Supplemental Digital Content is available in the text.
Key Words: programmed death-ligand 1 (PD-L1), non–small cell lung cancer (NSCLC), tumor-infiltrating immune cells, driver mutation, smoking
Abstract
Our study was to evaluate the concordance of programmed cell death-ligand 1 (PD-L1) expression between 22C3 and SP263 assay and explore the association of clinicopathologic features with expression of PD-L1 on both tumor cells (TC) and tumor-infiltrating immune cells (IC). We retrospectively assessed the PD-L1 expression in 305 patients with lung adenocarcinoma or adenosquamous carcinoma by 22C3 and SP263 assay. The association of PD-L1 expression by 22C3 assay with clinicopathologic features was also analyzed. The prevalence of PD-L1 expression by 22C3 assay was 20.7% with a ≥50% cutoff and 46.6% with a ≥1% cutoff. The concordance rates between 2 PD-L1 assays while using 1%, 5%, 25%, and 50% positive TC as the cutoffs were 91.8%, 93.1%, 95.1% and 99.0%, respectively. For PD-L1 expression on IC, the concordance rate was 93.4% using a 1% cutoff. According to the results of 22C3 assay, high PD-L1 expression (using a ≥50% cutoff) on TC was significantly associated with smoking, advanced stage disease, and KRAS mutation. PD-L1 expression on IC was significantly associated with smoking and KRAS mutation. PD-L1 expression on TC and IC were both significantly associated with average number of cigarettes smoked ≥20 per day. The 22C3 and SP263 assays were highly concordant for assessment of PD-L1 expression on TC and IC. Patients with KRAS mutation and smoking history, particularly those having a large number of cigarettes smoked per day, were more likely to have PD-L1 expression on both TC and IC.
Lung cancer is the leading cause of cancer-related death worldwide.1 Although targeted molecular therapy has recently greatly improved the clinical course for patients with non–small cell lung cancer (NSCLC) having common driver mutations, the prognosis of patients with NSCLC who do not harbor driver oncogene mutations remains poor.2 Immunotherapy targeting the programmed cell death-1 (PD-1) and the programmed cell death-ligand 1 (PD-L1) pathway has recently emerged as an effective and promising treatment for advanced NSCLC. Appropriate biomarker selection is thereby essential to improve immunotherapy efficacy. The KEYNOTE-024 trial demonstrated that in patients with advanced NSCLC and PD-L1 expression on at least 50% of tumor cells (TC), pembrolizumab was associated with significantly longer progression-free and overall survival and with fewer adverse events than platinum-based chemotherapy.3 Thus, PD-L1 expression on TC has become an important biomarker in selecting patients for first-line immunotherapy. Recent studies showed that PD-L1 expression on tumor-infiltrating immune cells (IC) could also influence the immune response, which may serve as a supplementary diagnostic factor for PD-L1 expression on TC to predict the response to PD-1/PD-L1 blockade immunotherapy.4,5
Several PD-L1 diagnostic tests have been developed specifically for use with individual therapies. The test for pembrolizumab uses the Dako 22C3 assay and the Ventana SP263 assay is in development for use with durvalumab.6,7 Each assay uses different antibody clones, immunohistochemistry (IHC) protocols, scoring algorithms, and cutoffs for PD-L1 positivity. PD-L1 assays are not standardized, and use of different assay methods could lead to inappropriate treatment selection. It is important to compare the analytic performance of PD-L1 diagnostic assays to allow appropriate interpretation of their use with respect to treatment selection. In the current study, we compared the analytical performance of PD-L1 22C3 PharmDx with that of Ventana PD-L1 SP263 and evaluated the association between clinicopathologic features and PD-L1 expression on both TC and IC in a large cohort of NSCLC patients using the PD-L1 IHC 22C3 PharmDx.
MATERIALS AND METHODS
Patients
We retrospectively analyzed 305 patients with lung adenocarcinoma or adenosquamous carcinoma who were diagnosed and treated at Cancer Hospital, Chinese Academy of Medical Science, Beijing, China between July 2016 and April 2017. Clinicopathologic features, including age, sex, smoking history, histology, pathologic TNM stage (the American Joint Committee on Cancer eighth edition of the lung cancer staging system), EGFR mutation status, KRAS mutation status, ALK fusion status, and ROS1 fusion status were studied. In addition, detailed assessments of smoking variables were also analyzed, including average number of cigarettes smoked per day, total smoking duration, and cumulative pack-years. This study was approved by our institutional review board of Cancer Hospital, Chinese Academy of Medical Science.
