Abstract
In this paper, a poly (glycine) modified carbon paste electrode (PGMCPE) for sensitive determination of Tartrazine (Tz) was developed. The electrochemical behaviors of Tz at the PGMCPE were investigated by cyclic voltammetry, differential voltammetry and the results showed that the polymer film on electrode exhibited excellent electrocatalytic activity for the electrochemical oxidation of Tz in phosphate buffer solution, pH 7 (PBS). The influencing factors containing a supporting electrolyte, pH of the solution, deposition potential, amount of Tz and scan rate were investigated. The sensor exhibited two linear behavior in the range of 1 × 10−6 to 2.7 × 10−5 mol L−1 and 3.5 × 10−5 to 8.7 × 10−5 mol L−1 for Tz (correlation coefficients: 0.991 and 0.995 respectively) with detection limit (LOD) of 2.83 × 10−7 mol L−1, limit of quantification (LOQ) 9.4 × 10−7 mol L−1 and detection sensitivity (2.0452 μA/μM), for Tz. The results show that the biosensor is sensitive and useful for the determination of Tz.
Keywords: Electrochemistry, Analytical chemistry, Food analysis
1. Introduction
Tartrazine is one of the most common colorants used in a wide range of foods products. Today, it is a typical added substance found in sustenances, drinks, medications, nutrient enhancements, beauty care products, toiletries, and other non-nourishment items [1]. Usually colorants have the maximum limit allowed is 200–500 parts per million with few exception of lower limits selected types of foods. Tartrazine regularly causes unfriendly responses, for example, intermittent urticaria, angioedema, and asthma and is as often as possible embroiled in conduct issues [2]. The most well-known side effects connected with tartrazine affectability are urticaria and asthma [3] but, like with any food or chemical, symptoms are very individual specific. Synthesised colorants usually contain azo compounds are hurtful to human wellbeing. Consequently, the controlled utilize and the exact examination of their substance in items are essential [4].
Few methods proposed for detection and determination of Tz in foods like Stopped-flow kinetic analysis [5], voltammetric analysis [6, 7], visible spectrophotometry [8] thin-layer chromatography [9, 10], capillary electrophoresis [11, 12, 13], superior high-performance liquid chromatography [14, 15, 16, 17, 18] in biological samples. These published methods are known to utilize risky solvents, invest long examination energy, and sometimes it is important to make sample pretreatments. Then again, the combination of simple methodologies, like cyclic voltammetric strategies with polymer modified electrodes, represents a quick, simple and modest methodology for the determination of Tz.
To date, the ability to identify Tz with high sensitivity is as yet a noteworthy focus of electroanalytical research. The significant issue of estimating Tz is the very low concentration (micromolar and nanomolar) and the huge abundances of interfering substances. In order to resolve this issue, chemically modified electrodes have been produced for enhancing the sensitivity and selectivity [19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32]. These modified electrodes decrease the oxidative over the potential for some biomolecules, thus increasing the resolution of the biomolecules oxidation signal. As of late, polymer electrodes, as a result of their stability and high catalytic ability [29, 30, 31, 32, 33], have formed one of the slanting fields of research.
Electropolymerization is one of the cheap but powerful methods focusing on selective modification of various types of electrodes with desired matrices. Numerous polymers have mostly been utilized in the improvement of biosensors [29, 30]. Poly (glycine) film has excellent stability, better reproducibility, large active sites, homogeneity in electrochemical deposition and firm adherence to the modified electrode surface.
We report a novel, basic, exact and sensitive cyclic voltammetric method utilizing a polymer modified carbon paste electrode for the determination of Tz was reported. The technique was applied effectively to Tz in the real sample. The work was carried out to provide a low capital cost, economical.
2. Materials and methods
2.1. Reagents
Tz, Silicone oil, disodium phosphate, monosodium phosphate, and Glycine were obtained from Himedia chemicals, Bangalore, India and were used as received. The preparation of aqueous solution was done with double distilled deionized water. The stock solution of Tz (25 × 10−4 M), Glycine (25 × 10−3 M) were in the double distilled water. Phosphate buffer solution was prepared by mixing the suitable amount of 0.2 M monosodium phosphate and 0.2 M disodium phosphate.
2.2. Apparatus
Cyclic voltammetry experiments were performed in analytical systems model EA-201 Chemilink system. A conventional three-electrode system was used with a carbon paste electrode (3 mm diameter CPE), a KCl-saturated calomel reference electrode (SCE), and a platinum electrode as a counter electrode.
2.3. Preparation of bare carbon paste electrode
The BCPE (bare carbon paste electrode) was prepared by mixing in Graphite powder (150 Mesh) and silicone oil in a ratio of 70:30 % w/w [33]. The paste was then packed into the cavity of a homemade electrode and smoothed out on a weighing paper.
