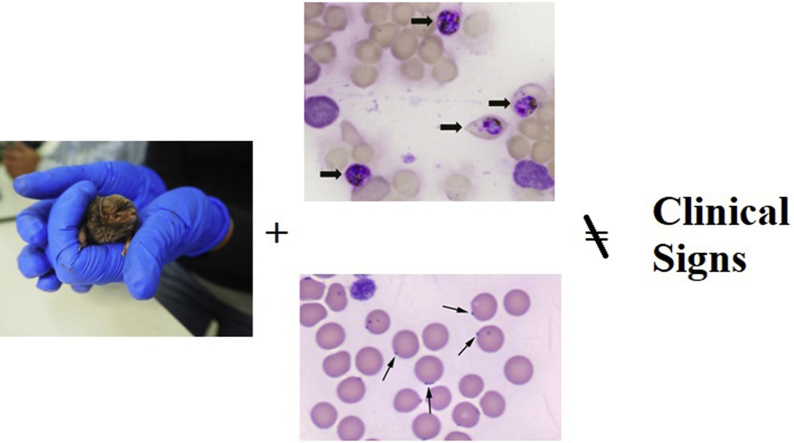
Abstract
While bats are often viewed as carriers of infectious disease agents, little research has been conducted on the effects these pathogens may have on the bat populations themselves. The southern bent-winged bat (Miniopterus orianae bassanii) is a critically endangered subspecies endemic to south-eastern Australia. Population numbers of this bat have declined over the past 50 years, but the reasons for this are unclear. As part of a larger study to determine if disease could be a contributing factor to this decline, southern bent-winged bats from several locations in Victoria and South Australia were captured and examined for the presence of the blood parasite, Polychromophilus melanipherus, and haemoplasmas (Mycoplasma sp.). Results were compared with those obtained from populations of the more common, partially sympatric, eastern bent-winged bat (Miniopterus orianae oceanensis) from three different locations in Victoria. Both organisms were found in both subspecies (prevalence of P. melanipherus 60% by PCR for southern bent-winged bats compared with 46% for eastern bent-winged bats; prevalence of haemoplasmas 10% for southern bent-winged bats compared with 8% for eastern bent-winged bats), with no association between the probability of infection, body weight, abnormal blood parameters or any other indicators of ill health. However, Victorian southern bent-winged bats had heavier burdens of P. melanipherus than both the South Australian southern bent-winged bats and eastern bent-winged bats. Further investigations are required to determine if these differences are impacting population health.
Keywords: bats, Haemoplasma, Miniopterus orianae bassanii, Miniopterus orianae oceanensis, Parasites, Polychromophilus melanipherus
Graphical abstract
Highlights
-
•
Polychromophilus melanipherus not associated with ill health in southern or eastern bent-winged bats.
-
•
Haemoplasma infection not associated with ill health in southern or eastern bent-winged bats.
-
•
Greater prevalence and intensity of Polychromophilus melanipherus infection in Victorian southern bent-winged bats.
1. Introduction
Historically, infectious disease agents and parasites were rarely considered as causing significant extinction-threatening processes (McCallum, 2012). Diverse host communities, i.e. those with high biodiversity, may inhibit the spread of parasites if the number of susceptible species is diluted by a large number of non-susceptible species (Civitello et al., 2015), but anthropogenic impacts have caused dramatic declines in biodiversity worldwide. Australian declines are eight times the global average (Wilting et al., 2017). It has been suggested that this loss of biodiversity is at least partly responsible for the increase in the number of emerging diseases (Woolhouse and Gowtage-Sequeria, 2005).
While some parasitic infections have obvious clinical effects, e.g. avian malaria (Atkinson, 2008) even apparently subclinical infections can still exert negative effects on their host. Anthelmintic treatment of wild common eider ducks (Somateria mollissima) infected with gastrointestinal helminths but showing no clinical signs, resulted in markedly increased survival (Wobeser, 2008). A study of migrating passerines found that haemosporidian infections extended the time of migration, more heavily infected birds arriving later than less infected birds despite no difference in their physical condition (Emmenegger et al., 2018).
As well as these more subtle effects, parasites, including haemoparasites, cause major diseases of humans (e.g. malaria infections resulted in an estimated 445,000 deaths in 2016 (World Health Organisation, 2017)) and agricultural animals (e.g. diseases caused by Babesia and Anaplasma cost the Australian cattle industry close to $16 million dollars per annum (McLeod and Kristjanson, 1999)). Wildlife is also impacted. For example, haemoparasites have been implicated in major species population declines, such as trypanosome infections which were linked to the extinction of Maclear's rat (Rattus macleari) on Christmas Island (Wyatt et al., 2008), and avian malaria which contributed to the extinction of up to 23 endemic Hawaiian forest bird species (Atkinson, 2008).
Bats are known to be infected with a number of different haemoparasite species, including trypanosomes (Mackie et al., 2017), Babesia vesperuginis (Corduneanu et al., 2017) and four genera of haemosporidians: Plasmodium, Nycteria, Hepatocystis and Polychromophilus (Garnham, 1966; Megali et al., 2011; Schaer et al., 2013). The first three haemosporidian genera only occur in the tropics of Asia and Africa, while Polychromophilus also occurs in temperate regions of Europe, the United States and Australia (Garnham, 1973a; Megali et al., 2011). There are five species within the Polychromophilus genus: P. corradetti has been found in the greater long-fingered bat (M. inflatus) and P. adami has been found in the least long-fingered bat (M. minor minor), both from Africa. Polychromophilus deanei has been found in Pallas's long-tongued bat (Glossophaga soricine) and the black myotis (Myotis nigricans) in Brazil (Garnham et al., 1971), while P. melanipherus is reportedly only found in bats of the genus Miniopterus (Megali et al., 2011). An early report claimed to have found this parasite in eastern long-eared bats (Nyctophilus bifax), eastern cave bats (Vespadelus troughtoni (previously V. pumilus)) and Semon's leaf-nosed bats (Hipposideros semoni) in Australia (Mackerras, 1959). However, it has since been shown that morphological characteristics alone are unreliable when it comes to differentiating haemosporidians (Megali et al., 2011). Garnham (1973b) reclassified the parasite found in the aforementioned bats as P. murinus, which is found in vespertilionid bats.
South-eastern Australia is home to two subspecies of the large bent-winged bat (Miniopterus orianae) (Richards and Reardon, 2008). The southern bent-winged bat (M. orianae bassanii) occurs only in south-western Victoria and south-eastern South Australia (SA). There are two maternity caves in Victoria, near Warrnambool and Cape Bridgewater, and one near Naracoorte in SA. In the last 50 years, the size of the Naracoorte population of southern bent-winged bats has declined from an estimated 200,000 in the 1950s to 20,000 in 2009 (DELWP, 2017). The Warrnambool population declined from approximately 15,000 to 10,000 over the same time period (DELWP, 2017). The subspecies was listed as critically endangered under the Environment Protection and Biodiversity Conservation Act in 2007. The eastern bent-winged bat (M. orianae oceanensis) is more common and widespread, being distributed along the east coast of Australia (Richards and Reardon, 2008). Although numbers appear to be stable, the subspecies is listed as vulnerable in Victoria due to the use of just a single maternity site.
Disease has been suggested as a possible cause for the declines in southern bent-winged bat populations (DELWP, 2017). Despite this, there has been only one published disease investigation of this subspecies (McLelland et al., 2013), which did not examine blood-borne parasites.
Polychromophilus melanipherus has been recorded from presumptive eastern bent-winged bats in NSW and Queensland (Dew and McMillan, 1970; Mackerras, 1959). Bat flies, which are common, haematophagous external parasites of bats, are the accepted intermediate host (Dew and McMillan, 1970; Gardner and Molyneux, 1988; Mackerras, 1959; Obame-Nkoghe et al., 2016). The life cycle for P. murinus has been described (Gardner and Molyneux, 1988) and is assumed to be similar for P. melanipherus. Sporogony occurs within the bat fly, oocysts forming in the midgut and sporozoites invading the salivary glands. These are then injected into the bat host. Schizogony takes place in the bone marrow, spleen, lungs, kidneys and liver, releasing pigmented gametocytes which invade erythrocytes (Fig. 1). These are then taken up by bat flies when they are feeding on their host, and the cycle repeats itself (Gardner and Molyneux, 1988; Garnham, 1973b; Mackerras, 1959). Prevalence and infection intensity in bats were reported to be seasonal, lowest in April and highest in August (Dew and McMillan, 1970). As P. melanipherus is a haemoparasite, it may be a potential cause of erythrocyte destruction and anaemia.