Detection of Driver Mutation
EGFR and KRAS mutation status were evaluated using amplification refractory mutation system polymerase chain reaction. For EGFR mutational analysis, 4 exons that code for the tyrosine kinase domain of the EGFR gene (exons 18–21) were examined. For the mutational analysis of KRAS, 2 exons of KRAS gene (codons 12, 13) were examined.
For the ALK fusion, IHC was carried out on an automated Ventana Benchmark XT stainer, using the prediluted Ventana anti-ALK (D5F3) Rabbit monoclonal primary antibody. Each case was also stained with a matched Rabbit Monoclonal Negative Control Ig antibody. Binary scoring system was adopted for evaluating the staining results. Presence of strong granular cytoplasmic staining in TC (any percentage of positive TC) was deemed to be ALK-positive, whereas absence of strong granular cytoplasmic staining in TC was deemed to be ALK-negative.
For the ROS1 fusion, IHC was performed on 4-μm thick FFPE tissue sections using clone D4D6 rabbit monoclonal antibody. ROS1 IHC was scored using the scoring system as follows: 0 for no staining; 1+ for faint cytoplasmic reactivity without any background in any percentage of cells; 2+ for cytoplasmic staining in 0%–50% of TC; and 3+ for cytoplasmic staining in >50% of TC. We performed fluorescence in situ hybridization (FISH) analysis using the Vysis LSI ROS1 Dual color, Break Apart Rearrangement Probe, to verify all positive IHC staining cases. Samples were considered to be FISH positive if >15% of the scored TC had split 1 or both ROS1 5' and 3' probe signals or had isolated 5' signals. The results of FISH analysis were considered to be the final results for discrepant cases between FISH analysis and IHC.
IHC Analysis of PD-L1
Tissue sections (4-mm thick) were cut from formalin-fixed, paraffin-embedded blocks containing representative tumors (tumor samples were from surgical resections or core needle biopsies) and processed for PD-L1 IHC. The presence of at least 100 viable TC was required for the specimen to be considered adequate for quantification of PD-L1 expression.
Two PD-L1 IHC assays, 22C3 and SP263, were carried out for all 305 patients possessing adequate specimens according to the manufacturer’s protocol. PD-L1 IHC 22C3 pharmDx was performed on the DAKO Autostainer Link 48, whereas PD-L1 SP263 assay was conducted on the automated Ventana Benchmark XT stainer. TC showing either partial or complete cell membrane staining for PD-L1 were evaluated as positive cells. Tumor proportion score (TPS) was used to evaluate PD-L1 expression on TC, which was the percentage of PD-L1-positive TC showing partial or complete membrane staining in the overall tumor sections. PD-L1 expression on IC was assessed as the proportion of tumor area occupied by PD-L1-positive IC of any intensity. All slides were assessed by 2 experienced pathologists. In cases of disagreement, the slides were reviewed by all 2 observers together to achieve consensus.
Statistical Analysis
Given that previous studies8,9 have suggested the interchangeability of these 2 PD-L1 IHCs and PD-L1 IHC 22C3 pharmDx assay is the only approved companion diagnostic for the treatment of NSCLC with the PD-1-targeted monoclonal antibody pembrolizumab, the following analysis was performed according to the results of 22C3 assay. Associations between PD-L1 expression and clinicopathologic variables were evaluated statistically by the Fisher exact test. A multivariable analysis was performed using a logistic regression model to investigate the association of PD-L1 expression with clinicopathologic characteristics. Statistical tests were 2-sided, and the significance level for all analyses was set at P<0.05. Statistics were performed using SPSS software (version 20.0 of SPSS, Chicago, IL).
RESULTS
Patients Characteristics
A total of 305 patients with NSCLC were collected in the present study, including 301 adenocarcinomas and 4 adenosquamous carcinomas. The median age was 60 years (range, 34–82 y). In total, 126 (41.3%) patients were male, and 96 (31.5%) patients were smokers. A total of 167 patients (54.8%) had stage I disease, 51 patients (16.7%) had stage II disease, 80 patients (26.2%) had stage III disease, and 7 patients (2.3%) had IV disease. All patients were analyzed for presence of EGFR and KRAS mutation and for ALK and ROS1 fusion: this analysis included 138 (45.2%) with EGFR mutations, 39 (12.8%) with KRAS mutations, 19 (6.2%) ALK fusions, and 12 (3.9%) ROS1 fusions. Supplementary Table 1 (Supplemental Digital Content 1, http://links.lww.com/JIT/A516) shows clinicopathologic characteristics according to the status of PD-L1 expression on TC and IC by 22C3 and SP263 assay.
