Abstract
Purpose
Modern neuroimaging techniques allow investigating brain structures and substances involved in the pathophysiology of mental disorders, trying to find new markers of these disorders. To better understanding of the pathophysiology and differential diagnosis of schizophrenia and bipolar disorder, this study was conducted to assess the neurochemical alterations in the frontal and temporal lobes in hospitalized patients with schizophrenia and bipolar disorder.
Methods
Twenty-one subjects with schizophrenia (paranoid and differentiated types), 16 subjects with bipolar I disorder (manic, depressive, and mixed episode), and 20 healthy subjects were studied. Magnetic resonance (MR) imaging and proton resonance magnetic spectroscopy (1H MRS) were performed on a 1.5 T scanner. Voxels of 8 cm3 were positioned in the left frontal and left temporal lobes.
Results
Glx/H2O (GABA, glutamine, and glutamate/nonsuppressed water signal) ratios were significantly increased in the left temporal lobe in schizophrenia, but not in bipolar disorder, compared with controls. Cho/H2O (choline/nonsuppressed water signal) ratios in the left frontal lobe had a tendency to increase in bipolar disorder and schizophrenia, relative to controls. A lower temporal lobe NAA/H2O ratio in mixed than in manic and depressive episode of bipolar patients was also found. No other significant differences were found among three studied groups as regards NAA, Cr, and mI ratios.
Conclusions
Our results partially confirm the role of a glutamatergic system in schizophrenia, however, only in a temporal lobe. We also point to the importance of the choline-containing compounds (marker of cellular density) in the frontal lobe of patients suffering from bipolar disorder and schizophrenia. We also found the deleterious effect of mixed bipolar episode on the integrity and functioning of the temporal lobe. Glutamatergic left temporal spectroscopic changes may potentially help in differential diagnosis of schizophrenia from bipolar disorder.
1. Introduction
The etiology of schizophrenia and bipolar disorder has not been yet well recognized. Over 100 years ago, Kraepelin proposed dementia praecox and manic-depressive psychosis as two separate diseases. Nevertheless, these two disorders share several clinical features such as psychotic symptoms, typical onset in young adults and earlier in males, as well as the frequent occurrence of life events prior to the onset or relapse [1]. Fischer and Carpenter suggest that overlapping features such as psychotic symptoms are not decisive in differential diagnosis and in each disorder are rather a syndrome, not a disease entity [2]. Furthermore, the genetic studies show that schizophrenia and bipolar disorder share certain susceptibility genes, e.g., CACNA1C, NT5C2, and CCDC68 [3]. The genetic risk for schizophrenia is associated with gray matter volume deficits in the bilateral fronto-striato-thalamic and left lateral temporal regions, whereas the genetic risk for bipolar disorder is specifically associated with gray matter deficits only in the right anterior cingulated gyrus and ventral striatum [4].
Modern neuroimaging techniques allow investigating not only brain structures but also substances involved in pathophysiology of mental disorders. Proton magnetic resonance spectroscopy (1H MRS) is a useful tool that detects brain metabolites such as NAA (N-acetylaspartate), a marker for neuronal integrity or neuronal-glial homeostasis; Glx (GABA, glutamine, and glutamate); Cho (choline containing compounds), a measure of cellular density; Cr (creatine plus phosphocreatine), a marker of cellular energy level; and mI (myo-inositol), a marker of brain osmotic balance and glial cells [5, 6]. It is known that not only MRI brain imaging or neurophysiological measures but also the neurochemical changes detected by MRS may be biomarkers of schizophrenia and other neuropsychiatric disorders, e.g., posttraumatic stress disorder, antidepressant treatment response, or binge drinking [7–10].