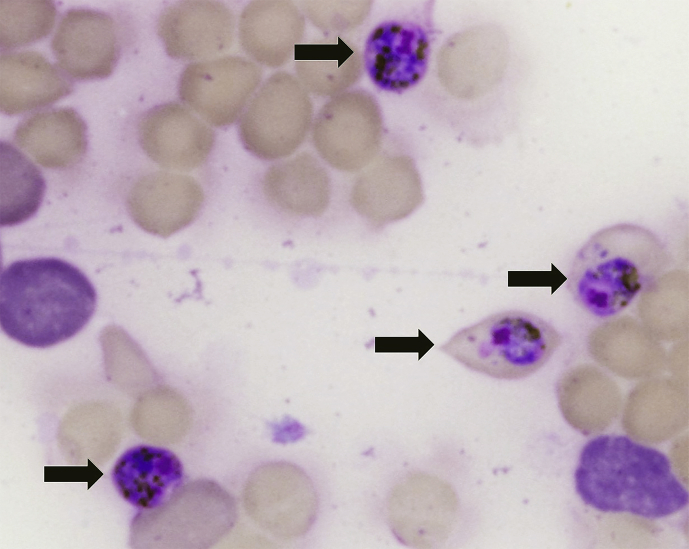
Fig. 1.
Blood smear showing Polychomophilus melanipherus gametocytes within erythrocytes (Arrows). Diff Quik stain. x 400 magnification.
Haemotropic mycoplasmas, or haemoplasmas, are cell wall-less, gram negative, non-acid-fast bacteria that cannot be cultured, are distributed worldwide and reside on the surface of mammalian erythrocytes. They were originally classified as rickettsia in the genera Haemobartonella and Eperythrozoon, but molecular work has confirmed their identity as mycoplasmas (Messick and Harvey, 2012). Haemoplasma infection has been reported in a wide range of hosts including humans (Steer et al., 2011), dogs (Compton et al., 2012), cats (Messick, 2004), turtles (Jarred et al., 2018), white-tailed deer (Odocoileus virginianus) (Maggi et al., 2013), Darwin's fox (Lycalopex fulvipes) (Cabello et al., 2013), Japanese badgers (Meles meles anakuma) (Harasawa et al., 2014), raccoon dogs (Nyctereutes procyonoides viverrinus) (Harasawa et al., 2014) and bats (Ikeda et al., 2017; Mascarelli et al., 2014; Millan et al., 2015). They have been associated with haemolytic anaemia, ill thrift and infertility, but infections are also frequently asymptomatic (Messick and Harvey, 2012; Cabello et al., 2013; Maggi et al., 2013; Millan et al., 2015). The status of Australian bats regarding haemoplasma infections and their potential pathogenicity is unknown.
The aims of this study were to survey southern bent-winged bats for P. melanipherus and haemoplasmas and compare the results with the more common eastern bent-winged bats. We hypothesised that P. melanipherus and haemoplasmas would be associated with signs of anaemia and would be more prevalent in southern bent-winged bats. This work was part of a larger disease investigation involving viruses (Holz et al., 2018), fungi and ectoparasites.
2. Material and methods
2.1. Study population and sites
Sampling was undertaken during summer (January–February), autumn (March–April) and early spring (September), between April 2015 and March 2017. Trapping for southern bent-winged bats occurred at the Naracoorte breeding cave, but, because of the difficult access to the breeding cave near Warrnambool, no trapping occurred there. Instead, those southern bent-winged bats were trapped at nearby non-breeding caves (Allansford (38.3861° S, 142.5931° E) and Portland (38.3609° S, 141.6041° E) 1 and 2). Eastern bent-winged bats were trapped at abandoned mines at Christmas Hills (37.6515° S, 145.3173° E) and Eildon (37.2343° S, 145.8976° E) and the only Victorian breeding cave near Lakes Entrance (37.8511° S, 147.9958° E) in eastern Victoria. Due to concerns that members of the public may enter caves and disturb the critically endangered southern bent-winged bats, this paper uses a generic description of the cave locations, rather than the specific name of each cave.
2.2. Sample collection
Individuals were caught as they flew out of the caves/mines, using harp traps (Austbat, Bairnsdale, Victoria (Tidemann and Woodside, 1978),) set at dusk at the entrances. Traps were monitored continually with the bats either left in the harp trap bag, or transferred in small numbers to cloth bags, prior to sampling. All bats were examined for any external signs of disease, aged as juveniles or adults (based on the presence or absence of a cartilaginous core at the metacarpal-phalangeal joint (Brunet-Rossinni and Wilkinson, 2009)), their sex was determined, forearm length measured from carpus to elbow, and weighed.
For blood collection, bats were placed in dorsal recumbency and a maximum of 90 μl blood collected from the median vein, located over the ventral aspect of the humerus (Olsson and Woods, 2008). Ethanol 70% was used to wipe down the skin surface over the vein. A solution of EDTA was drawn into a 0.5 ml insulin syringe (Becton, Dickinson and Company, New Jersey, USA). The EDTA solution was then expelled, coating the interior of the syringe with anticoagulant. Blood was collected in the syringe, a drop was expressed onto a glass slide and a smear made immediately. If sufficient blood was able to be collected for molecular testing, the remainder was placed in a labelled Eppendorf tube (Eppendorf South Pacific Pty Ltd, North Ryde, Australia), which was held at 4 °C prior to transport back to the laboratory.
Packed cell volume (PCV) and red cell count (RCC) were determined on an XT-2000i haematology analyser (Sysmex, Kungsbacka, Sweden). Left over blood was frozen at −20 °C prior to DNA extraction and PCR assay.
During the course of the study, seven dead bats were examined opportunistically (six from the Naracoorte cave and one from the Warrnambool breeding cave). Bats were necropsied and examined histologically. The causes of death were trauma unrelated to the study (five cases) and unknown (two cases). Tissue samples (lung, liver, spleen) were collected for the PCR detection of P. melanipherus.
2.3. Laboratory methods
Blood smears were air dried and stained with Diff Quik stain (Thermofisher, Riverstone, Australia). Each slide was scanned for 10 min under x 100 oil immersion for the presence of P. melanipherus gametocytes. The number of gametocytes found over this time period was recorded. For each slide, ten monolayer blood fields were also examined for the presence of polychromatophilic erythrocytes (PE). These were counted and divided by ten to obtain a mean polychromatophilic erythrocyte count per oil immersion field (Briggs and Bain, 2016).
For molecular testing for P. melanipherus, blood and organ samples were thawed to room temperature. DNA was extracted using a QIAamp DNA Mini Kit (Qiagen, Melbourne, Australia) according to manufacturer's instructions. Extracted DNA was used as a template in a PCR, using primers designed to amplify a 787 bp nucleotide sequence of the mitochondrial cytochrome b gene: DW2/F (5′-TAATGCCTAGACGTATTCCTGATTATCCAG-3′) and DW4/R (5′-TGTTTGCTTGGGAGCTGTAATCATAATGTG-3′) (Duval et al., 2012). PCR cycles conditions were: 5 min at 94 °C, followed by 40 repetitive cycles of 30 s at 94 °C, 90 s at 59 °C (the annealing temperature was decreased from 60 °C as this modification yielded more obvious bands on the agarose gel) and 90 s at 72 °C followed by a final 10 min extension at 72 °C. PCR negative controls containing no DNA template were also included. PCR products were visualised on an agarose gel. Positive samples underwent DNA purification (QIAquick Gel Extraction Kit, Qiagen, Melbourne, Australia) and Sanger sequencing (Big Dye Terminator version 3.1, Applied Biosystems, Melbourne, Australia) to confirm their identity.
