCs were identified by the low expression of CD45 and by CD45-dim and by the double expression of CD34 and VEGFR2 (Figure 1), as previously described [20, 31, 32].
Figure 1.
Analysis of EPC by flow cytometry: (a) labeling with CD3-PerCP for identification of lymphocytes, (b) lymphocytic gate, (c) identification of cells with CD45-dim, and (d) after identification of item (c), selection of those with labeling for CD34 and VEGFR2 (Q2).
The detection of all antibodies was performed by a flow cytometer (FacsCanto I, BD Biosciences, San Diego, CA, USA). The gated data of CD3+ (T lymphocytes), CD45-PE-Cy7, CD34-FITC, and VEGFR2PE were presented as cells per 900,000 events.
2.4. Measurement of Brachial Artery FMD
Ultrasound-based measurements of brachial artery reactivity were performed according to the guidelines of the International Brachial Artery Reactivity Task Force [33]. The assessment of vascular reactivity was always carried out by the same examiner who was blinded to the group allocation. The brachial artery was assessed and measured in longitudinal section just above the antecubital fossa using a high-resolution ultrasound system (Sequoia Echocardiography System, version 6.0, Acuson, Siemens, Vernon, CA, USA) equipped with a multifrequency linear transducer (7–12 MHz) to produce two-dimensional images. The techniques used to evaluate the changes of FMD and nitrate-mediated dilation (NMD) after physical and pharmacological stimulation, respectively, are described elsewhere [34].
2.5. Pulse Wave Velocity (PWV)
As a surrogate marker of subclinical atherosclerosis, arterial stiffness was noninvasively measured by the PWV of the carotid and femoral arteries. PWV was carried out by the same examiner who was blinded to the group allocation using the Complior SP equipment (Artech Medical, Pantin, France) and then analyzed by appropriate software.
2.6. Statistical Analysis
Mean and standard deviation, median, and interquartile range or frequencies (proportion) were calculated for each variable, as appropriate. The Kolmogorov-Smirnov statistical test was used to investigate the normal distribution of data. Comparisons of continuous variables were performed using Student's t-test and the Mann-Whitney U test, for normal and skewed data, respectively. Comparisons of proportions were performed using chi-squared analysis or Fisher's exact test, as appropriate. Among the variables that evaluated endothelial function, FMD was the only one that showed normal distribution. Thus, generalized linear models (GLMs) were performed with normal or gamma distribution, according to the variable characteristics. For the assessment of FMD, the model was adjusted to the following variables: age, gender, peripheral artery disease, and use of acetylsalicylic acid and antihypertensive drugs; for the evaluation of SDF-1α: age, gender, and use of acetylsalicylic acid; and for the EPC assessment: use of acetylsalicylic acid, lipid-lowering agents, and CD3 type. P values < 0.05 were considered statistically significant. All statistical analysis was performed using the SPSS for Windows (SPSS 19.0, Chicago, IL, USA). The sample size was calculated based on previous study by Wong et al. [35]. For this calculation, a conservative approach was adopted and was performed using the Gpower 3.1.2 software. Assuming a difference in EPCs and FMD of 50%, a total of 74 subjects, 37 in each group, were required to reach a level of significance of 5% and a power of 80%.
3. Results
Characteristics of the CKD patients according to the presence (CKD-DM group) or absence (CKD group) of diabetes are listed in Tables 1 and 2. There was a predominance of elderly hypertensive men in both groups.
Table 1.
Clinic characteristics of the study population.