Concordance Between PD-L1 22C3 and SP263 Assays
The distribution of PD-L1 expression score on TC and IC was shown in Figures 1 and 2. The concordance between PD-L1 SP263 and 22C3 staining on TC and IC are shown in Figure 3. For PD-L1 expression on TC, the concordance rates while using 1%, 5%, 25%, and 50% as cutoff values were 91.8%, 93.1%, 95.1%, and 99.0%, respectively. For PD-L1 expression on IC, the concordance rate was 93.4% using 1% as cutoff value. Generally, PD-L1 TPS of 22C3 assay was higher than that of SP263 assay in most discrepant cases at different cutoff values.
FIGURE 1.
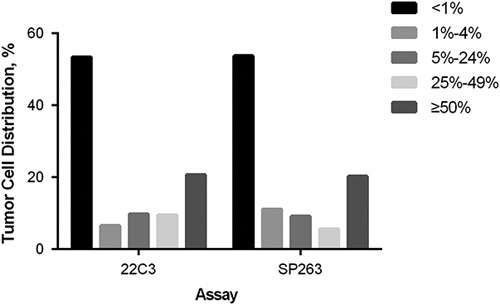
The distribution of PD-L1 expression score on tumor cells.
FIGURE 2.
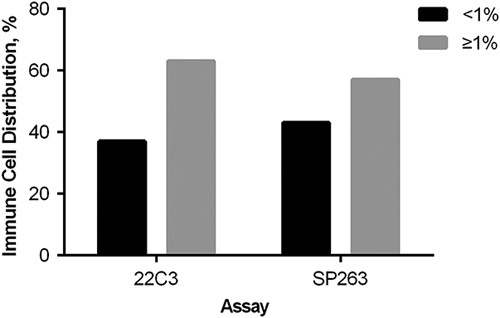
The distribution of PD-L1 expression score on immune cells.
FIGURE 3.
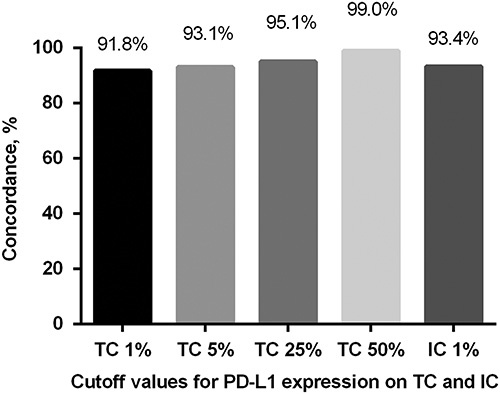
Concordance between PD-L1 SP263 and 22C3 staining on TC and IC. IC indicates immune cell; TC, tumor cell.
Clinicopathologic Correlation of PD-L1 Expression on TC and IC
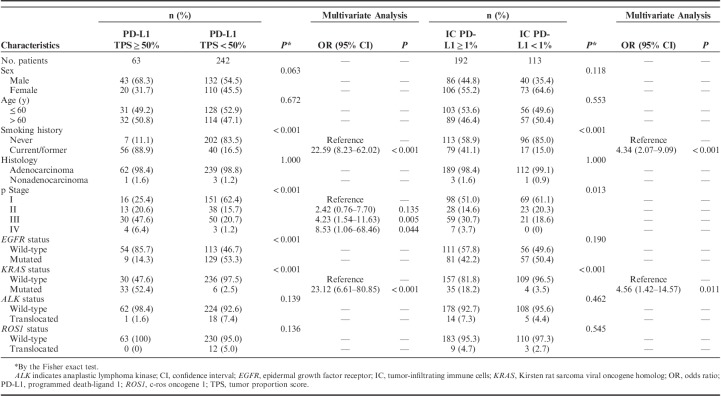
Clinicopathologic correlation of PD-L1 expression on TC and IC was examined by the Fisher exact test and multivariable analysis (Table 1). In total, 63 patients (20.7%) were positive for PD-L1 expression on TC when the cutoff value was set at 50%. Smoking, advanced stage disease, KRAS mutation, and wild-type EGFR status significantly correlated with PD-L1 TPS of ≥50% (all P<0.001). Multivariate analysis showed that smoking [hazard ratio (HR)=22.59, 95% confidence interval (CI), 8.23–62.02, P<0.001], advanced stage disease (III vs. I: HR=4.23, 95% CI, 1.54–11.63, P=0.005; IV vs. I: HR=8.53, 95% CI, 1.06–68.46, P=0.044, respectively), and KRAS mutation (HR=23.12, 95% CI, 6.61–80.85, P<0.001) were independent predictors of PD-L1 TPS of ≥50%. In total, 192 patients (63.0%) were positive for PD-L1 expression on IC. Smoking and KRAS mutation were found to be associated with significantly positive expression of PD-L1 on IC (all P<0.001). Multivariate analysis revealed that smoking and KRAS mutation remained independent predictors for positive expression of PD-L1 on IC (HR=4.34, 95% CI, 2.07–9.09, P<0.001 and HR=4.56, 95% CI, 1.42–14.57, P=0.011, respectively).