The same extracted DNA samples were used as templates in two haemoplasma PCRs designed to amplify a 1380 bp region of the 16S rRNA gene. Amplification was performed using two sets of oligonucleotides: HemMycop16S-41s (5′-GYATGCMTAAYACATGCAAGTCGARCG-3′) and HemMyco16S-938as (5′-CTCCACCACTTGTTCAGGTCCCCGTC-3′); HemMycop16S-322s (5′-GCCCATATTCCTACGG GAAGCAGCAGT-3′) and HemMycop16S-1420as (5′-GTTTGACGGGCGGTGTGTACAAGACC-3′). These were used as they targeted different regions of the 16S rRNA gene, increasing the likelihood of detecting haemoplasma DNA (Maggi et al., 2013). PCR cycle conditions were: 2 min at 94 °C, followed by 55 cycles of 15 s at 94 °C, 15 s at 68 °C and 18 s at 72 °C, followed by a final 1 min extension at 72 °C (Ikeda et al., 2017). PCR negative controls containing no DNA template were also included. PCR products were visualised on an agarose gel. Positive samples underwent DNA purification (QIAquick Gel Extraction Kit, Qiagen, Melbourne, Australia) and Sanger sequencing (Big Dye Terminator version 3.1, Applied Biosystems, Melbourne, Australia) to confirm their identity.
2.4. Sequence and phylogenetic analyses
Polychromophilus and haemoplasma sequences were submitted to GenBank and analysed with Geneious 7.1 software (Biomatters Ltd, Auckland, New Zealand). They were compared with published nucleotide sequences in the GenBank database (NCBI, 2016) using the BLAST-N algorithm (Altschul et al., 1990). Nucleotide phylogenetic trees were generated using MrBayes v3.2.6 (Huelsenbeck and Ronquist, 2001) with four heated chains, a chain length of 1,000,000, sampling every 1000 iterations, and a burn in of 10%. Model selection was undertaken using ModelGenerator v0.85 (Keane et al., 2006). Models used were the general time reversible model (Tavare, 1986) and the Hasegawa Kishino Yano model (Hasegawa et al., 1985), each with gamma-distributed rate variation across sites, for Polychromophilus and haemoplasma respectively.
2.5. Statistical analyses
A range of potential internal and external predictor variables were screened for association with infection with P. melanipherus and haemoplasma, using univariable logistic regression and calculating Odds Ratios. Odds Ratios (OR) indicate the odds of an event happening versus it not happening, and are thus in this context used to assess whether the probability of infection is associated with a specific predictor variable, and are considered significant when their 95% confidence interval does not include 1. The variables examined included location (grouped as South Australian southern bent-winged bat, Victorian southern bent-winged bat and Victorian eastern bent-winged bat), body weight, sex, age (adult or juvenile), PE, PCV, RCC and absence/presence of co-infection with P. melanipherus and haemoplasma (internal factors). Season (spring, summer, autumn) was the only external factor included. Residuals were examined to confirm that model assumptions were met. All factors significant at p < 0.20 were subsequently included in a multivariable logistic regression model, using backward stepping. The final model only included those variables significant at p < 0.05; again, residuals were examined to confirm that model assumptions were met. All statistics were performed using Minitab 18 (Minitab, USA).
3. Results
In total, 274 blood smears (one smear/bat) were examined for the presence of P. melanipherus (Table 1) and polychromatophilic erythrocytes (Table 2). No other blood parasites were seen. Where sufficient blood was available, PCV and RCC were also measured (Table 2). Polychromophilus melanipherus was detected in 36.5% (n = 274) of smears (Table 1). Prevalence by location group was 57.4% for Victorian southern bent-winged bats (n = 61), 33.6% for South Australian southern bent-winged bats (n = 128) and 25.9% (n = 85) for eastern bent-winged bats. After univariable screening for P. melanipherus, sex, PE, haemoplasma, location group and season were significant at the p < 0.20 level. When these variables were placed in multivariable models, location group was the only significant predictor (p < 0.05) of P. melanipherus infection (p = 0.004). Victorian southern bent-winged bats were more likely than South Australian southern bent-winged bats (Odds Ratio = 2.0, 95% Confidence Interval = 1.1, 3.7) and eastern bent-winged bats (Odds Ratio = 3.1, 95% Confidence Interval = 1.6, 6.2) to be infected with P. melanipherus. There was no significant difference between eastern bent-winged bats and South Australian southern bent-winged bats (Odds Ratio = 0.6, 95% Confidence Interval = 0.4, 1.1).
Table 1.
Prevalence of Polychromophilus melanipherus and haemoplasmas in southern and eastern bent-winged bats by blood smear and/or PCR. All bats are adults unless otherwise indicated. n = sample size. NT = not tested due to insufficient blood volume collected. Intensity of infection = mean number of organisms observed/bat following a 10 min scan of the slide under oil immersion.
| Location | Date |
Polychromophilus melanipherus |
Haemoplasma |
||
|---|---|---|---|---|---|
| Prevalence (%) |
Intensity of infection (smear) | Prevalence (%) |
|||
| Blood smear (n) | PCR (n) | PCR (n) | |||
| Southern bent-winged bats | |||||
| Allansford | Sep 2015 | 58 (19) | NT | 2.9 ± 4.6 | NT |
| Male | 75 (8) | NT | 1.7 ± 2.4 | NT | |
| Female | 45 (11) | NT | 3.7 ± 5.6 | NT | |
| Portland 1 | Sep 2016 | 54 (26) | 90 (10) | 4.2 ± 7.0 | 21 (14) |
| Male | 50 (10) | 100 (3) | 2.9 ± 4.1 | 50 (4) | |
| Female | 56 (16) | 86 (7) | 5.1 ± 8.4 | 10 (10) | |
| Portland 2 | Feb 2017 | 63 (16) | 100 (3) | 1.5 ± 1.6 | 33 (3) |
| Male | 83 (12) | 100 (3) | 2.0 ± 1.5 | 33 (3) | |
| Female | 0 (4) | NT | 0 | NT | |
| Naracoorte | Jan 2016 | 41 (59) | 88 (8) | 1.1 ± 2.1 | 17 (6) |
| Male | 36 (28) | 100 (4) | 1.0 ± 2.4 | 0 (3) | |
| Female | 45 (31) | 75 (4) | 1.2 ± 1.8 | 33 (3) | |
| Naracoorte | Sep 2016 | 28 (69) | 42 (36) | 0.8 ± 2.1 | 3 (35) |
| Male | 35 (34) | 54 (13) | 1.2 ± 2.6 | 0 (14) | |
| Female | 20 (35) | 30 (23) | 0.5 ± 1.2 | 5 (21) | |
| Mean | 41 (189) | 60 (57) | 1.6 ± 3.6 | 10 (58) | |
| Male | 47 (92) | 74 (23) | 1.5 ± 2.6 | 7 (30) | |
| Female | 36 (97) | 47 (34) | 1.8 ± 4.3 | 14 (28) | |
| Eastern bent-winged bats | |||||
| Christmas Hills | April 2015 | 20 (10) | NT | 0.6 ± 1.6 | NT |
| Male | 25 (4) | NT | 1.3 ± 2.5 | NT | |
| Female | 17 (6) | NT | 0.2 ± 0.4 | NT | |
| Christmas Hills | Sep 2015 | 35 (17) | 67 (6) | 1.2 ± 2.7 | 25 (4) |
| Male | 33 (12) | 100 (3) | 1.3 ± 3.1 | 50 (2) | |
| Female | 40 (5) | 33 (3) | 0.8 ± 1.1 | 0 (2) | |
| Eildon | Sep 2016 | 25 (36) | 57 (14) | 0.5 ± 1.2 | 8 (13) |
| Male | 18 (11) | 75 (4) | 0.3 ± 0.6 | 25 (4) | |
| Female | 28 (25) | 50 (10) | 0.6 ± 1.3 | 0 (9) | |
| Lakes Entrance | March 2017 | 23 (22)* | 13 (8)# | 0.5 ± 1.0 | 0 (8)# |
| Male | 0 (6) | 0 (1) | 0 | 0 (3) | |
| Female | 45 (16) | 14 (7) | 0.6 ± 1.1 | 0 (5) | |
| Mean | 26 (85) | 46 (28) | 0.6 ± 1.6 | 8 (25) | |
| Male | 21 (33) | 75 (8) | 0.7 ± 2.1 | 22 (9) | |
| Female | 29 (52) | 35 (20) | 0.6 ± 1.2 | 0 (16) | |
* Six of these bats were juveniles. # Two of these bats were juveniles. All were negative.