2.4. Preparation of the PGMCPE
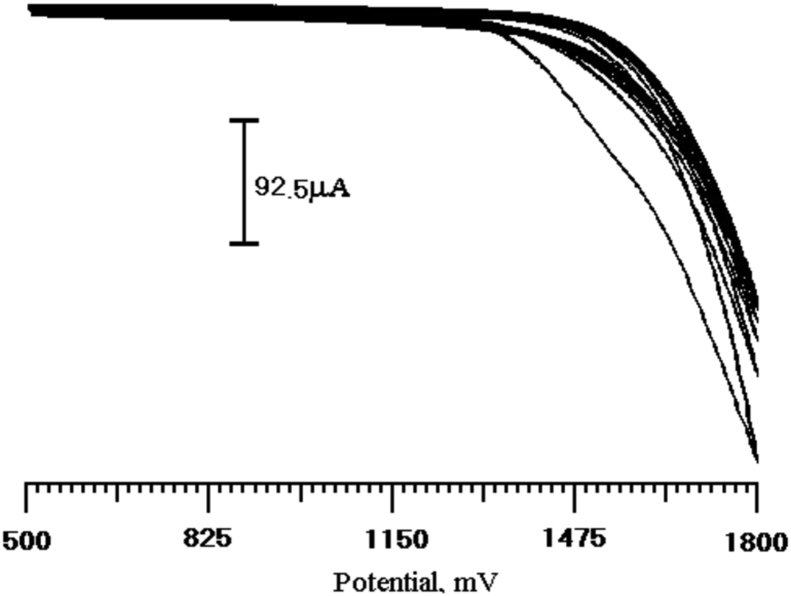
BCPE was electrochemically activated by using ten times cyclic potential sweeps in the range of 500 to 1800 mV in a PBS solution (pH 5.7) at a scan rate of 100 mVs−1 shown in Fig. 1. The modified electrode was fabricated in 1mM glycine under the same conditions as those in the electrode activation procedure. After electropolymerization, the modified electrode was rinsed thoroughly with distilled water.
Fig. 1.
Continuous 10 cyclic voltammograms for the electrochemical polymerization of 1 × 10−3 M glycine on a CPE in 0.2M PBS (5.7 pH) at the scan rate 100 mV/s.
3. Results and discussion
3.1. Morphological characterization of BCNTPE and PGMCPE
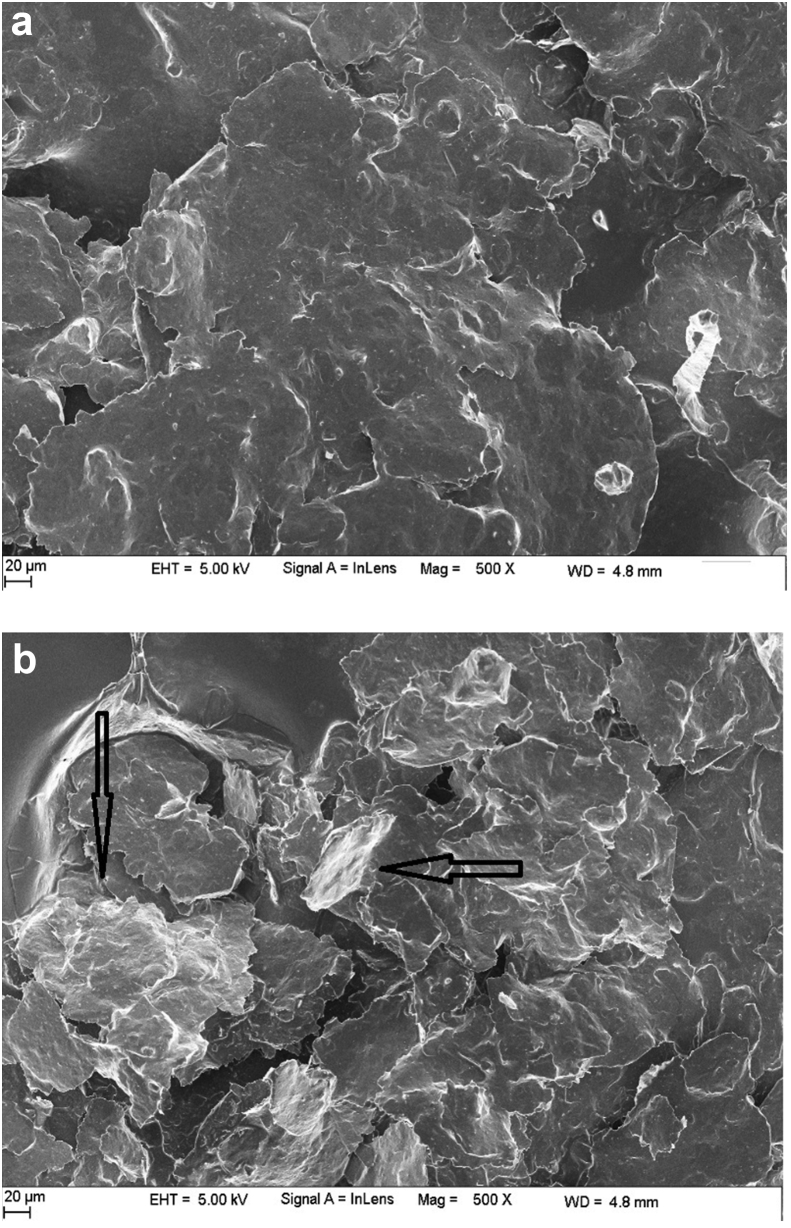
The surface morphologies of BCPE and PGMCPE characterized by, field emission scanning electron microscopy (FESEM) are shown in Fig. 2a and b, respectively. Fig. 2b clearly shows the formation of poly (glycine) layer on the CPE surface can be confirmed by the deposited poly (glycine) clusters on the surface.