| CKD group (n = 37) | CKD-DM group (n = 37) | P | |
|---|---|---|---|
| Age, years | 65.9 ± 13.9 | 64.1 ± 9.9 | 0.54 |
| Male, n (%) | 21 (56.8%) | 22 (59.5%) | 0.814 |
| Hypertension, n (%) | 35 (97.2%) | 34 (94.4%) | 0.555 |
| Chronic kidney disease etiology, n (%) | <0.001 | ||
| Diabetes | 0 (0%) | 33 (89.2%) | |
| Undetermined | 19 (51.4%) | 1 (2.7%) | |
| Hypertension | 7 (18.9%) | 0 | |
| Glomerulopathy | 5 (13.5%) | 0 | |
| Others | 6 (16.2%) | 3 (8.1%) | |
| Cardiovascular disease, n (%) | 12 (32.4%) | 19 (51.4%) | 0.099 |
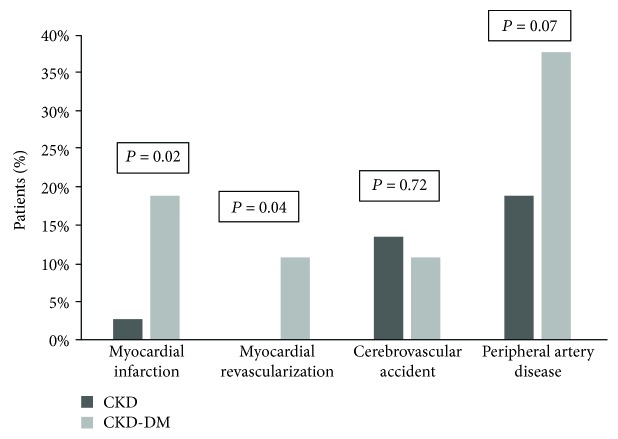
| Myocardial infarction | 1 (2.7%) | 7 (18.9%) | 0.025 |
| Myocardial revascularization | 0 (0%) | 4 (10.8%) | 0.04 |
| Cerebrovascular accident | 5 (13.5%) | 4 (10.8%) | 0.722 |
| Peripheral artery disease | 7 (18.9%) | 14 (37.8%) | 0.071 |
| Drugs, n (%) | |||
| ACEI/ARB | 27 (75%) | 33 (91.7%) | 0.058 |
| Calcium channel blockers | 27 (75%) | 17 (47.2%) | 0.016 |
| Diuretics | 25 (69.4%) | 34 (94.4%) | 0.006 |
| Vasodilator | 2 (5.6%) | 7 (19.4%) | 0.075 |
| Lipid-lowering agents | 24 (68.6%) | 30 (85.7%) | 0.088 |
| Acetylsalicylic acid | 14 (38.9%) | 27 (73%) | 0.003 |
| Systolic blood pressure, mmHg | 130 (125–140) | 140 (123.5–158.5) | 0.214 |
| Diastolic blood pressure, mmHg | 80 (70–90) | 77 (70–84) | 0.343 |
| Body mass index, kg/m2 | 27.7 ± 4.7 | 31.4 ± 5.7 | 0.004 |
| Waist-hip circumference ratio | 0.97 ± 0.07 | 1.00 ± 0.06 | 0.032 |
ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker.
Table 2.
Laboratorial characteristics of the study population.
| CKD group (n = 37) | CKD-DM group (n = 37) | P | |
|---|---|---|---|
| Creatinine, mg/dl | 2.33 ± 0.65 | 2.22 ± 0.65 | 0.442 |
| CKD-EPI, ml/min/1.73 m2 | 24 (21–34.5) | 28 (23.5–35.5) | 0.267 |
| Albuminuria, μg/min (24 h) | 42.1 (11.5–131.7) | 132.3 (39.5–767.4) | 0.014 |
| Glucose, mg/dl | 88 (85–92) | 142 (80–206) | 0.003 |
| Hemoglobin A1c, % | 5.6 (5.3–5.9) | 8.4 (7.2–9.9) | <0.001 |
| Total cholesterol, mg/dl | 163 (151–187) | 183 (141–217) | 0.141 |
| HDL cholesterol, mg/dl | 52 (42–61) | 46 (40–53) | 0.074 |
| LDL cholesterol, mg/dl | 90 (70–105) | 103 (71–123) | 0.113 |
| Triglycerides, mg/dl | 128 (90–183) | 176 (126–305) | 0.002 |
| Ionized calcium, mmol/l | 1.31 ± 0.06 | 1.29 ± 0.06 | 0.095 |
| Phosphate, mg/dl | 3.5 ± 0.6 | 3.6 ± 0.6 | 0.338 |
| Alkaline phosphatase, U/l | 67 (60–86) | 80 (66–95) | 0.036 |
| Bicarbonate, mmol/l | 24.8 ± 2.8 | 26.9 ± 3.4 | 0.005 |
| Parathyroid hormone, pg/ml | 163 (96–240) | 167 (117–210) | 0.948 |
| Hemoglobin, g/dl | 13.5 ± 1.6 | 13.7 ± 1.6 | 0.607 |
| Pulse wave velocity, m/s | 8.5 ± 1.8 | 10.3 ± 1.7 | <0.001 |
HDL = high-density lipoprotein; LDL = low-density lipoprotein.
