Abstract
Background
Cholangiocarcinoma (CCA) is a rare, lethal cancer with 5-year survival of less than 10%. Although incidence rates have been increasing in the United States, ethnic variations in survival have not been investigated. We examined multi-ethnic variation in overall survival (OS) and CCA-specific survival (CSS) using data from the population-based Surveillance Epidemiology and End Results (SEER) program in the 4-year period after introduction of gemcitabine/cisplatin (GC) as treatment for CCA, compared with prior years.
Methods
The study included data from 5,616 advanced, intrahepatic CCA cases reported in SEER between 1990 and 2013. Multivariable-adjusted hazard ratios (HR) and 95% confidence intervals (CIs) were calculated to examine OS and CSS by ethnicity, age, gender and in the pre- and post-GC era (1990–2000, 2001–2009 vs. 2010–2013).
Results
Compared to non-Hispanic Whites, Hispanics had poorer 3-year OS (HR 1.11, 95% CI: 1.03–1.20) and 3-year CSS (HR 1.15, 95% CI: 1.05–1.25). Similarly, non-Hispanic Blacks had 3-year OS (HR 1.21, 95% CI: 1.10–1.34) and 3-year CSS (HR 1.21, 95% CI: 1.09–1.35). Males and older patients had shorter survival compared to females and younger patients. OS and CSS were both improved for patients’ post-advent of GC. Statistically significant improvement in CSS pre- and post-advent of GC was noted in non-Hispanic Whites, while Hispanics actually had worsened survival.
Conclusions
Hispanics and non-Hispanic Blacks have worse survival after diagnosis with advanced, intrahepatic CCA. Further studies are needed to determine determinants of poor survival among these groups.
Keywords: Cholangiocarcinoma (CCA), Surveillance Epidemiology and End Results (SEER), race, survival, gemcitabine, cisplatin
Introduction
Cholangiocarcinoma (CCA) is an uncommon malignancy arising from epithelial cells lining the biliary tree and has a rapidly fatal course, with a 5-year survival rate less than 10% (1,2). Although relatively rare in Western countries, it is highly prevalent in Latin America and East Asia (3). In the United States (US), CCA accounts for approximately 2% of all new cancer diagnoses and has been rising in incidence over the last few decades (4-10). The clinical presentation of CCA is variable and dependent on the location of the primary tumor (i.e., intrahepatic versus perihilar versus distal extrahepatic); however, most patients present with unresectable or metastatic disease, which is associated with worse prognosis (11). Major risk factors for CCA include primary sclerosing cholangitis, bile duct stones/hepatolithiasis, liver fluke infection, bile duct cysts, inflammatory bowel disease (IBD), hepatitis C, hepatitis B, cirrhosis, obesity, diabetes, alcohol, smoking, and genetic polymorphisms, all factors considered associated with inflammationpalme (8,12-15). Given the potential association of some of these risk factors with lifestyle and demographic variability as well as the rising incidence and high mortality of CCA, a better understanding of the populations at risk for CCA and the factors determining outcome is warranted (16,17). Furthermore, there are limited therapeutic options for this aggressive disease. The combination of gemcitabine and cisplatin is considered standard of care based on the ABC-02 trial, which showed this combination to be superior to single-agent gemcitabine with respect to both median overall survival (OS) as well as progression-free survival (PFS), across all anatomic subtypes (intrahepatic, extrahepatic, hilar, gallbladder) of the disease (15). An effect of this paradigm shift in CCA management on various population subgroups has not been reported previously. While some geographic and racial variability in the incidence of CCA has been reported previously, these studies were rather limited, not taking into account the current US demographics with increasing racial-ethnic minorities and did not report on any existing outcome disparities (18,19). We, therefore, conducted a comprehensive analysis of CCA from the Surveillance Epidemiology and End Results (SEER) database with the aim of examining CCA incidence and survival over time in relation to age, gender, and race-ethnicity, as well as the impact of available therapeutics.
Methods
Data were obtained from the National Cancer Institute’s SEER program. The SEER database has been described in detail (www.seer.cancer.gov). In brief, the SEER registry includes data from 18 population-based cancer registries from Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Los Angeles, San Jose-Monterey, greater California, Seattle-Puget Sound, Utah, the Alaska Native Tumor Registry, Kentucky, Louisiana, New Jersey, rural Georgia and the greater Georgia area. Data reported in SEER covers up to 28% of the general population in and is reflective of the sociodemographic patterns in the US.
OS was defined as the time period from intrahepatic CCA diagnosis to death from any cause. If patients were still alive, OS was censored at date of last follow-up or December 31, 2013, whichever came first. CCA-specific survival (CSS) was calculated from diagnosis to death from CCA. Data on patients who died of causes other than CCA was censored at the time of their death. Information on year of diagnosis, registry identification (ID), age at diagnosis, sex, race, marital status, survival time and cause of death were available in the SEER database.