Fig. 2.
FESEM images of (a) BCPE (b) PGMCPE.
3.2. Electrochemical oxidation of Tz
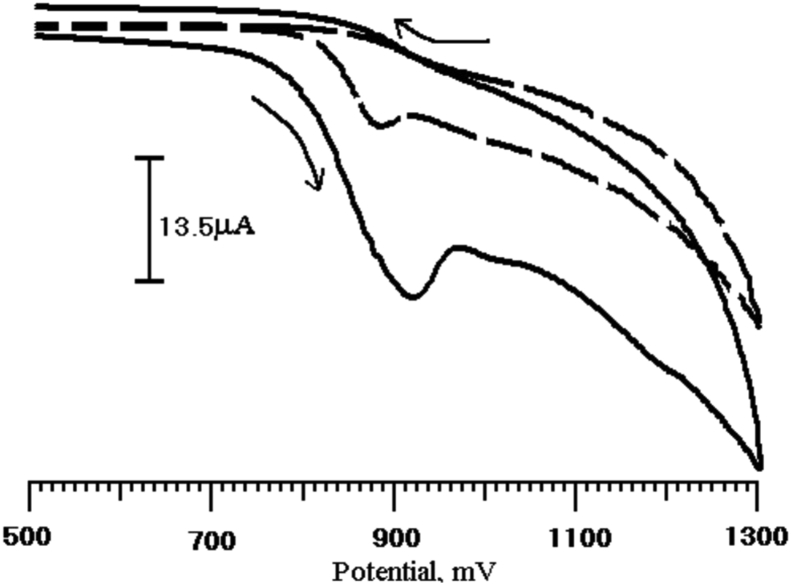
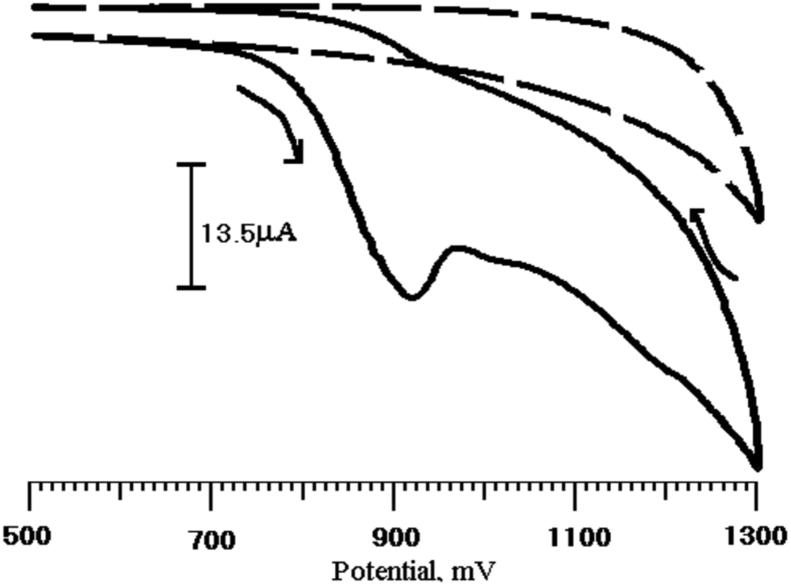
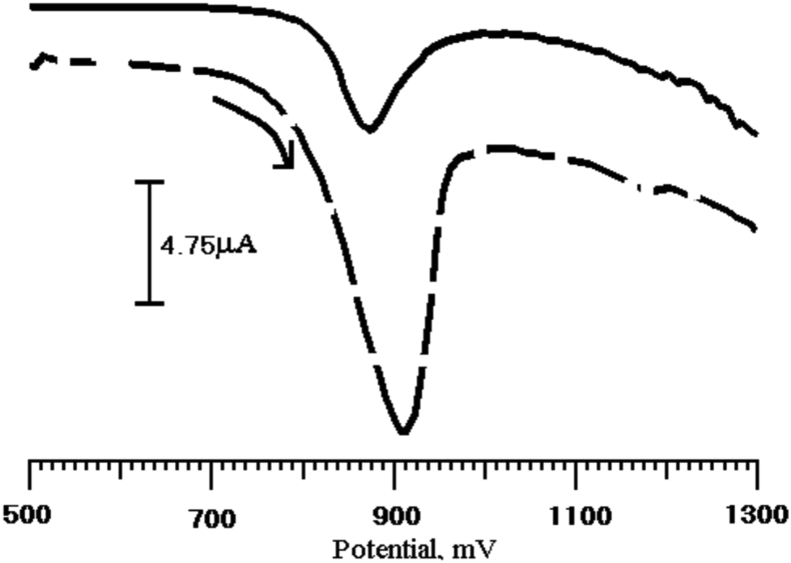
The electrocatalytic behavior of the BCPE and PGMCPE toward Tz oxidation were studied to determine how the sensor affected the electrochemical detection of Tz. Cyclic voltammograms were measured for the electrodes immersed in a pH 7 PBS containing Tz (1 × 10−4 M), as shown in Fig. 3 at PGMCPE, Tz exhibited a good electrochemical response, comparable to BCPE. The oxidation potential and current of Tz at BCPE were 883 mV, 11.32 μA and PGMCPE shows 918 mV, 30.46 μA, respectively. These results indicate that PGMCPE can accelerate the rate of Tz electron transfer. In order to test the electrocatalytic activity of the Tz, the cyclic voltammetric responses of a PGMCPE in the absence and presence of Tz (1 × 10−4 M), in a PBS (pH 7) at a scan rate of 100 mVs−1 are shown in Fig. 4. No current peaks are observed in the potential range from 500 to 1300 mV in the absence of Tz, whereas in its presence a characteristic crossover is observed around 910 mV together with a well-defined peak.