When compared to the CKD group, patients with CKD-DM showed a higher prevalence of previous myocardial infarction, myocardial revascularization, and a trend for more peripheral artery disease (Figure 2). Diabetic patients received more diuretic and acetylsalicylic acid but less calcium channel blocker. There was no difference in the use of ACEI/ARB or lipid-lowering drugs. The CKD-DM group had higher proportion of obese individuals (21 (57%) vs. 9 (24%); P = 0.004). Of note, only 2 patients in the CKD group had waist-hip circumference ratio within normal range. A higher prevalence of patient with uncontrolled systolic blood pressure was observed among CKD-DM patients (20 (54) vs. 11 (30) %; P = 0.034).
Figure 2.
Previous cardiovascular disease according to the groups.
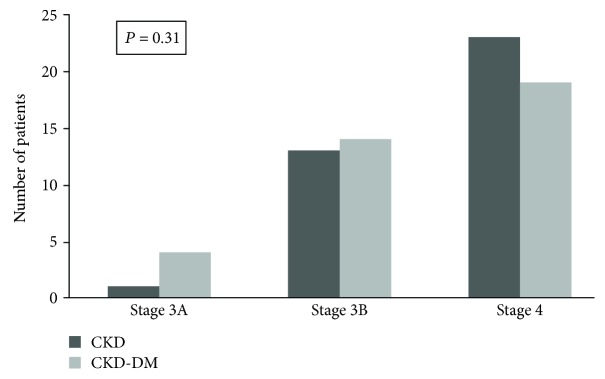
Renal function as well as the distribution of patients according to CKD stages did not differ between groups (Figure 3). As expected, the CKD-DM group had higher albuminuria, glucose, HbA1c, and triglyceride levels. There was a trend towards lower HDL in this group. Bicarbonate was significantly higher in the CKD-DM group, although the supplementation of bicarbonate was similar in both groups. Alkaline phosphatase was significantly higher in the CKD-DM group; however, only 3 patients (2 of CKD-DM group) presented serum levels above the normal range. There was no difference in hemoglobin (Hb) concentration between the groups, and all patients had Hb greater than 10 mg/dl. The PWV was higher in the CKD-DM group as well as the proportion of patients with increased values (21 (60) vs. 8 (22) %; P = 0.001).
Figure 3.
Distribution of patients based on stages of CKD.
EPC number was higher in the CKD-DM group compared to CKD. The FMD was similar, showing low values in both groups. Of note, 10% of the patients in each group failed to display any dilation during the test. No difference in SDF-1α and serum or urinary NO between the groups was observed (Table 3).
Table 3.
Endothelial dysfunction markers in CKD and CKD-DM groups.
| CKD group (n = 37) | CKD-DM group (n = 37) | P | |
|---|---|---|---|
| FMD, % | 2.68 ± 3.11 | 2.95 ± 3.69 | 0.737 |
| NMD, % | 11.51 ± 6.05 | 9.26 ± 5.47 | 0.104 |
| EPC, % | 0.25 (0.1–0.6) | 0.60 (0.3–0.9) | 0.009 |
| SDF-1α, pg/ml | 3730 (2915–4830) | 3430 (2695–4770) | 0.699 |
| Serum nitric oxide, μmol/l | 390.5 (296.5–568.8) | 387.5 (241.5–613.8) | 0.641 |
| 24 h urinary nitric oxide, μmol | 3432 (1593–5521) | 3336 (1213–5896) | 0.734 |
FMD = flow-mediated dilation; NMD = nitrate-mediated dilation; EPC = endothelial progenitor cell; SDF-1α = stromal cell-derived factor 1.
When the sample was divided based on CKD stages, there was no difference in endothelial parameters (EPC, SDF-1α, serum, and urinary NO and FMD) (Table 4).
Table 4.