The meta-analysis of 1H MRS studies in schizophrenia revealed lower NAA level in the brain of patients with schizophrenia, primarily in the hippocampus, and in the gray and white matter of the frontal lobe [11]. Also, the systematic review of 1H MRS findings in bipolar disorder revealed that NAA levels were lower in euthymic bipolar patients in the frontal lobe and hippocampus and Cho/Cr ratios were higher in the basal ganglia of euthymic patients [12]. Glutamate/glutamine levels were higher in all mood states compared to controls. The combined meta-analyses of neurometabolite alterations in both disorders: schizophrenia and bipolar disorder, revealed that NAA levels were affected in schizophrenia and bipolar disorder [13]. The most consistent findings were decreased NAA levels in the basal ganglia and frontal lobe for schizophrenia, but only in the basal ganglia for bipolar disorder. Cho and Cr levels were not altered in either disorder. There are only few studies which directly compare the spectroscopic measures in both disorders [14–20]. These sparse spectroscopy studies report evidence of decreased neuronal integrity in cortical gray matter in both disorders [21]. In order to get better understanding of (to clarify) the pathophysiology of schizophrenia and bipolar disorder, this study was conducted to assess the neurochemical alterations in the frontal and temporal lobes in hospitalized patients with schizophrenia and bipolar disorder.
2. Material and Methods
2.1. Subjects
Twenty-one subjects with schizophrenia, 16 subjects with bipolar I disorder, and 20 healthy subjects were studied. Two recruited bipolar patients were unable to tolerate the magnetic resonance scanning, and they were not included to the studied group. All patients were hospitalized in the Department of Psychiatry of the Medical University of Białystok and in other inpatient wards of the Psychiatric Hospital in Choroszcz. The diagnosis of schizophrenia and bipolar disorder was made according to the ICD-10 and DSM-IV criteria. The schizophrenic subjects were paranoid type (n = 17), and undifferentiated type (n = 4). In this group, the clinical symptoms were assessed by the battery of psychiatric measures: Positive and Negative Syndrome Scale (PANSS) [22], Calgary Depression Scale for Schizophrenia (CDSS) [23], and Clinical Global Impression (CGI) [24]. The bipolar patients were in manic episode (n = 6), depressive episode (n = 5), and mixed episode (n = 5). In these patients, the mental state was assessed by the clinical scales: the Montgomery-Asberg Depression Rating Scale (MADRS) [25] and the Young Mania Rating Scale (YMRS) [26]. There were no significant age differences between groups (Table 1). Disease duration was similar in the schizophrenia and bipolar groups. Schizophrenic patients were receiving neuroleptics, and bipolar patients were treated with mood stabilizers, neuroleptics, and antidepressants due to their clinical condition. The study exclusion criteria were as follows: central nervous system organic damage confirmed in a routine neurological and MR examinations, active alcohol and other psychoactive substance dependence, and contraindications to conduct MR examinations. The subjects signed a written approval to participate in the study, in accordance with the protocol approved by the Local Bioethical Committee.
Table 1.
Clinical data for patients with schizophrenia and bipolar disorder, and controls (means ± SD).
| Schizophrenia N = 21 |
Bipolar disorder N = 16 |
Controls N = 20 |
P value | |
|---|---|---|---|---|
| Age (years) | 37.76 ± 8.04 | 43.69 ± 11.00 | 36.95 ± 7.41 | 0.11a |
| Females/males | 11/10 | 11/5 | 10/10 | 0.48b |
| Disease duration (years) | 13.33 ± 8.27 | 8.78 ± 8.37 | — | NSc |
| PANSS | 86.28 ± 11.02 | — | — | |
| CDSS | 4.52 ± 4.52 | — | — | |
| CGI | 4.52 ± 0.68 | — | — | |
| MADRS | 14.00 ± 12.95 | |||
| YMRS | 11.06 ± 8.49 |
aKruskal-Wallis test. bChi-square test. cMann–Whitney U test. NS: nonsignificant; PANSS: Positive and Negative Syndrome Scale; CDSS: Calgary Depression Scale for Schizophrenia; CGI: Clinical Global Impression; MADRS: Montgomery-Asberg Depression Rating Scale; YMRS: Young Mania Rating Scale.