Fig. 3.
(a) Cyclic voltammograms of Tz (1 × 10−4 M) in 0.2 M phosphate buffer solution of pH 7 at BCPE (dashed line) and PGMCPE (solid line).
Fig. 4.
A typical cyclic voltammograms of PGMCPE with Tz (1 × 10−4 M) (solid line) and without 1 × 10−4 M Tz (dashed line) at pH 7 PBS.
3.3. The reproducibility, repeatability, and stability of the PGMCPE
In order to investigate the stability of the PGMCPE, the CVs for Tz (1 × 10−4 M) in 0.2 M pH 7 PBS was tested over 15 days. When cyclic voltammograms were recorded after the electrode was stored in the atmosphere at room temperature, the peak potential for oxidation of Tz was unchanged, and the oxidation current showed that 7% decrease. The sensor repeatability is measured by repeated measurement of 1 × 10−4 mol L−1 Tz. It indicates an aceptable RSD of 3.7 % under four determinations under optimum conditions. Four electrodes prepared in the same way, an acceptable reproducibility was obtained for the determination of 1 × 10−4 mol L−1 Tz, indicating good reproducibility of the preparation method.
3.4. Differential pulse voltammetric measurements
Fig. 5 depicts the oxidation of Tz at a BCPE (solid line) and PGMCPE (dashed line). The electrochemistry of Tz on the BCPE only showed a small oxidation peak at about 870 mV. However, at the PGMCPE, a sharp peak at 911 mV was observed, indicating the PGMCPE showed good electrocatalytic activity towards the oxidation of Tz.
Fig. 5.
DPVs of a solution containing Tz (1 × 10−4 M) in 0.2 M PBS (pH 7) at the BCPE (solid line), the PGMCPE (dashed line).
3.5. Scan rate effect
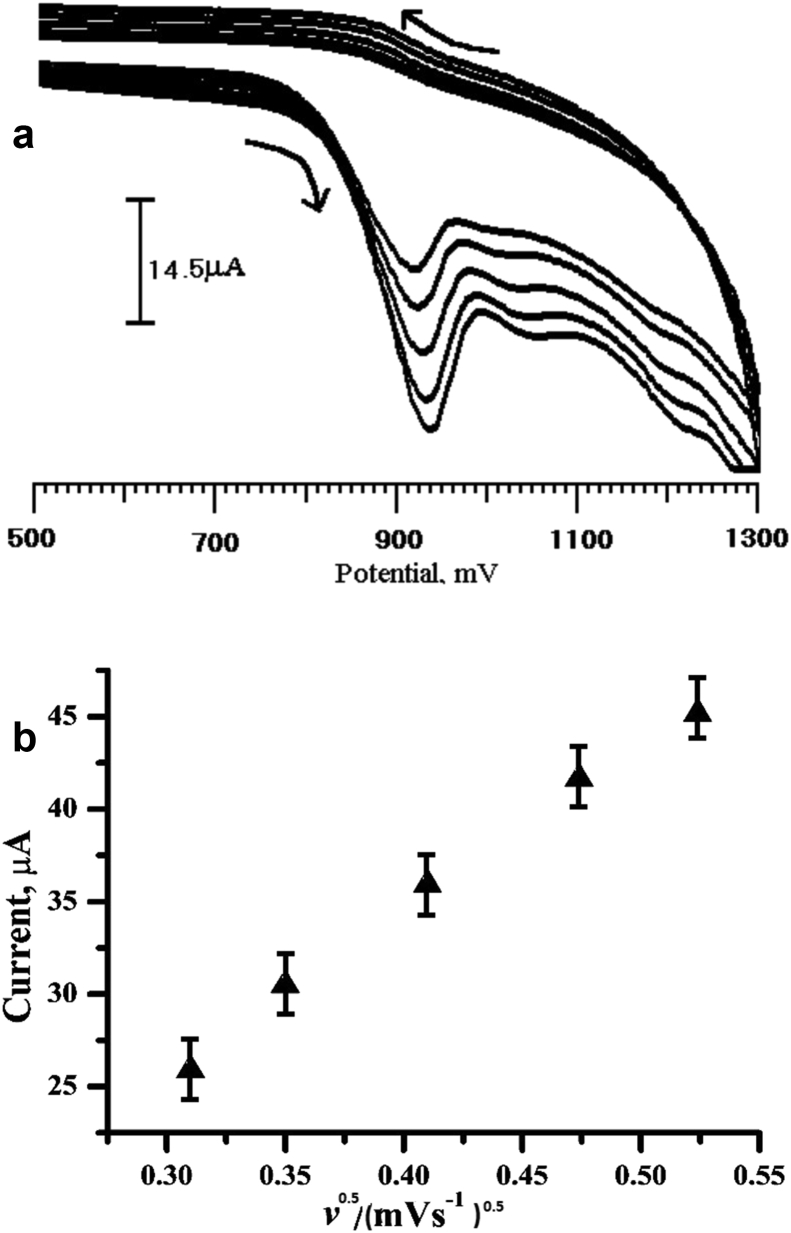
The effect of scan rate on the anodic peak current of Tz was studied as shown in Fig. 6a. As the scan rate increased, the oxidation peak current (Ipa) increased. The Ipa was directly proportional to the square root of scan rate ν1/2 over the range of 100–275 mVs−1, which suggested a surface-controlled process on the modified electrode surface [25, 26, 27]. The linear regression equation was Ipa (μA) = 89.846 + 1.33653 ν1/2 (mV s−1)1/2, with a correlation coefficient of 0.997 (Fig. 6b).