Table 2.
Haematological parameters of southern and eastern bent-wing bats from southern Australia. n = sample size. NT = not tested due to insufficient blood volume collected. PE = polychromatophilic erythrocytes. No/OIF = mean number of PE observed/oil immersion field. PCV = packed cell volume. RCC = red cell count.
| Location | Date | PE No/OIF (n) |
PCV % (n) |
RCC x 1012/L (n) |
|---|---|---|---|---|
| Southern bent-winged bats | ||||
| Allansford | Sep 2015 | 21.0 ± 14.6 (19) | NT | NT |
| Portland 1 | Sep 2016 | 13.4 ± 4.4 (26) | 50.8 ± 3.5 (14) | 12.9 ± 1.0 (14) |
| Portland 2 | Feb 2017 | 11.9 ± 3.2 (16) | 43.8 ± 2.5 (8) | 11.7 ± 1.5 (8) |
| Naracoorte | Jan 2016 | 11.0 ± 2.7 (59) | NT | NT |
| Naracoorte | Sep 2016 | 13.2 ± 4.4 (69) | 50.8 ± 6.0 (57) | 12.9 ± 1.6 (57) |
| Mean Total | 13.2 ± 6.4 (189) | 50.2 ± 5.9 (79) | 12.8 ± 1.5 (79) | |
| Eastern bent-winged bats | ||||
| Christmas Hills | April 2015 | 9.6 ± 2.7 (10) | NT | NT |
| Christmas Hills | Sep 2015 | 7.8 ± 3.5 (17) | 50.8 ± 6.6 (4) | NT |
| Eildon | Sep 2016 | 11.2 ± 6.0 (36) | 55.4 ± 5.4 (11) | 13.8 ± 1.8 (11) |
| Lakes Entrance | March 2017 | 11.5 ± 3.6 (22) | NT | NT |
| Mean Total | 10.4 ± 4.8 (85) | 53.8 ± 6.1 (15) | ||
For 85 individuals, it was possible to collect sufficient blood to examine smears and conduct PCR for P. melanipherus. PCR detected P. melanipherus in 55% of these samples, whereas the organism was observed in only 38% of smears from the same bats. Six bats were positive on blood smear examination but negative by PCR, while 21 bats were positive by PCR but negative on blood smear examination.
Organs tested from the seven dead bats were all negative for P. melanipherus by PCR. No evidence of P. melanipherus infection was found in any of the organs examined histologically.
After completing the Polychromophilus PCR, sufficient blood remained to run the haemoplasma PCR on a total of 83 samples (Table 1). Of these, 9.6% (8/83) were positive for haemoplasma DNA. Prevalence by location group was 23.5% (4/17) for Victorian southern bent-winged bats, 4.9% (2/41) for South Australian southern bent-winged bats and 8.0% (2/25) for eastern bent-winged bats (Table 1). After univariable screening for haemoplasma, sex, P. melanipherus and location group were significant at the p < 0.20 level. After placing these variables into a multivariable model, however, there were no significant (p < 0.05) predictors of haemoplasma infection. Nonetheless, the Odds Ratio for haemoplasma infection in Victorian southern bent-winged bats compared to South Australian southern bent-winged bats was 6.0, with a 95% confidence interval of 0.98–36.6, strongly suggestive of a trend for the Victorian location group of this subspecies to be more likely to be infected than the South Australian one.
Of the 32 P. melanipherus and 18 haemoplasma DNA samples submitted for sequencing results suitable for further analysis were obtained from seven P. melanipherus (five southern bent-winged bats: D23 (GenBank Accession Number MK088162), D26 (GenBank Accession Number MK088163), D33 (GenBank Accession Number MK088166), D39 (GenBank Accession Number MK088165), and N42 (GenBank Accession Number MK088164) and two eastern bent-winged bats: E4 (GenBank Accession Number MK088168) and E19 (GenBank Accession NumberMK088167)) samples and one haemoplasma (a Victorian southern bent-winged bat: D19 (GenBank Accession Number MK088169)) PCR positive sample. Polychromophilus melanipherus and haemoplasma sequences were compared with sequences in GenBank to determine the published sequence with the highest nucleotide identity. The maximum percentage predicted nucleotide identity to other known Polychromophilus spp. was found to range from 95% (JN990709.1 and JN990711.1 for bat D33) to 99% (JN990711.1 for bat E4). Sequences from bats D33, D39, E4, E19 and N42 also showed a high percentage identity to two P. murinus haplotypes (JN990712.1 and JN990713.1), ranging from 94% for bat D33 up to 97% for bat E4.
A phylogenetic tree demonstrating the relationship of the P. melanipherus sequences (D23, D26, E4 and N42) with closely related P. melanipherus haplotypes and P. murinus sequences from bats, along with representatives from other haemosporidian groups, is presented in Fig. 2. Sequences D33, D39 and E19 were too short to include. They overlapped the other sequences by about 100 bases in a region with little variation. Since they were near identical to the longer reads they were removed from the phylogenetic analysis. Extended attempts to sequence the DNA failed due to low concentrations of insufficient quality to include in the phylogenetic tree.
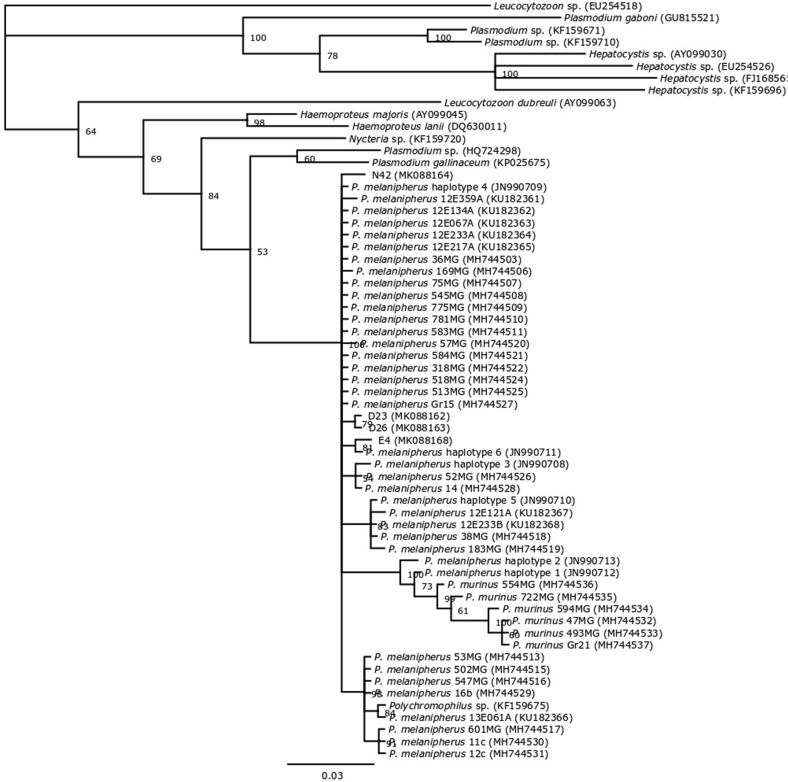
Fig. 2.