TABLE 1.
Association Analysis for Clinicopathologic Features and PD-L1 Expression (22C3) on Tumor Cells and IC
Association Between Cigarette Smoking and PD-L1 Expression on TC and IC
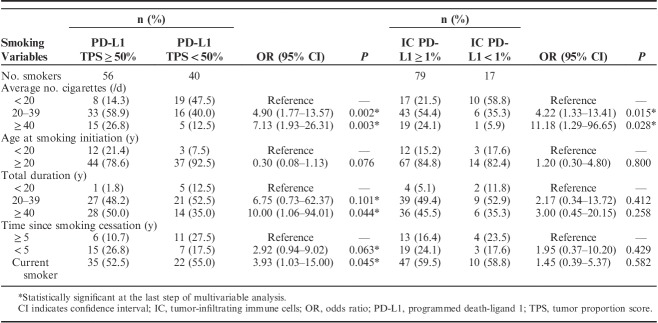
Association of detailed smoking variables including average number of cigarettes smoked per day, age at smoking initiation, total duration of smoking, and time since smoking cessation with PD-L1 expression on TC and IC was shown in Table 2.
TABLE 2.
Associations Between Cigarette Smoking and PD-L1 Expression (22C3) on Tumor Cells and IC
For PD-L1 expression on TC, multivariate analysis indicated that a significant association was found between PD-L1 TPS of ≥50% and average number of cigarettes smoked ≥20 per day (average number of cigarettes smoked 20–39/d vs. average number of cigarettes smoked <20/d: HR=4.90, 95% CI, 1.77–13.57, P=0.002; average number of cigarettes smoked ≥40/d vs. average number of cigarettes smoked <20/d: HR=7.13, 95% CI, 1.93–26.31, P=0.003), total duration of smoking ≥40 years (total duration of smoking ≥40 y vs. total duration of smoking <20 y: HR=10.00, 95% CI, 1.06–94.01, P=0.044) and current smoking status (current smoking status vs. time since smoking cessation ≥5 y: HR=3.93, 95% CI, 1.03–15.00, P=0.045). For PD-L1 expression on IC, in multivariate analysis, only average number of cigarettes smoked ≥20 per day was significantly associated with PD-L1 positivity (average number of cigarettes smoked 20–39/d vs. average number of cigarettes smoked <20/d: HR=4.22, 95% CI, 1.33–13.41, P=0.015; average number of cigarettes smoked ≥40/d vs. average number of cigarettes smoked <20/d: HR=11.18, 95% CI, 1.29–96.65, P=0.028).
DISCUSSION
Blockade of immune checkpoints has recently demonstrated efficacy in patients with advanced NSCLC. An appropriate biomarker that can select patients for immunotherapy is of vital important. In the KEYNOTE-024 study, advanced NSCLC patients with PD-L1 expression on at least 50% of TC could benefit more from pembrolizumab monotherapy than the standard platinum-based chemotherapy.3 Therefore, NCCN guidelines recommended PD-L1 expression on TC as an important biomarker in the selection of advanced NSCLC patients for first-line pembrolizumab monotherapy.10 Various IHC assays have been developed to assess the expression of PD-L1, and different antibodies, clones, platforms, score systems, and cutoff values have been introduced for and linked to a specific inhibitor. It is imperative to assess the analytical comparability of different PD-L1 IHC assays. In the current study, we evaluated the PD-L1 expression by 22C3 assay and SP263 assay in NSCLC patients and focused on the correlation of PD-L1 expression with clinicopathologic features.