Fig. 6.
(a) Cyclic voltammograms of Tz (1 × 10−4 M) at the PGMCPE in pH 7 PBS at various scan rates. From: 100,125, 175, 225 and 275 mV/s. (b) Plot of the peak current of Tz as a function of the square root of scan rate.
To determine the number of electron transfer for an irreversible reaction, the peak potential can be demonstrated by this equation [34] Ep = b/2 × Log (v) + K where v is the scan rate, b is the Tafel slope, and K is a constant value. The plot of Ep versus log (v) was drawn, and the slope of this plot is 55 mV. Therefore, the Tafel slope turn into 110 mV/decade, α is assumed to be 0.50, the results indeed suggest one-electron (na 1.07 ∼ = 1) transfer in this reaction.
3.6. Effect of pH
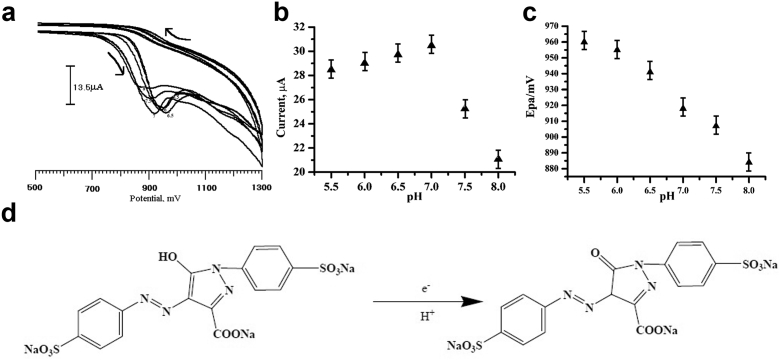
The pH value of the supporting electrolyte has a significant influence on the voltammetric behavior of Tz at the PGMCPE was shown in Fig. 7a. The effect of pH on the oxidation behavior of Tz was investigated. The oxidation peak current increased when pH was in the range of 5.5–8. However, it decreased when pH was more than 7 shown in Fig. 7b. Therefore, pH 7 was chosen for further experiments. The peak potentials were shifted from 28.46 mV to 21.08 mV with increasing pH of the solution from 5.5 to 8. The potential diagram was constructed by plotting the graph of Epa vs. pH of the solution, and it was shown in Fig. 7c and the linear regression equation was found to be Epa (mV) = 1138.48–31.257 pH (30). According to the above results, the electrode process for Tz on the PGMCPE can be expressed as shown in Fig. 7d [35].
Fig. 7.
(a) Cyclic voltammograms obtained at the PGMCPE in 0.2 M PBS in pH values, (a) 5.5 (b) 6 (c) 6.5 (d) 7 (e) 7.5 (f) 8 containing Tz (1 × 10−4 M) (b) Plot of anodic peak current vs. pH (5.5–8.0) of Tz (1 × 10−4M) at the PGMCPE (c) Plot of Epc vs pH for Tz (d) The scheme of oxidation of Tz.