Phylogenetic tree demonstrating the relationship between the mitochondrial cytochrome b gene of seven Polychromophilus melanipherus isolates detected using PCR in Victorian southern bent-winged bats (D23, D26, D33 and D39), South Australian southern bent-winged bats (N42) and eastern bent-winged bats (E4 and E19) and a selection of other haematozoa. The tree was generated using MrBayes v3.2.6 (Huelsenbeck and Ronquist, 2001) with four heated chains, a chain length of 1,000,000, sampling every 1000 iterations, and a burn in of 10%. The model used was the general time reversible model (Tavare, 1986) with gamma-distributed rate variation across sites. Numbers represent branch support values as posterior probability. GenBank accession numbers are located to the right of each organism.
The D19 haemoplasma sequence shared a 98% identity with a range of uncultured Mycoplasma species (KM538691.1, KM538692.1, KM538696.1, KM538697.1 and KM538698.1), all previously recorded from Schreibers' bats (M. schreibersii) in Spain (Millan et al., 2015). D19 also shared a 96% identity with a sequence found in a Spanish long-fingered bat (Myotis capaccinii) (KM538693.1) (Millan et al., 2015), 92% identity with a sequence identified in little brown bats (Myotis lucifugus) (KF713538.1) from the USA (Mascarelli et al., 2014) and 89% identity to sequences found in Pallas's mastiff bats (Molossus molossus) (KY356751.1) from Brazil (Ikeda et al., 2017).
A phylogenetic tree demonstrating the relationship of the haemoplasma sequence type (D19) with closely related bat haemoplasmas and a selection of haemoplasmas found in other species is presented in Fig. 3.
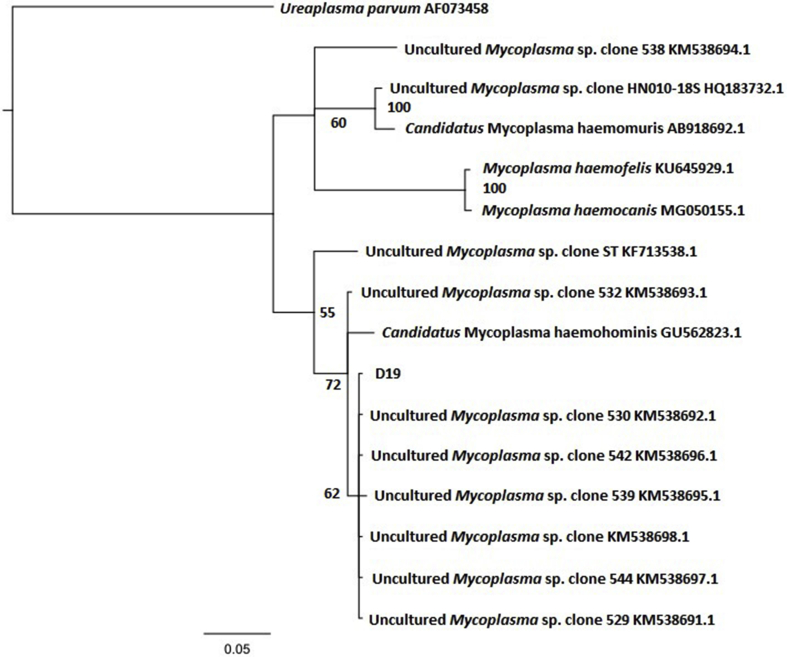
Fig. 3.
Phylogenetic tree demonstrating the relationship between the 16S rRNA gene of a haemoplasma isolate detected using PCR in a Victorian southern bent-winged bat (D19) and a selection of other haemoplasmas. The tree was generated using MrBayes v3.2.6 (Huelsenbeck and Ronquist, 2001) with four heated chains, a chain length of 1,000,000, sampling every 1000 iterations, and a burn in of 10%. The model used was the Hasegawa Kishino Yano model (Hasegawa et al., 1985) with gamma-distributed rate variation across sites. Numbers represent branch support values as posterior probability. GenBank accession numbers are located to the right of each organism.
4. Discussion
This study measured the prevalence of two haemoparasites in two subspecies of bent-winged bats from south-eastern Australia. Polychromophilus melanipherus was found in over a third of all samples, with a higher prevalence in Victorian southern bent-winged bats than the other two groups. This parasite had previously been identified in bent-winged bats from New South Wales (Dew and McMillan, 1970), with a prevalence ranging from 0 to 83%. In contrast to our study where there was no association with time of year, Dew and McMillan (1970) found a lower prevalence in April and higher in August. A study from Gabon also failed to detect any statistically significant difference in prevalence, the parasite being detected equally during both the wet and dry seasons (Duval et al., 2012).
Microscopic examination of blood smears was compared with molecular methods for the detection of P. melanipherus. Prevalence rates ranged from 38% for smears compared with 55% for PCR. This is comparable to other studies which detected prevalence rates of 18–67% on smears and 63–89% by PCR (Duval et al., 2012). Prevalence estimates of blood parasites are typically greater using PCR than blood smear examination (Duval et al., 2012; Garamszegi, 2010; Ndao et al., 2004; Teal et al., 2012; Wangai et al., 2011). In the current study, 21 bats tested negative by smear examination but were PCR positive, indicating the greater likelihood of false negatives occurring when blood smears alone are used. The accuracy of blood smear examination is dependent on the skill of the microscopist, the number of optical fields examined and the intensity of the parasitaemia (Garamszegi, 2010; Valkiunas et al., 2008). Unfortunately, identification by morphological characteristics seen on blood smears cannot be used to reliably differentiate haemoparasite species due to differences seen both within and between species (Garnham, 1966, 1973b; Megali et al., 2011). The current study also demonstrated that, while PCR was better able to detect P. melanipherus positive bats, it may not be perfect with six bats testing negative by PCR despite organisms that were morphologically consistent with P. melanipherus being observed on blood smear. This could have been the result of using a poor quality blood sample, as it was difficult to obtain sufficient blood to run the PCR in a number of instances. It is also possible that the organisms seen on the blood smears were not P. melanipherus, as morphology is not a reliable means of species identification (Megali et al., 2011).
The P. melanipherus DNA sequenced in this study showed close association with previously sequenced P. melanipherus from Schreibers' bent-winged bats from Switzerland (Witsenburg et al., 2012), bent-winged bats (Miniopterus spp.) from Madagascar ((Ramasindrazana et al., 2018) greater long-fingered bats from Gabon (Duval et al., 2012) and Villier's long-fingered bats (M. villiersi) from Guinea (Schaer et al., 2013) (Fig. 2). As demonstrated in this and other studies, cytochrome b molecular diversity is high in Polychromophilus spp. compared with other related parasites, such as Plasmodium (Duval et al., 2012). Current research indicates that Polychromophilus likely had a bird or reptile infecting Plasmodium ancestor. At some point in its evolutionary past it switched to a mammalian host (bats) and also switched vectors from Culicidae (mosquitoes) to Nycteribiidae (bat flies) (Witsenburg et al., 2012). This is speculated to have occurred before the Miniopteridae and Vespertilionidae family diversifications (Duval et al., 2012). Once these two families arose, the parasites continued to undergo independent evolution, which gave rise to P. melanipherus in the Miniopteridae and P. murinus in the Vespertilionidae. Previous work reported a clear genetic distinction between P. melanipherus and P. murinus (Witsenburg et al., 2012). This distinction is supported by the sequences identified in the current study.
Polychromophilus melanipherus schizogony occurs in the liver, lung and spleen (Garnham, 1973b), which were examined histologically and tested by PCR for seven dead bats. No evidence of infection was found but, as no blood was collected from these bats, it was not possible to verify that these were true negatives.
To date, the significance of the parasite on bat health is unknown. In a European study, intensity of infection in Daubenton's bats (Myotis daubentonii), with P. murinus, was inversely correlated with body weight (Witsenburg et al., 2014). However, unlike some other haematozoan infections, there was no indication of anaemia. This was speculated to be because Polychromophilus, unlike Plasmodium, does not undergo schizogony in the blood. Therefore, infection should lead to the destruction of fewer erythrocytes (Witsenburg et al., 2014). In the present study P. melanipherus infections did not correlate with lower body weights. There was also no significant association with infection and numbers of polychromatophilic erythrocytes, red cell count or packed cell volume, all possible indicators of anaemia. Polychromatophilic erythrocyte numbers were high compared with other mammals (ranging from zero in cattle, sheep, goats and horses up to two cells per oil immersion field in dogs and pigs (Harvey, 2012)), which can be indicative of a regenerative anaemia (Clark, 2004), but bats normally have high counts (10 cells per oil immersion field for eastern bent-winged bats and 13 cells per oil immersion field for southern bent-winged bats) due to the short longevity of their erythrocytes (Riedesel, 1977; Schinnerl et al., 2011; Valdivieso and Tamsitt, 1971).