For the present study, and similar to prior studies, we extracted data on all histologically confirmed CCA cases limited to the intrahepatic CCA subtype among adults (≥18 years) reported to SEER between 1990 and 2013 using the International Classification of Disease for Oncology, 3rd edition (ICD-O3) topography codes C22.1 thus limiting ascertainment bias. We limited the population to those patients with stage 4 disease only as this is the patient population to whom treatment with gemcitabine and cisplatin applies. The SEER database provides demographic and some clinical information, which includes data on age at diagnosis, gender, race/ethnicity [non-Hispanic White (NHW), non-Hispanic Black (NHB), non-Hispanic Asian and Pacific Islander (API), Native American/American Indian (NA/AI), and Hispanic], year of diagnosis, cancer stage at diagnosis, and survival data. However, specific treatment information, such as chemotherapy regimen, is not available in the data set. We excluded cases that received a diagnosis at death certificate or autopsy, did not have any follow-up records (survival time code of 0 months), as well as those lacking data on age at diagnosis, sex, or race/ethnicity. Incidence rates were calculated per 100,000 based on the 2010 standard US population as described (www.seer.cancer.gov). Incidence rates were calculated for each gender and for subgroups defined by age (<50, 50–70, >70 years), pre- and post-gemcitabine/cisplatin era (1990–2000, 2001–2009, 2010–2013), and by race/ethnicity as listed above. The incidence rates were calculated using the numerators from the specific population from the SEER data as numerators and the corresponding proportion in the 2010 standard US population as the denominator. We also calculated average annual percent changes (AAPC) in incidence rates over time, as described (20). In addition, we assessed relative risk of overall mortality and CCA-specific mortality of diagnosis, using Cox proportional hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazards assumption was tested prior to fitting the hazard models based on visual inspection of log-log survival curves, and none of the study variables violated the proportionality assumption. We calculated both univariate and multivariable-adjusted HRs, with adjustment for age, sex, race/ethnicity, and cancer stage at diagnosis. Further, we assessed survival patterns using Kaplan-Meier method. Survival was calculated per SEER definitions. All statistical tests were two-sided and P values lower than 0.05 were considered statistically significant. All analyses were performed in SAS® version 9.4 (SAS Inc., Cary, NC, USA).
Results
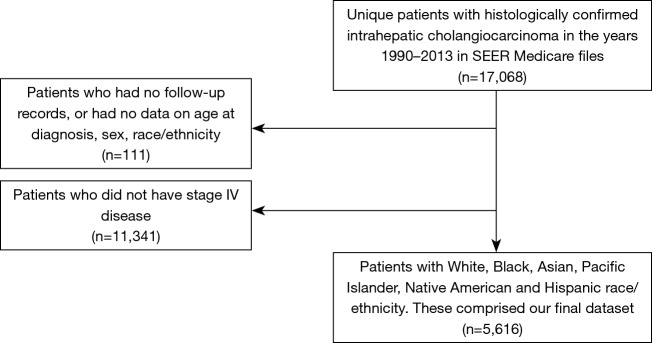
A total of 5,616 patients with the diagnosis of stage 4 CCA were identified in the SEER database after excluding patients missing information regarding sex, age, year of diagnosis, and ethnicity information (Figure 1). Distributions of the study population per the factors explored in our survival analyses are shown in Table 1. The median age at diagnosis for men and women was 65 (range, 12–97) and 67 (range, 21–102) years, respectively. The most numerous age cohort was patients 50–70 years (49.5%) and only 11.0% patients were younger than 50 years of age at the time of diagnosis.
Figure 1.
Consort diagram.
Table 1. Overall survival in cholangiocarcinoma by patient demographic characteristics.