2.2. MRI and 1H MRS
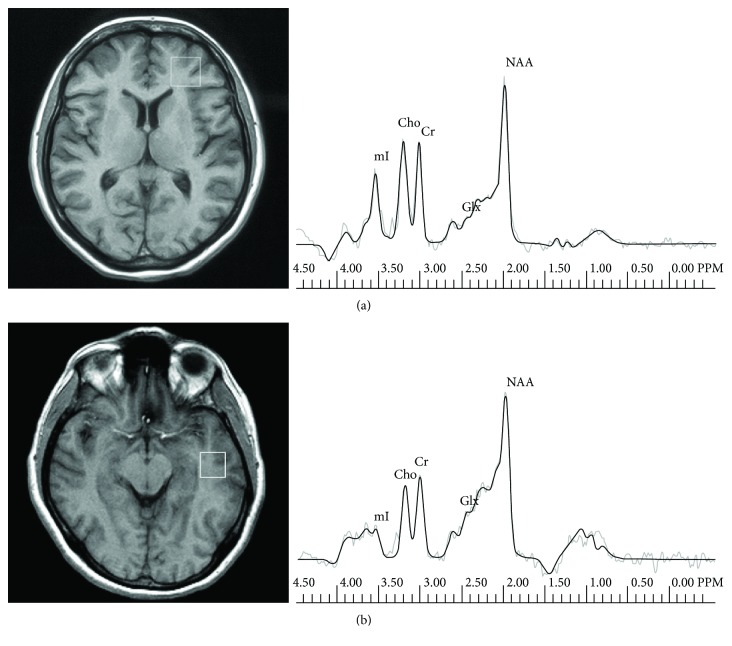
MR imaging and MR spectroscopy examinations were performed at the Department of Radiology, Medical University of Białystok, on a 1.5 T scanner (Picker Eclipse, Picker International Inc., Highlands Heights, OH, USA) by means of a standard circularly polarized head coil. T1-weighted FAST scans and conventional FSE T2-weighted series were obtained [27]. 1H MR spectroscopy examinations were carried out by means of single voxel PRESS (point-resolved single voxel localized spectroscopy) with the following parameters: TR = 1500 ms, TE = 35 ms (TE1 = ~17 ms), and nex = 192 and 3906 KHz bandwidth. Voxels of 2 × 2 × 2 cm3 were positioned in the regions of the left frontal lobe and left temporal lobe. A trained investigator located voxels by eye by means of T1-weighted sections in sagittal, coronal, and axial planes, and the inclusion of CSF was minimized. The left frontal lobe voxel was localized in the region which included the superior and middle frontal gyri and the above anterior horns of the lateral ventricles and is comprised most of all white matter and cortex. The left temporal lobe voxel was localized in the region which included the middle and inferior temporal gyri (Figure 1). Next, the signal over the voxel was shimmed to within a linewidth of 3 to 7 Hz and the transmitter pulse power was optimized by automated procedures. The MOIST (Multiply Optimized Insensitive Suppression Train) method was applied in order to suppress the signal from water [28]. The software package via 2.0C provided by Picker was used to analyse spectroscopic data. The 1H MRS data were zero-filled to 8192 points, and residual water resonances were removed using time-domain high-pass filtering. Exponential to Gaussian transformation was applied as a time-domain apodizing Gaussian filter. Next, data were Fourier transformed and phase corrected. After the application of a Legendre polynomial function to approximate the baseline, an automated curve fitting was performed using an iterative, nonlinear least-square fitting procedure by means of the Levenberg-Marquardt algorithm. Line shapes of the simulated peaks used in the fitting process were fixed with 85% Gaussian and 15% Lorentzian fractions. The following metabolites were assessed: NAA (N-acetylaspartate) at 2.01 ppm, Glx (GABA, glutamine, and glutamate) in the area from 2.11 ppm to 2.45 ppm, Cho (choline-containing compounds) at 3.22 ppm, Cr (creatine plus phosphocreatine) at 3.03 ppm, and mI (myo-inositol) at 3.56 ppm (Figure 1). Then metabolite to creatine ratios were analysed as well; the ratio of metabolites to nonsuppressed water signal was calculated according to the following formula: metabolite area × 1000/nonsuppressed water area.