Victorian southern bent-winged bats had a greater prevalence of P. melanipherus infections than South Australian southern bent-winged bats or eastern bent-winged bats. As parasite burdens tend to be higher in individuals with poorer immune responses (Christe et al., 2000, 2007; Lourenço and Palmeirim, 2008), this could be an indication of some type of immune compromise in the Victorian southern bent-winged bat population. The source of this is open to speculation but could be related to the fact that southern bent-winged bats occupy a more disturbed environment than eastern bent-winged bats. The Victorian southern bent-winged bat environment contains less than 18% of the pre-European vegetation (Glenelg Hopkins Catchment Management Authority, 2013), while less than 6% of the original South Australian bent-winged bat habitat remains (South East Natural Resources Management Board, 2010). This is in comparison with the eastern bent-winged bat habitat which remains 45–62% intact (Victorian Environmental Assessment Council, 2010).
Other factors that may suppress the immune system include exposure to organochlorine pesticides (Faroon et al., 2002). Victorian southern bent-winged bats have been found to have greater levels of pesticides compared with South Australian southern bent-winged bats (Mispagel et al., 2004).
There are three published bat haemoplasma surveys, which found a variable prevalence of haemoplasmas in bats: 4–14% across a range of bat species tested in Brazil (Ikeda et al., 2017), 47% of little brown bats from the USA (Mascarelli et al., 2014), and 42% of Schreibers’ bats from Spain (Millan et al., 2015). No clinical signs were attributed to any of the infections. Infection rates in this study ranged from 0 to 33% and were also not associated with any clinical effects.
It is not surprising that the single sequenced haemoplasma from this study, detected in a southern bent-winged bat, showed the closest relationship (98% identity) with previously identified haemoplasmas found in Schreibers’ bent-winged bats, as the two bat species are closely related and, until recently, considered to be the same species (Cardinal and Christidis, 2000). The sequence also showed a 96% identity with a human haemoplasma isolate (GU562823.1) (Fig. 3) that was a cause of haemolytic anaemia and pyrexia (Steer et al., 2011). While the patient was diagnosed in Britain, she had recently returned from a vacation in Australia, and the authors speculated that the infection may have been zoonotic. The species specificity of haemoplasmas is not clear with several veterinary haemoplasmas, such as Mycoplasma suis (Yuan et al., 2009), M. haemofelis (dos Santos et al., 2008) and M. ovis (Sykes et al., 2010) having also been recorded from humans. Blood sucking arthropods, such as mosquitoes and midges, have been implicated in haemoplasma transmission (Messick, 2004) and could act as vectors between species.
5. Conclusions
Based on the results from the present study, P. melanipherus and haemoplasma infections do not appear to be having a deleterious effect on their hosts. There was no obvious association with low body weight or anaemia. PCR detected more P. melanipherus infected bats than blood smear examination and this is the recommended method for any future prevalence studies. More Victorian southern bent-winged bats appear to be infected with P. melanipherus, which is possibly indicative of some sort of underlying stress. Future research should focus on obtaining a broader sample size for haemoplasma testing and the investigation of a wider range of blood parasites, such as Bartonella (Ikeda et al., 2017) and trypanosomes (Mackie et al., 2017) to determine what effect, if any, they may be having on population health.
Permits
Samples were collected with approval from the Faculty of Veterinary and Agricultural Science Animal Ethics Committee, University of Melbourne, Victoria (ethics approval 1513456.1), Department of Environment, Land, Water and Planning, Victoria (permit number 0007644), Wildlife Ethics Committee, South Australia (permit number 37/2015) and the Department of Environment, Water and Natural Resources, South Australia (permit number Q26488-1).
Conflicts of interest
The authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to thank the Holsworth Wildlife Research Endowment (HOLSW2016-R1-F109), Wildlife Disease Association Australasia, Australian Government’s Threatened Species Discretionary Grants Program, Department of the Environment, Land, Water and Planning Victoria, Karst Conservation Fund and David Middleton for providing generous financial support for this project. The lead author is supported by an Australian Postgraduate Award scholarship. The authors acknowledge the valuable assistance provided by Amanda Bush, Phillip Clark, Abdul Jabbar, David McLelland, and the numerous volunteers involved with the bat trapping and sampling trips.
References
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Atkinson C.T. Avian malaria. In: Atkinson C.T., Thomas N.J., Hunter D.B., editors. Parasitic Diseases of Wild Birds. Wiley-Blackwell; Ames: 2008. pp. 35–53. [Google Scholar]
- Briggs C., Bain B.J. Basic haematological techniques. In: Bain B.J., Bates I., Laffan M.A., editors. Dacie and Lewis Practical Haematology. twelfth ed. Elsevier; London: 2016. pp. 23–56. [Google Scholar]
- Brunet-Rossinni A.K., Wilkinson G.S. Methods for age estimation and the study of senescence in bats. In: Kunz T.H., Parsons S., editors. Ecological and Behavioral Methods for the Study of Bats. Johns Hopkins University Press; Baltimore: 2009. pp. 315–325. [Google Scholar]
- Cabello J., Altet L., Napolitano C., Sastre N., Hidalgo E., Davila J.A., Millan J. Survey of infectious agents in the endangered Darwin's fox (Lycalopex fulvipes): high prevalence and diversity of hemotrophic mycoplasmas. Vet. Microbiol. 2013;167:448–454. doi: 10.1016/j.vetmic.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Cardinal B.R., Christidis L. Mitochondrial DNA and morphology reveal three geographically distinct lineages of the large bentwing bat (Miniopterus schreibersii) in Australia. Aust. J. Zool. 2000;48:1–19. [Google Scholar]
- Christe P., Arlettaz R., Vogel P. Variation in intensity of a parasitic mite (Spinturnix myoti) in relation to the reproductive cycle and immunocompetence of its bat host (Myotis myotis) Ecol. Lett. 2000;3:207–212. [Google Scholar]
- Christe P., Glaizot O., Evanno G., Bruyndonckx N., Devevey G., Yannic G., Patthey P., Maeder A., Vogel P., Arlettaz R. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J. Anim. Ecol. 2007;76:703–710. doi: 10.1111/j.1365-2656.2007.01255.x. [DOI] [PubMed] [Google Scholar]
- Civitello D.J., Cohen J., Fatima H., Halstead N.T., Liriano J., McMahon T.A., Ortega C.N., Sauer E.L., Sehgal T., Young S., Rohr J.R. Biodiversity inhibits parasites: broad evidence for the dilution effect. Proc. Natl. Acad. Sci. U.S.A. 2015;112:8667–8671. doi: 10.1073/pnas.1506279112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P. CSIRO Publishing; Collingwood: 2004. The Leukocytes, Haematology of Australian Mammals; pp. 47–76. [Google Scholar]