| Characteristic | N | Median survival (months) | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | Overall P value | HR (95% CI) | P value | Overall P value | ||||
| Sex | 0.002 | <0.001 | |||||||
| Female | 2,820 | 4 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| Male | 2,796 | 3 | 1.09 (1.03–1.15) | 0.002 | 1.13 (1.07–1.19) | <0.001 | |||
| Age cohort, years | <0.001 | <0.001 | |||||||
| <50 | 618 | 6 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| 50–70 | 2,780 | 4 | 1.22 (1.11–1.34) | <0.001 | 1.23 (1.12–1.35) | <0.001 | |||
| >70 | 2,218 | 2 | 1.67 (1.52–1.83) | <0.001 | 1.71 (1.56–1.89) | <0.001 | |||
| Year | <0.001 | <0.001 | |||||||
| 1990–2000 | 891 | 3 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| 2001–2009 | 2,585 | 3 | 0.94 (0.87–1.01) | 0.10 | 0.92 (0.85–1.00) | 0.04 | |||
| 2010–2013 | 2,140 | 4 | 0.82 (0.75–0.89) | <0.001 | 0.81 (0.74–0.88) | <0.001 | |||
| Race/ethnicity | 0.02 | <0.001 | |||||||
| Non-Hispanic White | 3,577 | 3 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| Non-Hispanic Black | 485 | 2 | 1.13 (1.02–1.25) | 0.01 | 1.21 (1.10–1.34) | <0.001 | |||
| Non-Hispanic Asian or Pacific Islander | 658 | 4 | 0.92 (0.85–1.01) | 0.07 | 0.94 (0.86–1.03) | 0.19 | |||
| Non-Hispanic American Indian/Alaska Native | 48 | 3 | 1.02 (0.76–1.37) | 0.90 | 1.08 (0.81–1.45) | 0.60 | |||
| Hispanic (all races) | 848 | 3 | 1.04 (0.96–1.12) | 0.40 | 1.11 (1.03–1.20) | 0.009 | |||
Gender and survival
The survival analyses showed that males had shorter median OS than females (3 vs. 4 months), and in the multivariable-adjusted model, males had worse OS as compared to females (HR 1.13, 95% CI: 1.07–1.19, P<0.001) (Table 1). CCA-specific survival also resulted in a slightly higher mortality for men (HR 1.10, 95% CI: 1.03–1.16) (Table 2).
Table 2. Cause-specific survival in cholangiocarcinoma by patient demographic characteristics.
| Characteristic | N | Median survival (months) | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | Overall P value | HR (95% CI) | P value | Overall P value | ||||
| Sex | 0.04 | 0.004 | |||||||
| Female | 2,820 | 5 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| Male | 2,796 | 4 | 1.07 (1.00–1.13) | 0.04 | 1.10 (1.03–1.16) | 0.004 | |||
| Age cohort, years | <0.001 | <0.001 | |||||||
| <50 | 618 | 7 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| 50–70 | 2,780 | 5 | 1.14 (1.04–1.26) | 0.008 | 1.17 (1.06–1.29) | 0.003 | |||
| >70 | 2,218 | 4 | 1.39 (1.25–1.54) | <0.001 | 1.44 (1.30–1.59) | <0.001 | |||
| Year | <0.001 | <0.001 | |||||||
| 1990–2000 | 891 | 3 | 1.00 (ref) | 1.00 (ref) | |||||
| 2001–2009 | 2,585 | 4 | 0.91 (0.84–0.99) | 0.03 | 0.90 (0.82–0.97) | 0.01 | |||
| 2010–2013 | 2,140 | 6 | 0.79 (0.72–0.86) | <0.001 | 0.78 (0.71–0.85) | <0.001 | |||
| Race/ethnicity | 0.02 | <0.001 | |||||||
| Non-Hispanic White | 3,577 | 5 | 1.00 (ref) | – | 1.00 (ref) | – | |||
| Non-Hispanic Black | 485 | 3 | 1.15 (1.04–1.29) | 0.01 | 1.21 (1.09–1.35) | <0.001 | |||
| Non-Hispanic Asian or Pacific Islander | 658 | 5 | 0.96 (0.87–1.06) | 0.41 | 0.98 (0.89–1.08) | 0.65 | |||
| Non-Hispanic American Indian/Alaska Native | 48 | 5 | 1.02 (0.73–1.42) | 0.91 | 1.06 (0.76–1.48) | 0.72 | |||
| Hispanic (all races) | 848 | 4 | 1.09 (1.00–1.19) | 0.05 | 1.15 (1.05–1.25) | 0.002 | |||
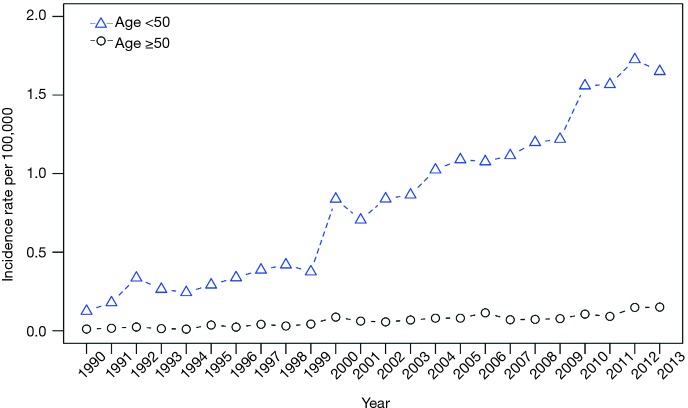
Age-specific incidence rates
We also examined the changes in CCA incidence rate over time among those <50 years old versus those ≥50 years old, and found an average rate of increase in CCA incidence of 10.90% among those <50 years and 11.15% among those ≥50 years, with an overall rate of increase from 1990 to 2013 of 12.25% (Figure 2). We note that this represents does not represent a significantly higher rate of incidence in those >50 compared with those <50 years of age.