The prevalence of PD-L1 expression by 22C3 assay was 20.7% with a ≥50% cutoff and 46.6% with a ≥1% cutoff in our cohort, which was lower than that reported in the KEYNOTE studies (KETNOTE-001, 010, and 024)3,11,12 (20.7% vs. 28% using a ≥50% cutoff, and 46.6% vs. 66% using a ≥1% cutoff).13 The discrepancy in prevalence of PD-L1 expression may be attributed to several factors including ethnicity, histologic subtypes, pathologic stage, smoking status, driver mutation status. Many studies have investigated the concordance and interchangeability of different PD-L1 assays, most of which displayed good concordance between 22C3 assay and SP263 assay.9,14–17 Consistent with the previous findings, our results showed high concordance between 22C3 assay and SP263 assay. In our study, the concordance rate of PD-L1 expression on TC all exceeded 90% at a cutoff of 1%, 5%, 25%, and 50%, and a higher level of cutoff value was associated with a higher concordance rate. In addition, our data showed that the PD-L1 TPS of 22C3 assay was higher than that of SP263 assay in most discrepant cases at 4 different cutoff values. Given that the PD-L1 IHC 22C3 pharmDx assay is the only approved companion diagnostic for the treatment of NSCLC with the PD-1-targeted monoclonal antibody pembrolizumab, using SP263 assay detecting PD-L1 expression to select appropriate patients for first-line therapy with pembrolizumab may lead to neglect some proper patients. Although SP142 assay was developed to detect PD-L1 expression on IC with high specificity and sensitivity,18 22C3 and SP263 assay are also able to stain IC and allow evaluation of the contingent of positive IC. In contrast to PD-L1 expression on TC, assessment of PD-L1 expression on IC showed great variability and poor concordance between assays in most previous studies.8,15,19 However, we assessed PD-L1 expression on IC using 22C3 and SP263 assay and demonstrated that the analytical performances of 22C3 and SP263 were highly concordant in IC staining.
Many studies have investigated the association between various clinicopathologic features and PD-L1 expression on TC in NSCLC.20–23 In the present study, we analyzed the clinicopathologic correlation with PD-L1 expression on TC and multivariate analysis revealed that smoking, advanced stage, and KRAS mutation were independent predictors for PD-L1 high expression on TC, which was consistent with previous studies.17,24,25 Dong et al,24 reported that KRAS-mutated tumors manifested exclusive increased expression of PD-L1 and prominently increased mutation burden. In the study by Tseng et al,17 smokers were more likely to present PD-L1, where higher smoking intake was associated with a higher PD-L1-positive rate. Chen et al25 showed that advanced TNM stage was associated with higher PD-L1 expression in patients with NSCLC. In addition, the chronic inflammation induced by exposure to tobacco carcinogens involves upregulation of interferon-γ that is known to induce increased expression of PD-L1.26,27 However, the influence of PD-L1 expression on IC on the immune response remained unclear and few reports focused on the correlation between clinicopathologic characteristics and PD-L1 expression on IC. In the KEYNOTE-001 study, 3 of 28 NSCLC patients with PD-L1 TPS of <1% achieved an overall response upon pembrolizumab treatment and 54.8% of NSCLC patients with a PD-L1 TPS of at least 50% did not respond to pembrolizumab.11 Therefore, PD-L1 expression alone on TC seems to be not reliable enough to select patients who might respond to PD-1/PD-L1 inhibitors. A recent study suggested that coexpression of PD-L1 on TC and IC by SP142 IHC assay independently predicted improved overall survival with atezolizumab.4 Another study indicated that high expression of PD-L1 on tumor-infiltrating lymphocytes (especially CD25+ CD4+ T cells) in the tumor microenvironment can serve as a diagnostic factor to supplement PD-L1 expression in TC and predict the response to PD-1/PD-L1 blockade immunotherapy in NSCLC.5 These studies showed that expression of PD-L1 on IC positively correlated with outcomes of treatment with PD-1/PD-L1 inhibitors. In our study, we revealed that smoking and KRAS mutation were significantly associated with PD-L1 expression on IC in multivariate analysis. Collectively, our findings suggested that smoking and KRAS mutation were not only significantly associated with PD-L1 expression on TC, but also with PD-L1 expression on IC. Furthermore, we analyzed the relation between detailed smoking variables and PD-L1 expression and found that a large number of cigarettes smoked per day, long duration of smoking, and current smoking status were significantly associated with PD-L1 high expression on TC. For PD-L1 expression on IC, only a large number of cigarettes smoked per day was significantly associated with PD-L1 positivity. These findings indicated that a large number of cigarettes smoked per day was a common independent factor related to PD-L1 expression on both TC and IC.