- Compton S.M., Maggi R.G., Breitschwerdt E.B. Candidatus Mycoplasma haematoparvum and Mycoplasma haemocanis infections in dogs from the United States. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:557–562. doi: 10.1016/j.cimid.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Corduneanu A., Hrazdilova K., Sandor A.D., Matei I.A., Ionica A.M., Barti L., Ciocanau M.A., Mantoiu D.S., Coroiu I., Hornok S., Fuehrer H.P., Leitner N., Bago Z., Stefke K., Modry D., Mihalca A.D. Babesia vesperuginis, a neglected piroplasmid: new host and geographical records, and phylogenetic relations. Parasites Vectors. 2017;10:598. doi: 10.1186/s13071-017-2536-3. 510.1186/s13071-13017-12536-13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELWP . Department of Environment; Land, Water and Planning, Melbourne: 2017. National Recovery Plan for the Southern Bent-wing Bat Miniopterus Schreibersii Bassanii Draft. [Google Scholar]
- Dew B.B., McMillan B. Seasonal variation of Polychromophilus melanipherus (Sporozoa:Haemoproteidae) in the bent-winged bat Miniopterus schreibersii (chiroptera) in New South Wales. Parasitology. 1970;61:161–166. [Google Scholar]
- dos Santos A.P., dos Santos R.P., Biondo A.W., Dora J.M., Goldani L.Z., de Oliveira S.T., de Sa Guimaraes A.M., Timenetsky J., de Morais H.A., Gonzalez F.H., Messick J.B. Hemoplasma infection in HIV-positive patient, Brazil. Emerg. Infect. Dis. 2008;14:1922–1924. doi: 10.3201/eid1412.080964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval L., Mejean C., Maganga G.D., Makanga B.K., Mangama Koumba L.B., Peirce M.A., Ariey F., Bourgarel M. The chiropteran haemosporidian Polychromophilus melanipherus: a worldwide species complex restricted to the family Miniopteridae. Infect. Genet. Evol. 2012;12:1558–1566. doi: 10.1016/j.meegid.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Emmenegger T., Bauer S., Hahn S., Müller S.B., Spina F., Jenni L. Blood parasites prevalence of migrating passerines increases over the spring passage period. J. Zool. 2018 [Google Scholar]
- Faroon O., Harris M.O., Llados F., Swarts S., Sage G., Citra M., Gefell D. Toxicological profile for DDT, DDE and DDD. In: Services U.D.o.H.a.H., editor. Agency for Toxic Substances and Disease Registry; Atlanta: 2002. p. 497. [Google Scholar]
- Garamszegi L.Z. The sensitivity of microscopy and PCR-based detection methods affecting estimates of prevalence of blood parasites in birds. J. Parasitol. 2010;96:1197–1203. doi: 10.1645/GE-2531.1. [DOI] [PubMed] [Google Scholar]
- Gardner R.A., Molyneux D.H. Polychromophilus murinus: a malarial parasite of bats: life-history and ultrastructural studies. Parasitology. 1988;96(Pt 3):591–605. doi: 10.1017/s0031182000080215. [DOI] [PubMed] [Google Scholar]
- Garnham P.C. Polychromophilus species in insectivorous bats. Trans. R. Soc. Trop. Med. Hyg. 1973;67:2–3. doi: 10.1016/0035-9203(73)90253-8. [DOI] [PubMed] [Google Scholar]
- Garnham P.C. The zoogeography of Polychromophilus and description of a new species of a gregarine (Lankesteria galliardi) Ann. Parasitol. Hum. Comp. 1973;48:231–242. doi: 10.1051/parasite/1973482231. [DOI] [PubMed] [Google Scholar]
- Garnham P.C., Lainson R., Shaw J.J. A contribution to the study of the haematozoon parasites of bats. A new mammalian haemopCroteid, Polychromophilus deanei n. sp. Mem. Inst. Oswaldo Cruz. 1971;69:119–127. doi: 10.1590/s0074-02761971000100009. [DOI] [PubMed] [Google Scholar]
- Garnham P.C.C. Blackwell Scientific Publications; Oxford: 1966. Polychromophilus Melanipherus and Polychromophilus Murinus, Malaria Parasites and Other Haemosporidia; pp. 916–926. [Google Scholar]
- Glenelg Hopkins Catchment Management Authority . 2013. Glenelg Hopkins Regional Catchment Strategy 2013-2019, Hamilton; p. 84. [Google Scholar]
- Harasawa R., Orusa R., Giangaspero M. Molecular evidence for hemotropic Mycoplasma infection in a Japanese badger (Meles meles anakuma) and a raccoon dog (Nyctereutes procyonoides viverrinus) J. Wildl. Dis. 2014;50:412–415. doi: 10.7589/2013-09-229. [DOI] [PubMed] [Google Scholar]
- Harvey J.W. Saunders; St. Louis: 2012. Evaluation of Erythrocytes, Veterinary Hematology: a Diagnostic Guide and Color Atlas; pp. 49–121. [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J. Mol. Evol. 1985;22:160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Holz P.H., Lumsden L.F., Druce J., Legione A.R., Vaz P., Devlin J.M., Hufschmid J. Virus survey in populations of two subspecies of bent-winged bats (Miniopterus orianae bassanii and oceanensis) in south-eastern Australia reveals a high prevalence of diverse herpesviruses. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquist F. MRBAYES: bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Ikeda P., Seki M.C., Carrasco A.O.T., Rudiak L.V., Miranda J.M.D., Goncalves S.M.M., Hoppe E.G.L., Albuquerque A.C.A., Teixeira M.M.G., Passos C.E., Werther K., Machado R.Z., Andre M.R. Evidence and molecular characterization of Bartonella spp. and hemoplasmas in neotropical bats in Brazil. Epidemiol. Infect. 2017;145:2038–2052. doi: 10.1017/S0950268817000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarred J., Lewbart G.A., Stover K., Thomas B., Maggi R., Breitschwerdt E.B. Identification of hemotropic mycoplasmas in an eastern box turtle (Terrapene carolina carolina) and a yellow-bellied slider (Trachemys scripta scripta) from North Carolina, USA. J. Wildl. Dis. 2018;54:371–374. doi: 10.7589/2017-07-153. [DOI] [PubMed] [Google Scholar]
- Keane T.M., Creevey C.J., Pentony M.M., Naughton T.J., McLnerney J.O. Assessment of methods for amino acid matrix selection and their use on empirical data shows that ad hoc assumptions for choice of matrix are not justified. BMC Evol. Biol. 2006;6 doi: 10.1186/1471-2148-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço S., Palmeirim J.M. Which factors regulate the reproduction of ectoparasites of temperate-zone cave-dwelling bats? Parasitol. Res. 2008;104:127–134. doi: 10.1007/s00436-008-1170-6. [DOI] [PubMed] [Google Scholar]
- Mackerras M.J. The haematozoa of Australian mammals. Aust. J. Zool. 1959;7:105–135. [Google Scholar]
- Mackie J.T., Stenner R., Gillett A.K., Barbosa A., Ryan U., Irwin P.J. Trypanosomiasis in an Australian little red flying fox (Pteropus scapulatus) Aust. Vet. J. 2017;95:259–261. doi: 10.1111/avj.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi R.G., Chitwood M.C., Kennedy-Stoskopf S., DePerno C.S. Novel hemotropic Mycoplasma species in white-tailed deer (Odocoileus virginianus) Comp. Immunol. Microbiol. Infect. Dis. 2013;36:607–611. doi: 10.1016/j.cimid.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Mascarelli P.E., Keel M.K., Yabsley M., Last L.A., Breitschwerdt E.B., Maggi R.G. Hemotropic mycoplasmas in little brown bats (Myotis lucifugus) Parasites Vectors. 2014;7 doi: 10.1186/1756-3305-7-117. 117, 110.1186/1756-3305-1187-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum H. Disease and the dynamics of extinction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367:2828–2839. doi: 10.1098/rstb.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLelland D.J., Reardon T., Bourne S., Dickason C., Kessell A., Boardman W. Outbreak of skin nodules associated with Riouxgolvania beveridgei (Nematoda: muspiceida) in the southern bentwing bat (Miniopterus schreibersii bassanii), South Australia. J. Wildl. Dis. 2013;49:1009–1013. doi: 10.7589/2012-11-288. [DOI] [PubMed] [Google Scholar]
- McLeod R., Kristjanson P. International Livestock Research Institute; Nairobi: 1999. Economic Impact of Ticks and Tick-borne Diseases to Livestock in Africa, Asia and Australia; p. 99. [Google Scholar]