Figure 1.
Voxel placement in the frontal lobe with a corresponding representative proton spectrum of a patient with bipolar disorder (a) and voxel placement in the temporal lobe with a corresponding representative proton spectrum of a patient with schizophrenia (b).
2.3. Statistical Analysis
Due to the small size of each group, we performed nonparametric tests. Demographic and clinical data were compared across groups using the Mann–Whitney U test, chi-square test, and Kruskal-Wallis test. When the group factor in the Kruskal-Wallis test was significant, post hoc paired comparisons were made. Also, the relationship between metabolic data and age with Spearman's correlation was analysed. Statistical analysis was performed using Statistica 10.0 PL, StatSoft Polska Ltd. The level of significance P was assumed to be below 0.05.
3. Results
Except for a higher NAA/H2O ratio of the temporal lobe in manic than in mixed (p = 0.007) and in the depressive than in mixed (p = 0.031) episode of bipolar disorder, we found no other differences in any of the studied metabolites between different episodes of bipolar disorder. We also found no differences in any of the studied metabolites between paranoid and undifferentiated types of schizophrenia. Therefore, we included manic, depressive, and mixed episode as a one bipolar group and a paranoid and undifferentiated schizophrenia as a one schizophrenia group to the main statistical analyses.
The left frontal and temporal lobe MRS metabolite ratios are presented in Table 2. Results of the Kruskal-Wallis test showed a significant overall group effect for the Glx/H2O ratio in the left temporal lobe (p = 0.02). The post hoc tests revealed that Glx/H2O ratios were significantly increased only in schizophrenic patients as compared to controls (p = 0.02).
Table 2.
Metabolite ratios in the frontal and temporal lobes for patients with schizophrenia and bipolar disorder and controls (means ± SD).
| Metabolite ratios | Patients with schizophrenia N = 21 |
Patients with bipolar disorder N = 16 |
Controls N = 20 |
P value |
|---|---|---|---|---|
| Left frontal lobe | ||||
| NAA/Cr | 1.75 ± 0.22 | 1.75 ± 0.32 | 1.74 ± 0.21 | NS |
| Glx/Cr | 1.98 ± 0.29 | 1.93 ± 0.56 | 2.17 ± 0.39 | NS |
| Cho/Cr | 0.99 ± 0.17 | 0.99 ± 0.12 | 0.90 ± 0.17 | NS |
| mI/Cr | 0.76 ± 0.26 | 0.78 ± 0.19 | 0.71 ± 0.25 | NS |
| NAA/H2O | 0.47 ± 0.08 | 0.47 ± 0.08 | 0.46 ± 0.06 | NS |
| Glx/H2O | 0.54 ± 0.08 | 0.50 ± 0.12 | 0.56 ± 0.10 | NS |
| Cho/H2O | 0.26 ± 0.04 | 0.27 ± 0.05 | 0.23 ± 0.04 | 0.04 |
| Cr/H2O | 0.27 ± 0.02 | 0.26 ± 0.05 | 0.26 ± 0.03 | NS |
| mI/H2O | 0.20 ± 0.07 | 0.21 ± 0.05 | 0.18 ± 0.06 | NS |
| Left temporal lobe | ||||
| NAA/Cr | 1.83 ± 0.37 | 1.73 ± 0.36 | 1.78 ± 0.35 | NS |
| Glx/Cr | 2.24 ± 0.44 | 2.18 ± 0.58 | 2.24 ± 0.66 | NS |
| Cho/Cr | 0.98 ± 0.17 | 0.97 ± 0.20 | 1.00 ± 0.20 | NS |
| mI/Cr | 0.73 ± 0.22 | 0.73 ± 0.27 | 0.72 ± 0.24 | NS |
| NAA/H2O | 0.45 ± 0.11 | 0.39 ± 0.07 | 0.40 ± 0.08 | NS |
| Glx/H2O | 0.62 ± 0.07 | 0.53 ± 0.14 | 0.54 ± 0.15 | 0.02 |
| Cho/H2O | 0.25 ± 0.06 | 0.23 ± 0.07 | 0.23 ± 0.06 | NS |
| Cr/H2O | 0.25 ± 0.06 | 0.24 ± 0.04 | 0.23 ± 0.05 | NS |
| mI/H2O | 0.18 ± 0.06 | 0.17 ± 0.06 | 0.17 ± 0.07 | NS |
Kruskal-Wallis test. NS: nonsignificant; NAA: N-acetylaspartate; Glx: GABA, glutamine, and glutamate; Cho: choline-containing compounds; Cr: creatine plus phosphocreatine; mI: myo-inositol.