Figure 2.
Age cohorts and incidence rates.
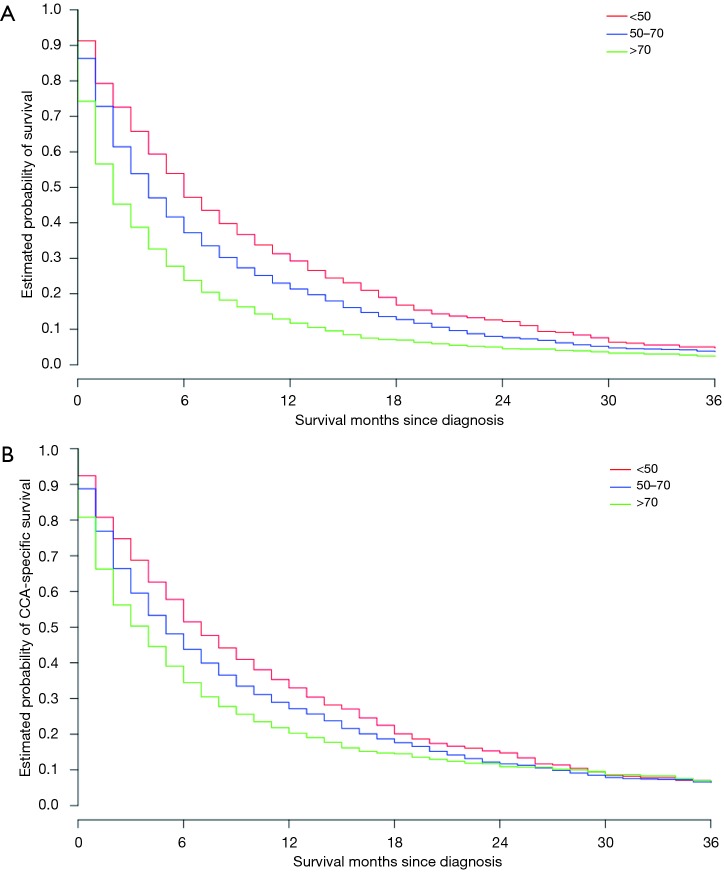
Age and survival
Age at diagnosis significantly impacted median OS (Figure 3A) with those younger than 50 years having a median OS of 6 months, those age 50–70 years having a median OS of 4 months, and those older than 70 years old having a median OS of only 2 months. In the multivariable-adjusted analysis, increasing age at diagnosis was associated with increased risk of all-cause mortality, with HRs (95% CIs) for age group 50–70 years and >70 years of 1.23 (95% CI: 1.12–1.35; P<0.001) and 1.71 (95% CI: 1.56–1.89; P<0.001), respectively (Table 1). A similar pattern was seen for CSS as well, with the multivariable-adjusted HRs (95% CIs) for age group 50–70 years and >70 years being 1.17 (95% CI: 1.06–1.29; P=0.003) and 1.44 (95% CI: 1.30–1.59; P<0.001), respectively (Table 2, Figure 3B).
Figure 3.
Age and survival. (A) Age cohorts and overall survival; (B) age cohorts and cholangiocarcinoma (CCA)-specific survival.
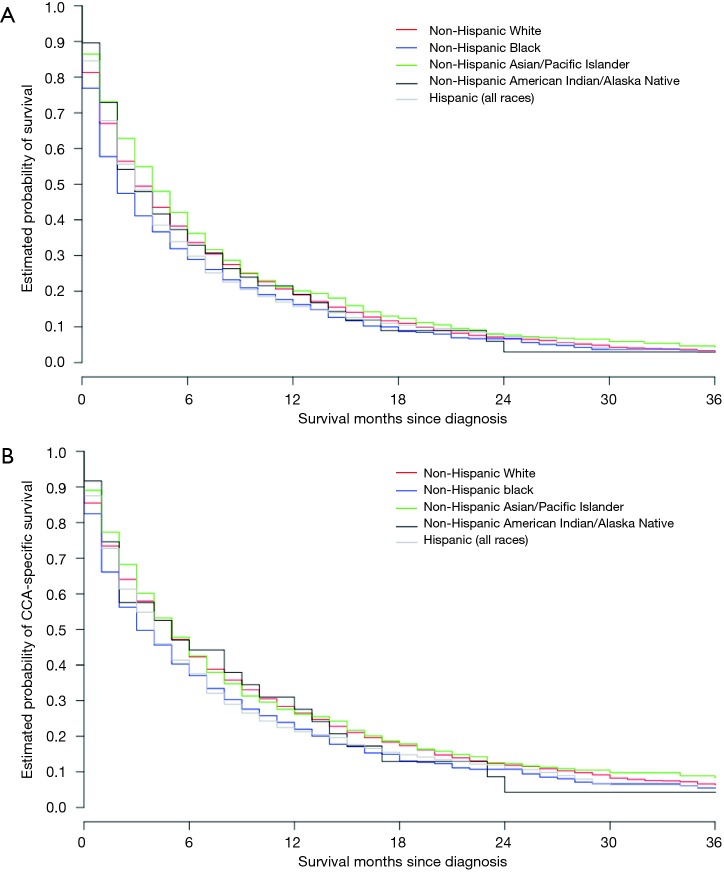
Race-ethnicity and survival
We found a significant impact of patient race-ethnicity on both the median OS (Figure 4A) and also median CSS (Figure 4B). Median OS for all racial-ethnic subgroups ranged between 2 and 4 months (Table 1). We found that the median OS was significantly worse for NHB (HR 1.21, 95% CI: 1.10–1.34, P<0.001) and Hispanics (HR 1.11, 95% CI: 1.03–1.20, P=0.009), the two largest racial-ethnic minorities in the US, as compared to the reference population of NHW. Median CSS for these subgroups ranged between 3 and 5 months with similar significant differences including worse median CSS for NHB (HR 1.15, 95% CI: 1.04–1.29, P=0.01), and Hispanics (HR 1.09, 95% CI: 1.00–1.19, P=0.05) compared to NHW (Table 2, Figure 4B).