3.7. Calibration plot and limit of detection
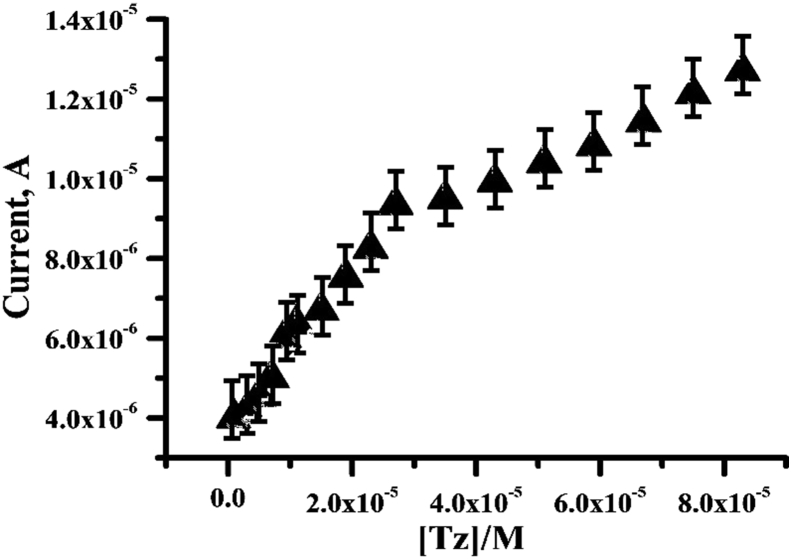
Under these optimum experimental conditions, the influence of Tz concentration on the CV signal was studied. The experimental results showed that the peak current increased with the increase in the concentration of Tz (see Fig. 8). The voltammetric calibration curve exhibited (due to concentration increases) two linear behavior in the range of 1 × 10−6 mol L−1 to 2.7 × 10−5 mol L−1 and 3.5 × 10−5 mol L−1 to 8.7 × 10−5 mol L−1 for Tz (correlation coefficients: 0.991 and 0.995, respectively) and regression equations were Ipa (A) = 3.7342 × 10−6 + 0.20452C, the detection limit (LOD) of 2.83 × 10−7 mol L−1 (S/N = 3), limit of quantification (LOQ) 9.4 × 10−7 mol L−1 and detection sensitivity (2.0452 μA/μM) for Tz [33]. The presented method in this work showed remarkable advantages such as sharp waves with relatively large peak currents and low backgrounds, which can be conducted to improve the sensitivity and detection limit in analytical determinations. The detection limit compared with other reported works [4, 7, 16, 36] are summarized in Table 1.
Fig. 8.
Calibration plot for the determination of Tz at the PGMCPE in pH 7 PBS with the scan rate 100 mV/s.
Table 1.
The comparison of detection limit with different methods for the determination of Tz.
3.8. Analytical applications
The applicability of a PGMCPE to the determination of Tz in some food samples bought from the local market at Madikeri, India was examined. There are two different samples were analyzed including candy and soft drink. Concentrations were measured by applying the calibration plot using the standard addition method. The recovery rate was calculated to be 94.5 to 98.2%, and the sample was conducted five times, and the relative standard deviation (RSD) was found lower than 1%, showing good accuracy and feasible. The recovery values indicate the excellent accuracy of the proposed method. From above experimental results, it is evident that this method has great potential for the determination of trace amounts of these compounds in biological systems or pharmaceutical preparations.
4. Conclusions
In this work, a sensitive and efficient method, based on the electrocatalytic oxidation of Tz at PGMCPE, is described. The CPE surface modified with poly glycine showed the good electrocatalytic response for the oxidation of Tz. The effect of Tz concentration, polymerisation cycle, scan rate, pH, DPV and stability have been investigated in detail. Under the optimal conditions, PGMCPE electrode displays a low detection limit detection limit and limit of quantification of 2.83 × 10−7 mol L−1, 9.4 × 10−7 mol L−1 for Tz respectively. Besides, the proposed method was used for the determination of Tz in commercial foods using CV methods and successfully obtained close value. The stability, reproducibility, and repetitive usability exhibited by the proposed modified electrode.