Additionally, results of the Kruskal-Wallis test showed a significant overall group effect for the Cho/H2O ratio in the left frontal lobe (p = 0.04). The post hoc tests revealed only a trend toward a significant increase in the frontal Cho/H2O ratio of patients with bipolar disorder and schizophrenia relative to controls (p = 0.07 and p = 0.08, respectively), and no significant differences between the bipolar and schizophrenia groups. No significant differences were found among the three studied groups as regards NAA, Cr, and mI ratios.
The age of bipolar patients was inversely significantly correlated with the left frontal NAA/H2O ratio (R Spearman = −0.636; p = 0.01). The age of controls significantly correlated with the left frontal Glx/H2O ratio (R = 0.486; p = 0.04).
4. Discussion
In our study, we found an increase in Glx/H2O ratios in the left temporal lobe in schizophrenic patients relative to controls. Glutamate is the most abundant amino acid in the brain and in the spectral peaks is often grouped together with glutamine and GABA because of their overlaps [5]. Öngür et al. [16] examined the glutamine/glutamate ratio in acute mania, schizophrenia, and controls. In this study, the glutamine/glutamate ratio was significantly higher in the anterior cingulate cortex and parieto-occipital cortex in bipolar disorder, but also, there was a trend toward the significance in schizophrenia, compared with healthy controls. In another study, Atagün et al. [19] observed that glutamate levels were lower in left Heschl's gyrus and the planum temporale for schizophrenia at trend levels compared to healthy participants and glutamate and NAA levels were lower in euthymic bipolar patients when compared to controls. In the review regarding glutamate-related abnormalities in mood disorders [29], a highly consistent pattern of Glx reductions in major depressive disorder and elevations in bipolar disorder is pointed out. Also, depressive and manic episodes may be characterized by modulation of the glutamine/glutamate ratio in opposite directions. Our study group of bipolar patients consisted of manic, mixed, and depressive patients, and no differences in glutamatergic metabolites were found between these groups and between the bipolar group and controls. However, we found the lowest NAA/H2O ratio (marker of neuronal integrity) in the temporal lobe in the mixed episode of bipolar disorder, when compared to manic and depressive episodes. It suggests that of bipolar episodes, mostly, the mixed episode may potentially disturb the integrity of functioning of the temporal lobe. In our previous study, we found increased Glx level in the left temporal lobe in first-episode schizophrenic patients as compared to healthy controls [30]. Marsman et al. [31] in review revealed that frontal region glutamate is lower and glutamine is higher in patients with schizophrenia compared to healthy individuals. Our results therefore point to the importance of the glutamatergic system in schizophrenia; however, we observed its importance only in the temporal lobe.
We also observed a trend toward the increase in the frontal Cho/H2O ratios in patients with bipolar disorder and schizophrenia, compared to controls. The results of previous studies concerning Cho level among schizophrenia, bipolar, and controls are inconsistent. There were not found differences among these groups in the Cho/Cr ratio in the prefrontal cortex [14], in the anterior cingulate cortex, and he parieto-occipital cortex [16]. On the other hand, Sarramea Crespo et al. [15] observed in schizophrenia patients higher Cho/Cre ratios in the anterior cingulum as compared with controls and bipolar patients. Cho levels in the dorsolateral prefrontal cortex were noted to be lower in patients with schizoaffective and bipolar disorders compared to the control group, and there was no significant difference between the schizophrenic patients and the control group [18]. The findings from our study are consistent with previous studies in bipolar disorder in higher choline levels, reported in the hippocampus [32, 33], in the orbitofrontal cortex [33], and in the anterior cingulate [34]. An increase in the choline-containing compound signal reflects an increase in membrane turnover, myelination, or inflammation [5].