Figure 4.
Race/ethnicity and survival. (A) Comparison of race/ethnicity cohorts and overall survival; (B) comparison of race/ethnicity cohorts and cholangiocarcinoma (CCA)-specific survival.
Gemcitabine/cisplatin era and survival
Survival differences were compared between years of diagnosis 1990–2000, 2001–2009, 2010–2013, separated, at 2009, by the year of publication of the ABC-02 study and split into equal 4-year periods immediately surrounding 2009, to assess the potential impact of gemcitabine/cisplatin chemotherapy on outcomes in the population as a whole as well as in different racial subgroups (21). Median OS for those diagnosed between 1990 and 2000 and 2001 and 2009 versus those diagnosed between 2010 and 2013 was 3 versus 3 versus 4 months, respectively. OS was significantly better between 2010–2013, after the introduction of gemcitabine/cisplatin (HR 0.81, 95% CI 0.74–0.88, P<0.001) as compared with 1990–2000 and 2001–2009 (Table 1). Similarly, median CSS for those diagnosed between 2010–2013 was significantly better at 6 months, as compared to 4 and 3 months for those diagnosed between 1990 and 2000 and 2001 and 2009 (HR 0.78, 95% CI: 0.71–0.85, P<0.001) (Table 2).
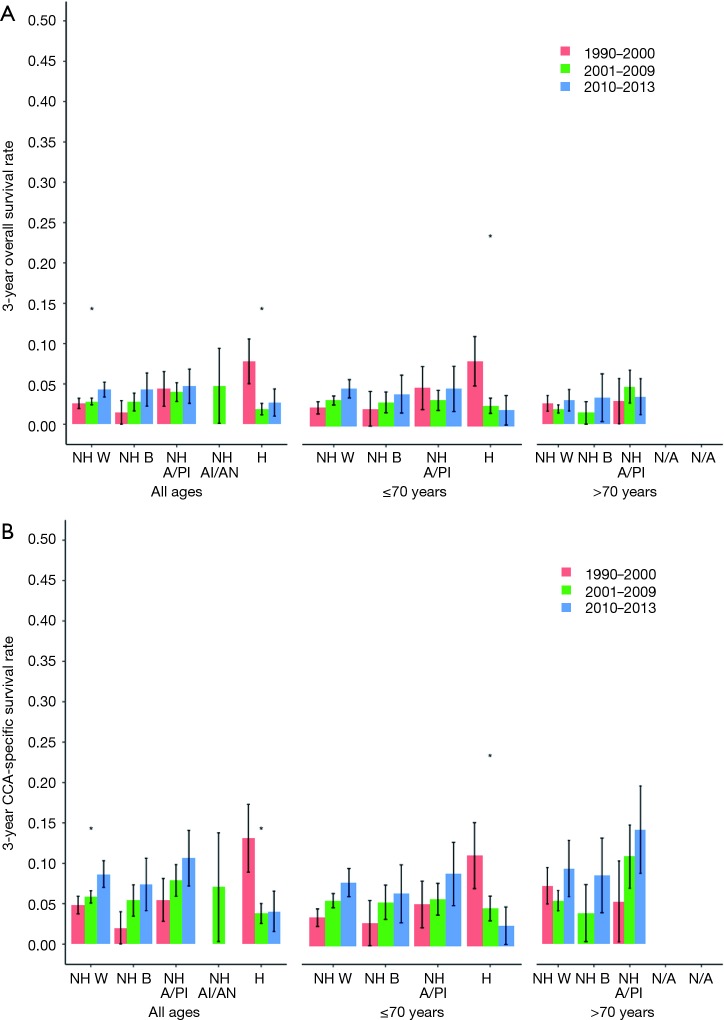
Overall and CCA-specific 3-year survival were analyzed before and after the advent of gemcitabine/cisplatin chemotherapy within ethnic subgroups and by age as well (Figure 5A,B). Among all groups, but for Hispanics, survival has been improving over time since 1990. In patients of all ages, only the NHW cohort had statistically significant improvement in both overall and CCA-specific survival. In patients younger than 70 years of age, a significant change in survival after the advent of gemcitabine/cisplatin was only noted in the Hispanic cohort, with a worsening of survival, rather than an improvement. A trend in improving survival was indeed noted in the NHW and NHB cohorts. In those older than 70 years, no particular ethnicity cohort had any significant improvement in survival after the advent of gemcitabine/cisplatin.