Declarations
Author contribution statement
J. G. Manjunatha: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by VGST, Bangalore (Research Project. No. KSTePS/VGST - KFIST (L1)2016-2017/GRD-559/2017-18/126/333,21/11/2017) and Mangalore University (Research Project order No. MU/DEV/2014-15/D3 D.t:30.04.2016).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Moutinho I.L.D., Bertges L.C., Assis R.V.C. Prolonged use of the food dye tartrazine (FD&C yellow n°5) and its effects on the gastric mucosa of Wistar rats. Braz. J. Biol. 2007;67:141–145. doi: 10.1590/s1519-69842007000100019. PMID: 17505761. [DOI] [PubMed] [Google Scholar]
- 2.Williams C.C. Clinical spectrum of adverse reactions to tartrazine. J. Asthma. 1985;22:139–143. doi: 10.3109/02770908509073132. PMID: 3894321. [DOI] [PubMed] [Google Scholar]
- 3.Dipalma J.R. Tartrazine sensitivity. Am. Fam. Physician. 1990;42:1347–1350. PMID: 2239641. [PubMed] [Google Scholar]
- 4.Sha O., Zhu X., Feng Y., Weixing M. Determination of sunset yellow and tartrazine in food samples by combining ionic liquid-based aqueous two-phase system with high performance liquid chromatography. J. Anal. Methods. Chem. 2014;2014:1–8. doi: 10.1155/2014/964273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenone A.V., Culzoni M.J., Marsili N.R., Goicoeche H.C. Determination of tartrazine in beverage samples by stopped-flow analysis and three-way multivariate calibration of non-linear kinetic-spectrophotometric data. Food Chem. 2013;138:1928–1935. doi: 10.1016/j.foodchem.2012.11.126. [DOI] [PubMed] [Google Scholar]
- 6.Kobun Rovina, Siddiquee Shafiquzzaman, Shaarani Sharifudin Md. Sensitive determination of tartrazine (E 102) based on chitosan/nanoparticles/MWCNTs modified gold electrode in food and beverage products. Trans. Sci. Technol. 2016;3:176–180. [Google Scholar]
- 7.Song Y.Z., Xu J.M., Lv J.S., Zhong H., Ye Y., Xie J.M. Electrochemical reduction of tartrazine at multiwalled carbon nanotube pyrolytic graphite electrode. Indian J. Chem. 2010;49:1030–1034. [Google Scholar]
- 8.Berzas J.J., Rodríguez Flores J., Villaseñor Llerena M.J., Farinas Rodriguez N. Spectrophotometric resolution of ternary mixtures of tartrazine, patent blue V and indigo carmine in commercial products. Anal. Chim. Acta. 1999;391:353–364. [Google Scholar]
- 9.Oka H., Ikai Y., Kawamura N., Yamada M., Inoue H., Ohno T., Inagaki K., Kuno A., Yamamoto N. Simple method for the analysis of food dyes on reversed phase thin layer plates. J. Chromatogr. 1987;411:437–444. doi: 10.1016/s0021-9673(00)93996-7. PMID: 3443633. [DOI] [PubMed] [Google Scholar]
- 10.Soponar F., Mot A.C., Sarbu C. Quantitative determination of some food dyes using digital processing of images obtained by thin-layer chromatography. J. Chromatogr. A. 2008;1188:295–300. doi: 10.1016/j.chroma.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 11.Dossi N., Toniolo R., Susmel S., Pizzariello A., Bontempelli G. Simultaneous RP-LC determination of additives in soft drinks. Chromatographia. 2006;63:557–562. [Google Scholar]
- 12.Dossi N., Toniolo R., Pizzariello A., Susmel S., Perennes F., Bontempelli G. A capillary electrophoresis microsystem for the rapid in-channel amperometric detection of synthetic dyes in food. J. Electroanal. Chem. 2007;601:1–7. [Google Scholar]
- 13.Dossi N., Piccin E., Bontempelli G., Carrilho E., Wang J. Rapid analysis of azo-dyes in food by microchip electrophoresis with electrochemical detection. Electrophoresis. 2007;28:4240–4246. doi: 10.1002/elps.200700208. [DOI] [PubMed] [Google Scholar]
- 14.Minioti K.S., Sakellariou C.F., Thomaidis N.S. Determination of 13 synthetic food colorants in water-soluble foods by reversed-phase highperformance liquid chromatography coupled with diode-array detector. Anal. Chim. Acta. 2007;583:103–110. doi: 10.1016/j.aca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Pereira Alves S., Brum D.M., de Andrade É.C.B., Netto A.D.P. Determination of synthetic dyes in selected foodstuffs by high performance liquid chromatography with UV–DAD detection. Food Chem. 2008;107:489–496. [Google Scholar]
- 16.Vachirapatama N., Mahajaroensiri J., Visessanguan W. Identification and determination of seven synthetic dyes in foodstuffs and soft drinks on monolithic C18 column by high performance liquid chromatography. J. Food Drug Anal. 2008;16:77–82. [Google Scholar]
- 17.Yang Y., Yin J., Shao B. Simultaneous determination of five aluminium lake dyes in chewing gum by HPLC with photodiode array detection. Food. Addit. Contam. Part A. 2011;28:1159–1167. doi: 10.1080/19440049.2011.584910. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka N., Ichihashi K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode arry detection. Talanta. 2008;74:1408–1413. doi: 10.1016/j.talanta.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Beitollahi H., Karimi-Maleh H., Khabazzadeh H. Epinephrine in the Presence of norepinephrine using carbon paste electrode modified with carbon nanotubes and novel 2-(4-Oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N′-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008;80:9848–9851. doi: 10.1021/ac801854j. [DOI] [PubMed] [Google Scholar]