Endothelial dysfunction markers based on CKD stages.
| CKD 3 | CKD 4 | P | |
|---|---|---|---|
| FMD, % | 2.78 ± 3.26 | 2.85 ± 3.55 | 0.930 |
| EPC, % | 0.4 (0.2–1.0) | 0.3 (0.1–0.7) | 0.180 |
| SDF-1α, pg/ml | 3535 (2935–4927) | 3800 (2697–4472) | 0.739 |
| Serum nitric oxide, μmol/l | 414.0 (294.0–581.2) | 347.0 (260.0–586.9) | 0.659 |
| 24 h urinary nitric oxide, μmol | 3121 (2224–6348) | 3149 (1010–4976) | 0.252 |
CKD 3 = chronic kidney disease stage 3; CKD 4 = chronic kidney disease stage 4; FMD = flow-mediated dilation; EPC = endothelial progenitor cell; SDF-1α = stromal cell-derived factor 1.
4. Discussion
The present study has demonstrated a high prevalence of endothelium dysfunction in CKD patients regardless the presence of diabetes. All the endothelial dysfunction markers, but EPC number, were similar in CKD patients with and without diabetes.
Few studies, including dialysis and nondialysis patients, demonstrated a similar number of EPCs in CKD patients with and without DM [36–38]. In contrast with these studies, our results showed that CKD-DM patients had higher EPC number. This unexpected finding could be related to the fact that all diabetic patients were using insulin, which is known to increase the EPC number [39, 40]. Moreover, one could hypothesize that this elevated number of EPC reflects a better activity of the endogenous vascular repair system. However, our CKD-DM patients had high prevalence of cardiovascular disease and an inadequate arterial stiffness, revealed by the increased PWV. Based on that, we could speculate that the EPC could be dysfunctional or the increased number might be insufficient to repair the vessels.
It is well known that diabetic patients have EPC dysfunction [20, 41] mainly due to hyperglycemia [41, 42]. High glucose level leads to an increasing of advanced glycation end products, reactive oxygen species, and inflammatory cytokines, factors that could induce EPC dysfunction [12, 20, 43]. Likewise, there are substantial data indicating EPC dysfunction in CKD patients [16, 44]. Uremic environment causes a deficient NO production, which leads to a decreased EPC mobilization [38]. Additionally, uremic toxins were found to cause EPC dysfunction by inhibiting migratory activity, adhesion to matrix proteins and to endothelial cells [38, 45]. Corroborating with that, studies have suggested that the reduction of uremic toxins by kidney transplantation improves EPC function [46, 47]. Unfortunately, we did not evaluate EPC function in the present study.
Other factor that could be related to the endothelial dysfunction observed in our CKD patients is the EPC resistance. Herbrig et al. observed that chronically elevated SDF-1α levels result in impair EPC homing to sites of vascular damage, indicating EPC resistance [46]. Of note, Jie et al. demonstrated that SDF-1α is increased in CKD patients due to its reduced renal clearance [19]. On the other hand, in diabetic patients, SDF-1α concentration has been shown to be decreased. The diminished concentration of SDF-1α, observed in that diabetic population, was associated to the reduction of EPC releasing to the damaged vessels [48]. In the present study, SDF-1α levels were high in both CKD and CKD-DM groups, which may suggest that uremia effect on SDF-1α overcomes that of diabetes. Supporting this hypothesis, serum and urinary NO and FMD values were similar in both groups. In agreement with this finding, previous studies, including nondialysis [49] and dialysis [50] patients, were not able to demonstrate that the presence of diabetes had influenced FMD, even after adjustments for confounding factors.
Some limitations of this study should be acknowledged, such as the relative small sample size, the absence of a healthy control group, and its cross-sectional design. Nevertheless, to the best of our knowledge, this is the first study designed to investigate the additive effect of diabetes on the endothelial function of CKD patients.
5. Conclusion
Endothelial dysfunction is frequent in CKD patients, and an additive effect of diabetes cannot be implicated, suggesting the predominant role of uremia in this condition.