Several limitations have to be taken into account while interpreting the results of our study. Firstly, the use of antipsychotic medication can influence the brain metabolism as measured by the 1H MRS [35]. Antipsychotic treatment may increase NAA levels during the -time observation; however, this effect may disappear in longer observation. Also, glutamate measures are decreasing along with the duration of the disease [35]. It was also observed that the treatment of mania with olanzapine may increase choline level [36] or antidepressant use in bipolar disorder is correlated with lower choline levels [34]. The lithium treatment may also influence lower Glx levels and increased myo-inositol in grey matter [37]. Secondly, we did not make segmentation within the volume of interest. This procedure was not available in our study, and thus, we cannot exclude the possibility of small regional variations in metabolite concentration [38]. Nevertheless, the use of nonsuppressed water signal as an internal standard enhances the meaning of our results since reduction in Cr levels is observed in schizophrenia, but not in bipolar disorder, as compared to controls [16]. Then the age is an important factor influencing metabolite ratios, mostly NAA [39]. Also, in our study, we observed the effect of the age on frontal NAA ratios but only on bipolar patients (negative correlation). The meta-analysis of 1H MRS studies found that glutamate and glutamine levels in the frontal region decrease at a faster rate with the age of patients with schizophrenia as compared with healthy controls [31]. In our study, frontal Glx ratios positively correlated with age only in controls, but not in patients.
5. Conclusion
Our results point to the role of the temporal glutamatergic system in schizophrenia and choline-containing compounds in both bipolar disorder and schizophrenia. The lowest NAA/H2O ratio in the mixed episode of bipolar disorder suggests the deleterious effect of mixed episode on the integrity and functioning of the temporal lobe. Especially, glutamatergic spectroscopic changes may potentially help in the differential diagnosis of bipolar disorder from schizophrenia. The observed alterations in neurometabolites may be involved in the pathogenesis of bipolar disorder and schizophrenia.
Acknowledgments
This research was supported by a grant from the Medical University of Białystok (grant no. 113-477262).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors of the present manuscript declare no conflict of interests.
Authors' Contributions
Beata Galińska-Skok wrote the main body of the paper, and Napoleon Waszkiewicz and Agata Szulc critically revised and corrected it. Aleksandra Małus, Beata Konarzewska, and Anna Rogowska-Zach helped in literature search, writing, critical revision, correction, and preparation to submission. Robert Milewski did the statistical analyses. Eugeniusz Tarasów did the magnetic resonance imaging and spectroscopy examinations. All authors have approved the final version of the article.