Figure 5.
Age, ethnicity, and survival rates by time period. (A) Age, ethnicity and 3-year overall survival before and after the advent of gemcitabine/cisplatin; (B) age, ethnicity and 3-year cholangiocarcinoma-specific survival before and after the advent of gemcitabine/cisplatin. NH, non-Hispanic; W, Whites; B, Blacks; A/PI, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native; H, Hispanic; N/A, not available.
Conclusions
To our knowledge, this is the largest analysis of a population-based database exploring outcome disparities in patients with advanced intrahepatic CCA from different ethnicity subgroups with a specific focus on race/ethnicity-related treatment outcomes. Previous studies have reported variations in population-based database derived outcomes of intrahepatic CCA patients, but outcomes among various ethnicities and age groups, related in particular to available standard treatments, such as gemcitabine/cisplatin, have not been evaluated (18,19).
Disease heterogeneity by ethnicity has been reported in many malignant disorders, though this is most often posed in terms of African-American versus Caucasian status. In recent years incidence and outcome disparities in cancer have been reported in other racial-ethnic minorities as well, including Hispanics and Asians, the two fastest growing minorities in the US (20). While one recent study does explore differences in CCA mortality amongst Asians, African-Americans and whites, and demonstrates increasing mortality especially for African-Americans, a deeper understanding of CCA outcomes is lacking for patients of other ethnicities (18). Recent studies do continue to explore incidence trends related to both intrahepatic and extrahepatic CCA among a variety of ethnicity groups in SEER, though some varying results are seen (21-23). Further information would be invaluable and can clearly be used for exploring healthcare access and utilization trends, in addition to racial-ethnic differences in treatment effects.
The commonly acknowledged clinical factors that may serve as predictors for survival include intrahepatic satellite lesions, lymph node invasion, and distant metastasis. Our analysis shows for the first time, to our knowledge, that males with advanced intrahepatic CCA are suffering from worse OS (P<0.001), and also CSS (P=0.004). Other epidemiologic studies have suggested a risk of higher CCA related mortality in males, thought to be due to increased risk of hepatitis C, cirrhosis and primary sclerosing cholangitis (24-26).
We show that older age at diagnosis of advanced intrahepatic CCA (>70 years) is associated with significantly worse overall and CSS (P<0.001 and P<0.001, respectively) in multivariate analysis. This may be due to less aggressive therapeutic approaches employed for older patients who have more comorbidities, later diagnosis given delayed workup due to comorbidities, or also possibly due to a difference in disease biology at older age.
Recent studies, in colorectal cancer for example, have noted alarmingly rising incidence rates in younger age groups (27,28). We analyzed the incidence of advanced intrahepatic CCA in patients within the SEER database, comparing those >50 years of age with younger patients <50 years old. We noted that, for the entire cohort of all ethnicities, there has been an increased incidence rate of CCA in patients >50, in addition to those <50 years of age. Certainly, in relation to CCA, the rise in incidence may be in part attributable to greater awareness of the disease and categorization of liver lesions as CCA rather than, for example, “carcinoma of unknown primary”. While young-onset disease may have a small familial component, the majority of cases are quite likely sporadic. The rising incidence rates in this cohort of young patients signals relatively recent changes in exposures that influence risk. Established lifestyle factors associated with CCA include obesity, metabolic syndrome, alcohol abuse and tobacco use may likely be contributing to the rise in incidence. However, epidemiologic data regarding any associations between CCA risk and lifestyle factors, such as high consumption of processed meats and sugars and low levels of physical activity, is lacking.
A comprehensive evaluation of ethnicity-based survival was also conducted in this study. NHB and Hispanics had significantly worse median OS than whites while API had a trend toward better median OS. Certainly, one may surmise that access to care, in regard to NHB and Hispanics, may be at least part of the reason for the detriment in survival, although a difference in disease biology is also plausible. We also noted a significantly worse CSS among NHB and Hispanics as compared to the reference population of NHW.