Acknowledgments
The authors thank Silvia Regina Manfredi, Victória Cabral S M de Godoy, and Caren Cristina Grabulosa for helping in the execution of this study, as well as Pedro Barbosa for his contribution in the English correction.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Lozano R., Naghavi M., Foreman K., et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System. National Institutes of Health. Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases; 2015. 2015 USRDS annual data report: epidemiology of kidney disease in the United States. [Google Scholar]
- 3.Molitch M. E., Adler A. I., Flyvbjerg A., et al. Diabetic kidney disease: a clinical update from Kidney Disease: Improving Global Outcomes. Kidney International. 2015;87(1):20–30. doi: 10.1038/ki.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuttle K. R., Bakris G. L., Bilous R. W., et al. Diabetic kidney disease: a report from an ADA consensus conference. Diabetes Care. 2014;37(10):2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Afkarian M., Katz R., Bansal N., et al. Diabetes, kidney disease, and cardiovascular outcomes in the Jackson Heart Study. Clinical Journal of the American Society of Nephrology. 2016;11(8):1384–1391. doi: 10.2215/CJN.13111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidney Disease: Improving Global Outcomes (KDIGO) Work. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney International. 2009;76(Supplement 113):S1–S2. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese G., Solini A., Bonora E., et al. Chronic kidney disease in type 2 diabetes: lessons from the Renal Insufficiency and Cardiovascular Events (RIACE) Italian Multicentre Study. Nutrition, Metabolism, and Cardiovascular Diseases. 2014;24(8):815–822. doi: 10.1016/j.numecd.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Henderson A. Endothelial dysfunction: a reversible clinical measure of atherogenic susceptibility and cardiovascular inefficiency. International Journal of Cardiology. 1997;62(Supplement 1):S43–S48. doi: 10.1016/S0167-5273(97)00212-X. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 10.Jourde-Chiche N., Dou L., Cerini C., Dignat-George F., Brunet P. Vascular incompetence in dialysis patients--protein-bound uremic toxins and endothelial dysfunction. Seminars in Dialysis. 2011;24(3):327–337. doi: 10.1111/j.1525-139X.2011.00925.x. [DOI] [PubMed] [Google Scholar]
- 11.Wheeler D. C. Cardiovascular disease in patients with chronic renal failure. The Lancet. 1996;348(9043):1673–1674. doi: 10.1016/S0140-6736(05)65816-3. [DOI] [PubMed] [Google Scholar]
- 12.Calles-Escandon J., Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocrine Reviews. 2001;22(1):36–52. doi: 10.1210/edrv.22.1.0417. [DOI] [PubMed] [Google Scholar]
- 13.Malyszko J. Mechanism of endothelial dysfunction in chronic kidney disease. Clinica Chimica Acta. 2010;411(19-20):1412–1420. doi: 10.1016/j.cca.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Zhang K., Yin F., Lin L. Circulating endothelial cells and chronic kidney disease. BioMed Research International. 2014;2014:7. doi: 10.1155/2014/364738.364738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams S. B., Cusco J. A., Roddy M. A., Johnstone M. T., Creager M. A. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 1996;27(3):567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz M. I., Saglam M., Caglar K., et al. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. American Journal of Kidney Diseases. 2006;47(1):42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Rigato M., Avogaro A., Fadini G. P. Levels of circulating progenitor cells, cardiovascular outcomes and death. Circulation Research. 2016;118(12):1930–1939. doi: 10.1161/CIRCRESAHA.116.308366. [DOI] [PubMed] [Google Scholar]
- 18.Lu C. L., Leu J. G., Liu W. C., et al. Endothelial progenitor cells predict long-term mortality in hemodialysis patients. International Journal of Medical Sciences. 2016;13(3):240–247. doi: 10.7150/ijms.14209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jie K. E., Zaikova M. A., Bergevoet M. W. T., et al. Progenitor cells and vascular function are impaired in patients with chronic kidney disease. Nephrology, Dialysis, Transplantation. 2010;25(6):1875–1882. doi: 10.1093/ndt/gfp749. [DOI] [PubMed] [Google Scholar]