References
- 1.Murray R. M., Sham P., van Os J., Zanelli J., Cannon M., McDonald C. A developmental model for similarities and dissimilarities between schizophrenia and bipolar disorder. Schizophrenia Research. 2004;71(2-3):405–416. doi: 10.1016/j.schres.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Fischer B. A., Carpenter W. T. Will the Kraepelinian dichotomy survive DSM-V? Neuropsychopharmacology. 2009;34(9):2081–2087. doi: 10.1038/npp.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee K. W., Woon P. S., Teo Y. Y., Sim K. Genome wide association studies (GWAS) and copy number variation (CNV) studies of the major psychoses: what have we learnt? Neuroscience & Biobehavioral Reviews. 2012;36(1):556–571. doi: 10.1016/j.neubiorev.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 4.McDonald C., Bullmore E. T., Sham P. C., et al. Association of genetic risks for schizophrenia and bipolar disorder with specific and generic brain structural endophenotypes. Archives of General Psychiatry. 2004;61(10):974–984. doi: 10.1001/archpsyc.61.10.974. [DOI] [PubMed] [Google Scholar]
- 5.Ross A. J., Sachdev P. S. Magnetic resonance spectroscopy in cognitive research. Brain Research Reviews. 2004;44(2-3):83–102. doi: 10.1016/j.brainresrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Dager S. R., Corrigan N. M., Richards T. L., Posse S. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Topics in Magnetic Resonance Imaging. 2008;19(2):81–96. doi: 10.1097/RMR.0b013e318181e0be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weickert C. S., Weickert T. W., Pillai A., Buckley P. F. Biomarkers in schizophrenia: a brief conceptual consideration. Disease Markers. 2013;35(1):9. doi: 10.1155/2013/510402.510402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt U., Kaltwasser S. F., Wotjak C. T. Biomarkers in posttraumatic stress disorder: overview and implications for future research. Disease Markers. 2013;35(1):54. doi: 10.1155/2013/835876.835876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labermaier C., Masana M., Müller M. B. Biomarkers predicting antidepressant treatment response: how can we advance the field? Disease Markers. 2013;35(1):31. doi: 10.1155/2013/984845.984845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waszkiewicz N., Galińska-Skok B., Nestsiarovich A., et al. Neurobiological effects of binge drinking help in its detection and differential diagnosis from alcohol dependence. Disease Markers. 2018;2018:9. doi: 10.1155/2018/5623683.5623683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steen R. G., Hamer R. M., Lieberman J. A. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30(11):1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 12.Yildiz-Yesiloglu A., Ankerst D. P. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2006;30(6):969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Kraguljac N. V., Reid M., White D., et al. Neurometabolites in schizophrenia and bipolar disorder – a systematic review and meta-analysis. Psychiatry Research. 2012;203(2-3):111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molina V., Sánchez J., Sanz J., et al. Dorsolateral prefrontal N-acetyl-aspartate concentration in male patients with chronic schizophrenia and with chronic bipolar disorder. European Psychiatry. 2007;22(8):505–512. doi: 10.1016/j.eurpsy.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Sarramea Crespo F., Luque R., Prieto D., et al. Biochemical changes in the cingulum in patients with schizophrenia and chronic bipolar disorder. European Archives of Psychiatry and Clinical Neuroscience. 2008;258(7):394–401. doi: 10.1007/s00406-008-0808-9. [DOI] [PubMed] [Google Scholar]
- 16.Öngür D., Jensen J. E., Prescot A. P., et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biological Psychiatry. 2008;64(8):718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Öngür D., Prescot A. P., Jensen J. E., Cohen B. M., Renshaw P. F. Creatine abnormalities in schizophrenia and bipolar disorder. Psychiatry Research. 2009;172(1):44–48. doi: 10.1016/j.pscychresns.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalaycı D., Özdel O., Sözeri-Varma G., Kıroğlu Y., Tümkaya S. A proton magnetic resonance spectroscopy study in schizoaffective disorder: comparison of bipolar disorder and schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2012;37(1):176–181. doi: 10.1016/j.pnpbp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Atagün M. I., Şıkoğlu E. M., Can S. S., et al. Investigation of Heschl’s gyrus and planum temporale in patients with schizophrenia and bipolar disorder: a proton magnetic resonance spectroscopy study. Schizophrenia Research. 2015;161(2-3):202–209. doi: 10.1016/j.schres.