One of the most significant recent therapeutic advances made in the management of CCA, with a demonstrated improvement in patient survival, is the advent of the gemcitabine/cisplatin regimen as the standard first-line therapy for patients with advanced disease. However, studies to date have reported outcomes in predominantly white patient populations. This, of course, is not representative of the true US population effected by the disease, nor of survival outcomes in these varied racial population subgroups. One study did examine survival comparing 494 patients in the ABC-02 study done in the United Kingdom and those in BT22 study performed in Japan (29). No significant differences in survival were noted.
To analyze the impact of the advent of gemcitabine/cisplatin upon survival, we divided patients into 3 cohorts, based on the date of publication of the seminal ABC-02 study in 2010, to evaluate survival trends over time: patients diagnosed between 1990 and 2000, 2001 and 2009, and 2010 and 2013. For the study population as a whole, median CSS for those diagnosed from 2010 onwards was significantly better (P<0.001). In our analysis of outcomes based on ethnicity group, we noted generally improving survival across time in all groups, with a statistically significant improvement in CSS pre- and post-advent of gemcitabine/cisplatin in NHW (P<0.001). Interestingly, statistically significant worsening of survival was noted in Hispanics over all ages and in those younger than 70. More specifically, among those younger than 70 years of age no statistically significant improvements were seen in any cohorts, but a trend toward improved survival was noted in NHW, NHB, and NH API groups. In those older than 70 years old, no statistically significant improvements were seen in any cohorts, although not enough data was available for some of these groups and time frames, including the Hispanic and AI/AN cohorts. Similar findings have been reported in analysis of other cancer subtypes from the SEER database and are inherent to population-based analysis where large-scale trends rather than absolute numbers have been considered more significant (21). This finding of ethnicity-based differences in treatment outcomes, among the largest CCA patient population studied, is the first to be reported to our knowledge and can serve as a basis for studying healthcare access and utilization trends and response to treatment by patient subgroups, among others. The significant worsening of survival especially seen in the Hispanic cohort deserves further in-depth study. Other contributing factors besides the gemcitabine/cisplatin chemotherapy are, of course, also at play, such as access to care, patient and cultural preferences, and improvements in supportive care.
The major strength of our study is the use of a large population-based data set with maximum representation of the current racial-ethnic makeup of current US population. We have reported CSS as well as OS for five different patient subgroups, which may help to elucidate the interplay of factors that may dictate variability in overall and disease-specific outcomes in CCA. We acknowledge a limitation here in that survival dates and times in SEER data are not exact. Cancer registries generally report only the month and year and number of whole months of survival to the SEER program in an effort to limit patient identifiability and ensure privacy. Due to the nature of our source data, we were also unable to include some relevant clinical variables, in addition to variables relating to access to care, patient preference, and biologic features of the disease in our analysis. As an example, specific treatment details, such as chemotherapy regimen details, are not available in the SEER database.
Given this lack of more specific data we use a specific time point as a surrogate for the introduction of a particular treatment. This is based on an assumption that CCA patients primarily received gemcitabine/cisplatin after 2009. To circumvent this assumption, we are currently validating our findings in ongoing studies utilizing other databases where more specific treatment information is readily available. Longer follow-up in the era of newer drugs/treatment regimens, as well as an analysis of healthcare utilization data will help better understand the impact of these agents across various ethnic subgroups. Additionally, our analysis may be further improved if we included additional comparative data from patients with other anatomic subtypes of CCA—extrahepatic, gallbladder. Emergence of data regarding genomic subtypes of CCA (IDH1/2, FGFR2, MSI, BRAF) will also need to be included in large, public databases for future analyses of ethnicity-based differences in outcomes. Currently available institutional and cooperative group/consortium databases may currently enable the beginnings of such an analysis. Nevertheless, studies such as this one include information relating to ethnic disparities. This proves to include important descriptions of disease characteristics and ultimately inform us regarding the management needs of specific patient populations as well as optimal triaging of healthcare resources. Our analysis helps to delineate these disparities in CCA patients and is hypothesis-generating. Further systematic analysis is required to determine the exact causes and devise strategies to overcome these disparities.
Acknowledgements
Statistical support was provided by Mayo Clinic Florida Focused Research Teams Program.
Footnotes
Conflicts of Interest: Kabir Mody: (I) research support: Senwha Biociences Inc; Tracon Pharmaceuticals; Genentech; Astrazeneca/Medimmune; Arqule, Inc; Agios; Taiho Oncology; Boston Biomedical; Ipsen; (II) consulting/advisory board: Eisai Co, Ltd.; Bayer Pharmaceuticals; Sikander Ailawadhi: (I) research support: Pharmacyclics, Inc; (II) consulting/advisory board: Takeda Oncology; Novartis Pharmaceuticals; Amgen; Tanios Bekaii-Saab: (I) research support: Boston Biomedical; Bayer; Celgene; Merrimack; (II) consulting/advisory board: Taiho; Bayer; Boehringer Ingelheim; Merrimack; Glenmark; Amgen; Genentech. The other authors have no conflicts of interest to declare.