- 20.Chen L. L., Yu F., Zeng T. S., Liao Y. F., Li Y. M., Ding H. C. Effects of gliclazide on endothelial function in patients with newly diagnosed type 2 diabetes. European Journal of Pharmacology. 2011;659(2-3):296–301. doi: 10.1016/j.ejphar.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 21.Ozuyaman B., Ebner P., Niesler U., et al. Nitric oxide differentially regulates proliferation and mobilization of endothelial progenitor cells but not of hematopoietic stem cells. Thrombosis and Haemostasis. 2005;94(4):770–772. doi: 10.1160/TH05-01-0038. [DOI] [PubMed] [Google Scholar]
- 22.Sabatier F., Camoin-Jau L., Anfosso F., Sampol J., Dignat-George F. Circulating endothelial cells, microparticles and progenitors: key players towards the definition of vascular competence. Journal of Cellular and Molecular Medicine. 2009;13(3):454–471. doi: 10.1111/j.1582-4934.2008.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled A., Grabovsky V., Habler L., et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. The Journal of Clinical Investigation. 1999;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian S., Liu C., Aviv A., et al. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(9):2100–2105. doi: 10.1161/ATVBAHA.114.303579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehta N. N., Matthews G. J., Krishnamoorthy P., et al. The Chronic Renal Insufficiency Cohort (CRIC) Study investigators; higher plasma CXCL12 levels predict incident myocardial infarction and death in chronic kidney disease: findings from the Chronic Renal Insufficiency Cohort Study. European Heart Journal. 2014;31:2115–2122. doi: 10.1093/eurheartj/eht481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ras R. T., Streppel M. T., Draijer R., Zock P. L. Flow-mediated dilation and cardiovascular risk prediction: a systematic review with meta-analysis. International Journal of Cardiology. 2013;168(1):344–351. doi: 10.1016/j.ijcard.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 27.Raitakari O. T., Celermajer D. S. Flow-mediated dilatation. British Journal of Clinical Pharmacology. 2000;50(5):397–404. doi: 10.1046/j.1365-2125.2000.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuczmarski J. M., Darocki M. D., DuPont J. J., et al. Effect of moderate-to-severe chronic kidney disease on flow-mediated dilation and progenitor cells. Experimental Biology and Medicine. 2011;236(9):1085–1092. doi: 10.1258/ebm.2011.011008. [DOI] [PubMed] [Google Scholar]
- 29.Lin C. J., Wu C. J., Wu P. C., et al. Indoxyl sulfate impairs endothelial progenitor cells and might contribute to vascular dysfunction in patients with chronic kidney disease. Kidney & Blood Pressure Research. 2016;41(6):1025–1036. doi: 10.1159/000452604. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone M. T., Creager S. J., Scales K. M., Cusco J. A., Lee B. K., Creager M. A. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88(6):2510–2516. doi: 10.1161/01.CIR.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 31.Van Craenenbroeck E. M., Van Craenenbroeck A. H., van Ierssel S., et al. Quantification of circulating CD34+/KDR+/CD45dim endothelial progenitor cells: analytical considerations. International Journal of Cardiology. 2013;167(5):1688–1695. doi: 10.1016/j.ijcard.2012.10.047. [DOI] [PubMed] [Google Scholar]
- 32.Fadini G. P., Losordo D., Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circulation Research. 2012;110(4):624–637. doi: 10.1161/CIRCRESAHA.111.243386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Corretti M. C., Anderson T. J., Benjamin E. J., et al. International Brachial Artery Reactivity Task Force; guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39(2):257–265. doi: 10.1016/S0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 34.Giribela C. R. G., Melo N. R., Silva R. C. G., et al. A combined oral contraceptive containing drospirenone changes neither endothelial function nor hemodynamic parameters in healthy young women: a prospective clinical trial. Contraception. 2012;86(1):35–41. doi: 10.1016/j.contraception.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Wong C. Y., Yiu K. H., Li S. W., et al. Fish-oil supplement has neutral effects on vascular and metabolic function but improves renal function in patients with type 2 diabetes mellitus. Diabetic Medicine. 2010;27(1):54–60. doi: 10.1111/j.1464-5491.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 36.Westerweel P. E., Hoefer I. E., Blankestijn P. J., et al. End-stage renal disease causes an imbalance between endothelial and smooth muscle progenitor cells. American Journal of Physiology. Renal Physiology. 2007;292(4):F1132–F1140. doi: 10.1152/ajprenal.00163.2006. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez-Ayala E., Yao Q., Holmén C., Lindholm B., Sumitran-Holgersson S., Stenvinkel P. Imbalance between detached circulating endothelial cells and endothelial progenitor cells in chronic kidney disease. Blood Purification. 2006;24(2):196–202. doi: 10.1159/000090519. [DOI] [PubMed] [Google Scholar]