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atagün M. I., Şıkoğlu E. M., Soykan Ç., et al. Perisylvian GABA levels in schizophrenia and bipolar disorder. Neuroscience Letters. 2017;637:70–74. doi: 10.1016/j.neulet.2016.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birur B., Kraguljac N. V., Shelton R. C., Lahti A. C. Brain structure, function, and neurochemistry in schizophrenia and bipolar disorder – a systematic review of the magnetic resonance neuroimaging literature. NPJ Schizophrenia. 2017;3(1):p. 15. doi: 10.1038/s41537-017-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay S. R., Fiszbein A., Opler L. A. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 23.Addington D., Addington J., Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. The British Journal of Psychiatry. 1993;163(Suppl.22):39–44. doi: 10.1192/S0007125000292581. [DOI] [PubMed] [Google Scholar]
- 24.Guy W. ECDEU Assessment Manual for Psychopathology. Rockville, MD, USA: US Department of Heath, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976. [Google Scholar]
- 25.Montgomery S. A., Asberg M. A new depression scale designed to be sensitive to change. The British Journal of Psychiatry. 1979;134(4):382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 26.Young R. C., Biggs J. T., Ziegler V. E., Meyer D. A. A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 27.Szulc A., Konarzewska B., Galinska-Skok B., et al. Proton magnetic resonance spectroscopy measures related to short-term symptomatic outcome in chronic schizophrenia. Neuroscience Letters. 2013;547:37–41. doi: 10.1016/j.neulet.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 28.Murdoch J. B., Lampman D. A. Beyond WET and DRY: optimized pulses for water suppression. Proceedings of the Twelfth Annual Meeting, Society of Magnetic Resonance in Medicine; 1993; New York, NY, USA. p. p. 1191. [Google Scholar]
- 29.Yüksel C., Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biological Psychiatry. 2010;68(9):785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szulc A., Galinska B., Czernikiewicz A., et al. Glutamatergic system dysfunction in schizophrenia. A proton magnetic resonance spectroscopy (1H MRS) study. Polish Journal of Radiology. 2004;69(1):33–36. [Google Scholar]
- 31.Marsman A., van den Heuvel M. P., Klomp D. W. J., Kahn R. S., Luijten P. R., Hulshoff Pol H. E. Glutamate in schizophrenia: a focused review and meta-analysis of 1H-MRS studies. Schizophrenia Bulletin. 2013;39(1):120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atmaca M., Yildirim H. Altered neurochemical ingredient of hippocampus in patients with bipolar depression. Depression Research and Treatment. 2012;2012:6. doi: 10.1155/2012/485249.485249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senaratne R., Milne A. M., MacQueen G. M., Hall G. B. C. Increased choline-containing compounds in the orbitofrontal cortex and hippocampus in euthymic patients with bipolar disorder: a proton magnetic resonance spectroscopy study. Psychiatry Research. 2009;172(3):205–209. doi: 10.1016/j.pscychresns.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Moore C. M., Breeze J. L., Gruber S. A., et al. Choline, myo-inositol and mood in bipolar disorder: a proton magnetic resonance spectroscopic imaging study of the anterior cingulate cortex. Bipolar Disorders. 2000;2(3.2) Part 2:207–216. doi: 10.1034/j.1399-5618.2000.20302.x. [DOI] [PubMed] [Google Scholar]
- 35.Szulc A., Galinska-Skok B., Waszkiewicz N., Bibulowicz D., Konarzewska B., Tarasow E. Proton magnetic resonance spectroscopy changes after antipsychotic treatment. Current Medicinal Chemistry. 2013;20(3):414–427. doi: 10.2174/0929867311320030013. [DOI] [PubMed] [Google Scholar]
- 36.DelBello M. P., Cecil K. M., Adler C. M., Daniels J. P., Strakowski S. M. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31(6):1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 37.Friedman S. D., Dager S. R., Parow A., et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biological Psychiatry. 2004;56(5):340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Auer D. P., Wilke M., Grabner A., Heidenreich J. O., Bronisch T., Wetter T. C. Reduced NAA in the thalamus and altered membrane and glial metabolism in schizophrenic patients detected by 1H-MRS and tissue segmentation. Schizophrenia Research. 2001;52(1-2):87–99. doi: 10.1016/s0920-9964(01)00155-4. [DOI] [PubMed] [Google Scholar]
- 39.Krukowski P., Podgórski P., Guziński M., Szewczyk P., Sąsiadek M. Change of the brain magnetic resonance spectroscopy metabolite’s ratios with aging. Medical Science Monitor. 2010;16(Supplement 1):19–23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.