- 20.Tajik S., Ali Taher M., Beitollahi H. Application of a new ferrocene-derivative modified-graphene paste electrode for simultaneous determination of isoproterenol, acetaminophen and theophylline. Sens. Actuators. B Chem. 2014;197:228–236. [Google Scholar]
- 21.Taleat Z., Mazloum Ardakani M., Naeimi H., Beitollahi H., Nejati M., Reza Zare H. Electrochemical behavior of ascorbic acid at a 2,2¢-[3,6-Dioxa- 1,8-octanediylbis(nitriloethylidyne)]-bis-hydroquinone carbon paste electrode. Anal. Sci. 2008;24:1039–1044. doi: 10.2116/analsci.24.1039. [DOI] [PubMed] [Google Scholar]
- 22.Mehdi Foroughi M., Beitollahi H., Tajik S., Hamzavi M., Parvan H. Hydroxylamine Electrochemical sensor based on a modified carbon nanotube paste electrode: application to determination of hydroxylamine in water samples. Int. J. Electrochem. Sci. 2014;9:2955–2965. [Google Scholar]
- 23.Mazloum-Ardakani M., Ganjipour B., Beitollahi H., Kazem Amini M., Mirkhalaf F., Naeimi H., Nejati-Barzoki Maryam. Simultaneous determination of levodopa, carbidopa and tryptophan using nanostructured electrochemical sensor based on novel hydroquinone and carbon nanotubes: application to the analysis of some real samples. Electrochim. Acta. 2011;56:9113–9120. [Google Scholar]
- 24.Mohammadi S., Beitollahi H., Mohadesi A. Electrochemical behaviour of a modified carbon nanotube paste electrode and its application for simultaneous determination of epinephrine, uric acid and folic acid. Sens. Lett. 2013;11:388–394. [Google Scholar]
- 25.Beitollahi H., Raoof J.B., Karimi-Maleh H., Hosseinzadeh Rahman. Electrochemical behavior of isoproterenol in the presence of uric acid and folic acid at a carbon paste electrode modified with 2,7-bis(ferrocenyl ethyl)fluoren-9-one and carbon nanotubes. J. Solid. State Electr. 2012;16:1701–1707. [Google Scholar]
- 26.Akhgar M.R., Beitollahi H., Salari M., Karimi-Maleh H., Zamani H. Fabrication of a sensor for simultaneous determination of norepinephrine, acetaminophen and tryptophan using a modified carbon nanotube paste electrode. Anal. Methods. 2012;4:259–264. [Google Scholar]
- 27.Alghamdi Ali F. Electrochemical oxidation behavior of hydrochlorothiazide on a glassy carbon electrode and its voltammetric determination in pharmaceutical formulations and biological fluids. J. Food Drug Anal. 2014;22:363–369. doi: 10.1016/j.jfda.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjunatha J.G., Deraman M., Basri N.H., N S., Nor M., Talib I.A., Ataollahi N. Sodium dodecyl sulfate modified carbon nanotubes paste electrode as a novel sensor for the simultaneous determination of dopamine, ascorbic acid, and uric acid. C. R. Chimie. 2014;17:465–476. [Google Scholar]
- 29.Manjunatha J.G., Deraman M., Basri N.H., Talib I.A. Selective detection of dopamine in the presence of uric acid using polymerized phthalo blue film modified carbon paste electrode. Adv. Mater. Res. 2014;895:447–451. [Google Scholar]
- 30.Manjunatha J.G., Deraman M., Basri N.H., Talib I.A. Fabrication of poly (Solid Red A) modified carbon nano tube paste electrode and its application for simultaneous determination of epinephrine, uric acid and ascorbic acid. Arab. J. Chem. 2014 [Google Scholar]
- 31.Manjunatha J.G., Deraman M., Basri N.H. Electrocatalytic detection of dopamine and uric acid at poly (Basic blue b) modified carbon nanotube paste electrode. Asian J. Pharm.Clin. Res. 2015;8:48–53. [Google Scholar]
- 32.Manjunatha J.G. Poly (Nigrosine) modified electrochemical sensor for the determination of dopamine and uric acid: a cyclic voltammetric study. Int. J. Chem. Tech. Res. 2016;9:136–146. [Google Scholar]
- 33.Manjunatha J.G. A novel poly (glycine) biosensor towards the detection of indigo carmine: a voltammetric study. J. Food Drug Anal. 2017 doi: 10.1016/j.jfda.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim-Nezhad Ghasem, Khorablou Zeynab, Zamani Maryam, Dorraji Parisa Seyed, Alamgholiloo Mahdieh. Voltammetric sensor for tartrazine determination in soft drinks using poly (p-aminobenzenesulfonic acid)/zinc oxide nanoparticles in carbon paste electrode. J. Food Drug Anal. 2017;25:293–301. doi: 10.1016/j.jfda.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu X., Lu L., Leng J., Yu Y., Wang W., Jiang M., Bai L. An enhanced electrochemical bplatform based on graphene oxide and multi-walled carbon nanotubes nanocomposite for sensitive determination of Sunset Yellow and Tartrazine. Food Chem. 2016;190:889–895. doi: 10.1016/j.foodchem.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 36.Zeynali K.A., Aleshi M. Electrochemical modification of glassy carbon electrode by Bismuth-chitosan nanosheets for electrocatalytic reduction and determination of tartrazine. Port. Electrochim. Acta. 2014;32:369–379. [Google Scholar]