- 38.Choi J. H., Kim K. L., Huh W., et al. Decreased number and impaired angiogenic function of endothelial progenitor cells in patients with chronic renal failure. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(7):1246–1252. doi: 10.1161/01.ATV.0000133488.56221.4a. [DOI] [PubMed] [Google Scholar]
- 39.Fadini G. P., de Kreutzenberg S. V., Mariano V., et al. Optimized glycaemic control achieved with add-on basal insulin therapy improves indexes of endothelial damage and regeneration in type 2 diabetic patients with macroangiopathy: a randomized crossover trial comparing detemir versus glargine. Diabetes, Obesity & Metabolism. 2011;13(8):718–725. doi: 10.1111/j.1463-1326.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 40.António N., Fernandes R., Soares A., et al. Reduced levels of circulating endothelial progenitor cells in acute myocardial infarction patients with diabetes or pre-diabetes: accompanying the glycemic continuum. Cardiovascular Diabetology. 2014;13(1):p. 101. doi: 10.1186/1475-2840-13-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yue W. S., Lau K. K., Siu C. W., et al. Impact of glycemic control on circulating endothelial progenitor cells and arterial stiffness in patients with type 2 diabetes mellitus. Cardiovascular Diabetology. 2011;10(1):p. 113. doi: 10.1186/1475-2840-10-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yiu K. H., Tse H. F. Specific role of impaired glucose metabolism and diabetes mellitus in endothelial progenitor cell characteristics and function. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(6):1136–1143. doi: 10.1161/ATVBAHA.114.302192. [DOI] [PubMed] [Google Scholar]
- 43.Hamed S., Brenner B., Roguin A. Nitric oxide: a key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type 2. Cardiovascular Research. 2011;91(1):9–15. doi: 10.1093/cvr/cvq412. [DOI] [PubMed] [Google Scholar]
- 44.Brunet P., Gondouin B., Duval-Sabatier A., et al. Does uremia cause vascular dysfunction? Kidney & Blood Pressure Research. 2011;34(4):284–290. doi: 10.1159/000327131. [DOI] [PubMed] [Google Scholar]
- 45.Herbrig K., Pistrosch F., Oelschlaegel U., et al. Increased total number but impaired migratory activity and adhesion of endothelial progenitor cells in patients on long-term hemodialysis. American Journal of Kidney Diseases. 2004;44(5):840–849. doi: 10.1016/S0272-6386(04)01083-2. [DOI] [PubMed] [Google Scholar]
- 46.Herbrig K., Gebler K., Oelschlaegel U., et al. Kidney transplantation substantially improves endothelial progenitor cell dysfunction in patients with end-stage renal disease. American Journal of Transplantation. 2006;6(12):2922–2928. doi: 10.1111/j.1600-6143.2006.01555.x. [DOI] [PubMed] [Google Scholar]
- 47.de Groot K., Bahlmann F. H., Bahlmann E., Menne J., Haller H., Fliser D. Kidney graft function determines endothelial progenitor cell number in renal transplant recipients. Transplantation. 2005;79(8):941–945. doi: 10.1097/00007890-200504270-00012. [DOI] [PubMed] [Google Scholar]
- 48.Jose T., Inzucchi S. E. Cardiovascular effects of the DPP-4 inhibitors. Diabetes & Vascular Disease Research. 2012;9(2):109–116. doi: 10.1177/1479164111436236. [DOI] [PubMed] [Google Scholar]
- 49.Takenaka T., Takane H., Kikuta T., Watanabe Y., Suzuki H. Statin improves flow-mediated vasodilation in chronic kidney diseases. International Journal of Hypertension. 2013;2013:9. doi: 10.1155/2013/876865.876865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S., Ryu J. H., Kim S. J., Ryu D. R., Kang D. H., Choi K. B. The relationship between magnesium and endothelial function in end-stage renal disease patients on hemodialysis. Yonsei Medical Journal. 2016;57(6):1446–1453. doi: 10.3349/ymj.2016.57.6.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.