The present study has several limitations. First, it was a single-institutional retrospective study. Second, no patients in this study received PD-1/PD-L1 inhibitors, for which we cannot evaluate the predictive power of the 2 PD-L1 IHC assays. Third, area of staining rather than individual count (in percentage) of positive cells was applied when scoring PD-L1 positivity on IC.
In conclusion, our study showed high concordance between 22C3 assay and SP263 assay, when assessing PD-L1 expression on TC and IC. Patients with KRAS mutation and smoking history, particularly those having a large number of cigarettes smoked per day, were more likely to have PD-L1 expression on both TC and IC.
CONFLICTS OF INTEREST/FINANCIAL DISCLOSURES
Supported by the grant 2016YFC0905400 from the National Key R&D Program of China, Beijing Municipal Science & Technology Commission No. LC2015A13, CAMS Initiative for Innovative Medicine (CAMS-2017-I2M-4-002), and CAMS Initiative for Innovative Medicine (CAMS-2017-I2M-2-003).
All authors have declared that there are no financial conflicts of interest with regard to this work.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Oxnard GR, Binder A, Janne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. [DOI] [PubMed] [Google Scholar]
- 4.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 5.Wu S-P, Liao R-Q, Tu H-Y, et al. Stromal PD-L1+regulatory T cells and PD-1+CD8+ T cells define the response of different subsets of non-small-cell lung cancer to PD-1/PD-L1 blockade immunotherapy. J Thorac Oncol. 2018;13:521–532. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. Dako PD-L1 IHC 22C3 pharmDx. 2015. Available at: www.accessdata.fda.gov/cdrh_docs/pdf15/P150013c.pdf. Accessed August 10, 2016.
- 7.Rebelatto MC, Midha A, Mistry A, et al. Development of a programmed cell death ligand-1 immunohistochemical assay validated for analysis of non-small cell lung cancer and head and neck squamous cell carcinoma. Diagn Pathol. 2016;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Büttner R, Gosney JR, Skov BG, et al. Programmed death-ligand 1 immunohistochemistry testing: a review of analytical assays and clinical implementation in non-small-cell lung cancer. J Clin Oncol. 2017;35:3867–3876. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti A, Barberis M, Franco R, et al. Multicenter comparison of 22C3 PharmDx (Agilent) and SP263 (Ventana) assays to test PD-L1 expression for NSCLC patients to be treated with immune checkpoint inhibitors. J Thorac Oncol. 2017;12:1654–1663. [DOI] [PubMed] [Google Scholar]
- 10.Ettinger DS, Wood DE, Aisner DL, et al. NCCN clinical practice guidelines in oncology: non-small cell lung cancer, version 9. 2017. Available at: www.nccn.org/professionals. Accessed September 28, 2017.
- 11.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 12.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal C, Abreu DR, Felip E, et al. Prevalence of PD-L1 expression in patients with non-small cell lung cancer screened for enrollment in KEYNOTE-001, -010, and -024. Ann Oncol. 2016;27:359–378.26658890 [Google Scholar]
- 14.Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol. 2017;12:208–222. [DOI] [PubMed] [Google Scholar]
- 15.Tsao MS, Kerr K, Yatabe Y, et al. Blurprint2: PD-L1 immunohistochemistry comparability study in real-life, clinical samples. J Thorac Oncol. 2017;12 (11S2):S1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan AWH, Tong JHM, Kwan JSH, et al. Assessment of programmed cell death ligand-1 expression by 4 diagnostic assays and its clinicopathological correlation in a large cohort of surgical resected non-small cell lung carcinoma. Mod Pathol. 2018;30:1381–1390. [DOI] [PubMed] [Google Scholar]
- 17.Tseng JS, Yang TY, Wu CY, et al. Characteristics and predictive value of PD-L1 status in real-world non-small cell lung cancer patients. J Immunother. 2018;41:292–299. [DOI] [PubMed] [Google Scholar]
- 18.Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PDL1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol. 2016;11:1879–1890. [DOI] [PubMed] [Google Scholar]
- 22.Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer. 2014;50:1361–1369. [DOI] [PubMed] [Google Scholar]
- 23.Ji Mei, Liu Yan, Li Qing, et al. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016;17:407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Z-Y, Zhong WZ, Zhang X-C, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23:3012–3024. [DOI] [PubMed] [Google Scholar]
- 25.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori. 2012;98:751–755. [DOI] [PubMed] [Google Scholar]
- 26.Conway EM, Pikor LA, Kung SHY, et al. Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193:116–130. [DOI] [PubMed] [Google Scholar]
- 27.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.