References
- 1.Marzioni M, Invernizzi P, Candelaresi C, et al. Human cholangiocarcinoma development is associated with dysregulation of opioidergic modulation of cholangiocyte growth. Dig Liver Dis 2009;41:523-33. 10.1016/j.dld.2008.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Facts & Figures, 2018. American Cancer Society, 2018. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html
- 3.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 4.Saha SK, Zhu AX, Fuchs CS, et al. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016;21:594-9. 10.1634/theoncologist.2015-0446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayala D, Blackstock AW. Effective treatment strategies for cholangiocarcinoma: the challenge remains. Gastrointest Cancer Res 2008;2:251-2. [PMC free article] [PubMed] [Google Scholar]
- 6.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. 10.1053/jhep.2001.25087 [DOI] [PubMed] [Google Scholar]
- 7.Patel T. Cholangiocarcinoma--controversies and challenges. Nat Rev Gastroenterol Hepatol 2011;8:189-200. 10.1038/nrgastro.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol 2012;57:69-76. 10.1016/j.jhep.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol 2010;2:407-16. 10.4251/wjgo.v2.i11.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvaro D, Crocetti E, Ferretti S, et al. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis 2010;42:490-5. 10.1016/j.dld.2009.10.009 [DOI] [PubMed] [Google Scholar]
- 11.Anderson CD, Pinson CW, Berlin J, et al. Diagnosis and treatment of cholangiocarcinoma. Oncologist 2004;9:43-57. 10.1634/theoncologist.9-1-43 [DOI] [PubMed] [Google Scholar]
- 12.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84. 10.1002/hep.24351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48:308-21. 10.1002/hep.22310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirschfield GM, Karlsen TH, Lindor KD, et al. Primary sclerosing cholangitis. Lancet 2013;382:1587-99. 10.1016/S0140-6736(13)60096-3 [DOI] [PubMed] [Google Scholar]
- 15.Ren HB, Yu T, Liu C, et al. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control 2011;22:837-47. 10.1007/s10552-011-9754-3 [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. 10.3322/caac.21332 [DOI] [PubMed] [Google Scholar]
- 17.Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol 2004;40:472-7. 10.1016/j.jhep.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 18.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int 2006;26:1047-53. 10.1111/j.1478-3231.2006.01350.x [DOI] [PubMed] [Google Scholar]
- 19.Altekruse SF, Petrick JL, Rolin AI, et al. Geographic variation of intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma, and hepatocellular carcinoma in the United States. PLoS One 2015;10:e0120574. 10.1371/journal.pone.0120574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ailawadhi S, Aldoss IT, Yang D, et al. Outcome disparities in multiple myeloma: a SEER-based comparative analysis of ethnic subgroups. Br J Haematol 2012;158:91-8. 10.1111/j.1365-2141.2012.09124.x [DOI] [PubMed] [Google Scholar]
- 21.Mosadeghi S, Liu B, Bhuket T, et al. Sex-specific and race/ethnicity-specific disparities in cholangiocarcinoma incidence and prevalence in the USA: An updated analysis of the 2000-2011 Surveillance, Epidemiology and End Results registry. Hepatol Res 2016;46:669-77. 10.1111/hepr.12605 [DOI] [PubMed] [Google Scholar]
- 22.Antwi SO, Mousa OY, Patel T. Racial, Ethnic, and Age Disparities in Incidence and Survival of Intrahepatic Cholangiocarcinoma in the United States; 1995-2014. Ann Hepatol 2018;17:274-85. 10.5604/01.3001.0012.0929 [DOI] [PubMed] [Google Scholar]
- 23.Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci 2014;59:3103-10. 10.1007/s10620-014-3276-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaib YH, El-Serag HB, Davila JA, et al. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology 2005;128:620-6. 10.1053/j.gastro.2004.12.048 [DOI] [PubMed] [Google Scholar]
- 25.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology 2009;136:1134-44. 10.1053/j.gastro.2009.02.038 [DOI] [PubMed] [Google Scholar]
- 26.Toy E, Balasubramanian S, Selmi C, et al. The prevalence, incidence and natural history of primary sclerosing cholangitis in an ethnically diverse population. BMC Gastroenterol 2011;11:83. 10.1186/1471-230X-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109. doi: . 10.1093/jnci/djw322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 29.Valle JW, Furuse J, Jitlal M, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol 2014;25:391-8. 10.1093/annonc/mdt540 [DOI] [PubMed] [Google Scholar]