- Megali A., Yannic G., Christe P. Disease in the dark: molecular characterization of Polychromophilus murinus in temperate zone bats revealed a worldwide distribution of this malaria-like disease. Mol. Ecol. 2011;20:1039–1048. doi: 10.1111/j.1365-294X.2010.04905.x. [DOI] [PubMed] [Google Scholar]
- Messick J.B. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet. Clin. Pathol. 2004;33:2–13. doi: 10.1111/j.1939-165x.2004.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Messick J.B., Harvey J.W. Hemotropic mycoplasmosis (Hemobartonellosis) In: Greene C.E., editor. Infectious Diseases of the Dog and Cat. fourth ed. Elsevier Saunders; St. Louis, Missouri: 2012. pp. 310–319. [Google Scholar]
- Millan J., Lopez-Roig M., Delicado V., Serra-Cobo J., Esperon F. Widespread infection with hemotropic mycoplasmas in bats in Spain, including a hemoplasma closely related to "Candidatus Mycoplasma hemohominis. Comp. Immunol. Microbiol. Infect. Dis. 2015;39:9–12. doi: 10.1016/j.cimid.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Mispagel C., Allinson M., Allinson G., Iseki N., Grant C., Morita M. DDT and metabolites residues in the southern bent-wing bat (Miniopterus schreibersii bassanii) of south-eastern Australia. Chemosphere. 2004;55:997–1003. doi: 10.1016/j.chemosphere.2003.12.008. [DOI] [PubMed] [Google Scholar]
- NCBI Database resources of the national center for biotechnology information. Nucleic Acids Res. 2016;44:D7–D19. doi: 10.1093/nar/gkv1290. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndao M., Bandyayera E., Kokoskin E., Gyorkos T.W., MacLean J.D., Ward B.J. Comparison of blood smear, antigen detection, and nested-PCR methods for screening refugees from regions where malaria is endemic after a malaria outbreak in Quebec, Canada. J. Clin. Microbiol. 2004;42:2694–2700. doi: 10.1128/JCM.42.6.2694-2700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obame-Nkoghe J., Rahola N., Bourgarel M., Yangari P., Prugnolle F., Maganga G.D., Leroy E.M., Fontenille D., Ayala D., Paupy C. Bat flies (Diptera: Nycteribiidae and Streblidae) infesting cave-dwelling bats in Gabon: diversity, dynamics and potential role in Polychromophilus melanipherus transmission. Parasites Vectors. 2016;9:333. doi: 10.1186/s13071-016-1625-z. 310.1186/s13071-13016-11625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A., Woods R. Bats. In: Vogelnest L., Woods R., editors. Medicine of Australian Mammals. CSIRO Publishing; Collingwood, Australia: 2008. pp. 465–502. [Google Scholar]
- Ramasindrazana B., Goodman S.M., Dsouli N., Gomard Y., Lagadec E., Randrianarivelojosia M., Dellagi K., Tortosa P. Polychromophilus spp. (Haemosporida) in Malagasy bats: host specificity and insights on invertebrate vectors. Malar. J. 2018;17:318. doi: 10.1186/s12936-018-2461-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G.C., Reardon T.B. Family Miniopteridae. In: Van Dyck S., Strahan R., editors. The Mammals of Australia. third ed. Reed New Holland; Sydney: 2008. pp. 503–510. [Google Scholar]
- Riedesel M.L. Blood physiology. In: Wimsatt W., editor. Biology of Bats. Academic Press; New York: 1977. pp. 485–517. [Google Scholar]
- Schaer J., Perkins S.L., Decher J., Leendertz F.H., Fahr J., Weber N., Matuschewski K. High diversity of West African bat malaria parasites and a tight link with rodent Plasmodium taxa. Proc. Natl. Acad. Sci. U.S.A. 2013;110:17415–17419. doi: 10.1073/pnas.1311016110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinnerl M., Aydinonat D., Schwarzenberger F., Voigt C.C. Hematological survey of common neotropical bat species from Costa Rica. J. Zoo Wildl. Med. 2011;42:382–391. doi: 10.1638/2010-0060.1. [DOI] [PubMed] [Google Scholar]
- South East Natural Resources Management Plan. Mt. Gambier; 2010. South East Natural Resources Management Board; p. 158. [Google Scholar]
- Steer J.A., Tasker S., Barker E.N., Jensen J., Mitchell J., Stocki T., Chalker V.J., Hamon M. A novel hemotropic Mycoplasma (hemoplasma) in a patient with hemolytic anemia and pyrexia. Clin. Infect. Dis. 2011;53:e147–151. doi: 10.1093/cid/cir666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J.E., Lindsay L.L., Maggi R.G., Breitschwerdt E.B. Human coinfection with Bartonella henselae and two hemotropic mycoplasma variants resembling Mycoplasma ovis. J. Clin. Microbiol. 2010;48:3782–3785. doi: 10.1128/JCM.01029-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavare S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986;17:57–86. [Google Scholar]
- Teal A.E., Habura A., Ennis J., Keithly J.S., Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J. Clin. Microbiol. 2012;50:903–908. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidemann C.R., Woodside D.P. A collapsible bat-trap and a comparison of results obtained with the trap and with mist-nets. Aust. Wildl. Res. 1978;5:355–362. [Google Scholar]
- Valdivieso D., Tamsitt J.R. Hematological data from tropical American bats. Can. J. Zool. 1971;49:31–36. doi: 10.1139/z71-007. [DOI] [PubMed] [Google Scholar]
- Valkiunas G., Iezhova T.A., Krizanauskiene A., Palinauskas V., Sehgal R.N., Bensch S. A comparative analysis of microscopy and PCR-based detection methods for blood parasites. J. Parasitol. 2008;94:1395–1401. doi: 10.1645/GE-1570.1. [DOI] [PubMed] [Google Scholar]
- Victorian Environmental Assessment Council . 2010. Remnant Native Vegetation Investigation Discussion Paper, East Melbourne; pp. 57–117. [Google Scholar]
- Wangai L.N., Karau M.G., Njiruh P.N., Sabah O., Kimani F.T., Magoma G., Kiambo N. Sensitivity of microscopy compared to molecular diagnosis of P. falciparum: implications on malaria treatment in epidemic areas in Kenya. Afr. J. Infect Dis. 2011;5:1–6. doi: 10.4314/ajid.v5i1.66504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting H.C., Schipper A.M., Bakkenes M., Meijer J.R., Huijbregts M.A. Quantifying biodiversity losses due to human consumption: a global-scale footprint analysis. Environ. Sci. Technol. 2017;51:3298–3306. doi: 10.1021/acs.est.6b05296. [DOI] [PubMed] [Google Scholar]
- Witsenburg F., Salamin N., Christe P. The evolutionary host switches of Polychromophilus: a multi-gene phylogeny of the bat malaria genus suggests a second invasion of mammals by a haemosporidian parasite. Malar. J. 2012;11(53) doi: 10.1186/1475-2875-11-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsenburg F., Schneider F., Christe P. Epidemiological traits of the malaria-like parasite Polychromophilus murinus in the Daubenton's bat Myotis daubentonii. Parasites Vectors. 2014;7:566. doi: 10.1186/s13071-014-0566-7. 510.1186/s13071-13014-10566-13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobeser G. Australian Registry of Wildlife Health; Sydney: 2008. An Overview of Health and Disease, Diagnostic Pathology of the Diseases of Aquatic, Aerial and Terrestrial Wildlife; pp. 6–15. [Google Scholar]
- Woolhouse M.E., Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . 2017. World Malaria Report, Luxembourg; p. 160. [Google Scholar]
- Wyatt K.B., Campos P.F., Gilbert M.T., Kolokotronis S.O., Hynes W.H., DeSalle R., Ball S.J., Daszak P., MacPhee R.D., Greenwood A.D. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003602. e3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C.L., Liang A.B., Yao C.B., Yang Z.B., Zhu J.G., Cui L., Yu F., Zhu N.Y., Yang X.W., Hua X.G. Prevalence of Mycoplasma suis (Eperythrozoon suis) infection in swine and swine-farm workers in Shanghai, China. Am. J. Vet. Res. 2009;70:890–894. doi: 10.2460/ajvr.70.7.890. [DOI] [PubMed] [Google Scholar]